Abstract
Straight-chain aliphatic carboxylic acids are valuable biomolecules with wide applications in various industries. Microbial biosynthesis offers an effective and environmentally friendly approach to the production of bioproducts from renewable feedstock materials. This book chapter reviews the current research progress of microbial biosynthesis of straight-chain aliphatic carboxylic acids. The biosynthesis pathways for exemplary products and associated engineering efforts for production improvement are highlighted to demonstrate the research status in this promising and developing field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microbial biosynthesis
- Straight-chain aliphatic carboxylic acids
- Metabolic engineering
- Renewable feedstocks
- Native producers
- Heterologous hosts
1 Overview
Straight-chain aliphatic carboxylic acids are a group of important molecules with various chemical properties and a wide range of industrial values. Traditionally, many of these molecules have large global markets and are produced mainly from the petroleum industry. However, large-scale production of these products often requires high reaction temperature and harsh conditions, consumption of nonrenewable crude oil, and use of reactant/catalytic materials that are not environmentally friendly. In recent years, microbial biosynthesis has been developed as a robust alternative technology for the production of a variety of bioproducts. The biosynthesis processes recruit microbes to convert renewable feedstocks to desired products through enzymatic reactions, which can be conducted under mild conditions without using any harsh chemicals. As such, microbial biosynthesis offers great advantages in process costs, sustainability, and environmental friendliness.
To date, a variety of microbes, either natural producers or metabolically engineered heterologous hosts, have been widely used for biosynthesis of straight-chain aliphatic carboxylic acids from renewable feedstocks; these include popularly used bacteria (e.g., Escherichia coli, Corynebacterium glutamicum), yeast (e.g., Saccharomyces cerevisiae, Yarrowia lipolytica), and microalgae (e.g., Phaeodactylum tricornutum). Assisted by the advances of related research disciplines, such as synthetic biology, metabolic engineering, and directed evolution, there is a boom of studies on microbial biosynthesis of straight-chain aliphatic carboxylic acids in recent years. Although the bioproduction performance for many of these molecules is still not yet ready for meeting the requirements of industrial production, the accumulated knowledge, techniques, and experience pave the way for its practical applications in the future.
This chapter provides a summary of the research status of microbial biosynthesis of straight-chain aliphatic carboxylic acids. In particular, we review representative groups of acids with high research and industrial values. The biosynthesis pathway development, metabolic engineering strategies adopted for biosynthesis improvement, and recent achievements of large-scale bioproduction are discussed. The challenges and opportunities for future development are also discussed.
2 Biosynthesis of Straight-Chain Aliphatic Carboxylic Acids
2.1 C1 Formic Acid
Formic acid is a single carbon organic acid and has a long history of being used in different industries. For example, it is a food preservative and antibacterial agent, and it has been used as an important intermediate for the synthesis of other valuable chemicals. What makes formic acid biosynthesis special is that this molecule can be produced from carbon dioxide. As such, it carries strong research significance for green-house gas fixation.
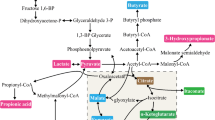
CO2 can be reduced to formic acid by formate dehydrogenase using NADH or electrode-derived electrons as the reducing power (Choe et al. 2014; Le et al. 2018). Alternatively, the reaction can be carried out by other enzymes such as carbon dioxide reductase using hydrogen gas as the reducing power (Schuchmann and Müller 2013; Eguchi et al. 1985; Alissandratos et al. 2014). The CO2 reduction is also part of the Wood–Ljungdahl pathway that is used for other acid biosynthesis as discussed in the sections below (Fig. 1). Compared with other organic acids, the research and application of formic acid bioproduction is very limited. This is mainly because of the low efficiency of the bioconversion process due to technical difficulties such as CO2 mass transfer limitations. To date, only a small number of microbes, such as A. woodii, E. coli, and S. oneidensis, have been investigated for formic acid bioproduction. For example, one recent study reported the 20 mM formic acid biosynthesis from CO2, and the production was improved when HCO3− was used as the carbon substrate.
2.2 C2 Acetic Acid
Acetic acid is an industrially important compound that has been widely used for the production of synthetic polymers (e.g., polyvinyl acetate), photographic film materials (e.g., cellulose acetate), organic solvent (e.g., ethyl acetates), vinegar, and other products.
Acetic acid biosynthesis has been well studied. There have been extensive efforts for the utilization of various microbes for acetic acid production. The main microbes recruited for the bioproduction are native producers, namely acetic acid bacteria. So far, a few different pathways have been discovered and utilized for acetic acid biosynthesis. The first pathway is ethanol oxidation in which acetyl-CoA, a central metabolite, is converted to ethanol and subsequently undergoes two consecutive oxidation reactions catalyzed by alcohol dehydrogenase and aldehyde dehydrogenase, respectively (Fig. 1). Since the pathway precursor ethanol is derived from glycolysis, strong metabolic flux can be attracted to acetic acid formation and the overall biosynthesis performance is high. For example, using this pathway, acetic acid bacteria have been adopted for high-level bioproduction of acetic acid at industrially relevant scales (Gullo et al. 2014). On the other hand, acetyl-CoA can be converted to acetate phosphate and then acetic acid. This route adopted the Wood–Ljungdahl pathway for fixation of CO2 to acetic acid (Ragsdale and Pierce 2008). Specifically, this pathway comprises two branches that convert CO2 to formic acid and CO, respectively, which are then combined to generate acetyl-CoA and acetic acid. Notably, the reducing equivalent needed for Wood–Ljungdahl pathway can be provided by glycolysis and subsequent conversion of acetyl-CoA to acetic acid. As such, the coupling of Wood–Ljungdahl pathway with glycolysis/acetyl-CoA conversion will lead to the formation of three acetic acid molecules from one glucose molecule, eliminating the CO2 emission.
Besides the Wood–Ljungdahl pathway, it has been found that CO2 can be fixed to make acetic acid through the glycine synthase pathway. Notably, it has been found that the Wood–Ljungdahl pathway and the glycine synthase pathway are not totally independent; instead, they are functionally interconnected for CO2 fixation (Song et al. 2020).
Large-scale cultivation has increased the acetic acid biosynthesis level to dozens of grams per liter in many reports (Vidra and Németh 2018). One challenge for acetic acid bioproduction is product toxicity. To this end, it is important to increase the resistance of the recruited microbial host (Nakano et al. 2004) or remove acetic acid in situ using bioprocess engineering techniques (Karekar et al. 2020).
2.3 C3 Propionic Acid
Propionic acid is one of the top value-added compounds from biomass (Werpy and Petersen 2004). It is a Generally Recognized as Safe (GRAS) compound with various important chemical properties. Its derivatives have been widely used as food preservatives, personal care products, and pharmaceutical substances. Moreover, propionic acid can be used as a building block molecule for the synthesis of many other industrially important molecules. The biosynthesis of this C3 organic acid has been well studied and widely achieved using various microbes (Ranaei et al. 2020; Eş et al. 2017).
Several pathways have been discovered for propionic acid biosynthesis. For example, succinate in the TCA cycle can be decarboxylated to make propionic acid via propionyl-CoA. This pathway also called Wood–Werkman cycle, is a popular pathway used by many native producers. Another well-studied pathway uses lactic acid to produce propionic acid via lactoyl-CoA and acryloyl-CoA. In addition, malonyl-CoA of the fatty acid pathway can be converted to 3-hydroxyproionic acid which is then used to generate propionic acid. Acetyl-CoA can be combined with pyruvate to make propionic acid via citramalate. Some simple amino acids, such as valine, isoleucine, threonine, and methionine, can also be converted to propionic acid through a series of enzymatic reactions.
Propionic acid can be produced by a variety of microbes, such as several species of Propionibacterium and Veillonella. Most of the previous studies focused on using bioprocess engineering techniques, e.g., manipulation of the cultivation conditions, to improve the bioproduction performance (Ranaei et al. 2020; Eş et al. 2017; Gonzalez-Garcia et al. 2017). To this end, extensive efforts have been made for cultivation optimization, which led to high-level production. For example, 97 g/L propionic acid production with a yield of 0.54 g/g glucose was achieved using a Propionibacterium acidipropionici mutant strain (Zhang and Yang 2009). Also, a variety of different carbon substrates, such as glucose, glycerol, and whey lactose, have been used for propionic acid biosynthesis (Ranaei et al. 2020). It is noteworthy that most of the native producers are difficult to modify using genetic or metabolic engineering tools. So far, only a few microbes, such as P. acidipropionici and P. jensenii have been genetically engineered for propionic acid biosynthesis. As such, strain evolution by random mutagenesis and genome shuffling is an alternative approach for production improvement. On the other hand, there are an increasing number of studies using non-native producers, such as model microbe E. coli, for propionic acid biosynthesis (Akawi et al. 2015; Kandasamy et al. 2013). These microbes are more genetically tractable and thus can be rationally engineered for bioproduction. Yet, the bioproduction performance of these microbes so far is still not comparable with the native producers.
2.4 C4 (1) Butyric Acid
Butyric acid has been widely used in food and pharmaceutical industries (Wang et al. 2016); it is also an important precursor for many valuable chemicals such as butanol (biofuel) and cellulose acetate butyrate (thermoplastic) (Richter et al. 2012; Zhang et al. 2009). Although butyric acid has an unpleasant odor, its derivatives are popular fragrance molecules (Jiang et al. 2018).
Butyric acid biosynthesis has been established in a variety of different microbes. The development of bioproduction using sustainable materials, such as lignocellulose, industrial byproducts, and municipal waste, has also been the focus of recent studies. Generally, the first step of using sugar-based feedstocks is to generate simple carbon substrates such as glucose or xylose. The carbon substrates are converted to pyruvate and further to acetyl-CoA through the central metabolism. Acetyl-CoA is then converted to butyryl-CoA by several enzymes, including thiolase and butyryl-CoA dehydrogenase. From butyryl-CoA, butyric acid is produced either through the phosphotransbutyrylase(PTB)-butyrate kinase(BUK) pathway or by the CoA-transferase(CTF) (Jiang et al. 2018) (Fig. 1). The competing pathways include the conversion from pyruvate to lactate, from acetyl-CoA to acetate and ethanol, and the generation of butanol from butyryl-CoA.
The main butyric acid-producing microbes are Clostridium, Butyrivibrio, Butyribacterium, Eubacterium, and Fusobacterium (Duncan et al. 2002; Pituch et al. 2013). Clostridium, which has been genome-sequenced and widely studied, is promising for industrial applications due to its ability of using various feedstocks and high production performance (Dwidar et al. 2012). For example, Clostridium tyrobutyricum ATCC 25755 was able to use xylose to produce 57.9 g/L butyric acid with a yield of up to 0.59 g/g (Zhu and Yang 2004). When using glucose as the substrate, the production of butyric acid reached 73.38 g/L with a yield of 0.46 g/g (Song et al. 2010). However, the difficulties associated with genetic engineering of Clostridium remain a big challenge. E. coli, has also been metabolically engineered to produce 10 g/L butyric acid from glucose with a high product selectivity (butyric acid/acetic acid ratio: 143 g/g) (Saini et al. 2014). The highest production by E. coli is 14.3 g/L, although the yield is still low (0.11 g/g glucose). This is probably due to the poor butyric acid tolerance (Jawed et al. 2016). Thermobifida fusca, a microbe with robust cellulases and hemicelluloses for lignocellulose degradation, has been metabolically engineered to express an exogenous acetate CoA-transferase to produce butyric acid from lignocellulose. Grown on 90 g/L corn stover as the substrate, such engineered T. fusca achieved a production of 17.1 g/L butyric acid (Deng et al. 2015).
2.5 C4 (2) Isobutyric Acid
Isobutyric acid, also known as 2-methylpropanoic acid, is one critical chemical for the production of plastics, resins, pharmaceuticals, and food industry products. Traditional manufacturing of isobutyric acid relies on the oxidation of petroleum-derived isobutyraldehyde.
For the isobutyric acid biosynthesis, glucose can enter the central metabolism to produce pyruvate and then isobutyraldehyde, which is converted to isobutyric acid by alcohol dehydrogenase. Using this pathway, engineered E. coli was reported to produce 11.7 g/L isobutyric acid (Zhang et al. 2011). Moreover, by deleting the competing isobutanol pathway and overexpressing additional enzymes for isobutyraldehyde conversion to isobutyric acid, a high production yield of 0.39 g/g glucose was achieved (Xiong et al. 2015). After the process scale-up and cultivation condition optimization, the production was boosted to 90 g/L (Xiong et al. 2015). Other microbes, such as Pseudomonas, were also metabolically engineered to produce isobutyric acid, although the production performance was relatively low (Lang et al. 2014).
2.6 C5 Valeric Acid
Valeric acid, also called pentanoic acid, has long been used as a food additive or flavoring agent. Valeric acid has an unpleasant odor, but many of its esters are pleasant-smelling chemicals and therefore popularly used in perfumes and cosmetics industries. In addition, valeric acid and its derivatives can be used for making biofuel additives and bioplastics.
Valeric acid biosynthesis is under development, as there are only limited efforts for biosynthesis studies compared with other straight-chain carboxylic acids. This is largely due to the difficulties associated with engineering the biosynthesis pathway(s) and relatively limited industrial values of this C5 molecule. It has been reported that valeric acid can be biosynthesized from pyruvate through an engineered pathway (Dhande et al. 2012). Such an artificial pathway uses threonine, 2-ketobutyrate, and 2-ketovalerate as the intermediates and has been successfully reconstituted in E. coli for 2.58 g/L production from glucose. Alternatively, valeric acid can be biosynthesized through the combination of propionyl-CoA and acetyl-CoA, which generates a series of intermediates including ketovaleryl-CoA, trans-2-pentenoyl-CoA, and valeryl-CoA and finally results in the formation of valeric acid. Using this designed pathway, 398 mg/L valeric acid was produced from 10 g/L glycerol (Tseng and Prather 2012). Notably, there are also efforts using simple organic acids as the substrates for microbial biosynthesis. For example, propionate and methanol were used for valeric acid production in an anaerobic chain elongation open-culture bioreactor (De Smit et al. 2019). Large-scale production of valeric acid using engineering microbes has not been extensively studied, yet.
2.7 C6 (1) Caproic Acid
Caproic acid, also called hexanoic acid, is an oily acrid liquid with wide application in different industries. For example, it can be used as an antimicrobial agent, food additive, lubricating agent, etc. Moreover, it is a building block molecule for the chemical synthesis of various downstream products. Its esters are particularly useful for food and pharmaceutical industries.
A variety of natural anaerobic bacteria, such as Clostridium kluyveri, Megasphaera elsdenii, and Eubacterium limosum, are capable of producing caproic acid (Cavalcante et al. 2017). The caproic acid biosynthesis uses the mechanism of carboxylic acid chain elongation, which is essentially a reverse β-oxidation pathway. Specifically, acetyl-CoA is used as the starting molecule for the carboxylic acid chain formation, which proceeds via intermediate metabolites such as acetoacetyl-CoA, butyryl-CoA, and hexanoyl-CoA. Since many intermediates are involved in the pathway, biosynthesis requires a large number of enzymes, which are mostly active in the native producers. Lactic acid and ethanol are often used as electron donors for this biosynthesis process under anaerobic conditions. For example, ethanol can be converted to acetic acid to generate the energy needed for biosynthesis; acetic acid is subsequently used as the carbon source for the caproic acid chain formation. Notably, a similar pathway can be used for butyric acid as discussed in the section above.
Many caproic acid biosynthesis studies utilize the methods that are less commonly used for other carboxylic acid bioproduction (e.g., open cultures, mixed cultures, unsterile conditions). For example, it has been reported that, using a microbiome obtained from a fermentation pit, the caproic acid bioproduction reached 23 g/L under optimized conditions (Zhu et al. 2015). Most of the current studies rely on the use of a mixed substrate, such as acetic acid and ethanol, for high bioproduction.
2.8 C6 (2) Adipic Acid
Adipic acid, also named hexanedioic acid, is the most important dicarboxylic acid with a huge global market. This C6 molecule has a carboxyl group at each end of the linear chain, providing special chemical properties (e.g., pKa of 4.43 and 5.41) and outstanding industrial values. For example, adipic acid is a precursor for the manufacturing of commodity chemical nylon. It is also used as a food ingredient and a material for the production of polyurethanes.
There are two major pathways for adipic acid biosynthesis. The first pathway is oxidation of caproic acid (discussed in the section above) via the fatty acid ω-oxidation pathway. Via this pathway, the production of more than 50 g/L adipic acid was achieved using an industrial yeast strain (Beardslee and Picataggio 2012). Another pathway is the combination of acetyl-CoA and succinyl-CoA to generate 3-oxoadipyl-CoA which is subsequently converted to 3-hydroxyadipyl-CoA, 5-carboxy-2-pentenyl-CoA, adipyl-CoA, and finally adipic acid. This is also called the reverse adipate degradation pathway. In a recent report, 2.23 g/L adipic acid was produced from 50 g/L glucose in a T. fusca strain harboring this pathway (Deng and Mao 2015). Similarly, Cheong et al. biosynthesized 2.5 g/L adipic acid from succinyl-CoA and acetyl-CoA using engineered E. coli (Cheong et al. 2016).
In addition, there are other adipic acid production routes that use both biosynthesis and chemical reactions. For example, glucaric acid and cis,cis-muconic acid, both of which can be obtained from simple carbon substrates by microbial biosynthesis, can be converted to adipic acid using catalyzed hydrogenation reactions. Notably, cis,cis-muconic acid can also be produced from lignin through depolymerization reactions and enzymatic aromatic degradation, which greatly expands the source for adipic acid production. These pathways have been recruited for the production using different microbes (Kruyer and Peralta-Yahya 2017; Deng et al. 2016). However, depending on the starting materials and the adopted biosynthesis conditions, the production efficiency varies significantly between them.
2.9 C7 Heptanoic Acid
Heptanoic acid, or enanthic acid, is an odd-chain acid with an unpleasant odor. It can be used for the production of biodiesel, antibiotic agents, fragrances, and bioplastics.
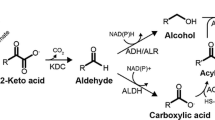
Compared with other acids, there are only a few potential routes for heptanoic acid biosynthesis. The main route is through the fatty acid biosynthesis mechanism (Fig. 2). However, instead of acetyl-CoA, this pathway uses propionyl-CoA as the starting molecule; and by incorporation of C2 extension unit, it results in the formation of C5 and then C7 acids. Some microbes have the enzymes needed for the utilization of this pathway and thus have been used for microbial bioproduction.
It should be noted that, due to the nature of the heptanoic acid biosynthesis pathway, many microbial biosynthesis processes tend to produce a mixture of different medium-chain carboxylic acids. For example, a mixed culture cultivated in an upflow anaerobic filter reactor was engineered to produce valerate, caproate, and heptanoate all at the gram per liter scale (Grootscholten et al. 2013). Co-production of multiple acid products is also very common for the mono-culture of one single microbe. Jeon and coworkers utilized the culture of a Megasphaera strain and produced 5.7 g/L pentanoic acid, 9.7 g/L hexanoic acid, 3.2 g/L heptanoic acid, and 1.2 g/L octanoic acid (Jeon et al. 2016). However, the efforts for high production of heptanoic acid as the specific main bioproduct are still limited. Also, separating heptanoic acid from other byproducts presents an additional challenge of high-level bioproduction.
2.10 Medium-Chain Straight Aliphatic Carboxylic Acids
2.10.1 Medium-Chain Fatty Acids
The definition of medium-chain fatty acids, i.e., the length of the carbon chain, varies in different reports. For this work, medium-chain fatty acids refer to the C8-C12 fatty acids. Medium-chain saturated fatty acids possess stronger hydrophobicity than the short-chain acids discussed above. As such, they have distinct chemical properties and industrial values.
C8
Octanoic acid (caprylic acid) is an oily liquid with very low water solubility and light odor. The main use of octanoic acid is to produce esters that can be used in perfume and dye industries. Octanoic acid itself has antimicrobial activities and thus has been used as a disinfectant. In addition, as a medium-chain fatty acid, it can be taken as a dietary supplement.
C9
Nonanoic acid (pelargonic acid) is a naturally occurring fatty acid found in many plants. It is mainly used as an herbicide and blossom thinner in agriculture and an additive for personal-care products. Its synthetic esters are commonly used for food flavoring.
C10
Caprici acid (decanoic acid or decylic acid) is a C10 saturated fatty acid. Its esters are often used for the production of perfumes, lubricants, food additives, and plastics. In addition, it can be linked to pharmaceutical molecules to generate prodrugs with better lipophilicity that is particularly useful for drug injection.
C11
Undecylic acid (undecanoic acid) is a highly hydrophobic compound with poor water solubility. It is also a good antifungal agent.
C12
Lauric acid (dodecanoic acid) is a twelve-carbon fatty acid with special chemical properties that makes it an effective surfactant. It is historically used for the manufacturing of soaps, detergents, pesticides, and cosmetics.
For medium-chain acids, biosynthesis often utilizes the fatty acid biosynthesis pathway, which is a ubiquitous process in many organisms and has been extensively studied in the open literature. Specifically, acetyl-CoA and malonyl-CoA (also derived from acetyl-CoA) are the substrates for fatty acid biosynthesis. A set of repetitive decarboxylative Claisen condensation reactions are used for carbon chain elongation via ACP-intermediates. Meanwhile, enzymatic reactions, such as keto-reduction, dehydration, and enoyl reduction, take place on the extending chain and result in the formation of saturated or unsaturated fatty acids.
For medium-chain acid biosynthesis using the fatty acid biosynthesis pathway, the key challenge is how to control the length of the chain, i.e., how to stop the chain elongation process at the proper timing to make the desired products (Heil et al. 2019). To this end, it is important to engineer the reaction specificity of the enzymes involved in the chain elongation steps. On the other hand, despite the efforts in engineering the pathway enzymes, it is almost entirely unavoidable that some fatty acid byproducts, either straight or branched, are co-produced by the recruited microbial host. This, therefore, presents a challenge for the products’ downstream separation and purification. In fact, compared with short-chain acid, medium-chain fatty acid biosynthesis using microbial hosts has not been extensively studied. Nevertheless, there have been attempts to explore this area, which are nicely summarized in some review articles (Sarria et al. 2017). For example, octanoic acid is biosynthesized by adding a C2 unit (from acetyl-CoA) to C6 hexanoic acid. Tan et al. reported that, using metabolically engineered E. coli, the fatty acid biosynthesis pathway can be manipulated to produce octanoic acid from glucose. Several engineering strategies, such as computational strain design, pathway gene regulation, medium optimization, were adopted. The highest production reached 1 g/L with greater than 70% selectivity using a fed-batch culture. In another report, it was found that the rapid, irreversible elongation of fatty acid acyl-ACP precursors is a critical issue for medium-chain fatty acid biosynthesis. As such, a key pathway enzyme, ketoacyl synthase, was engineered for degradation using an exogenous inducer. This strategy ingeniously slowed down the chain elongation and improved the production of medium-chain fatty acid in engineered E. coli. Based on these efforts, the authors were able to achieve comprehensive production of all even- and odd-chain-length fatty acids with high efficiency (Torella et al. 2013). On the other hand, yeast, such as S. cerevisiae and Y. lipolytica, has also been recruited for octanoic acid biosynthesis by using the eukaryotic fatty acid biosynthesis enzymes (Leber and Da Silva 2014; Rutter et al. 2015; Rigouin et al. 2017).
Alternatively, biosynthesis can be achieved using the reverse β-oxidation pathway, as illustrated in Fig. 3. The pathway allows the use of acetyl-CoA as a direct substrate for chain elongation, bypassing the ATP-requiring conversion to malonyl-CoA in the regular fatty acid biosynthesis pathway. Similar to the fatty acid biosynthesis pathway, the chain length control for this pathway is challenging, and the biosynthesis efforts often lead to the production of acid mixtures. For example, Wu et al. utilized the reverse β-oxidation pathway to produce medium-chain fatty acids in E. coli (Wu et al. 2017). In particular, the rate-limiting steps of the pathway were identified and addressed using metabolic engineering strategies. After the step-wise optimization, the production reached 3.8 g/L from glucose. Notably, the products contained a mixture of C6, C8, and C10 fatty acids, due to the limitation of the specificity of the pathway enzymes. The reverse β-oxidation pathway has also been established in S. cerevisiae for the production of medium-chain fatty acids (Lian and Zhao 2015).
It should be noted that there is a lack of successful studies for converting simple carbon materials to a specific medium-chain saturated fatty acid without byproduct acids. The separation of the desired acid products thus needs to overcome considerable technical challenges to isolate them from byproducts with similar chemical properties. In this sense, more research efforts should be dedicated with ingenious design and effective engineering approaches.
2.10.2 Medium-Chain Dicarboxylic Acids
Medium-chain dicarboxylic acids are widely used for the manufacturing of valuable industrial products, including detergents, surfactants, lubricants, perfumes, and pharmaceuticals. Moreover, the presence of the two carboxyl groups makes them outstanding materials for the production of various synthetic polymers, such as nylon, polyamides, and polyesters.
There are a few different pathways that can be used for microbial biosynthesis of medium-chain dicarboxylic acids. For the first pathway, medium-chain fatty acids can be hydroxylated at the ω position (the terminal carbon of the aliphatic chain) by ω-specific enzymes, generating ω-hydroxy fatty acids (Song et al. 2014). Subsequently, the terminal carbon is further oxidized by appropriate alcohol and aldehyde dehydrogenases to produce medium-chain dicarboxylic acids. This route can be coupled with the regular fatty acid biosynthesis pathway or the reverse β-oxidation pathway to enable the de novo bioproduction from simple carbon substrates such as glucose and glycerol. For example, Bowen et al. used a bioinformatics approach to identify key enzymes such as a ω-specific cytochrome P450 enzyme and selected alcohol and aldehyde dehydrogenases, which were then utilized to effectively oxidize medium-chain fatty acids at the terminal carbon to generate corresponding alcohol, aldehyde, and eventually dicarboxylic acids. This pathway was introduced into E. coli and integrated with the engineered fatty acid biosynthesis, which enabled the conversion of glucose to medium-chain α, ω-dicarboxylic acids (Bowen et al. 2016; Haushalter et al. 2017). The de novo production reached 600 mg/L after optimization of the cultivation conditions. Similarly, S. cerevisiae has been engineered to produce medium-chain α, ω-dicarboxylic acids through functional expression of a heterologous cytochrome P450 and cytochrome reductase. Coupled with the engineered fatty acid pathway, this approach resulted in the de novo dicarboxylic acid production from simple renewable sugars (Han et al. 2017).
Alternatively, some medium-chain dicarboxylic acids can be biosynthesized using non-decarboxylative Claisen condensation and β-reduction reactions, which have been established in E. coli (Cheong et al. 2016). To this end, selected functionalized primers and functionalized extender units (e.g., analogs of fatty acid biosynthesis precursors) were combined to generate the desired α, ω-dicarboxylic acid products. By selection of the pathway enzymes with desired specificity and activities, suberic acid (C8) and sebacic acid (C10) has been successfully produced from glycerol. Notably, a similar strategy was also used for the adipic acid biosynthesis, as discussed in the previous section.
Straight-chain aliphatic dicarboxylic acids can also be produced by direct oxidation of alkanes using the engineered microbial alkane degradation pathway. For example, it has been found that a wide range of microbes is able to oxidize different alkanes to carboxylic acids (Wentzel et al. 2007). However, this pathway is promiscuous and can generate a variety of different products beside carboxylic acids. Also, the bioconversion efficiency is relatively low and hard to control. As such, this pathway is not popularly adopted for making straight-chain aliphatic dicarboxylic acids.
2.11 Long Straight-Chain Aliphatic Carboxylic Acids
2.11.1 Long-Chain Fatty Acids (>C12)
Long-chain fatty acids are essential constituent components of lipids and play an important role in cell membrane integrity. They are also critical substances for cellular energy storage. In addition, they carry other essential biological functions, such as gene expression regulation, protein modification, metabolic signaling, etc. Long-chain fatty acids, either saturated or unsaturated, can be used in various industries. For example, many of them have high nutraceutical values and thus have been widely used as food supplements.
Most long-chain fatty acids studied by the research communities are straight acids. As such, their biosynthesis can use the regular straight-chain fatty acid biosynthesis pathway (Fig. 2). Similar to the discussion above, due to the issue of chain elongation and termination control, the products are usually a mixture of products with different lengths. Recently, there have been a lot of new achievements in using various microbes as the host of biosynthesis. For example, Yarrowia lipolytica, a non-pathogenic native oleaginous yeast, has been widely utilized and engineered for fatty acid biosynthesis due to its outstanding native capability for lipid formation (Liu et al. 2021; Ledesma-Amaro and Nicaud 2016). In fact, a variety of engineering tools and strategies have been developed for this microbe, making it great a platform host for the biosynthesis of fatty acids and other related product biosynthesis. It should be also noted that bioproduction scale-up based on the use of Y. lipolytica is also straightforward, offering important benefits for industrially relevant production. It has been reported that, by blocking fatty acids’ peroxisomal uptake and conversion to lipid, Y. lipolytica can produce 2.3 g/L free fatty acids in shake flask, and the long-chain fatty acid content was improved significantly (Ghogare et al. 2020). In another study, Y. lipolytica’s glycerol metabolism was eliminated to direct more flux toward the lipid pathway. When combined with other engineering strategies, 2 g/L free fatty acids, mostly C16-C24 fatty acids, were produced from glucose (Yuzbasheva et al. 2018). Y. lipolytica has also been used for the production of other unusual carboxylic acid products, such as polyunsaturated fatty acids (Gemperlein et al. 2019).
Long-chain fatty acid biosynthesis in E. coli has also been achieved (Lu et al. 2008). For example, by integrating the selected plant genes into E. coli’s fatty acid biosynthesis pathway, Kassab et al. established the production of long-chain fatty acids with yields greater than 200 mg/g dried cell weight (Kassab et al. 2019). Lu et al. reported the production of 2.5 g/L fatty acids (C12 and above) by engineering the native fatty acid biosynthesis and competing pathways and optimizing the resulting strain’s cultivation conditions. Moreover, microalgae are another efficient workhorse for fatty acid biosynthesis. For example, through engineering glucose-6-phosphate, Xue et al. reprogrammed and improved the fatty acid bioproduction profile in oleaginous microalga Phaeodactylum tricornutum (Xue et al. 2017). Notably, microalgae can directly use photosynthesis to fix CO2 and produce desired bioproducts, offering an intriguing route for renewable bioproduction. However, challenges, such as the development of genetic engineering tools and environmental concerns of using genetically modified organisms, need to be addressed (Blatti et al. 2013; Tang et al. 2020).
The reverse β-oxidation pathway is an alternative route for long-chain fatty acid bioproduction. In fact, it has been reported that, by controlling the expression of different thioesterases of this pathway, several long-chain fatty acids were produced in engineered E. coli (Dellomonaco et al. 2011). Further, individual functional units of the pathway were characterized in vitro and in vivo, which provided new insights for long-chain fatty acid biosynthesis.
2.11.2 Long-Chain Dicarboxylic Acids
Long-chain dicarboxylic acids are versatile raw materials used in cosmetic and plastic industries for the manufacturing of polymers, lubricants, perfumes, UV blocker in sun cream, and other products. Current production of long-chain dicarboxylic acids mostly relies on chemical synthesis. However, the formation of unwanted byproducts and multistep purification processes make the cost of production rise dramatically with the length of the carbon chain. Efficient biosynthesis provides a cost-effective manner for long-chain dicarboxylic acid production from renewable substrates.
Similar to their medium-chain counterparts, long-chain dicarboxylic acids can be produced by oxidation of long-chain alkanes, fatty acids, or fatty acid esters. To this end, alkane can be first oxidized to fatty alcohol by selected hydroxylase complexes. Fatty alcohol is further oxidized to fatty aldehyde by fatty alcohol oxidase, followed by the oxidation of the aldehyde group to carboxyl group at both ends of the carbon chain. The first step of the series of the oxidation reaction is also known as the rate-limiting step, as the corresponding enzyme is composed of hard-to-express Cytochrome P450 (CYP) monooxygenase and a NADPH-CYP reductase.
The conversion of fatty acid follows a similar pathway, although the oxidation only occurs at one end of the carbon chain. In an industrial yeast strain Candida tropicalis, four genes encoding isozymes of the acyl-CoA oxidase involved in the β–oxidation pathway were disrupted to direct more alkane and fatty acid substrates to enter the ω–oxidation pathway. Further overexpression of P450 and NADPH-cytochrome reductase genes of the pathway increased the productivity, and dicarboxylic acids ranging from C12 to C22 were biosynthesized with high conversion (80–100%) from dodecane or methyl myristate (Picataggio et al. 1992). Another study showed that knocking out the carnitine acetyltransferase (CAT) gene, which is responsible for transporting the acetyl-CoA into the mitochondrion for the TCA cycle, resulted in a 21% increase of dicarboxylic acids production in Candida tropicalis (Cao et al. 2006).
E. coli has also been engineered to produce long-chain α, ω-dicarboxylic acids from fatty acids by utilization of a CYP450-dependent ω-oxidation pathway (Sathesh-Prabu and Lee 2015). To reduce the damage to the cells by H2O2, an undesired byproduct of the terminal oxidation, thiourea was added to the cell culture. In addition, 5-aminolevulinic acid was supplemented as a heme precursor to enhance the activity of a hemi-dependent monooxygenase of the pathway. Based on these strategies, the production of 159 mg/L of C12 dicarboxylic acids and 410 mg/L of C14 dicarboxylic acids was achieved. For biosynthesis using fatty acid ester, Lee et al. genetically engineered yeast Wickerhamiella sorbophila to block the β-oxidation pathway and enabled the bioconversion of methyl laurate to dodecanedioic acid (Lee et al. 2018).
3 The Challenges and Future Directions
3.1 Product Toxicity
Despite the efforts of establishing effective biosynthesis pathways in selected microbial hosts, straight-chain aliphatic acid biosynthesis is often limited by the product toxicity (Warnecke and Gill 2005). In fact, for most of the compounds discussed above, high-level accumulation in the cell culture almost unavoidably leads to the inhibition of microbial growth and other metabolic activities. To address this challenge, many innovative strategies have been developed and implemented in recent years. For example, product secretion can largely help reduce the toxic products’ intracellular accumulation. Zhu et al. metabolically engineered S. cerevisiae for medium-chain fatty acid biosynthesis and conducted directed evolution for a membrane transporter to enhance the secretion of medium-chain fatty acids (Zhu et al. 2020). In addition, strain adaptive laboratory evolution was also utilized to improve the robustness of S. cerevisiae, leading to the production of greater than 1 g/L extracellular medium-chain fatty acids.
Another popularly used strategy is in situ removal of the toxic compounds from the culture during the cultivation process (De Brabander et al. 2021). For example, organic solvents can be added to the cell culture during the cultivation process to extract the toxic product from the aqueous phase, which also enriches the product in the organic phase and facilitates the downstream purification efforts. It has been reported that, using an alamine 336 and oleyl alcohol mixture as the extractant, hexanoic acid produced by Megasphaera elsdenii was effectively removed from the cell culture, which improved the cell growth and the bioproduction performance (Choi et al. 2013). Membrane-supported reactive extraction and resin adsorption have also been used to achieve the in situ removal of the toxic metabolic products and byproducts (Gössi et al. 2020; Ataei and Vasheghani-Farahani 2008).
Strain evolution is also a viable option for increasing the cell resistance to the product toxicity. Fletcher et al. found that S. cerevisiae could be adapted to low pH and high concentration of lactic acid by utilizing evolutionary paths to develop the tolerance (Fletcher et al. 2017). Notably, the strain adaption is largely dependent upon the cultivation conditions, such as carbon source, the product type, the recruited microbial species, etc.
3.2 Genetic Engineering Tools for Microbial Biosynthesis
Model microbes, such as E. coli and S. cerevisiae, have been widely used for the biosynthesis of straight-chain aliphatic carboxylic acids (Abbott et al. 2009; Liu and Jarboe 2012; Yu et al. 2011). Their rich engineering toolkits allow for advanced bioproduction manipulation. However, these microbes are only recruited for the biosynthesis of a portion of the carboxylic acid products. For many other microbes, reliable genetic engineering tools are still largely under-developed. As such, they are highly difficult to engineer for improving biosynthesis capabilities. This includes not only the alteration of the native pathways but also the introduction of heterologous enzymes from different organisms. One typical example is propionic acid producer Propionibacterium. Despite its wide application for propionic acid bioproduction, genetic engineering protocols for this microbe are still not well established.
This situation presents an outstanding challenge and calls for the development of sophisticated biotechniques. For example, clustered regularly interspaced short palindromic repeats (CRISPR) are expected to provide new and powerful tools to enable the engineering of the target microbes that are hard to engineer before. On the other hand, utilizing microbial consortia or co-cultures adds a new dimension to address the current challenge, as it extends the biosynthesis capability of one microbe by supplementing one or more biosynthesis partners (Brenner et al. 2008). Specifically, one hard-to-engineer microbe can utilize its native biosynthesis capability to generate a particular pathway intermediate/precursor, whereas another microbe(s) with established genetic tools can be used to convert the intermediate/precursor to the desired final product. This strategy enables biosynthesis that cannot be achieved using either strain alone.
3.3 Efficiency of the Biosynthesis Pathways
Microbial biosynthesis performance is largely dependent on the efficiency of the selected pathways. Straight-chain aliphatic carboxylic acid biosynthesis often involves both endogenous and heterologous enzymes of the recruited microbial hosts. As such, it is challenging to ensure coordinated expression and functions of all pathway enzymes. In fact, it is quite common that the biosynthesis efficiency is restricted by the low activities of one or few pathway enzymes or by distraction of metabolic flux into the competing pathways.
Resolution of these issues requires the adoption of new techniques and methods. For example, transcriptomics and metabolomics analyses will play an important role in identifying the limiting steps of the biosynthesis pathway. Consequently, protein engineering can be adopted to improve the bioconversion efficiency of a particular enzyme responsible for the limiting step. On the other hand, the metabolic flux distribution at the branching points between the desired and competing pathways can be controlled by manipulating the activities of the corresponding enzymes through directed enzyme evolution or the utilization of alternative enzymes from other organisms.
It should be noted that dynamic control of the pathway precursor supply and consumption is another robust approach for improving the overall biosynthesis efficiency. For instance, Xu et al. established a metabolic regulation network to control malonyl-CoA consumption and supply and significantly improved the fatty acid biosynthesis in E. coli (Xu et al. 2014). Also, modular co-culture engineering, which modularizes the biosynthesis pathway for expression in different strains or species in one culture, offers another perspective for pathway balancing and bioproduction enhancement (Zhang and Wang 2016). For this, there have been increasing efforts for the biosynthesis of various biomolecules using modular co-culture engineering, which lays a foundation for straight-chain aliphatic carboxylic acid production. Last but not least, large-scale cultivation using advanced bioreactor techniques can be utilized to improve the overall biosynthesis performance at the industrially relevant levels. This is especially important for establishing high substrate consumption, high cell density, and high product production processes. It is also a necessary step for the industrial application of any biosynthesis systems.
4 Concluding Remarks
Biosynthesis of straight-chain aliphatic carboxylic acids has been attracting significant research interest in recent years. This chapter highlights representative members of this group of biomolecules to demonstrate the potential of this vigorous field. Based on the above discussions, identifying the right microbe is a critical step for biosynthesis. In fact, many microbes natively have strong power for producing certain straight-chain aliphatic carboxylic acids. These native producers have been extensively recruited in the past several decades, which has led to considerable application success. On the other hand, model organisms, such as E. coli and S. cerevisiae, can be genetically and metabolically engineered to accommodate heterologous pathways or a hybrid of native and heterologous pathways to achieve biosynthesis of the desired acid products.
Another key factor for successful biosynthesis is the establishment of proper pathways. In fact, the choice of feasible biosynthesis pathways is largely dependent upon the type of the end products. For the relatively small straight-chain acids, there are multiple independent pathways available for biosynthesis. For the relatively large acids, the choices of the feasible pathways are much limited. In fact, long-chain acids are almost entirely produced from the fatty acid biosynthesis pathway.
After the selection of the pathway, biosynthesis optimization needs to be pursued to meet the need of potential industrial production. To this end, enhancing the efficiency of biosynthesis pathways plays a critical role. Currently, many pathway enzymes (from same or different organisms) with desired functions can be identified from protein or genome databases and assembled in a selected microbial host for effective biosynthesis, which has resulted in tremendous success in the past. For future studies, the increasing availability of genome sequences of various organisms, fueled by DNA sequencing technologies, will make strong and positive impacts. Moreover, advances in protein engineering research, e.g., directed evolution, will promote the emergence of enzymes with needed diversity and catalytic activities.
On the other hand, balancing the distribution of metabolic resource between the target pathway, competing pathways, and the background metabolism, is essential for improving the biosynthesis performance. However, controlling such a balance is often times highly complicated, as suggested by previous studies (Jones et al. 2015). Therefore, innovative engineering strategies, such as biosensor-based dynamic control, need to be adopted to attract metabolic flux into the target pathway and reduce the byproduct formation. It should be noted that computational approaches, such as genome-scale modeling can also be utilized to facilitate biosynthesis system optimization. For example, there have been efforts for modeling the fatty acid biosynthesis behaviors, which provides important perspectives for engineering the global metabolism for supporting the desired biosynthesis (Youngquist et al. 2012). Collectively, innovations in various fields of science and technology will catalyze new and powerful development of straight-chain aliphatic carboxylic acid biosynthesis.
References
Abbott DA, Zelle RM, Pronk JT, Van Maris AJA (2009) Metabolic engineering of Saccharomyces cerevisiae for production of carboxylic acids: current status and challenges. FEMS Yeast Res 9:1123–1136
Akawi L, Srirangan K, Liu X et al (2015) Engineering Escherichia coli for high-level production of propionate. J Ind Microbiol Biotechnol 42:1057–1072
Alissandratos A, Kim HK, Easton CJ (2014) Formate production through carbon dioxide hydrogenation with recombinant whole cell biocatalysts. Bioresour Technol 164:7–11
Ataei SA, Vasheghani-Farahani E (2008) In situ separation of lactic acid from fermentation broth using ion exchange resins. J Ind Microbiol Biotechnol 35:1229–1233
Beardslee T, Picataggio S (2012) Bio-based adipic acid from renewable oils. Lipid Technol 24:223–225
Blatti JL, Michaud J, Burkart MD (2013) Engineering fatty acid biosynthesis in microalgae for sustainable biodiesel. Curr Opin Chem Biol 17:496–505
Bowen CH, Bonin J, Kogler A et al (2016) Engineering Escherichia coli for conversion of glucose to medium-chain ω-hydroxy fatty acids and α,ω-dicarboxylic acids. ACS Synth Biol 5:200–206
Brenner K, You L, Arnold FH (2008) Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol 26:483–489
Cao Z, Gao H, Liu M, Jiao P (2006) Engineering the acetyl-CoA transportation system of Candida tropicalis enhances the production of dicarboxylic acid. Biotechnol J 1:68–74
Cavalcante WDA, Leitão RC, Gehring TA et al (2017) Anaerobic fermentation for n-caproic acid production: a review. Process Biochem 54:106–119
Cheong S, Clomburg JM, Gonzalez R (2016) Energy-and carbon-efficient synthesis of functionalized small molecules in bacteria using non-decarboxylative Claisen condensation reactions. Nat Biotechnol 34:556–561
Choe H, Joo JC, Cho DH et al (2014) Efficient CO2-reducing activity of NAD-dependent formate dehydrogenase from Thiobacillus sp. KNK65MA for formate production from CO2 gas. PLoS One 9:e103111
Choi K, Jeon BS, Kim BC et al (2013) In situ biphasic extractive fermentation for hexanoic acid production from sucrose by Megasphaera elsdenii NCIMB 702410. Appl Biochem Biotechnol 171:1094–1107
De Brabander P, Uitterhaegen E, Verhoeven E et al (2021) In situ product recovery of bio-based industrial platform chemicals: a guideline to solvent selection. Fermentation 7:26
De Smit SM, De Leeuw KD, Buisman CJN, Strik DPBTB (2019) Continuous n-valerate formation from propionate and methanol in an anaerobic chain elongation open-culture bioreactor. Biotechnol Biofuels 12:132
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 476:355–359
Deng Y, Ma L, Mao Y (2016) Biological production of adipic acid from renewable substrates: current and future methods. Biochem Eng J 105:16–26
Deng Y, Mao Y (2015) Production of adipic acid by the native-occurring pathway in Thermobifida fusca B6. J Appl Microbiol 119:1057–1063
Deng Y, Mao Y, Zhang X (2015) Driving carbon flux through exogenous butyryl-CoA: acetate CoA-transferase to produce butyric acid at high titer in Thermobifida fusca. J Biotechnol 216:151–157
Dhande YK, Xiong M, Zhang K (2012) Production of C5 carboxylic acids in engineered Escherichia coli. Process Biochem 47:1965–1971
Duncan SH, Barcenilla A, Stewart CS et al (2002) Acetate utilization and butyryl coenzyme A (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol 68:5186–5190
Dwidar M, Park JY, Mitchell RJ, Sang BI (2012) The future of butyric acid in industry. Sci World J 2012:9
Eguchi SY, Nishio N, Nagai S (1985) Formic acid production from H2 and bicarbonate by a formateutilizing methanogen. Appl Microbiol Biotechnol 22:148–151
Eş I, Khaneghah AM, Hashemi SMB, Koubaa M (2017) Current advances in biological production of propionic acid. Biotechnol Lett 39:635–645
Fletcher E, Feizi A, Bisschops MMM et al (2017) Evolutionary engineering reveals divergent paths when yeast is adapted to different acidic environments. Metab Eng 39:19–28
Gemperlein K, Dietrich D, Kohlstedt M et al (2019) Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases. Nat Commun 10:4055
Ghogare R, Chen S, Xiong X (2020) Metabolic engineering of oleaginous yeast Yarrowia lipolytica for overproduction of fatty acids. Front Microbiol 11:1717
Gonzalez-Garcia RA, McCubbin T, Navone L et al (2017) Microbial propionic acid production. Fermentation 3:21
Gössi A, Burgener F, Kohler D et al (2020) In-situ recovery of carboxylic acids from fermentation broths through membrane supported reactive extraction using membrane modules with improved stability. Sep Purif Technol 241:116694
Grootscholten TIM, Steinbusch KJJ, Hamelers HVM, Buisman CJN (2013) High rate heptanoate production from propionate and ethanol using chain elongation. Bioresour Technol 136:715–718
Gullo M, Verzelloni E, Canonico M (2014) Aerobic submerged fermentation by acetic acid bacteria for vinegar production: process and biotechnological aspects. Process Biochem 49:1571–1579
Han L, Peng Y, Zhang Y et al (2017) Designing and creating a synthetic omega oxidation pathway in Saccharomyces cerevisiae enables production of medium-chain α, ω-dicarboxylic acids. Front Microbiol 8:2184
Haushalter RW, Phelan RM, Hoh KM et al (2017) Production of odd-carbon dicarboxylic acids in Escherichia coli using an engineered biotin-fatty acid biosynthetic pathway. J Am Chem Soc 139:4615–4618
Heil CS, Wehrheim SS, Paithankar KS, Grininger M (2019) Fatty acid biosynthesis: chain-length regulation and control. Chembiochem 20:2298–2321
Jawed K, Mattam AJ, Fatma Z et al (2016) Engineered production of short chain fatty acid in Escherichia coli using fatty acid synthesis pathway. PLoS One 11:e0160035
Jeon BS, Choi O, Um Y, Sang BI (2016) Production of medium-chain carboxylic acids by Megasphaera sp. MH with supplemental electron acceptors. Biotechnol Biofuels 9:129
Jiang L, Fu H, Yang HK et al (2018) Butyric acid: applications and recent advances in its bioproduction. Biotechnol Adv 36:2101–2117
Jones JA, Toparlak TD, Koffas MAG (2015) Metabolic pathway balancing and its role in the production of biofuels and chemicals. Curr Opin Biotechnol 33:52–59
Kandasamy V, Vaidyanathan H, Djurdjevic I et al (2013) Engineering Escherichia coli with acrylate pathway genes for propionic acid synthesis and its impact on mixed-acid fermentation. Appl Microbiol Biotechnol 97:1191–1200
Karekar SC, Srinivas K, Ahring BK (2020) Continuous in-situ extraction of acetic acid produced by Acetobacterium woodii during fermentation of hydrogen and carbon dioxide using Amberlite FPA53 ion exchange resins. Bioresour Technol Rep 12:100568
Kassab E, Fuchs M, Haack M et al (2019) Engineering Escherichia coli FAB system using synthetic plant genes for the production of long chain fatty acids. Microb Cell Factories 18:163
Kruyer NS, Peralta-Yahya P (2017) Metabolic engineering strategies to bio-adipic acid production. Curr Opin Biotechnol 45:136–143
Lang K, Zierow J, Buehler K, Schmid A (2014) Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production of isobutyric acid and other secondary metabolites. Microb Cell Factories 13:2
Le QAT, Kim HG, Kim YH (2018) Electrochemical synthesis of formic acid from CO2 catalyzed by Shewanella oneidensis MR-1 whole-cell biocatalyst. Enzym Microb Technol 116:1–5
Leber C, Da Silva NA (2014) Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnol Bioeng 111:347–358
Ledesma-Amaro R, Nicaud JM (2016) Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res 61:40–50
Lee H, Han C, Lee HW et al (2018) Development of a promising microbial platform for the production of dicarboxylic acids from biorenewable resources. Biotechnol Biofuels 11:310
Lian J, Zhao H (2015) Reversal of the β-oxidation cycle in Saccharomyces cerevisiae for production of fuels and chemicals. ACS Synth Biol 4:332–341
Liu H, Song Y, Fan X et al (2021) Yarrowia lipolytica as an oleaginous platform for the production of value-added fatty acid-based bioproducts. Front Microbiol 11:608662
Liu P, Jarboe LR (2012) Metabolic engineering of biocatalysts for carboxylic acids production. Comput Struct Biotechnol J 3:e201210011
Lu X, Vora H, Khosla C (2008) Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab Eng 10:333–339
Nakano S, Fukaya M, Horinouchi S (2004) Enhanced expression of aconitase raises acetic acid resistance in Acetobacter aceti. FEMS Microbiol Lett 235:315–322
Picataggio S, Rohrer T, Deanda K et al (1992) Metabolic engineering of Candida tropicalis for the production of long-chain dicarboxylic acids. Nat Biotechnol 10:893–899
Pituch A, Walkowiak J, Banaszkiewicz A (2013) Butyric acid in functional constipation. Prz Gastroenterol 8:295–298
Ragsdale SW, Pierce E (2008) Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta Proteins Proteomics 1784:1873–1898
Ranaei V, Pilevar Z, Khaneghah AM, Hosseini H (2020) Propionic acid: method of production, current state and perspectives. Food Technol Biotechnol 58:115–127
Richter H, Qureshi N, Heger S et al (2012) Prolonged conversion of n-butyrate to n-butanol with Clostridium saccharoperbutylacetonicum in a two-stage continuous culture with in-situ product removal. Biotechnol Bioeng 109:913–921
Rigouin C, Gueroult M, Croux C et al (2017) Production of medium chain fatty acids by Yarrowia lipolytica: combining molecular design and TALEN to engineer the fatty acid synthase. ACS Synth Biol 6:1870–1879
Rutter CD, Zhang S, Rao CV (2015) Engineering Yarrowia lipolytica for production of medium-chain fatty acids. Appl Microbiol Biotechnol 99:7359–7368
Saini M, Wang ZW, Chiang CJ, Chao YP (2014) Metabolic engineering of Escherichia coli for production of butyric acid. J Agric Food Chem 62:4342–4348
Sarria S, Kruyer NS, Peralta-Yahya P (2017) Microbial synthesis of medium-chain chemicals from renewables. Nat Biotechnol 35:1158–1166
Sathesh-Prabu C, Lee SK (2015) Production of long-chain α,ω-dicarboxylic acids by engineered Escherichia coli from renewable fatty acids and plant oils. J Agric Food Chem 63:8199–8208
Schuchmann K, Müller V (2013) Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science 342:1382–1385
Song H, Eom MH, Lee S et al (2010) Modeling of batch experimental kinetics and application to fed-batch fermentation of clostridium tyrobutyricum for enhanced butyric acid production. Biochem Eng J 53:71–76
Song JW, Lee JH, Bornscheuer UT, Park JB (2014) Microbial synthesis of medium-chain α,ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long-chain fatty acids. Adv Synth Catal 356:1782–1788
Song Y, Lee JS, Shin J et al (2020) Functional cooperation of the glycine synthasereductase and Wood-Ljungdahl pathways for autotrophic growth of Clostridium drakei. Proc Natl Acad Sci U S A 117:7516–7523
Tang DYY, Yew GY, Koyande AK et al (2020) Green technology for the industrial production of biofuels and bioproducts from microalgae: a review. Environ Chem Lett 18:1967–1985
Torella JP, Ford TJ, Kim SN et al (2013) Tailored fatty acid synthesis via dynamic control of fatty acid elongation. Proc Natl Acad Sci U S A 110:11290–11295
Tseng HC, Prather KLJ (2012) Controlled biosynthesis of odd-chain fuels and chemicals via engineered modular metabolic pathways. Proc Natl Acad Sci U S A 109:17925–17930
Vidra A, Németh Á (2018) Bio-produced acetic acid: a review. Period Polytech Chem Eng 62:245–256
Wang J, Lin M, Xu M, Yang ST (2016) Anaerobic fermentation for production of carboxylic acids as bulk chemicals from renewable biomass. In: Advances in Biochemical Engineering/Biotechnology. pp 323–363
Warnecke T, Gill RT (2005) Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Factories 4:25
Wentzel A, Ellingsen TE, Kotlar HK et al (2007) Bacterial metabolism of long-chain n-alkanes. Appl Microbiol Biotechnol 76:1209–1221
Werpy T, Petersen G (2004) Top value added chemicals from biomass: volume I—Results of screening for potential candidates from sugars and synthesis gas. Office of Scientific and Technical Information (OSTI). Off Sci Tech Inf 69
Wu J, Zhang X, Xia X, Dong M (2017) A systematic optimization of medium chain fatty acid biosynthesis via the reverse beta-oxidation cycle in Escherichia coli. Metab Eng 41:115–124
Xiong M, Yu P, Wang J, Zhang K (2015) Improving engineered Escherichia coli strains for high-level biosynthesis of isobutyrate. AIMS Bioeng 2:60–74
Xu P, Li L, Zhang F et al (2014) Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci U S A 111:11299–11304
Xue J, Balamurugan S, Li DW et al (2017) Glucose-6-phosphate dehydrogenase as a target for highly efficient fatty acid biosynthesis in microalgae by enhancing NADPH supply. Metab Eng 41:212–221
Youngquist JT, Lennen RM, Ranatunga DR et al (2012) Kinetic modeling of free fatty acid production in Escherichia coli based on continuous cultivation of a plasmid free strain. Biotechnol Bioeng 109:1518–1527
Yu C, Cao Y, Zou H, Xian M (2011) Metabolic engineering of Escherichia coli for biotechnological production of high-value organic acids and alcohols. Appl Microbiol Biotechnol 89:573–583
Yuzbasheva EY, Mostova EB, Andreeva NI et al (2018) A metabolic engineering strategy for producing free fatty acids by the Yarrowia lipolytica yeast based on impairment of glycerol metabolism. Biotechnol Bioeng 115:433–443
Zhang A, Yang ST (2009) Engineering Propionibacterium acidipropionici for enhanced propionic acid tolerance and fermentation. Biotechnol Bioeng 104:766–773
Zhang C, Yang H, Yang F, Ma Y (2009) Current progress on butyric acid production by fermentation. Curr Microbiol 59:656–663
Zhang H, Wang X (2016) Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng 37:114–121
Zhang K, Woodruff AP, Xiong M et al (2011) A synthetic metabolic pathway for production of the platform chemical isobutyric acid. ChemSusChem 4:1068–1070
Zhu X, Tao Y, Liang C et al (2015) The synthesis of n-caproate from lactate: a new efficient process for medium-chain carboxylates production. Sci Rep 5:14360
Zhu Y, Yang ST (2004) Effect of pH on metabolic pathway shift in fermentation of xylose by Clostridium tyrobutyricum. J Biotechnol 110:143–157
Zhu Z, Hu Y, Teixeira PG et al (2020) Multidimensional engineering of Saccharomyces cerevisiae for efficient synthesis of medium-chain fatty acids. Nat Catal 3:64–74
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhuang, L., Liu, Y., Zhang, H. (2022). Microbial Biosynthesis of Straight-Chain Aliphatic Carboxylic Acids. In: Rehm, B.H.A., Wibowo, D. (eds) Microbial Production of High-Value Products. Microbiology Monographs, vol 37. Springer, Cham. https://doi.org/10.1007/978-3-031-06600-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-06600-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06599-6
Online ISBN: 978-3-031-06600-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)