Abstract
Taking into account the benign nature of craniopharyngiomas, the main method of treatment is the resection of the tumor. However, the tendency of these tumors to invade critical structures (such as optic pathways, the hypothalamic–pituitary system, the Willis circle vessels) often limits the possibility of a radical surgery.
Craniopharyngiomas of the third ventricle represent the greatest challenge for surgery. After radical surgery, hypothalamic disorders often occur, including not only obesity but also cognitive, emotional, mental, and metabolic disturbances. Metabolic disorders associated with damage to the hypothalamus progress after surgery and lead to impaired functions of the internal organs. This process is irreversible and, in many cases, becomes the direct cause of mortality. The life expectancy of patients with the surgically affected hypothalamus is significantly shorter than in patients with preserved diencephalic function. The incidence of hypothalamic disorders after surgery can reach 40%.
Even with macroscopically total resection, craniopharyngiomas can recur in 10–30% of cases, and in the presence of tumor remnants and with no further radiation treatment, the risk of recurrence significantly increases to up to 50–85% according to various studies. For this reason, the observation of patients with residual tumors after surgery is an incorrect strategy.
Radiation therapy significantly improves progression-free survival (PFS), and the use of stereotactic irradiation techniques ensures conformity of irradiation of tumor remnants with a complicated shape and location (Iwata H et al., J Neurooncol 106(3):571–577, 2012; Aggarwal et al., Pituitary 16(1):26–33, 2013; Savateev et al., Zh Vopr Neirokhir Im N N Burdenko 81(3):94–106; 2017), which potentially reduces the risk of undesirable postradiation effects. Therefore, the quality of life in patients with craniopharyngiomas infiltrating the hypothalamus is significantly higher after non-radical operations with subsequent stereotactic radiation than after a total or subtotal removal.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Cranipharyngiomas offer the most baffling problem which confronts the neurosurgeon (H. Cushing, 1932)
Craniopharyngioma still remains a frustrating and disappointing lesion because of its inaccessibility (D. Matson, 1969)
To this day the surgical management of craniopharyngiomas remains a challenging experience to every neurologic surgeon (R. Rand, 1985)
There is perhaps no other primary brain tumor that evokes more passion, emotion, and, as a result, controversy than does the craniopharyngioma (James T. Ruthka, 2002)
Current treatment strategies are debated, ranging from radical surgical strategies … to limited surgical approaches … focused on quality of life (Stephanie Puget, 2019)
Ninety years have passed, but the craniopharyngioma is still an unsolved problem…
3.1 Introduction
Craniopharyngiomas are rare histologically benign embryonic neoplasms of the sellar/parasellar region that represent 0.5–2.5 cases per one million, that is, 2–5% of all primary brain tumors in adults [1] and 5.6–13% in children [2]. Most often, craniopharyngiomas appear in the age groups of 5–14 years and 50–74 years [3]. These tumors form the largest group of non-glial tumors in children. CP contribute to up to 56% all tumors of the chiasmo-sellar region in children [2]. In 2011, Nielsen et al. performed a meta-analysis of 15 epidemiological studies of CP (with total of 1232 patients). According to these data, the incidence was 1.34 (1.24–1.46) per one million people and 1.44 (1.33–1.56) per one million children [4].
3.2 Our Experience
During last 40 years, more than 2000 patients with craniopharyngiomas were operated in the National Burdenko Neurosurgical Center (Moscow).
Since 2005 till 2015, more than 350 children (0–18 years) with craniopharyngiomas were operated and more than 200 patients have been treated in the Department of Radiotherapy and Radiosurgery. From April 2009 to January 2015, 68 patients were irradiated with the CyberKnife.
For the comparative analysis of the treatment results of surgery and radiotherapy, 155 patients with known follow-up (mediana = 7.1 years), endocrinological, ophthalmological, quality of life investigations performed before and after surgery, MRI before, after treatment, and during follow-up were analyzed. Two hundred and ten surgical procedures including transcranial microsurgery (135), endonasal endoscopy (65), and Ommaya reservoir (10) were done. Radiotherapy was performed in 75 patients in this group.
The majority of transcranial surgery was performed by the first author.
3.3 Embriology
CPs develop from the embryonic remnants of the craniopharyngeal duct (Rathke’s pouch), connecting primary digestive tract and hypophysis during the process of pituitary gland formation. Stem cells deposited along its migratory path play a crucial role in CP formation [5].
3.4 Biology
There are two histological types of CP: adamantinomatous and papillary. Adamantinomatous CP are diagnosed both in children and adults (with two peaks 4–14 years and 40–65 years), while papillary CP are found only in adult age (50–60 years old).
Papillary craniopharyngiomas. Papillary CP usually harbor somatic BRAF V600E mutations. These tumors are composed of solid sheets of well-differentiated squamous epithelial cells interrupted by cores of fibro-vascular stroma. Keratinization and calcification are usually absent. Tumors are generally encapsulated and separated from surrounding brain tissue and are typically solid.
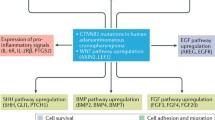
Adamantinomatous craniopharyngiomas. Adamantinomatous CP are driven by somatic mutations in CTNNB1 (which encodes β-catenin) and drastically improve β-catenin stability (Fig. 3.1b). Tumors consist of epithelial lobules. Cells at the periphery are palisaded, whereas internally situated cells are loosely textured (“stellate” reticulum). Extensive keratinization and calcification are common. Tumors are locally invasive with glial “pseudocapsule” and are predominantly cystic.
β-Catenin destruction complex under normal conditions (a), with a CTNNB1 exon 3 missense mutation (b), and with an APC truncation (c). Normally, β-catenin destruction complex enables a β-TrCP ubiquitin ligase to mark β-catenin for proteasome-mediated degradation. This relies upon β-catenin being phosphorylated by specific amino acid residues encoded in exon 3 (a, blue circles). This mechanism fails if a missense mutation is introduced to or adjacent to these sites (b). Alternatively, a truncating mutation may render APC incapable of stabilizing the destruction complex, which allows cytoplasmic β-catenin to escape even when the exon 3 is intact (c). In both scenarios, β-catenin becomes free to reach the nucleus and induce the expression of its target genes. S33, S38, T41, and S45 denote amino acid residues and their position in the protein sequence (S for serine and T for threonine). APC adenomatous polyposis coli, AXIN axin1, CK1 casein kinase 1 alpha 1, CTNNB1 catenin beta 1, GSK-3β glycogen synthase kinase 3 beta, βTrCP beta-transducin repeat containing E3 ubiquitin protein ligase
Recently, we performed whole exome sequencing (WES) in two half-sisters with adamantinomatous craniopharyngioma-produced evidence for the APC gene dysfunction as a novel tumorigenic driver in adamantinomatous CP (Fig. 3.1c). CTNNB1 remained intact, while the disruption of both APC alleles in the tumor strongly suggested the role of a second hit event [6].
Glial capsule of craniopharyngioma. Tumor cells may penetrate into surrounding brain tissue of thalamus and hypothalamus. Gliotic reactive tissue at the tumor boarder may facilitate the resection, but on the other hand provide false impression of a gross total removal (Fig. 3.2).
High expression of GHR (growth hormone receptor) is associated with shorter duration of postoperative stable disease in patients with craniopharyngioma. If high GHR expression is found in the surgical specimens of craniopharyngiomas, GH supplementation should be introduced more carefully [7].
3.5 Classification
Many classifications were proposed to reflect the biological peculiarities of CP and to facilitate the surgical planning.
Choux, Raybaud (1991) proposed the classification of these tumors based on their relation to the sellar, pituitary stalk, and third ventricle: intracellar, infundibular, intraventricular, and global.
Kassam et al. [8] in his classification based on the anatomical relation of tumor to infundibulum define the preinfundibular craniopharyngiomas (Type I), transinfundibular (Type II), and retroinfundibular (Type IIIa, b) [8].
Puget et al. [9] advocated classification based on the functional relation of the tumor to hypothalamus. Thus, she outlines the tumors with no hypothalamic involvement and thus no possible surgical hypothalamic damage (Grade 0); cases where hypothalamus is displaced by the tumor and minimal hypothalamic damage is possible (Grade 1)—and with hypothalamic involvement and severe possible hypothalamic damage (Grade 2) [9].
In the Burdenko National Neurosurgical Center, the classification based on the embriological origin is used. Thus, the following groups are distinguished: endosuprasellar, pituitary stalk, and intraextraventricular (Fig. 3.3).
Endosuprasellar craniopharyngiomas (Fig. 3.4) take their origin in the embryonic remnants of the craniopharyngeal duct in the anterior hypophysis. Thus, it primarily grows in the sella, destroys the hypophysis, and may expand to the suprasellar region. Diaphragm may be distended and become an external layer of a tumor capsule or may be penetrated when tumor grows along the stalk. Hypothalamus is displaced but not destroyed. These tumors usually manifest with the signs of hormone pituitary deficiency followed by visual disturbances.
Pituitary stalk craniopharyngiomas (Fig. 3.5) have an origin in the pituitary stalk and primarily destroy it. They grow above the diaphragm and beneath the third ventricle floor and minimally invade the hypothalamus. Tumor capsule is separated from the brain tissue by pia mater and from vessels—by arachnoidea. The major part of these tumors are cystic and may grow anteriorly under the frontal lobe (antesellar growth), posteriorly (retrosellar growth) into the prepontine cystern, and laterally to the middle fossa. The first sign is usually visual loss.
Intraextraventricular craniopharyngiomas (third ventricle CP) (Fig. 3.6) originate in the floor of the III ventricle and severely destroy the hypothalamus. This type of CP typically manifests with the signs of raised intracranial pressure.
3.6 Surgery
Surgery is still the first step in almost all the cases [10,11,12,13,14,15,16,17,18]. But today the surgical challenge is not only the visual function, not only the tumor control, not yet the endocrine function, but prevention of hypothalamic disturbances and restauration of an acceptable quality of life [19,20,21,22].
The main task of a surgeon, and at the same time the main challenge, is the identification of hypothalamus during surgery and assessment of the degree of its involvement in order to prevent its damage. It is much easier to totally remove CP than to perform subtotal removal and to leave a small fragment of a tumor capsula specially near the hypothalamic area. Unfortunately, we cannot monitor the hypothalamus!
Because of the unacceptably high complications rate and the lack of 100% prevention of recurrence following radical tumor resection, there has been a growing advocacy for less-invasive tumor resection with adjuvant therapy [9, 23].
We outline 3 grades of radicality of tumor removal: total—when there are no visible remnants on MRI (performed 24–48 h after surgery); subtotal—with flat remnants of capsula less than 0.5 cm2 (volume can’t be estimated precisely); and partial with volumetric 3D part of the tumor.
In our series, the gross total removal (GTR) was achieved in 40% of patients, subtotal (STR)—in 26%, partial (PR)—in 19%, endonasal endoscopic evacuation—in 11%, and aspiration via Ommaya reservoir—in 4% of patients.
Primary surgery has significantly better survival rate (due to more radical removal) than secondary one: 43% 5-PFS, vs. 19% 5-PFS (Gehan’s Wilcoxon: p = 0.03; Log-Rank: p = 0.05).
Localization of a tumor has no influence on recurrent free survival. There was no significant difference between groups of patients according to surgical approach; nevertheless, for the endosuprasellar tumors, recurrence free survival with transcranial approach is worse; may be due to worse visualization of the endosellar region.
Location of tumor remnants has minor influence on recurrence free survival rates. Despite the absence of significant difference, we see more rapid relapses in cases with endosellar remnants (medium RFS is 7.2 months vs. other localizations—15.0 months, Mann–Whitney; p = 0.0053). Thorough follow-up or immediate adjuvant radiotherapy should be kept in mind.
3.6.1 Endosuprasellar Craniopharyngiomas
The best option for the removal of endosuprasellar craniopharyngiomas is the endoscopic transnasal (transsphenoidal) approach (Figs. 3.4, 3.7 and 3.8). If the tumor has an anterior extension and trancranial approach is impossible (due to short optic nerves), the anterior extended transsphenoidal approach may be used (Figs. 3.9 and 3.10). If the transnasal approach is impossible (in young children, absence of sinus sphenoidalis), the transcranial subfrontal approach is indicated with endoscopic assistance, which gives the possibility to view, and manipulated in the sella with angled optics and instruments (Fig. 3.11).
Hypothalamus is usually far from the operating field and remains intact. The visual nerves in the majority of cases are long (which makes possible the removal of a tumor via subchiasmal approach).
The origin of a tumor is inside the sella, thus remnants of pituitary stalk may be seen on the upper pole of the tumor.
3.6.2 Pituitary Stalk Craniopharyngiomas (Suprasellar-Extraventricular)
The whole set of approaches were proposed for the removal of suprasellar and intraventricular tumors (Fig. 3.12).
Usually suprasellar-extraventricular CP has large cysts which facilitate surgical actions. In cases of anterior extension, a tumor is clearly seen just below the frontal lobes (Figs. 3.13 and 3.14). The ACA and AcoA are on the posterior pole of the tumor. The subfrontal approach is indicated in such cases. Visual nerves may be rather long what makes possible to remove a tumor subchiasmatically (Fig. 3.15).
In cases of suprasellar-lateral growth, pterional approach may be used. The position of the bifurcation of ICA which may be disclocated, compressed, or covered by the tumor should be kept in mind (It is extremely dangerous to manipulate above the ACA even if the tumor capsula is clearly seen as it is possible to damage the tiny perforating arteries that go across it (Figs. 3.16 and 3.17).
The most difficult situation occurs in CPs with retrosellar growth (Fig. 3.18). In these cases, the combination of subfrontal and retrosygmoid approach may be useful for the removal of both parts of the tumor.
Infundibulum is usually stretched and displaced upwards and located on the upper pole of the tumor. Capsula of suprasellar CP due to its origin is covered by the arachnoidea and even though there may be tight adhesions, it is usually feasible to find the cleavage between the capsula and infundibulum and remove tumor with minimal damage to hypothalamus.
Visual nerves and a chiasm are usually tightly attached to the tumor capsula and here is the second place where the remnants of a capsula may be left in order to preserve visual functions.
The origin of a tumor is at the pituitary stalk but above the sella, thus its remnants may be seen on the inferior (basal) surface of the tumor. In the very rare cases, it is feasible to maintain its anatomical integrity, but function is unfortunately lost.
There are three pathological components in the tumor: solid, liquid, and calcified. The latter can be represented as a huge coral-like structure which is impossible to divide into parts. It can be a source of relapse and such patients need irradiation.
3.6.3 Intraextraventricular (III Ventricle) Craniopharyngiomas
Intraextraventricular CP are located both on the scull base predominantly behind the chiasm and in the III ventricle (Figs. 3.6, 3.19 and 3.20).
The variety of basal approaches may be used for the removal of the inferior part of the tumor (Fig. 3.12).
Subchiasmal approach is the least useful as the chiasm usually shifted anteriorly and pressed to the scull base.
Translaminaterminalis approach may be used for the removal of the anterior intraventricular part of the tumor, but it is rather narrow as the distance between the chiasm and ACoA may be very small [24]. In addition, it requires the large traction of the frontal lobes (Fig. 3.21). There is a variant of this approach above the AcoA up to anterior commissure [18].
An approach through the optico-carotid triangle allows to remove the retrochiasmal part of a tumor, but it is limited by the chiasm, ICA and ACoA (Fig. 3.22). Despite the narrow space, an approach may be enlarged by gentle shift of the chiasm and ICA; an arterial spasm which often seen during surgery has no clinical significance and need no treatment.
All manipulations lateral to ICA should be very carefully done in order not to damage the a. choroidea ant. and III nerve.
Trancallosal approach (Fig. 3.23) is possible in patients with hydrocephalus. There are three ways to reach the cavity of the III ventricle: through foramen Monro, subchoroideal, and transfornical.
An access via the enlarged foramen Monro (Fig. 3.24) allows to view the whole cavity of the III ventricle except the most anterior part. Ventricle roof, aqueductus Sylvii, midbrain, basilar artery, and Lilienkvist membrane are easily seen after the gross total removal of a craniopharyngioma. The dorsum sella is the most anterior structure seen in the operating field. The anterior view is limited because of risk of columna fornicis damage which leads to fixation amnesia (Fig. 3.25). Nevertheless, transcallosal approach is the method of choice for the resection of large tumors occupying the III ventricle.
Subchoroideal access lays through the fissure between thalamus and tela choroidea and permits view of the most posterior part of the III ventricle up to the pineal region.
Transfornical approach performed by the longitudinal splitting of fornix could provide an ideal access to all parts of the III ventricle, but it is feasible only in cases of huge tumors pushing apart the bodies of the fornix. Otherwise, we have a great risk to damage it.
Transcortical approach is much easier than transcallosal one, but it is more “lateral” and cannot provide an adequate view of the ipsilateral part of the III ventricle.
In order to reach both basal and intraventricle part of the tumor, it is useful to combine basal and transcallosal approaches.
Hypothalamus is located on both sides of a tumor as an “equator” and can be hardly differentiated from tumor capsule. As this type of CP arises in the III ventricle floor, it doesn’t separate from the brain tissue by arachnoidea and thus may have digitations into hypothalamus (Fig. 3.2). That’s why, it is better to leave some remnants in this area in order to preserve the diencephalic functions.
3.7 Endoscopy-Assisted Microsurgery
Endoscope assistance during microsurgical removal of craniopharyngiomas may be performed for initial inspection of operating field, especially its deepest part and areas “around the corner”. During removal of the tumor, it provides better illumination of the operating field, additional magnification, and manipulations beyond the microscope view. In the end of the surgery, it allows to find the remnants of the tumor, to look for, and stop the possible hemorrhage in remote places.
Endoscope assistance may be performed in air or water environment.
In air environment, endoscope-assisted microsurgery is performed with a special assistant endoscope, microscope, and conventional microinstruments. For the key-hole neurosurgery, an assistant endoscope with special “para”endoscopic instruments or traditional endoscope with two working canals and endoscopic instruments may be used.
In water environment, the routine one or two working canals endoscope with endoscopic instruments may be useful.
In cases of craniopharyngioma surgery (Figs. 3.15, 3.22, 3.26 and 3.27), an endoscope allows to view the suprasellar area under the frontal lobes, the retrochiasmal space, the endosellar area, to easily identify the pituitary stalk, ACA and ACoA hidden behind the tumor, remove the endosellar part of a tumor via the subfrontal approach, and even reach the retrosellar part. The special application of this method is in the course of transcallosal approach where it makes possible the removal of the tumor anterior to foramen Monro (columna fornicis).
For endoscopic ultrasound aspiration, the Gaab endoscope, Söring endoscopic US-aspirator, water pump, bipolar endoscopic coagulation, and holding arm are necessary. For the safety of this procedure, it is necessary to maintain the balance of irrigation and suction. Irrigation is performed with the water pump through the canal of endoscope and suction—through US-aspirator. As a result, we have the clear view even in a case of bleeding! (Fig. 3.28).
In cases of cystic CP (especially polycystic ones), endoscopy may be used for fenestration of all cysts and installation of only one catheter for intracavitary treatment or controlling the cysts volume during radiotherapy (Figs. 3.29 and 3.30).
3.8 Radiation Therapy
3.8.1 Historical Note
Attempts to use radiotherapy (RT) for the treatment of CP have been undertaken since the beginning of the twentieth century. The first results of irradiation of patients with CP, published by Carpenter in 1937, were unsatisfactory and the authors concluded that “tumors of the pituitary stalk can be resistant to radiation exposure” [25].
However, in 1950, Love et al. got good results of irradiation after partial removal of the tumor [26]. Subsequently, conventional fractionated RT has begun to be used more often in patients with CP.
Kramer published the first report of RT for pediatric CP in the Royal Marsden Hospital in 1961. Between March 1952 and March 1954, six children and four adults underwent stereotactic aspiration of cyst with subsequent conventional radiotherapy. The tumor dose delivered by radiotherapy varied from 5000 R in 37 days to 6550 R in 57 days in the children, and from 5580 R in 51 days to 6950 R in 39 days in the adults. One adult patient died in 2 years of intercurrent disease, but all six children were alive and free from tumor relapse over 6.5–7 years [27].
In 1993, Rajan et al. published their results: 77 patients after non-radical surgical removal underwent a course of radiation therapy to a total dose of about 56 Gy in 1950–1986. 5-year and 10-year PFS was 83% and 79%, respectively [28].
Later with the development of technology stereotactic irradiation techniques appeared. The use of radiation therapy with incomplete removal of CP allowed to increase a progression-free survival up to 75–90% [28,29,30,31,32].
Currently, only stereotactic irradiation techniques should be used in CP, including stereotactic radiosurgery (SRS), standard fractionated, and hypofractionated RT [33].
3.8.2 Single-Fraction Radiosurgery
For the treatment of small residual tumor or relapses of CP, a stereotactic radiosurgery (SRS) can be used with a relatively high dose of radiation during a single fraction. Through this radiation technique, the dose outside the target decreases sharply without substantial damage to healthy brain tissue [34].
It was shown that PFS after radiosurgery with CP is equivalent to survival after fractionated stereotactic RT. The 5-year PFS of patients who received SRS immediately after surgery or for recurrence of craniopharyngioma was 56.7–91.6%, while the 5- and 10-year OS were 86–97% and 88–91%, respectively [35,36,37,38]. The weighted average value of the 5-year PFS, estimates on the data from several studies (231 patients) [35, 37,38,39], was 67%. It should be noted that most relapses occur outside the volume of radiosurgical irradiation.
No significant differences in the efficacy of SRS and fractionated RT were disclosed by Jeon et al. after an analysis of 50 observations [39]. The volume of tumor smaller than 1.6 cm3 and the dose more than 14.5 Gy [35] were found out by Xu et al. [35] as prognostic factors for better tumor control. The authors considered the absence of a cystic component of the tumor and the minimum number of surgical procedures before radiation as additional factors associated with a good response of CP to SRS [35].
3.8.3 Image-Guided Radiosurgery and Hypofractionated Radiotherapy
Stereotactic navigation during radiosurgery can be performed with a frame (Gamma Knife, Novalis, etc.) or using frameless navigation (CyberKnife). There may be difficulties with fixing the frame in young children, patients after surgery, especially after large bifrontal approaches, while CyberKnife has no such disadvantage. Besides that, frameless navigation provides the possibility of multiple uniform positioning of the patient, which allows to perform the hypofractional irradiation. The doses used by CyberKnife are similar to those using the Gamma Knife [40, 41] and the geometric accuracy is higher than 0.5 mm [42,43,44].
During last 20 years, the hypofractionation mode in the treatment of brain tumors was actively introduced into practice. This process was facilitated by the usage of robotic linear accelerator for the frameless stereotaxis as CyberKnife, in which modes of radiosurgery or hypofractionated radiotherapy may be carried out.
Hypofractionated radiation therapy which is performed in 2–10 fractions has a set of advantages in comparison with the standard course of radiotherapy and radiosurgery. First, unlike radiosurgical treatment, it is feasible to irradiate patients with relatively large tumors located close to or inside vital structures such as visual pathways, brainstem, pituitary gland, and hypothalamus. On the other hand, according to the biology of the tumor, the hypofractionation is similar to radiosurgery. Second, in the treatment of patients with cystic CP, fast fractionation mode which takes from 3 to 5 days allows to avoid an increase in the tumor cyst during radiotherapy and, thus not to lose the tumor borders beyond the limits of the radiation volume, which can happen with the standard course of radiotherapy (6-week duration). Third, from the radiobiological point of view, hypofractionation mode let us target the hypoxic cells.
The hypofractionation regimen uses a single dose (SD) more than 3 Gy. Irradiation in this mode can be carried out at many linear electron accelerators. However, in the majority of publications devoted to hypofractionated RT, the CyberKnife was used.
In the situation of compression of the optic chiasm by the tumor, Lee et al. reported no complications and a 90% tumor control with preservation of visual functions in 11 patients with craniopharyngiomas after treatment with CyberKnife (total dose was 20–25 Gy in 3–5 fractions) [41].
Iwata et al. analyzed results of hypofractionated radiotherapy (2–5 fractions with marginal dose of 13–25 Gy) by CyberKnife in 40 patients with craniopharyngiomas and reported the 85% PFS with a median follow-up of 3 years (tumor volume was 0.09–20.8 cm3, the hypofractionation regimens 8 Gy × 2 fractions, 7 Gy × 3 fractions, and 5 Gy × 5 fractions were used). The author noted a temporary increase of cystic component after treatment in nine patients, but no serious complications were reported [40].
Presently, the dose of 25–27.5 Gy with hypofractionated irradiation is considered to be optimal for craniopharyngiomas, as tolerant doses to critical structures [33].
We recommend to include both solid and cystic components of the tumor in the GTV when planning radiation therapy. Any tumor capsula fragments in the operative field as well as cyst walls and all calcifications visible on high resolution MRI and CT scans should be incorporated in GTV.
It is helpful to use a preoperative MR and CT scan in addition to MRI and CT studies at the time of radiation therapy, taking into consideration the data of the surgical protocol. It is recommended to use CTV equal to GTV, and PTV is formed as CTV plus 1–2 mm margin for SRS or hypofractionated SRT.
For cases with a small GTV when the position of a tumor border allows to exclude optic chiasm and the hypothalamus from the PTV, single-fraction SRS is recommended. Hypofractionated SRT can be used even in cases where critical structures are inside the tumor.
3.8.4 Standard Fractionated Radiotherapy
For standard fractionated radiotherapy, dose per fraction is less than 2.2 Gy (for WBRT—up to 3.0 Gy). Stereotactic technology of irradiation provides full coverage of tumor and minimal dose to healthy tissue and vessels. It allows to reduce risk of side effects [45,46,47,48,49].
5-year PFS after SRT for CP with total dose 50–54 Gy exceeds 73% [50]. Regime after analysis of 56 patients with 17 years median follow-up after SRT demonstrated that recurrence risk increased to 44% with dose less than 54 Gy in contrast with 16% with dose more than 54 Gy [51]. Currently, total dose for standard fractionates stereotactic radiotherapy is usually 54 Gy, delivered in 30 fractions.
According to the experience of the National Burdenko Neurosurgical Center, 5-year PFS after SRT with total dose 54 Gy for partial resected CP or it’s relapse is 83.6%. It is recommended to include in CTV all solid and cystic parts of tumor and tumor bed which may contain small residual fragments of tumor.
3.9 Results of Surgical Treatment and Irradiation
As it was mentioned above, the main task of a craniopharyngioma treatment is the avoidance of recurrence on one hand and prevention of hypothalamic disturbances on the other hand. That’s why it is so important to compare two main methods of treatment: radical surgery and subtotal surgery with irradiation.
3.9.1 Recurrence-Free Survival
Conservative surgery is associated with higher recurrence rates with an average rate of 75%. Limited surgery and RT have demonstrated results equivalent to radical surgery alone. Recent studies report at least 90% disease control with 5-year follow-up [52].
Radiotherapy dramatically increases the survival rate in non-total surgery (Fig. 3.31). The degree of radicality does not matter, but almost all non-totally removed CP (partial and subtotal) recover sooner or later! We obtained the exactly equal results of GTR and non-GTR + radiotherapy (Fig. 3.32).
Another important question is the time when to start radiotherapy in cases of small remnants: either to start immediately after surgery or to wait for the first signs of recurrence. The timing of post-surgery RT remains controversial [53]. Several studies demonstrated no significant difference in progression-free survival after early postoperative versus late radiotherapy for tumor regrowth [54]. In contrast, others found fewer craniopharyngioma relapse with early radiotherapy given as initial therapy when compared with those received late radiotherapy for recurrence [55]. Our investigation shows that PFS rates after adjuvant and salvage radiotherapy are equal (Fig. 3.33).
From April 2009 to January 2020, more than 200 patients were irradiated. All of them had previous surgery, 2/3 of them—by transcranial and 1/3—by transnasal approaches.
Radiosurgery was performed in patients with small tumors (<5 cm3) and sufficient distance to optic pathways, or for tumors in contact with the optic nerve on the side of the blind eye. Median dose was 16 Gy. Median target volume—1.7 сm3 (0.07–5.1). 5-year PFS was 85%.
Patients with larger tumors (>5 сm3) and/or with a tumor adjacent to critical structures were irradiated with hypofractionated regimen. The following regimens were used: 3 fr. × 7 Gy (total dose 21 Gy), 5 fr. × 5 Gy (total dose 25 Gy), and 5 fr. × 5.5 Gy (total dose 27.5 Gy). Median tumor volume was 3.1 cm3 (0.25–15.3). After hypofractionated SRT for CP, 5-year PFS was 91%. After irradiation with a dose of 27.5 Gy for 5 fractions, PFS was higher and reached 96%.
Standard fractionated SRT was performed for patients with the largest tumors, with infiltration of the optic nerves and in the presence of multiple small fragments of the capsule along the tumor bed after surgery. Median dose was 54 Gy. 10-year PFS reached 82%.
The median follow-up was 48.4 months (1.4–95.1 months). Progression of cystic component was observed in 4.4% patients (which required aspiration of the cyst or its surgical removal). Progression of the solid component of the tumor was absent. Average 5-year PFS after all types of SRT and SRS (86%) was higher than after partial or subtotal removal of CP without subsequent irradiation (19%), p < 0.00001, and equivalent to 5-year PFS after total removal of the tumor (79%), p = 0.4.
3.9.2 Surgical and Radiation Morbidity
Although RT now is more commonly used in the management of childhood onset CP, it may carry considerable morbidity and mortality. Endocrinopathies, vasculopathies, visual deterioration, neurocognitive impairment, and second malignancies (1.9% at 10 years) are registered following RT for these tumors [52]. However, the vast majority of studies of side effects of radiation treatment reflect the risks associated with the use of conventional RT—a method which is currently no longer used in CP. Stereotactic irradiation methods provide much lower doses on critical structures minimizing complications.
Thus, with surgery and stereotactic radiation therapy, only visual, endocrine, and cognitive complications should be monitored.
3.9.3 Visual Deficits
In the majority of patients (80%) after surgery, visual functions remain stable or improve (Fig. 3.34). Only in 20% of patients, vision deterioration was detected. These cases are related to situations where capsule was firmly attached to the visual pathways, or to an attempt to resect a solid calcification behind the chiasm. The main risk factor for visual deterioration is the low vision before surgery. The worse the vision was before surgery, the greater was the risk of its further decline.
It is believed that the maximal tolerance single dose to the visual pathways is 10–12 Gy [29, 56]. The risk of radiation damage to the visual pathways is associated with fraction dose and total dose. According to the published data, radiation injury to the optic nerves is observed in 1–2% of patients who received a dose of 50 Gy or more with conventional irradiation and more often is observed in patients who had previously shown visual disturbances [28, 57].
Among patients who received 50–55 Gy with 1.8 Gy per fraction, the risk of visual deficit is less than 2.5% [58,59,60]. However, the frequency of this complication significantly increases at doses of 55–60 Gy [51, 61]. After stereotactic radiosurgery and hypofractionated RT, damage to the optic nerves was noted only in a few cases [41, 62].
The same results were supported in our investigations (Fig. 3.35).
3.9.4 Endocrinological Dysfunctions
To estimate an influence of surgery and radiotherapy on endocrine functions, it is necessary to compare the state of endocrinological functions before and after treatment in the long-term follow-up period.
3.9.4.1 Endocrine Disorders at the Time of Diagnosis
Tumor embryogenesis determines its close approximation to hypothalamus–pituitary complex and high incidence of endocrine disorders in CP patients. Inflammation could be an additional factor in the formation of hypopituitarism, particularly GH deficiency through IL-1α-induced pituitary fibrosis [63].
The first clinical symptoms of CP include headache, vomiting, visual loss, and endocrine disorders: growth retardation, delayed puberty in elder children, excessive thirst and polyuria, weight gain, and fatigue.
According to analysis of 411 pediatric patients [64], the most frequent symptom before diagnosis was headache—50%; the combination of headache and growth failure was most frequent (18%). The median time between appearance of first sign of disease until diagnosis was 12 months, with a range of 0.01–96 months. Stunt growth and weight gain were the symptoms observed with the longest duration of history. A combination of headache, visual impairment, decreased growth velocity, weight gain, and polydipsia and/or polyuria is highly specific for childhood CP and should lead to further investigation [65].
Endocrine disorders include anterior pituitary deficiencies, diabetes insipidus, and weight gain/obesity. At the time of CP diagnosis, at least one endocrine deficit is presented in 40–87% of patients [66,67,68].
GH deficiency is the most common deficit in children occurring in up to 75% of patients at the time of diagnosis [69]. The main symptom is decreased growth velocity (less than 4 cm/year), resulting in short stature. Other signs include decreased muscle mass and strength, increased subcutaneous and visceral fat mass, and dyslipidemia.
Gonadotropin deficiency (LH and FSH) is the most common deficit in adolescents occurring in up to 50–70% of patients resulting in arrested puberty in younger children, amenorrhea, or erectile dysfunction in elder adolescents.
Secondary hypothyroidism (TSH deficiency) is present in 21–42% of cases. Symptoms include weight gain, dry skin, dry and brittle hair, fatigue, cold intolerance, and bradycardia.
Secondary adrenal insufficiency (ACTH deficiency) occurs in 20–25% of patients at the time of diagnosis and can present with fatigue, myalgias, arthralgias, weakness, and hypoglycemia and hyponatremia due to glucocorticoid deficiency.
Diabetes insipidus (ADH deficiency) occurs in 17–28% of patients and may present with polydipsia and polyuria with low urine osmolality.
Obesity/weight gain is the third most common clinical endocrine abnormality associated with CP. Hypothyroidism, GH deficiency, and direct hypothalamic injury can contribute to obesity and weight gain. Obesity and weight gain are reported in about 20% of presenting patients.
Hypopituitarism can be easily diagnosed using basal hormone levels except GH and ACTH deficiency, which may require the stimulation tests. Preoperative assessment includes detecting of basal levels of TSH/T4, cortisol, LH/FSH, sex steroids, cortisol, IGF-1 levels, urinary output, and osmolality. Obtained test results allow for perioperative hormone replacement as necessary and may be helpful in CP topography detection.
Some studies have reported about higher incidence of pituitary dysfunction at the diagnosis in childhood onset CP, than in adult onset [66,67,68].
According to our data of 155 CP pediatric patients, endocrine deficit depends on the tumor location. Patients with endosellar CP had more prominent pituitary deficiency before surgery (20% panhypopituitarism) than patients with suprasellar CP (5% panhypopituitarism). In patients with endosellar CP, the incidence of GH deficiency before surgery was 98%, TSH deficiency 74.5%, ACTH deficiency 67.4%, gonadotropin deficiency 86.7%, diabetes insipidus 25.9%, and 20% had panhypopituitarism. In patients with suprasellar CP, the incidence of GH deficiency before surgery was 81.5%, TSH deficiency 18.2%, ACTH deficiency 14.9%, gonadotropin deficiency 88.5%, diabetes insipidus 10.5%, and 5% had panhypopituitarism [70]. Preoperative assessment of endocrine function can be useful in the detection of CP location and surgery planning.
3.9.4.2 Endocrine Disorders After Surgery and Radiotherapy
Hypothalamic–pituitary dysfunction may result from the tumor itself or its surgical or radiation treatment; however, additional dysfunction may develop in the months to years following initial treatment.
3.9.4.2.1 Pituitary Insufficiency
Majority of clinical studies provide data on the deterioration of endocrine function upon treatment at the follow-up up to 95–100% of pediatric onset СP. Patients have at least one endocrine deficiency and about 50% children are obese [66,67,68, 70, 71].
According to our data, 80% patients after surgery had panhypopituitarism and diabetes insipidus [70]. Therefore, RT as a risk factor for endocrine deficiency is important only in 20% of patients with partially preserved endocrine functions.
Nevertheless, hormone deficit before and after surgery depends on the localization of a tumor. It increases nearly to the same extent in all types of tumors, but the initial state is much worse in endosuprasellar tumors (Fig. 3.36a–c).
The majority of studies showed that the incidence of anterior pituitary insufficiency is similar in patients treated with surgery alone compared to those who had surgery and RT [68]. The dynamics of endocrine functions after combined treatment in our series were estimated only in patients with partial hormone deficit after surgery before irradiation. There was a minor deterioration of hormone state after radiation therapy due only to the increased ACTH deficiency [70] (Fig. 3.36d).
It was shown that an average single dose of 15 Gy or less to adenohypophysis does not cause hypothyroidism and hypogonadism, and a single dose of 18 Gy or less does not cause hypocortisolism [72].
The rate of radio-induced endocrinopathies is a dose-dependent parameter. With conventional irradiation with a dose of more than 60 Gy, the new endocrine deficiency was detected in more than 80% of patients and with dose of 54–60 Gy—in 36% [73]. Now the doses greater than 60 Gy are not used in RT.
Xu et al. showed that SRS in patients with endosellar pituitary adenoma causes pituitary deficiency up to 30% of irradiated patients within 3 years, developing in the majority of cases somatotropic insufficiency and hypothyroidism, less often hypogonadism, and even less often hypocorticism.
Diabetes insipidus is not typical for RT/SRS and appears in the majority of cases due to tumor regrowth, rather than to radiation damage [52].
3.9.4.2.2 Hormonal Substitutional Therapy
Adequate hormonal substitutional therapy can restore normal pituitary physiology. The impact of hypopituitarism on the QoL is low [74], so preservation of pituitary function isn’t the goal of the surgery.
Substitutional therapy includes GH therapy in GH deficiency, l-thyroxine in hypothyroidism, hydrocortisone in adrenal insufficiency, and desmopressin in diabetes insipidus. As ACTH deficiency can be life-threatening, substitution should be initiated as soon as a deficit is confirmed. Hypogonadism is usually compensated by sex steroids replacement in both sexes; LH, FSH analogs, or pulsatile gonadotropin-releasing hormone may be recommended in fertility induction.
Despite GH deficiency, some CP patients after tumor resection have normal or accelerated growth. Insulin and leptin presumably play a leading role in this phenomenon.
Patients with pituitary insufficiency have higher rates of cardiovascular complications and mortality in comparison to the general population [75], so follow-up and proper hormonal replacement therapy in CP patients are crucially important. GH substitution is safe in terms of its possible effect on the risk of tumor recurrence [76] and may have a positive effect on body composition and lipid profile. Compensation of hypogonadism with low-dose estrogens instead of contraceptives in female and avoiding of glucocorticoid overreplacement may decrease cardiovascular morbidity and mortality.
3.9.4.2.3 Hypothalamic Dysfunction and Obesity
Another factor worsening quality of life and increasing the risks of metabolic syndrome, cardiovascular disease, and contributing to early mortality is weight gain/obesity.
In 1996, De Vile et al. for the first time have shown that hypothalamic lesions positively correlated with weight gain after tumor resection [77].
Hypothalamic area is a relatively small structure of 100 mm3 volume, containing several nuclei and tracts which have highly diverse molecular, functional, and structural organization, providing important functions which can be summarized to maintain homeostasis. It includes regulation of endocrine, metabolic, autonomic, emotional, and behavioral functions, including neurohormonal control of the pituitary gland, hemodynamic, water and electrolyte metabolism, energy supply, body weight and eating behavior, sexual/reproduction functions, sleep cycles, body temperature regulation, and cognitive and memory function .
The symptoms of hypothalamic dysfunction may include rapid weight gain/obesity (in rare cases cachexia), emotional and behavioral changes, disturbed circadian rhythm (daytime sleepiness), body temperature disturbances (hyper/hypothermia), imbalances in regulation of thirst, hemodynamic homeostasis (heart rate, and/or blood pressure), memory and cognitive impairment, and in rare cases urinary/fecal incontinence.
At the time of CP diagnosis, signs of hypothalamic dysfunction may present in about 20–35% of childhood patients, but its incidence dramatically increases following surgical treatment up to 50–70% [69].
Obesity. Patients usually rapidly have increased weight within the first year after surgery due to impairment of satiety regulation and hyperphagia. In contrast to ordinary obesity, caloric restriction and lifestyle modification have usually low effect to prevent or treat the hypothalamic obesity due to their multifactorial origin.
The pathogenesis of hypothalamic obesity involves the inability of adequate transduction of afferent hormonal signals of adiposity, in effect mimicking a state of CNS starvation [78]. It is associated with several endocrine dysfunctions, such as hyperleptinemia, hyperinsulinemia, decreased metabolic rate due to suppression of sympathetic nervous system activity, impaired 11β-hydroxysteroid dehydrogenase-1 activity, and dysregulation of melatonin and oxytocin secretion. Furthermore, increased daytime sleepiness, reduced physical activity, chronic apathy, and psychosocial problems may also contribute to the obesity development.
Despite theoretical understanding in mechanisms of hypothalamic obesity, effective medical treatment has not yet been developed.
In our investigations, it was shown that radicality of tumor removal and its location plays a crucial role in deterioration of hypothalamic dysfunction. Body mass index (BMI) as a marker of a diencephalic damage is much higher in patients with total surgery (Kolmogorov–Smirnov = 0.25, 160, p < 0.01) (Table 3.1) BMI also correlates with tumor location: it is significantly higher in patients with III ventricle craniopharyngiomas (p = 0.0252) (Table 3.2).
The role of radicality of tumor removal and the importance of preservation of hypothalamic area may be illustrated on two very similar cases of intraextraventricular craniopharyngiomas. In the first case (Fig. 3.37), the CP was removed totally with unavoidable hypothalamic damage which resulted in panhypopituitarism, diabetes insipidus, obesity, body temperature disturbances, memory and cognitive impairment, and limited socialization. In another case, the tumor was resected subtotally and a small piece of capsula was intentionally left at infundibulum which allowed to avoid hypothalamic disturbances. Stereotactic irradiation prevented recurrence without further deterioration of endocrine functions (Fig. 3.38).
3.9.4.2.4 Quality of Life
Hypothalamic obesity is the major factor, negatively influencing QoL in CP patients. In the multinational KRANIOPHARYNGEOM 2007 trial, CP patients treated with gross total resection resulting in hypothalamic lesions presented with significantly lower self- and parent-assessed QoL during the follow-up of 3 years after surgery than patients treated with incomplete resection with or without radiotherapy [79].
Since the main factor determining the decline in the quality of life in CP patients is hypothalamus dysfunction over the last two decades, the morbidity associated with hypothalamic function has become the focus of CP outcome. Planning of surgery strategy and avoiding irreversible hypothalamic damage became key goals in the treatment and have led to a trend towards more conservative surgery, aiming to preserve hypothalamic structures, with a greater reliance on postoperative radiation treatment [80].
Recent studies demonstrate that gross total resection associates with higher morbidity and mortality compared to subtotal resection and RT [81].
Despite the trend towards hypothalamus-sparing surgery in patients with initial hypothalamic tumor involvement and as a result, decrease of the frequency of hypothalamic obesity, about half of the patients are morbidly obese [82].
In our study on 155 CP pediatric patients in cases of endosellar CP, the degree of tumor resection didn’t influence QoL. In patients with suprasellar CP, QoL score was significantly higher after partial resection/Omaya followed by RT, than after gross total resection (Fig. 3.39).
3.9.5 Сognitive and Neuropsychological Dysfunction
After radiation treatment of tumors of sellar and parasellar localization, cognitive deficiency was observed in the 1990s [83, 84]. The correlation between cognitive impairment and exposure of large area of the brain in conventional RT is well known. The invention of stereotactic RT and SRS decreased the frequency and severity of cognitive disturbances [28, 51, 85,86,87,88].
Merchant (2006) analyzed IQ in 27 patients with craniopharyngiomas before and after stereotactic RT. Follow-up during 48 months showed a significant difference in the IQ between patients younger and older than the age of 7.4 at the time of RT. Moreover, the intelligence level of children younger than 7.4 years after RT decreased linearly over time, while in older patients it remained almost stable [86].
Kiehna together with Merchant analyzed in 2010 32 articles concerning RT in children with craniopharyngiomas and noted that IQ remains stable during 5 years after conformal RT with a possible subsequent decrease [52]. The following negative prognostic factors for cognitive functions were found: an age less than 7 years old at the time of RT, female gender, presence of hydrocephalus, large cystic component, traumatic surgery, and diabetes insipidus before surgery [52].
3.10 Conclusions
We advocate the treatment of CrPh according to the degree of hypothalamic involvement estimated by localization of tumor and confirmed at surgery (Fig. 3.40).
To the low-risk group, we refer the patients with endosupresellar and suprasellar extraventricular tumors. There is no hydrocephalus in such patients as there are no CSF occlusion in the III ventricle area. Due to their origin, these tumors don’t infiltrate the hypothalamus and the safe gross total removal may be performed.
In the high-risk group, there are patients with third ventricle craniopharyngiomas (intraextraventricular) with related hydrocephalus and hypothalamic syndrome. These tumors usually invade the hypothalamic area and cannot be totally removed without its damage.
Despite the MRI data, the precise relation of CP to hypothalamus may be revealed only during surgery.
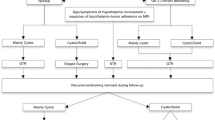
In cases of GTR, we recommend observation with MRI investigations every 6 months at least during 2 years, then once a year. In patients older than 7 years with subtotal removal, the stereotactic radiotherapy is indicated.
If the purely cystic recurrence is diagnosed, the intracavitary treatment may be performed.
The use of stereotactic radiotherapy or radiosurgery in a setting of presence of a residual tumor, tumor relapse, or progression of craniopharyngioma significantly increases the disease-free survival after non-radical surgery to a level similar to that obtained after total resection of the tumor. SRT and SRS after non-radical surgery are safer for visual function preservation than total removal of the craniopharyngioma.
Stereotactic irradiation in patients with CP rarely exacerbates hormonal deficiency (6.7% of cases). Hypofractionated SRT and SRS do not lead to a worsening of the diencephalic disorders. In patients with CP that infiltrates the third ventricle, the quality of life is higher after non-radical operations followed by stereotactic radiation than after total or subtotal removal of the tumor.
References
Samii M, Tatagiba M. Craniopharyngioma. In: Kaye AH, Laws Jr ER, editors. Brain tumors: an encyclopedic approach. New York: Churcill Livingstone; 1995. p. 873–94.
Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17(9):503–11.
Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89(4):547–51.
Nielsen EH, Feldt-Rasmussen U, Poulsgaard L, Kristensen LO, Astrup J, Jorgensen JO, et al. Incidence of craniopharyngioma in Denmark (n = 189) and estimated world incidence of craniopharyngioma in children and adults. J Neurooncol. 2011;104(3):755–63.
Martinez-Barbera JP, Andoniadou CL. Concise review: paracrine role of stem cells in pituitary tumors: a focus on adamantinomatous craniopharyngioma. Stem Cells. 2016;34(2):268–76.
Gorelyshev A, Mazerkina N, Medvedeva O, Vasilyev E, Petrov V, Ryzhova M, et al. Second-hit APC mutation in a familial adamantinomatous craniopharyngioma. Neuro Oncol. 2020;22(6):889–91.
Ogawa Y, Watanabe M, Tominaga T. Prognostic factors of craniopharyngioma with special reference to autocrine/paracrine signaling: underestimated implication of growth hormone receptor. Acta Neurochir. 2015;157(10):1731–40.
Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108(4):715–28.
Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106(1 Suppl):3–12.
Elliott RE, Wisoff JH. Surgical management of giant pediatric craniopharyngiomas. J Neurosurg Pediatr. 2010;6(5):403–16.
Gorelyshev S. Surgical treatment of craniopharyngiomas of the III ventricle in children. The dissertation for the degree of candidate of medicine doctor. Moscow: USSR Academy of Medical Sciences; 1989.
Konovalov AN, Gorelyshev SK. Surgical treatment of anterior third ventricle tumours. Acta Neurochir. 1992;118(1–2):33–9.
Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI. Aggressive surgical management of craniopharyngiomas in children. J Neurosurg. 1992;76(1):47–52.
Konovalov AN, Vikhert TM, Korshunov AG, Gorelyshev SK. Evaluation of the radicalness of the removal of craniopharyngioma of the 3d ventricle in children and the possible sources of their continued growth and recurrence. Zh Vopr Neirokhir Im N N Burdenko. 1988;6:7–12.
Elliott RE, Jane JA Jr, Wisoff JH. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery. 2011;69(3):630–43; discussion 43.
Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg. 1999;90(2):237–50.
Zuccaro G. Radical resection of craniopharyngioma. Childs Nerv Syst. 2005;21(8–9):679–90.
Shi XE, Wu B, Fan T, Zhou ZQ, Zhang YL. Craniopharyngioma: surgical experience of 309 cases in China. Clin Neurol Neurosurg. 2008;110(2):151–9.
Barajas MA, Ramirez-Guzman G, Rodriguez-Vazquez C, Toledo-Buenrostro V, Velasquez-Santana H, del Robles RV, et al. Multimodal management of craniopharyngiomas: neuroendoscopy, microsurgery, and radiosurgery. J Neurosurg. 2002;97(5 Suppl):607–9.
Albright AL, Hadjipanayis CG, Lunsford LD, Kondziolka D, Pollack IF, Adelson PD. Individualized treatment of pediatric craniopharyngiomas. Childs Nerv Syst. 2005;21(8–9):649–54.
Schoenfeld A, Pekmezci M, Barnes MJ, Tihan T, Gupta N, Lamborn KR, et al. The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neurooncol. 2012;108(1):133–9.
Muller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5(1):75.
Tomita T, Bowman RM. Craniopharyngiomas in children: surgical experience at children’s memorial hospital. Childs Nerv Syst. 2005;21(8–9):729–46.
Rhoton AL Jr, Yamamoto I, Peace DA. Microsurgery of the third ventricle: part 2: operative approaches. Neurosurgery. 1981;8(3):357–73.
Carpenter RC, Chamberlin GW, Frazier CH. The treatment of hypophyseal stalk tumors by evacuation and irradiation. Am J Roentgenol. 1937;38:162–77.
Love JG, Marshall TM. Craniopharyngiomas. Surg Gynecol Obstet. 1950;90(5):591–601.
Kramer S, McKissock W, Concannon JP. Craniopharyngiomas: treatment by combined surgery and radiation therapy. J Neurosurg. 1961;18:217–26.
Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, et al. Craniopharyngioma—a long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26(1):1–10.
Aggarwal A, Fersht N, Brada M. Radiotherapy for craniopharyngioma. Pituitary. 2013;16(1):26–33.
Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, et al. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 1993;27(2):189–95.
Habrand JL, Ganry O, Couanet D, Rouxel V, Levy-Piedbois C, Pierre-Kahn A, et al. The role of radiation therapy in the management of craniopharyngioma: a 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys. 1999;44(2):255–63.
Van Effenterre R, Boch AL. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97(1):3–11.
Golanov AV, Savateev AN, Trunin YY, Antipina NA, Nikitin KV, Konovalov AN. Craniopharyngiomas. In: Conti A, Romanelli P, Pantelis E, Soltys SG, Cho YH, Lim M, editors. CyberKnife NeuroRadiosurgery. Cham: Springer; 2020.
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316–9.
Xu Z, Yen CP, Schlesinger D, Sheehan J. Outcomes of gamma knife surgery for craniopharyngiomas. J Neurooncol. 2011;104(1):305–13.
Hasegawa T, Kobayashi T, Kida Y. Tolerance of the optic apparatus in single-fraction irradiation using stereotactic radiosurgery: evaluation in 100 patients with craniopharyngioma. Neurosurgery. 2010;66(4):688–94; discussion 94–5.
Niranjan A, Kano H, Mathieu D, Kondziolka D, Flickinger JC, Lunsford LD. Radiosurgery for craniopharyngioma. Int J Radiat Oncol Biol Phys. 2010;78(1):64–71.
Kobayashi T, Kida Y, Mori Y, Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103(6 Suppl):482–8.
Jeon C, Kim S, Shin HJ, Nam DH, Lee JI, Park K, et al. The therapeutic efficacy of fractionated radiotherapy and gamma-knife radiosurgery for craniopharyngiomas. J Clin Neurosci. 2011;18(12):1621–5.
Iwata H, Tatewaki K, Inoue M, Yokota N, Baba Y, Nomura R, et al. Single and hypofractionated stereotactic radiotherapy with CyberKnife for craniopharyngioma. J Neurooncol. 2012;106(3):571–7.
Lee M, Kalani MY, Cheshier S, Gibbs IC, Adler JR, Chang SD. Radiation therapy and CyberKnife radiosurgery in the management of craniopharyngiomas. Neurosurg Focus. 2008;24(5):E4.
Iwata H, Sato K, Tatewaki K, Yokota N, Inoue M, Baba Y, et al. Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro Oncol. 2011;13(8):916–22.
Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP. An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery. 2003;52(1):140–6. discussion 6–7
Antypas C, Pantelis E. Performance evaluation of a CyberKnife G4 image-guided robotic stereotactic radiosurgery system. Phys Med Biol. 2008;53(17):4697–718.
Cocchi U. Radiotherapy of brain tumors; therapeutic results and complications. Strahlentherapie. 1957;15(Sonderbd. 37):317–55.
Fike JR, Cann CE, Turowski K, Higgins RJ, Chan AS, Phillips TL, et al. Radiation dose response of normal brain. Int J Radiat Oncol Biol Phys. 1988;14(1):63–70.
Delattre JY, Poisson M. Neurologic complications of brain radiotherapy: contribution of experimental studies. Bull Cancer. 1990;77(7):715–24.
Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35(6):659–63.
Amaral F. Current knowledge on major complications after whole brain radiotherapy or radiosurgery. Acta Med Port. 2010;23(1):85–94.
Clark AJ, Cage TA, Aranda D, Parsa AT, Sun PP, Auguste KI, et al. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst. 2013;29(2):231–8.
Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol. 1993;27(1):13–21.
Kiehna EN, Merchant TE. Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus. 2010;28(4):E10.
Kortmann RD. Different approaches in radiation therapy of craniopharyngioma. Front Endocrinol (Lausanne). 2011;2:100.
Stripp DC, Maity A, Janss AJ, Belasco JB, Tochner ZA, Goldwein JW, et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58(3):714–20.
Lin LL, El Naqa I Leonard JR, Park TS, Hollander AS, Michalski JM, , et al. Long-term outcome in children treated for craniopharyngioma with and without radiotherapy. J Neurosurg Pediatr 2008;1(2):126–130.
Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(1):43–50.
Brada M, Thomas DG. Craniopharyngioma revisited. Int J Radiat Oncol Biol Phys. 1993;27(2):471–5.
Combs SE, Thilmann C, Huber PE, Hoess A, Debus J, Schulz-Ertner D. Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Cancer. 2007;109(11):2308–14.
Merchant TE. Craniopharyngioma radiotherapy: endocrine and cognitive effects. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):439–46.
Minniti G, Saran F, Traish D, Soomal R, Sardell S, Gonsalves A, et al. Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiother Oncol. 2007;82(1):90–5.
Varlotto JM, Flickinger JC, Kondziolka D, Lunsford LD, Deutsch M. External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys. 2002;54(2):492–9.
Chiou SM, Lunsford LD, Niranjan A, Kondziolka D, Flickinger JC. Stereotactic radiosurgery of residual or recurrent craniopharyngioma, after surgery, with or without radiation therapy. Neuro Oncol. 2001;3(3):159–66.
Mao J, Qiu B, Mei F, Liu F, Feng Z, Fan J, et al. Interleukin-1alpha leads to growth hormone deficiency in adamantinomatous craniopharyngioma by targeting pericytes: implication in pituitary fibrosis. Metabolism. 2019;101:153998.
Hoffmann A, Boekhoff S, Gebhardt U, Sterkenburg AS, Daubenbuchel AM, Eveslage M, et al. History before diagnosis in childhood craniopharyngioma: associations with initial presentation and long-term prognosis. Eur J Endocrinol. 2015;173(6):853–62.
Muller HL. The diagnosis and treatment of craniopharyngioma. Neuroendocrinology. 2020;110(9–10):753–66.
Wijnen M, van den Heuvel-Eibrink MM, Janssen JA, Catsman-Berrevoets CE, Michiels EM, van Veelen-Vincent MC, et al. Very long-term sequelae of craniopharyngioma. Eur J Endocrinol. 2017;176(6):755–67.
Jazbinsek S, Kolenc D, Bosnjak R, Faganel KB, Zadravec ZL, Jenko BB, et al. Prevalence of endocrine and metabolic comorbidities in a national cohort of patients with craniopharyngioma. Horm Res Paediatr. 2020;93(1):46–57.
Hussein Z, Glynn N, Martin N, Alkrekshi A, Mendoza N, Nair R, et al. Temporal trends in craniopharyngioma management and long-term endocrine outcomes: a multicentre cross-sectional study. Clin Endocrinol (Oxf). 2021;94(2):242–9.
Muller HL. Craniopharyngioma. Endocr Rev. 2014;35(3):513–43.
Mazerkina NA, Konovalov AN, Gorelyshev SK, Semenova ZB, Krasnova TS, Tenedieva VD. Endocrine disorders in craniopharyngiomas in children: dependence on the site of a tumor. Zh Vopr Neirokhir Im N N Burdenko. 2008;1:23–9.
Tiulpakov AN, Mazerkina NA, Brook CG, Hindmarsh PC, Peterkova VA, Gorelyshev SK. Growth in children with craniopharyngioma following surgery. Clin Endocrinol (Oxf). 1998;49(6):733–8.
Xu Z, Lee Vance M, Schlesinger D, Sheehan JP. Hypopituitarism after stereotactic radiosurgery for pituitary adenomas. Neurosurgery. 2013;72(4):630–7.
Regine WF, Kramer S. Pediatric craniopharyngiomas: long term results of combined treatment with surgery and radiation. Int J Radiat Oncol Biol Phys. 1992;24(4):611–7.
Tang B, Xie S, Huang G, Wang Z, Yang L, Yang X, et al. Clinical features and operative technique of transinfundibular craniopharyngioma. J Neurosurg. 2019;133(1):119–28.
Bulow B, Hagmar L, Eskilsson J, Erfurth EM. Hypopituitary females have a high incidence of cardiovascular morbidity and an increased prevalence of cardiovascular risk factors. J Clin Endocrinol Metab. 2000;85(2):574–84.
Alotaibi NM, Noormohamed N, Cote DJ, Alharthi S, Doucette J, Zaidi HA, et al. Physiologic growth hormone-replacement therapy and craniopharyngioma recurrence in pediatric patients: a meta-analysis. World Neurosurg. 2018;109:487–96.
De Vile CJ, Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, et al. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg. 1996;85(1):73–81.
Lustig RH. Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment. Front Endocrinol (Lausanne). 2011;2:60.
Hoffmann A, Warmth-Metz M, Gebhardt U, Pietsch T, Pohl F, Kortmann RD, et al. Childhood craniopharyngioma—changes of treatment strategies in the trials KRANIOPHARYNGEOM 2000/2007. Klin Padiatr. 2014;226(3):161–8.
Cossu G, Jouanneau E, Cavallo LM, Elbabaa SK, Giammattei L, Starnoni D, et al. Surgical management of craniopharyngiomas in adult patients: a systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir. 2020;162(5):1159–77.
Marcus HJ, Rasul FT, Hussein Z, Baldeweg SE, Spoudeas HA, Hayward R, et al. Craniopharyngioma in children: trends from a third consecutive single-center cohort study. J Neurosurg Pediatr. 2019;25(3):242–50.
van Iersel L, Meijneke RW, Schouten‐van Meeteren AY, Reneman L, de Win MM, van Trotsenburg AP, et al. The development of hypothalamic obesity in craniopharyngioma patients: a risk factor analysis in a well-defined cohort. Pediatr Blood Cancer. 2018;65(5):e26911.
Grattan-Smith PJ, Morris JG, Shores EA, Batchelor J, Sparks RS. Neuropsychological abnormalities in patients with pituitary tumours. Acta Neurol Scand. 1992;86(6):626–31.
Peace KA, Orme SM, Sebastian JP, Thompson AR, Barnes S, Ellis A, et al. The effect of treatment variables on mood and social adjustment in adult patients with pituitary disease. Clin Endocrinol (Oxf). 1997;46(4):445–50.
Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, et al. Craniopharyngioma: the St. Jude Children’s Research Hospital experience 1984–2001. Int J Radiat Oncol Biol Phys. 2002;53(3):533–42.
Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006;104(2 Suppl):94–102.
Pemberton LS, Dougal M, Magee B, Gattamaneni HR. Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol. 2005;77(1):99–104.
Savateev AN, Trunin YY, Mazerkina NA. Radiotherapy and radiosurgery in treatment of craniopharyngiomas. Zh Vopr Neirokhir Im N N Burdenko. 2017;81(3):94–106.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gorelyshev, S., Savateev, A.N., Mazerkina, N., Medvedeva, O., Konovalov, A.N. (2022). Craniopharyngiomas: Surgery and Radiotherapy. In: Di Rocco, C. (eds) Advances and Technical Standards in Neurosurgery. Advances and Technical Standards in Neurosurgery, vol 45. Springer, Cham. https://doi.org/10.1007/978-3-030-99166-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-99166-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99165-4
Online ISBN: 978-3-030-99166-1
eBook Packages: MedicineMedicine (R0)