Abstract
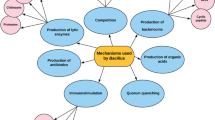
One of the activities of probiotics is their ability to control the onset of infectious diseases. The most common mechanism is the production of substances that inhibit microbial growth, including bacteriocins and organic acids. These substances are synthesised as a mechanism of competition for nutrients and adhesion sites. Although the range of bacteriocin-producing bacteria is broad, few putative probiotics are used in commercial aquaculture. This chapter reviews the latest research on pathogen-antagonistic microorganisms. After bacteriocidal activity, one of the most outstanding properties of probiotics is their ability to activate the immune response. The use of probiotics as a pathogen biocontrol mechanism is also compared with other strategies, such as the use of medicinal plants, immunostimulants and vaccines. Despite the existence of a great diversity of microorganisms with probiotic potential, a deeper understanding of their safety in animals, including humans, and the environment is required, so that they can be used on an industrial scale in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Disease outbreaks in aquaculture are traditionally treated with antibiotics and chemotherapeutics. To decrease the use of these drugs, alternative strategies have been developed for improving fish health in aquaculture systems whilst reducing the potential spread of antimicrobial resistance (Gudmundsdóttir and Björnsdóttir 2007; Nayak 2010; Dawood et al. 2019). One of the most common activities of probiotics is the ability to control infectious diseases. The most common mechanism is the production of substances like bacteriocins, which inhibit microbial growth. Bacteriocins are a heterogeneous group of antimicrobial peptides with the ability to kill closely related microorganisms (narrow spectrum) or a wide range of microorganisms (broad spectrum) (Gálvez et al. 2014). Bacteriocins are synthesised by many bacteria as a mechanism of competition for nutrients and adhesion sites. They act at low concentrations, and may be biodegraded and digested by animals, which is not harmful to health. Probiotics may also produce and release organic acids and hydrogen peroxides to defend the host against the invasion of pathogens (Gaspar et al. 2018). Furthermore, probiotics control pathogen virulence by inhibiting their communication systems (by quorum sensing). Interference with the quorum sensing signal, called quorum quenching, might offer a new alternative for preventing and/or treating bacterial infections via inhibition of virulence factor expression and biofilm formation (Kim et al. 2018).
To use probiotics as a control mechanism for infectious disease, the benefits and drawbacks of their use must be compared with those of other disease control systems, such as immunostimulants, medicinal plants or vaccines. On the other hand, in the process of selecting a probiotic, it is necessary to evaluate which pathogens it can affect, since the antimicrobial range of action depends on the antimicrobial substances it releases.
2 Probiotics Effective Against Aquaculture Diseases
There is a wide range of pathogenic microorganisms whose growth has been affected by potentially probiotic bacteria, either in in vitro experiments or in animal tests.
Most probiotics put forward as biological control agents in aquaculture belong to the lactic acid bacteria (Lactobacillus), and to the Vibrio and Bacillus genera (Hoseinifar et al. 2018). Table 1 summarises some recent research on probiotics and their effect against some aquaculture pathogens. Unlike probiotics used in terrestrial animals, a large number of Gram-negative bacteria have been proposed for use in aquaculture. The number of species with probiotic potential is very high and includes strains of species that are even described as pathogenic (Arijo et al. 2008; Allameh et al. 2017; Medina et al. 2020; Wang et al. 2020). Several probiotic species have caused disease outbreaks in the aquaculture industry, including Vibrio sp. and Weissella sp. (Figueiredo et al. 2012). This implies a limitation of the use of these strains, since a probiotic strain useful for one fish species could be pathogenic for another animal especially if virulence genes are acquired. For example, Vagococcus lutrae has been used as a probiotic for seabream and seabass, but it has been observed to cause skin lesions in warm-blooded animals (Fu et al. 2020). On the other hand, there is also the possibility of plasmid transfer between pathogens and potential probiotics, which could give the probiotic virulence factors (van Reenen and Dicks 2011). This can be dangerous in the case of transmission of antibiotic resistance genes between probiotics and pathogens (Patel et al. 2012), which is why, in fact, legal provisions limit the use of probiotics to very few species. For example, the European Regulation (EC) No 178/2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority (EFSA) and laying down procedures in matters of food safety (art. 14 and 15), and the European Regulation (EU) 68/2013 about feed additives. In the absence of a list of authorised microorganisms, the Qualified Presumption of Safety (QPS) list of the EFSA is taken as a reference for their safe use in food, a list that is periodically reviewed (Herman et al. 2019). The list includes as safe microorganisms Gram-positive bacteria, i.e. Bacillus, Bifidobacterium, Carnobacterium, Lactobacillus, Leuconostoc and Streptococcus. However, there are no Gram-negative bacteria listed as safe to use as a living organism. This legal limitation implies that future research will have to focus on observing the potential adverse effects of probiotics proposed for use in aquaculture, otherwise it will not be possible to use all these probiotics in the aquaculture industry.
3 Probiotics Compared with Other Disease Control Measures
3.1 Probiotics verses Non-specific Immunostimulants
The concept of immunostimulation first appeared in 1970 as part of the vaccination process, and was later followed by the concept of probiotics (Portalès and Clot 2006). Indeed, it is difficult to separate the concept of immunostimulation from vaccination, as immunostimulants have been administered in combination with vaccines as adjuvants for boosting the immune response (Anderson 1992). However, they have been used independently since the 1980s (Olivier et al. 1985; Siwicki 1987). The use of immunostimulants for the prevention of diseases in fish culture has been extended since the beginning of the 1990s when these products were considered a new promising treatment against diseases (Kitao et al. 1987; Siwicki 1989; Anderson 1992). Anderson (1992) defined ‘immunostimulant’ as a chemical substance, drug, stressor or action that elevates the non-specific defence mechanism or the specific immune response. This is because an innate immune response is initiated upon recognition of pathogen-associated molecular patterns (PAMPs) (Wangkahart et al. 2019), molecules that mimic some cellular or extracellular pathogenic bacterial components. Immunostimulant agents were first used with whole bacteria, such as Cryptosporidium parvum, and later used with high molecular weight substances (LPS or peptidoglycans) (Werner 1986). Therefore, the link with the effect of probiotic bacteria is very close.
The first immunostimulant product developed was Ribomunyl® in 1980, and its composition was based on proteoglycans from Klebsiella pneumoniae and purified ribosomes from pathogens (Dussourd d’Hinterland et al. 1980). One decade later, immunostimulants began to be used in the aquaculture industry, and are now based on biological and/or synthetic compounds (Siwicki et al. 1994). Synthetic substances include compounds, such as Levamisole (Olivier et al. 1985) or FK-565 (Kitao and Yoshida 1986). Meanwhile, Mehana et al. 2015 classified the biological substances in bacterial derivatives, polysaccharides, animal and plant extracts, nutritional factors, such as vitamins and hormones, cytokines and others. All of them may be effective in preventing diseases when administered alone, without the need to be coupled with a vaccine (Hungin et al. 2018), or use of antibiotics and chemotherapeutics. Also, they are widely applied to improve fish welfare and production (Mehana et al. 2015).
Immunostimulants exert a non-specific response, including macrophage and phagocytic activity, killing activity, reactive oxygen species (ROS), chemiluminescent response, and humoral response, which includes increases in serum complement, lysozyme and immune substances associated with non-specific and specific immune responses (Gannam and Schrock 1999). Meanwhile, probiotics exert their mode of action in many aspects of fish physiology (Tapia-Paniagua et al. 2012; Soltani et al. 2019), including the immune system, microbiota, nutrition, growth, maturation or reproductive aspects (Irianto and Austin 2002; Gatesoupe 2008; Zorriehzahra et al. 2016; Chauhan and Singh 2019).
The benefits of immunostimulants assayed in vivo include increased survival when affected by viral, bacterial and parasitic diseases, growth enhancement, increased antibody production following vaccination and increased lysozyme levels (Barman and Nen 2013; Wang et al. 2017; Dawood et al. 2018). Also, these products may be obtained from a natural source in large amounts, such as glucans from yeast or chitosan from arthropods, which are low-cost ingredients. However, the use of immunostimulants has some disadvantages: (i) some of the molecules have a high cost and limited efficiency; (ii) the memory component developed by these substances and the duration of the immune response is very short or unknown; (iii) they are not effective against all diseases; (iv) overdoses of some products can induce immunosuppression or toxicity (Bullock et al. 2000). Sometimes the mode of action and effects are not clearly defined, or the effects of long-term oral administration remain unclear. Other authors claim that the benefits described are numerous, but theoretical. For example, in larvae culture, there is controversy between authors that defend that the early use of immunostimulants in fish larvae can induce immune tolerance (Bricknell and Dalmo 2005). However, large quantities of live probiotic cells may interfere with the associated eco-systems (Sharifuzzaman et al. 2011), or the risk of lateral gene transfer of antibiotic resistance genes (Gueimonde et al. 2013; Sharma et al. 2016; Tan et al. 2016). This is why new strategies are being set up, such as the use of microbial cellular components with immunostimulant effects on fish (Kum and Sekki 2011; Giri et al. 2015, 2018).
Some bacterial derivatives are considered to be immunostimulants (Giri et al. 2015). Examples include, but are not limited to, muramyl dipeptide (N-acetyl-muramyl-L-alanyl-D-isoglutamine, MDP), derived from Mycobacterium lipopolysaccharide (LPS; Kodama et al. 1993) that is a cell wall component of Gram-negative bacteria (Neumann 1995; Nya and Austin 2010); Freund’s complete adjuvant (FCA) that contains killed Mycobacterium butyricum (Sakai 1999); V. anguillarum whole cell inactivated vaccine [= bacterin] (Norqvist et al. 1989), Clostridium butyricum and Achromobacter stenohalis cells and other components, such as flagellin (Wangkahart et al. 2019) or cell wall proteins of Kocuria SM1 and Rhodococcus SM2 (Sharifuzzaman et al. 2011); bacterial DNA (Giri et al. 2015) and unmethylated CpG dinucleotides (Jørgensen et al. 2001).
The efficacy of immunostimulants and probiotics depends on the effective dose, exposure time and, in some cases, the feeding regime of each type of fish. For example, in Atlantic salmon, injection with a high dose of glucans (100 mg/kg) led to absence of protection for 1 week, but maximum benefits occurred after 3–4 weeks, whilst the injection of a low dose (2–10 mg/kg) gives protection for only one week (Kum and Sekki 2011). There are three main ways to deliver immunostimulants: (i) injection, (ii) immersion and oral uptake and (iii) bioencapsulation. The advantages and limitations are similar to those of probiotics. Injection is not usual when administering probiotics, but immunostimulants provide potent immunisation and can be administered in large fish. It is, however, a complicated task, which is costly and is highly stressful for the animals. Immersion and oral uptake are the simplest methods, making it possible to treat many fish of any size at the same time. However, the substances can lose activity due to their dilution in water, and it is difficult to measure the amount of feed ingested by the fish. The potency is not as high as with the injection route, and large amounts of immunostimulants are needed to achieve good protection. Currently, bioencapsulation is a good alternative, since it protects against the digestive system and environmental conditions. Table 2 shows the effects of probiotics compared with immunostimulants.
3.2 Probiotics verses Medicinal Plant Products
Medicinal plants comprise herbs, seaweeds, herbal extracted compounds, spices, commercial plant-derived products and traditional Chinese herbs (Van Hai 2015). There is growing interest in the use of medicinal herbs in aquaculture because of their promising effects, and they look like a promising alternative method for controlling fish diseases (Van Hai 2015; Abarike et al. 2018b). Plants have been reported to produce various effects, such as growth promotion, appetite stimulation, immunostimulation, and to have antipathogenic properties in aquaculture (Citarasu 2010; Reverter et al. 2014; Bulfon et al. 2015; Awad and Awaad 2017). The mode of action of these plants and their derivatives is attributed to the presence of many bioactive compounds, such as alkaloids, steroids, phenolics, tannins, terpenoids, saponins, glycosides and flavonoids (Harikrishnan et al. 2011a; Mendam et al. 2015).
Plants may be administered as a whole or in parts (leaf, root, bark, fruit), and can either be used fresh or as herbal extract preparations with different solvents (water, methanol, ethanol, chloroform) (Kim et al. 2011; Pan et al. 2013; Fridman et al. 2014; Hu et al. 2014; Zhang et al. 2014; Thanigaivel et al. 2015; Zhou et al. 2016). Their effects are variable amongst fish species, and depend mainly on different factors, such as route of administration, dosage and time (Zakęś et al. 2008; Harikrishnan et al. 2011a; Bulfon et al. 2015). Like other immunostimulants, medicinal plants and their extracts may be administered via injection (Harikrishnan et al. 2011a), bathing/immersion (Çek et al. 2007) or oral administration (Wang et al. 2015), which is the most practical and commonly used in aquaculture (Pourmoghim et al. 2015; Bilen et al. 2016; Öz et al. 2018). The review performed by Bulfon et al. (2015) presented a great variety of different dosages including up to 25% of the diet, although the most common doses ranged from 0.01 and 0.5%. However, there is not any positive correlation between dosage and its effect on the immune response (Jian and Wu 2004). Similarly, the length of feeding time is fundamentally important. To date, studies with medicinal plants and/or their bioactive compounds have involved different feeding durations, ranging from 1 to 16 weeks (Awad and Awaad 2017), but the basis for choosing these periods is often unclear.
One of the main problems of using medicinal plants as a chemotherapeutic is that the biological activity and chemical compositions of plants and extracts vary according to their characteristics (location, age, climate, cultivars, temperature and growth regulators) and sampling methods (plant part, drying, distillation and storage) (Wang et al. 2014). The antimicrobial activity of a plant against bacteria is determined by its mechanism of action, which is determined by the chemical composition (Chouhan et al. 2017; Cui et al. 2019). Thus, differing antimicrobial activities of plants with different chemical profiles are expected. In this sense, in vitro studies evaluating the cytotoxicity and the antibacterial effects of herbs have examined several bacterial fish pathogens (Vaseeharan et al. 2013; Alizadeh Behbahani and Imani Fooladi 2018; Da Cunha et al. 2018; Assane et al. 2020), highlighting their potential use for controlling bacterial disease in cultured fish.
A key aspect for proposing a natural substance as an antimicrobial agent is whether it has active compounds that may be toxic for the host. There have been reports that some plants and their major components are toxic for different animals (Malekmohammad et al. 2019), including fish (Spanghero et al. 2019; Tavares-Dias 2018).
The administration of medicinal plants for disease control in aquaculture may be achieved singly or in combination with other plants. Some studies show that medicinal plants (such as Allium sativum, Azadirachta indica, Curcuma longa, Ocimum basilicum, Ocimum sanctum, Cinnamomum zeylanicum, Juglans regia, Mentha piperita, Radix astragalus and Radix angelicae) enhance growth, immune responses and survival against a wide range of pathogen infections in farmed fish, such as O. mykiss, L. calcarifer, C. carpio and Pseudosciaena crocea (Jian and Wu 2003; Harikrishnan et al. 2009; Nya and Austin, 2009a, 2009b; Mohamad and Abasali 2010; Talpur and Ikhwanuddin 2012; Talpur et al. 2013; Awad and Awaad 2017; Stratev et al. 2018; Hayatgheib et al. 2020; Kuebutornye and Abarike 2020).
Medicinal plants may be incorporated with a probiotic. Thus, fenugreek seed (Trigonella foenum graecum) in combination with probiotic strains B. licheniformis, L. plantarum and B. subtilis enhanced growth performance, skin mucosal immunity response, humoral immune response and the expression of immune-associated genes of gilthead seabream (Sparus aurata) after three weeks of a feeding regime (Bahi et al. 2017; Guardiola et al. 2017). A diet enriched with Scutellaria baicalensis, and/or Lactobacillus sakei BK19 in rock bream, O. fasciatus, demonstrated that the maximum protection against Edwardsiella tarda was recorded in the mixed (plant + probiotic) diet group (Harikrishnan et al. 2011b). The synergistic effect of M. piperita and the probiotic Bacillus coagulans improved the growth performance, nutritional physiology and resistance of Indian carp (Catla catla) when challenged against A. hydrophila (Bhatnagar and Saluja 2019). The effect of herbal‐probiotic mixtures of Astragalus membranaceus, Angelica sinensis, Crataegus hupehensis and probiotics B. subtilis and B. lincheniformis improved growth and enhanced immune responses and survival of Nile tilapia (O. niloticus) when challenged against S. agalactiae (Abarike et al. 2018b). Moreso, in O. niloticus, a mixture of Chinese medicinal herbs and probiotics (Bacillus, Lactobacillus and Yeast) enhanced growth performance, innate immune response and antibacterial activity against E. tarda (Hwang et al. 2019).
There are some advantages and disadvantages when using probiotics instead of medicinal plants. On the one hand, probiotics may colonise the gut and adhere to the epithelial surface, and consequently interfere with the adhesion of pathogens (Zorriehzahra et al. 2016). Furthermore, they can consume the nutrients that are essential for the growth of a number of pathogens (Brown 2011). However, safety regulations and marketing authorizations are very restrictive regarding the use of live microorganisms. Conversely, medicinal plants are easily accessible and economical, and there is no need for significant investment in their biotechnological development, which is also an encouraging factor for large scale usage in aquaculture. Moreover, although plant products have a natural origin, and most of these medicinal plants do not represent a hazard for human health, animal health, or the environment (Stratev et al. 2018), some constituents are unstable (e.g. they are photo- and/or thermo-labile) (Burt 2004). Finally, little is known regarding the interaction of the plants with the host microbiota.
In contrast to plant extracts and the other protein-based antimicrobial preservatives, bacteriocins, produced by some probiotic bacteria tolerate high thermal stress and are active over a wide pH range, remaining effective at fairly low concentrations (Wang et al. 2019c).
3.3 Probiotics verses Vaccines
Modern vaccines can be classified as killed, attenuated, DNA, synthetic peptide, recombinant vector, genetically modified and subunit vaccines, but although whole vaccines showed a better advantage than other types (Assefa and Abunna 2018), all showed disadvantages, especially with regard to the route of administration. Although it is a very efficient for achieving protection against pathogens, the intraperitoneal inoculation of vaccines combined with adjuvants (Harikrishnan et al. 2011c) may be the cause of stress, feed intake reduction (Lillehaug 2014), lesions such as inflammation, deformities and granulomas (Berg et al. 2006), and growth alterations (SØrum and Damsgård 2004; Berg et al. 2007). In addition, staff with experience in the application of this type of vaccines is required. On the other hand, the oral vaccination route is favoured because of its ease of administration, but not all fish can eat/take the same amount of antigen so it may not provide a uniform protection. It may also become more expensive if it is necessary to protect the antigen by encapsulation (Vallejos-Vidal et al. 2014).
Probiotics may be used to reduce disease outbreaks in aquaculture. Some probiotics are characterised by their antagonistic activity against pathogens or the stimulation of the fish immune response, including the production of specific antibodies. Immune cross-reactions amongst phylogenetically-related bacteria are widely documented, and they play an important role in protection against pathogens (Medina et al. 2020). Some vaccines use non-pathogenic microorganisms that contain antigens similar to those of pathogenic strains (Brunt and Austin 2005; Brunt et al. 2007; Arijo et al. 2008; Abbass et al. 2010). If a probiotic shares antigens with a certain pathogen, it could produce antibodies with a cross-reaction to that pathogen. Therefore, a probiotic with these characteristics could be used in a similar way to a live vaccine.
The ability of probiotic bacteria administered through diet to modulate the innate and adaptive immune system of farmed fish has been reported (Brunt and Austin 2005; Nayak 2010; Hemaiswarya et al. 2013; Foey and Picchietti 2014), even when some probiotic microorganisms were supplied as heat-killed cells (Biswas et al. 2013). There is information that a probiotic strain of E. faecium increased the transcription of genes encoding complement system, lysozyme activity, protease activity and proinflammatory cytokines in specimens of P. olivaceus infected with L. garvieae (Kim et al. 2013). On the other hand, significant increases in T lymphocytes (Romano et al. 2007; Picchietti et al. 2009), granulocytes (Sharma et al. 2013), and immunoglobulins (Sharifuzzaman and Austin 2010; Neissi et al. 2013; Xing et al. 2013) have been reported in farmed fish receiving probiotics, and include D. labrax, Rachycentron canadum and O. mykiss. However, different studies have reported the ability of the subcellular components obtained from probiotics to exert an immunostimulant effect on the specific and non-specific immune responses of farmed fish (Arijo et al. 2008; Chi et al. 2014; Giri et al. 2015, 2018). All these studies strongly suggested that probiotics may be used as adjuvants in aquaculture. In this sense, the reduction of the side effects of vaccines administered with adjuvants is a challenging goal for fish vaccination (Dadar et al. 2017), and the use of probiotics as potential adjuvants is a very interesting possibility, especially because they can be easily administered through the diet as spores (Soltani et al. 2019), freeze-dried (Tapia-Paniagua et al. 2015) and using some type of encapsulation (Martínez Cruz et al. 2012; Rosas-Ledesma et al. 2012). Another interesting aspect in comparison with vaccines is that the use of the probiotic is not limited by the size of fish, because they have been supplied in all growth stages even during larviculture (Lobo et al. 2014).
However, new terms, such as postbiotic, have emerged that imply that bacterial viability is not an essential requirement for health benefits. Postbiotics are soluble factors resulting from the metabolic activity of a probiotic or any released molecule capable of conferring beneficial effects to the host in a direct or indirect way (Tsilingiri et al. 2012), and include a wide range of compounds (Aguilar-Toalá et al. 2018; Ang et al. 2020). In human and veterinary uses, postbiotics have shown beneficial health effects (Nakamura et al. 2016; Compare et al. 2017) indicating a high capacity to modulate different organs and tissues in the host, inducing several biological responses such as an immune response (Kearny et al. 2015), and suggesting that they could mimic the health effects of probiotics.
Therefore, the use of postbiotics may represent a valid and safer alternative to avoid risks linked to live probiotic bacteria for treating many diseases, and the scientific evidence of their beneficial health effects is increasing (Haileselassie et al. 2016; Nakamura et al. 2016; Compare et al. 2017; Zółkiewicz et al. 2020). However, especially in the case of aquaculture, the information on the application of postbiotics is limited (Lieke et al. 2020; Ang et al. 2020), and mainly focused on Gram-positive microorganisms. Studies on the relationship between the immune system and postbiotics can be very relevant, because they could imply a more efficient application of probiotics.
4 Conclusion and Suggestions for Further Work
In conclusion, there is a wide range of probiotics that has been studied for the control of infectious diseases. Probiotics have shown the ability to act against pathogens at the same level as other treatments, such as immunostimulants, medicinal plants and vaccines. However, most probiotics are not legally recognised for use in aquaculture. This represents a limitation for the commercial use of the strains studied. More research is needed to demonstrate that the wide range of probiotics used experimentally are safe for farmed fish, other animals (including humans) and the environment in general.
References
Abarike ED, Cai J, Lu Y, Yu H, Chen L, Jian J, Tang J, Jun L, Kuebutornye FKA (2018) Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 82:229–238. https://doi.org/10.1016/j.fsi.2018.08.037
Abarike ED, Jian J, Tang J, Cai J, Yu H, Lihua C, Jun L (2018) Influence of traditional Chinese medicine and Bacillus species (TCMBS) on growth, immune response and disease resistance in Nile tilapia, Oreochromis Niloticus. Aquaculture Res 49(7):2366–2375. https://doi.org/10.1111/are.13691
Abbass A, Sharifuzzaman SM, Austin B (2010) Cellular components of probiotics control Yersinia ruckeri infection in rainbow trout, Oncorhynchus mykiss (Walbaum): Cellular components of probiotics. J Fish Dis 33:31–37
Abomughaid MM (2020) Isolation and identification of some Probiotic bacteria and their potential role in improving immune response and resistance of Nile tilapia (Oreochromis niloticus) in comparison with a commercial product. Int J Microbiol 8865456. https://doi.org/10.1155/2020/8865456
Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro AF, González-Córdoba AF, Vallejo-Córdoba B, Hernández-Mendoza A (2018) Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol 75:105–114
Alizadeh Behbahani B, Imani Fooladi AA (2018) Evaluation of phytochemical analysis and antimicrobial activities Allium essential oil against the growth of some microbial pathogens. Microb Pathog 114:299–303. https://doi.org/10.1016/j.micpath.2017.11.055
Allameh SK, Ringø E, Yusoff FM, Daud HM, Ideris A (2017) Dietary supplement of Enterococcus faecalis on digestive enzyme activities, short-chain fatty acid production, immune system response and disease resistance of Javanese carp (Puntius gonionotus, Bleeker 1850). Aquac Nutr 23(2):331–338. https://doi.org/10.1111/anu.12397
Anderson DP (1992) Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annu Rev Fish Dis 2:281–307. https://doi.org/10.1016/0959-8030(92)90067-8
Ang CY, Sano M, Dan S, Leelakriangsak M, Lal TM (2020) Postbiotics applications as infectious disease control agent in aquaculture. Biocontrol Sci 25(1):1–7
Arijo S, Brunt J, Chabrillón M, Díaz-Rosales P, Austin B (2008) Subcellular components of Vibrio harveyi and probiotics induce immune responses in rainbow trout, Oncorhynchus mykiss (Walbaum), against V. harveyi. J Fish Dis 31:579–590
Arun C, Singh R (2019) Study of Enterococcus faecium strain LF3(1) as potential probiotics for Cyprinus carpio to prevent Pseudomonas aeruginosa infection. Res J Biotechnol 14(6):122–129
Assane IM, Valladão GMR, Pilarski F (2020) Chemical composition, cytotoxicity and antimicrobial activity of selected plant-derived essential oils against fish pathogens. Aquac Res. https://doi.org/10.1111/are.14935
Assefa A, Abunna F (2018) Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet Med Int 5432497
Awad E, Awaad A (2017) Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol 67:40–54. https://doi.org/10.1016/j.fsi.2017.05.034
Bahi A, Guardiola FA, Messina C, Mahdhi A, Cerezuela R, Santulli A, Bakhrouf A, Esteban MA (2017) Effects of dietary administration of fenugreek seeds, alone or in combination with probiotics, on growth performance parameters, humoral immune response and gene expression of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 60:50–58. https://doi.org/10.1016/j.fsi.2016.11.039
Barman D, Nen P (2013) Immunostimulants for aquaculture health management. J Marine Sci Res Dev 03(03):1–11. https://doi.org/10.4172/2155-9910.1000134
Berg A, Rodseth O, Tangeras A, Hansen T (2006) Time of vaccination influences development of adhesions, growth hand spinal deformities in Atlantic salmon Salmo salar. Dis Aquat Org 69:239
Berg A, Rødseth OM, Hansen T (2007) Fish size at vaccination influence the development of side-effects in Atlantic salmon (Salmo Salar L.). Aquaculture 265:9–15
Bhatnagar A, Saluja S (2019) Synergistic effects of autochthonous probiotic bacterium and Mentha piperita diets in Catla catla (Hamilton, 1822) for enhanced growth and immune response. Fish Aquatic Sci 22(1):16. https://doi.org/10.1186/s41240-019-0130-7
Bilen S, Altunoglu YC, Ulu F, Biswas G (2016) Innate immune and growth promoting responses to caper (Capparis spinosa) extract in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 57:206–212. https://doi.org/10.1016/j.fsi.2016.08.040
Biswas G, Korenaga H, Nagamine R, Takayama H, Kawahara S, Takeda S, Kikuchi Y, Dashnyam B, Kono T, Sakai M (2013) Cytokine responses in the Japanese pufferfish (Takifugu rubripes) head kidney cells induced with heat-killed probiotics isolated from the Mongolian dairy products. Fish Shellfish Immunol 34:1170–1177
Bricknell I, Dalmo R (2005) The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol 19(5):457–472. https://doi.org/10.1016/j.fsi.2005.03.008
Brown M (2011) Modes of action of probiotics: recent developments. J Anim Vet Adv 10(14):1895–1900. https://doi.org/10.3923/javaa.2011.1895.1900
Brunt J, Austin B (2005) Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 28:693–701
Brunt J, Newaj-Fyzul A, Austin B (2007) The development of probiotics for the control of multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 30:573–579
Bulfon C, Volpatti D, Galeotti M (2015) Current research on the use of plant-derived products in farmed fish. Aquac Res 46(3):513–551. https://doi.org/10.1111/are.12238
Bullock G, Blazer V, Tsukuda S, Summerfelt S (2000) Toxicity of acidified chitosan for cultured rainbow trout (Oncorhynchus mykiss). Aquaculture 185(3–4):273–280. https://doi.org/10.1016/S0044-8486(99)00359-2
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol 94:223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Çek Ş, Turan F, Atik E (2007) Masculinization of convict cichlid (Cichlasoma nigrofasciatum) by immersion in Tribulus terrestris extract. Aquacult Int 15(2):109–119. https://doi.org/10.1007/s10499-006-9071-0
Chauhan A, Singh R (2019) Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 77(2):99–113. https://doi.org/10.1007/s13199-018-0580-1
Chen Y, Li J, Xiao P, Li GY, Yue S, Huang J, Zhu WY, Mo ZL (2016) Isolation and characterization of Bacillus spp. M001 for potential application in turbot (Scophthalmus maximus L.) against Vibrio anguillarum. Aquac Nutr 22(2):374–381. https://doi.org/10.1111/anu.12259
Chen SW, Liu CH, Hu SY (2019) Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 84:695–703. https://doi.org/10.1016/j.fsi.2018.10.059
Chen X, Xie J, Liu Z, Yin P, Chen M, Liu Y, Tian L, Niu J (2020) Modulation of growth performance, non-specific immunity, intestinal morphology, the response to hypoxia stress and resistance to Aeromonas hydrophila of grass carp (Ctenopharyngodon idella) by dietary supplementation of a multi-strain probiotic. Comp Biochem Physiol Part-c: Toxicol Pharmacol 231:108724. https://doi.org/10.1016/j.cbpc.2020.108724
Chi C, Jiang B, Yu X-B, Liu T-Q, Xia L, Wang G-X (2014) Effects of three strains of intestinal autochthonous bacteria and their extracellular products on the immune response and disease resistance of common carp, Cyprinus carpio. Fish Shellfish Immunol 36:9–18
Chouhan S, Sharma K, Guleria S (2017) Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 4(3):58. https://doi.org/10.3390/medicines4030058
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult Int 18(3):403–414. https://doi.org/10.1007/s10499-009-9253-7
Cui H, Zhang C, Li C, Lin L (2019) Antibacterial mechanism of oregano essential oil. Ind Crops Prod 139:111498. https://doi.org/10.1016/j.indcrop.2019.111498
Compare D, Rocco A, Coccoli P, Angrisani D, Sgamato C, Iovine B, Salvatore U, Nardone G (2017) Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol 17(1):53. https://doi.org/10.1186/s12876-017-0605-x
Da Cunha JA, Heinzmann BM, Baldisserotto B (2018) The effects of essential oils and their major compounds on fish bacterial pathogens—a review. J Appl Microbiol 125(2):328–344. https://doi.org/10.1111/jam.13911
Dadar M, Dhama K, Vakharia VN, Hoseinifar SH, Karthike K, Ruchi Tiwari R, Khandia R, Munjal A, Salgado-Mirandah C, Josh S (2017) Advances in aquaculture vaccines against fish pathogens: global status and current trends. Rev Fish Sci Aquac 25:184–217
Dawood MAO, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10(4):950–974. https://doi.org/10.1111/raq.12209
Dawood MAO, Koshio S, Abdel-Daim MM, Van Doan H (2019) Probiotic application for sustainable aquaculture. Rev Aquac 11(3):907–924. https://doi.org/10.1111/raq.12272
Di J, Chu Z, Zhang S, Huang J, Du H, Wei Q (2019) Evaluation of the potential probiotic Bacillus subtilis isolated from two ancient sturgeons on growth performance, serum immunity and disease resistance of Acipenser dabryanus. Fish Shellfish Immunol 93:711–719. https://doi.org/10.1016/j.fsi.2019.08.020
Dias JAR, Abe HA, Sousa NC, Couto MVS, Cordeiro CAM, Meneses JO, Cunha FS, Mouriño JLP, Martins ML, Barbas LAL, Carneiro PCF, Maria AN, Fujimoto RY (2018) Dietary supplementation with autochthonous Bacillus cereus improves growth performance and survival in tambaqui Colossoma macropomum. Aquac Res 49(9):3063–3070. https://doi.org/10.1111/are.13767
Dussourd d’Hinterland L, Normier G, Durand J (1980) Ribosomal vaccines: preparation of subcellular fractions. Arzneimittelforschung 30(1a):126–132
European Union. Commission Regulation (EC) No 178/2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Official Journal of the European Union
European Union. Commission Regulation (EU) No 68/2013 of 16 January 2013 on the Catalogue of feed materials. Off J Euro Union
Faeed M, Kasra R, Pourkazemi M, Darboee M, Haghighi S (2016). Effect of the probiotic Enterococcus faecium on hematological and non-specific immune parameters and disease resistance in zander (Sander lucioperca). Iranian J Fish Sci 15(4). http://jifro.ir/article-1-2470-en.html
Farias THV, Levy-Pereira N, Alves L, Dias D, Tachibana L, Pilarski F, Belo MA, Ranzani-Paiva MJT (2016) Probiotic feeding improves the immunity of pacus, Piaractus mesopotamicus, during Aeromonas hydrophila infection. Anim Feed Sci Technol 211:137–144. https://doi.org/10.1016/j.anifeedsci.2015.11.004
Feng J, Chang X, Zhang Y, Yan X, Zhang J, Nie G (2019) Effects of Lactococcus lactis from Cyprinus carpio L. as probiotics on growth performance, innate immune response and disease resistance against Aeromonas hydrophila. Fish Shellfish Immunol 93:73–81. https://doi.org/10.1016/j.fsi.2019.07.028
Figueiredo HC, Costa FA, Leal CA, Carvalho-Castro GA, Leite RC (2012) Weissella sp. outbreaks in commercial rainbow trout (Oncorhynchus mykiss) farms in Brazil. Vet Microbiol 156(3–4):359–366. https://doi.org/10.1016/j.vetmic.2011.11.008
Foey A, Picchietti S (2014) Immune defenses of teleost fish. In: Merrifield D, Ringø E (eds) Aquaculture nutrition: gut health, probiotics and prebiotics. John Wiley & Sons, Ltd., Chichester, UK. http://dx.doi.org/https://doi.org/10.1002/9781118897263.ch2
Foysal MJ, Alam M, Kawser AQMR, Hasan F, Rahman MM, Tay CY, Prodhan MSH, Gupta SK (2020) Meta-omics technologies reveals beneficiary effects of Lactobacillus plantarum as dietary supplements on gut microbiota, immune response and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 520:734974. https://doi.org/10.1016/j.aquaculture.2020.734974
Fridman S, Sinai T, Zilberg D (2014) Efficacy of garlic based treatments against monogenean parasites infecting the guppy (Poecilia reticulata (Peters)). Vet Parasitol 203(1–2):51–58. https://doi.org/10.1016/j.vetpar.2014.02.002
Fu S, Xia W, Wang Q, Rahman MM, Hao J, Ye S, Liu Y, Li R (2020) Genomic characterization and pathogenicity analysis of the probiotic Vagococcus lutrae strain VL-18 causing severe skin lesions in warm-blooded animals. Aquaculture 523:1–8. https://doi.org/10.1016/j.aquaculture.2020.735166
Gannam AL, Schrock RM (1999) Immunostimulants in fish diets. J Appl Aquac 9(4):53–89. https://doi.org/10.1300/J028v09n04_06
Gálvez A, Burgos MJG, López, RL, Pulido RP (2014) Natural antimicrobials for food biopreservation. In: Springer briefs in food, health, and nutrition. Springer, New York, NY, pp 3–14
Gatesoupe F-J (2008) Updating the importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. J Mol Microbiol Biotechnol 14(1–3):107–114. https://doi.org/10.1159/000106089
Gaspar C, Donders GG, Palmeira-de-Oliveira R, Queiroz JA, Tomaz C, Martinez-de-Oliveira J, Palmeira-de-Oliveira A (2018) Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Expr 8(1):153. https://doi.org/10.1186/s13568-018-0679-z
Giri SS, Sen SS, Chi C, Kim HJ, Yun S, Park SC, Sukumaran V (2015) Effect of cellular products of potential probiotic bacteria on the immune response of Labeo rohita and susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol 46(2):716–722. https://doi.org/10.1016/j.fsi.2015.08.012
Giri SS, Chi C, Jun JW, Park SC (2018) Use of bacterial subcellular components as immunostimulants in fish aquaculture. Rev Aquac 10(2):474–492. https://doi.org/10.1111/raq.12182
Gobi N, Vaseeharan B, Chen JC, Rekha R, Vijayakumar S, Anjugam M, Iswarya A (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Guardiola FA, Bahi A, Bakhrouf A, Esteban MA (2017) Effects of dietary supplementation with fenugreek seeds, alone or in combination with probiotics, on gilthead seabream (Sparus aurata L.) skin mucosal immunity. Fish Shellfish Immunol 65:169–178. https://doi.org/10.1016/j.fsi.2017.04.014
Gudmundsdóttir BK, Björnsdóttir B (2007) Vaccination against atypical furunculosis and winter ulcer disease of fish. Vaccine 25(30):5512–5523. https://doi.org/10.1016/j.vaccine.2007.02.009
Gueimonde M, Sánchez B, de los Reyes-Gavilán C.G, Margolles A. (2013) Antibiotic resistance in probiotic bacteria. Front Microbiol 4. https://doi.org/10.3389/fmicb.2013.00202
Gupta A, Gupta P, Dhawan A (2016) Paenibacillus polymyxa as a water additive improved immune response of Cyprinus carpio and disease resistance against Aeromonas hydrophila. Aquac Rep 4:86–92. https://doi.org/10.1016/j.aqrep.2016.07.002
Haileselassie Y, Navis M, Vu N, Qazi KR, Rethi B, Sverremark-Ekström E (2016) Postbiotic modulation of retinoic acid imprinted mucosal-like dendritic cells by Probiotic Lactobacillus reuteri 17938 in vitro. Front Immunol 7:96. https://www.frontiersin.org/article/https://doi.org/10.3389/fimmu.2016.00096
Harikrishnan R, Balasundaram C, Kim MC, Kim JS, Han YJ, Heo MS (2009) Innate immune response and disease resistance in Carassius auratus by triherbal solvent extracts. Fish Shellfish Immunol 27(3):508–515. https://doi.org/10.1016/j.fsi.2009.07.004
Harikrishnan R, Balasundaram C, Heo MS (2011a) Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317(1–4):1–15. https://doi.org/10.1016/j.aquaculture.2011.03.039
Harikrishnan R, Kim MC, Kim JS, Balasundaram C, Heo MS (2011b) Protective effect of herbal and probiotics enriched diet on haematological and immunity status of Oplegnathus fasciatus (Temminck & Schlegel) against Edwardsiella tarda. Fish Shellfish Immunol 30(3):886–893. https://doi.org/10.1016/j.fsi.2011.01.013
Harikrishnan R, Balasundaram C, Heo MS (2011c) Fish health aspects in grouper aquaculture. Aquaculture 320(1–2):1–21
Hayatgheib N, Moreau E, Calvez S, Lepelletier D, Pouliquen H (2020) A review of functional feeds and the control of Aeromonas infections in freshwater fish. Aquacult Int 28(3):1083–1123. https://doi.org/10.1007/s10499-020-00514-3
Hemaiswarya S, Raja R, Ravikumar R, Carvalho IS (2013) Mechanism of action of probiotics. Braz Arch Biol Technol 56:113–119
Herman L, Chemaly M, Cocconcelli PS, Fernandez P, Klein G, Peixe L, Prieto M, Querol A, Suarez JE, Sundh I, Vlak J, Correia S (2019) The qualified presumption of safety assessment and its role in EFSA risk evaluations: 15 years past. FEMS Microbiol Lett 366(1):fny260. https://doi.org/10.1093/femsle/fny260
Hoseinifar SH, Sun YZ, Wang A, Zhou Z (2018) Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol 9(OCT):2429. https://doi.org/10.3389/fmicb.2018.02429
Hu Y, Ji J, Ling F, Chen Y, Lu L, Zhang Q, Wang G (2014) Screening medicinal plants for use against Dactylogyrus intermedius (Monogenea) infection in goldfish. J Aquat Anim Health 26(3):127–136. https://doi.org/10.1080/08997659.2014.902872
Hwang Y-S, Bang SJ, Kang TY, Choi JH, Jung SM, Kang IS, Jeon SY, Park KH, Choi S (2019) The dietary effect of medicinal herbs extract and multiple probiotics mixture on the growth performance, innate immune response and antibacterial activity of Nile tilapia Oreochromis niloticus. J Fish Pathol 32(1):9–20. https://doi.org/10.7847/jfp.2019.32.1.009
Hungin APS, Mitchell CR, Whorwell P, Mulligan C, Cole O, Agreus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Seifert B, Wensaas KA, Winchester C, de Wit N (2018) Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther 47(8):1054–1070. https://doi.org/10.1111/apt.14539
Interaminense JA, Vogeley JL, Gouveia CK, Portela RWS, Oliveira JP, Andrade HA, Peixoto SM, Soares RB, Buarque DS, Bezerra RS (2018) In vitro and in vivo potential probiotic activity of Bacillus subtilis and Shewanella algae for use in Litopenaeus vannamei rearing. Aquaculture 488:114–122. https://doi.org/10.1016/j.aquaculture.2018.01.027
Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25(11):633–642. https://doi.org/10.1046/j.1365-2761.2002.00422.x
Jian J, Wu Z (2003) Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture 218(1–4):1–9. https://doi.org/10.1016/S0044-8486(02)00192-8
Jian J, Wu Z (2004) Influences of traditional Chinese medicine on non-specific immunity of Jian Carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 16(2):185–191. https://doi.org/10.1016/S1050-4648(03)00062-7
Jinendiran S, Nathan AA, Ramesh D, Vaseeharan B, Sivakumar N (2019) Modulation of innate immunity, expression of cytokine genes and disease resistance against Aeromonas hydrophila infection in goldfish (Carassius auratus) by dietary supplementation with Exiguobacterium acetylicum S01. Fish Shellfish Immunol 84:458–469. https://doi.org/10.1016/j.fsi.2018.10.026
Jørgensen JB, Johansen A, Stenersen B, Sommer A-I (2001) CpG oligodeoxynucleotides and plasmid DNA stimulate Atlantic salmon (Salmo salar L.) leucocytes to produce supernatants with antiviral activity. Dev Comp Immunol 25(4):313–321. https://doi.org/10.1016/S0145-305X(00)00068-9
Kazuń B, Małaczewska J, Kazuń K, Żylińska-Urban J, Siwicki AK (2018) Immune-enhancing activity of potential probiotic strains of Lactobacillus plantarum in the common carp (Cyprinus carpio) fingerling. J Vet Res (poland) 62(4):485–492. https://doi.org/10.2478/jvetres-2018-0062
Kearney SC, Dziekiewicz M, Feleszko W (2015) Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann Allergy, Asthma Immunol: off Pub Am Coll Allergy, Asthma, Immunol 114(5):364–369. https://doi.org/10.1016/j.anai.2015.02.008
Kim J-S, Harikrishnan R, Kim M-C, Jang I-S, Kim D-H, Hong S-H, Balasundaram C, Heo M-S (2011) Enhancement of Eriobotrya japonica extracts on non-specific immune response and disease resistance in kelp grouper Epinephelus bruneus against Vibrio carchariae. Fish Shellfish Immunol 31(6):1193–1200. https://doi.org/10.1016/j.fsi.2011.10.015
Kim YR, Kim EY, Choi SY, Hossain MT, Oh R, Heo WS, Lee JM, Cho YC, Kong IS (2013) Effect of a probiotic strain, Enterococcus faecium, on the immune responses of olive flounder (Paralichthys olivaceus). J Microbiol Biotechnol 22:526–529
Kim D-H, Subramanian D, Park S-H, Jang Y-H, Heo M-S (2017) Assessment and potential application of the probiotic strain, Bacillus amyloliquefaciens JFP2, isolated from fermented seafood-jeotgal in flounder Paralichthys olivaceus Juveniles. Israeli J Aquac-Bamidgeh. http://www.siamb.org.il
Kim J, Kim J, Kim Y, Oh S, Song M, Choe JH, Whang K-Y, Kim KH, Oh S (2018) Influences of quorum-quenching probiotic bacteria on the gut microbial community and immune function in weaning pigs. Anim Sci J 89:412–422. https://doi.org/10.1111/asj.12954
Kitao T, Yoshida Y (1986) Effect of an immunopotentiator on Aeromonas salmonicida infection in rainbow trout (Salmo gairdneri). Vet Immunol Immunopathol 12(1–4):287–296. https://doi.org/10.1016/0165-2427(86)90132-7
Kitao T, Yoshida T, Anderson DP, Dixon OW, Blanch A (1987) Immunostimulation of antibody-producing cells and humoral antibody to fish bacterins by a biological response modifier. J Fish Bio 31(sA):87–91. https://doi.org/10.1111/j.1095-8649.1987.tb05298.x
Kodama H, Hirota Y, Mukamoto M, Baba T, Azuma I (1993) Activation of rainbow trout (Oncorhynchus mykiss) phagocytes by muramyl dipeptide. Dev Comp Immunol 17(2):129–140. https://doi.org/10.1016/0145-305X(93)90023-J
Krishnaveni G, Vignesh S, Vidhyalakshmi N, Vijay V, Ramesh U (2020) Effects of dietary supplementation of Lactobacillus fermentum URLP18 on growth, innate immunity and survival against Aeromonas hydrophila ATCC 7966 challenge in freshwater fish Cyprinus carpio (common carp). Aquac Res 1–17. https://doi.org/10.1111/are.14974
Kuebutornye FKA, Abarike ED (2020) The contribution of medicinal plants to tilapia aquaculture: a review. Aquacult Int 28(3):965–983. https://doi.org/10.1007/s10499-020-00506-3
Kuebutornye FKA, Wang Z, Lu Y, Abarike ED, Sakyi ME, Li Y, Xie CX, Hlordzi V (2020) Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol 97:83–95. https://doi.org/10.1016/j.fsi.2019.12.046
Kum C, Sekki S (2011) The immune system drugs in fish: immune function, immunoassay, drugs. In: Aral EF (ed) Recent advances in fish farms. InTech. https://doi.org/10.5772/26869
Lee S, Katya K, Park Y, Won S, Seong M, Hamidoghli A, Bai SC (2017) Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 61:201–210. https://doi.org/10.1016/j.fsi.2016.12.035
Lee C, Han JE, Kim JE, Kim SH, Kim JW, Eun JS, Lee KJ (2019) Effect of dietary supplementation of Bacillus spp. on growth performance, and resistance of pacific white shrimp (Litopenaeus vannamei) to acute hepatopancreatic necrosis disease. Israeli J Aquaculture—Bamidgeh 71:10. https://evols.library.manoa.hawaii.edu/handle/10524/61067
Lee C, Cha J, Kim M, Shin J, Woo SH, Kim SH, Kim JW, Ji S, Lee K (2020) The effects of dietary Bacillus subtilis on immune response, hematological parameters, growth performance, and resistance of juvenile olive flounder (Paralichthys olivaceus) against Streptococcus iniae. J World Aquaculture Soc 51(2):551–562. https://doi.org/10.1111/jwas.12680
Li J, Wu ZB, Zhang Z, Zha JW, Qu SY, Qi XZ, Wang GX, Ling F (2019) Effects of potential probiotic Bacillus velezensis K2 on growth, immunity and resistance to Vibrio harveyi infection of hybrid grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀). Fish Shellfish Immunol 93:1047–1055. https://doi.org/10.1016/j.fsi.2019.08.047
Li Y, Hu S, Gong L, Pan L, Li D, Cao L, Khan TA, Yang Y, Peng Y, Ding X, Yi G, Liu S, Xia L (2020) Isolating a new Streptomyces amritsarensis N1–32 against fish pathogens and determining its effects on disease resistance of grass carp. Fish Shellfish Immunol 98:632–640. https://doi.org/10.1016/j.fsi.2019.10.038
Lieke T, Meinelt T, Hoseinifar SH, Pan B, Straus DL, Steinberg CEW (2020) Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev Aquac 12:943–965
Lillehaug A (2014) Vaccination strategies and procedures. In: Gudding R, Lillehaug A, Evensen Ø (eds) Fish vaccination, vol 9780470674550. Wiley Blackwell, pp 140–152. https://doi.org/10.1002/9781118806913.ch12
Lin HL, Shiu YL, Chiu CS, Huang SL, Liu CH (2017) Screening probiotic candidates for a mixture of probiotics to enhance the growth performance, immunity, and disease resistance of Asian seabass, Lates calcarifer (Bloch), against Aeromonas hydrophila. Fish Shellfish Immunol 60:474–482. https://doi.org/10.1016/j.fsi.2016.11.026
Lin YS, Saputra F, Chen YC, Hu SY (2019) Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol 86:410–419. https://doi.org/10.1016/j.fsi.2018.11.047
Liu CH, Wu K, Chu TW, Wu TM (2018) Dietary supplementation of probiotic, Bacillus subtilis E20, enhances the growth performance and disease resistance against Vibrio alginolyticus in parrot fish (Oplegnathus fasciatus). Aquacult Int 26(1):63–74. https://doi.org/10.1007/s10499-017-0189-z
Lobo C, Moreno-Ventas X, Tapia-Paniagua S, Rodríguez C, Moriñigo MA, García de La Banda I (2014) Dietary probiotic supplementation (Shewanella putrefaciens Pdp11) modulates gut microbiota and promotes growth and condition in Senegalese sole larviculture. Fish Physiol Biochem 40:295–309
Maji UJ, Mohanty S, Pradhan A, Maiti NK (2017) Immune modulation, disease resistance and growth performance of Indian farmed carp, Labeo rohita (Hamilton), in response to dietary consortium of putative lactic acid bacteria. Aquacult Int 25(4):1391–1407. https://doi.org/10.1007/s10499-017-0122-5
Malekmohammad K, Rafieian-Kopaei M, Sardari S, Sewell RDE (2019) Toxicological effects of Mentha piperita (peppermint): a review. Toxin Rev 1–15. https://doi.org/10.1080/15569543.2019.1647545
Martínez Cruz P, Ibáñez AL, Monroy Hermosillo OA, Ramírez HC (2012) Use of probiotics in aquaculture. Int Sch Res Not 916845:13. https://doi.org/10.5402/2012/916845
Medina A, Moriñigo MA, Arijo S (2020) Selection of putative probiotics based on antigen-antibody cross-reaction with Photobacterium damselae subsp. piscicida and Vibrio harveyi for use in Senegalese sole (Solea senegalensis). Aquac Rep 17:100366. https://doi.org/10.1016/j.aqrep.2020.100366
Meidong R, Khotchanalekha K, Doolgindachbaporn S, Nagasawa T, Nakao M, Sakai K, Tongpim S (2018) Evaluation of probiotic Bacillus aerius B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla-mong Pangasius bocourti. Fish Shellfish Immunol 73:1–10. https://doi.org/10.1016/j.fsi.2017.11.032
Mehana E, Rahmani A, Aly S (2015) Immunostimulants and fish culture: an overview. Ann Res Rev Biol 5(6):477–489. https://doi.org/10.9734/ARRB/2015/9558
Mendam K, Kavitha B, Naik SJK (2015) Natural sources used for treatment and prevention of filariasis. World J Pharm Pharm Sci 3:1634–1652
Mohamad S, Abasali H (2010) Effect of plant extracts supplemented diets on immunity and resistance to Aeromonas hydrophila in common carp (Cyprinus carpio). Agric J 5(2):119–127. https://doi.org/10.3923/aj.2010.119.127
Mohammadian T, Nasirpour M, Tabandeh MR, Heidary AA, Ghanei-Motlagh R, Hosseini SS (2019) Administrations of autochthonous probiotics altered juvenile rainbow trout Oncorhynchus mykiss health status, growth performance and resistance to Lactococcus garvieae, an experimental infection. Fish Shellfish Immunol 86:269–279. https://doi.org/10.1016/j.fsi.2018.11.052
Mohammadian T, Jangaran-Nejad A, Mesbah M, Shirali T, Malekpouri P, Tabandeh MR (2020) Effect of Lactobacillus casei on innate immunity responses and Aeromonas hydrophila resistance in Shabot. Tor Grypus Probiotics Antimicrob Proteins 12(1):224–235. https://doi.org/10.1007/s12602-018-9510-z
Nakamura F, Ishida Y, Sawada D, Ashida N, Sugawara T, Sakai M, Fujiwara S (2016) Fragmented lactic acid bacteria cells activate peroxisome proliferator-activated receptors and ameliorate dyslipidemia in obese mice. J Agric Food Chem 64:2549–2559
Nandi A, Banerjee G, Dan SK, Ghosh K, Ray AK (2018) Evaluation of In Vivo Probiotic efficiency of Bacillus amyloliquefaciens in Labeo rohita challenged by pathogenic strain of Aeromonas hydrophila MTCC 1739. Probiotics Antimicrob Proteins 10(2):391–398. https://doi.org/10.1007/s12602-017-9310-x
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Neissi A, Rafiee G, Nematollahi M, Safari O (2013) The effect of Pediococcus acidilactici bacteria used as probiotic supplement on the growth and non-specific immune responses of green terror, Aequidens rivulatus. Fish Shellfish Immunol 35:1976–1980
Neumann N (1995) Macrophage activating factor(s) secreted by mitogen stimulated goldfish kidney leukocytes synergize with bacterial lipopolysaccharide to induce nitric oxide production in teleost macrophages. Dev Comp Immunol 19(6):473–482. https://doi.org/10.1016/0145-305X(95)00032-O
Nguyen TL, Park CI, Kim DH (2017) Improved growth rate and disease resistance in olive flounder, Paralichthys olivaceus, by probiotic Lactococcus lactis WFLU12 isolated from wild marine fish. Aquaculture 471:113–120. https://doi.org/10.1016/j.aquaculture.2017.01.008
Noor-Ul H, Haokun L, Junyan J, Xiaoming Z, Dong H, Yunxia Y, Shouqi X (2020) Dietary supplementation of Geotrichum candidum improves growth, gut microbiota, immune-related gene expression and disease resistance in gibel carp CAS III (Carassius auratus gibelio). Fish Shellfish Immunol 99:144–153. https://doi.org/10.1016/j.fsi.2020.02.001
Norqvist A, Hagström A, Wolf-Watz H (1989) Protection of rainbow trout against vibriosis and furunculosis by the use of attenuated strains of Vibrio anguillarum. Appl Environ Microbiol 55(6):1400–1405. https://doi.org/10.1128/AEM.55.6.1400-1405.1989
Nya EJ, Austin B (2009a) Use of dietary ginger, Zingiber officinale Roscoe, as an immunostimulant to control Aeromonas hydrophila infections in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32(11):971–977. https://doi.org/10.1111/j.1365-2761.2009.01101.x
Nya EJ, Austin B (2009b) Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32(11):963–970. https://doi.org/10.1111/j.1365-2761.2009.01100.x
Nya EJ, Austin B (2010) Use of bacterial lipopolysaccharide (LPS) as an immunostimulant for the control of Aeromonas hydrophila infections in rainbow trout Oncorhynchus mykiss (Walbaum). J Appl Microbiol 108(2):686–694. https://doi.org/10.1111/j.1365-2672.2009.04464.x
Olivier G, Evelyn TPT, Lallier R (1985) Immunity to Aeromonas salmonicida in coho salmon (Oncorhynchus kisutch) induced by modified Freund’s complete adjuvant: Its non-specific nature and the probable role of macrophages in the phenomenon. Dev Comp Immunol 9(3):419–432. https://doi.org/10.1016/0145-305X(85)90005-9
Öz M, Dikel S, Durmus M (2018) Effect of black cumin oil (Nigella sativa) on the growth performance, body composition and fatty acid profile of rainbow trout (Oncorhynchus mykiss). Iranian J Fish Sci 17(4):713–724. https://doi.org/10.22092/ijfs.2018.116826
Pan T, Yan M, Chen S, Wang X (2013) Effects of ten traditional Chinese herbs on immune response and disease resistance of Sciaenops ocellatus (Actinopterygii: Perciformes: Sciaenidae). Acta Ichthyol Piscat 43(1):41–49. https://doi.org/10.3750/AIP2013.43.1.06
Patel AR, Shah NP, Prajapati JB (2012) Antibiotic resistance profile of lactic acid bacteria and their implications in food chain. World J Dairy Food Sci 7(2):202–211. https://doi.org/10.5829/idosi.wjdfs.2012.7.2.1113
Picchietti S, Fausto AM, Randelli E, Carnevali O, Taddei AR, Buonocore F, Scapigliati G, Abelli L (2009) Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immunol 26(3):368–376. https://doi.org/10.1016/j.fsi.2008.10.008
Portalès P, Clot J (2006) Immunostimulants revisited: focus on the pharmacology of ribomunyl. BioDrugs 20(2):81–84. https://doi.org/10.2165/00063030-200620020-00002
Pourmoghim H, Haghighi M, Sharif M (2015) Effect of dietary inclusion of Origanum vulgare extract on non-specific immune responses and hematological parameters of rainbow trout (Oncorhynchus mykiss). Bull Environ Pharm Life Sci 4(3):33–39
Pourgholam MA, Khara H, Safari R, Sadati MAY, Aramli MS (2017) Hemato-immunological responses and disease resistance in siberian sturgeon Acipenser baerii fed on a supplemented diet of Lactobacillus plantarum. Probiotics Antimicrob Proteins 9(1):32–40. https://doi.org/10.1007/s12602-016-9229-7
Ramesh D, Souissi S, Ahamed TS (2017) Effects of the potential probiotics Bacillus aerophilus KADR3 in inducing immunity and disease resistance in Labeo rohita. Fish Shellfish Immunol 70:408–415. https://doi.org/10.1016/j.fsi.2017.09.037
Ramesh D, Souissi S (2018) Effects of potential probiotic Bacillus subtilis KADR1 and its subcellular components on immune responses and disease resistance in Labeo rohita. Aquac Res 49(1):367–377. https://doi.org/10.1111/are.13467
Reda RM, El-Hady MA, Selim KM, El-Sayed HM (2018) Comparative study of three predominant gut Bacillus strains and a commercial B. amyloliquefaciens as probiotics on the performance of Clarias gariepinus. Fish Shellfish Immunol 80:416–425. https://doi.org/10.1016/j.fsi.2018.06.031
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433:50–61. https://doi.org/10.1016/j.aquaculture.2014.05.048
Romano N, Rossi F, Abelli L, Caccia E, Piergentili R, Mastrolia L, Randelli E, Buonocore F (2007) Majority of TcRbeta+ T-lymphocytes located in thymus and midgut of the bony fish, Dicentrarchus labrax (L.). Cell Tissue Res 329(3):479–489. https://doi.org/10.1007/s00441-007-0429-z
Rosas-Ledesma P, León-Rubio JM, Alarcón FJ, Moriñigo MA, Balebona MC (2012) Calcium alginate capsules for oral administration of fish probiotic bacteria: assessment of optimal conditions for encapsulation. Aquac Res 43:106–116
Safari R, Adel M, Lazado CC, Caipang CMA, Dadar M (2016) Host-derived probiotics Enterococcus casseliflavus improves resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss) via immunomodulation. Fish Shellfish Immunol 52:198–205. https://doi.org/10.1016/j.fsi.2016.03.020
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172(1–2):63–92. https://doi.org/10.1016/S0044-8486(98)00436-0
Schaeck M, Duchateau L, Van den Broeck W, Van Trappen S, De Vos P, Coulombet C, Boon N, Haesebrouck F, Decostere A (2016) Vibrio lentus protects gnotobiotic sea bass (Dicentrarchus labrax L.) larvae against challenge with Vibrio harveyi. Vet Microbiol 185:41–48. https://doi.org/10.1016/j.vetmic.2016.01.024
Sharifuzzaman SM, Austin B (2010) Development of protection in rainbow trout (Oncorhynchus mykiss, Walbaum) to Vibrio anguillarum following use of the probiotic Kocuria SM1. Fish Shellfish Immunol 29:212–216
Sharifuzzaman SM, Abbass A, Tinsley JW, Austin B (2011) Subcellular components of probiotics Kocuria SM1 and Rhodococcus SM2 induce protective immunity in rainbow trout (Oncorhynchus mykiss, Walbaum) against Vibrio anguillarum. Fish Shellfish Immunol 30(1):347–353. https://doi.org/10.1016/j.fsi.2010.11.005
Sharma P, Ram C, Sihag CR, Gahlawat SK (2013) Relative efficacy of two probiotics in controlling the Epizootic Ulcerative Syndrome disease in Mrigal (Cirrhinus mrigala Ham). J Fish Aquat Sci 8:305–322
Sharma P, Tomar SK, Sangwan V, Goswami P, Singh R (2016) Antibiotic esistance of Lactobacillus sp. isolated from commercial probiotic preparations: antibiotic-resistant Lactobacillus. J Food Saf 36(1):38–51. https://doi.org/10.1111/jfs.12211
Siwicki A (1987) Immunomodulating activity of levamisole in carp spawners, Cyprinus carpio L. J Fish Biol 31(1):245–246. https://doi.org/10.1111/j.1095-8649.1987.tb05325.x
Siwicki AK (1989) Immunostimulating influence of levamisole on nonspecific immunity in carp (Cyprinus carpio). Dev Comp Immunol 13(1):87–91. https://doi.org/10.1016/0145-305X(89)90021-9
Siwicki AK, Anderson DP, Rumsey GL (1994) Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol 41(1–2):125–139. https://doi.org/10.1016/0165-2427(94)90062-0
Soltani M, Abdy E, Alishahi M, Mirghaed AT, Hosseini-Shekarabi P (2017) Growth performance, immune-physiological variables and disease resistance of common carp (Cyprinus carpio) orally subjected to different concentrations of Lactobacillus plantarum. Aquacult Int 25(5):1913–1933. https://doi.org/10.1007/s10499-017-0164-8
Soltani M, Ghosh K, Hoseinifar SH, Kumar V, Lymbery AJ, Roy S, Ringø E (2019) Genus Bacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev Fish Sci Aquac 27(3):331–379. https://doi.org/10.1080/23308249.2019.1597010
Sookchaiyaporn N, Srisapoome P, Unajak S, Areechon N (2020) Efficacy of Bacillus spp. isolated from Nile tilapia Oreochromis niloticus Linn. on its growth and immunity, and control of pathogenic bacteria. Fish Sci 86(2):353–365. https://doi.org/10.1007/s12562-019-01394-0
Sørum U, Damsgard B (2004) Effects of anaesthetisation and vaccination on feed intake and growth in Atlantic salmon (Salmo salar L.). Aquaculture 232:333–341
Spanghero DBN, Spanghero ECADM, Pedron JDS, Chagas EC, Chaves FCM, Zaniboni-Filho E (2019) Peppermint essential oil as an anesthetic for and toxicity to juvenile silver catfish. Pesquisa Agropecuária Bras 54:00367. https://doi.org/10.1590/s1678-3921.pab2019.v54.00367
Srisapoome P, Areechon N (2017) Efficacy of viable Bacillus pumilus isolated from farmed fish on immune responses and increased disease resistance in Nile tilapia (Oreochromis niloticus): laboratory and on-farm trials. Fish Shellfish Immunol 67:199–210. https://doi.org/10.1016/j.fsi.2017.06.018
Stratev D, Zhelyazkov G, Noundou XS, Krause RWM (2018) Beneficial effects of medicinal plants in fish diseases. Aquacult Int 26(1):289–308. https://doi.org/10.1007/s10499-017-0219-x
Sun Y, He M, Cao Z, Xie Z, Liu C, Wang S, Guo W, Zhang X, Zhou Y (2018) Effects of dietary administration of Lactococcus lactis HNL12 on growth, innate immune response, and disease resistance of humpback grouper (Cromileptes altivelis). Fish Shellfish Immunol 82:296–303. https://doi.org/10.1016/j.fsi.2018.08.039
Talpur AD, Ikhwanuddin M (2012) Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch). Aquaculture 364–365:6–12. https://doi.org/10.1016/j.aquaculture.2012.07.035
Talpur AD, Ikhwanuddin M, Ambok Bolong AM (2013) Nutritional effects of ginger (Zingiber officinale Roscoe) on immune response of Asian sea bass, Lates calcarifer (Bloch) and disease resistance against Vibrio harveyi. Aquaculture 400–401:46–52. https://doi.org/10.1016/j.aquaculture.2013.02.043
Tan HY, Chen SW, Hu SY (2019) Improvements in the growth performance, immunity, disease resistance, and gut microbiota by the probiotic Rummeliibacillus stabekisii in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 92:265–275. https://doi.org/10.1016/j.fsi.2019.06.027
Tan LT-H, Chan K-G, Lee L-H, Goh B-H (2016) Streptomyces bacteria as potential probiotics in aquaculture. Front Microbiol 7:79. https://doi.org/10.3389/fmicb.2016.00079
Tapia-Paniagua ST, Díaz-Rosales P, León-Rubio JM, García de La Banda I, Lobo C, Alarcón FJ, Chabrillón M, Rosas-Ledesma P, Varela JL, Ruiz-Jarabo I, Arijo S, Esteban MA, Martínez-Manzanares E, Mancera JM, Balebona MC, Moriñigo MA (2012) Use of the probiotic Shewanella putrefaciens Pdp11 on the culture of Senegalese sole (Solea senegalensi, Kaup 1858) and gilthead seabream (Sparus aurata L.). Aquac Int 20(6):1025–1039. https://doi.org/10.1007/s10499-012-9509-5
Tapia-Paniagua ST, Vidal S, Lobo C, García de la Banda I, Esteban MA, Balebona MC, Moriñigo MA (2015) Dietary administration of the probiotic SpPdp11: effects on the intestinal microbiota and immune-related gene expression of farmed Solea senegalensis treated with oxytetracycline. Fish Shellfish Immunol 46:449–458
Tavares-Dias M (2018) Current knowledge on use of essential oils as alternative treatment against fish parasites. Aquat Living Res 31:13. https://doi.org/10.1051/alr/2018001
Thanigaivel S, Chandrasekaran N, Mukherjee A, Thomas J (2015) Investigation of seaweed extracts as a source of treatment against bacterial fish pathogen. Aquaculture 448:82–86. https://doi.org/10.1016/j.aquaculture.2015.05.039
Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G (2012) Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut 61(7):1007–1015
Vallejos-Vidal E, Reyes-Lopez F, MacKenzie S (2014) Immunostimulant diets and oral vaccination In Fish. In: Austin B, Newaj-Fyzul A (eds) Diagnosis and control of diseases of fish and shellfish. Oxford, John Wiley & Sons, Ltd, pp 77–89
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture 446:88–96. https://doi.org/10.1016/j.aquaculture.2015.03.014
van Reenen CA, Dicks LM (2011) Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: What are the possibilities? a review. Arch Microbiol 193(3):157–168. https://doi.org/10.1007/s00203-010-0668-3
Vaseeharan B, Sivalingam M, Palaniappan R (2013) Inhibitory activity of essential oils from medicinal plants against Pseudomonas sp. isolated from aquatic environments. Aquac Res 45(1):97–105. https://doi.org/10.1111/j.1365-2109.2012.03208.x
Wang M, Chittiboyina A, Avonto C, Parcher J, Khan I (2014) Comparison of current chemical and stereochemical tests for the identification and differentiation of Pelargonium oils: analytical data for (-)-(1S, 4R, 5S)-guaia-6,9-diene and (-)-(7R,10S)-10-EPI-γ-eudesmol. Rec Nat Prod 80(10):360–372. https://doi.org/10.1055/s-0034-1382645
Wang JL, Meng XL, Lu RH, Wu C, Luo YT, Yan X, Li XJ, Kong XH, Nie GX (2015) Effects of Rehmannia glutinosa on growth performance, immunological parameters and disease resistance to Aeromonas hydrophila in common carp (Cyprinus carpio L.). Aquaculture 435:293–300. https://doi.org/10.1016/j.aquaculture.2014.10.004
Wang W, Sun J, Liu C, Xue Z (2017) Application of immunostimulants in aquaculture: current knowledge and future perspectives. Aquac Res 48(1):1–23. https://doi.org/10.1111/are.13161
Wang C, Liu Y, Sun G, Li X, Liu Z (2019) Growth, immune response, antioxidant capability, and disease resistance of juvenile Atlantic salmon (Salmo salar L.) fed Bacillus velezensis V4 and Rhodotorula mucilaginosa compound. Aquaculture 500:65–74. https://doi.org/10.1016/j.aquaculture.2018.09.052
Wang YC, Hu SY, Chiu CS, Liu CH (2019) Multiple-strain probiotics appear to be more effective in improving the growth performance and health status of white shrimp, Litopenaeus vannamei, than single probiotic strains. Fish Shellfish Immunol 84:1050–1058. https://doi.org/10.1016/j.fsi.2018.11.017
Wang J, Zhang S, Ouyang Y, Li R (2019) Current developments of bacteriocins, screening methods and their application in aquaculture and aquatic products. Biocatal Agric Biotechnol 22(October):101395. https://doi.org/10.1016/j.bcab.2019.101395
Wang X, Yang Y, Huyoke MM (2020) Risks associated with enterococci as probiotics. Food Res Int 129:108788. https://doi.org/10.1016/j.foodres.2019.108788
Wangkahart E, Secombes CJ, Wang T (2019) Studies on the use of Flagellin as an immunostimulant and vaccine adjuvant in fish aquaculture. Front Immunol 9:3054. https://doi.org/10.3389/fimmu.2018.03054
Werner G (1986) Synthetic immunostimulants (excluding sulfur-containing compounds). Comp Immunol Microbiol Infect Dis 9(2–3):131–136. https://doi.org/10.1016/0147-9571(86)90004-4
Xia Y, Lu M, Chen G, Cao J, Gao F, Wang M, Liu Z, Zhang D, Zhu H, Yi M (2018) Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis Niloticus. Fish Shellfish Immunology 76:368–379. https://doi.org/10.1016/j.fsi.2018.03.020
Xia Y, Wang M, Gao F, Lu M, Chen G (2020) Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim Nutr 6(1):69–79. https://doi.org/10.1016/j.aninu.2019.07.002
Xing C-F, Hu H-H, Huang J-B, Fang H-C, Kai Y-H, Wu Y-C, Chi S-C (2013) Diet supplementation of Pediococcus pentosaceus in cobia (Rachycentron canadum) enhances growth rate, respiratory burst and resistance against photobacteriosis. Fish Shellfish Immunol 35:1122–1128
Yamashita MM, Pereira SA, Cardoso L, de Araujo AP, Oda CE, Schmidt ÉC, Bouzon ZL, Martins ML, Mouriño JLP (2017) Probiotic dietary supplementation in Nile tilapia as prophylaxis against streptococcosis. Aquac Nutr 23(6):1235–1243. https://doi.org/10.1111/anu.12498
Yi CC, Liu CH, Chuang KP, Chang YT, Hu SY (2019) A potential probiotic Chromobacterium aquaticum with bacteriocin-like activity enhances the expression of indicator genes associated with nutrient metabolism, growth performance and innate immunity against pathogen infections in zebrafish (Danio rerio). Fish Shellfish Immunol 93:124–134. https://doi.org/10.1016/j.fsi.2019.07.042
Zakęś Z, Kowalska A, Demska-Zakęś K, Jeney G, Jeney Z (2008) Effect of two medicinal herbs (Astragalus radix and Lonicera japonica) on the growth performance and body composition of juvenile pikeperch [Sander lucioperca (L.)]. Aquac Res 39(11):1149–1160. https://doi.org/10.1111/j.1365-2109.2008.01977.x
Zhang XP, Li WX, Ai TS, Zou H, Wu SG, Wang GT (2014) The efficacy of four common anthelmintic drugs and traditional Chinese medicinal plant extracts to control Dactylogyrus vastator (Monogenea). Aquaculture 420–421:302–307. https://doi.org/10.1016/j.aquaculture.2013.09.022
Zhang D, Gao Y, Ke X, Yi M, Liu Z, Han X, Shi C, Lu M (2019) Bacillus velezensis LF01: in vitro antimicrobial activity against fish pathogens, growth performance enhancement, and disease resistance against streptococcosis in Nile tilapia (Oreochromis niloticus). Appl Microbiol Biotechnol 103(21–22):9023–9035. https://doi.org/10.1007/s00253-019-10176-8
Zhang HP, Dong WL, Chen L, Wang YM, Muhammad I, Ju AQ, Shan XF, Ma HX, Kong L (2020) Effects of dietary Lactobacillus plantarum C20015 on growth, immunity, and disease resistance in koi carp. Aquacult Int 28(5):1797–1809. https://doi.org/10.1007/s10499-020-00558-5
Zhou S, Li WX, Wang YQ, Zou H, Wu SG, Wang GT (2016) Anthelmintic efficacies of three common disinfectants and extracts of four traditional Chinese medicinal plants against Gyrodactylus kobayashii (Monogenea) in goldfish (Carassius auratus). Aquaculture 466:72–77. https://doi.org/10.1016/j.aquaculture.2016.09.048
Zhu C, Yu L, Liu W, Jiang M, He S, Yi G, Wen H, Liang X (2019) Dietary supplementation with Bacillus subtilis LT3-1 enhance the growth, immunity and disease resistance against Streptococcus agalactiae infection in genetically improved farmed tilapia, Oreochromis Niloticus. Aquac Nutr 25(6):1241–1249. https://doi.org/10.1111/anu.12938
Zółkiewicz J, Marzec A, Ruszczyṅski M, Feleszk W (2020) Postbiotics—a step beyond pre- and probiotics. Nutrients 12:2189. https://doi.org/10.3390/nu12082189
Zorriehzahra MJ, Delshad ST, Adel M, Tiwari R, Karthik K, Dhama K, Lazado CC (2016) Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review. Vet Q 36(4):228–241. https://doi.org/10.1080/01652176.2016.1172132
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
García-Márquez, J., Tapia-Paniagua, S., Moriñigo, M.Á., Arijo, S. (2022). Probiotics for Controlling Infectious Diseases. In: Austin, B., Sharifuzzaman, S. (eds) Probiotics in Aquaculture. Springer, Cham. https://doi.org/10.1007/978-3-030-98621-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-98621-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98620-9
Online ISBN: 978-3-030-98621-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)