Abstract
Probiotic effect of a consortium of putative lactic acid bacteria on Labeo rohita was investigated with emphasis on growth performance, immune response, and disease resistance against Aeromonas hydrophila. Fish were fed either a lactic acid bacteria-supplemented diet or a control diet for a period of 30 days. At the end of the experiment, probiotic fed group showed a significant improvement in weight gain percentage, specific growth rate, and feed conversion ratio along with increased respiratory burst activity of blood phagocytes and serum antiprotease activity level. Quantitative real-time PCR showed significant upregulation of IL-10 gene in kidney, intestine, and liver of probiotic-treated group, whereas TNF-α gene was significantly upregulated only in liver and intestine. HSP70 gene was significantly upregulated in intestine but downregulated in liver on day 15. Challenge with Aeromonas hydrophila on day 30 of probiotic feeding showed a significant increase in survival percentage of treated (93.33 %) over the control group (33.33 %). Further challenge after 20 and 40 days of withdrawal of probiotic showed higher survival percentage (60 and 40 %, respectively) in withdrawn group compared to control although difference was statistically insignificant. The consortium of putative probionts may serve simultaneously as an immunomodulating feed additive useful for disease protection and growth enhancer in eco-friendly freshwater aquaculture practices. However, feeding at regular interval with probiotic supplemented diet is suggested for a prolonged immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The complex microbial ecology of the gastrointestinal tract of fish interacts with the internal and external environment and has an important influence on its health and disease. The manipulation of the gut microbiota through dietary supplementation of beneficial microbes like lactic acid bacteria (LAB) has been useful for better growth, digestion, immunity, and disease resistance of the host (Nayak 2010; Dimitroglou et al. 2011). It is a successful novel approach not only from nutritional point of view but also as an alternate viable therapeutic modality to overcome the adverse effects of antibiotics and drugs in aquaculture (Nayak 2010).
Probiotics are live microorganisms which when administered in adequate amount confer a health benefit on the host (Food and Agriculture Organization of the United Nations 2001). It has been suggested that the efficacy of probiotics is highest in the host species from which they are isolated because such strains perform better as they have already adhered to the gut wall of the fish and are well adapted to compete with the pathogens (Ghosh et al. 2007). Moreover, functionality of a multistrain or multispecies probiotic could be more effective and more consistent than that of a monostrain probiotic because mixed cultures may contain bacteria that complement each other’s health effect and thus have synergistic probiotic properties (Timmerman et al. 2004; Nayak 2010). However, a very few studies have contemplated the use of combinations of more than three probiotic species at the same time in the diet or culture environment and their effect on fish (Irianto and Austin 2002; Geng et al. 2012; Standen et al. 2015).
Rohu, Labeo rohita (Hamilton), one of the most important freshwater species of Asian region, is intrinsically sensitive to various stressors and subsequently susceptible to infectious agents during normal aquaculture production (Choudhury et al. 2005). The Gram-negative bacterium, Aeromonas hydrophila, is recognized as one of the most important freshwater fish pathogens responsible for major bacterial diseases like hemorrhagic septicemia, infectious dropsy, tropical ulcerative disease, and fin rot adversely affecting all vital organs and subsequently leading to high mortality in a wide variety of freshwater fish species (Sahu et al. 2011). This bacterium can also behave as a secondary opportunistic pathogen, by assailing already compromised or stressed hosts (Reyes-Becerril et al. 2011). A therapeutic agent must, therefore, regulate the severe inflammatory immune response, while promoting elimination of the pathogen by the immune system. In this context, microbial interventions like LAB have been proposed as a nonpathogenic bacteriotherapeutic means of modulating immune phenotype expression and they may provide important regulatory signals to the immune system that influence systemic as well as local patterns of immunoreactivity (Panigrahi et al. 2007). Strains of LAB have shown to modulate production of cytokines like tumor necrosis factor (TNF-α) and IL-10 (Panigrahi et al. 2007; Perez-Sanchez et al. 2011; Gioacchini et al. 2014) which play an important role in host defense mechanisms in response to bacterial colonization or invasion. Recent studies have also shown the effect of dietary probiotics on expression of HSP70 genes in fish which contributes in establishing a cytoprotective state by appropriate protection of protein structures, stopping apoptotic mechanisms (Rollo et al. 2006) and strengthening the immune system (Liu et al. 2013).
In the present study, growth performance, disease resistance, and immune modulation of rohu in response to a dietary consortium of putative probionts containing strains of Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus brevis, and Pediococcus pentosaceus isolated from intestines of different freshwater fish were investigated. Further, possible protective effect against challenge infection at different time interval after withdrawal of probiotic feed was considered.
Materials and methods
Bacterial strains
L. plantarum SM16 (GenBank accession no. KJ690731), L. plantarum SM33 (GenBank accession no. KJ690748), L. fermentum SM51 (GenBank accession no. KJ729045), L. brevis SM56 (GenBank accession no. KJ729050), and P. pentosaceus SM64 (GenBank accession no. KJ729058) isolated from intestines of freshwater fish rohu, L. rohita (Hamilton), catla, Catla catla (Hamilton), mrigal, Cirrhinus mrigala (Hamilton), silver carp, Hypopthalmichthys molitrix (Valenciennes), and grass carp, Ctenopharyngodon idella (Valenciennes), and identified by biochemical reactions and 16S rDNA gene sequencing were selected as potential probiotics. These strains were chosen from a pool of 76 isolates because of their maximum positive in vitro probiotic characteristics, which include resistance to pH and bile, positive antagonism against freshwater fish pathogen A. hydrophila, and detection of bacteriocin gene (Table 1) (Maji et al. 2016). The strains were grown anaerobically in de Man, Rogosa, and Sharpe (MRS) broth (HiMedia) at 37 °C. Stock cultures stored at −80 °C were prepared from overnight cultures to which 30 % (vol/vol) glycerol (HiMedia) was added just prior to freezing.
Safety of probiotic strains
Ninety apparently healthy, rohu fingerlings of average weight (20.06 ± 0.15 g) obtained from the farm of Central Institute of Freshwater Aquaculture (CIFA), Kausalyaganga, Bhubaneswar were divided into six equal groups including five experimental and one control group in three replicates (each of five fish) and distributed randomly among eighteen 100-L tank. The fish were acclimatized for 2 weeks. Throughout the experiment, all the fish were fed the basal diet (formulation and chemical composition is shown in Table 2) and maintained in well-aerated freshwater at 27 ± 1 °C with 25 % water exchange every day. For determination of the safety of probiotic strains, 100 μL of culture suspension with concentration 109 CFU mL−1 of each strain was injected intraperitoneally into the respective groups of fish, whereas control group was injected with normal saline solution (NSS). All the groups of fish were observed for 10 days for any evidence of disease.
Diet formulation and probiotic supplementation
The basal diet (Table 2) served as the control diet. The experimental diet was prepared using the basal formulation with supplementation of probiotic strains. For this, the five selected probiotic LAB strains were grown overnight in MRS broth at 37 °C. The cultures were centrifuged at 3300×g for 30 min. The pellets were washed twice in NSS and resuspended in NSS. The absorbance at 600 nm was adjusted to 0.5 which corresponded to 109 CFU mL−1. The probiotic cells were added at equal proportions (1:1:1:1:1) and thoroughly mixed with the basal diet to make a final concentration of 109 CFU g−1 of diet. Pellets were made and air dried at room temperature. Basal feed (without probiotic strains) and the experimental feed were stored in clean plastic containers at 4 °C. The feed was prepared twice weekly.
The survival of the supplemented bacteria in the diet was assessed following storage at 4 °C for 1 month. One gram of the diet was homogenized in 9.0 mL saline, serial dilutions were prepared, and 0.1 mL was spread onto triplicate plates of MRS agar. The colonies were counted after incubation for 48 h at 37 °C (Aly et al. 2008).
Feeding experiment
Rohu fingerlings were procured from the farm of CIFA, Kausalyaganga, Bhubaneswar. The fish had not been vaccinated nor exposed to disease and were healthy without any symptoms of infection. All the fish were fed the basal diet during 2 weeks of acclimatization. The experiment used twelve 500-L tanks with six replicate tanks per treatment, and 300 fish of uniform size (19.72 ± 0.18 g) were randomly distributed into the tanks (25 fish per tank). Among the six replicate tanks of each treatment, three replicate tanks were used for sampling and the remaining three replicate tanks were used for growth measurement and challenge test. During the feeding trial, the fish were fed twice daily (9.00 and 15.00 h) with or without probiotics according to the treatment groups, control group and compound probiotic group, at the rate of 3 % of body weight for 30 days following which probiotic diet was withdrawn and basal diet was fed to fish in all treatment groups for next 40 days. The amount of diet consumed was determined by daily recovery of excess feed, which was then dried and weighed. Daily feed was adjusted every 7 days by batch weighing following a 24-h starvation period. All the fish were maintained in well-aerated freshwater at 27 ± 1 °C with 25 % water exchange every day. Basic physicochemical parameters of water were measured every week, and the pH, dissolved oxygen, alkalinity, nitrite, ammonia, and nitrate values were 7.4 ± 0.83, 5.8 ± 0.73, 171 ± 0.3, 0.014 ± 0.1, 0.17 ± 0.029, and 0.08 ± 0.009 mg L−1, respectively, with very little fluctuations.
Survival and growth study
The dead fish in each tank was counted daily and fish survival percentage was determined as per the formula:
At the end of the 30-day trial, fish were batch weighed and growth performances were assessed in terms of weight gain percent (WG %), specific growth rate (SGR), and feed conversion ratio (FCR) as per the formulae:
Innate immune response
Five fish were randomly collected from each three replicates (n = 5 in triplicate) of control and treatment tank on day 15 and day 30 of experiment. The fish were anesthetized with MS222 (Argent Chemical, Redmond, USA), bled by caudal venipuncture, and blood was collected. An aliquot of blood was heparinized (50 IU/mL), and the remaining part was allowed to clot at room temperature and kept at 4 °C for 4 h. The heparinized blood samples were used (within 1 h of collection) for measurement of nitro blue tetrazolium (NBT) activity. The serum (from clotted blood) was separated by centrifugation at 5800×g for 5 min at 4 °C. The serum samples collected were stored at −80 °C until further analysis.
Respiratory burst activity
Reactive oxygen radical production during respiratory burst activity was assayed by the reduction of NBT to formazan following Anderson and Siwicki (1995) and as described previously (Sahoo et al. 2011). The extent of NBT reduced was measured at an optical density of 540 nm of the supernatant using dimethyl formamide as the blank.
Antiprotease activity
Serum antiprotease assay was done as described previously (Ellis 1990; Sahoo et al. 2011). Briefly, 10 μL of test serum was incubated with 100 μL trypsin (type 1 from bovine pancreas) for 30 min at 25 °C along with two blanks (110 μL PBS) and three references (10 μL PBS + 100 μL trypsin). Each of the reaction mixture was further incubated with 1 mL casein for 15 min. The reactions were terminated with the addition of 500 μL of 10 % trichloroacetic acid (TCA). The samples were centrifuged at 10,551×g for 5 min, and optical density of the supernatants at 280 nm was measured. Percent inhibition was calculated using the following formula:
Immune-related gene expressions
Three fish from each of the three replicates (n = 3 in triplicates) were sampled on day 0, day 15, and day 30 for analysis of immunity and HSP70 gene expression in kidney, liver, and intestine. To reduce individual variation of gene expression, the sampled fish organs from each tank were pooled and homogenized using a glass homogenizer (He et al. 2011; He et al. 2013). The tissues were collected, frozen in liquid nitrogen, and stored at −80 °C until further use.
RNA extraction, cDNA synthesis, and real-time PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol and was treated with RNase-Free DNase I (Fermentas, Germany). The total RNA (1 μg) was used for cDNA synthesis, by using Revert Aid™ First Strand cDNA synthesis kit (Fermentas) following the manufacturer’s protocol. The quantitative real-time PCR (qRT-PCR) analyses for TNF-α, IL-10, HSP70, and the housekeeping gene (β-actin) were performed in triplicate for each cDNA sample using Light Cycler-480 SYBR Green I kit (Roche Diagnostics) in a Light Cycler 480 RT-PCR instrument (Roche Diagnostics) as per manufacturer’s instructions. The primers used are mentioned in Table 3 (He et al. 2011; Swain et al. 2011). The following real-time PCR program was employed: initial denaturation for 5 min at 95 °C, 40 cycles of a three segmented amplification and quantification program [denaturation for 10 s at 94 °C; annealing for 10 s at 54 °C (IL-10)/55 °C (TNF-α)/58 °C (HSP70); elongation for 20 s at 72 °C], a melting step by slow heating from 64 to 95 °C with continuous fluorescence measurement, and a final cooling step to 42 °C. After the run, cycle threshold (C T) values were acquired using the Abs Quant/2nd derivative Max method of the real-time machine (Roche Light Cycler, LC 480). Negative control PCR containing RNA templates were included for each sample to rule out the possibility of genomic DNA contamination. Relative mRNA levels of target gene were normalized to β-actin expression for each sample. The efficiency corrected relative quantification of mRNA was determined using the advanced relative quantification method (E-method) provided in the software (Roche Diagnostics), and gene expression levels were calculated by the 2−ΔΔC T comparative threshold cycle (C T) method (Livak and Schmittgen 2001).
Challenge test
The challenge test was conducted in triplicate with five fish from each group (n = 5 in triplicate) on day 30, day 50, and day 70 of the experiment. The virulent strain of A. hydrophila was obtained from culture repository of Fish Health Management Division, CIFA. The bacterial isolate was cultured in brain heart infusion broth (HiMedia) at 37 °C for 16 h and diluted to log10 7.0 CFU mL−1 in phosphate-buffered saline. Fish were injected intraperitoneally with 0.25 mL of diluted culture (10LD50 dose approximately as calculated by Reed and Muench method 1938). The fish were observed for 10 days for mortality, and post-challenge survival percentage was calculated for both the groups. The cause of mortality was confirmed by re-isolating the bacteria from the kidney of 10 % dead fish as described by Kumari et al. (2003).
Statistical analysis
Statistical analysis was conducted using the computerized software Statistical Package for Social Sciences (SPSS) version 18.0. Data are reported as means ± SE. Student’s t test was used to determine the significant difference in growth parameters. Differences were considered significant at P < 0.05. For the rest of all the parameters, differences between means were determined and compared by multiple comparison test (Duncan). All tests used P < 0.05 as a significance level.
Results
Safety of the probiotic strains
There were neither any signs of disease and pathological alterations nor any mortality observed in the probiotic injected rohu during 10 days of post-challenge examination. This confirms the safety and nonpathogenicity of the probiotic strains used in freshwater fish rohu.
Shelf-life of the feed, survival, and growth performance
The LAB level in the probiotic feed stored at 4 °C after 1 month was found to be 108 CFU g−1. Both the control and probiotic-treated group showed cent percent survival. There was significant (P < 0.05) improvement in the final weight, weight gain percent, SGR, and FCR of probiotic-treated group in comparison to the control group at the end of the 30 days of experiment (Table 4).
Innate immune response
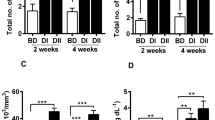
For each parameter, the mean value and standard error were calculated separately for the control and the probiotic-treated group. The respiratory burst activity of blood phagocytes and serum antiprotease activity level were significantly (P < 0.05) higher in the probiotic-treated group compared to the control group both at day 15 and day 30 of the experiment (Fig. 1a, b).
Immune-related gene expressions
The effects of dietary probiotic on expression of genes encoding TNF-α, IL-10, and HSP70 were examined.
No significant effect was detected for TNF-α gene expression in the kidney. However, in the intestine, the dietary probiotic caused a significant (P < 0.05) upregulation of TNF-α gene expression gradually from day 0 to day 15 and then to day 30. In the liver, the TNF-α gene expression was significantly (P < 0.05) upregulated at day 30 compared to initial day 0 and day 15 (Fig. 2a).
In the kidney, IL-10 gene expression was significantly downregulated (P < 0.05) at day 15 relative to day 0 and day 30, whereas in the intestine, the dietary probiotic caused a gradual significant (P < 0.05) upregulation of IL-10 gene expression from day 0 to day 30. Similarly, in the liver, IL-10 gene expression increased significantly (P < 0.05) at day 15 and day 30 relative to initial level at day 0 (Fig. 2b).
No significant effect was detected for HSP70 gene expression in the kidney. However, in the intestine, HSP70 gene expression was significantly (P < 0.05) higher at day 15 in comparison to day 0 and day 30, whereas in the liver, the dietary probiotic caused a significant (P < 0.05) downregulation of HSP70 gene expression at day 15 relative to day 0 and day 30 (Fig. 2c).
Challenge study
The first challenge experiment conducted against A. hydrophila after 30 days of probiotic feeding showed a significant (P < 0.05) increase in the post-challenge survival percentage of the probiotic treated group (93.33 %) than the control group (33.33 %). Further challenge studies carried out after 20 days and 40 days of probiotic feed withdrawal showed a higher percentage of survival (60 and 40 %, respectively) in comparison to the control, although difference was statistically insignificant (P > 0.05) (Fig. 3).
Post-challenge survival percentage of Labeo rohita after challenge with A. hydrophila on day 30 of feeding probiotic supplemented diet and after 20 days (day 50 of the experiment) and 40 days (day 70 of experiment) of withdrawal of probiotic supplemented diet. Columns with different letters are significantly different (P < 0.05)
Discussion
There is a general consensus that probiotics from autochthonous source have a greater chance of competing with resident microbes and becoming predominant within a short period of intake (Nayak, 2010). The strategy of isolating probiotics from the gut of mature animals and then use in immature animals of the same species has been successfully applied in fish (Picchietti et al. 2009; Perez-Sanchez et al. 2011; Beck et al. 2015). Moreover, multistrain or multispecies probiotics are considered to be more effective than monostrain probiotics. There is conclusive evidence that, in higher vertebrates, adequately designed multistrain or multispecies probiotic formulations possess health-promoting effects that are lacking in monospecies probiotic diets (Timmerman et al. 2004; Salinas et al. 2008). Some of the proposed mechanisms include greater survival, growth, viability or adhesion to mucosal surfaces of one species in the presence of another species, the production of different enzymes or other proteins, the creation of a probiotic niche, and additive/synergistic effects of strain-specific properties (Salinas et al. 2008). Also in aquaculture, a few studies have shown the multispecies formulation to be more effective than any of the single-bacteria experimental diets (Aly et al. 2008; Salinas et al. 2008; Beck et al. 2015). These results suggest that, as in humans, the appropriate design of multispecies probiotics can have synergistic positive effects on fish health. Hence, in the present study, a consortium of putative probionts consisting different strains of L. plantarum, L. fermentum, L. brevis, and P. pentosaceus was used as a dietary supplement to evaluate its efficacy on growth performance, immune modulation, and disease resistance in rohu.
Identifying probiotic characteristics of the putative LAB strains by in vitro studies forms the basis for selection of functional probiotics for future in vivo applications (Srinu et al. 2013). According to the guidelines of the evaluation of probiotic organisms reported by a joint FAO/WHO working group, acid tolerance and bile tolerance tests are the two most important in vitro tests used for evaluation of probiotic organisms (Mirlohi et al. 2009). Moreover, in vitro antagonistic activity against pathogenic bacteria and detection of various bacteriocin genes (bacteriocin production) are highly desirable probiotic traits that enable the establishment and persistence of the producing strains within the gastrointestinal tract and, as such, would offer potential alternatives to traditional antibiotics with respect to controlling pathogens within the gut and overcoming complications such as the proteolytic degradation of orally delivered antimicrobial peptides during gastric transit (O’Shea et al. 2011). The five strains used in the present study showed the best (maximum) in vitro probiotic characteristics among the 76 strains of LAB previously isolated from intestines of various freshwater fish (Maji et al., 2016) and were thus considered for further in vivo study as dietary probiotic candidates for sustainable and environmental friendly aquaculture.
For aquaculture applications, the dose of probiotics usually varies from 106 to 1010 CFU g−1 feed (Nayak et al. 2010). While a high dose may have deleterious effects and fail to provide adequate protection against challenge study, a lower dose may not be suitable for colonization of adequate number of bacteria in the gastrointestinal tract of fish and fail to achieve the required level of immunity (Nikoskelainen et al. 2001; Son et al. 2009; Saini et al. 2014). Also during storage of feed, a decrease in viability may further decrease the count of bacteria below efficacy level. Hence, in the present study, consortium of probiotic at a concentration of 109 CFU g−1 of diet (with equal proportions of 1:1:1:1:1 of different strains of probiotics) was used and was found to be successful. The formulated probiotic-supplemented diet significantly (P < 0.05) increased the final weight, body weight gain percent, and SGR and also significantly (P < 0.05) decreased the FCR in the treated groups than the untreated control. This is in consistent with earlier findings where combination of two or more probiotics in the diet resulted in better growth performance in fish (Geng et al. 2012; Mohapatra et al. 2012; Giri et al. 2014). This could be attributed to better feed utilization and improved nutrient absorption and digestive activity by enhancing the synthesis of vitamins, cofactors, and enzymatic activity by the probiotics in the diet (Aly et al. 2008). There was cent percent survival observed in both the groups as also reported by other researchers (He et al. 2011; Standen et al. 2013). This may probably be due to good environmental conditions, high husbandry care, and highly nutritive value diet maintained throughout the experiment (Standen et al. 2013). Probiotic products are usually standardized based on the presumption that the culture viability is a reasonable measure of their activity. In the present study, the LAB level in the probiotic feed stored at 4 °C after 1 month was found to be 108 CFU g−1, a value indicating a better shelf-life of feed and still adequate to be used as a probiotic in the diet and confirmed earlier findings (Aly et al. 2008; Utiswannakul et al. 2011).
The innate immune parameters, viz., respiratory burst and antiprotease activities, constitute the first line of defense and are of major importance in combating infections of fish. The NBT assay is used to determine the respiratory burst activity, especially of neutrophils and monocytes and as such is a very good indicator of the health status or the immunization effectiveness in fish (Sahoo et al. 2005; Aly et al. 2008). Antiprotease present in the fish plasma acts as a protease inhibitor and helps in restricting the ability of the pathogen to produce proteolytic enzymes that aid in the breakdown of host tissues (Eslamloo et al. 2013). In the present study, both the NBT activity and antiprotease activity increased significantly when rohu were fed the consortium of probiotics. Previous studies have shown that dietary compound probiotics enhanced such innate immunity in the fish (Aly et al. 2008; Geng et al. 2012; Eslamloo et al. 2013; Giri et al. 2014; Beck et al. 2015).
Probiotics cause immune modulation by inducing immune regulatory responses mediating a control of the balance between pro- and anti-inflammatory cytokines which play a leading role in nonspecific immune responses. Though there are a very few reports on effect of dietary probiotic mixture on cytokine expressions in fish (Beck et al. 2015), studies on mice have shown therapeutic potentials of multiple probiotic formulation by regulating various pro- and anti-inflammatory cytokines (Lavasani et al. 2010; Pagnini et al. 2010). In the present study, the expression of pro-inflammatory cytokine TNF-α in intestine and liver was significantly (P < 0.05) upregulated with administration of the probiotic consortium. Earlier reports have also indicated an increase in the expression of TNF-α cytokine in fish fed with probiotic-supplemented diet (He et al. 2011; Standen et al. 2013; Gioacchini et al. 2014; Beck et al. 2015). The pro-inflammatory cytokine TNF-α, which is commonly used as a biomarker for immune regulation, is an acute phase protein that induces an inflammatory response and initiates a cascade of cytokines which subsequently recruits macrophages and neutrophils to the site of inflammation or colonization (Panigrahi et al. 2007). The present study also reported an upregulation of IL-10 gene expression on probiotic feeding. As an autoregulatory mediator, IL-10 has important regulatory effects on immunological and inflammatory responses because of its capacity to inhibit the production of pro-inflammatory cytokines by monocytes (Perez-Sanchez et al. 2011). Hence, it is assumed that in order to modulate immune response, IL-10 expression was significantly (P < 0.05) increased in the immune organs (liver and intestine) (He et al. 2011; Perez-Sanchez et al. 2011).
The dietary administration of probiotic consortium had different effect on HSP70 gene expression levels in different organs of fish. The HSP70 gene expression level was upregulated in the intestine at day 15 but reduced thereafter, whereas in the liver, its expression was downregulated at day 15. No significant effect was detected for HSP70 gene expression in the kidney. Also similar to our studies, Liu et al. (2013) reported different expression level of HSP70 gene in intestine, kidney, and spleen of tilapia on administration of different strains of Lactobacillus probiotic diet. Studies have also shown that the HSP70 gene expression level was upregulated in the liver at day 10 but reduced thereafter; no effects were seen in intestine and kidney of koi carp treated with the probiotic (He et al. 2011). HSP70 was selected with consideration of its wide involvement in protection of protein structures and stopping apoptotic mechanisms (Rollo et al. 2006). A higher HSP70 level indicates a greater potentiality to respond to the stressful conditions possibly present in fish farms. HSP70 has also been implicated in both innate and adaptive immunity (Daugard et al. 2007; Zhang et al. 2015). In the present study, it was observed that there was a well-coordinated expression of HSP70 and TNF-α gene in various organs. For example, there was no significant expression of HSP70 and TNF-α in the kidney, whereas in the intestine, a higher level of expression of HSP70 and TNF-α gene was observed on day 15. Similarly, in liver, at day 15, downregulation of HSP70 gene was observed and no significant difference in the expression level of TNF-α was seen and both the genes upregulated on day 30 of probiotic treatment. It appears that HSP70 regulated the expression of TNF-α and is consistent with previous findings on the regulatory effect of grass carp HSP70 on pro-inflammatory cytokine production (Zhang et al. 2015).
Higher expression of the cytokine genes combined with the results of other innate immune parameter tested overall implies that fish fed probiotic mixture diet are in an immunologically elevated state which may aid in resisting pathogenic insults by improving the immune readiness (Beck et al. 2015). This was most likely responsible for the protection and increased survival of probiotic fed rohu against A. hydrophila challenge conducted after 30 days of probiotic feeding. Similar findings have been reported where combination of two or more probiotics in the diet provided higher level of protection against pathogens like A. hydrophila (Aly et al. 2008; He et al. 2011; Parthasarthy and Ravi 2011; Giri et al. 2014), Streptococcus iniae (Beck et al. 2015), and Vibrio harveyi (Geng et al. 2012) possibly by eliciting the nonspecific immune responses.
In order to observe the effect of withdrawal of probiotics on duration of protection, further challenge studies were carried out after 20 and 40 days of probiotic withdrawal. Though a higher percentage of survival was observed in the probiotic withdrawn group in comparison to the control, it was statistically insignificant. Sharifuzzaman and Austin (2010) also reported a steady decline in the relative percent survival of rainbow trout from 87 to 36 % on every week challenge with Vibrio anguillarum for 5 weeks on withdrawal of probiotic feed. It is likely that, as a consequence of withdrawal of the probiotic feed, there was a gradual decline in the population of probiotic bacteria in the gut of rohu and a subsequent decrease in the immune response, as also reported in previous studies on rainbow trout (Nikoskelainen et al. 2003; Panigrahi et al. 2005) and brown trout (Balcazar et al. 2007). However, during the feeding trial of 30 days, the immune regulatory genes in the intestine were significantly affected by application of dietary probiotic mixture. This indicates that the direct association between the probiotic and host epithelium is not always a prerequisite to induce localized effects which ultimately induce systemic immune responses (Perez-Sanchez et al. 2011). It is therefore suggested that along with in vitro assays like adhesion to epithelial cells, growth within mucus, pathogen antagonism, etc., the preliminary selection criteria of the strains should also include certain other methods such as the assessment of relevant immune regulatory gene expression of intestinal mucosal cells and lymphocytes after exposure to probiotic cell wall components and extracellular products (Medina et al. 2007; Perez-Sanchez et al. 2011).
The present study shows successful application of dietary consortium of putative multi-strain probionts as a growth promoter and immune stimulator in rohu and consequently conferring better protection against A. hydrophila by regulating the expression of immune regulatory genes. However, feeding at regular interval with probiotic supplemented diet is suggested to maintain the immunity for a prolonged period. Further investigations should focus on duration of feeding interval of probiotic and subsequent protective immune response in fish.
References
Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25:128–136
Anderson DP, Siwicki AK (1995) Basic hematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian aquaculture II. Fish health section. Asian Fisheries Society, Manila, pp 185–202
Balcazar JL, de Blas I, Ruiz-Zarzuela I, Vendrell D, Calvo AC, Marquez I, Girones O, Muzquiz JL (2007) Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout (Salmo trutta). Br J Nutr 97:522–527
Beck BR, Kim D, Jeon J, Lee SM, Kim HK, Kim OJ, Lee JI, Suh BS, Do HK, Lee KH, Holzapfel WH, Hwang JY, Kwon MG, Song SK (2015) The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralicthys olivaceus). Fish Shellfish Immunol 42:177–183
Choudhury D, Pal AK, Sahu NP, Kumar S, Das SS, Mukherjee SC (2005) Dietary yeast RNA supplementation reduces mortality by Aeromonas hydrophila in rohu (Labeo rohita L.) juveniles. Fish Shellfish Immunol 19:281–291
Daugard M, Rohde M, Jaattela M (2007) The heat shock protein 70 family: highly homologous 24 proteins with overlapping and distinct functions. FEBS Lett 581:3702–3710
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels C, Guroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production—a Mediterranean perspective. Fish Shellfish Immunol 30:1–16
Ellis AE (1990) Serum antiproteases in fish. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, Fair Haven, NJ, pp 95–99
Eslamloo K, Akhavan SR, Henry MA (2013) Effects of dietary administration of Bacillus probiotics on the non-specific immune responses of tinfoil barb, Barbonymus schwanenfeldii (Actinopterygii: Cypriniformes: Cyprinidae). Acta Icthyol Piscat 43:211–218
Food and Agriculture Organization of the United Nations (FAO) (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. In the joint FAO/WHO expert consultation report on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria (October 2001)
Geng X, Dong X-H, Tan B-P, Yang Q-H, Chi S-Y, Liu H-Y, Liu X-Q (2012) Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquac Nutr 18:46–55
Ghosh S, Sinha A, Sahu C (2007) Isolation of putative probionts from the intestines of Indian major carps. Isr J Aquac Bamid 59:127–132
Gioacchini G, Giorgini E, Olivotto I, Maradonna F, Merrifield DL, Carnevali O (2014) The influence of probiotics on zebra fish Danio rerio innate immunity and hepatic stress. Zebrafish 11:98–106
Giri SS, Sukumaran V, Sen SS, Jena PK (2014) Effects of dietary supplementation of potential probiotic Bacillus subtilis VSG1 singularly or in combination with Lactobacillus plantarum VSG3 or/and Pseudomonas aeruginosa VSG2 on the growth, immunity and disease resistance of Labeo rohita. Aquac Nutr 20:163–171
He S, Liu W, Zhou Z, Mao W, Ren P, Marubashi T, Ringo E (2011) Evaluation of probiotic strain Bacillus subtilis C-3102 as a feed supplement for koi carp (Cyprinus carpio). J Aquac Res Development. doi:10.4172/2155-9546.S1-005
He S, Zhang Y, Xu L, Yang Y, Marubashi T, Zhou Z, Yao B (2013) Effects of dietary Bacillus subtilis C-3102 on the production, intestinal cytokine expression and autochthonous bacteria of hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Aquaculture 412-413:125–130
Irianto A, Austin B (2002) Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 25:333–342
Kumari J, Swain T, Sahoo PK (2003) Dietary bovine lactoferrin induces changes in immunity level and disease resistance in Asian catfish Clarias batrachus. Vet Immunol Immunopathol 94:1–9
Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Westrom B (2010) A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One 5:e9009. doi:10.1371/journal.pone.0009009
Liu W, Ren P, He S, Xu L, Yang Y, Zhou Z (2013) Comparison of adhesive gut bacteria composition, immunity and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains. Fish Shellfish Immunol 35:54–62
Livak KJ, Schmittgen DT (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2-ΔΔC T method. Methods 25:402–408
Maji UJ, Mohanty S, Mahapatra AS, Maiti NK (2016) Diversity and probiotic potentials of putative lactic acid bacteria for application in freshwater aquaculture. Turk J Fish Aquat Sci 16:805–818
Medina M, Izquierdo M, Ennahar S, Sanz Y (2007) Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol 150:531–538
Mirlohi M, Soleimanian-Zad S, Dokhani S, Sheikh-Zeinodin M, Abghary A (2009) Investigation of acid and bile tolerance of native Lactobacilli isolated from fecal samples and commercial probiotics by growth and survival studies. Iran J Biotech 7:233–240
Mohapatra S, Chakraborty T, Prusty AK, Das P, Paniprasad K, Mohanta KN (2012) Use of different microbial probiotics in the diet of rohu, Labeo rohita fingerlings: effects on growth, nutrient digestibility and retention, digestive enzyme activities and intestinal microflora. Aquac Nutr 18:1–11
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14
Nikoskelainen S, Ouwehand A, Salminen S, Bylund G (2001) Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture 198:229–236
Nikoskelainen S, Ouwehand AC, Bylund G, Salminen S, Lilius EM (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immunol 15:443–452
O’Shea EF, O’Connor PM, Raftis EJ, O’Toole PW, Stanton C, Cotter PD, Ross RP, Hill C (2011) Production of multiple bacteriocins from a single locus by gastrointestinal strains of Lactobacillus salivarius. J Bacteriol 193:6973–6982
Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F (2010) Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A 107:454–459
Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H (2005) The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 243:241–254
Panigrahi A, Kiron V, Satoh S, Hirono H, Kobayashi T, Sugita H, Puangkaew J, Aoki T (2007) Immune modulation and expression of cytokine genes in rainbow trout Oncorhynchus mykiss upon probiotic feeding. Dev Comp Immunol 31:372–382
Parthasarthy R, Ravi D (2011) Probiotic bacteria as growth promoter and biocontrol agent against Aeromonas hydrophila in Catla catla (Hamilton, 1822). Indian J Fish 58:87–93
Perez-Sanchez T, Balcazar JL, Merrifield DL, Carnevali O, Gioacchini G, de Blas I, Ruiz-Zarzuela I (2011) Expression of immune related genes in rainbow trout (Oncorhynchus mykiss) induced by probiotic bacteria during Lactococcus garvieae infection. Fish Shellfish Immunol 31:196–201
Picchietti S, Fausto AM, Randelli E, Carnevali O, Taddei AR, Buonocore F, Scapigliati G, Abelli L (2009) Early treatment with Lactobacillus delbruekii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immunol 26:368–376
Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497
Reyes-Becerril M, Tovar-Ramírez D, Ascencio-Valle F, Civera-Cerecedo R, Gracia-Lopez V, Barbosa-Solomieu V, Esteban MA (2011) Effects of dietary supplementation with probiotic live yeast Debaryomyces hansenii on the immune and antioxidant systems of leopard grouper Mycteroperca rosacea infected with Aeromonas hydrophila. Aquac Res 42:1676–1686
Rollo A, Sulpizi R, Nardi M, Silvi S, Orpianesi C, Caggiano M, Cresci A, Carnevali O (2006) Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish Physiol Biochem 32:167–177
Sahoo PK, Kumari J, Mishra BK (2005) Non-specific immune responses in juveniles of Indian major carps. J Appl Ichthyol 21:151–155
Sahoo PK, Rauta PR, Mohanty BR, Mahapatra KD, Saha JN, Rye M, Eknath AE (2011) Selection for improved resistance to Aeromonas hydrophila in Indian major carp Labeo rohita: survival and innate immune responses in first generation of resistant and susceptible lines. Fish Shellfish Immunol 31:432–438
Sahu I, Das BK, Marhual N, Samanta M, Mishra BK, Eknath AE (2011) Toxicity of crude extracellular products of Aeromonas hydrophila on rohu, Labeo rohita (Ham.). Indian J Microbiol 51:515–520
Saini VP, Ojha ML, Gupta MC, Nair P, Sharma A, Luhar V (2014) Effect of dietary probiotic on growth performance and disease resistance in Labeo rohita (Ham.) fingerlings. Int J Fish Aquat Stud 1:07–11
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MA (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 25:114–123
Sharifuzzaman SM, Austin B (2010) Development of protection in rainbow trout (Oncorhynchus mykiss, Walbaum) to Vibrio anguillarum following use of the probiotic Kocuria SM1. Fish Shellfish Immunol 29:212–216
Son VM, Chang CC, Wu MC, Guu YK, Chiu CH, Cheng W (2009) Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol 26:691–698
Srinu B, Madhava Rao T, Mallikarjuna Reddy PV, Kondal Reddy K (2013) Evaluation of different lactic acid bacterial strains for probiotic characteristics. Vet World 6:785–788
Standen BT, Rawling MD, Davies SJ, Castex M, Foey A, Gioacchini G, Carnevali O, Merrifield DL (2013) Probiotic Pediococcus acidilactici modulates both localized intestinal and peripheral-immunity in tilapia (Oreochromis niloticus). Fish Shellfish Immunol 35:1097–1104
Standen BT, Rodiles A, Peggs DL, Davies SJ, Santos GA, Merrifield DL (2015) Modulation of the intestinal microbiota and morphology of tilapia, Oreochromis niloticus, following the application of a multi-species probiotic. Appl Microbiol Biotechnol 99:8403–8417
Swain B, Basu M, Samanta M (2011) Cloning of interleukin-10 gene in the Indian major carp, Labeo rohita (Hamilton 1822) and its functional characterization following Aeromonas hydrophila infection. Indian J Fish 58:39–47
Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beyen AC (2004) Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int J Food Microbiol 96:219–233
Utiswannakul P, Sangchai S, Rengpipat S (2011) Enhanced growth of black tiger shrimp Penaeus monodon by dietary supplementation with Bacillus (BP11) as a probiotic. J Aquac Res Dev 3. doi: 10.4172/2155-9546.S1-006
Zhang A, Guo Y, Zhang S, Fan X, Wang X, Zhou X, Yang K, Zhou H (2015) Cytokine effects and cellular signaling pathways of grass carp HSP70 in head kidney leukocytes. Fish Shellfish Immunol 46:550–556
Acknowledgements
Financial assistance for this work from the Application of Microorganisms in Agriculture and Allied Sectors (AMAAS), National Bureau of Agriculturally Important Microorganisms (NBAIM) project of Indian Council of Agricultural Research, New Delhi, India is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Maji, U.J., Mohanty, S., Pradhan, A. et al. Immune modulation, disease resistance and growth performance of Indian farmed carp, Labeo rohita (Hamilton), in response to dietary consortium of putative lactic acid bacteria. Aquacult Int 25, 1391–1407 (2017). https://doi.org/10.1007/s10499-017-0122-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0122-5