Abstract
The ACL is the primary restraint to tibial internal rotation. Secondary stabilizers, including the ITB, ALL, anterolateral capsule, and lateral meniscus, provide additional rotational control. When the ACL is injured, these secondary stabilizers may also be damaged, leading to anterolateral rotatory instability. This is particularly concerning in patients with large pivot shifts. Biomechanical studies have demonstrated that ACLR alone may not restore knee IR kinematics. When combined with ACLR, anterolateral ligament reconstruction (ALLR) has been shown in some studies to restore stability, though there is a risk of overconstraint. Multiple techniques for ALLR exist, but the most important keys are to avoid femoral tunnel convergence and fixing the graft in external rotation or with significant anisometry, which may lead to overtightening or loss of motion. Clinical outcome studies appear promising, but only short- to mid-term data exists. Lateral extra-articular tenodesis procedures may also be considered, which are discussed in another chapter. Both techniques have unique advantages and disadvantages.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anterolateral rotatory instability
- Anterolateral ligament reconstruction
- High-grade pivot shift
- Anterolateral procedures
- Lateral extra-articular tenodesis
Background
Despite the overall success of anterior cruciate ligament reconstruction (ACLR procedure), failure rates remain unacceptably high, ranging from 1.1% to 14% [1, 2]. Despite anatomically placed femoral and tibial tunnels, 1.7–7.7% of ACLR patients are thought to fail due to persistent rotational instability [3,4,5]. This realization has led researchers to investigate the concept of tibial rotational restraint. This includes the role of the anterior cruciate ligament (ACL) as a primary stabilizer, along with several lateral-sided structures serving as important secondary stabilizers. Interest in anterolateral rotatory instability (ALRI) of the knee, and its possible responsibility for a percentage of ACLR failures, was further heighted following renewed interest in the anterolateral complex (ALC), specifically the anterolateral ligament (ALL).
Credit for the original description of what is now considered the ALL (or mid-third capsular ligament) belongs to Paul Segond, who described an avulsion fracture (now referred to as the eponymous “Segond fracture”) in 1879, along the anterolateral aspect of the tibia [6]. At the location of the fracture, Segond noted the presence of a “pearly, resistant, fibrous band, which invariably showed extreme amounts of tension during forced internal rotation.” [6] Despite occasional reference to an anterolaterally based knee structure in the literature, it was not named until 2012, when Vincent et al. published their anatomical work [7]. However, popularization of the ALL, and the ensuing controversy around its function (and existence), is often credited to Claes et al., who published a detailed anatomic description of the ALL based on a series of cadaveric dissections [8]. Since the “rediscovery” of the ALL, significant research has been done on its structure, function, and potential implications in ALRI, specifically as it relates to ACLR and ACLR failures. This chapter will provide an overview of the ALL, including relevant anatomy and the biomechanical role of the ALL with knee stabilization. It will explore the indications for ALL reconstruction (ALLR), particularly in the setting of revision ACLR, delineate patient work-up after an ACL injury (or failed ACLR), and describe the surgical techniques for ALLR. It will conclude with outcomes and complications of the procedure based on a review of the literature and considerations regarding the choice between an ALLR and a lateral extra-articular tenodesis (LET).
Anatomy of the Anterolateral Complex of the Knee
The complexity of the lateral knee anatomy has led to challenges regarding structure identification and contributed to the opacity around the ALL definition. While controversy still exists, a layer-by-layer approach to the anterolateral knee helps elucidate its various components, including the layers of the iliotibial band (ITB), ALL, and anterolateral joint capsule (Table 16.1).
Iliotibial Band
The ITB is a thickening of deep fascia, intimately connected with the tensor fasciae latae (TFL) anteriorly and the gluteus maximus posteriorly. It extends laterally from the iliac crest to the tibia, with connecting fibers to the femur along its length. The complexity of lateral and anterolateral knee anatomy is due to the various layers of the ITB and the difficulty in distinguishing them from the other components of the ALC. The ITB is made up of the superficial ITB (sITB), Kaplan fibers, and deep ITB (dITB).
The sITB originates, as a continuation of the TFL and gluteus maximum fascia, with attachments along the intermuscular septum to the linea aspera of the femur [9]. It then courses laterally, with its posterior fibers reinforcing the fascia of the anterior aspect of the biceps femoris. Anteriorly, it curves obliquely, merging with the fascia of the vastus lateralis and partially inserting on the lateral aspect of the patella (forming the iliopatellar band fascia) [10]. Distally, the sITB inserts at Gerdy’s tubercle . An important component of the sITB is the Kaplan fibers, which connect the sITB to the distal femoral metaphysis, primarily at the lateral femoral supraepicondylar region [11].
The dITB originates posterior and medial (deep) to the sITB along the lateral intermuscular septum, in the vicinity of the Kaplan fibers. It courses anteromedially, to combine with the sITB distal to the lateral femoral epicondyle, ultimately inserting on Gerdy’s tubercle [12].
The capsulo-osseous layer represents the deepest (most medial) layer of the ITB. The layer begins proximally, contiguous with the fascia of the plantaris and lateral gastrocnemius muscle. It then runs deep and slightly posterior to the sITB , merging with the sITB and dITB distally at Gerdy’s tubercle [12].
Anterolateral Ligament and Anterolateral Capsule
The anterolateral joint capsule, as described by Hughston et al., consists of a superficial and deep layer, relative to the LCL [13]. The layers then become confluent anterior to the LCL [14]. The capsule can be further separated into anterior, mid-third, and posterior divisions [13]. The anterior division is thin without a femoral attachment; however, the mid-third capsular ligament is a discrete thickening, with femoral and tibial bony insertions which inserts on the lateral meniscus, forming the meniscofemoral and meniscotibial ligaments (coronary ligament) [15].
While it remains controversial, the evidence is increasingly pointing to the ALL as a ligamentous structure, separate from the capsule. However, there is considerable definitional overlap with the mid-third capsular ligament, and the question of whether these are two distinct structures is not yet settled [16]. A cadaver study by Getgood et al. found the ALL to be a ligamentous structure, which differentiated it from the surrounding capsule. It was not however a completely discrete ligament, the way the LCL is, however [17]. Accordingly, the ALL has been likened to the glenohumeral ligaments (GHL) of the shoulder, which are dense condensations of tissue that provide static joint stability [16]. Recent evidence points toward the ALL as a ligamentous structure embedded within capsular tissue.
The femoral origin of the ALL is perhaps one of the most controversial elements to its anatomic characterization and is largely responsible for the continued difficulty in identification. The femoral origin has variably been reported as anterior, directly on, or posterior and proximal to the lateral epicondyle [8, 18, 19]. Further research has led to an increasing consensus that the ligament primarily originates proximal and posterior to the lateral epicondyle [20]. This origin is most commonly posterior and proximal to the attachment site of the LCL, but it has been reported as anterior and distal as well [21, 22]. It has a large, fanlike footprint, which overlaps the LCL origin [23]. The diameter of the ALL at its femoral origin is 11.85 mm [24].
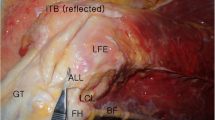
The ALL then courses anterolaterally, inserting onto the lateral aspect of the lateral meniscus, roughly at the junction between the anterior horn and body, for a mean length of 5.6 mm [25]. It then inserts onto the tibia, roughly halfway between Gerdy’s tubercle and the anterior aspect of the fibular head [8]. Cadaver dissection has found the insertion to be on average 24.7 mm posterior to the center of Gerdy’s tubercle and 26.1 mm anterior to the anterior margin of the fibular head [22]. The ALL has been found to be on average 9.5 mm distal to the joint line, just proximal to the insertion of the biceps femoris (Fig. 16.1) [22, 23].
The ALL is not an isometric ligament. Rather, the length of the ALL increases with progressive knee flexion (loosens). The critical clinical importance of this fact will be explored further in the surgical technique section [26].
Primary and Secondary Stabilizers of Tibial Rotation
To understand the concept behind, and potential value of, ALLR, one must understand the synergistic relationship between the ACL and the ALC in limiting tibial rotation (Table 16.2). Butler and colleagues introduced the concept of primary restraint. [27] This was further expanded upon, and defined by Andersen as, “cutting a primary restraint results in an increase in joint motion, whereas cutting a secondary restraint will result in an increase of joint motion only in the absence of the primary restraint.” [28] This notion was used as the foundation for future biomechanical studies establishing the significance of various restraints [29].
The Primary Stabilizer of Tibial Rotation: The Anterior Cruciate Ligament
Based on available evidence, the ACL is likely the primary stabilizer of tibial IR, particularly from 0 to 30° of flexion [30]. At flexion >30°, the ACL is likely still the chief restraint to tibial IR, but with a greater relative contribution of the ALC [ 28, 32,33,34,35]. Biomechanically, the mechanism of restraint is believed to be winding of the ACL bundles around each other, which loads the ligament and resists rotation [31].
Secondary Stabilizers
Evidence suggests the ITB is an important secondary stabilizer of tibial IR, particularly at higher flexion angles [36, 37]. The sITB provides more restraint at higher flexion angles, while the dITB contributes more at lower flexion angles [9, 36, 37]. Because the ACL resists more IR at extension, the dITB likely works synergistically at low flexion angles to assist the ACL in resisting rotation here. As the knee flexes, the sITB then takes over to help resist IR.
Additionally, based on available evidence, the ALL is an important secondary stabilizer to tibial IR, particularly in the ACL-deficient state. The ITB however may be a more important contributor to IR restraint, specifically the dITB at lower flexion angles and the sITB at higher flexion angles [1, 29, 35, 38,39,40,41,42,43,44]. Given this role, one can see how neglecting an anterolateral injury in the setting of an ACL rupture (or ACLR failure) could lead to increased stress on the ACL graft and risk future failure. With this in mind, the concept of a lateral-based extra-articular procedure has been re-visited, in the forms of lateral extra-articular tenodesis (LET) and anterolateral ligament reconstruction (ALLR).
Unlike the medial meniscus, the lateral meniscus does not contribute much to resisting anterior translation. It has, however, been shown both biomechanically and clinically to be an important secondary stabilizer to tibial IR [44,45,46,47].
Biomechanical Evidence for Anterolateral Ligament Reconstruction
Efficacy of ALLR
As ALLR is still a relatively nascent procedure, the majority of evidence of its efficacy comes from biomechanical studies [48,49,50,51,52]. These studies have attempted to evaluate the value of reconstructing the ALLR in the setting of ACLR, particularly in terms of additional IR restraint provided (Table 16.3). Conversely, several studies have demonstrated conflicting results, casting doubt on the ability of the ALLR to provide clinically relevant rotational stability. These studies reported not only a lack of overconstraint but a lack of restoration of native stability [1, 51,52,53].
Due to the mixed biomechanical and clinical results, a consensus has not yet been reached on the efficacy of ALLR. While most studies find it improves rotational stability, enough well-performed biomechanical investigations have found evidence to the contrary. Additionally, some studies that did find improved rotational control with ALLR found it only did so by simultaneously overconstraining the knee. For these reasons, a systematic review of biomechanical outcomes by DePhillipo et al. concluded only, “there is inconsistency in terms of femoral origin, flexion angle, and performance of the ALLR at this time” [53].
Femoral Origin for Graft Placement
There is also lack of agreement regarding the native femoral origin of the ALL, and it is of little surprise that various locations have been used for reconstruction. The 2017 DePhillipo systematic review found the six surgical technique articles, with the most common femoral origin site described being the posterior and proximal to the LCL origin (four studies) [54,55,56,57] vs two studies using a point anterior and distal to the LCL origin [58, 59].
Interestingly, in a comparison of four biomechanical outcome studies, two studies that did not find any overconstraint used the anterior and distal origin point [1, 48, 60, 61]. These studies also did not find any significant increase in rotational control following ALLR when using the anterior and distal origin. This indicates the anterior point likely cannot adequately tension the graft. Additional studies have demonstrated length changes depending on the location of the femoral origin, and accordingly the posterior and proximal position has been recommended for reconstruction [18, 26, 35, 62].
Knee Flexion Angle
The degree the graft is fixed may potentially affect efficacy of the procedure and may alter knee kinematics. While the optimal fixation angle has not yet been determined, the available evidence supports placing the graft with the knee in full extension (and with the leg in neutral rotation) is likely safest to prevent overconstraint [20, 49, 52, 60].
Graft Isometry
Unlike other ligament reconstructions where isometry is key, the ALL has surprisingly been found to be non-isometric [26, 63]. This lack of isometry should be considered when fixing the graft. During assessment, the graft should be slightly looser in flexion (because the native ligament lengthens with flexion) than in extension. If the graft is found to tighten with knee flexion, the femoral origin point may be too anterior and distal and should be revised to a more posterior and proximal position [20].
Indications
Currently, there are no consensus indications for an ALLR in the setting of an ACLR. This is due to the lack of sufficient clinical data with adequate follow-up to determine which patients benefit most from the procedure. However, several indications have been cited in the literature repeatedly (Table 16.4).
Irrespective of if it is a revision scenario or not, indications revolve around signs, or likely signs, of concomitant anterolateral injury. For instance, a high-grade pivot shift (defined as either 2+ or more typically 3+) is an indication for many authors. The higher pivot shift, however, is taken as a sign that secondary stabilizers must also be injured [16, 20, 56, 64]. Similarly, chronic ACL injuries are often considered for ALLR, as the chronic instability may weaken the secondary stabilizers [65]. Evidence of a Segond fracture has also been cited as an indication, as it represents an avulsion of the ALL off of the tibia [19, 56, 66]. Other indications include patients returning to high-risk sports/activities, such as those that require pivoting [20, 64], though this is more controversial than the other indications listed. Generalized hyperlaxity (measured via Beighton score) [67] should also prompt consideration for an ALLR, as the excess stress may be put on the graft without additional constraint [64].
Another factor is if a soft tissue or allograft is selected for ACLR, one may give stronger consideration to performing the ALLR, as it may help protect a potentially weaker graft (one that takes longer to incorporate without bone-to-bone healing). This concept is anecdotal and has not yet been studied.
In the revision setting, specifically, there is debate if ALLR should be performed in all (or the majority) of cases or if it should be more limited to revisions that also meet the above criteria [16]. More evidence is required before any definitive statements can be made.
Contraindications are also still being developed, but strong consideration should be given to not performing an ALLR in the setting of a multi-ligament knee injury (MLKI). Given that stiffness is one of the most common complications following MLKI reconstruction, avoiding a procedure that may lead to overconstraint is advisable. Additionally, by addressing the additional soft tissue injuries, the instability may improve, obviating the potential benefits of ALLR [64].
The authors’ definitive indications for ALLR in the setting of a revision ACL include a pivot shift of 2+ or greater, knee recurvatum (as an indication of laxity), and a young or contact/collision athlete. Other causes of laxity or risk increases, addressed at length in other chapters, must be remedied as well.
Patient Evaluation
History
While a thorough history should be taken for all patients, when considering an ALLR, several key points should be highlighted. In regard to the ALLR, the physician should try to understand the status of the patient’s anterolateral knee structures, their prior injury history, and their future risk.
The patient’s injury history/mechanism of injury should be determined, with specific attention to rotational mechanisms. Surgical history is crucial, including prior ACLR in the setting of revisions, including type of grafts, implants, and if any concomitant procedures were performed. The patient should be asked if their knee ever felt stable after the primary surgery. In regard to risk, the patient’s functional goals, occupation, and athletic activity should all be discussed.
Physical Examination
A standard knee and ligamentous exam should be performed. Special attention should again be paid to the lateral side of the knee for any signs of specific instability. For instance, a varus gait or thrust may suggest damaged lateral structures [64]. Additionally, a good pivot shift exam is essential to help assess the amount of ALRI; however, this typically can only be done properly under anesthesia. Finally, inspection of any prior surgical incisions should be performed to assist in surgical planning.
General laxity testing should also be performed to calculate a Beighton score [67]. Patients with elevated Beighton scores should be considered for ALLR.
Imaging
Standard knee x-rays, including an AP, lateral, and merchant/sunrise view, should be obtained at baseline. In addition to identifying other injuries, their main use in consideration of ALLR is to look for the presence of a Segond fracture, indicating ALL injury [66]. These can also be used to assess prior tunnel placement, tunnel lysis, and prior fixation. Additional radiographs that should be considered are full-length AP and lateral weightbearing views to assess for coronal and sagittal alignment. CT scans should also be obtained in the revision setting to help further assess tunnel lysis and tunnel placement.
Because the ALL is extra-articular, ultrasonography (US) has been proposed as a means of radiologic assessment. Furthermore, because the ligament tightens with internal rotation, it has been posited that ultrasound, which can dynamically evaluate structures, could be a cheap, effective method of investigating potential ALL injury [68]. Based on these principles, Cavaignac et al. conducted an anatomic dissection study using US to identify the ALL and reported excellent interrater agreement (Cohen k, 0.88–0.94) [68]. However, a similar study by Capo et al. using ten fresh-frozen cadaver knees and ultrasound found ultrasound to be unreliable and did not recommend its use in routine work-up [69]. More evidence is required at this time before a determination on the utility of US can be made in diagnosing ALL injuries.
Studies attempting to use magnetic resonance imaging (MRI) to identify the ALL, and characterize ALL injuries, have also had mixed results. Visualization of the ALL has been reported at 11–100% of patients (including cadaveric specimens) [70,71,72]. Though some studies have reported high sensitivities, most appear to be plagued by low-to-moderate inter- and intra-rater reliability. For example, a study by Hartigan et al. had two musculoskeletal radiologists visualize the ALL on 1.5 Tesla MRI [71]. Though they reported the ability to visualize the ALL in 100% of cases, one reviewer found the ALL to be torn in 26% of patients, while the second reviewer found the ALL to be torn in 62% of patients [71]. Of note, the tibia portion has been demonstrated to be the most consistently seen on MRI [73]. MRI appears to be a more promising modality to detect ALL injuries, though more research needs to be done, and protocols need to be improved before it can be considered a reliable diagnostic tool.
Surgical Procedure
Native ALL Properties and Graft Selection
An ideal graft choice would be one that mimics the properties of the native ALL as close as possible. Several studies have attempted to quantify the biomechanical properties of the native ALL in order to improve graft selection. The range of load to failure in these studies was 49.9 ± 14.62 N to 319.7 ±212.6 N [74, 75]. The stiffness of the ALL has been reported at 21 ± 8.2 N to 41.9 ± 25.7 N [74, 76, 77]. A consensus group led by Sonnery-Cottet reported the mean load to failure as “around 180 N” and the mean stiffness as 31 N/mm [20].
ALL Compared to Possible Grafts
The ideal graft for ALLR has not yet been determined, and multiple options have been described [23]. Kernkamp et al. described using an ITB-based autograft, by taking a free slip of it (similar to performing a modified Lemaire, but transecting the distal attachment) [78]. The most common graft used in the literature for both biomechanical and surgical technique articles is a gracilis graft (auto- and allo- have both been described) [54, 56, 59]. A tripled semitendinosus graft and a doubled gracilis graft have also been described [54]. Sonnery-Cottet et al. prefer a doubled gracilis tendon, but place the graft in an inverted Y formation, with two distal points of fixation in the tibia [55].
While limited comparative data exists regarding graft properties, Wytrykowski et al. compared the properties of the native ALL with the ITB and a two-strand gracilis graft in cadaveric knees. They found the ITB to be most consistent with the ALL; however, they did not describe their tissue harvesting technique [77]. Additionally, there is some concern about harvesting a portion of the ITB, which is a known important stabilizer to tibial IR, to reconstruct the ALL, with the goal of improving tibial IR (i.e., “robbing Peter to pay Paul”) [79,80,81].
Set-Up
The patient is placed supine on a standard operating table with the feet brought to the edge of the bed. An exam under anesthesia is performed to confirm the diagnosis. If work-up regarding ALRI was equivocal up to this point, results of the pivot test exam can be critical in deciding whether an ALLR is indicated. Following the exam, a tourniquet is placed high on the thigh. The operative extremity is secured with a leg holder, high on the thigh to allow lateral access. We prefer to use a semitendinosus allograft for reconstruction, sized to a 4 mm diameter. This is prepared on the back table while the diagnostic arthroscopy is performed.
Procedure
After the leg is prepped and draped in the usual sterile fashion, landmarks are marked on the skin. These include the lateral epicondyle, Gerdy’s tubercle, and the fibular head (Fig. 16.2). The ACLR (or revision ACLR) is performed first, up to and including drilling the femoral and tibial tunnels. Pearls and pitfalls of dealing with specific challenges of revision ACLR cases, such as graft choice, osteolysis, tunnel widening, prior implant management, etc., are covered in prior chapters. The reason for drilling the femoral and tibial tunnels (particularly the femoral tunnel) prior to the ALLR is so that the femoral tunnel can be visualized through the anteromedial portal while drilling the ALL femoral tunnel. However, with experience and proper positioning and angling of the tunnels, this becomes less necessary.
Following ACL tunnel drilling, we proceed with the ALLR portion. A guide pin is placed at the femoral origin of the ALL, approximately 8 mm proximal and 4 mm posterior to the lateral epicondyle at the anatomic origin (Fig. 16.3). This guide pin is directed anteriorly and proximally to avoid tunnel convergence as well as the femoral trochlea. As the guide pin is placed, the femoral tunnel for the ACLR is visualized for any signs of convergence from the guide pin. If the pin is seen on arthroscopy, it is redirected. Once the guide pin is in place, the ACL graft is fixed in the femoral tunnel.
For the ALLR, one can do either a percutaneous approach, which has the advantages of improved cosmesis and limited soft tissue insult, or a larger incision from just proximal to the lateral epicondyle to about the midway point between Gerdy’s and the anterior fibular head (tibial insertion point). The advantage of doing the open rather than percutaneous approach is it permits landmark palpation and direct visualization, which may better assist tunnel placement. The percutaneous approach is described below.
Following ACL graft fixation, a small skin incision is made over the femoral guidewire, and blunt dissection is carried out until bone is reached. A 4.5 mm reamer is used to overdrill the guidewire to a depth of 25 mm in preparation for a SwiveLock anchor (Arthrex, Naples, FL). The one limb of the graft is then inserted into the femoral tunnel and secured with the screw (Fig. 16.4). Sutures are tied over the anchor as back-up fixation. Other options for femoral fixation may include interference screw fixation [57].
Landmarks, including Gerdy’s tubercle and the fibular head, are again palpated for confirmation. A skin incision midway between the two and about 1 cm distal to the joint line is then made. Blunt dissection is performed until bone is reached. A guidewire is then passed from the anatomic ALL insertion in a slight superomedial orientation (Fig. 16.5). A 7.5 mm reamer is then used to overdrill the guidewire, again to a depth of 25 mm.
A clamp is passed from distal to proximal subcutaneously, and the suture on the distal limb of the grasp is fed to the clamp. The graft is then tunneled under the IT band and then subcutaneously (Fig. 16.6). At this point, the graft is provisionally fixed, and the knee is taken through a range of motion to assess graft isometry. Our preference is for the graft either to be isometric or to slightly loosen with flexion and tighten with extension (similar to the native ALL). If the graft position is felt to be adequate, a fork-tipped PEEK tenodesis screw (Arthrex, Naples, FL) is used to fix the graft to the tibia (Fig. 16.7). Anatomic ALLR tunnel position is critical for appropriate graft tension and function through knee range of motion (Fig. 16.8).
It is important to avoid overtension on the graft at any flexion angle. The ALL acts as a checkrein, and overtightening can lead to overconstraint. As the primary concern following this procedure is stiffness, we typically fix our grafts in 0–30 degrees of flexion and neutral rotation. It is critical to assess isometry prior to ultimate fixation; the graft should become looser in flexion. Pearls and pitfalls can be seen in Table 16.5.
Postoperative Protocol
As stiffness is one of the primary concerns with the addition of a lateral extra-articular procedure, we utilize a standard ACLR protocol. This includes weightbearing as tolerated with crutches and early range of motion as tolerated on postoperative day 1 while in the hinged knee brace (locked in extension for ambulation during the first week). Physical therapy focuses on quadriceps and hamstring strengthening and regaining range of motion, particularly extension.
After 2 weeks, crutches are weaned, and the brace can be discontinued once full extension without evidence of an extensor lag is reached, typically by 3–4 weeks post-op. The patient also begins closed-chain extension exercises and hamstring curls and use of a stationary bike.
At 3 months, the patient should achieve full, painless ROM. They can begin straight ahead running at this point. By 8 months, the patient may begin gradual return to athletic activity as tolerated with the goal of competitive play by 10 months.
Additional Case Examples
Case 1
A 44-year-old male had previously undergone an ACLR at an outside institution 11 years prior to presentation. He had been doing well until about 2 years prior to presentation (9 years status-post index ACLR) when he had a rotational injury associated with hemarthrosis. Since this incident, he has had recurrent swelling, pain, and recurrent instability. He also reported mechanical symptoms (clicking and catching).
On exam, the patient was noted to have a mild effusion, standing grossly physiologic valgus standing alignment, with range of motion of 0–135 degrees. For ligamentous assessment, he was found to have a 2B Lachman and a positive pivot shift. He otherwise was stable to posterior, varus, and valgus stress testing, as well as posterolateral corner testing. Dial test was also negative. Additionally, he had medial joint line tenderness and a positive McMurray test.
Imaging was notable primarily for a vertical femoral tunnel (Fig. 16.9). MRI confirmed ACL re-rupture, as well as a medial meniscal tear (Fig. 16.10). Given the patient’s symptoms, he was indicated for ACLR.
Case 1 Considerations
The femoral tunnel in this case is positioned anterior. This likely was ultimately responsible for the graft failure, given the poor rotational control associated with vertical tunnel placement. In terms of other possible failure etiologies, the patient did not have any unidentified concomitant ligamentous injuries or pathologic coronal or sagittal alignment. There was also a small meniscal tear, but one that appeared stable, without root compromise.
In this scenario, it was felt that in addition to creating a separate femoral tunnel in a more anatomic position, performing an ALLR would help provide further rotational control, improving stability and taking stress off the new graft.
For the ACL, a new femoral tunnel was drilled, and a large bone-patellar-bone allograft was used to address the tibial tunnel widening. The femoral and tibial sides were fixed with interference screws. The tibial fixation was backed up with a screw and washer (Fig. 16.11). For the ALLR, a semitendinosus allograft with suture anchor fixation was used following the ACLR.
At 5 years follow-up, the patient has not had any recurrent instability and returned to his prior level of activity (recreational basketball).
Case 2
A 23-year-old male presented with recurrent right knee pain and instability 6 months after a revision ACLR with BTB allograft at an outside institution (first failure was BTB autograft). He reports he began feeling unstable during a recent physical therapy session and had not yet returned to any sports.
On exam, he had a large effusion, and range of motion of 0–135°. For ligamentous testing, he had a 2B Lachman and positive pivot shift test. He otherwise did not have any instability to posterior, varus, or valgus stress or posterolateral corner testing. Dial test was also negative. He did not have any medial or lateral joint line tenderness.
Imaging revealed physiologic coronal alignment and tunnel widening of the femoral and tibial tunnels (Figs. 16.12, 16.13, and 16.14a, b). MRI demonstrated ACL graft rupture (Fig. 16.15). Due to recurrent instability symptoms in the setting of ACLR failure, the patient was indicated for revision surgery.
Case 2 Considerations
Similar to case 1, there were no other anatomic factors contributing to the patient’s ACLR failure. The patient’s coronal and sagittal alignments were within normal range, there were no missed concomitant injuries, and tunnel positions seemed appropriate. One possible source of failure in this patient is the use of an allograft in a patient under 30 years old.
With this in mind, it was decided to treat this patient in a staged fashion, due to the tunnel widening. The first stage included bone grafting of the femoral and tibial tunnels. Following confirmation of graft incorporation (Fig. 16.14c, d), the patient was taken for re-revision ACLR.
Given the lack of any other failure sources, it was felt the patient would benefit from a lateral extra-articular procedure to help improve rotational control. The patient was therefore indicated for an ALLR, in addition to the revision ACLR. A contralateral BTB autograft was utilized for the ACL. A semitendinosus allograft was utilized for the ALLR, which was secured with a knotless anchor device in the femur and a metal screw in the tibia.
At 6 years follow-up, the patient has returned to his prior level of activity, without any recurrent instability events or pain.
Case 3
A 20-year-old female collegiate basketball player that had undergone an L knee ACLR with hamstring autograft 14 months presented with recurrent instability after a non-contact pivoting injury during practice.
On exam, she had a small effusion and range of motion of 0–130°. For ligamentous testing, she had a 1B Lachman with significant guarding. It was not possible to perform a pivot shift due to this guarding. Otherwise, no instability was noted to posterior, varus, or valgus stress or posterolateral corner testing. Dial test was also negative. She also was noted to have lateral joint line tenderness and a positive lateral McMurray sign.
Imaging demonstrated a likely ACL re-tear on MRI (Fig. 16.16), though this was not definitive. Alignment films demonstrated a slight 2–3 valgus malalignment (Fig. 16.17).
Case 3 Considerations
The patient’s recurrent instability was strongly suggestive of re-tear; however, given the patients guarding on multiple exam attempts, and somewhat equivocal MRI, it was felt that diagnosis would be confirmed with an exam under anesthesia (EUA), followed by a diagnostic arthroscopy if the EUA was felt to be suggestive of a tear.
Under anesthesia, the patient was found to have a positive pivot shift and 2B Lachman, so the decision was made to proceed with diagnostic arthroscopy and tunnel bone grafting. Follow-up x-ray 3 months later confirmed graft consolidation (Fig. 16.18). The patient was indicated for revision ACLR 4 months after the bone grafting procedure. A partial lateral meniscectomy was also performed at this time.
In terms of options for this patient, while she did have some valgus malalignment, this was felt to be minimal (only 2–3°) and likely not contributory. She did not have any increased posterior tibial slope necessitating a closing wedge osteotomy. No other concomitant injuries were appreciated. Given she had already failed a prior soft tissue autograft, the patient was indicated for a BTB autograft ACLR. Similar to the cases above, given the lack of other etiologies for her failure, it was felt that this patient likely would benefit from additional rotational control in the form of a lateral extra-articular procedure. She was therefore indicated for an ALLR, in addition to ACLR.
One thing to always be cognizant of is the danger of overconstraint. It is important to avoid overtension on the graft at any flexion angle. Because the ALL only acts as a checkrein, overtightening it can lead to overconstraint. This can both limit ROM and increase contact pressures in the lateral compartment, leading to premature osteoarthritis. To avoid this, one should try to always fix the graft with the leg in neutral rotation, as opposed to external rotation, which may lead to overtightening. Additionally, the leg should be kept in 0–30°, which has been shown to result in the least overconstraint. Most importantly, it is critical to assess isometry prior to ultimate fixation; the graft should become looser in flexion.
At 5 years follow-up, the patient was able to return to sport and has not had any instability symptoms since her revision surgery.
All three cases above serve to illustrate the same principles. It is imperative to always evaluate any patient with an ACL tear, particularly in the revision setting, for sources of failure etiology. This may include alignment, concurrent injuries, tunnel position, graft choice, and graft fixation. Performing an anterolateral extra-articular tenodesis procedure, such as an ALLR, can help provide additional rotational stability, limiting the stress on ACL grafts. In a revision setting, when there is not another cause for failure, this can be a particularly useful tool to help minimize failure rate.
Clinical Outcomes
At the time of this writing, the only study evaluating outcomes in patients undergoing revision ACLR (and thus most relevant to this textbook chapter) is a 2019 paper by Lee et al. In a retrospective study of revision ACLR patients that underwent either isolated revision ACLR or combined revision ACLR with ALLR, the group found the combined ACLR ALLR group had significantly less patients with postoperative pivot shifts, significantly more patients returning to sport at the same level of activity pre-injury, and significantly higher IKDC [85]. The group theorized that the extra-articular procedures participate in a load-sharing effect with the ACLR, limiting stress on the ACLR and preventing delayed healing and ultimately failure [85].
Given the relative paucity of available evidence, particularly mid- and long-term studies, ALLR cannot be unequivocally recommended. In clinical studies, it does appear to improve stability and outcome scores and possibly reduce re-rupture rates [82,83,84]. However, no information exists regarding the effect on lateral compartment cartilage following combined ALLR. Until long-term studies on combined ACLR and ALLR patients can be reported, some question about the clinical effects of potential overconstraint will remain. A summary table of clinical outcome studies can be found in Table 16.6.
Complications
While no specific clinical complications have been reported in the literature, as discussed in the biomechanics section above, several studies have found evidence of overconstraint with both ALLR and LET procedures [49, 50, 52, 60]. Conversely, well-performed studies, like that by Noyes et al., have found ALLR to not even improve rotational laxity, let alone result in overconstraint [51]. Importantly, no clinical data about overconstraint exists. If ALLR does result in slight overconstraint, there is no information regarding the clinical effects of this.
There are two primary concerns with overconstraint from lateral extra-articular procedures. Overconstraint generally refers to reduced tibial internal rotation and anterior translation as compared to the intact state [49]. The first is that the constraint may limit knee range of motion. This is particularly evident, as the studies that did find evidence of overconstraint found it primarily at higher knee flexion angles [49, 50]. This could lead to knee stiffness. The other concern is the effect of constraint on the lateral compartment of the knee. With increased constraint, compartment pressures may increase, which could lead to accelerated degeneration. This has not been demonstrated yet in the literature, but is a very real theoretical concern. Until long-term clinical research can be performed, the value of increased stability with extra-articular procedures must be balanced against the risk of overconstraint.
If one elects to proceed with ALLR, steps should be taken to avoid the risk of overconstraint. This includes fixing the graft at a lower degree of knee flexion (either full extension or at most 30 degrees based on surgeon preference/patient laxity). It is also important to have the leg in neutral rotation (avoiding external rotation), which can also possibly lead to overconstraint [64, 65, 86]. After demonstrating restoration of knee kinematics with the ALLR in a cadaveric study, Smith et al. noted the importance of placing the femoral origin of the tunnel posterior and proximal to the LCL origin, and having the graft tighten in extension, while remaining slightly lax with flexion. They noted using this position, and fixing the graft in extension, ensures that the graft is tightest in extension where the pivot shift mechanism comes into play. Conversely, having the graft be tighter in flexion was shown to lead to overconstraint while simultaneously being less effective at resisting the pivot shift [86].
Other complications are related to the surgical technique itself. Femoral tunnel convergence can occur if the ALLR tunnel is not directed anterior and proximal. The surgeon may also use the arthroscope to view the ACL femoral tunnel while the femoral ALL tunnel is drilled. If any breach is noted, ALLR tunnel drilling should be stopped and redirected [55, 57]. Though relatively far from the zone of surgery, one must always be cognizant of the common peroneal nerve, initially deep and then posterior to the biceps femoris, until it wraps around the fibular neck. There are also the extra-surgical scar and potential hematoma from the lateral genicular artery.
Considerations for ALLR vs Modified Lemaire
When considering which lateral extra-articular procedure to use, it is important to note that the current state of literature is, at best, equivocal. While some advantages and disadvantages of each procedure have been offered, very little of how these biomechanical or theoretical concerns will clinical affect patients is actually known at this time.
Which procedure is biomechanically, or, more importantly, clinically, more effective is unknown at this time. ALLR may be more anatomic and may require less surgical insult [12, 20, 55, 86]. It also may be less prone to overconstraint [52]. However, it does not address a potentially injured ITB, does not benefit from the pulley effect of the LCL, and may be more expensive [29, 35, 64]. Ultimately, when directly compared, neither procedure has proven superior [49, 53]. The decision on which to use (if any at all) should be up to the discretion of the operating surgeon. A list of ALLR and LET considerations can be found in Table 16.7.
Conclusion
The ACL is the primary restraint to tibial internal rotation. Secondary stabilizers, including the ITB, ALL, anterolateral capsule, and lateral meniscus, provide additional restraint. When the ACL is injured, these secondary stabilizers may also be injured, particularly in patients with large pivot shifts. Biomechanical studies have demonstrated that ACLR alone may not restore knee IR kinematics. When combined with ACLR, ALLR has been shown in some studies to restore stability, though there is a risk of overconstraint. Multiple techniques for ALLR exist, but the most important keys are to avoid femoral tunnel convergence and fixing the graft in external rotation or significant knee flexion, which may lead to overtightening. Clinical outcome studies appear promising, but only short- to mid-term data exists.
References
Spencer L, Burkhart TA, Tran MN, et al. Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(9):2189–97.
Tanaka M, Vyas D, Moloney G, Bedi A, Pearle AD, Musahl V. What does it take to have a high-grade pivot shift? Knee Surg Sports Traumatol Arthrosc. 2012;20(4):737–42.
Hettrich CM, Dunn WR, Reinke EK, Group M, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534–40.
Hussein M, van Eck CF, Cretnik A, Dinevski D, Fu FH. Individualized anterior cruciate ligament surgery: a prospective study comparing anatomic single- and double-bundle reconstruction. Am J Sports Med. 2012;40(8):1781–8.
Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551–7.
Segond P-F. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Paris: Hachette Livre; 2017. p. 1879.
Vincent JP, Magnussen RA, Gezmez F, et al. The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):147–52.
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–8.
Kaplan EB. The iliotibial tract; clinical and morphological significance. J Bone Joint Surg Am. 1958;40-A(4):817–32.
Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotatory instability. Am J Sports Med. 1996;24(1):2–8.
Lobenhoffer P, Posel P, Witt S, Piehler J, Wirth CJ. Distal femoral fixation of the iliotibial tract. Arch Orthop Trauma Surg. 1987;106(5):285–90.
Musahl V, Herbst E, Burnham JM, Fu FH. The anterolateral complex and anterolateral ligament of the knee. J Am Acad Orthop Surg. 2018;26(8):261–7.
Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58(2):173–9.
Seebacher JR, Inglis AE, Marshall JL, Warren RF. The structure of the posterolateral aspect of the knee. J Bone Joint Surg Am. 1982;64(4):536–41.
Herbst E, Albers M, Burnham JM, et al. The anterolateral complex of the knee: a pictorial essay. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1009–14.
Musahl V, Getgood A, Neyret P, et al. Contributions of the anterolateral complex and the anterolateral ligament to rotatory knee stability in the setting of ACL injury: a roundtable discussion. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):997–1008.
Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3186–95.
Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: anatomy, length changes and association with the Segond fracture. Bone Joint J. 2014;96-B(3):325–31.
Zhang H, Qiu M, Zhou A, Zhang J, Jiang D. Anatomic anterolateral ligament reconstruction improves postoperative clinical outcomes combined with anatomic anterior cruciate ligament reconstruction. J Sports Sci Med. 2016;15(4):688–96.
Sonnery-Cottet B, Daggett M, Fayard JM, et al. Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament – deficient knee. J Orthop Traumatol. 2017;18(2):91–106.
Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606–15.
Kraeutler MJ, Welton KL, Chahla J, LaPrade RF, McCarty EC. Current concepts of the anterolateral ligament of the knee: anatomy, biomechanics, and reconstruction. Am J Sports Med. 2018;46(5):1235–42.
Daggett M, Ockuly AC, Cullen M, et al. Femoral origin of the anterolateral ligament: an anatomic analysis. Arthroscopy. 2016;32(5):835–41.
Helito CP, Bonadio MB, Soares TQ, et al. The meniscal insertion of the knee anterolateral ligament. Surg Radiol Anat. 2016;38(2):223–8.
Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–70.
Andersen HN, Dyhre-Poulsen P. The anterior cruciate ligament does play a role in controlling axial rotation in the knee. Knee Surg Sports Traumatol Arthrosc. 1997;5(3):145–9.
Kaplan DJ, Jazrawi LM. Secondary stabilizers of tibial rotation in the intact and anterior cruciate ligament deficient knee. Clin Sports Med. 2018;37(1):49–59.
Lorbach O, Pape D, Maas S, et al. Influence of the anteromedial and posterolateral bundles of the anterior cruciate ligament on external and internal tibiofemoral rotation. Am J Sports Med. 2010;38(4):721–7.
Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. J Bone Joint Surg Am. 2009;91(1):107–18.
Lipke JM, Janecki CJ, Nelson CL, et al. The role of incompetence of the anterior cruciate and lateral ligaments in anterolateral and anteromedial instability. A biomechanical study of cadaver knees. J Bone Joint Surg Am. 1981;63(6):954–60.
Engebretsen L, Wijdicks CA, Anderson CJ, Westerhaus B, LaPrade RF. Evaluation of a simulated pivot shift test: a biomechanical study. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):698–702.
Amis AA, Scammell BE. Biomechanics of intra-articular and extra-articular reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 1993;75(5):812–7.
Kittl C, El-Daou H, Athwal KK, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44(2):345–54.
Huser LE, Noyes FR, Jurgensmeier D, Levy MS. Anterolateral ligament and iliotibial band control of rotational stability in the anterior cruciate ligament-intact knee: defined by tibiofemoral compartment translations and rotations. Arthroscopy. 2017;33(3):595–604.
Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med. 1993;21(1):55–60.
Rasmussen MT, Nitri M, Williams BT, et al. An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med. 2016;44(3):585–92.
Sonnery-Cottet B, Lutz C, Daggett M, et al. The involvement of the anterolateral ligament in rotational control of the knee. Am J Sports Med. 2016;44(5):1209–14.
Bonanzinga T, Signorelli C, Grassi A, et al. Kinematics of ACL and anterolateral ligament. Part I: combined lesion. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1055–61.
Monaco E, Ferretti A, Labianca L, et al. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):870–7.
Ruiz N, Filippi GJ, Gagniere B, Bowen M, Robert HE. The comparative role of the anterior cruciate ligament and anterolateral structures in controlling passive internal rotation of the knee: a biomechanical study. Arthroscopy. 2016;32(6):1053–62.
Thein R, Boorman-Padgett J, Stone K, Wickiewicz TL, Imhauser CW, Pearle AD. Biomechanical assessment of the anterolateral ligament of the knee: a secondary restraint in simulated tests of the pivot shift and of anterior stability. J Bone Joint Surg Am. 2016;98(11):937–43.
Guenther D, Rahnemai-Azar AA, Bell KM, et al. The anterolateral capsule of the knee behaves like a sheet of fibrous tissue. Am J Sports Med. 2017;45(4):849–55.
Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38(8):1591–7.
Musahl V, Rahnemai-Azar AA, Costello J, et al. The influence of meniscal and anterolateral capsular injury on knee laxity in patients with anterior cruciate ligament injuries. Am J Sports Med. 2016;44(12):3126–31.
Hosseini A, Li JS, Gill TJt, Li G. Meniscus injuries alter the kinematics of knees with anterior cruciate ligament deficiency. Orthop J Sports Med. 2014;2(8):2325967114547346.
Nitri M, Rasmussen MT, Williams BT, et al. An in vitro robotic assessment of the anterolateral ligament, part 2: anterolateral ligament reconstruction combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(3):593–601.
Geeslin AG, Moatshe G, Chahla J, et al. Anterolateral knee extra-articular stabilizers: a robotic study comparing anterolateral ligament reconstruction and modified Lemaire lateral extra-articular tenodesis. Am J Sports Med. 2018;46(3):607–16.
Jette C, Gutierrez D, Sastre S, Llusa M, Combalia A. Biomechanical comparison of anterolateral ligament anatomical reconstruction with a semi-anatomical lateral extra-articular tenodesis. A cadaveric study. Knee. 2019;26(5):1003–9.
Noyes FR, Huser LE, Jurgensmeier D, Walsh J, Levy MS. Is an anterolateral ligament reconstruction required in ACL-reconstructed knees with associated injury to the anterolateral structures? A robotic analysis of rotational knee stability. Am J Sports Med. 2017;45(5):1018–27.
Inderhaug E, Stephen JM, Williams A, Amis AA. Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(2):347–54.
DePhillipo NN, Cinque ME, Chahla J, Geeslin AG, LaPrade RF. Anterolateral ligament reconstruction techniques, biomechanics, and clinical outcomes: a systematic review. Arthroscopy. 2017;33(8):1575–83.
Ferreira Mde C, Zidan FF, Miduati FB, Fortuna CC, Mizutani BM, Abdalla RJ. Reconstruction of anterior cruciate ligament and anterolateral ligament using interlinked hamstrings – technical note. Rev Bras Ortop. 2016;51(4):466–70.
Sonnery-Cottet B, Barbosa NC, Tuteja S, Daggett M, Kajetanek C, Thaunat M. Minimally invasive anterolateral ligament reconstruction in the setting of anterior cruciate ligament injury. Arthrosc Tech. 2016;5(1):e211–5.
Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239–44.
Chahla J, Menge TJ, Mitchell JJ, Dean CS, LaPrade RF. Anterolateral ligament reconstruction technique: an anatomic-based approach. Arthrosc Tech. 2016;5(3):e453–7.
Wagih AM, Elguindy AM. Percutaneous reconstruction of the anterolateral ligament of the knee with a polyester tape. Arthrosc Tech. 2016;5(4):e691–7.
Smith JO, Yasen SK, Lord B, Wilson AJ. Combined anterolateral ligament and anatomic anterior cruciate ligament reconstruction of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3151–6.
Schon JM, Moatshe G, Brady AW, et al. Anatomic anterolateral ligament reconstruction of the knee leads to overconstraint at any fixation angle. Am J Sports Med. 2016;44(10):2546–56.
Tavlo M, Eljaja S, Jensen JT, Siersma VD, Krogsgaard MR. The role of the anterolateral ligament in ACL insufficient and reconstructed knees on rotatory stability: a biomechanical study on human cadavers. Scand J Med Sci Sports. 2016;26(8):960–6.
Katakura M, Koga H, Nakamura K, Sekiya I, Muneta T. Effects of different femoral tunnel positions on tension changes in anterolateral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1272–8.
Kernkamp WA, Van de Velde SK, Hosseini A, et al. In vivo anterolateral ligament length change in the healthy knee during functional activities-a combined magnetic resonance and dual fluoroscopic imaging analysis. Arthroscopy. 2017;33(1):133–9.
Lau BC, Rames J, Belay E, Riboh JC, Amendola A, Lassiter T. Anterolateral complex reconstruction augmentation of anterior cruciate ligament reconstruction: biomechanics, indications, techniques, and clinical outcomes. JBJS Rev. 2019;7(11):e5.
Weber AE, Zuke W, Mayer EN, et al. Lateral augmentation procedures in anterior cruciate ligament reconstruction: anatomic, biomechanical, imaging, and clinical evidence. Am J Sports Med. 2019;47(3):740–52.
Vundelinckx B, Herman B, Getgood A, Litchfield R. Surgical indications and technique for anterior cruciate ligament reconstruction combined with lateral extra-articular tenodesis or anterolateral ligament reconstruction. Clin Sports Med. 2017;36(1):135–53.
Naal FD, Hatzung G, Muller A, Impellizzeri F, Leunig M. Validation of a self-reported Beighton score to assess hypermobility in patients with femoroacetabular impingement. Int Orthop. 2014;38(11):2245–50.
Cavaignac E, Wytrykowski K, Reina N, et al. Ultrasonographic identification of the anterolateral ligament of the knee. Arthroscopy. 2016;32(1):120–6.
Capo J, Kaplan DJ, Fralinger DJ, et al. Ultrasonographic visualization and assessment of the anterolateral ligament. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3134–9.
Taneja AK, Miranda FC, Braga CA, et al. MRI features of the anterolateral ligament of the knee. Skelet Radiol. 2015;44(3):403–10.
Hartigan DE, Carroll KW, Kosarek FJ, Piasecki DP, Fleischli JF, D’Alessandro DF. Visibility of anterolateral ligament tears in anterior cruciate ligament-deficient knees with standard 1.5-tesla magnetic resonance imaging. Arthroscopy. 2016;32(10):2061–5.
Porrino J Jr, Maloney E, Richardson M, Mulcahy H, Ha A, Chew FS. The anterolateral ligament of the knee: MRI appearance, association with the Segond fracture, and historical perspective. AJR Am J Roentgenol. 2015;204(2):367–73.
Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skelet Radiol. 2014;43(10):1421–7.
Rahnemai-Azar AA, Miller RM, Guenther D, et al. Structural properties of the anterolateral capsule and iliotibial band of the knee. Am J Sports Med. 2016;44(4):892–7.
Zens M, Feucht MJ, Ruhhammer J, et al. Mechanical tensile properties of the anterolateral ligament. J Exp Orthop. 2015;2(1):7.
Helito CP, Bonadio MB, Rozas JS, et al. Biomechanical study of strength and stiffness of the knee anterolateral ligament. BMC Musculoskelet Disord. 2016;17:193.
Wytrykowski K, Swider P, Reina N, et al. Cadaveric study comparing the biomechanical properties of grafts used for knee anterolateral ligament reconstruction. Arthroscopy. 2016;32(11):2288–94.
Kernkamp WA, van de Velde SK, Bakker EW, van Arkel ER. Anterolateral extra-articular soft tissue reconstruction in anterolateral rotatory instability of the knee. Arthrosc Tech. 2015;4(6):e863–7.
Fetto JF, Marshall JL. The natural history and diagnosis of anterior cruciate ligament insufficiency. Clin Orthop Relat Res. 1980;147:29–38.
Fox JM, Blazina ME, Del Pizzo W, Ivey FM, Broukhim B. Extra-articular stabilization of the knee joint for anterior instability. Clin Orthop Relat Res. 1980;147:56–61.
Ellison AE. Distal iliotibial-band transfer for anterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1979;61(3):330–7.
Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BH, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598–605.
Rosenstiel N, Praz C, Ouanezar H, et al. Combined anterior cruciate and anterolateral ligament reconstruction in the professional athlete: clinical outcomes from the scientific anterior cruciate ligament network international study group in a series of 70 patients with a minimum follow-up of 2 years. Arthroscopy. 2019;35(3):885–92.
Ibrahim SA, Shohdy EM, Marwan Y, et al. Anatomic reconstruction of the anterior cruciate ligament of the knee with or without reconstruction of the anterolateral ligament: a randomized clinical trial. Am J Sports Med. 2017;45(7):1558–66.
Lee DW, Kim JG, Cho SI, Kim DH. Clinical outcomes of isolated revision anterior cruciate ligament reconstruction or in combination with anatomic anterolateral ligament reconstruction. Am J Sports Med. 2019;47(2):324–33.
Smith PA, Thomas DM, Pomajzl RJ, Bley JA, Pfeiffer FM, Cook JL. A biomechanical study of the role of the anterolateral ligament and the deep iliotibial band for control of a simulated pivot shift with comparison of minimally invasive extra-articular anterolateral tendon graft reconstruction versus modified Lemaire reconstruction after anterior cruciate ligament reconstruction. Arthroscopy. 2019;35(5):1473–83.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kaplan, D.J., Mannino, B.J., Gonzalez-Lomas, G., Jazrawi, L.M. (2022). The Role of Anterolateral Procedures: Anterolateral Ligament Reconstruction. In: Alaia, M.J., Jones, K.J. (eds) Revision Anterior Cruciate Ligament Reconstruction. Springer, Cham. https://doi.org/10.1007/978-3-030-96996-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-96996-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96995-0
Online ISBN: 978-3-030-96996-7
eBook Packages: MedicineMedicine (R0)