Abstract
Purpose
Injury to the anterolateral ligament (ALL) of the knee has recently received attention as a potential risk factor for failure of anterior cruciate ligament reconstruction. However, evaluation of the anterolateral ligament is currently difficult, and radiologic data are sparse with regard to the normal appearance of this ligament. The purpose of the present study was to determine whether the ALL could be identified and visualized using ultrasonography.
Methods
Ten non-paired, fresh-frozen cadaveric knees underwent ultrasound by an experienced musculoskeletal radiologist using a Siemens S2000 Acuson Ultrasound machine with a 14-MHz linear transducer. After first identifying anatomical landmarks by palpation, a thin band of tissue originating in the vicinity of the fibular collateral ligament (FCL) origin was identified and followed up distally. The tibia was held at 30° of flexion and internally rotated to verify tightening of the structure. Under ultrasound guidance, 25-gauge hypodermic needles were placed at what were sonographically determined to be the origin and insertion points of the ligament. One-tenth of a CC of aniline blue dye was injected. The specimens were then dissected to confirm the presence and location of the ALL. If an ALL was found, distances between the epicentre of the injected dye and the actual origin and insertion points were calculated. Additionally, ligament length based on dissection images and ultrasound images was calculated.
Results
Eight of ten specimens had an anterolateral structure that originated from the lateral femoral epicondyle just posterior and superior to the origin of the FCL and inserted on the lateral plateau approximately halfway between Gerdy’s tubercle and the fibular head. The average length based on ultrasound was 3.8 cm (±.7; range 3.1–4.7) and 4.1 cm (±1.1; range 2.6–6.1) based on dissection. Length based on dissection and ultrasound had minimal agreement (ICC = .308; 95 % confidence interval .257–.382, p = .265). The average width of the structure on dissection was .8 cm (±.24; range .5–1.2). The mean distance from ultrasound-determined origin and insertion points to anatomical origin and insertion based on dissection was 10.9 mm (±2.9, range 7.0–15.8) and 12.5 mm (±5.7 range 3.2–19.3), respectively. Inter-observer reliability was excellent for all measurements based on dissection and ultrasound.
Conclusion
Ultrasound was unable to reliably identify the anterolateral structure from its femoral to tibial attachment sites. Distinguishing it from the posterior IT band and anterolateral capsule was challenging, and it is possible that the structure is a thickened band of fascia rather than a true ligament. As a clinical diagnostic tool, ultrasound likely offers little utility in the evaluation of the ALL for injury.
Level of evidence
IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The anterolateral ligament (ALL) was first described over 130 years ago by Dr. Paul Segond, who described a “pearly, resistant, fibrous band” associated with an avulsion fracture pattern of the anterolateral proximal tibia [5, 17]. Since its discovery, the little-studied structure has been referred to by various names, and its structure, function and even existence has been a matter of debate.
In 2013, Claes et al. demonstrated the presence of the anterolateral ligament in 40 out of 41 human cadaveric knees (97 %) [5]. Later studies have confirmed the presence of the ALL in over 95 % of human knees [20], most of which describe the structure as an extra-articular thickening of the lateral capsule that comes under tension with internal rotation [10, 20]. It has been postulated that the ALL plays a role in anterolateral rotational stability, and uncorrected ALL deficiency could be responsible for lingering rotational instability—most notably the positive pivot-shift—seen after ACL reconstruction [10].
Imaging assessment of the ALL has focused on MRI. Studies have reported successfully visualizing the ALL in 75–94 % of cases [3, 4, 10, 11]. Limited studies have investigated the use of ultrasound to visualize the ALL.
The purpose of the present study was to determine whether the ALL could be identified and visualized using ultrasonography. It was hypothesized that due to the extra-articular course of the ALL, it would be visible on ultrasound. Given the proposed importance of the ALL, successfully determining whether the structure can be imaged with non-invasive and cost-effective ultrasonographic methods would benefit both patients and the health system.
Materials and methods
Ten non-paired, fresh-frozen cadaveric knees were examined using ultrasonography. Knees with a history of surgery, ligamentous injury, or advanced osteoarthritis were excluded.
Ultrasound evaluation was performed by an experienced musculoskeletal radiologist, with over 20 years of experience performing musculoskeletal ultrasound, using a Siemens S2000 Acuson Ultrasound machine (Siemens Medical Systems, Mountain view, CA) and a 14-MHz linear transducer. The fibular head and Gerdy’s tubercle were first located by palpation. With the knee flexed to 60°, the fibular collateral ligament (FCL) was identified under ultrasound guidance and followed to its origin on the lateral femoral epicondyle. A thin band of tissue originating in the vicinity of the FCL origin was identified and followed up distally. The structure was carefully assessed to ensure it was a continuous structure and distinct from the IT band. Most often, it was found deep to the posterior margin of the IT band proximally, becoming posterior to the IT band moving distally and inserting on the tibia posterolateral to Gerdy’s tubercle. Once identified, the tibia was internally rotated with respect to the femur to verify the structure tightened or became more prominent. The structure was followed distally to its insertion on the tibia and proximally to its origin on the femoral condyle. Under ultrasound guidance, a 25-gauge hypodermic needle was placed at what the radiologist believed to be the origin and insertion points of the structure (Fig. 1). After needle placement, .1 CC of aniline blue dye was injected (Fig. 2). Images were taken of the ultrasound screen once the entire ligament was in view, and the needles were placed.
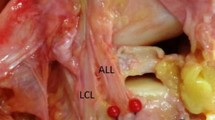
a Lateral view of a left dissected specimen demonstrating an intact ALL. Dye marks can be seen representing pin placement. A ruler is seen which was used for scale. b Anterior view of a different left dissected specimen. Osseous structures are outlined with dashed lines. LEC lateral femoral epicondyle, GT Gerdy’s tubercle, JC joint capsule, FCL fibular collateral ligament
After dye injection, the specimens were dissected to confirm the presence and location of the anterolateral ligament. The skin and subcutaneous tissues were removed to visualize the iliotibial band. The IT band was transected proximal to the femoral epicondyle and reflected inferiorly. With the ITB reflected, the ALL could be visualized, if present. Forced internal rotation of the knee while palpating the anterior lateral capsule was used if necessary, to enhance ALL prominence. Surrounding soft tissues were carefully removed until the origin and insertion points of the ALL could be clearly visualized, as well as the mark left from injection of the dye. We considered an ALL to be present when there was a clear area of palpable capsular thickening that tightened upon internal rotation of the tibia and was anatomically differentiable from the surrounding anterolateral capsule. There was substantial variability in this thickened tissue; in the most robust specimens, a plane could be easily developed with a Freer elevator that separated the thickened tissue from joint capsule. Pictures were taken of each dissection with a ruler for scale (Fig. 2a, b). As this was a cadaver study, no institutional review board (IRB) approval was required.
Measurement
Upon dissection, the distance between the dye epicentre and the actual origin and insertion of the ALL, as well as the length and width of the ligament, was measured directly, using a ruler.
For each specimen where an ALL was found on dissection, ligament length based on ultrasonography was also measured using saved US pictures taken at the time of pin placement. Because the course of the ligament was often curved, a string was used to ascertain the straight-line length of individual segments. Using the scale provided in each ultrasound image (e.g. 1 cm on screen=true length of 3 cm) for reference, this distance was used to calculate the length of each ligament.
All length and width measurements based on dissection and ultrasonography were taken by two raters at separate times using the same techniques. The more experienced rater’s measurements were used for final calculations.
Statistical analysis
Statistics were calculated using IBM SPSS version 23 (IBM, Armonk, NY). Descriptive statistics were generated, and inter-observer reliability was calculated for all measurements using a two-way random absolute agreement inter-class correlation coefficient (ICC) test. Agreement between length of the anterolateral structure based on ultrasound and dissection was also calculated using the ICC.
Results
On ultrasound, six of the ten specimens had an identifiable structure that originated from the lateral femoral epicondyle just posterior and superior to the origin of the FCL and inserted on the lateral plateau approximately halfway between Gerdy’s tubercle and the fibular head, and tightened with flexion and internal rotation of the knee—two were difficult to distinguish but we thought were present and two had no ALL. On dissection, eight of the ten specimens had a clearly identifiable anterolateral structure (the same eight specimens that had the structure on ultrasound).
The average length of the ALL based on ultrasound imaging was 3.8 cm (±.7; range 3.1–4.7) and 4.1 cm (±1.1; range 2.6–6.1) based on dissection (Table 1). Length based on dissection and ultrasound had minimal agreement (ICC = .31; 95 % confidence interval .26–.38, p = .27). The average of the absolute value of the difference between the length calculated from the dissection and the length calculated from the ultrasound was 1.3 cm (±.1; range .1–2.9). The average width of the structure on dissection was .8 cm (±.2; range .5–1.2).
The mean distance from ultrasound-determined origin and insertion points to anatomical origin and insertion points based on dissection was 10.9 mm (±2.9, range 7.0–15.8) and 12.5 mm (±5.7 range 3.2–19.3), respectively. Inter-observer reliability was excellent for all measurements based on dissection and ultrasound (Table 2).
Discussion
The most important finding of the present study is the ultrasound did not consistently correctly locate the origin and insertion points of the ALL. A secondary finding is the ALL was difficult to isolate from surrounding structures, even on dissection.
The ALL has been described as originating posterior to the lateral femoral epicondyle and has been reported as variably anterior [5, 20] or posterior [17] to the fibular collateral ligament (FCL). From there, the ALL courses anteroinferiorly, crossing the knee joint obliquely, and inserts into the proximal tibia midway between Gerdy’s tubercle and the anterior border of the fibular head [5, 10, 14, 16, 20].
The ALL is hypothesized to provide anterolateral rotational stability to the knee [5, 6, 14]. Disruption of the ALL then is thought to be partially responsible for the pivot-shift phenomenon in ACL-deficient knees [6, 10, 14, 16] and may help explain continued pivot-shifting following ACL reconstruction; however, it is worth noting that several studies have shown the iliotibial band to also play an important role in stabilizing the knee against internal rotation [12, 18].
Given the possible clinical impact of the ALL, evaluation of the structure may start to be incorporated into routine injury workup. Most investigations have focused on MRI, but this modality has drawbacks. It has been shown that the anterolateral ligament may not be completely visualized on MRI, in part because the oblique course of the structure prevents it from being visualized completely in one cut [9, 19]. Additionally, MRI may be prohibitively expensive and have limited availability in certain areas. Given the extra-articular course of the ALL, ultrasound could be a helpful modality, particularly in patients with known ACL tears. It is non-invasive and relatively inexpensive, and importantly, it allows for real-time, dynamic evaluation of the ligament under internal rotational stress. Although others have found great accuracy in detecting the anterolateral ligament [2, 13], this is the first paper, to our knowledge, to question the accuracy of ultrasound in assessing the anterolateral ligament of the knee.
In this study, a structure with a similar course to the one described for the ALL was identified in the majority of specimens on ultrasound, and the two specimens in which no ALL was seen on ultrasound matched those specimens in which no ALL was found on dissection. Contrary to the results found by Cavaignac et al., significant error was encountered in the finer ultrasonographic localization of this structure, with difficulty in viewing the entire course of the ligament from origin to insertion.
On dissection, an anterolateral structure was identified in eight of ten knees—within the reported range of ALL prevalence [4, 5, 8, 15, 19]. The structure did not appear to be contiguous with the meniscofemoral ligament in any samples. Though average length on ultrasound compared to dissection was not vastly different, it is clear based on the average absolute differences and poor ICC agreement between measuring methods that the ultrasound was unreliable, consistently over- or underestimating ligament length (see Table 1). On dissection, average length of the ligament was found to be 4.1 cm (±1.1 cm), which is comparable to other studies reporting average lengths ranging from 3.7–4.1 cm [1, 5, 8]. Interestingly, the ligament lengths measured on ultrasound were more consistent than what we found on dissection (lower standard deviation) despite the lower accuracy. This may be because the midsubstance was more distinguishable than the insertion/origin points on ultrasound, and thus, measurements may have incorrectly frequently only recorded the more visible midsubstance (even if measuring the ALL). This would result in a more consistent, albeit incorrect measurement. This could also explain the lower recorded lengths. Our distance discrepancy seems to represent greater inaccuracy in ultrasound’s ability to determine the origin and insertion of the ligament than that has been previously reported [2]. The differences in location between the insertion and origin points based on ultrasound compared to those found on dissection further indicate ultrasonography did not accurately localize the structure. Inter-observer reliability was consistently strong for all metrics, indicating that the discrepancy between ultrasound and dissection likely was not due to our measurement technique.
Claes et al. identified an ALL in 40/41 specimens on dissection [5], and Caterine et al. identified 19 of 19 ALL on dissection [1]. Several studies have reported the presence of an ALL in most knees on imaging [4, 8, 15, 19]. However, other studies have not been as definitive. Helito et al. only found an ALL in 51 % of the knees scanned, and of these, only 11 % were completely visible [8]. Porino et al. reported visualizing a structure along the lateral aspect of the knee connecting the distal femur with the proximal tibia, but were quick to note that the structure was inseparable from the FCL at the origin attachment site and did not have a reliable division with the anterior lateral capsule at the meniscofemoral attachment site, and the distal insertion was inseparable anteriorly from the posterior IT band [15]. They further noted that there was no reliable visualized plane separating the ALL and the immediately adjacent lateral capsule, which results in a single thin ill-defined structure as opposed to a discrete cordlike structure [15]. Goldman et al. found that abnormalities in course occur along the entire length of the ligament in MR scans of ACL-injured knees [7].
While identification of the ligament is clearly user-dependent, it is unlikely that the difference in our results could be explained by a difference in operators alone. Much of the challenge in ultrasonography of the ALL is likely due to its physical characteristics. It was particularly difficult to differentiate the ALL from both the deep IT band fascia and the surrounding anterolateral joint capsule on ultrasound. Unfortunately, neither of these structures are labelled on the ultrasound images provided by Cavaignac et al. despite running in close proximity. On dissection, we found substantial variability in the physical appearance of the ALL, ranging from a robust unique structure easily separated from the underlying capsule, to a simple thickening that could only be visualized and palpated with internal rotation of the tibia. Furthermore, the ALL origin often blended with the FCL origin, and the ALL insertion blended with the distal deep portion of IT band, making them difficult to see as distinct structures. The intimate association of the ALL with the deep IT band and joint capsule, as well as the close proximity of the origin of the FCL and insertion of the ITB, made identification difficult both radiologically and on dissection. Based on our results, we predict the use of ultrasound to aid in the diagnosis of ALL injury would be limited at best, and we cannot recommend routine use of ultrasound in the workup of rotational instability of the knee.
Some error in this study may be partially explained by experimental design. It was difficult to ensure the tip of the inserted needle was exactly in the origin or insertion and not passing through the structure. Also, diffusion of the injected dye may represent a source of error; however, a distinct epicentre of the injectate was prominent and identifiable in all cases, and still at a considerable distance from the dissected and isolated anterolateral ligament fibres.
The donor age and history of knee trauma in our specimens were unknown, and the potential for attenuation or rupture during the specimen donor’s lifetime is a consideration. In addition, one could argue that the use of one ultrasonographer could potentiate a source of bias.
Perhaps most importantly, there was substantial variability in the physical characteristics of the ALL. The structure often blended into the joint capsule proximally, distally or both, making identification challenging. Both on ultrasound and in dissection, a structure with a similar course as described in the previous studies was found, and this structure tightened with internal rotation. However, this structure had an inconsistent course, variable appearance, and intimate association with the deep IT band. It is possible that the structure dissected was a facial thickening of the deep IT band rather than a unique ligament, and we cannot unequivocally state we found a true ligament anterolaterally rather than a band of thickened facia.
Conclusion
Ultrasonographic visualization of the course of the anterolateral ligament from origin to insertion was difficult, and distinguishing it from the deep IT band and anterolateral capsule was challenging, and it is possible that the structure is a thickened band of IT fascia rather than a true ligament. As a clinical diagnostic tool, the ultrasound likely offers little utility in the evaluation of the anterolateral for injury.
References
Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A (2014) A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthroscopy 23:3186–3195
Cavaignac E, Wytrykowski K, Reina N, Pailhe R, Murgier J, Faruch M, Chiron P (2015) Ultrasonographic Identification of the anterolateral ligament of the knee. Arthroscopy 32:120–126
Cianca J, John J, Pandit S, Chiou-Tan FY (2014) Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil 93:186
Claes S, Bartholomeeusen S, Bellemans J (2014) High prevalence of anterolateral ligament abnormalities in magnetic resonance images of anterior cruciate ligament-injured knees. Acta Orthop Belg 80:45–49
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J (2013) Anatomy of the anterolateral ligament of the knee. J Anat 223:321–328
Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA (2014) The anterolateral ligament: anatomy, length changes and association with the segond fracture. Bone Joint J 96 B:325–331
Goldman AB, Pavlov H, Rubenstein D (1988) The Segond fracture of the proximal tibia: a small avulsion that reflects major ligamentous damage. Am J Roentgenol 151:1163–1167
Helito CP, Demange MK, Helito PVP, Costa HP, Bonadio MB, Pecora JR, Rodrigues MB, Camanho GL (2014) Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Ortop 50:214–219
Helito CP, Victor P, Helito P, Costa HP, Bordalo-rodrigues M, Pecora JR, Camanho GL (2014) MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1. 5-T scans. Skelet Radiol 43:1421–1427
Kennedy MI, Claes S, Fuso FAF, Williams BT, Goldsmith MT, Turnbull TL, Wijdicks CA, LaPrade RF (2015) The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med 43:1606–1615
Kosy JD, Mandalia VI, Anaspure R (2015) Characterization of the anatomy of the anterolateral ligament of the knee using magnetic resonance imaging. Skelet Radiol 44:1647–1653
Lutz C, Sonnery-Cottet B, Niglis L, Freychet B, Clavert P, Imbert P (2015) Behavior of the anterolateral structures of the knee during internal rotation. Orthop Traumatol Surg Res 101:523–528
Oshima T, Nakase J, Numata H, Takata Y, Tsuchiya H (2016) The knee ultrasonography imaging of the anterolateral ligament using real-time virtual sonography. Knee 23:198–202
Pomajzl R, Maerz T, Shams C, Guettler J, Bicos J (2015) A review of the anterolateral ligament of the knee: current knowledge regarding its incidence, anatomy, biomechanics, and surgical dissection. Arthroscopy 31:583–591
Porrino J, Maloney E, Richardson M, Mulcahy H, Ha A, Chew FS (2015) The anterolateral ligament of the knee: MRI appearance, association with the segond fracture, and historical perspective. Am J Roentgenol 204:367–373
Rasmussen MT, Nitri M, Williams BT, Moulton SG, Cruz RS, Dornan GJ, Goldsmith MT, LaPrade RF (2015) An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med 44(3):585–592
Segond P (1879) Recherches cliniques et expérimentales sur les épanchements sanguins du fenou par entorse. Progres Med 7:297–341
Sonnery-Cottet B, Lutz C, Daggett M, Dalmay F, Freychet B, Niglis L, Imbert P (2016) The involvement of the anterolateral ligament in rotational control of the knee. Am J Sports Med 44(5):1209–1214
Taneja AK, Miranda FC, Braga CAP, Gill CM, Hartmann LGC, Santos DCB, Rosemberg LA (2014) MRI features of the anterolateral ligament of the knee. Skelet Radiol 44:403–410
Van Der Watt L, Khan M, Rothrauff BB, Ayeni OR, Musahl V, Getgood A, Peterson D (2015) The structure and function of the anterolateral ligament of the knee: a systematic review. Arthroscopy 31(569–582):e3
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capo, J., Kaplan, D.J., Fralinger, D.J. et al. Ultrasonographic visualization and assessment of the anterolateral ligament. Knee Surg Sports Traumatol Arthrosc 25, 3134–3139 (2017). https://doi.org/10.1007/s00167-016-4215-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4215-x