Abstract
The most attractive aspect of metal foams is that they can combine the properties of metallic materials (e.g., strength, high melting temperature) with the properties of porous materials (e.g., light-weightiness, sound/heat energy absorption). This makes them favorable in aerospace applications where multifunctional lightweight materials are required since the amount of work done against inertial forces is larger. Within the scope of this chapter, after giving information about the primary and secondary processes of metal foams with open and closed-cells, some applications of these materials in aircraft and spacecraft structures are mentioned.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Light weighting is a target that almost all designers are responsible. By reducing the weight, energy efficiency increases due to the decreasing energy consumption. In addition, vehicle performance increases with increasing acceleration [1]. Material substitution is a method to achieve light weighting. For instance, mechanically, a material with higher specific strength (strength/density) (such as aluminum foam instead of polymer foam) can be used. Besides material substitution, lower weight constructions can be obtained by changing the shape (e.g., using a lattice structure) or by developing composite structures [2].
As an example, instead of a single steel plate, a composite construction with the same stiffness value and an 83% lower weight can be obtained by using two aluminum sheets with aluminum foam between them (AFS/aluminum foam sandwich). A similar reduction in weight (77%) can be achieved with a CFRP/carbon fiber-reinforced plastic composite structure. However, the cost of this material is approximately four times compared to that of AFS [1]. Also, AFS can have similar bending stiffness values as an aluminum honeycomb sandwich structure of the same weight [3].
There are many porous materials such as bone and wood in nature. Studies have been recorded since the mid-twentieth century on making metals porous by imitating nature. Developed methods can also be used in obtaining functionally graded structures because that the density of the products can to some extent be adjusted [4].
Porous metals attracted attention after being used against high shock-wave pressures in the 1950s, and studies on aluminum foam production for aircraft technology began. As a result of the increasing number of studies on porous metals, continuous improvements are achieved in production methods and material properties. There is a conference originated in Europe (MetFoam), dedicated to developments in this field and being held regularly since 1999. In addition to these improvements, the ability of porous metals to meet ever-rising and often conflicting expectations increases the interest in this material group [5]. As of 2019, when the total use of metal foams in different industries is approximately compared, the industries where they are used the most are automotive (31%) and aerospace/defense (20%) [6].
Although many other metals can be turned into a metal foam, still the most study is on aluminum and its alloys. Aluminum foam materials combine light weightiness, high mechanical energy absorption, good sound absorption, nonflammability, low thermal conductivity, and electromagnetic shielding [7].
Metal foams are classified as open cell if their cells are open to each other and closed cell if they are closed to each other (Fig. 2.1).
2.2 Primary Processing
Basically, there are possibilities of making metals porous while they are in four different forms (Table 2.1): Solid (powder, fiber, sphere), liquid (melt), gas (vapor), ion [10]. Closed-cell porous structures can only be formed by some liquid metal methods (Fig. 2.2). In these methods, it is the surface tension (which occurs in liquid state) that allows the formation of cells that are almost completely closed [11]. An exception to this situation is syntactic metal foams. Fully closed cells in these structures are generated with hollow, place-holder elements that remain in the structure after manufacturing [12]. Another exception is the studies on the production of closed/open-cell metal foams with the help of 3D printing technology [13], which has been started to be employed for metallic material manufacturing, too.
Expansion stages of a metal foam, monitored by X-ray radioscopy [14]. Reprinted by permission from AIP Publishing
Although many of the porous metals are commonly referred to as metal foam, “metal foam” literally defines materials formed by the dispersion of gas bubbles in liquid metal [10]. Even though aluminum is the most common material that is foamed; zinc, magnesium, gold, copper, tin, brass, lead, nickel, and even steel can also be made into this form [15].
The idea of producing metal foam materials with macroscopic cells was embodied in a series of patents received in the 1950s. There was a second activity in this area in the 1980s; since these dates, many scientific studies have been conducted and the results have been published, some companies have commercialized metal foam materials by establishing small-scale production lines [16]. It was only possible in the early 2000s for these materials to become a niche market where productions are made at 1000 kg/h capacities [10].
In closed-cell metal foam production methods (Table 2.2), where liquid metal is foamed, if a bubble formation mechanism (injecting gas or introducing a gas-releasing agent) is activated when the metal becomes molten, these methods are called direct foaming. If the bubbles are formed spontaneously due to the melting of a previously prepared structure (precursor), these methods are called indirect foaming [17]. In this second method, pores may start to emerge before the structure is completely melted, even if it is not desired [18].
Although naming indirect foaming method of metals as “PM/powder metallurgical foaming method” is common, this can be evaluated as a mumpsimus. The reason is that the similarity of two methods ends at the end of the consolidation stage. The next stage in indirect metal foaming is melting and no sintering stage is included.
Pore structure is predetermined in some porous metallic materials. For instance, in investment casting method, a metal replica of an open-cell polymer structure is produced. On the other hand, in metal foams, the pore structure is formed almost freely, even some control mechanisms have been developed. In addition, the pores are subject to deteriorations such as drainage (liquid flowing over the foam boundaries and accumulating at the bottom) and coalescence (loss of the foam boundaries).
There are some requirements for the foam structure to form and keep its form (Table 2.3). According to this, there is a need for a source to generate gas bubbles, a force to keep the bubbles in the structure, and a mechanism that stabilizes the bubbles.
The source which generates gas bubbles in molten metal is a mechanism that injects gas from a rotating propeller or a vibrating nozzle in the gas injection method. In this way, usually air, alternatively nitrogen or argon gas, is sent into the mixture in a fine and homogeneous manner. Another source of gas formation is the so-called blowing agents, which cause gas emergence when decomposed at high temperatures [19]. For a matrix of Al or its alloys, TiH2 and CaCO3 compounds are the most popular blowing agents. This type of gas formation can be used in both direct and indirect methods. In direct methods, it is ensured that the blowing agent is dispersed into the molten metal. In indirect methods, the blowing agent is added to the precursor material in a homogeneous manner before melting [20].
The gas bubbles formed tend to leave the molten metal rapidly due to the buoyant force. In order to prevent this in direct foaming methods, the viscosity of the liquid is increased. For this purpose, ceramic particles such as SiC, Al2O3, TiB2 are added to the molten metal [22]. Another method is to add elements that will form a ceramic compound (in situ ceramic particle formation). For this, the addition of elements such as Ca or Al in an oxygen-containing medium has yielded good results [11]. It has been observed that viscosity can be increased up to five times with the help of Ca addition [19].
These particles, which increase the viscosity, are also effective in stabilizing the bubbles. There is a correlation between viscosity and foam stability. However, viscosity is not a reason for foam stability; it is only considered to be an indication of the presence of hard particles. Many studies have shown that melts without hard particles do not form metal foam. It was also found that there is a proportionality between the oxide content and the total value of the inner surface areas of the cells. When the ratio of these particles remains too low, the surface tensions increase and the foam structure deteriorates. SiC has come to the fore with its ability to delay bubble coalescence for a long time, to cover foam films (boundaries that will become walls when solidified) without getting stuck in the bubbles, and to allow the formation of very thin foam films [16].
In some cases, the addition of blowing agent can both generate bubbles and lead to their stabilization. An example for this is the use of CaCO3 as a blowing agent for direct foaming of aluminum and its alloys. As a result of the reaction of Al (or Al alloy) and CaCO3, (besides the CO2 gas that provides foaming) hard particles such as CaO and Al2O3 are formed and act as stabilizers. Such a mechanism does not occur in the use of TiH2 blowing agent [23].
The indirect foaming method involves the compression of mixed powder materials (“pre-alloyed powder + blowing agent” or “alloying elements + blowing agent”). The purpose of this stage is to increase the density of the powder mixture (min. 94% of the theoretical density [24]) so that it is ensured that the blowing agent is embedded in the metal matrix and that it is made difficult for the bubbles to escape from the structure by reducing the porosity as much as possible [10]. Another function of the compression stage is to break down the oxide layers on the metal powders. As a result, the broken oxide particles increase the stability of the metal foam (when the metal becomes liquid); also, the breaking of the oxide shells on the powder metals improves the combining of powder particles [25]. Even the oxide content on as-received powders could be sufficient to obtain a successful expansion [26].
Although metal foam production methods do not involve very complex process steps, when the process parameters and the material content (alloying elements, hard particles, blowing agent, etc.) are not precisely controlled, the quality of the product obtained changes significantly [27]. As mentioned previously, the impact of the compression process on foaming in indirect foaming methods can be given as an example.
Another example is the need to ensure that gas bubbles or blowing agent particles sent into the liquid metal are dispersed homogeneously in direct foaming methods. One method developed to enable homogeneous distribution of the blowing agent is two-stage foaming. In one version of this method, the matrix metal is brought to a temperature above its solidus temperature but below the decomposition temperature of the blowing agent and mixing is performed. Afterward, foaming occurs when the intermediate product (can be called precursor material), obtained by cooling and shaping in molds, is brought to about melting temperature [10]. In another version (FORMGRIP), an Al-based matrix is brought to a temperature above its melting temperature and TiH2 (blowing agent) is mixed in pretreated (oxidized) condition, ensuring limited decomposition. Casting and cooling take place thereafter, yielding a precursor material. The second stage is similar to that of the former version [28]. In fact, these two two-stage foaming versions, which are modifications of direct foaming, turn into indirect foaming methods since the metal foam is produced by baking a precursor. In another method developed for homogeneous distribution, a eutectic metal alloy with a low melting temperature is liquidized and the blowing agent is added into this liquid and mixing is performed. After solidification, a structure is formed in which the blowing agent is embedded. This structure is added homogeneously into a suitable liquid metal (with a higher melting temperature) and foaming occurs, achieving the desired alloy foam. As a result, a more homogeneous foam structure is attained [10].
On the other hand, it is an advantage that the mixture can be prepared at room temperature before melting in the indirect method; but the decomposition of the blowing agent before the metal matrix melts is a problematic situation that needs improvement. For this reason, the melting temperature of the metal matrix and the decomposition temperature of the blowing agent are tried to be brought as close as possible [29]. Addition of alloying elements (lowering the melting temperature) and pretreatment of the blowing agent (providing oxidation by means of annealing, thus, increasing the decomposition temperature) are two examples for this purpose [30].
The properties such as type, size, and ratio of oxides that will provide stability should also be carefully selected. While the ratio is too low, it decreases the stability of the structure; while being too high makes the material difficult to expand by increasing the viscosity too much. In addition, using a very high oxide ratio increases the brittleness of the product obtained. While too small particle sizes make mixing difficult, too big sizes make it difficult to cover the bubbles and cause the stability to decrease [11].
After the maximum expansion is achieved, the foam structure deteriorates within seconds. For this reason, the foaming time is also an important parameter that needs to be carefully adjusted. The final density and foam structure, arising from the production parameters (foaming time, foaming temperature, mixing time, etc.), determine the mechanical, thermal, acoustic performances of the product.
Direct foaming methods are the most suitable ones for mass production. The materials produced in the form of sheets, slabs, or blocks are used as they are or after they are processed into the desired shape by secondary operations [10]. Alternatively, the desired shape can be obtained by guiding the material into a mold in the foaming stage (either by the action of the buoyant force [16] or by using a customized injection machine [31]). Indirect foaming method (foaming powder compact precursors) could also be used in mass production effectively by employing continuous furnaces, which would involve conveyors to transfer precursors from foaming to cooling stages [10].
Sandwich structures (e.g., AFS) can be produced by placing metal foam materials (in the form of slabs or sheets, produced by direct or indirect methods) between two metal sheets (aluminum or steel). One way to construct sandwich products is using adhesives [32]. In an alternative way, two metal sheets are rolled by placing a precursor material between them, subsequently, they are shaped by deep drawing if necessary, and finally, the precursor is foamed as a result of heating. This method forms a metallurgical bond between the sheets and the metal foam core [10].
An application applied in the indirect foaming method is to foam the precursor inside a hollow structure, which also results in a metallurgical bond [33]. Using precursor materials with a shape adapting the shape of the hollow structure ensures more successful filling of the cavities while foaming [10].
As stated previously, although open-cell metallic structures are not metal foams in the literal sense of the word, they are often considered in the metal foam material group. In addition to their functional properties, when compared to their alternatives, high strength, high melting point, and nonflammability properties make these materials preferred. Open-cell metal foams find applications in energy storage (e.g., battery electrodes, fuel cell membranes), thermal management (e.g., heat exchangers, radiative cooling systems), electromagnetic shielding, filtering, and so on [34].
Since the 1920s, a wide range of production processes has been developed for open-cell metal foams [35]. The intended application dictates the structure and the form of open-cell metal foam. Generally, to be produced in the desired form, open-cell metal foams need a pattern. Reticulated structures (sponges), space holders, and additive manufactured geometries are the most common patterns, which are made of mostly polymers. Additive manufacturing can even be used to produce directly open-cell metal foams themselves [9]. Some of the open-cell metal foam production methods will be described subsequently.
One of the common liquid state methods is investment casting, which generates a metallic copy of a polymer sponge. Wax could be used to increase the wall thicknesses of the sponge. The process is suitable for most of the metals [36]. It starts by trapping the polymer foam in a slurry, which will become the metal casting mold after dried. The material of this mold has to be heat resistant and should be selected such that the mold can easily be dissolved or dispersed at the end of the process. Then the composite structure, containing the polymer foam and the slurry, is dried and heated to remove the polymer foam by evaporation or melting, resulting in a structure with cavities. It serves as a mold in the metal casting stage. The mold may need to be fired to endure the casting temperature and pressure. In some evolved processes, these three operations can occur concurrently [37]. By the act of gravity, or in some cases by applying pressure, the target metal is filled into the mold cavities. Then the mold is dissolved with some chemicals or just water. Final product appears almost the same as the polymer sponge. The phases of the process are shown in Fig. 2.3. It is possible to obtain an open-cell metal foam with 80–97% porosity using this method [10].
Producing open-cell metal foams by investment casting [21]. Reprinted by permission from The Royal Society of Chemistry
Polymer foam in the method above may be replaced with lattice structure. Porous metals with such a regular lattice structure are also called periodic cellular metal. Polymer fragments may be glued together to form a lattice. The lattice can also be constructed on 3D printers with PLA, ABS, or other filaments. Since the structure of a lattice block is not stochastic like that of a polymer foam, it may be possible to manufacture metal foams with more consistent and repeatable mechanical properties. As a result of the regular pattern, it is quite easy to predict their behavior [38].
Producing open-cell metal foams with the method called supergravity infiltration is also a viable casting option (Fig. 2.4). The term “supergravity” represents the accelerations, which are much greater than gravitational acceleration (a >>> g), applied to the particles. In such a condition, mass and heat transfer, and reaction rates increase [39]. In supergravity infiltration method, supergravity fields are where the detrimental effect of surface tension in molten metal during infiltration can be minimized or even removed, yielding complete formation of the intended open-cell foam. The supergravity fields are generated by centrifuge effect. A mold made of plaster can be used. Increasing the gravity coefficient improves the product quality by allowing molten metal to penetrate into cavities more easily [40].
However, in this and similar methods, the metal foam can get deformed when releasing it from the mold. Using a dissolvable preform material such as NaCl is more practical. By leaching with water, the final foam can be obtained without damaging it [41].
One of the most cost-effective ways of open-cell metal foam production in liquid state is employing space holders. In this method, infiltration casting technique is used (Fig. 2.5). An essential step in this method is dissolving of the space holders in a solvent, which is water in most cases. Pearl grain sugar, saccharose [42], coarse grain salt, ultra-coarse granule sodium bicarbonate, tapioca starch [43], carbamide [44], potassium chloride [45, 46], potassium sorbate, potassium bromide [47], coarse ground calcium carbonate, magnesium sulfate can be used as space holders [9, 48,49,50]. To enable the molten metal to leak through the gaps; pressure, load, or vacuum may be used. The form and the volume of the porosity are generated by space holders. The contact points of the space holders provide the interconnections between the cells, which also make the removal of the space holders possible. A porosity of 65–80% can be achieved [51]. Space holders can cause corrosion, so it is critical to consider their compatibility with the metal foam material.
A process for producing open-cell metal foams by using space holders [52]. Reprinted by permission from Elsevier
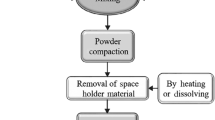
Solid-state processes for open-cell metal foam production include methods that combine powder metallurgy and the use of space holders (Fig. 2.6). First step is mixing the powders and space holders. The compression for the packaging process is then performed at a pressure that does not cause the space holders to break but slightly deforms them. If the space holders are polymers like cellulose, polyurethane, polyvinyl, or polystyrene [53], the compact is preheated to remove them from the structure before the sintering process, since evaporation of them at sintering temperature can cause structural damage [52]. If dissolvable space holders are preferred, materials mentioned in infiltration casting method could be used. The total porosity in this process is between 60 and 80% [10].
A process for producing open-cell metal foams by using sintering and space holders [55]. Reprinted by permission from Elsevier
Another powder-based process is the slurry method. The powder is transformed into a viscous slurry and coated on polymer reticulated sponge walls. The slurry is dried after being coated upon the sponge. At a proper temperature, the polymer is extracted and sintering is then performed to obtain a metal foam [54]. Since the structure is fragile, the operations should be carried out with caution. This process produces relatively poor metal foams, both in terms of strength and microstructure (Fig. 2.7).
A process to produce open-cell ASS/Austenitic Stainless Steel foams by slurry method [54]. Reprinted by permission from Elsevier
In vapor deposition method, a solid pattern structure is required to define the geometry of the metal foam. As polymer patterns, reticulated polyurethane foams or lattice blocks may be used. The basic principle is that metal particles in vapor phase condense on cold surfaces of a pattern structure in a vacuum chamber. There are several techniques to evaporate metals. Physical evaporation (resistance, inductive, arc, electron beam, laser techniques) or chemical evaporation can be utilized [9, 56]. The density and the application time of the metal vapor can be used to predict the thickness of the film. It is possible to produce nickel foams effectively by applying a coating at low temperatures (above 120 °C) using nickel tetracarbonyl gas, which decomposes at these temperatures and generates nickel (the coating material) and carbon monoxide [57]. After the coating process, the substrate is carefully removed (thermally or chemically). Performed concurrently or subsequently, a high-temperature sintering treatment is a common practice, aiming densification of the connections [58]. It is possible to obtain metal foams with a very high purity and cells with diameters of 0.4–6 mm. They have a macroscopic shape (Fig. 2.8) that is somewhat similar to that of electrodeposition foams, described subsequently. Incofoam is a commercially available nickel foam made in this manner [10].
A sample metal foam produced by vapor deposition [57]. Reprinted by permission from Wiley
Metal ions in an electrolyte solution are deposited on an open-cell polymeric foam in electrodeposition technique. The metal structure deposited replaces the polymer foam in the process. Metallic deposition methods do not work well with aluminum [9]. On the other hand, the electrodeposition method can be employed to coat an aluminum foam with copper [59]. The surface to be coated has to be conductive in order to achieve electrodeposition. Polymer pattern foams are covered in a graphite slurry or carbon black to obtain a thin conductive layer. Spraying the slurry onto the polymer pattern is another way to attain conductivity. The pattern foam acts as the cathode for the electrolyzed metal. In the electrolysis process, the metal is deposited on this cathode. As a final step, the polymer is removed from the finished product (Fig. 2.9). Generally, nickel or nickel–chromium alloys but also copper is preferred to be deposited in this way [60]. Retimet (Dunlop, UK), Celmet (Sumitomo Electric, Japan), and Recemat (SEAC, the Netherlands) are commercial foams produced by electrodeposition process [10].
A process for producing open-cell metal foams by electrodeposition. (This example does not include the common final step, namely, pattern removal) [61]. Under the Creative Commons license
Technological advances have resulted in significant improvements in additive manufacturing. Metal powders which are used in layered printing technology allow for direct printing of metal foams. Laser sintering or direct energy deposition with a powder bed may be used to produce metal foams. However, these processes are not effective in obtaining aluminum foams [62]. High reflectivity and thermal conductivity of aluminum make the operation complicated [9]. Studies have been conducted in which aluminum foams produced using laser powder bed fusion technique were compared to those produced by common methods [63]. Besides, patterns used in abovementioned open-cell foam production methods can be generated using additive manufacturing techniques. PLA, ABS, photosensitive resin, or another 3D printing material can be used.
For aluminum foams, Table 2.4 summarizes some representative properties.
2.3 Secondary Processing
Secondary operations of metal foams are of high significance since they help these materials to be applicable [68]. Thus, these processes, which turn metal foams into useful products, will be reviewed and the purpose of their use will be cited briefly.
Secondary operations of metal foams can be listed in five main groups:
-
Cutting
-
Forming
-
Joining
-
Finishing
-
Coating.
In cutting processes, it is desired that the metal foam maintains the form of its cells after the operation. The methods generally used in cutting metal foams can be given as band sawing, circular sawing, wire sawing, diamond sawing, chemical milling, EDM (electrical discharge machining), laser cutting, and water-jet cutting [67, 69,70,71,72]. While each method has some advantages, there are also some weaknesses and various difficulties. For instance, in EDM processes, it is possible to ensure that the cells do not deteriorate, but the cost and the speed of the operation should also be evaluated. As another example, if an open-cell metal foam press-fitted in a heat exchanger is considered, the wire-sawing method is more effective in reducing the macroscopic surface roughness (quality of the walls/struts regarding their distance to the cutting plane) compared to circular sawing, band sawing, and EDM (Fig. 2.10) [69].
Comparison of four cutting methods according to macroscopic surface roughness: Circular sawing (CS), wire sawing (WS), electrical discharge machining (EDM), band sawing (BS) [69]. Reprinted by permission from Elsevier
In a study where the laser cutting method was applied on 9 mm thick closed-cell aluminum foams, some problems due to overheating were encountered. However, it was concluded that laser cutting provided burr-free and parallel-sided cutting operations without damaging the cells [71].
As to forming, foaming of powder metal precursors in a mold or press-forming during foaming of precursors was carried out [73, 74]. Metal foam sheets can also be formed after foaming, in regard to ease of application and cost. To give the desired shape, various operations such as V-bending, roll forming, stamping, forging, and laser forming can be applied [32, 75,76,77]. Some forming process results for AFSs are given in Fig. 2.11.
Although bending, forging, and stamping processes are thought to deteriorate the foam structure substantially, foam core could maintain its cellular structure, if the process is applied carefully [32]. There are some potential defects that affect and limit the forming processes of foam sandwich panels. Core fracture/crack, delamination/debonding (at the core–face sheet interface), and face tearing can be listed as the most encountered ones (Fig. 2.12) [78, 79]. Joining techniques have an important role in the forming ability of metal foam sandwiches [80].
Various difficulties could be encountered when joining individual metal foams or metal foam sandwich panels. Generally, these difficulties arise from the deformation sensitivity of the foam and the limited surface area available for joining. Figure 2.13 shows the joint surface conditions for two sample joining operations.
For sandwich panels, three scenarios exist on joining: Joining two sandwich panels, joining a sandwich panel to another material, joining the face sheets with the foam core (adhesive or metallurgical joining). Joining the face sheets metallurgically with the foam core (e.g., by soldering) results in higher strengths against peeling, when compared to adhesive joining (Fig. 2.14). Some of the joining techniques (exemplified in Fig. 2.15) for metal foam structures in the literature are soldering, brazing, diffusion welding, friction stir welding, laser welding, bonding, and fastening (threaded, riveted, pinned) [78, 81,82,83,84,85,86,87,88,89,90,91].
Comparison of: (a) adhesive and (b) metallurgical joining [83]. Under the Creative Commons license
Some methods for joining metal foam structures [67]. Reprinted by permission from Elsevier
There are also innovative solutions such as joining two aluminum foam sheets by using a foamable filler (aluminum-silicon alloy), which was melted and foamed by applying concentrated solar energy [93]. Another example is a fluxless soldering method for closed-cell aluminum foams using surface self-abrasion (back and forth motion of the foams in the presence of a solder alloy between them) [92]. To choose an appropriate joining method, the load condition (axial tension/compression, bending, shear, peel, cleavage, or combined) and the material in use should be known. Results of tensile shear tests under oscillating and static loads for various joining methods applied to Al foam + metal sheet structures are given in Fig. 2.16.
Failure loads/stresses of Al foam + metal sheet structures for some joining methods [88]. Reprinted by permission from Wiley
Although welding or similar methods seem like ideal joining techniques for sandwich structures, they involve a heat input. Heat-applying methods can cause material structure to alter. Besides, these methods and also adhesive methods necessitate rigorous preparation steps. Other processes, such as inlays, require advanced manufacturing. These drawbacks could be eliminated by mechanical joining methods described in [89, 90]. In a study [90], the best mechanical joining solutions for sandwich structures have been proposed (Fig. 2.17).
The configurations proposed as the best ways for mechanical joining of sandwiches [90]. Reprinted by permission from SAGE
Some aluminum foam manufacturers claim that secondary operations are facilitated by manipulating the properties of the material. ALUHAB has been shown to be ideal for processes like slicing, milling, turning, and cogging since it does not disintegrate during the process. This type of metal foam is easy to drill, holds the screw steadily, and the wood screw can be driven into it without threading [94].
Burrs and other surface defects may occur as a result of the processes. These problems are a bit more complicated in metal foams. Because, when processed, negative burr formation (smearing) can be observed in metal foam structures. Surfaces with defects could cause the succeeding processes such as joining or coating to be unsuccessful. Edge quality is also an important property for product functionality, safety, price, and appearance. Increased or changed friction, increased wear on moving or stressed parts, interferences in assemblies, turbulent flow, and reduced formability are just a few of the issues which can be caused by poorly finished edges.
Generated burrs could get embedded into the cells of metal foam products, which would make it difficult to remove them. Therefore, achieving a surface with minimum burr formation is of high significance when machining metal foams. In this context, ultrasonic milling has been proposed to be an alternative to conventional milling, in a study [95] where ALUHAB specimens (a closed-cell aluminum foam, mentioned previously) were machined (Fig. 2.18).
Macroscopic comparison of conventional and ultrasonic milling in terms of smearing [95]. Reprinted by permission from Elsevier
Corrosion occurrence on metal foams may induce functionality losses. In some cases, electrical isolation or even aesthetic improvements may also be requested. For such reasons, metal foams may need to be coated with various materials and methods. The effect of coating on mechanical properties of metal foams was also investigated. For this purpose, closed-cell aluminum foam sheets were coated with various porcelain enamel coating systems and 4-point bending tests, also indentation tests were performed. The results indicated that the enamel coating strongly affected the Young’s modulus of elasticity of the foam sheets and enhance the stiffness of them. Bending tests showed that the cracks on the samples initiated at the points where the coating was thinner (Fig. 2.19) [96].
Crack initiations on enamel coated closed-cell aluminum foams [96]. Reprinted by permission from Taylor & Francis
Besides vitreous enamel coating mentioned previously, which provides resistance to both corrosive and thermal effects, an acrylic cataphoretic deposited paint, which has the ability to achieve a protective and homogeneous paint layer even with complex geometries, is also a viable option for metal foams. In a study, both these coatings prevented deterioration after an exposure of 850 h in accelerated corrosion tests. As to the uncoated samples, corrosion products were encountered after about 360 h. In the light of the findings, it is possible to conclude that both of the examined layers are ideal for protecting the aluminum foam [97].
2.4 Aircraft Applications
The use of aluminum foam material in the aircraft industry can bring along more efficient exploitation of material and energy resources, as well as the economic benefits of light weighting. Traditional parts such as stamped steel can be replaced with alternative parts generated using aluminum foam. These changes will serve the goal of lower fuel consumption, which is the priority of the aircraft [98]. For instance, every 1 kg of weight reduction in the fuselage provides a reduction of approximately one million Euros in 25 years in terms of fuel costs. This gain will increase as fossil fuel prices rise [50]. The use of aluminum, which was 81% by weight in the Boeing 747, decreased to 20% in the Boeing 787. The proportion of composites, on the other hand, has reached 50% [99].
In this context, sandwich composite structures, which can be shaped in three dimensions and contain metal foam core, can also fulfill the requirements of light weighting and safety [100]. Nowadays, besides aluminum honeycomb sandwich structures and aluminum + FRP/fiber-reinforced plastic structures in laminated form, AFS structures have also started to be used as flooring elements or interior fixture elements in aircrafts [1]. In addition, sandwich structures containing titanium foam and sandwich structures containing aluminum foam have been used by Boeing in the tail booms of aircrafts and helicopters [6].
Duocel foams, open-cell metal foams produced by ERG Aerospace Corporation, have some commercial implementations in aircraft industry. “Breather plugs” (used in Lockheed Martin C-130 Hercules military transport aircraft) for equalization of pressure during rapid changes in altitude and “air/oil separators” (used in Rolls Royce Trent 1000 turbofan engine of Boeing 787) for utilization in engine gearboxes are two examples (Fig. 2.20) [101].
Some commercial applications of Duocel (an open-cell metal foam) in aircraft industry: (a) In breather plugs and (b) in air/oil separators [101]. Reprinted by permission from ERG Aerospace Corporation
Multifunctional materials are economically advantageous. This advantage can reach the highest values in the aerospace applications [50]. In this context, sandwich structures, which contain metal foam and stand out with their low densities, not only meet mechanical requirements such as specific strength and energy absorption, but also have functions such as absorbing the vibration/sound and insulating heat, which are generated by the turbines. Their life-cycle costs are low; also recycling is possible for these materials [102]. It can be said that the long and costly validation of new materials and the current popularity of fiber-reinforced composites have slowed the spread of metal foam-containing materials in this industry [50].
The performance of AFS materials in mechanical energy absorption is remarkable due to the characteristic behavior of the aluminum foam material used in the core. While the aluminum foam material performing mechanical absorption, it operates at an almost constant stress value (plateau stress) during the large deformation experienced until it reaches its densification point. This feature makes it an effective material against crash and blast [102].
Where AFS materials can be used in the aerospace industry can be listed as follows: Luggage containers, premixed combustion chambers (as sound absorbers), heat sinks of electronic devices, aircraft elevators bringing aircrafts from the hangar to the takeoff area in aircraft carriers (as a structural element) [103], floor and drop ceilings, cargo pallets, passenger seat pallets [104]. In AFS panels, the face sheets and the core can be joined using adhesives or metallurgical bonding. For AFSs, using adhesives may be disadvantageous due to the maximum temperature resistance of approx. 220 °C, thermal expansion, moisture absorption, and low elasticity modulus [7].
In military aviation applications, aluminum foams (especially closed cell) can provide considerable improvements in the performance of some equipment. Examples are aircraft carrier deck, bulletproof composite armor, airborne buffer table, and lightweight missile manhole cover. When an AFS structure is employed as flight deck in an aircraft carrier (Fig. 2.21a), the rebound effect which occurs in aircraft landing is reduced by more than 50%. This secures a smooth glide and makes it possible that the tailhook of the aircraft engages the cable of the arresting gear system (located on the aircraft carrier) and the aircraft is arrested. It would also enhance the blast resistance and antimissile combat capability. AFSs can also be employed together with aluminum foam–filled columns, as shown in Fig. 2.21b. In heavy equipment airdrop missions, the equipment attached to a parachute is expected to land safely. Russian airborne equipment of 20 tons can be dropped in a smooth and safe manner by using a parachute and a buffer table involving aluminum foam–filled columns of 500 mm height [105].
Military applications: (a) Flight deck of an aircraft carrier and (b) buffer table for airborne equipment [105]. Under the Creative Commons license
Studies have been carried out on metal foam materials to absorb vibration and sound in aircraft. An active vibration control system has been introduced for an aerobatic aircraft wing using closed-cell aluminum foam structures in a study. They carried out both numerical and experimental tests building a scaled model (Fig. 2.22). The total wing was modeled virtually, including a velocity feedback control system. Two different materials were employed (glass fiber-reinforced epoxy and aluminum foam) as wing spars and compared with respect to the effectiveness of the controller. The use of aluminum foam for the wing’s spars decreased the wing’s settling time by 83% [106].
(a) Wing elements and (b) Al foam as substitute material for spars [106]. Under the Creative Commons license
The sound transmission loss characteristics of sandwich aircraft panels were examined in another research. They compared the performances of commonly used aerospace cores such as honeycomb, triangular, closed-cell aluminum foam, and Rohacell foam (a commercial PMI/PolyMethacrylimide based closed-cell foam) with various (aluminum, titanium, and epoxy carbon laminate) face sheets. It was stated that a desirable sound transmission loss level can be achieved by varying the density of foam and the type of face sheets [107].
Acoustic absorptive materials, withstanding high temperatures, were aimed to be developed in a study in order to reduce internal noise caused by turbo engines. As a result, two types of metallic materials were suggested: Open-cell metal foams and metallic fiber materials. It was determined that for open-cell foam, high porosity results in more space for sound waves to pass through the material, therefore, inducing less reflection and more absorption [108].
The use of open-cell foam materials as a means of reducing turbulent boundary-layer trailing edge noise has been suggested too (Fig. 2.23). The researchers studied the aeroacoustics of a porous trailing edge made of Ni–Cr–Al open-cell metal foam. A NACA 0018 airfoil was examined numerically with three different configurations of trailing edges: A baseline solid trailing edge, a completely porous trailing edge, and a blocked-porous version. They discovered two noise reduction mechanisms on a trailing edge of open-cell metal foam, allowing to improve the noise reduction capability even further [109]. Open-cell metal foams have also been used to control the flow and related aerodynamic noise in various other applications. They greatly decreased the aerodynamic noise produced by airfoils, tubes, aeroengines, and axial/centrifugal fans in experimental studies [110].
Porous trailing edge (TE) concept [109]. Reprinted by permission from Cambridge University Press
In aircrafts, metal foams can also be used against the effects of collisions with foreign objects. There is always a risk of a soft body impact (such as rubber) or a hard body impact (such as runway debris) in consequence of these collisions. Since the materials used on the exterior surfaces of aircrafts can vary depending on the area of use, different materials from metal to glass are studied in the researches about collisions with foreign objects [111]. In this context, especially for bird strikes, the aerospace industry has exploited cellular materials like metallic/organic foams and hollow sphere assemblies [112].
Due to their potential for severe damage and accidents, collisions between birds and an aircraft pose a significant threat to aviation, both in safety and cost issues. Average bird strike rates in civil aviation are given in Table 2.5. Large front components like the radome and nose, the leading edges, and the engines are being the target of the majority of bird strikes. Since 1960, 23 of the 30 accidents involving hull losses and casualties have occurred as a result of one or more engines struck by birds. An average of 101 h aircraft downtime per strike has been reported in the United States. Table 2.6 shows the reported repair and indirect costs arising from bird strikes in the United States from 1990 to 2018 [113].
Between 1990 and April 2021, the US Air Force recorded 244,341 bird/wildlife strikes on aircraft around the world, with a total cost of $779,892,099 for civilian and military aircraft [114]. The majority of bird strikes occurred while the aircrafts are takingoff or landing. To develop structures resisting these impacts, studies where the leading edges and leading edge flaps of the wings have been strengthened with closed-cell aluminum foam have been conducted (Fig. 2.24) [115]. In general, although the proportion of fiber-reinforced composites in aircraft fuselage construction has increased nowadays, metallic materials are still used in areas exposed to strikes by foreign objects [116].
(a) Leading edge and (b) an Al-foam-filled leading edge [117]. Reprinted by permission from Elsevier
An aircraft damaged from its radome shield is shown in Fig. 2.25. Although the radome shield was made of a honeycomb structure, it did not provide sufficient protection. In a study using AFS panels, experimental (by using real birds) and numerical investigations have been performed in order to determine the behavior of AFS panels against bird strike. The motivation of the study was that this type of a structure could be used to protect the radome bulkhead section from bird penetration into the cockpit. They tried various core (AlSi7Mg0.5 foam) thicknesses, core densities, and impact velocities (140–190 m/s) in order to compare the amount of penetration (Fig. 2.26) [111]. In another study, a gas gun was used to generate the impact of a bird strike [118].
Deformed states of sandwich panels after bird strike simulations (both experimental and numerical) for: (a) 0.15 g/cm3, (b) 0.3 g/cm3 core densities [111]. Reprinted by permission from Elsevier
Besides bird strikes, weather conditions can pose a threat to the safety of an aircraft. For instance, even a short transition from a hail zone can cause deteriorations such as depression, cracks, and holes. Therefore, important elements such as leading edge, radome, and engine can be damaged. In a numerical study, the mechanical performances of eight different sandwich structures were analyzed in a way that they were exposed to hail effect at different velocities. The core parts of sandwich structures consisted of different designs such as corrugated metal sheet, pyramidal lattice, and metal foam. As a result, the best design in terms of energy absorption has been the aluminum foam reinforced tetrahedral core [121].
A composite material comprising a stainless steel open-cell metal foam (S-S CMF) infused with a hydrophobic epoxy resin (Fig. 2.27) was introduced in a study. A vacuum-assisted thermal infusion process was employed. S-S CMF was produced using a powder metallurgy technique and infused thereafter. The final product was used as the leading edge of aircraft wings. The aim was to protect the edges from three-dimensional disturbances due to insect adhesion, ice accretion, and particle wear. This, in turn, would improve performance, safety, and fuel efficiency, with an overall product density similar to that of aluminum [122].
An image of an epoxy-infused S-S CMF composite developed [122]. Reprinted by permission from Elsevier
Another composite material involving a nonaluminum metal foam, but this time in sandwich form, was designed and produced to be used in aircrafts by a research group. It was obtained by a 17-4 PH/Precipitation-Hardened stainless steel open-cell foam (having high strength and high toughness) and two face sheets of the same material in dense form (Fig. 2.28). This composite structure was employed as an engine fan blade and compared to a solid titanium blade, with respect to vibration characteristics and design requirements. In the study, skin thickness and core volume were varied. The vibration analysis revealed that substantial weight/cost savings can be achieved without having unacceptable resonant frequencies. This innovative design exhibited a competitive performance while having a significantly lower cost and lightweight [123].
(a) A metal foam sandwich panel (17-4 PH stainless steel foam core + 17-4 PH face sheets). (b) The engine fan model where the sandwich panel was used and numerically analyzed [123]. Reprinted by permission from SAGE
As a different sort of exploitation, open-cell metal foams have been employed to generate electricity (micro-energy harvesting) from the surroundings of an aircraft. Based upon this idea, harvesting of thermal fluctuations in the fuselage during a flight and their transformation into electricity (by using thermoelectric effect) was modeled and simulated numerically, and reported by a researcher. The model involved a thermoelectric generator (TEG) and (as a heat reservoir) a phase change material (PCM) combined with each other, aiming to boost electricity generation. It was concluded that open-cell metallic foam inside the PCM enhanced the effective conductivity, allowed larger volumes of heat storage units, and increased the heat transfer between the TEG and PCM; thus, increased the electric power generation [124, 125].
2.5 Spacecraft Applications
Besides their multi-functionality, also the high temperatures and stresses in aerospace applications make metal foams feasible materials in spacecraft industry. Existing utilizations of the cylindrical shells involving metal foams in missile/spacecraft hulls, storage tanks, and nuclear reactor shells indicate the potential of these materials. There are many researches on buckling and vibration of these shell structures [4]. Metal foams are suitable to be used for mechanical energy absorbing in space vehicle landing pads and for reinforcement of load-bearing structures in satellites [10]. Moreover, metal foams could be employed in high-temperature applications such as transpiration-cooled rocket nozzles, heat shielding for exhaust [108], heat sinks, filtration, and PEM/proton-exchange membrane fuel cells [126]. Metal foams could play a greater role in the design of spacecrafts in the future [3].
For spacecrafts and satellites, there is always a collision risk with hypervelocity space debris and meteoroids. Critical components have to be protected against these impacts by using appropriate structures, without causing substantial increase in weight. In this context, hypervelocity impact tests of open-cell Al foams were performed in various studies, being supported by NASA and ESA [127].
When compared to metallic honeycombs, metal foams are competitive in mechanical performance and advantageous by not having channeling cells (which are impairing MMOD/micro-meteoroids and orbital debris shielding) [128]. The gas trapped in the porosities of a closed-cell foam could expand and burst the cell walls due to the changing ambient pressure in high amounts, during the spacecraft launch. These deformations could bring about unexpected results. Therefore, open-cell foams are better suited to the spacecraft components [127].
A modified version for the representative shielding element (against MMOD) of the ISS/International Space Station was developed employing an open-cell foam instead of honeycomb structure in a study. Total weight was kept equal with the original structure. The ballistic limit equation, which was derived by using the data from the hypervelocity impact tests conducted by the researchers, resulted that for 3 km/s, the critical projectile diameter increased by 15%. The performance gain was predicted to be higher with the increase in impact obliquity [129].
NASA published an article evaluating the shielding performance of sandwich panel configurations having open-cell aluminum foam core(s), comparing them with alternative sandwich panel configurations (aluminum honeycomb core, Trussgrid 3D aluminum honeycomb core, nonmetallic honeycomb core) (Fig. 2.29). The effects of foam parameters, core thickness, and face sheet thickness were examined, too. Also, a ballistic limit equation was developed for the foam core sandwich panels. According to this equation, at both normal and oblique strikes, the foam core panel resulted in a superior performance with respect to the honeycomb core panel [128].
The deformed states of three sandwich panel configurations, having a 2.0″ total thickness, after an impact of a 3.6 mm diameter projectile at ~6.8 km/s with 0°: With an open-cell Al foam core (40 pores per inches), with an Al honeycomb core, with a nonmetallic honeycomb core, respectively from left to right. (The upper images show the rear face sheet deformation) [128]. Courtesy of NASA
The effect of elevated temperatures on the penetration behavior of AFSs was evaluated in a numerical study. The developed model was validated with experiments and could be employed in aerospace structural designs. Numerical results showed that when increasing the temperature from room temperature to 300 °C, the decreases in energy absorption and peak force were 29.9% and 31.8%, respectively. Another result was that the decreases in these characteristics were linear when the numerical tests were carried out in various temperatures by increasing the temperature 100 °C each time [126].
Utilizing metal foam core sandwich panels, Pohltec Metalfoam manufactured a prototype of a conical adapter for the Ariane rocket. This prototype, having a diameter bigger than 4 m and including welded and curved AFS panels, exhibited an exceptional performance because of the good vibration damping property of AFS panels [130].
Ensuring an efficient heat dissipation in spacecrafts and space-based equipments (e.g., lunar probes) is a challenging issue. Heat sinks such as space radiators and water sublimators are employed for this task. Open-cell aluminum foam is an ideal solution for this type of applications [131].
An open-cell Al foam (Duocel) heat exchanger produced by ERG Aerospace Corporation has been used both for heat exchanging and air filtration in a space shuttle and ISS. Filtration was achieved by granulated chemicals supported by the Al foam so as to absorb and remove CO2 and moisture. The system rotates periodically to emit the CO2 and the moisture into space, therefore, functions without stopping servicing a long time. Duocel have also been utilized to provide heating and cooling uniformly in satellite cryogenic tanks, as well as isothermalization and baffling in solid cryogenic coolers of space-based infrared optical devices [50].
Radiation heat transfer is dominant in cooling of the systems located in spacecrafts. Passive cryogenic radiators having extended heat transfer surfaces are suitable for this condition. High porosity open-cell metal foam, possessing a low density and high surface area density, could be an alternative material (Fig. 2.30) to be used as a passive cryogenic radiator [132]. Interactive surface area for radiation is higher in open-cell metal foams, due to their high porosity. Heat transfer performances of a copper and an aluminum open-cell foam, each having a pore density of 20 PPI/Pores per Inch (with corresponding porosities of 94.9% and 90.3%, respectively) and intended to be used as passive cryogenic radiators, have been evaluated in a study. As a common practice, performance characteristic was taken as surface area/cooling capacity. According to the experimental and theoretical results, the aluminum foam was found to be more successful than the copper foam in cooling [133]. In a theoretical study, radiative cooling performance of aluminum foam fins (with a 20 PPI pore density and a 90% porosity) was concluded to be ten times higher, when compared to that of solid aluminum fins [134].
The concept of a radiative cooling system was designed and tested in a study [132]. Reprinted by permission from Taylor & Francis
Gamma-ray shielding in spacecrafts and satellites could be another application for metal foams. Open-cell metal foams have been proposed to be used instead of perforated beam screens, employed in high synchrotron radiation particle accelerators of the CERN Large Hydrogen Collider [135].
References
Gokhale, A. A., Prasad, E., & Basu, B. (2019). Light weighting for defense, aerospace, and transportation. Springer Nature.
Council, N. R. (2012). Application of lightweighting technology to military aircraft, vessels, and vehicles. National Academies Press.
Thoma, K., Schäfer, F., Hiermaier, S., & Schneider, E. (2004). An approach to achieve progress in spacecraft shielding. Advances in Space Research, 34(5), 1063–1075.
Toan Thang, P., Nguyen-Thoi, T., & Lee, J. (2020). Mechanical stability of metal foam cylindrical shells with various porosity distributions. Mechanics of Advanced Materials and Structures, 27(4), 295–303.
Selivanov, V. V., Silnikov, M. V., Markov, V. A., Popov, Y. V., & Pusev, V. I. (2021). Using highly porous aluminum alloys and honeycomb structures in spacecraft landing gear. Acta Astronautica, 180, 105–109.
Rajak, D. K., & Gupta, M. (2020). An insight into metal based foams: Processing, properties and applications. Springer.
Ubertalli, G., Ferraris, M., & Bangash, M. K. (2017). Joining of AL-6016 to Al-foam using Zn-based joining materials. Composites Part A: Applied Science and Manufacturing, 96, 122–128.
Wan, T., Liu, Y., Zhou, C., Chen, X., & Li, Y. (2021). Fabrication, properties, and applications of open-cell aluminum foams: A review. Journal of Materials Science and Technology, 62, 11–24.
Aluminium Foam. (2021). Retrieved from http://www.alupam.com/.
Banhart, J. (2001). Manufacture, characterisation and application of cellular metals and metal foams. Progress in Materials Science, 46(6), 559–632.
Banhart, J. (2000). Manufacturing routes for metallic foams. The Journal of The Minerals, Metals & Materials Society, 52, 22–27.
Szlancsik, A., Katona, B., Kemény, A., & Károly, D. (2019). On the filler materials of metal matrix syntactic foams. Materials, 12(12).
Atalay, S., Bayri, N., Kaya, H., İzgi, T., & Kolat, V. S. (2018). Al-Si-Mg foam produced by 3D printer. Adıyaman University Journal of Science, 8(1), 13–23.
Banhart, J., Stanzick, H., Helfen, L., & Baumbach, T. (2001). Metal foam evolution studied by synchrotron radioscopy. Applied Physics Letters, 78(8), 1152–1154.
Babcsán, N., Banhart, J., & Leitlmeier, D. (2003). Metal foams—Manufacture and physics of foaming. In Advanced metallic materials 2003: Proceedings of the international conference. Bratislava: Institute of Materials & Machine Mechanics Slovak Academy of Science.
Banhart, J. (2006). Metal foams: Production and stability. Advanced Engineering Materials, 8(9), 781–794.
Avinash, G., Harika, V., Sandeepika, C., Kumar, R., & Gupta, N. (2019). Porosity control in aluminium foams using different additives. Materials Today: Proceedings, 18, 1054–1057.
Rack, A., Helwig, H. M., Bütow, A., Rueda, A., Matijašević-Lux, B., Helfen, L., … Banhart, J. (2009). Early pore formation in aluminium foams studied by synchrotron-based microtomography and 3-D image analysis. Acta Materialia, 57(16), 4809–4821.
Banhart, J. (2000). Metallic foams: Challenges and opportunities. In Eurofoam2000 (pp. 13–20). MIT-Verlag Bremen.
Kevorkijan, V. (2010). Low cost aluminium foams made by CaCO3 particulates. Association of Metallurgical Engineers of Serbia AMES, 16(3), 205–219.
Kränzlin, N., & Niederberger, M. (2015). Controlled fabrication of porous metals from the nanometer to the macroscopic scale. Materials Horizons, 2(4), 359–377.
Uzun, A., & Turker, M. (2015). The effect Of B4C addition on pore morphology of the AlSi7 foams. Journal of the Faculty of Engineering and Architecture of Gazi University, 30(3), 523–532.
Soloki, A., & Esmailian, M. (2015). Carbonate-foaming agents in aluminum foams: Advantages and perspectives. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, 46(2), 1052–1057.
Kennedy, A. R. (2002). Effect of compaction density on foamability of Al-TiH2 powder compacts. Powder Metallurgy, 45(1), 75–79.
Ramirez, A. M. M., Vintila, R. R., & Drew, R. A. L. (2019). Morphology of aluminum alloy foams produced with dolomite via partial sintering of precursors. Materials, 12(10).
Asavavisithchai, S., & Kennedy, A. R. (2006). The effect of compaction method on the expansion and stability of aluminium foams. Advanced Engineering Materials, 8(9), 810–815.
Ensarioğlu, C., & Çakır, M. C. (2015). Investigating the Influence of Alloying Elements on the Foamability and the Morphology of Aluminum Foams. In The Advances in Materials and Processing Technologies (AMPT 2015). Madrid, Spain.
Gergely, V., Degischer, H. P., & Clyne, T. W. (2000). Recycling of MMCs and production of metallic foams. Comprehensive Composite Materials, 3, 797–820.
Matijasevic, B., & Banhart, J. (2006). Improvement of aluminium foam technology by tailoring of blowing agent. Scripta Materialia, 54(4 SPEC. ISS), 503–508.
Ensarioglu, C. (2014). Alüminyum Köpük Malzemelerin Toz Metalürjisi Yöntemi ile Üretiminde Üretim Parametrelerinin Malzemenin İşlenebilirligi Üzerindeki Etkilerinin İncelenmesi. Bursa Uludag University.
Koerner, C. (2008). Integral foam molding of light metals: Technology, foam physics and foam simulation. Springer.
Banhart, J., & Seeliger, H. W. (2008). Aluminium foam sandwich panels: Manufacture, metallurgy and applications. Advanced Engineering Materials, 10(9), 793–802.
Banhart, J. (2003). Aluminum foams: On the road to real applications. MRS Bulletin, 28(4), 290–295.
Shi, Z. L., & Szpunar, J. A. (2018). The introduction of thin open-cell metal foams and their wider engineering applications. Materials Science Forum, 933, 112–122.
Lefebvre, L. P., Banhart, J., & Dunand, D. C. (2008). Porous metals and metallic foams: Current status and recent developments. Advanced Engineering Materials, 10(9), 775–787.
Yamada, Y., Shimojima, K., Sakaguchi, Y., Mabuchi, M., Nakamura, M., Asahina, T., … Higashi, K. (1999). Processing of an open-cellular AZ91 magnesium alloy with a low density of 0.05 g/cm3. Journal of Materials Science Letters, 18, 1477–1480.
Fischer, S. F., Schüler, P., Fleck, C., & Bührig-Polaczek, A. (2013). Influence of the casting and mould temperatures on the (micro)structure and compression behaviour of investment-cast open-pore aluminium foams. Acta Materialia, 61(14), 5152–5161.
Carneiro, V. H., Rawson, S. D., Puga, H., Meireles, J., & Withers, P. J. (2020). Additive manufacturing assisted investment casting: A low-cost method to fabricate periodic metallic cellular lattices. Additive Manufacturing, 33.
Wen, X., Bao, Q., Guo, L., & Guo, Z. (2021). The introduction of super-gravity into optimization separation of bismuth and zinc from crude bismuth melt. Chemical Engineering and Processing—Process Intensification, 160.
Chang, K., Gao, J. T., Wang, Z., & Guo, Z. C. (2018). Manufacturing 3-D open-cell aluminum foam via infiltration casting in a super-gravity field. Journal of Materials Processing Technology, 252, 705–710.
Wang, Z., Gao, J., Chang, K., Meng, L., Zhang, N., & Guo, Z. (2018). Manufacturing of open-cell aluminum foams: Via infiltration casting in super-gravity fields and mechanical properties. RSC Advances, 8(29), 15933–15939.
Jakubowicz, J., Adamek, G., & Dewidar, M. (2013). Titanium foam made with saccharose as a space holder. Journal of Porous Materials, 20(5), 1137–1141.
Mansourighasri, A., Muhamad, N., & Sulong, A. B. (2012). Processing titanium foams using tapioca starch as a space holder. Journal of Materials Processing Technology, 212(1), 83–89.
Aida, S. F., Zuhailawati, H., & Anasyida, A. S. (2017). The effect of space holder content and sintering temperature of magnesium foam on microstructural and properties prepared by Sintering Dissolution Process (SDP) using carbamide space holder. Procedia Engineering, 184, 290–297.
Dehghan-Manshadi, A., Chen, Y., Shi, Z., Bermingham, M., StJohn, D., Dargusch, M., & Qian, M. (2018). Porous titanium scaffolds fabricated by metal injection moulding for biomedical applications. Materials, 11(9), 1–13.
Shbeh, M. M., & Goodall, R. (2016). Open pore titanium foams via metal injection molding of metal powder with a space holder. Metal Powder Report, 71(6), 450–455.
Mat Noor, F., Zain, M. I. M., Jamaludin, K. R., Hussin, R., Kamdi, Z., Ismail, A., … Taib, H. (2014). Potassium bromide as space holder for titanium foam preparation. Applied Mechanics and Materials, 465–466, 922–926.
Mondal, D. P., Barnwal, A., & Diwakar, V. (2017). Effect of strain rate and relative density on the compressive deformation of open cell Ti6Al alloy foam through P/M route. Journal of Applied Mechanical Engineering, 6(6).
Jain, H., Mondal, D. P., Gupta, G., & Kumar, R. (2021). Effect of compressive strain rate on the deformation behaviour of austenitic stainless steel foam produced by space holder technique. Materials Chemistry and Physics, 259.
García-Moreno, F. (2016). Commercial applications of metal foams: Their properties and production. Materials, 9(2), 20–24.
Kennedy, A. (2012, August). Porous metals and metal foams made from powders. Powder Metallurgy.
Wan, T., Liu, Y., Zhou, C., Ding, X., Chen, X., & Li, Y. (2021). Fabrication of high-porosity open-cell aluminum foam via high-temperature deformation of CaCl2 space-holders. Materials Letters, 284, 129018.
Zhu, J., Liu, J., & Yang, S. (2019). Ceramic foam filter and manufacturing method thereof.
Jain, H., Kumar, R., Gupta, G., & Mondal, D. P. (2020). Microstructure, mechanical and EMI shielding performance in open cell austenitic stainless steel foam made through PU foam template. Materials Chemistry and Physics, 241.
Zhao, Y. Y., & Sun, D. X. (2001). Novel sintering-dissolution process for manufacturing Al foams. Scripta Materialia, 44(1), 105–110.
Tian, Q. H., & Guo, X. Y. (2010). Electroless copper plating on microcellular polyurethane foam. Transactions of Nonferrous Metals Society of China (English Edition), 20, 283–287.
Poserin, V., Marcuson, S., Shu, J., & Wilkinson, D. S. (2004). CVD technique for Inco nickel foam production. Advanced Engineering Materials, 6(6), 454–459.
Queheillalt, D. T., Hass, D. D., Sypeck, D. J., & Wadley, H. N. G. (2001). Synthesis of open-cell metal foams by templated directed vapor deposition. Journal of Materials Research, 16(4), 1028–1036.
Antenucci, A., Guarino, S., Tagliaferri, V., & Ucciardello, N. (2015). Electro-deposition of graphene on aluminium open cell metal foams. Materials and Design, 71, 78–84.
Duan, D. L., Li, S., Ding, X. J., & Jiang, S. L. (2008). Preparation of Ni–Cr alloy foams by electrodeposition technique. Materials Science and Technology, 24(4), 461–466.
Felten, M., Fries, M., Pullen, A., Proud, W. G., & Jung, A. (2020). Investigation of strain-rate effects in Ni/PU hybrid foams under low-impact velocities. Advanced Engineering Materials, 22(7), 1–9.
Luo, H. (2019). Preparation of foamed aluminum and its application in China. Journal of Physics: Conference Series, 1347(1).
Matheson, K., Cross, K., Javahery, I., Plumb, J., & Spear, A. (2016). Comparison of conventional open-cell aluminum foam and its additively manufactured twin. Materials Science and Technology Conference and Exhibition 2016, MS and T 2016, 2, 745–752.
Gale, W. F., & Prorok, B. C. (2004). Non-conventional and emerging metallic materials. In Smithells metals reference book (Eighth.). Elsevier.
Marx, J., & Rabiei, A. (2017). Overview of composite metal foams and their properties and performance. Advanced Engineering Materials, 19(11), 1–13.
Akseli, I. (2005). The application of aluminum foam for the heat and noise reduction in automobiles. İzmir Institute of Technology.
Ashby, M. F., Evans, A. G., Fleck, N. A., Gibson, L. J., Hutchinson, J. W., & Wadley, H. N. G. (2000). Metal foams: A design guide.
Sığırtmaç, T., Çakır, M. C., Uğuz, A., & Ensarioğlu, C. (2012). Alüminyum Köpük Malzemelerin İkincil İşlemleri Ve Bu İşlemlerin Sonlu Elemanlar Yöntemi İle Modellenmesi. In 3. Ulusal Tasarım İmalat ve Analiz Kongresi (TİMAK) (pp. 160–174). Balıkesir, Turkey.
De Jaeger, P., T’Joen, C., Huisseune, H., Ameel, B., De Schampheleire, S., & De Paepe, M. (2012). Assessing the influence of four cutting methods on the thermal contact resistance of open-cell aluminum foam. International Journal of Heat and Mass Transfer, 55(21–22), 6142–6151.
Meng, K. P., Chai, C. G., Sun, Y. L., Wang, W., Wang, Q. Y., & Li, Q. M. (2019). Cutting-induced end surface effect on compressive behaviour of aluminium foams. European Journal of Mechanics/A Solids, 75, 410–418.
Yilbas, B. S. (2018). The laser cutting process. The laser cutting process: Analysis and applications. Elsevier.
Ozturk, M., & Dogan, B. (2019). Enhancement of heat exchangers with metal foams. World Journal of Environmental Research, 9(1), 15–28.
Banhart, J. (2013). Light-metal foams—History of innovation and technological challenges. Advanced Engineering Materials, 15(3), 82–111.
Hangai, Y., Ohashi, M., Nagahiro, R., Amagai, K., Tomaru, T., Utsunomiya, T., & Yoshkawa, N. (2019). Press forming of aluminum foam during foaming of precursor. Materials Transactions, 60(11), 2464–2469.
Changdar, A., & Chakraborty, S. S. (2021). Laser processing of metal foam—A review. Journal of Manufacturing Processes, 61, 208–225.
Paunoiu, V., Quadrini, F., Cataragiu, A., & Santo, L. (2015). Laser forming of aluminium metal foam panels. In The annals of “Dunarea De Jos” University Of Galati Fascicle V, Technologies in machine building.
Contorno, D., Filice, L., Fratini, L., & Micari, F. (2006). Forming of aluminum foam sandwich panels: Numerical simulations and experimental tests. Journal of Materials Processing Technology, 177(1–3), 364–367.
Zhang, J., Qin, Q., Han, X., & Ai, W. (2016). The initial plastic failure of fully clamped geometrical asymmetric metal foam core sandwich beams. Composites Part B: Engineering, 87, 233–244.
Weiss, M., Abeyrathna, B., & Pereira, M. (2018). Roll formability of aluminium foam sandwich panels. International Journal of Advanced Manufacturing Technology, 97(1–4), 953–965.
D’Urso, G., & Maccarini, G. (2012). The formability of aluminum foam sandwich panels. International Journal of Material Forming, 5(3), 243–257.
Chen, N., Feng, Y., Chen, J., Li, B., Chen, F., & Zhao, J. (2013). Vacuum brazing processes of aluminum foam. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Metal Materials and Engineering, 42(6), 1118–1122.
Khokhlov, M., Ishchenko, D., & Khokhlova, J. (2016). Peculiarities of forming diffusion bimetallic joints of aluminum foam with a monolithic magnesium alloy. Journal of Magnesium and Alloys, 4(4), 326–329.
Yao, C., Hu, Z., Mo, F., & Wang, Y. (2019). Fabrication and fatigue behavior of aluminum foam sandwich panel via liquid diffusion welding method. Metals, 9(5), 1–11.
Peng, P., Wang, K., Wang, W., Huang, L., Qiao, K., Che, Q., … Cai, J. (2019). High-performance aluminium foam sandwich prepared through friction stir welding. Materials Letters, 236, 295–298.
Bušić, M., Kožuh, Z., Klobčar, D., & Samardžić, I. (2016). Friction stir welding (FSW) of aluminium foam sandwich panels. Meta, 55(3), 473–476.
Banhart, J., Schmoll, C., & Neumann, U. (1998). Light-weight aluminium foam structures for ships. Proc. Conf. Materials in Oceanic Environment, 1, 55–63.
Nowacki, J., Krajewski, S., & Grabian, J. (2014). Problems of aluminum foam soldering. Przegląd Spawalnictwa—Welding Technology Review, 86(1).
Bernard, T., Bergmann, H. W., Haberling, C., & Haldenwanger, H. G. (2002). Joining technologies for Al-foam-Al-sheet compound structures. Advanced Engineering Materials, 4(10), 798–802.
Castanie, B., Bouvet, C., & Ginot, M. (2020). Review of composite sandwich structure in aeronautic applications. Composites Part C: Open Access, 1.
Feldhusen, J., Warkotsch, C., & Kempf, A. (2009). Development of a mechanical technology for joining sandwich elements. Journal of Sandwich Structures and Materials, 11(6), 471–486.
Joesbury, A. M. (2015). New approaches to composite metal joining. Cranfield University. Retrieved from https://dspace.lib.cranfield.ac.uk/handle/1826/10009
Wan, L., Huang, Y., Huang, T., Lv, Z., & Feng, J. (2016). Interfacial behavior and mechanical properties of aluminum foam joint fabricated by surface self-abrasion fluxless soldering. Journal of Alloys and Compounds, 671, 346–353.
Cambronero, L. E. G., Cañadas, I., Ruiz-Román, J. M., Cisneros, M., & Corpas Iglesias, F. A. (2014). Weld structure of joined aluminium foams with concentrated solar energy. Journal of Materials Processing Technology, 214(11), 2637–2643.
Babcsan, N., Beke, S., Makk, P., Szamel, G., & Kadar, C. (2014). Pilot production and properties of ALUHAB aluminium foams. Procedia Materials Science, 4(1997), 127–132.
Líska, J., Kun, K., & Líska, K. (2016). MMC materials ultrasonic machining and its economic aspects. Procedia Engineering, 149, 245–256.
Rossi, S., Bergamo, L., Calovi, M., & Fontanari, V. (2019). Effect of enamel coatings on the mechanical properties of aluminium foams. Mechanics of Advanced Materials and Structures, 26(13), 1130–1139.
Rossi, S., Fedel, M., Da Col, L., Deflorian, F., & Petrolli, S. (2017). Coatings to increase the corrosion behaviour of aluminium foam. Surface Engineering, 33(6), 405–409.
Huang, Y., Gong, J., Lv, S., Leng, J., & Li, Y. (2012). Fluxless soldering with surface abrasion for joining metal foams. Materials Science and Engineering A, 552, 283–287.
Gloria, A., Montanari, R., Richetta, M., & Varone, A. (2019). Alloys for aeronautic applications: State of the art and perspectives. Metals, 9(6), 1–26.
Duan, X., Dai, Z., Xu, R., Mao, R., & Song, B. (2019). The preparation methods and application of aluminum foam. In Light metals 2019 (pp. 501–504).
ERG Aerospace Applications. (2020). Retrieved from http://ergaerospace.com/applications.
Raeisi, S., Kadkhodapour, J., & Tovar, A. (2019). Mechanical properties and energy absorbing capabilities of Z-pinned aluminum foam sandwich. Composite Structures, 214, 34–46.
Lu, T. (2002). Ultralight porous metals: From fundamentals to applications. Acta Mechanica Sinica/Lixue Xuebao, 18(5), 457–479.
Oana, B., Florea, R. M., Buz, A., Roman, C., & Carcea, I. (2013). Manufacturing and characterization of stabilized aluminum foams. International Journal of Modern Manufacturing Technologies, V(1), 17–24.
Yao, G.-C., Luo, H.-J., & Cao, Z.-K. (2015). The manufacturing technology of aluminum foam material and some special equipments. In Proceedings of the 2015 international conference on material science and applications (ICMSA) (pp. 869–874). Atlantis.
Ali, A. A., Mahmood, H. Y., & Saeed, M. W. S. (2018). Implementation of vibration suppression on an aircraft wing using velocity feedback controller. Association of Arab Universities Journal of Engineering Sciences, 25(2), 45–64.
Arunkumar, M. P., Pitchaimani, J., Gangadharan, K. V., & Lenin Babu, M. C. (2017). Sound transmission loss characteristics of sandwich aircraft panels: Influence of nature of core. Journal of Sandwich Structures and Materials, 19(1), 26–48.
Paun, F., Gasser, S., & Leylekian, L. (2003). Design of materials for noise reduction in aircraft engines. Aerospace Science and Technology, 7(1), 63–72.
Teruna, C., Manegar, F., Avallone, F., Ragni, D., Casalino, D., & Carolus, T. (2020). Noise reduction mechanisms of an open-cell metal-foam trailing edge. Journal of Fluid Mechanics, 898.
Xu, C., Mao, Y., & Hu, Z. (2018). Numerical study of pore-scale flow and noise of an open cell metal foam. Aerospace Science and Technology, 82–83, 185–198.
Hanssen, A. G., Girard, Y., Olovsson, L., Berstad, T., & Langseth, M. (2006). A numerical model for bird strike of aluminium foam-based sandwich panels. International Journal of Impact Engineering, 32(7), 1127–1144.
Heimbs, S. (2012). Energy absorption in aircraft structures. In 1st international workshop on hydraulic equipment and support systems for mining (IWHEM), pp. 1–10. Retrieved from http://www.heimbs-online.de/Heimbs_2012_IWHEM.pdf.
Metz, I. C., Ellerbroek, J., Mühlhausen, T., Kügler, D., & Hoekstra, J. M. (2020). The bird strike challenge. Aerospace, 7(3), 1–20.
FAA Wildlife Strike Database. (2021). Retrieved from https://wildlife.faa.gov/search.
Liu, J., Li, Y., Yu, X., Gao, X., & Liu, Z. (2018). Design of aircraft structures against threat of bird strikes. Chinese Journal of Aeronautics, 31(7), 1535–1558.
Why are the B787 leading edges made from aluminum? (2020). Retrieved from https://aircrafttechnic.com/aviation_technology/why-are-the-b787-leading-edges-made-from-aluminum.
Reglero, J. A., Rodríguez-Peárez, M. A., Solórzano, E., & de Saja, J. A. (2011). Aluminium foams as a filler for leading edges: Improvements in the mechanical behaviour under bird strike impact tests. Materials and Design, 32(2), 907–910.
Zhou, J., Liu, J., Zhang, X., Yan, Y., Jiang, L., Mohagheghian, I., … Charalambides, M. N. (2019). Experimental and numerical investigation of high velocity soft impact loading on aircraft materials. Aerospace Science and Technology, 90, 44–58.
5 May 2015—Nevsehir (Turkey) THY B737. (2021). The Aviation Herald. Retrieved from http://avherald.com/h?article=485d4116.
3 December 2015 Bandar Seri Begawan bird strike. (2021). The Aviation Herald. Retrieved from http://avherald.com/h?article=490d1e37.
Li, S., Jin, F., Zhang, W., & Meng, X. (2016). Research of hail impact on aircraft wheel door with lattice hybrid structure. Journal of Physics: Conference Series, 744(1).
Robbins, S. J., Marx, J., Grady, Z. A., Rabiei, A., & Palmieri, F. L. (2019). Polymer-filled metal foams for contamination resistant aircraft leading edges.
Min, J. B., Ghosn, L. J., & Lerch, B. A. (2015). A study for stainless steel fan blade design with metal foam core. Journal of Sandwich Structures and Materials, 17(1), 56–73.
Madruga, S. (2021). Modeling of enhanced micro-energy harvesting of thermal ambient fluctuations with metallic foams embedded in Phase Change Materials. Renewable Energy, 168, 424–437.
Madruga, S. (2019). Thermoelectric energy harvesting in aircraft with porous phase change materials. IOP Conference Series: Earth and Environmental Science, 354(1).
Xi, H., Tang, L., Luo, S., Liu, Y., Jiang, Z., & Liu, Z. (2017). A numerical study of temperature effect on the penetration of aluminum foam sandwich panels under impact. Composites Part B: Engineering, 130, 217–229.
Zhang, X., Wang, R., Liu, J., Li, X., & Jia, G. (2018). A numerical method for the ballistic performance prediction of the sandwiched open cell aluminum foam under hypervelocity impact. Aerospace Science and Technology, 75, 254–260.
Ryan, S., & Christiansen, E. L. (2015). Hypervelocity impact testing of aluminum foam core sandwich panels (NASA/TM–2015–218593). Houston, TX, USA.
Ryan, S., Hedman, T., & Christiansen, E. L. (2010). Honeycomb vs. foam: Evaluating potential upgrades to ISS module shielding. Acta Astronautica, 67(7–8), 818–825.
Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., & Miller, R. (2018). Foam films and foams: Fundamentals and applications. CRC Press. Taylor & Francis Group.
Gao, L., Li, Y., Xu, H., Zhang, X., Yuan, M., & Ning, X. (2019). Numerical investigation on heat-transfer and hydromechanical performance inside contaminant-insensitive sublimators under a vacuum environment for spacecraft applications. Energies, 12(23).
Dixit, T., & Ghosh, I. (2018). Experimental and numerical modeling of metal foam passive radiator at low temperatures. Experimental Heat Transfer, 31(5), 425–435.
Dixit, T., & Ghosh, I. (2017). Cooling capacity of high porosity open-cell metal foams as passive cryogenic radiators. Cryogenics, 84, 81–88.
Tisha, D., & Indranil, G. (2017). Prospective of employing high porosity open-cell metal foams in passive cryogenic radiators for space applications. IOP Conference Series: Materials Science and Engineering, 171, 012048.
Dixit, T., & Ghosh, I. (2016). Radiation heat transfer in high porosity open-cell metal foams for cryogenic applications. Applied Thermal Engineering, 102, 942–951.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ensarioglu, C., Bakirci, A., Koluk, H., Cakir, M.C. (2022). Metal Foams and Their Applications in Aerospace Components. In: Kuşhan, M.C., Gürgen, S., Sofuoğlu, M.A. (eds) Materials, Structures and Manufacturing for Aircraft. Sustainable Aviation. Springer, Cham. https://doi.org/10.1007/978-3-030-91873-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-91873-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91872-9
Online ISBN: 978-3-030-91873-6
eBook Packages: EnergyEnergy (R0)