Abstract
Despite great advances in modeling and cancer therapy using optimal control theory, tumor heterogeneity and drug resistance are major obstacles in cancer treatments. Since recent biological studies demonstrated the evidence of tumor heterogeneity and assessed potential biological and clinical implications, tumor heterogeneity should be taken into account in the optimal control problem to improve treatment strategies. Here, first we study the effects of two different treatment strategies (i.e., symmetric and asymmetric) in a minimal two-population model to examine the long-term effects of these treatment methods on the system. Second, by considering tumor adaptation to treatment as a factor of the cost function, the optimal treatment strategy is derived. Numerical examples show that optimal treatment decreases tumor burden for the long-term by decreasing rate of tumor adaptation over time.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Optimal control theory has been applied to reduce tumor burden when treatment is applied to the system [1,2,3]. In general, these methods proposed mathematical models and focused on identifying the optimal treatment regime or strategy that can drive the tumor population to a desired level so as to penalize excessive usage of the drug or minimize drug resistance [4]. For instance, in [1], the authors considered cancer therapy with application of one drug and determined the optimal regime that minimized the tumor burden while maintaining the normal cell population above a prescribed level. In other studies, the optimal drug adjustment is proposed to minimize the number of cancerous cells by considering different controlled combinations of administering the chemotherapy agents [2] or a mathematical model of tumor-immune interactions with chemotherapy is proposed [3].
Despite recent advances in modeling and cancer therapy using optimal control theory, tumor heterogeneity continues to be a major barrier for the successful treatment of cancer [5]. Many biological studies reported experimental evidence for the existence of heterogeneity, discussed their impact on management of cancer and assessed potential biological and clinical implications [5,6,7]. Some studies proposed mathematical models to consider different cell population dynamics [8,9,10,11]. For instance, in [8], the authors proposed a state transition model of tumor cells and demonstrated different cell transition behavior across treatments to indicate how a tumor responds to treatments and is responsible for resistance.
To bridge the gap between the optimal control problem for minimizing tumor burden and understanding of tumor adaptation, tumor heterogeneity has been taken into account as an optimal control problem; an ordinary differential equation (ODE) model, which consists of sensitive and resistant cells to a certain drug, is proposed to determine drug administration schedules in order to avoid resistant population be dominant [12]. Although the authors considered reducing both resistant and sensitive sub-populations in their cost function, they did not explicitly consider drug-imposed selective pressures with respect to tumor heterogeneity. In [13], cell traits are considered to model how a resistant cell responds to a certain drug and are taken into account as levels of resistance in the cost function. The authors also reported that maximum tolerable dosage is not a good treatment strategy as it may lead to increase resistant cell population. In recent study [9], the authors modeled long-term effects of two different drug treatment methods; symmetric treatment method in which sub-population kill is equal and asymmetric treatment method that sub-population kill is unequal. Then, they performed simulation studies to analyze the effects of each parameter on therapeutic efficacy. Although they performed systematic simulation study with the sensitivity analysis by sweeping parameters to interrogate the effects of different drug-imposed selective pressures on long-term therapeutic outcome, it is limited to draw a fundamental understanding of the effect of differential selective pressure. Selective pressure is the influence exerted by drugs to promote one group of sub-population over another that may shift tumor heterogeneity distribution and generate resistance cells to the drug.
In this paper, motivated by [9], we first focus on a fundamental and principled understanding of the effect of differential selective treatments since they result in different tumor reduction rates over time and thus affect therapeutic outcome. Second, we formulate an optimal control problem to penalize a rate of tumor adaptation while minimizing tumor burden. Numerical simulations are introduced to demonstrate how tumor heterogeneity affects long-term effects with and without considering effects of differential selective treatments.
2 Background: Differential Selective Pressure Affects Long-Term Therapeutic Outcome
In the previous study [9], a simple two-population model has been studied to find out long-term effects of two different treatment regimes and demonstrated simulation result by showing the long-term effect of differential-imposed selective treatments. Such models are useful to show the general behaviour of biological systems. Herein, we summarize their work since we extend this study by focusing more theoretical analyses.
A minimal two-population was modeled as \((x_1, x_2)\) with distinctive growth rates \((k_1, k_2)\) and drug killing rates \((\alpha _1, \alpha _2)\) respectively [9]. The kinetics of the two sub-populations were modeled using a simple ODE for exponential growth as follows:
where drug treatment (d) is a Heaviside step function as shown in Fig. 1. In the problem setting [9], in order to examine long-term effects of two different treatment regimes, the authors assumed the same initial overall tumor growth and tumor reduction for the first treatment cycle (i.e., from \(t_1^{on}\) and \(t_1^{off}\) where \(t_1^{on} \) and \(t_1^{off}\) represent the start time point and the end time point of the first treatment respectively) of both symmetric and asymmetric treatment conditions. Thus, the boundary and constraint prior to treatment are followed by:
where \(x_1(0)\) and \(x_2(0)\) represent the initial sub-population sizes respectively and \(k_s\) represents a single overall growth rate. Thus, during the initial untreated growth phase of the tumor, the total tumor size is equivalent to a single overall growth rate.
Similarly, the boundary and constraint following first round of drug treatment satisfy the following condition which confirms that cell population is the same after the first treatment cycle:
where \(\varDelta T \triangleq (t_1^{off}-t_1^{on})\) represents treatment time interval and is assumed to be constant in this paper and \(\alpha _s\) represents the overall killing rate. Thus, after the first treatment, the differential killing of the sub-populations of asymmetric treatment should result in equivalent overall tumor burden reduction of symmetric treatment as per overall growth rate (\(k_s\)) and killing rate (\(\alpha _s\)). These constraints make sure that treatment methods have the same effects after first treatment cycle and then long-term effect can be evaluated after that. A simulation result showed that symmetric treatment (i.e., the same killing effect on the different tumor cell types) is more effective than asymmetric treatment (i.e., different killing effect on the different tumor cell types) as shown in Fig. 1.
3 Differential-Imposed Selective Treatments Result in Different Tumor Reduction Rates
In this section, motivated by the simulation study [9], we provide a theoretical analysis to interrogate the effects of different drug-imposed selective pressures and further consider how to integrate this information into treatment design. First, we consider a tumor reduction after each round in symmetric treatment.
Definition 1
A tumor reduction (TR) rate after each round can be defined as follows:
where \(TR_{k}\) represents a tumor reduction rate of the \(k^{th}\) drug cycle, \(x(t_k^{on})\) and \(x(t_{k}^{off})\) represent total tumor population at time step \(t_k^{on}\) and \(t_{k}^{off}\) respectively as shown in Fig. 1.
Lemma 1
For symmetric treatment (i.e., equal selective treatment), a tumor reduction after each round will be constant over time.
Proof
where \(\varDelta T \triangleq t_k^{off} - t_k^{on}\) is assumed to be constant over k and for symmetric treatment we assume that \(k_1 - \alpha _1 = k_2 - \alpha _2 = k_s - \alpha _s \) (i.e., tumor reduction is equal). Therefore, for symmetric treatment, a tumor reduction rate is constant as follows:
Next, we consider a tumor reduction rate in asymmetric treatment case.
Lemma 2
For asymmetric treatment (i.e., differential selective treatments), a tumor reduction rate after each round will decrease over time, i.e., \(TR_{k} ^{asym} > TR_{k+1}^{asym}\).
We need to show \( TR_{k}-TR_{k+1}>0 \) for asymmetric treatment. Tumor population can be calculated by solving Eq. (1) and the final inequality we need to prove is as follows: \(x (t_{k+1}^{off})\cdot x (t_k^{on})-x (t_k^{off}) \cdot x (t_{k+1}^{on})>0 \) and then we simply have the following to prove:
By simplifying this, we need to show whether \((k_2 - \alpha _2> k_1 - \alpha _1 ) \cdot (2 k_2 - \alpha _2 > 2k_1 - \alpha _1) \) is true. We will prove this by contradiction.
Proof
(Suppose not) \((k_2 - \alpha _2> k_1 - \alpha _1 ) \cdot (2 k_2 - \alpha _2 > 2k_1 - \alpha _1) \) is false. Then we consider two cases: A) \(k_2 - \alpha _2 > k_1 - \alpha _1\) and \( 2 k_2 - \alpha _2 \le 2k_1 - \alpha _1 \) or B) \(k_2 - \alpha _2 < k_1 - \alpha _1\) and \(2 k_2 - \alpha _2 \ge 2k_1 - \alpha _1\). Note that we do not have the equality condition (\(k_2 - \alpha _2 = k_1 - \alpha _1\)) as we consider asymmetric treatment case here.
From the boundary condition and constraint (i.e., the same initial overall tumor growth and tumor reduction for the first treatment), we have the following conditions:
where the first equation represents the same initial tumor burden and the second equation represents the same initial efficacy. If we rearrange and use compositions (i.e., divided by the total population) and divided by \(\exp (\varDelta T)\)):
where \(p_i^0 = \frac{x_i(0) }{ x_1(0) + x_2(0)}\), \(\sum _i p_i^0 = 1\) and we have the following:
Then, we have two cases: 1) \(2 k_1 - \alpha _1 > k_1 + k_s - \alpha _s \) and \( k_2 + k_s - \alpha _s > 2 k_2 - \alpha _2 \) or 2) \(2 k_1 - \alpha _1 < k_1 + k_s - \alpha _s \) and \( k_2 + k_s - \alpha _s < 2 k_2 - \alpha _2\). Note that we consider asymmetric condition and thus do not consider when the equation is equal to zero since it results in \(k_1 -\alpha _1 = k_s - \alpha _s = k_2 - \alpha _2\). Then we simply have the followings:
Also, we have
Since this should hold in general (i.e., for any \((p_1^0, p_2^0)\)), we could consider the case where \(k_1 = k_s = k_2\). Then, it is simple to show contradiction from the assumption, for instance, for case A), \( 2 k_2 - \alpha _2 > 2 k_1 - \alpha _1 \) (contradiction, \(\because \) \(2k_2 - \alpha _2 \le 2 k_1 - \alpha _1\)). Similarly, for case B), \( 2k_1 - \alpha _1 > 2 k_2 - \alpha _2\) (contradiction, \(\because \) \(2 k_2 - \alpha _2 \ge 2 k_1 - \alpha _1\)).
We consider the rate of change in tumor sensitivity (or rate of tumor adaptation) by taking the slope of the percent tumor reduction values for successive doses. In other words, the greater the decrease in tumor reduction, the more negative the rate of change in tumor sensitivity. We can define a rate of tumor adaptation, which refers to how quickly the population of composition changes, by taking the absolute value of this metric [9].
Definition 2
A rate of tumor adaption (TA) is defined as follows:
where \(TR_k\) and \(TR_{k+1}\) represent tumor reduction at the \(k^{th}\) and \((k+1)^{th}\) round of treatment.
Based on Lemma 1, this value for symmetric treatment is equal to zero. On the other hand, for asymmetric treatment regime, \(TR_{k+1}^{asym}\) is smaller than \(TR_k^{asym}\) and thus a rate of tumor adaptation increases; From Lemma 2, since \(TR_k^{asym}\) is always greater than \(TR_{k+1}^{asym}\), the greater the difference between \(TR_k^{asym}\) and \(TR_{k+1}^{asym}\), the value of tumor adaptation rate increases and thus the effectiveness of drug killing decreases.
Lemma 3
For symmetric treatment, a rate of tumor adaptation is zero but for asymmetric treatment, a rate of tumor adaption is positive (i.e., tumor reduction decreases for successive doses).
Proof
by Definition 2 and Lemma 1 and 2.
Theorem 1
With the same initial overall tumor size at the time of treatment and the same initial efficacy on the overall tumor, differential-imposed selective pressures on the individual sub-populations (i.e., asymmetric treatment) results in higher tumor burden in the long-term compared to symmetric treatment.
Proof
\(TR_1^{sym} = TR_1^{asym} \) by assumption (i.e., the same initial efficacy on the overall tumor) and Lemma 3 (i.e., a tumor reduction rate is constant in symmetric treatment but decreases over time in asymmetric treatment).
Thus, in the case where two different regimes (i.e., symmetric and asymmetric treatment) have the same initial efficacy on the overall tumor, differential selective pressures on the individual sub-populations lead to different drug sensitivities and result in long-term therapeutic outcome. Now the question is how we could use such results to design treatment strategy for controlling such system. To address this, we consider differential selective pressures as a factor of the cost function in the following section.
4 Differential Selective Pressures as a Factor of the Cost Function
Motivated by the effects of distinct drug selective pressures on long-term tumor response, we consider how to use this principled concept in treatment design that ultimately minimize relapse. In this section, we formulate an optimal control problem to enable better design of therapeutics by considering differential selective pressures as a factor of the cost function.
We consider a general form
where \(N_i\) represents the population of the i-th cell type. Then, we define a composition rate:
where \(N_T(t) = \sum _{j=1}^m N_j(t) \). The rate of composition change is as follows:
Lemma 4
For symmetric treatment, sub-population composition does not change over time.
Proof
For symmetric treatment, we have \( k_i - \alpha _i d = k_j - \alpha _j d\) where \(i \ne j\).
Thus, symmetric treatment condition guarantees \(\dot{p}_i(t) = 0~ \forall i\) (i.e., sufficient condition). To show that it is a necessary condition for \( \dot{p}_i(t) = 0 ~\forall i\), we consider the following lemma:
Lemma 5
If the following holds: \(\forall i\), if \(k_i - \alpha _i d - \sum _{j=1}^m (k_j - \alpha _j d) \cdot p_j(t) =0 \) (i.e., \(\dot{p}_i(t) = 0\)), then \(k_i - \alpha _i d = k_j - \alpha _j d \) where \(i \ne j\)).
Proof
(by induction)
Assuming that it is true for m, i.e., \(\forall i = \{1, \cdots m \} \), \(k_i - \alpha _i d - \sum _{j=1}^m (k_j - \alpha _j d) \cdot p_j(t) =0 \) implies \(k_i -\alpha _i d = k_j - \alpha _j d\) where \(i\ne j\). Then, we prove that it is true for \(m+1\):
Rearranging this equation:
Using the assumption that \( (k_i - \alpha _i d ) \cdot ( \sum _{j=1, j\ne i} ^ {m} p_j(t) ) = \sum _{j=1, j\ne i} ^ {m} (k_j - \alpha _j d) \cdot p_j(t) \) implies \(k_i - \alpha _i d = k_j - \alpha _j d \) where \(i\ne j \) and \(i = \{ 1, \cdots , m\}\). Then, we have
where \(i \ne m+1\) and thus \(k_i - \alpha _i d = k_{m+1} - \alpha _{m+1} d\).
Theorem 2
To avoid increasing rate of tumor adaptation, we need to satisfy \(\forall i, (k_i - \alpha _i d) = (k_j - \alpha _j d) \) where \(i\ne j\), i.e., conserve sub-population composition over time.
Proof
Now we define the objective function in the following form:
In this equation \(r_i\), \(q_i\) and \(s_j\) denote weighting factors of total population, population during treatment and control effort respectively. Then the optimization problem can be described with the constraints \( k_i -\alpha _i d = k_j - \alpha _j d\) for all i where \(i \ne j\) to avoid increasing rate of tumor adaptation and thus ultimately minimize tumor burden in the long term:
where we also consider the maximum drug effect (\(\alpha _{max}\)) as inequality conditions. By solving the optimization problem, we minimize the overall tumor burden while maintaining sub-population composition in order to minimize tumor adaptation.
5 Numerical Simulation Results and Discussion
In this section, we consider numerical simulations to demonstrate the effects of drug selective pressure by solving the optimization problem. To demonstrate this, we consider the system of equations (1) and solve optimization problem using Lagrangian method:

where \(\mu _i\), \(l_i\), \(b_i\) represent Lagrangian multiplier for equality condition and inequality condition respectively. Here \(S_i=1\) if \(\alpha _i-\alpha _{max}>0\) and \(S_i=0\) if \(\alpha _i-\alpha _{max} \le 0\). Similarly \(V_i=1\) if \(\alpha _i<0\) and \(V_i=0\) if \(\alpha _i \ge 0\). In a simple two-population model, the objective function is as follows:
Herein, we consider optimization variable \(\alpha _1\) as constant value for the simplicity. By increasing Lagrangian multipliers, equality and inequality conditions hold. In simulation study, we consider optimization problems with and without the equality constraint to demonstrate how penalizing different selective pressures affects tumor adaptation, sub-population composition changes and long term effect of treatment. We consider three different scenarios: 1) the same initial sub-populations with the same growth rate, 2) different initial sub-populations with the same growth rate, and 3) the same initial sub-populations with different growth rates.
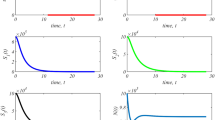
Simulation result when the initial condition and growth rates are the same for both sub-populations. (Left) Top figure shows the overall tumor population dynamics, middle figure shows sub-population dynamics and bottom figure shows sub-population ratio (\(\max (s_1, s_2) / \min (s_1, s_2))\). (Right) Tumor reduction (TR) rate after each round of treatment where TR is constant over time when tumor adaptation rate is considered in the objective function (top) and TR decreases over time when the tumor adaptation rate is not considered in the cost function (bottom).
Figure 2 (left) shows the first scenario with and without penalizing different selective pressures. The parameters in this case are as follows: \(x_1(0)=x_2(0)=0.5\), \(k_s=k_1=k_2=0.1\), \(\varDelta T=4\), \(\alpha _s=0.22\), \(\alpha _{max}=1\) and \(\alpha _2\) is obtained using Eq. (3) for no constraint case. Total tumor burden without constraint is higher than total tumor burden with constraint; In Fig. 2 (left-top), the red line shows the total population dynamics without considering constraint and we observe that sub-population composition changes over multiple rounds of drug treatment as shown in Fig. 2 (left-middle, bottom) and tumor reduction decreases after each round of treatment as shown in Fig. 2 (right-bottom). On the other hand, by conserving sub-population composition or rate of tumor adaptation, total tumor burden decreases more as shown in Fig. 2 (top) and tumor reduction does not change over time in successive drug treatment as shown in Fig. 2 (right-top). Note that sub-population ratio is conserved over time as shown in Fig. 2 (bottom) and thus tumor adaptation is zero.
Two additional simulation studies were performed to see different initial sub-population condition and the effect of different growth rate. Figure 3 (left) shows the effect of different initial sub-population conditions. All the parameters are the same as the previous case except the initial condition \(x_1(0)=0.65\) and \(x_2(0)=0.35\). Total tumor burden decreases more with constraint as shown in Fig. 3 (left-top) and sub-population ratio does not change over time as shown in Fig. 3 (left-bottom).
Figure 3 (right) shows the case with different growth rate (\(k_s=0.09\), \(k_1=0.11\)) where \(k_2\) is obtained by using equation (2). Total tumor burden decreases more by penalizing differential selective pressure as shown in Fig. 3 (right-top). Note that sub-population composition does not change when drug treatment is applied to the system but when drug is off, sub-population composition changes due to the different growth rates as shown in Fig. 3 (right-bottom) due to the different growth rates.
Throughout numerical simulation studies, we demonstrated that the constraint in the optimization problem enables to penalize different selective pressures and thus reduce the tumor burden by reducing long-term drug resistance or tumor adaptation.
6 Conclusion
In this paper, we consider tumor heterogeneity and selective pressure on sub-populations in the treatment design. By conserving sub-populations, we minimize tumor adaptation and thus reduce the long-term tumor burden. In future work, we will consider a more general form instead of using a simple two-population model to take mutations or cross-talk between each population into account which might decrease drug efficacy.
References
Matveev, A.S., Savkin, A.V.: Application of optimal control theory to analysis of cancer chemotherapy regimens. Syst. Control Lett. 46(5), 311–321 (2002)
Oke, S.I., Matadi, M.B., Xulu, S.S.: Optimal control analysis of a mathematical model for breast cancer. Math. Comput. Appl. 23(2), 21 (2018)
de Pillis, L.G., et al.: Chemotherapy for tumors: An analysis of the dynamics and a study of quadratic and linear optimal controls. Math. Biosci. 209(1), 292–315 (2007)
Boldrini, J.L., Costa, M.I.: Therapy burden, drug resistance, and optimal treatment regimen for cancer chemotherapy. Math. Med. Biol. 17(1), 33–51 (2000)
El-Sayes, N., Vito, A., Mossman, K.: Tumor heterogeneity: a great barrier in the age of cancer immunotherapy. Cancers 13(4), 806 (2021)
Martelotto, L.G., Ng, C.K., Piscuoglio, S., Weigelt, B., Reis-Filho, J.S.: Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 16(3), 1–11 (2014)
Marusyk, A., Polyak, K.: Tumor heterogeneity: causes and consequences. Biochim. Biophy. Acta (BBA)-Rev. Cancer 1805(1), 105–117 (2010)
Chapman, M.P., Risom, T., Aswani, A.J., Langer, E.M., Sears, R.C., Tomlin, C.J.: Modeling differentiation-state transitions linked to therapeutic escape in triple-negative breast cancer. PLoS Comput. Biol. 15(3), e1006840 (2019)
Sun, D., Dalin, S., Hemann, M.T., Lauffenburger, D.A., Zhao, B.: Differential selective pressure alters rate of drug resistance acquisition in heterogeneous tumor populations. Sci. Rep. 6(1), 1–13 (2016)
Zhao, B., Hemann, M.T., Lauffenburger, D.A.: Intratumor heterogeneity alters most effective drugs in designed combinations. Proc. Natl. Acad. Sci. 111(29), 10 773–10 778 (2014)
Zhao, B., Pritchard, J.R., Lauffenburger, D.A., Hemann, M.T.: Addressing genetic tumor heterogeneity through computationally predictive combination therapy. Cancer Discov. 4(2), 166–174 (2014)
Carrère, C.: Optimization of an in vitro chemotherapy to avoid resistant tumours. J. Theoret. Biol. 413, 24–33 (2017)
Ledzewicz, U., Wang, S., Schättler, H., André, N., Heng, M.A., Pasquier, E.: On drug resistance and metronomic chemotherapy: a mathematical modeling and optimal control approach. Math. Biosci. Eng. 14(1), 217 (2017)
Acknowledgement
This work was supported in part by the National Cancer Institute (U54CA209988).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this paper
Cite this paper
Ghodsi Asnaashari, T., Chang, Y.H. (2021). Strategies to Reduce Long-Term Drug Resistance by Considering Effects of Differential Selective Treatments. In: Bebis, G., Gaasterland, T., Kato, M., Kohandel, M., Wilkie, K. (eds) Mathematical and Computational Oncology. ISMCO 2021. Lecture Notes in Computer Science(), vol 13060. Springer, Cham. https://doi.org/10.1007/978-3-030-91241-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-91241-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91240-6
Online ISBN: 978-3-030-91241-3
eBook Packages: Computer ScienceComputer Science (R0)