Abstract
Understanding the biological effects of laser irradiation on human skin is a key to avoiding complications in laser treatment. The molecular effects of different ablative and non-ablative laser treatments on human skin cells—especially the direct effects on epidermal keratinocytes and dermal fibroblasts—are not yet fully understood. Therefore, to supplement the previous findings, which mostly came from clinical observations and histological examinations of patient skin biopsies, a novel in vitro 3D skin model for the investigation of effects of laser irradiation on human skin was developed, which for the first time enables a standardized investigation of time-dependent molecular changes after laser treatment. Using this 3D model system, morphological and molecular changes caused directly by fractional ablative CO2- or Er:YAG or non-ablative Er:glass laser treatment in human keratinocytes and fibroblasts at different points in time.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Biological Effects of Laser Treatment on the Human Skin
1.1 Ablative CO2 Laser
The CO2 laser has been increasingly used in dermatological surgery in recent decades and has become the gold standard in the treatment of a large number of skin changes. The development of the fractionated application has led to further aesthetic dermatological indications for CO2 laser and also offers the possibility of using it to rejuvenate light-aged skin and to revise scars. The ablative therapy of the skin by the fractionated CO2 laser leads, depending on the amount of energy used, to an erosion of the epidermis and parts of the upper dermis. Controlled thermal treatment of the skin induces wound healing stimulation, which in turn leads to the remodeling of the corium and reepithelialization of the epidermis.
Fractional CO2 laser systems have been successfully used to treat atrophic acne scars and in burn scars, skin rejuvenation, and laser-assisted drug delivery (LADD). The ablative CO2 laser treatment can also reduce the risk of non-melanocytic skin cancer after UV exposure. The first clinical investigations dealt with whether laser treatment leads to molecular changes in the human skin. Here, there was evidence of the resulting regulation of the expression of heat shock proteins and molecules associated with the extracellular matrix and collagen network. In these studies, however, mostly nonstandardized patient biopsies were examined using different techniques. The biopsies were always performed at the same time. Time-dependent effects of laser treatment on human skin have therefore not been fully elucidated so far.
Therefore, in one of our investigations organotypic human 3D skin models were treated with a fractionated, nonsequential, ultra-pulsed CO2 laser with different energy doses. In order to assess the morphological changes, histological examinations were subsequently performed at various points in time and microarray, and qRT-PCR analyses were performed to investigate the molecular effects of the laser treatment (Fig. 10.1). This showed that the fractionated CO2 laser treatment in the skin models led to dose-dependent morphological changes and an almost complete restoration of the epidermis 5 days after irradiation. On day 5 after laser treatment with an absorbed dose of 100 mJ/cm2, microarray analysis showed upregulation of genes associated with tissue remodeling and wound healing (e.g., COL12A1 and FGF7) as well as proinflammatory genes involved in immune response (e.g., CXCL12 and CCL8) and proteins of the heat shock family (e.g., HSPB3). In addition, the regulation of mRNA expression of matrix metalloproteinases (e.g., MMP3) and epidermal differentiation markers (e.g., LOR) decreased. These data were confirmed by an independently performed RT-PCR analysis; the regulation of CXCL12 was demonstrated at the protein level. In summary, the results showed that ablative CO2 laser treatment leads to morphological changes and regulation of the expression of various genes associated with epidermal differentiation, inflammation, and dermal remodeling. Interestingly, these results were even seen when no inflammatory mononuclear cells were present.
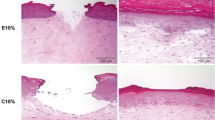
(a, b) Molecular effects of CO2-laser in a 3D skin model. (a) Histological sections of the laser-treated model show the wound healing process over a period of 5 days. (b) Chip-based gene expression analysis shows up- and downregulation of differentially expressed genes on day 5 after laser treatment with 100 mJ/cm2
1.2 Er:YAG Ablative Laser
Clinically, fractionated Er:YAG lasers are successfully used in indications similar to fractionated CO2 laser-assisted drug delivery (LADD) which is currently the focus of particular attention. It can significantly strengthen established methods, such as photodynamic therapy, in the future, especially for the treatment of actinic keratoses (grade II and III). It has already been described that treatment with the fractionated Er:YAG laser exerts an effect on the dermal extracellular matrix, which also leads to the formation of new collagen. One theory says that this effect could be explained by the intensity of the treatment and the resulting micro wounds or thermal effects. However, the exact underlying molecular effects remain unclear.
Therefore, the organotypic human 3D skin models with microlens array and different parameters were investigated at the same cumulative energy doses. To assess the morphological changes, histological examinations (Fig. 10.2) as well as microarray and qRT-PCR analyses were performed at different points in time analogous to the previous examination with the fractionated CO2 laser (Fig. 10.3). The 3D skin models were treated with different energy doses and settings recommended by the manufacturer. The following modes were selected: N10% (12 pulses, absorbed dose 5 J/cm2 pulse length 300 μs); E10% (6 pulses, energy density 10 J/cm2pulse length 300 μs); C10% (15 pulses, energy density 4 J/cm2pulse length 100 μs); and W25% (15 pulses, energy density 4 J/cm2pulse length 1000 μs). The cumulative energy dose of the laser treatment of the skin model was 60 J in all cases used.
This study showed that the fractionated Er:YAG laser treatment in the skin models resulted in dose-dependent morphological changes and an almost complete restoration of the epidermis 3 days after treatment. At the same time, there was a significant increase in mRNA expression of MMPs and their inhibitors (e.g., MMP1, MMP2, MMP3, TIMP1, and TIMP2) as well as chemokines (e.g., CXCL1, CXCL2, CXCL5, and CXCL6) and cytokines (e.g., IL-6, IL-8, and IL-24). In contrast, mRNA expression of epidermal differentiation markers such as keratin 4, filaggrin 1, filaggrin 2, and loricrin as well as antimicrobial peptides such as S100A7A, S100A9, and S100A12 was decreased. Interestingly, certain laser parameters (E10%; W25%) in these studies were associated with significantly higher gene regulation, while other parameters (N10%; C10%) did not show this effect. Pulse lengths of 1000 μs tended to lead to a stronger gene expression and showed a direct effect on the expression of collagen 1A2, 5A2, and 6A2. This can possibly be explained by the fact that this mode can also reach deeper dermal tissue layers and that the long pulse times also generate thermal effects that could play a role in the formation of collagen. Antimicrobial peptides such as S100A7 have antifibrotic effects and are significantly reduced in the tissue of keloids. The use of the Er:YAG laser seems to be rather unfavorable due to the described observations.
The study shows that time-dependent differences in gene expression exist, depending on the laser used and the settings used, which would allow the biological effect to be used more specifically or even predicted in the future.
1.3 Non-Ablative Er:Glass Laser
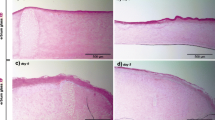
The Er:glass laser is used clinically, e.g., for scar treatment and for the treatment of stretch marks with the aim of achieving a deep dermal remodeling. Clinical improvements were also observed in melasma, female hair loss, and acne vulgaris. Studies of gene expression by a cDNA microarray 5 days after treatment of a 3D model showed increased regulation of epidermal differentiation markers such as loricrin and filaggrin 1 and 2 (Fig. 10.4). Proinflammatory chemokines such as CXCL1, CXCL2, CXCL5, and CXCL6 as well as interleukin IL-8 were predominantly decreased, as was caspase 14, which is involved in terminal differentiation of keratinocytes and thus in dermal remodeling.
(a, b) Molecular effects of the Er:glass laser with XD and XF lenses in a 3D skin model. (a) Histological sections of the laser-treated model show the wound healing process over a period of 5 days. (b) Chip-based gene expression analysis shows the up- and downregulation of differentially expressed genes on days 3 and 5 after laser treatment
It is known that chemokines (such as CXCLs) have chemotactic and activating functions on neutrophil granulocytes, which are particularly active in the acute inflammatory phase. The downregulation of chemokine expression as well as proinflammatory interleukins such as IL-6 and IL-8 shown in these investigations results in anti-inflammatory and differentiation-promoting effects for the Er:glass laser, which are important for the treatment of keloids or hypertrophic scars.
MMPs are also involved in the remodeling of the extracellular matrix, with this study showing an increased expression of MMP9 at mRNA and protein level. It is known that MMP9 can also be induced by compression therapy, which is beneficial in the treatment of keloids or hypertrophic scars. MMP3 is said to be responsible for fibroblast contraction and increased angiogenesis. Own investigations showed a decreased expression of MMP3 on mRNA and protein level. It follows from this that treatment with Er:glass lasers also has effects on the remodeling of the dermal extracellular matrix, which is mediated, among other things, by fine regulation of the expression of MMPs and chemokines.
1.3.1 Bottom Line
The molecular effect of fractionated nonsequential laser treatments on human skin has not yet been fully understood. Using a newly developed standardized 3D skin model, the effects of different laser systems and their settings on both skin morphology and gene expression during wound healing could be investigated in more detail. In vitro studies have shown that CO2 laser treatment of the 3D skin models led to histological changes and to the regulation of the expression of various genes associated with epidermal differentiation, inflammation, and dermal remodeling. In particular, the proinflammatory effect of the ablative CO2 laser systems seems to be advantageous in the treatment of atrophic or burn scars. Investigations into the biological effect of the ablative Er:YAG lasers showed that these lasers induce different gene regulations through different modes, which reveal similar proinflammatory properties as the fractionated CO2 lasers and are therefore also unsuitable for the treatment of keloids. In contrast to this the Er:glass laser with its anti-inflammatory and differentiation-promoting effect is better suited for the treatment of keloids or hypertrophic scars. Altogether, the studies on the standardized 3D skin model allow a better insight into the molecular effects of the different laser systems and thus a better understanding of the respective application possibilities. In the future, this could be used in a targeted manner to better predict treatment success.
Suggested Reading
Amann PM, Marquardt Y, Steiner T, et al. Effects of non-ablative fractional erbium glass laser treatment on gene regulation in human three-dimensional skin models. Lasers Med Sci. 2016;31:397–404.
Bullard KM, Mudgett J, Scheuenstuhl H, et al. Stromelysin-1-deficient fibroblasts display impaired contraction in vitro. J Surg Res. 1999;84:31–4.
Cho SB, Lee SJ, Cho S, et al. Non-ablative 1550-nm erbium-glass and ablative 10 600-nm carbon dioxide fractional lasers for acne scars: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol. 2010;24:921–5.
Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598–605.
Eckhart L, Declercq W, Ban J, et al. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol. 2000;115:1148–51.
Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35(3):171–5.
Gauglitz GG, Bureik D, Zwicker S, et al. The antimicrobial peptides psoriasin (S100A7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts. Skin Pharmacol Physiol. 2015;28:115–23.
Guimaraes PA, Haddad A, Sabino Neto M, et al. Striae distensae after breast augmentation: treatment using the nonablative fractionated 1550-nm erbium glass laser. Plastic Reconstr Surg. 2013;131:636–42.
Gye J, Ahn SK, Kwon JE, et al. Use of fractional CO2 laser decreases the risk of skin cancer development during ultraviolet exposure in hairless mice. Dermatol Surg. 2015;41:378–86.
Hantash BM, Bedi VP, Kapadia B, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39:96–107.
Helbig D, Paasch U. Molecular changes during skin aging and wound healing after fractional ablative photothermolysis. Skin Res Technol. 2011;17:119–28.
Helbig D, Mobius A, Simon JC, et al. Heat shock protein 70 expression patterns in dermal explants in response to ablative fractional phothothermolysis, microneedle, or scalpel wounding. Wounds. 2011;23:59–67.
Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before-after cohort study, with long-term follow-up. Ann Surg. 2014;260:519–29; discussion 529–32.
Ko DY, Jeon SY, Kim KH, et al. Fractional erbium: YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529–39.
Lee GY, Lee SJ, Kim WS. The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:1450–4.
Majid I, Imran S. Fractional CO2 laser resurfacing as monotherapy in the treatment of atrophic facial acne scars. J Cutan Aesthet Surg. 2014;7:87–92.
Moneib H, Tawfik AA, Youssef SS, et al. Randomized split-face controlled study to evaluate 1550-nm fractionated erbium glass laser for treatment of acne vulgaris—an image analysis evaluation. Dermatol Surg. 2014;40:1191–200.
Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49–60.
Orringer JS, Rittié L, Baker D, et al. Molecular mechanisms of nonablative fractionated laser resurfacing. Br J Dermatol. 2010;163:757–68.
Orringer JS, Rittie L, Hamilton T, et al. Intraepidermal erbium:YAG laser resurfacing: impact on the dermal matrix. J Am Acad Dermatol. 2011;64:119–28.
Orringer JS, Sachs DL, Shao Y, et al. Direct quantitative comparison of molecular responses in photodamaged human skin to fractionated and fully ablative carbon dioxide laser resurfacing. Dermatol Surg. 2012;38:1668–77.
Puri N. A study on fractional erbium glass laser therapy versus chemical peeling for the treatment of melasma in female patients. J Cutan Aesthet Surg. 2013;6:148–51.
Reno F, Grazianetti P, Stella M, et al. Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Arch Dermatol. 2002;138:475–8.
Sardana K, Manjhi M, Garg VK, et al. Which type of atrophic acne scar (ice-pick, boxcar, or rolling) responds to nonablative fractional laser therapy? Dermatol Surg. 2014;40:288–300.
Schmitt L, Amann PM, Marquardt Y, et al. Molecular effects of fractional ablative erbium:YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med Sci. 2017;32:805–14.
Sklar LR, Burnett CT, Waibel JS, et al. Laser assisted drug delivery: a review of an evolving technology. Lasers Surg Med. 2014;46:249–62.
Taudorf EH, Danielsen PL, Paulsen IF, et al. Non-ablative fractional laser provides long-term improvement of mature burn scars—a randomized controlled trial with histological assessment. Lasers Surg Med. 2015;47:141–7.
Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262–9.
Tretti Clementoni M, Galimberti M, Tourlaki A, et al. Random fractional ultrapulsed CO2 resurfacing of photodamaged facial skin: long-term evaluation. Lasers Med Sci. 2013;28:643–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Schmitt, L., Huth, S., Baron, J.M. (2022). Understanding the Biological Effects of Ablative and Non-Ablative Laser Systems in the Skin as Key to Avoiding Complications. In: Kautz, G. (eds) Energy for the Skin. Springer, Cham. https://doi.org/10.1007/978-3-030-90680-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-90680-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90679-5
Online ISBN: 978-3-030-90680-1
eBook Packages: MedicineMedicine (R0)