Abstract
Exposure to challenging experiences during development, such as reduced parental care and food availability, can have profound effects on the adult phenotype with far-ranging consequences for individual performance. Traditionally, such early-life adversities have been assumed to lead to detrimental consequences for health and survival. Growing empirical evidence, however, pin point that early-life stress exposure can also promote adaptive coping mechanisms of resistance and resilience, and have beneficial long-lasting effects. Developmental timing, type, and severity of early-life stress exposure are hypothesized to be key features underlying subsequent phenotypic outcomes. In this book chapter, we provide an overview of the main molecular mechanisms and signals that may be driving the emergence of subsequent stress vulnerability or resilience. We focus on the actions of glucocorticoid hormones in shaping adult physiological stress responses, and in organizing key cellular and molecular mechanisms underlying senescence and life-history evolution, including telomeres, oxidative stress, and epigenetics. Finally, we critically appraise and identify gaps in our current knowledge and provide directions for future research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The responses of an organism to early-life environmental conditions can have long-term effects on morphology, physiology, and behavior, potentially persisting for the whole lifespan and beyond one generation (Monaghan 2008). Organisms are currently exposed to growing environmental pressures including increased urbanization, habitat fragmentation, and climate changes due to global warming (Loarie et al. 2009; Bellard et al. 2012). Understanding how these challenges influence individual’s life history trajectories, and over what life stage effects are most likely to result in long-lasting phenotypic changes is a major research priority (Romero et al. 2015; Slavich 2016).
Animals evolved endogenous systems to appropriately respond to stressful conditions and return to homeostasis as fast as possible. In vertebrates, stress responses involve a highly conserved suite of molecular, physiological, and behavioral changes that are essential for promoting immediate survival strategies (Wingfield et al. 1998; Sapolsky 2000). But here the inevitable question—what is “stress”? Hans Selye, the “father of stress,” once said, “Everybody knows what stress is, but nobody really knows what it is” (Selye 1973). The scientific definition of “stress” continues to be hotly debated. This is mainly due to the difficulties in rigorously defining the stimuli causing stress exposure (“stressors”), the emergency responses activated by these stimuli, and the pathological consequences associated with overstimulation of the emergency responses (Mcewen and Wingfield 2003; Romero et al. 2009). In this book chapter, we use the term “stress” to broadly refer to the activation of conserved stress response systems, i.e., neuroendocrine, endocrine, and metabolic responses to noxious stimuli, or stressors, to maintain or recover physiological homeostasis. We refer to “early-life stress” to indicate different kinds of challenges or adversities sexually immature/developing individuals might be exposed to, including, but not limited to, nutritional restrictions, limited parental resources, social competition, predation pressures, extreme temperatures, or pollutants (Romero et al. 2015; Sapolsky 2015).
Epidemiological evidence showed substantial links between various forms of early-life stressors, including intrauterine growth restriction, harsh socio-economic conditions, and increased propensity to the emergence of adult-diseased phenotypes (Barker et al. 1990, 1993; Cottrell and Seckl 2009). A notorious example is the study of the long-term effects of the Dutch Hunger winter in 1944–1945 (in which daily rations were limited up to 1000 kilocalories per day). Individuals exposed to the famine during the pre- and peri-natal period had increased risk to develop obesity, diabetes, and coronary heart disease in adulthood (Ravelli et al. 1999; Roseboom et al. 2001; Painter et al. 2005, 2006). Moreover, individuals exposed to prenatal stressors were found to be at increased risk of neurodevelopmental and behavioral health issues, such as depression, schizophrenia, and autism spectrum disorder (Khashan et al. 2008; Kinney et al. 2008; Markham and Koenig 2011). These studies contributed enormously to the foundation of the Developmental Origins of Health and Disease (DOHaD)” hypothesis (originally termed “Fetal Origins of Adult Disease”—Hales et al. 1991). DOHaD postulates that adverse conditions experienced during the pre- and early post-natal period lead to subsequent increased morbidity and mortality. There is, however, a growing body of empirical work on a range of taxa, especially within the fields of eco-devo research, suggesting that developmental stress can also result in long-lasting phenotypic adaptations that may promote resilience, thus increase capability to cope with subsequent stressors (reviews: Monaghan 2008; Sih 2011; Langenhof and Komdeur 2018). These studies challenge the predominantly biased negative connotation of early-life stress on fitness outcomes and open empirical plausibility through which certain stressors might optimize individual coping strategies depending on future environmental circumstances (Gluckman et al. 2005, 2007).
We still have a poor understanding of the endogenous mechanisms through which exposure to developmental stress might lead to positive or negative fitness outcomes. Two key aspects in this context are: Which processes embed early-life experiences into molecular changes and signals? Which are the main molecular mechanisms regulating such organizational effects and how do they alter subsequent stress vulnerability and resilience? Endocrine systems are undoubtedly excellent candidates as modulators of developmental plasticity. Hormones influence a large number of processes across the entire lifespan and their pleiotropic effects can mediate variation in life histories. The influence of hormones on phenotypic traits is known to be particularly powerful during early development when they exert organizational effects on physiology or anatomy with long-lasting consequences on subsequent adult behaviors and lifestyles (Arnold 2009; Nugent et al. 2012). Glucocorticoid hormones, controlled by the Hypothalamic–Pituitary–Adrenal (or Interrenal) axis (HPA axis), are key mediators of the vertebrate stress response and fundamental candidates linking coping behaviors to environmental challenges, such as inclement weather and food availability (Sapolsky 1992; de Bruijn and Romero 2018). Thus, changes in the functioning of the HPA axis, for instance through a re-setting of HPA axis sensitivity during ontogeny, are thought to be a key mechanism underlying the links between early-life adversity and long-term health and adult-disease risk (Welberg and Seckl 2001; Seckl 2004; Meaney et al. 2007; Cottrell and Seckl 2009; Harris and Seckl 2011). Although other hormones have also substantial effects on the phenotype programming (e.g., sex steroids and thyroid hormones), we purposely focus on glucocorticoids because (i) we have a larger body of experimental work in both laboratory and wild settings, and (ii) emerging evidence suggests a role of these hormones in organizing important cellular mechanisms underlying senescence and life-history evolution, such as telomere dynamics and oxidative stress (Price et al. 2013; Angelier et al. 2018; Ridout et al. 2018). Telomeres shorten with age in many studied organisms with steep declines often being observed during early development (Heidinger et al. 2012; Angelier et al. 2018). Importantly, telomere length and rates of telomere shortening appear to be in some circumstances good predictors of individual’s quality and subsequent longevity (Cawthon et al. 2003; Heidinger et al. 2012; Wilbourn et al. 2018). Moreover, telomeres are influenced by various developmental stressors associated with changes in growth trajectories or parental care (Boonekamp et al. 2014; Marchetto et al. 2016; Monaghan and Ozanne 2018), and exposure to environmental stressors that cause oxidative stress fosters telomere attrition (Hau et al. 2015; Reichert and Stier 2017; Casagrande and Hau 2019). Thus, telomere length and dynamics have been considered to act as biomarkers of “biological age” and of exposure to environmental challenges. Oxidative stress refers to any changes in cellular oxidative status, which involve oxidation products (oxidative damage), nonenzymatic and enzymatic antioxidants, or repair mechanisms, which may potentially impinge on fitness-related metrics or on molecular mechanisms driving senescence, such as telomere length (Costantini 2019). Measurements of telomere dynamics and oxidative status markers have usually been used to trace the effects of challenging developmental conditions. However, they might also be important modulators of cellular signalling, thus they could potentially orchestrate some of the programming effects of early-life stress.
In this book chapter, we focus on the three inter-linked key endogenous mechanisms that could orchestrate the organizational effects of early-life stress: HPA axis functioning, telomere dynamics and oxidative stress, and epigenetic changes. We focus on mammals and birds in particular due to the larger body of literature, but the mechanisms and theories we discussed are valid across all vertebrate taxa.
2 Roles of Developmental System, Timing, and Stressor Type
Early-life stress experiments in animals allow for well-directed environmental manipulations during specific phases of pre- and/or post-natal development. Although the effects of early-life stress are examined in numerous different species including livestock and nonhuman primates (e.g., Abbott et al. 2008; Reynolds et al. 2010), most of the experimental studies use rodents as model systems. This is primarily due to feasibility as rodents are easy to house and handle together with much lower costs compared to primates.
As mammals depend upon the mother during prenatal development and also need intensive postnatal maternal care for normal development, early-life stress paradigms are typically based upon manipulations of maternal physiology and behavior. By cross-fostering of pups to control mothers or nursery rear it can be established whether the found effects are caused by particular pre- and/or postnatal events (Glover et al. 2010). In prenatal models, maternal stress or glucocorticoid administration is transferred via the placenta from mother to the developing fetus (Seckl 2001). In rats and mice, prenatal stress is typically imposed by restraint of the pregnant dam or administration of exogenous glucocorticoids during pregnancy. The mother is the key figure of early postnatal development in mammals as well. In postnatal stress models, stress experienced by the offspring is typically caused by manipulations of maternal behavior. In rodents, for instance, maternal care not only involves lactation but also offering of an adequate nest and specific behaviors, such as nursing, licking, and grooming, that provide important sensory input to the pups. Postnatal stress paradigms therefore most often involve prevention or disturbance of maternal care via temporary maternal separation from the offspring or allocating the dam with insufficient nesting material. Accordingly, the most common approaches of postnatal stress exposure are the maternal separation model and providing limiting nesting material. Importantly, maternal separation protocols vary greatly among studies depending on the frequency and duration of the separation episode as well as the specific age for separation. A review on the different experimental maternal separation protocols is beyond the scope of the present chapter. However, as a general rule, the greater is the frequency and duration of the separation episodes the greater the severity of the stress exposure (Parker and Maestripieri 2011).
More recently, birds have been employed as study systems to assess how early-life stress shapes an individual’s life history strategy within different eco-physiological contexts. Being egg-laying species, they offer the possibility to experimentally tease out pre- versus post-natal effects. In addition the reduced physiological intimacy between the developing bird and the mother as compared to mammals allows minimizing potential compensatory effects of the parents on the growing offspring (Love and Williams 2008; Spencer et al. 2009; Henriksen et al. 2011; Schoech et al. 2011; Marasco et al. 2012; Zimmer et al. 2013). One of the mostly used prenatal stress paradigms in birds is direct glucocorticoid injection in the yolk of the fertile egg soon after laying. Work in different species found that maternally derived yolk glucocorticoids reflect female condition and the environment to which females are exposed to at reproduction and during egg formation (Hayward and Wingfield 2004; Saino et al. 2005). Postnatal stress paradigms in the avian literature are in general more varied than in mammalian models. Apart from direct glucocorticoid treatment that is generally accomplished through oral dosing (Spencer et al. 2009), implants (Hayward and Wingfield 2004), or dermal patches (Wada and Breuner 2008), researchers have also used manipulations of brood size, sibling competition, ectoparasites exposure, predator cues, and food availability (reviewed by Schoech et al. 2011; Crino and Breuner 2015) as a way to increase stress levels in a developing bird within ecologically relevant contexts. Importantly, in highly precocial birds, such as domestic chickens and quails in which eggs are artificially incubated in the lab and no maternal care is provided to the chicks, the effects of postnatal stressors can be assessed excluding the possibility that parents would compensate for them as known to happen in rodent models.
Early stress paradigms are various, using different types of stressors with different intensity/duration and at different developmental stages. In this context, the comparisons of stress effects using direct hormonal administration of glucocorticoids or indirect manipulations of developmental stress exposure (e.g., changes in food availability and/or parental care) are often discussed. As argued in Crino and Breuner (2015), direct glucocorticoid treatment offers high control of the amount of stress applied and influences one component of a complex internal system. On the other side, indirect manipulations offer less experimental control as they alter multiple components of a complex pathway (regulation of energy availability) but are likely to be a better representation of naturally relevant conditions. For example, as a direct glucocorticoid injection in the yolk, exposing laying females to environmental stressors can induce an increase of corticosterone levels in their eggs, but higher variations among differing egg hormonal contents can also occur in relation to individual sensitivity of females to stressors or the matching between stressor timing and egg formation (Henriksen et al. 2011). However, when using indirect paradigms, other egg components could be modulated by the individual stress levels of the females, including yolk androgens/gestagens levels or albumin/yolk mass that influence embryo’s development and take part in the general mechanisms involved in prenatal programming effects (Guibert et al. 2011; Henriksen et al. 2011). Today, direct and indirect stress protocols are considered complementary methods, each one exploring different facets of early-stress processes. Comparing and interpreting results from studies that used direct versus indirect manipulations of stress exposure are useful but not straightforward due to multiple factors differing among them, including species and population life-histories, housing conditions, duration/intensity/timing of the specific stress paradigms. Despite not always possible, performing studies exploring the phenotypic effects induced by different stressor types can improve result interpretation (Crino and Breuner 2015).
The developmental timing in which stress exposure is experienced is another determining factor for its long-term effects. In their recent review, Berghänel et al. (2017), for instance, showed that the timing of prenatal maternal stress across mammal species determines growth trajectories in the offspring. Only if offspring were exposed to prenatal stress early in pregnancy, accelerated growth patterns probably as part of a faster life history strategy have been found (e.g., Dmitriew 2011; Schöpper et al. 2012; Berghänel et al. 2016), whereas prenatal stress in later pregnancy was rather associated with reduced pre- and post-natal growth (e.g., Merlot et al. 2013; Rooke et al. 2015). In addition, it has often been reported that prenatal stressors lead to different effects on stress physiology, brain, and behavior compared to postnatal stressors (e.g., Macri and Wuerbel 2006; Lupien et al. 2009; Marasco et al. 2012, 2016; Zimmer et al. 2013; Andersen 2015). The stage in which stress exposure occurs is tightly interconnected with the severity of adversity and the degree of development at birth. It is generally held that the earlier stress exposure takes place and/or the longer its duration, the more severe would be its long-term phenotypic consequences (Lindstrom 1999; Monaghan 2008; Lupien et al. 2009; Danese and McEwen 2012). For example, in the Japanese quail exposure to prenatal stress had stronger effects than postnatal stress in terms of long-term changes in physiological stress reactivity and stress-related behaviors (Zimmer et al. 2013, 2017), as well as transcriptome profiles in the brain (Marasco et al. 2016). However, postnatal stressors can also lead to long-lasting phenotypic changes in various species, both birds and mammals (e.g., Liu et al. 1997; Spencer and Verhulst 2007; Banerjee et al. 2012). These contrasting effects may be explained by interspecies temporal differences in the brain development along the precocial-altricial spectrum. In precocial species that produce relatively mature offspring at birth/hatching, maximal brain growth, and neuroendocrine maturation take place in utero/ovo. By contrast, in altricial species producing immature offspring at birth/hatching, brain developmental processes are comparatively delayed with substantial brain growth occurring during postnatal developmental stages. In addition, in line with the “developmental hypothesis” (Schwabl 1999), the timecourse of HPA axis responsiveness can markedly differ depending on the mode of development, thus on the capacity of the young animal to cope with and avoid stressors across the different stages of postnatal growth. For instance, in several studied bird and mammalian species, adrenocortical capacity to respond to stressors develops much earlier in precocial juveniles compared to altricial juveniles (reviewed by Brown and Spencer 2013).
3 Potential Life-Long Mechanisms of Early-Life Stress for Adverse or Positive Organismal Outcomes
3.1 Reprogramming of the HPA Axis
Glucocorticoid hormones, controlled by the Hypothalamic–Pituitary–Adrenal axis (HPA axis), are one of the major components of the stress response (Sapolsky 1992; Stratakis and Chrousos 1995; Wingfield et al. 1998; Sapolsky et al. 2000)—see Box 4.1 for a description of the principal systems regulating the stress response. Contrary to adrenaline and noradrenaline, glucocorticoids can easily cross the blood–brain barrier and bind to corticosteroid receptors in the brain (mainly glucocorticoid receptors and mineralocorticoid receptors) to influence brain function and cellular energetic signalling (Reul and Dekloet 1985; Datson et al. 2001). Hence, glucocorticoid hormones are thought to be ideal candidates for mediating the long-lasting changes of early-life stress.
Box 4.1 The Stress Response
All vertebrate species elicit highly conserved, relatively nonspecific, behavioral, and physiological changes upon exposure to stressors. Within seconds to hours upon the perception of stressful cues, two endocrine systems are activated, one involving catecholamines, such as adrenaline (acting within seconds) from the adrenal medulla, and the other involving glucocorticoids (acting within minutes) secreted from the adrenal cortex (Stratakis and Chrousos 1995). The fastest component of the stress response, best known as the “fight or flight response” triggers a variety of physiological changes, including increased cardiovascular tone and respiration rate prompting the body for immediate reactions and muscular action (Cannon 1929). As shown in the Fig. 4.1, within minutes upon perception of a stressor, two neuropeptides from the paraventricular nucleus of the hypothalamus, corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP), act synergistically to stimulate the secretion of adrenocorticotropic hormone (ACTH) from corticotroph cells in the anterior pituitary gland. ACTH is then transported via the systemic circulation to the adrenal cortex, where it stimulates the production and secretion of glucocorticoids (corticosterone in majority of amphibians, reptiles, and birds, and cortisol in majority of mammals—Harvey et al. 1984). The increased glucocorticoids in the circulation initiate an array of metabolic and behavioral effects that stimulate hepatic gluconeogenesis, inhibit glucose uptake by peripheral tissues and suppress inflammation and several immune reactions to maintain body homeostasis (Munck et al. 1984). The HPA axis is tightly regulated over time via negative feedback loops (indicated in the figure below by the sign −) on mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) in the brain and anterior pituitary. Under acute stress conditions, feedback mechanisms operate efficiently and the effects of elevated glucocorticoids are only short-term (within hours). In the brain, MR have a higher affinity than GR for glucocorticoids. Therefore, at basal concentrations of glucocorticoids, MR are occupied whereas GR remain largely unoccupied. During acute stress, there is increased occupation of GR. Hippocampal and hypothalamic MR are thought to be primarily involved in feedback regulation during basal secretion, while GR become important during stressful conditions (de Kloet et al. 1998; Matthews 2002; McEwen 2007). Under chronic stressful conditions, feedback mechanisms are impaired causing prolonged activation of the HPA axis, with potential detrimental consequences on brain functioning and body processes (Sapolsky 1996). Chronic stress may also reduce activity of the HPA axis under given circumstances. For example, chronically stressed female starlings had lower baseline corticosterone concentrations and lower reproductive success than unstressed females (Cyr and Romero 2007).
Hypothalamic–Pituitary–Adrenal (HPA) axis. Figure reproduced from Boonstra (2004) with permission of Oxford University Press
Substantial body of work shows that a variety of adversities are consistently associated with long-lasting changes in the functioning of the HPA axis and long-term health diseases (Welberg and Seckl 2001; Seckl 2004; Meaney et al. 2007; Cottrell and Seckl 2009; Harris and Seckl 2011). The general assumption is that early-life stress leads to a hyper-responsive stress phenotype with exaggerated circulating glucocorticoids, enhanced anxiety, and depression-like behaviors (reviews: Maniam et al. 2014; Agorastos et al. 2019). For instance, studies in rodents reported reduced glucocorticoid receptor levels in the hippocampus, attenuated negative feedback, and increased glucocorticoid response to stress in terms of both peak levels and duration of the response (Henry et al. 1994; Barbazanges et al. 1996; Szuran et al. 2000; Green et al. 2011; Bingham et al. 2013). Studies in rats and primates further showed that high glucocorticoid exposure during prenatal life caused elevated basal glucocorticoid levels later in life (Levitt et al. 1996; Welberg et al. 2001; de Vries et al. 2007) although other studies found unaffected basal glucocorticoid levels (review: van Bodegom et al. 2017). Increased adult stress reactivity in response to different stress-related treatments have also been experimentally demonstrated in some studied bird species, such as captive zebra finches, domestic chickens, and Japanese quails (e.g., Hayward and Wingfield 2004; Spencer et al. 2009; Banerjee et al. 2012; Haussmann et al. 2012) though, as in mammals, results are quite variable (Henriksen et al. 2011).
It has been suggested that early-life adversity merely constraints development and leads to underperforming adult phenotypes whatever the environmental conditions. In ecological studies, this idea refers to the “silver spoon hypothesis” (Grafen 1988; van de Pol and Verhulst 2006; Monaghan 2008;—Fig. 4.2a). In support of this hypothesis, there are studies conducted in various species including mammals and birds highlighting associations between early-life adversities and reduced fitness-related proxies, including shortened lifespans and reduced reproductive performance (Metcalfe and Monaghan 2001; Spencer et al. 2010; Monaghan et al. 2012; Tung et al. 2016). Could we then conclude that the optimal early-life experience should always be one of low stress exposure? Researchers noted, to their surprise, that exposure to early-life stress can at times, or for some individuals, have beneficial, rather than negative effects, such as increased growth rates or better reproductive performance (e.g., Schöpper et al. 2012; Dantzer et al. 2013; Crino et al. 2014). From an evolutionary perspective, adjusting the responsiveness to stressful events in response to early-life adversities by programming of the HPA axis could be adaptive if this would lead to phenotypes better able to cope with environmental conditions that are more likely to be experienced later life. This view is the basic concept of the “environmental matching hypothesis” (sometimes also termed as “predictive adaptive response”—Gluckman and Hanson 2007; Gluckman et al. 2007; Horton 2005; Fig. 4.2b). According to this hypothesis, the developing organism responds to environmental signals by a lasting alteration of physiological regulatory circuits, most notably the HPA axis, in order to be better adapted to its current and expected future environment. A heightened HPA response and increased anxiety, for instance, while usually considered maladaptive, can be highly adaptive in an environmental context characterized by adversity and unpredictability. A mismatch between environmental conditions experienced in early development and later life, however, is suggested maladaptive and may increase the risk of earlier mortality (Gluckman et al. 2007, 2010; Horton 2005). In line with this, individuals with a history of childhood adversity exhibited a dampened HPA axis in response to acute stress in adulthood (Elzinga et al. 2008), whereas a mismatch between childhood and adult environments was found to increase the vulnerability to psychopathology (Nederhof and Schmidt 2012; Fine et al. 2014). In addition, although in humans and primates, an increased risk to develop psychopathology after early-life exposure to traumatic stress has been reported, some studies also found a higher degree of resilience in terms of active coping with stressful conditions experienced in later life (Lyons and Parker 2007; Zozulya et al. 2008). Evidence for the match/mismatch hypothesis also comes from rodent studies. In rodents as in mammals in general, the mother plays a central role in the context of early-life programming because the environmental cues predictive of the future environment are primarily transferred to the offspring via the maternal physiology and behavior. In rats and mice specifically, the amount of licking and grooming represents an important cue. In accordance with this hypothesis, Champagne (2008) and Bagot et al. (2009) showed that adult offspring of mothers providing only a low amount of licking and grooming exhibited poor cognitive performance (low LG mothers) in a low-stress context. But under stressful conditions, cognitive performance of adult offspring of low LG mothers was superior to the performance of adult offspring that had received a high amount of licking and grooming and showed impaired cognitive performance in such a high-stress context (Champagne 2008; Bagot et al. 2009). Developmental programming effects associated with environmental matching cues might be enhanced when similar stressors are experienced across multiple developmental life stages within the same individuals. Some evidence for this comes from a study in the Japanese quail in which birds exposed to both pre- and post-natal stress-related treatments (prenatal corticosterone injection and unpredictable food availability, respectively) were more explorative and risk-taking in a novel (presumably stressful) environment, compared to the birds that were exposed to stress only as embryos or as chicks (Zimmer et al. 2013). One of the main criticisms about the studies conducted so far in support of the existence of predictive adaptive responses is that the vast majority of the work has been carried out in captive animals and humans, and often exposed to artificial or extreme stressors that may hardly represent evolutionary relevant conditions (see Berghänel et al. 2016 for a discussion on this aspect).
Diagram of (a) silver spoon, (b) environmental matching, and (c) inoculation models. Panels a and b are redrawn from Pigeon et al. (2019)
Indeed the actual severity of early-life adversity is likely to be an important contributory factor regulating subsequent stress resilience. Suggestions for this comes from research in humans highlighting that a history of some early-adversity can foster subsequent resilience compared to individuals with a high history of adversity but also to people with no history of adversity. For instance, moderately stressful events during childhood had been associated with decreased cardiovascular responses to stressful laboratory tests (Boyce et al. 1995), lower levels of anxiety (Edge et al. 2009), diminished cortisol activity (Elzinga et al. 2008; Gunnar et al. 2009), lower post-traumatic stress symptoms and distress (Seery et al. 2010). Research in mammalian laboratory models supported and extended these findings. For instance, short-term exposure to certain early-life stressors (intermittent social and/or maternal separations, high-demand foraging conditions) in rats and squirrel monkeys has been shown to attenuate anxiety-like behavior and diminish HPA axis reactivity compared to individuals raised under less stressful conditions (Parker and Maestripieri 2011). Individuals with an enhanced efficiency of the negative feedback would be able to bring glucocorticoids faster back to baseline levels upon exposure to challenging events and, therefore, have reduced probability to suffer from potential harmful effects of chronic glucocorticoid exposure (Taff et al. 2018; Zimmer et al. 2019). Taken together, these findings suggest nonlinear associations, probably U- or J-shaped associations, between early-life stress and later life resilience (Parker and Maestripieri 2011; Russo et al. 2012).
Little is known about the mechanisms that promote the development of stress resilience. As early handling in rodents is known to increase maternal licking and grooming (Liu et al. 1997), it was first hypothesized that the development of stress resilience was predominantly maternally mediated (maternal mediation hypothesis—Caldji et al. 2000; Plotsky and Meaney 1993). However, seminal experiments by Parker and colleagues in squirrel monkeys, a model in which brief intermittent maternal separation stress does not lead to changes in maternal behavior, demonstrate that it is stress exposure per se, rather than maternal care, to have a key role (Parker et al. 2006). These studies supported the alternative “stress-inoculation hypothesis” (Fig. 4.2c), which is based on the notion that mild-to-moderate stress exposure is necessary for the development of appropriate emotion regulation and subsequent stress resilience (Parker and Maestripieri 2011; Romeo 2015). This concept is related to that of “hormesis,” a type of dose–response relationship with low dose inhibition and high dose stimulation of organism performance (see Chap. 2 in this book), which might complement the inoculation model.
In the inoculation model, resilience arises from intermittent exposure to early-life stressors that are not overwhelming, but just challenging enough to transiently activate the HPA axis (Parker et al. 2005, 2006). The mechanisms leading to a resilient phenotype are likely to involve life-long changes in the brain and pituitary gland, which might be associated with increases in glucocorticoid and/or mineralocorticoid receptors (Zimmer and Spencer 2014; Sapolsky 2015; Marasco et al. 2016). Glucocorticoid receptor signalling has a key role in the regulation of HPA axis negative feedback (Cornelius et al. 2018; Dickens et al. 2009). The immunophilin FKBP5, a glucocorticoid receptor cofactor with inhibitory effect on glucocorticoid activity, is associated with individual differences in HPA axis negative feedback efficiency (Touma et al. 2011; Häusl et al. 2021) as well as altered risks of anxiety and post-traumatic stress disorder (Touma et al. 2011; Hariri and Holmes 2015). A recent study performed by Zimmer et al. (2021) in wild house sparrows (Passer domesticus) showed that reduced mRNA expression of FKBP5 in the hypothalamus was associated with higher HPA axis flexibility (i.e., within-individual, rapid and reversible change in HPA regulation in response to challenges) and improved stress coping capacities in terms of exploratory disposition, neophobia, and body mass maintenance. Although FKBP5 is sensitive to early-life conditions (review: Zimmer et al. 2020), whether this marker could capture long-term changes in physiological stress resilience and fitness outputs remains to be tested, offering a very exciting question to address in future research.
3.2 Telomere Dynamics and Oxidative Stress
There is considerable evidence across a wide range of vertebrate taxa that dynamics in telomere length and oxidative stress are two key cellular mechanisms that affect organism performance (Monaghan et al. 2018; Costantini 2019). Given the profound and long-lasting effects of glucocorticoids on physiological homeostasis and their properties to translate environmental stimuli into molecular responses, some authors suggested that they might be key molecular links between environmental quality and both telomere dynamics and oxidative stress (Costantini et al. 2011; Angelier et al. 2018). However, these hypotheses have been poorly explored so far in the context of early-life phenotypic programming.
In 2013, Marasco et al. (2013) provided experimental evidence for a role of early-life exposure to glucocorticoids in affecting some aspects of adult oxidative status. Marasco et al. (2013) used an experimental setting including four groups: pre- and postnatal untreated birds; prenatal corticosterone-treated and postnatal untreated birds; prenatal untreated and postnatal corticosterone-treated birds; pre- and postnatal corticosterone-treated birds. The manipulation of prenatal stress levels involved the injection of eggs of Japanese quail (Coturnix japonica) with 8.5 ng of corticosterone dissolved in peanut oil. The postnatal stress treatment involved the administration to chicks of one mealworm (Tenebrio molitor) per day injected with 45 μg (between 5 and 15 days of age) or 90 μg (between 16 and 19 days of age) of corticosterone dissolved in peanut oil. Both pre- and post-natal treatments with corticosterone were chosen in order to increase corticosterone within the age-specific physiological ranges of the study species. The effects of the experiment were then tested on four markers of oxidative status, analyzed in red blood cells collected at 64 days of age and in the brain (cerebellum and midbrain) at 69–73 days of age (Marasco et al. 2013). In red blood cells, there was no effect on the antioxidant enzyme superoxide dismutase nor on the marker of oxidative damage protein carbonyls. The activity of the antioxidant enzyme glutathione peroxidase was higher in all the corticosterone-treated birds than in controls, but there was an additive effect in birds that experienced both the pre- and post-natal treatment. Finally, a marker of nonenzymatic antioxidant capacity was lower in corticosterone-treated birds than in controls. All the markers of oxidative status were not affected in the midbrain; by contrast, in the cerebellum the glutathione peroxidase was marginally higher in the three corticosterone-treated groups and the nonenzymatic antioxidant capacity was lower in the birds that experienced both the pre- and the post-natal treatment than those that experienced only one of the two treatments. Overall, this experimental work suggested that increased exposure to corticosterone in ovo influenced the adult oxidative phenotype, possibly through direct effects on cell metabolism, gene expression, or growth rate. Importantly, the nature of effects depended on the interaction between pre- and post-natal environments, suggesting a certain degree of plasticity in the regulation of oxidative status and providing some support to the environmental matching paradigm, at least for certain aspects of oxidative status.
These interactive effects of early-life challenges on oxidative status were later shown using unpredictable food supply (which generally leads to increases in plasma corticosterone—e.g., Pravosudov et al. 2001; Marasco et al. 2018) in another precocial bird species, the gray partridge (Perdix perdix) (Homberger et al. 2013). Birds had higher blood antioxidant capacity when they experienced no stress in both the pre- and post-natal stages of life, and had lower antioxidants when experienced food stress only after hatching. By contrast, the production of free radicals in blood was not influenced by the stress regime, suggesting that trophic stress affected only some aspects of the antioxidant machinery. It is important to highlight that alterations of the HPA axis activity are one effect of unpredictable food supply (e.g., Lynn et al. 2003; Wingfield 2003). Thus, the results from Homberger et al. (2013) strengthen the hypothesis of Marasco et al. (2013) that the adult oxidative status will depend to some degree on the precocial exposure to different amounts of glucocorticoids. However, it appears to give more support to the silver spoon model because antioxidant capacity was preserved only when birds did not experience any stress both in early- and in adult-life.
It is unknown if these long-term effects on oxidative status have any fitness consequences. The strategy of depositing glucocorticoids into the eggs may be adaptive if any physiological costs for the chicks are lower than the benefits. This may be especially true for chickens and quail, as well as for other precocial species. Compared with altricial chicks, precocial chicks leave the nest soon after hatching and rely less on maternal care. Therefore, they have to be programmed to survive almost on their own very soon in life. Glucocorticoids may be important promoters of survival because they enhance fear and vigilance behaviors, so allowing precocial chicks to avoid predators or to stay close to their siblings (Hayward and Wingfield 2004; Janczak et al. 2007). Moreover, chickens and quail are short-lived species; therefore, they might have been programmed to prioritize investment in growth and reproduction at the expense of investment in protection against oxidative stress. Although these are still almost unexplored questions, a few studies suggested that the link between early-life exposure to glucocorticoids and oxidative status might be relevant for later fitness outcomes and for adjusting the phenotype to environmental challenges of the Anthropocene. Zimmer and Spencer (2015) showed that pre-natal experimental exposure to glucocorticoids may be associated with a higher cost of reproduction in terms of oxidative stress in the Japanese quail. Using the brown trout, Birnie-Gauvin et al. (2017) evaluated the short-term (2 weeks) and long-term (4 months over winter) effects of exogenous cortisol manipulations (as well as relevant shams and controls) on the oxidative status of wild juveniles. Cortisol caused an increase of the antioxidant glutathione in red blood cells over a two-week period and appeared to reduce glutathione over winter (Birnie-Gauvin et al. 2017). By contrast, cortisol treatment did not affect the ratio between reduced and oxidized glutathione nor a marker of antioxidant capacity. Importantly, over winter survival in the stream was associated with low levels of glutathione, suggesting that oxidative stress might be a mechanism by which elevated early-life exposure to cortisol causes negative physiological consequences (Birnie-Gauvin et al. 2017). Flores et al. (2019) evaluated the effect of traffic noise (traffic noise group vs. rural noise group) on baseline levels of corticosterone and stress responses in chicks of the Japanese quail. They observed (i) similar baseline levels of corticosterone in both experimental groups, (ii) a trend towards higher stress response in the traffic noise group, (iii) higher levels in red blood cells of the key intracellular antioxidant glutathione in the traffic noise group, and (iv) a negative effect of stress response on glutathione in the traffic noise treatment.
As compared to research on the link between early-life stress and oxidative status, much less is known for the long-term effects of early-life stress on adult pattern of change in telomere length. This is particularly unfortunate because in the majority of vertebrates studied so far, the highest rates of telomere shortening are observed during early development (Heidinger et al. 2012; Monaghan and Ozanne 2018). Studies in wild birds, including European shags (Phalacrocorax aristotelis) and great tits (Parus major), demonstrated that experimental exposure to corticosterone during early postnatal development fostered developmental telomere shortening (Herborn et al. 2014; Casagrande et al. 2020). However, a recent study in wild yellow-legged gulls showed that pre-natal corticosterone exposure led to telomere elongation and upregulated telomerase activity in the juveniles (Noguera et al. 2020). Many factors could explain differences among studies, such as the developmental timing in which stress exposure was experienced and the time in which telomere measurements were made. The severity of the stress exposure is likely to be especially important. An experimental study in the domestic chicken (Gallus domesticus) performed by Haussmann and collaborators (2012) showed that only very high prenatal glucocorticoid exposure increased developmental telomere loss, while a low prenatal corticosterone exposure had no effect (Haussmann et al. 2012). Importantly, in the latter study, only the high prenatal corticosterone exposed birds showed clear signs of HPA axis hyper-responsiveness compared to the other two treatment groups (Haussmann et al. 2012). It is thus possible that modest or brief activations of the HPA axis during development may trigger telomere repair mechanisms including up-regulated telomerase activity, while more severe or chronic stress exposure downregulate telomerase activity. Plausibility for such nonlinear inoculation-like effects comes from studies in rodents showing that a brief exposure to certain environmental stressors can rapidly increase telomerase activity (Beery et al. 2012; Epel and Lithgow 2014). We need more ecologically relevant experimental designs to further explore the links between early-life exposure to different amounts of glucocorticoids, telomeres, and oxidative status. We also suggest exploring if glucocorticoid-induced effects are associated with changes in mitochondrial metabolism (Casagrande et al. 2020). This is particularly intriguing because, on one side, mitochondria are one main source of prooxidant generation in organisms, and, on the other side, they produce the molecule ATP, which provides energy for growth and development.
3.3 Epigenetic Mechanisms Regulated by Glucocorticoids
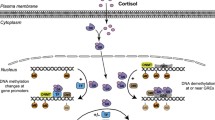
Rapidly growing evidence suggests that the underlying mechanisms through which early-life conditions are biologically embedded and may exert lifelong effects, involve epigenetic processes (see Chap. 1 for a comprehensive review about the understandings of the term “epigenetics” and related molecular mechanisms). This is because the epigenome regulates gene expression in a cell and tissue-specific manner. Thus, without modifying the genome itself, the epigenetic machinery determines the actual phenotypic outcome by regulating what is transcribed from the genome. Second, the epigenome is responsive to environmental influences, providing the biological basis for the interplay between environmental cues and the genome. Epigenetic remodelling caused by early-life experiences, therefore, serves as an ideal mechanism for developmental plasticity. Third, epigenetic modifications are stable and steadily transferred from one cell generation to the next. In this way, the epigenome facilitates long-lasting modifications of gene expression patterns caused by early-life environmental signals and, therefore, from an evolutionary perspective, provides a means to “fine-tune” the phenotype to forecast future conditions. Indeed, numerous studies have shown that early-life experiences can induce epigenetic modifications that cause persistent changes in gene expression patterns and thus exert long-term effects on phenotypic outcomes. Although identifying epigenetic modifications associated with any phenotypic outcome alone does not imply causality, numerous studies have provided strong evidence for a functional relationship through the analyses of mRNA expression (e.g., McGowan et al. 2009; Labonte et al. 2012).
Genes regulating the HPA axis are prime candidates for investigating how early-life stress can be biologically embedded by epigenetic modifications. Accordingly, one of the most renowned examples of epigenetic programming examined the effects of maternal care on epigenetic remodeling of genes involved in HPA axis function. This series of studies by Weaver, Meaney, Szyf, and colleagues demonstrated in rats, how natural differences in maternal behavior can lead to epigenetic programming inducing life-long changes in offspring behavior and physiology (Weaver et al. 2004; Meaney and Szyf 2005). The authors demonstrated that high levels of maternal licking and grooming during the first week of life resulted in higher mRNA expression of the glucocorticoid receptor gene in the offspring hippocampus caused by lower DNA methylation and higher histone acetylation of the glucocorticoid receptor promoter exon 17. The methylation difference was located at the binding site of the transcription factor nerve growth factor inducible A (NGFI-A), where in offspring of low licking mothers, methylation levels are high, impeding transcription factor binding and thus glucocorticoid receptor gene expression. The alteration of DNA methylation in response to maternal licking and grooming remained stable into adulthood, leading to life-long changes in HPA axis function of the offspring. In offspring of high licking mothers, higher hippocampal glucocorticoid receptor expression increased negative feedback sensitivity of the HPA axis, which ultimately resulted in lower endocrine and behavioral responses to stress and reduced fearfulness in the presence of a stressor such as a novel environment. Cross-fostering experiments (offspring of high LG mothers were fostered by low LG mothers and vice versa) demonstrated that indeed the differences in maternal care caused the epigenetic modifications and thus determined the offspring phenotype. This research provided strong evidence for a causality between early-life epigenetic programming and phenotypic outcome in adulthood as the epigenetic alterations induced by maternal behavior and its effects on gene expression and stress response in the offspring could be reversed in adulthood by central infusion of either methionine, affecting DNA methylation, or a histone deacetylase inhibitor, affecting histone acetylation (Weaver et al. 2004, 2006). In addition, these findings were also extended to humans. McGowan et al. (2009), for instance, showed that methylation of the human homologue of the hippocampal glucocorticoid receptor promotor region was increased and mRNA expression reduced in suicide victims that had a history of childhood abuse.
Genes, however, do not act independently. Even though the candidate gene approach is valuable as it has first shed light into the epigenetic mechanisms underlying programming effects of early-life experiences, the impact of early-life experiences is broader, involving numerous genes in a tissue-specific manner (e.g., Marasco et al. 2016). The advent of epigenome-wide association studies and transcriptomics now facilitates a more realistic analysis of epigenetic modifications induced by early-life experiences on the basis of the whole genome rather than a limited set of candidate genes. Interestingly, a recent study by Taff et al. (2019) on free-living female tree swallows (Tachycineta bicolor) showed associations between differentially methylated regions across the genome with stress resilience to handling (i.e., the ability to terminate the glucocorticoid stress response through negative feedback). The latter study thus indicates that global methylation patterns may predict stress coping abilities and possibly fitness consequences in natural settings, and might act as a useful biomarker of stress resilience. Taff et al. (2019) hypothesized that the differentially methylated regions identified in their study in relation to stress physiology might be due to early-life programming effects. At least to a certain extent, support for this explanation comes from a study showing that zebra finches raised in broods of different sizes (thus likely experiencing different early-life stress exposure levels due to changes in food availability and sibling competition) showed consistent hypo- or hypermethylation across the genome (Sheldon et al. 2018). Future experimental studies are, however, needed in order to determine whether large-scale regulation of methylation patterns in early-life is a causal driver of subsequent stress reactivity and coping abilities.
4 Conclusions and Future Directions
A large body of evidence from epidemiological and animal experiments clearly shows that exposure to early-life stress can have a remarkable influence on adult lifestyle and health outcomes. Detailed studies carried out in model organisms demonstrated that the HPA axis is likely to be a key physiological system underlying the programming effects of early-life adversity (Fig. 4.3). However, there has been increasing recognition that such effects operate at multiple biological scales and encompass more pervasive cellular and molecular changes. Current evidence suggests that measurements of telomere dynamics, oxidative status, and transcriptome/epigenetic networks are relevant mechanisms and markers to trace the long-lasting effects of early-life experience on performance and fitness-related proxies. However, whether these markers can be considered as main modulatory signals orchestrating some of the programming effects of early-life stress remains to be determined. Carefully designed experiments, for instance, manipulating an organism’s oxidative status during growth (e.g., by increasing generation of pro-oxidants or decreasing antioxidants along a low-high stress severity gradient) is now within reach in most ecological settings (Koch and Hill 2017) and would be very useful in this context. In addition, the ongoing advances in “omics” approaches constitute an exciting opportunity to characterize, and potentially manipulate, conserved transcriptome pathways and epigenetic mechanisms influenced by a particular level of stress exposure and to identify target brain structures in which such changes effectively operate and lead to long-term differences in stress susceptibility versus resilience.
Conceptual model of early-life stress programming. Exposure to stress during pre- and post-natal stages of development leads to increased exposure to glucocorticoid hormones. Elevated developmental glucocorticoids can lead to long-lasting changes in the Hypothalamic–Pituitary–Adrenal (or Interrenal) axis (HPA axis) activity (likely to play a central role in the shaping of phenotypic trajectories) and other molecular mechanisms underlying aging and life-history evolution including transcriptome and epigenenome regulation, oxidative status, and telomere dynamics. The phenotypic effects of early-life stress depend on an organism’s genetic background and on its trans-generational history, as well as on the developmental timing in which stress exposure occurs and specific features of the challenge/s (type, frequency, intensity, and duration). Interactive effects among these factors would determine subsequent resilience or vulnerability to later life challenges, and thus explain inter-individual variation in organismal and fitness outcomes of stressed-exposed phenotypes
The studies reviewed throughout this chapter clearly highlight that early-life stress does not necessarily lead to undesired adverse outcomes in adulthood. While extreme and/or prolonged stressors do often impair brain development, increase susceptibility to later life morbidities, and lower survival prospect of an organism, newer research suggest that milder forms of stress exposure, such as brief maternal separation or moderate physiological elevation of developmental glucocorticoids, can instead increase the range of tolerable stress for the organism and potentially ameliorate later life performance and delay aging processes. Yet, we have limited experimental data that explicitly manipulated the severity of stress exposure of differing types of early-life challenges and examined subsequent changes in relevant molecular/physiological pathways and fitness outcomes. Plus, stressor type and severity are likely to be interconnected with other biological features which need to be carefully considered when planning experiments, especially developmental timing of stress exposure, species-specific developmental strategies, and the later life environmental conditions experienced throughout an organism’s lifecycle (Fig. 4.3). Another aspect often overlooked in experimental planning is that a considerable inter-individual variability in the ontogeny of the stress response is merely attributable to genetic predisposition factors or to the trans-generational history of the study population (McIlwrick et al. 2016; McCormick et al. 2017—Fig. 4.3). As a consequence, similar or even the same early-life challenge could have major negative consequences for one individual or population, and have negligible or even positive effects in another. Understanding the relative contribution of all these factors on the biological embedding of early-life stress is a critical step forward in order to better define how just the right type and magnitude of stress inoculation can promote resilience processes and potentially shape phenotypes with better suited coping mechanisms to maximize fitness outputs.
References
Abbott DH, Zhou R, Bird IM, Dumesic DA, Conley AJ (2008) Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. Endocr Dev 13:145–158
Agorastos A, Pervanidou P, Chrousos GP, Baker DG (2019) Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front Psych 10:118–118
Andersen SL (2015) Exposure to early adversity: points of cross-species translation that can lead to improved understanding of depression. Dev Psychopathol 27:477–491
Angelier F, Costantini D, Blévin P, Chastel O (2018) Do glucocorticoids mediate the link between environmental conditions and telomere dynamics in wild vertebrates? A review. Gen Comp Endocrinol 256:99–111
Arnold AP (2009) The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 55:570–578
Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joëls M (2009) Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem 92:292–300
Banerjee SB, Arterbery AS, Fergus DJ, Adkins-Regan E (2012) Deprivation of maternal care has long-lasting consequences for the hypothalamic-pituitary-adrenal axis of zebra finches. Proc R Soc B Biol Sci 279:759–766
Barbazanges A, Piazza PV, Lemoal M, Maccari S (1996) Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 16:3943–3949
Barker DJP, Bull AR, Osmond C, Simmonds SJ (1990) Fetal and placental size and risk of hypertension in adult life. Br Med J 301:259–262
Barker DJP, Hales CN, Fall CHD, Osmond C, Phipps K, Clark PMS (1993) Type 2 (non-insulin-dependent) diabetes-mellitus, hypertension and hyperlipemia (syndrome-X)—relation to reduced fetal growth. Diabetologia 36:62–67
Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES (2012) Chronic stress elevates telomerase activity in rats. Biol Lett 8:1063–1066
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377
Berghänel A, Heistermann M, Schülke O, Ostner J (2016) Prenatal stress effects in a wild, long-lived primate: predictive adaptive responses in an unpredictable environment. Proc R Soc B Biol Sci 283:20161304
Berghänel A, Heistermann M, Schülke O, Ostner J (2017) Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc Natl Acad Sci U S A 114:E10658–e10666
Bingham BC, Sheela Rani CS, Frazer A, Strong R, Morilak DA (2013) Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior. Psychoneuroendocrinology 38:2746–2757
Birnie-Gauvin K, Peiman KS, Larsen MH, Aarestrup K, Willmore WG, Cooke SJ (2017) Short-term and long-term effects of transient exogenous cortisol manipulation on oxidative stress in juvenile brown trout. J Exp Biol 220:1693–1700
Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, Verhulst S (2014) Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc R Soc B Biol Sci 281:20133287
Boonstra R (2004) Coping with changing northern environments: the role of the stress axis in birds and mammals. Integr Comp Biol 44:95–108
Boyce WT et al (1995) Psychobiologic reactivity to stress and childhood respiratory illnesses: results of two prospective studies. Psychosom Med 57:411–422
Brown GR, Spencer KA (2013) Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience 249:115–128
Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ (2000) The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22:219–229
Cannon WB (1929) Bodily changes in pain, hunger, fear and rage: recent researches into the function of emotional excitement. Appleton & Co, New York
Casagrande S, Hau M (2019) Telomere attrition: metabolic regulation and signalling function? Biol Lett 15:20180885
Casagrande S et al (2020) Increased glucocorticoid concentrations in early life cause mitochondrial inefficiency and short telomeres. J Exp Biol 223:jeb222513
Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Champagne FA (2008) Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol 29:386–397
Cornelius JM, Perreau G, Bishop VR, Krause JS, Smith R, Hahn TP, Meddle SL (2018) Social information changes stress hormone receptor expression in the songbird brain. Horm Behav 97:31–38
Costantini D (2019) Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. J Exp Biol 222
Costantini D, Marasco V, Møller A (2011) A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B 181:447–456
Cottrell EC, Seckl J (2009) Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3:19
Crino OL, Breuner CW (2015) Developmental stress: evidence for positive phenotypic and fitness effects in birds. J Ornithol 156:389–398
Crino OL, Prather CT, Driscoll SC, Good JM, Breuner CW (2014) Developmental stress increases reproductive success in male zebra finches. Proc R Soc B Biol Sci 281:20141266
Cyr NE, Michael RL (2007) Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen Comp Endocrinol 151(1):82–89. https://doi.org/10.1016/j.ygcen.2006.12.003
Danese A, McEwen BS (2012) Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 106:29–39
Dantzer B, Newman AE, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG (2013) Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340:1215–1217
Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E (2001) Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci 14:675–689
de Bruijn R, Romero LM (2018) The role of glucocorticoids in the vertebrate response to weather. Gen Comp Endocrinol 269:11–32. https://doi.org/10.1016/j.ygcen.2018.07.007
de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301
de Vries A et al (2007) Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest 117:1058–1067
Dickens M, Romero LM, Cyr NE, Dunn IC, Meddle SL (2009) Chronic stress alters glucocorticoid receptor and mineralocorticoid receptor mRNA expression in the European Starling (Sturnus vulgaris) brain. J Neuroendocrinol 21:832–840
Dmitriew C (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86:97
Edge MD, Ramel W, Drabant EM, Kuo JR, Parker KJ, Gross JJ (2009) For better or worse? Stress inoculation effects for implicit but not explicit anxiety. Depress Anxiety 26:831–837
Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P (2008) Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology 33:227–237
Epel ES, Lithgow GJ (2014) Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci 69:S10–S16
Fine R, Zhang J, Stevens HE (2014) Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry 19:641–651
Flores R, Penna M, Wingfield JC, Cuevas E, Vásquez RA, Quirici V (2019) Effects of traffic noise exposure on corticosterone, glutathione and tonic immobility in chicks of a precocial bird. Conserv Physiol 7:coz061
Glover V, O’Connor TG, O’Donnell K (2010) Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev 35:17–22
Gluckman PD, Hanson MA (2007) Developmental plasticity and human disease: research directions. J Intern Med 261:461–471
Gluckman PD, Hanson MA, Spencer HG (2005) Predictive adaptive responses and human evolution. Trends Ecol Evol 20:527–533
Gluckman PD, Hanson MA, Beedle AS (2007) Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol 19:1–19
Gluckman PD, Hanson MA, Mitchell MD (2010) Developmental origins of health and disease: reducing the burden of chronic disease in the next generation. Genome Med 2:14
Grafen A (1988) On the uses of data on lifetime reproductive success. In: Reproductive success. T. Clutton-Brock, Cambridge
Green MK et al (2011) Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience 192:438–451
Guibert F et al (2011) Unpredictable mild stressors on laying females influence the composition of Japanese quail eggs and offspring’s phenotype. Appl Anim Behav Sci 132:51–60
Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ (2009) Moderate versus severe early life stress: associations with stress reactivity and regulation in 10-12-year-old children. Psychoneuroendocrinology 34:62–75
Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD (1991) Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303:1019–1022
Hariri AR, Holmes A (2015) Finding translation in stress research. Nat Neurosci 18:1347–1352
Harris A, Seckl J (2011) Glucocorticoids, prenatal stress and the programming of disease. Horm Behav 59:279–289
Harvey S, Phillips JG, Rees A, Hall TR (1984) Stress and adrenal-function. J Exp Zool 232:633–645
Hau M et al (2015) Repeated stressors in adulthood increase the rate of biological ageing. Front Zool 12:4
Häusl AS et al (2021) The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice. Mol Psychiatry 26:3060
Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM (2012) Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc B Biol Sci 279:1447–1456
Hayward LS, Wingfield JC (2004) Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol 135:365–371
Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A 109:1743–1748
Henriksen R, Rettenbacher S, Groothuis TGG (2011) Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci Biobehav Rev 35:1484–1501
Henry C, Kabbaj M, Simon H, Lemoal M, Maccari S (1994) Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult-rats. J Neuroendocrinol 6:341–345
Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P (2014) Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc R Soc B Biol Sci 281:20133151
Homberger B, Jenni-Eiermann S, Roulin A, Jenni L (2013) The impact of pre- and post-natal contexts on immunity, glucocorticoids and oxidative stress resistance in wild and domesticated grey partridges. Funct Ecol 27:1042–1054
Horton TH (2005) Fetal origins of developmental plasticity: animal models of induced life history variation. Am J Hum Biol 17:34–43
Janczak AM, Torjesen P, Palme R, Bakken M (2007) Effects of stress in hens on the behaviour of their offspring. Appl Anim Behav Sci 107:66–77
Khashan AS et al (2008) Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry 65:146–152
Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E (2008) Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord 38:481–488
Koch RE, Hill GE (2017) An assessment of techniques to manipulate oxidative stress in animals. Funct Ecol 31:9–21
Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G (2012) Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiatry 72:41–48
Langenhof MR, Komdeur J (2018) Why and how the early life environment affects development of coping behaviours. Behav Ecol Sociobiol 72:34
Levitt NS, Lindsay RS, Holmes MC, Seckl JR (1996) Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 64:412–418
Lindstrom J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Liu D et al (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277:1659–1662
Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD (2009) The velocity of climate change. Nature 462:1052–1055
Love OP, Williams TD (2008) Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Horm Behav 54:496–505
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
Lynn SE, Breuner CW, Wingfield JC (2003) Short-term fasting affects locomotor activity, corticosterone, and corticosterone binding globulin in a migratory songbird. Horm Behav 43:150–157
Lyons DM, Parker KJ (2007) Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress 20:423–433
Macri S, Wuerbel H (2006) Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav 50:667–680
Maniam J, Antoniadis C, Morris MJ (2014) Early life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front Endocrinol 5:73–73
Marasco V, Robinson J, Herzyk P, Spencer KA (2012) Pre- and post-natal stress in context: effects on the stress physiology in a precocial bird. J Exp Biol 215:3955–3964
Marasco V, Spencer KA, Robinson J, Herzyk P, Costantini D (2013) Developmental post-natal stress can alter the effects of pre-natal stress on the adult redox balance. Gen Comp Endocrinol 191:239–246
Marasco V, Herzyk P, Robinson J, Spencer KA (2016) Pre- and post-natal stress programming: developmental exposure to glucocorticoids causes long-term brain-region specific changes to transcriptome in the precocial Japanese quail. J Neuroendocrinol. https://doi.org/10.1111/jne.12387
Marasco V, Boner W, Heidinger B, Griffiths K, Monaghan P (2018) Environmental conditions shape the temporal pattern of investment in reproduction and survival. Proc R Soc B Biol Sci 285(1870):20172442
Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, Yao R, Haussmann MF (2016) Prenatal stress and newborn telomere length. Am J Obstet Gynecol 215:94.e91–94.e98
Markham JA, Koenig JI (2011) Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology 214:89–106
Matthews SG (2002) Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab 13:373–380
McCormick GL, Robbins TR, Cavigelli SA, Langkilde T (2017) Ancestry trumps experience: transgenerational but not early life stress affects the adult physiological stress response. Horm Behav 87:115–121. https://doi.org/10.1016/j.yhbeh.2016.11.010
McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904
Mcewen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43:2–15
McGowan PO et al (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348
McIlwrick S, Rechenberg A, Matthes M, Burgstaller J, Schwarzbauer T, Chen A, Touma C (2016) Genetic predisposition for high stress reactivity amplifies effects of early-life adversity. Psychoneuroendocrinology 70:85–97. https://doi.org/10.1016/j.psyneuen.2016.04.023
Meaney MJ, Szyf M (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 7:103–123
Meaney MJ, Szyf M, Seckl JR (2007) Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med 13:269–277
Merlot E, Quesnel H, Prunier A (2013) Prenatal stress, immunity and neonatal health in farm animal species. Animal 7:2016–2025
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc B Biol Sci 363:1635–1645
Monaghan P, Ozanne SE (2018) Somatic growth and telomere dynamics in vertebrates: relationships, mechanisms and consequences. Philos Trans R Soc Lond Ser B Biol Sci 373
Monaghan P, Heidinger BJ, D'Alba L, Evans NP, Spencer KA (2012) For better or worse: reduced adult lifespan following early life stress is transmitted to breeding partners. Proc R Soc B Biol Sci 279:709–714
Monaghan P, Eisenberg DTA, Harrington L, Nussey D (2018) Understanding diversity in telomere dynamics. Philos Trans R Soc B Biol Sci 373:20160435
Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5:25–44
Nederhof E, Schmidt MV (2012) Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav 106:691–700
Noguera JC, da Silva A, Velando A (2020) Egg corticosterone can stimulate telomerase activity and promote longer telomeres during embryo development. Mol Ecol. https://doi.org/10.1111/mec.15694
Nugent BM, Tobet SA, Lara HE, Lucion AB, Wilson ME, Recabarren SE, Paredes AH (2012) Hormonal programming across the lifespan. Horm Metab Res 44:577–586
Painter RC, Roseboom TJ, Bleker OP (2005) Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 20:345–352
Painter RC et al (2006) Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr 84:322–327
Parker KJ, Maestripieri D (2011) Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev 35:1466–1483
Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM (2005) Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry 57:848–855
Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM (2006) Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci U S A 103:3000–3005
Pigeon G, Loe LE, Bischof R, Bonenfant C, Forchhammer M, Irvine RJ, Ropstad E, Stien A, Veiberg V, Albon S (2019) Silver spoon effects are constrained under extreme adult environmental conditions. Ecology 100:e02886
Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res 18:195–200
Pravosudov VV, Kitaysky AS, Wingfield JC, Clayton NS (2001) Long-term unpredictable foraging conditions and physiological stress response in mountain chickadees (Poecile gambeli). Gen Comp Endocrinol 123(3):324–331
Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR (2013) Telomeres and early life stress: an overview. Biol Psychiatry 73:15–23
Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP (1999) Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 70:811–816
Reichert S, Stier A (2017) Does oxidative stress shorten telomeres in vivo? A review. Biol Lett 13:20170463
Reul JMHM, Dekloet ER (1985) 2 receptor systems for corticosterone in rat-brain: microdistribution and differential occupation. Endocrinology 117:2505–2511
Reynolds LP et al (2010) Developmental programming: the concept, large animal models, and the key role of uteroplacental vascular development. J Anim Sci 88:E61–E72
Ridout KK et al (2018) Early life adversity and telomere length: a meta-analysis. Mol Psychiatry 23:858–871
Romeo RD (2015) Perspectives on stress resilience and adolescent neurobehavioral function. Neurobiol Stress 1:128–133
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model - a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389
Romero LM, Platts SH, Schoech SJ, Wada H, Crespi E, Martin LB, Buck CL (2015) Understanding stress in the healthy animal - potential paths for progress. Stress 18:491–497
Rooke JA, Arnott G, Dwyer CM, Rutherford KMD (2015) The importance of the gestation period for welfare of lambs: maternal stressors and lamb vigour and wellbeing. J Agric Sci 153:497–519
Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP (2001) Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol 185:93–98
Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ (2012) Neurobiology of resilience. Nat Neurosci 15:1475–1484
Saino N, Romano M, Ferrari RP, Martinelli R, Moller AP (2005) Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool A Comp Exp Biol 303A:998–1006
Sapolsky RM (1992) Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D (eds) Behavioral endocrinology. MIT Press, Cambridge, MA, pp 287–324
Sapolsky RM (1996) Why stress is bad for your brain. Science 273:749–750
Sapolsky RM (2015) Stress and the brain: individual variability and the inverted-U. Nat Neurosci 18:1344–1346
Sapolsky RM (2000) Stress hormones: good and bad. Neurobiol Dis 7(5):540–542. https://doi.org/10.1006/nbdi.2000.0350
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Schoech SJ, Rensel MA, Heiss RS (2011) Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr Zool 57:514–530
Schöpper H, Klaus T, Palme R, Ruf T, Huber S (2012) Sex-specific impact of prenatal stress on growth and reproductive parameters of Guinea pigs. J Comp Physiol B 182:1117–1127
Schwabl H (1999) Developmental changes and among-sibling variation of corticosterone levels in an altricial avian species. Gen Comp Endocrinol 116:403–408
Seckl JR (2001) Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol Cell Endocrinol 185:61–71
Seckl JR (2004) Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151:U49–U62
Seery MD, Holman EA, Silver RC (2010) Whatever does not kill us: cumulative lifetime adversity, vulnerability, and resilience. J Pers Soc Psychol 99:1025–1041
Selye H (1973) The evolution of the stress concept: the originator of the concept traces its development from the discovery in 1936 of the alarm reaction to modern therapeutic applications of syntoxic and catatoxic hormones. Am Sci 61:692–699
Sheldon EL, Schrey AW, Ragsdale AK, Griffith SC (2018) Brood size influences patterns of DNA methylation in wild zebra finches (Taeniopygia guttata). Auk 135:1113–1122
Sih A (2011) Effects of early stress on behavioral syndromes: an integrated adaptive perspective. Neurosci Biobehav Rev 35:1452–1465
Slavich GM (2016) Life stress and health: a review of conceptual issues and recent findings. Teach Psychol 43:346–355
Spencer KA, Verhulst S (2007) Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata). Horm Behav 51:273–280
Spencer KA, Evans NP, Monaghan P (2009) Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinology 150:1931–1934
Spencer KA, Heidinger BJ, D'Alba LB, Evans NP, Monaghan P (2010) Then versus now: effect of developmental and current environmental conditions on incubation effort in birds. Behav Ecol 21:999–1004
Stratakis CA, Chrousos GP (1995) Neuroendocrinology and pathophysiology of the stress system. Stress 771:1–18
Szuran TF, Pliska V, Pokorny J, Welzl H (2000) Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav 71:353–362
Taff CC, Zimmer C, Vitousek MN (2018) Efficacy of negative feedback in the HPA axis predicts recovery from acute challenges. Biol Lett 14:20180131
Taff CC, Campagna L, Vitousek MN (2019) Genome-wide variation in DNA methylation is associated with stress resilience and plumage brightness in a wild bird. Mol Ecol 28:3722–3737
Touma C et al (2011) FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry 70:928–936
Tung J, Archie EA, Altmann J, Alberts SC (2016) Cumulative early life adversity predicts longevity in wild baboons. Nat Commun 7:11181
van Bodegom M, Homberg JR, Henckens M (2017) Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 11:87
van de Pol M, Verhulst S (2006) Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am Nat 167:766–773
Wada H, Breuner CW (2008) Transient elevation of corticosterone alters begging behavior and growth of white-crowned sparrow nestlings. J Exp Biol 211:1696–1703
Weaver ICG et al (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854
Weaver ICG, Meaney MJ, Szyf M (2006) Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A 103:3480–3485
Welberg LAM, Seckl JR (2001) Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 13:113–128
Welberg LAM, Seckl JR, Holmes MC (2001) Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience 104:71–79
Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ (2018) The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Philos Trans R Soc B Biol Sci 373:20160447
Wingfield JC (2003) Control of behavioural strategies for capricious environments. Anim Behav 66:807–816
Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD (1998) Ecological bases of hormone-behavior interactions: the “emergency life history stage”. Am Zool 38:191–206
Zimmer C, Spencer KA (2014) Modifications of glucocorticoid receptors mRNA expression in the hypothalamic-pituitary-adrenal axis in response to early life stress in female Japanese quail. J Neuroendocrinol 26:853–860
Zimmer C, Spencer KA (2015) Reduced resistance to oxidative stress during reproduction as a cost of early life stress. Comp Biochem Physiol A Mol Integr Physiol 183:9–13
Zimmer C, Boogert NJ, Spencer KA (2013) Developmental programming: cumulative effects of increased pre-hatching corticosterone levels and post-hatching unpredictable food availability on physiology and behaviour in adulthood. Horm Behav 64:494–500
Zimmer C, Larriva M, Boogert NJ, Spencer KA (2017) Transgenerational transmission of a stress-coping phenotype programmed by early life stress in the Japanese quail. Sci Rep 7:46125
Zimmer C, Taff CC, Ardia DR, Ryan TA, Winkler DW, Vitousek MN (2019) On again, off again: acute stress response and negative feedback together predict resilience to experimental challenges. Funct Ecol 33:619–628
Zimmer C, Hanson HE, Wildman DE, Uddin M, Martin LB (2020) FKBP5: a key mediator of how vertebrates flexibly cope with adversity. Bioscience 70:1127–1138
Zimmer C, Hanson HE, Martin LB (2021) FKBP5 expression is related to HPA flexibility and the capacity to cope with stressors in the house sparrow. Horm Behav 135:105038
Zozulya AA, Gabaeva MV, Sokolov OY, Surkina ID, Kost NV (2008) Personality, coping style, and constitutional neuroimmunology. J Immunotoxicol 5:221–225
Acknowledgment
VM was supported by a FWF Der Wissenschaftsfonds Lise Meitner Fellowship (#M2520-B29). We thank Renate Hengsberger for her support with the reference formatting.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Huber, S., Costantini, D., Houdelier, C., Marasco, V. (2022). Early-Life Stress Drives the Molecular Mechanisms Shaping the Adult Phenotype. In: Costantini, D., Marasco, V. (eds) Development Strategies and Biodiversity. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-90131-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-90131-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90130-1
Online ISBN: 978-3-030-90131-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)