Abstract
Circadian rhythms are maintained by the interaction of external environmental cues with body’s molecular clock machinery and help to optimize physiological functions by temporally coordinating them at the cellular, tissue, organ and behavioural level of an organism. Feeding-fasting pattern is one of the most important external cues that affect the robustness of the biological rhythms. Upon aging due to loss of indispensable neurons in the master clock- Suprachiasmatic nucleus (SCN), these rhythms get compromised and so does the temporal coordination thus leading to various age-related pathologies. Irregular eating-fasting patterns can also temporally disrupt the coordination between metabolism and physiology, leading to the onset of many chronic diseases and early aging. Hence, avoiding irregular feeding-fasting habits and maintaining strong rhythmic cycles following optimum amplitude and phase of rhythms can help in healthy aging and preventing diseases such as sleep disorders, cardiovascular, metabolic disorders, diabetes, obesity, breast cancer, inflammation, hypertension, neurodegeneration etc. Therefore, synchronizing the external cues and timings of signals from master clock along with time restricted eating patterns can help in sustaining a robust circadian clock. Such synchronization will help in preventing the diseases and improving their prognosis. In this chapter, we aim to discuss the role of diet in restoration of age-induced circadian dysfunction.
Z.A. Khan and M.S. Khan—Equal Contribution.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Circadian rhythms are evolutionarily conserved biological rhythms in almost all organisms ranging from archaea to mammals. Circadian (Latin: circa – approximately; diēm– day) rhythms are approximately 24 hour(h) rhythmic oscillations that regulate the physiology of an organism at molecular and behavioural level (Whitehead et al. 2009; Edgar et al. 2012). The adaptation to external environmental conditions using clock system allows an organism to predict changes and give an advantage to survival (Kondratov et al. 2006). Many diseases are associated with disruptions in the circadian clock like psychological disorders, sleep disorders, metabolic disorders, cardiovascular diseases, cancer, insomnia, fatigue, disorientation and hormonal profile shifts in night shift workers etc. (Hofman et al. 2006; Gibson et al. 2009). During aging, there is a shift in both amplitude and phases of circadian rhythm (Gibson et al. 2009; Jagota 2012).
Circadian rhythms have evolved as an adaptation to the oscillations found in the environment and to get entrained by them, notably to the day-night cycle (Jagota 2006). In the case of mammals, the main circadian rhythm regulator is SCN containing ~20,000 neurons located in the hypothalamic region. It acts as a timekeeper in controlling and synchronizing the circadian period of many physiological and behavioral functions and entraining them to 24 h light and dark cycles (Jagota et al. 2000; Jagota 2006; Takahashi et al. 2017). Three major pathways, Retino-hypothalamic tract (RHT), Geniculo-hypothalamic tract (GHT), and Retino-raphe pathway (RRP) receive the information from the environment through several cues such as photic (light) and non-photic cues (food, sound, humidity etc.). All these pathways diverge from the retinal ganglion cells before they enter into the SCN. Among these three, RHT is a photic pathway that has a direct synaptic contact of retinal ganglionic cells with the SCN; GHT is an indirect photic pathway where the retina conveys input signals to Intergeniculate Nucleus (IGL) via a separate branch of RHT that overlaps with the RHT terminals in the SCN; RRP is the third major input pathway which participates in the non-photic regulation of the SCN, where neuronal fibres from raphe nuclei end in the core region of the SCN (Jagota 2012).The auto-regulatory transcriptional and translational feedback loops drive the coordinated expression of genes such as (Clock)-circadian locomotor output cycles kaput, (Bmal1)-Brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1), (Per1)-Period1, (Per2)-Period2, (Per3)-Period3, (Cry1)-Cryptochrome1, (Cry2)-Cryptochrome2, (Ror)-retinoic acid-related orphan nuclear receptors, and (Rev-Erb)-reverse erythroblastosis virus etc. at both levels (Takahashi 2017).
The SCN plays the role of central clock or the relay centre of information. It regulates the release of neurohormone melatonin, the messenger of time from pineal gland. Melatonin then synchronizes the peripheral clocks with the central clock (Vriend and Rieter 2014). Every mammalian cell is autonomous and has its own clock machinery constituting the peripheral clock system controlled by the SCN through both sympathetic as well as parasympathetic pathways (Kalsbeek et al. 2010). The endogenous rhythms of clock gene expression have been reported by researchers in cell culture of peripheral clocks like liver, adrenal glands, hormones like adrenocorticotropic hormone (ACTH) and glucocorticoids (Cailotto et al. 2005; Mahoney et al. 2010).
2 Food as an Important Clock Regulator
Food has been reported as an important non-photic zeitgeber (ZT) or time giver to synchronize an organism's biological rhythms and shifting the timing of food leads to the shift in the activity of an organism (Carneiro et al. 2012). In SCN lesioned mice, food restriction has demonstrated to induce locomotor behaviours as well as temperature rhythms (Froy et al. 2010). Food timings entrain circadian clocks in different brain regions and most peripheral organs, thereby synchronizing their daily rhythms. This entrainment of peripheral clocks to mealtime is accomplished by multiple feeding-related signals, including absorbed nutrients and metabolic hormones, acting in parallel or series in a tissue-specific manner. Signals that synchronize circadian clock in the brain with feeding time are presumed to generate the circadian rhythms of food-anticipatory activity (FAA) that emerge when food is restricted to a fixed daily mealtime. Such FAA is regulated and paced by the food-entrainable oscillator (FEO) located outside the SCN (Flores et al. 2016; Chaudhari et al. 2017). Several timekeeping mechanisms involved in the FAA occurring before food intake include neuronal activation, molecular clock entrainment, hormonal cues, and metabolic regulation (Tahara et al. 2013; Challet 2019). The synchronization between food entrainable clock and central clock system is driven by a complex mechanism including humoral and enzymatic regulatory pathway (Challet 2019). β-hydroxybutyrate (β-OHB) synthesized in the liver during fasting conditions has been proposed to be a key candidate molecule in those pathways. Apart from being an energy source, β-OHB has many cellular signalling actions and participates in FAA modulation and its production is clock regulated involving Per2 (Newman et al. 2017).
3 Food and Melatonin Hormone
The synthesis and release of melatonin from the pineal gland is under regulation of the SCN. The photic cues are received by the SCN clock and relayed to pineal gland through a multi-synaptic pathway. Melatonin is a “night-time hormone” in all the animals (diurnal, nocturnal and crepuscular) irrespective of their activity niche and thus called messenger of darkness (Jagota et al. 2012; 2019). Melatonin is synthesised from serotonin through N-acetylation followed by methylation reaction in the presence of arylalkylamine N-acetyltransferase (AANAT; rate limiting enzyme) and hydroxyl indole-O-methyl-transferase (HIOMT) respectively (Jagota et al. 2012). Endogenous melatonin rhythm modulates feeding and fasting behaviour and anticipation of meal time. Melatonin cues modulate the phase and amplitudes of various hormonal rhythms including rhythms of cortisol and insulin (Challet et al. 2019). Reduced levels of melatonin has been associated with aging (Reddy and Jagota 2015). Melatonin is an important hormetin (Jagota et al. 2019), and its manipulation with dietary interventions in elderly may lead to restoration of circadian clock functions and improvement of health and wellbeing.
4 Circadian Dysfunction with Aging
Aging is an inevitable unidirectional natural process in the lifespan of an organism. The disruption of the endogenous nature of the molecular clock, deteriorated temporal synchronisation of the oscillators and an overall change in the physiology dampens the circadian rhythm as the organism ages (Jagota et al. 2000; Manoogiana and Panda 2017; Jagota et al. 2019). A phase advance in body temperature and melatonin secretion rhythms has been associated with aging in humans. Alterations in the daily rhythms of clock genes (Mattam and Jagota 2014), serotonin metabolism (Kalyani and Jagota 2008; 2010; Reddy and Jagota 2015), antioxidant enzymes (Manikonda and Jagota 2012), leptin (Reddy and Jagota 2014), nitric oxide (NO), and Suppressor of cytokine signaling (Socs) (Vinod and Jagota 2016; 2017) expression has been reported from our laboratory. Additionally, alterations in daily rhythms of Sirtuin1 (Sirt1), Nuclear factor erythroid 2–related factor 2 (Nrf2), Rev-erbα and inflammatory markers including Nfkb1, Tumor necrosis factor alpha (Tnf-α), Interleukin-6 (Il-6), Toll-like receptor 4 (Tlr4) and Toll-like receptor 9 (Tlr9) affecting circadian timing system (CTS) were observed with aging (Thummadi and Jagota 2019; Kukkemane and Jagota 2020). As endogenous rhythms dampen and deteriorate with age, the contribution by the external cues and its timing starts playing an increasingly critical role in maintaining the amplitude and phase of an organism’s circadian clock (Manoogian and Panda 2017; Jagota et al. 2020).
5 Circadian Rhythms, Metabolism and Homeostasis with Aging
The circadian clock controls energy metabolism and maintains homeostasis in peripheral tissues through the controlled expression of various metabolic hormones like leptin, ghrelin, secretin etc. involved in different metabolic pathways. This helps in maintaining the normal physiological functions and healthy aging (Green et al. 2008; Froy 2009).The circadian clock is itself under metabolic regulation and the disturbances induced by a nutrient imbalance results in circadian dysfunction (Chaix et al. 2014). Many hormones such as insulin, glucagon adiponectin, corticosterone, and ghrelin have been found to show circadian expression and oscillation (Ando et al. 2005; Yang et al. 2006). The temporal regulation of insulin which shows a peak in its production at 1700 h and a nadir at 0400 h is achieved by both the patterns of feeding-fasting and SCN signalling (Sadacca et al. 2011; Vieira et al. 2015). Leptin, a well-known appetite repressor also shows circadian rhythmicity in its expression. The removal of the SCN abolishes the rhythmicity of its secretion in rodents leading to uncontrolled feeding behaviour, obesity, pathologies, and early aging effects suggesting the role of the clock system in aging, metabolism and maintaining homeostasis (Kalra et al. 2003). The receptors for leptin and ghrelin have been demonstrated in SCN (Yi et al. 2006; Zigman, et al. 2006) thus, establishing a direct link between the main circadian clock (SCN) and metabolism (Prosser et al. 2003). Metabolism is also influenced by the microbiome which makes an important component of the gastrointestinal tract (GIT). The microbiome shows rhythmic oscillations in its composition as per the requirement in GIT for proper metabolism. Erratic feeding patterns can dampen the taxonomic diversity and disrupt the oscillating rhythm of the microbiome, contributing to metabolic disorders like intestinal dysbiosis, obesity, and early aging (Voigt et al. 2016). Forced feeding-fasting patterns achieved by different strategies like intermittent fasting (IF), periodic fasting (PF), and calorie restriction (CR) have been shown to restore some of these oscillations to normal and achieve healthy aging (Zarrinpar et al. 2014).
6 Chrononutrition: Timing of Food as a Therapeutic Intervention
Due to demanding work pressure and changing food habits, unhealthy and unscheduled meals including junk food have now become a part of our lifestyle. Such unscheduled meals with unhealthy amounts of sugar, salts, caffeine, processed meat, fats and an inadequate intake of fruits, green leafy vegetables, cereals etc. are the major risk factors for developing age-related pathologies and accelerate the aging process (Micha et al. 2017). Patterns of feeding and fasting can potentially contribute to the development of chronic pathologies and thus have an influential impact on human health and onset of diseases (Zarrinpar et al. 2014). Although clock oscillators in our body can recuperate from mild alteration in our daily feeding times, chronic imbalanced and unscheduled feeding behaviour results in untimed cues, circadian dysfunction and disease pathologies later in life with unhealthy aging (Asher and Sassone-Corsi 2015; Lopez-Minguez et al. 2019). Thus, the temporal attributes of food and its role in health and disease are as vital as the qualitative and quantitative nutritional aspects (Gupta et al. 2017; Kant et al. 2018).
6.1 Food, Energy Metabolism, Circadian Dysfunction, and Aging
Various mitochondrial rate-limiting enzymes are rhythmically expressed (Neufeld-Cohen et al. 2016). In circadian mutant mice, enforced feeding-fasting patterns can reinstate rhythmic expression of some of metabolites, such as Acylcarnitine carrier protein and Acyl CoA Dehydrogenase (Manoogian and Panda 2016; Neufeld-Cohen et al. 2016). Mice with a deleted exon-19 of the Clock gene shows an altered feeding rhythm, over-eating, obesity and other metabolic syndromes like high blood leptin, lipids and glucose levels (Turek et al. 2005). Such mutant (ClockΔ19) mice also showed a decrease in the expression of hypothalamic peptides like ghrelin and orexin, which are important for energy balance (Turek et al. 2005). Per2 mutations abolished rhythmicity for glucocorticoids, feeding patterns and caused obesity (Yang et al. 2009).
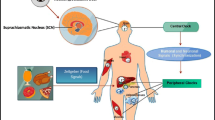
Aging leads to significant disruption in adipogenesis by affecting circadian components, Bmal1, Rev-erbα causing multiple pathologies like obesity, cardiovascular diseases and decline in longevity (Duez et al. 2008). Peroxisome proliferator-activated receptor α (PPARα) is important for transcriptional activation of Bmal1 and genes responsible for the metabolism of lipids and glucose (Lefebvre et al. 2006; Lamia et al. 2008). Impairment of PPARα leads to defective fatty acid oxidative pathways, enhanced inflammatory response, oxidative stress and renal fibrosis and impaired energy metabolism (Adnan 2007). Adenosine 5′-monophosphate–activated protein kinase (AMPK), an energy sensor of cells integrates the circadian clock with metabolism by regulating the response to feeding and modulating NAD+ levels and SIRT1 activity (Hardie et al. 2006; Canto et al. 2009). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a transcriptional co-activator of Bmal1 and Rev-erbα also regulates energy metabolism. Disrupted expressions of AMPK and PGC-1α leads to abnormal daily diurnal rhythms, body temperature energy imbalance, metabolic disorders, multiple pathologies and early aging (Grimaldi et al. 2007). Another important protein found to display a link between metabolism and the circadian clock of mammals is SIRT1. The influence of nutrient state and the circadian clock on insulin sensitivity is via SIRT1 (Bass and Takahashi 2010). It is an NAD+ dependent histone deacetylase that plays an important role in extending the life span in yeast, Caenorhabditis elegans, Drosophila and mice (Mair et al. 2008; Canto et al. 2009). SIRT1 can interact with CLOCK directly and deacetylate BMAL1 and PER2 in cultured fibroblasts (Asher et al. 2008; Nakahata et al. 2008). The information on the food, circadian clock, and metabolic factors discussed here has been compiled as a schematic diagram in Fig. 19.1.
Simplified schematic representation of the link between circadian clock, metabolism and aging. Aging leads to significant disruption in glucose and lipid metabolism by affecting circadian components like Bmal1, Clock, Rev-erbα etc. and metabolic components like Peroxisome proliferator-activated receptor α (PPARα) and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). Also, feeding and fasting cycle, controlled by Adenosine 5′-monophosphate–activated protein kinase (AMPK), with the involvement of NAD+, and SIRT1 gets desynchronised upon aging. This age induced desynchronization between clock and metabolism leads energy imbalance, metabolic disorders and accelerated aging.
 = Abolition of rhythms; (+) = Induces the expression
= Abolition of rhythms; (+) = Induces the expression
7 Various Dietary Interventions: Circadian Clock, Aging and Longevity
Dietary intervention is an ancient practice and various researchers using experimental models from invertebrates (C. elegans) to mammals (rat/mice) have demonstrated mean life-span extension by using different strategies of dietary interventions (Di Francesco et al. 2018). Such strategies (Table 19.1) achieved either by fasting for a certain period or reducing the calorie intake are discussed below.
7.1 Restricted Feeding (RF)
Restricting food to a particular time of the day while still ensuring nutritional adequacy is called restricted feeding (RF) (Froy et al. 2010). RF controls the FAA, which includes corticosterone secretion, gastrointestinal motility and activity of digestive enzymes before meals (Stephan et al. 2002). RF is dominant over the SCN and able to drive the rhythms in clock mutant rodents and animals with lesioned SCN regardless of photic cues (Hara et al. 2001; Stephan 2002). But in some cases, RF only affects the clock system in peripheral tissues such as the heart, kidney, pancreas and liver, causing their uncoupling from the SCN, followed by metabolic disorders, pathologies and early aging (Schibler et al. 2003; Hirota et al. 2004). This suggests the nutritional regulation of clock oscillators in peripheral tissues and its involvement in metabolic disorders and aging (Lin et al. 2008). Damiola et al. 2000 demonstrated when the food availability is made normal, the SCN, whose phase remains unaffected, resets the peripheral oscillators and synchronization of physiology. Per2 mutant mice do not show wheel-running food anticipation (Mistlberger 2006). In one of the interesting studies, the survival time of mice inoculated with osteosarcoma was prolonged under RF (Wu et al. 2004). RF has been found to modify the expression of genes responsible for carcinogenesis and tumor progression such as c-myc and p53 (Filipski et al. 2005) but whether RF affects life span in a real sense or not is still not clear.
7.2 Intermittent Fasting (IF)
IF is a cyclic pattern of eating and fasting, one of the regimen of IF is alternate day fasting (ADF), where food is available ad-libitum every alternate day (Froy et al. 2010). Two more variations of IF include: Periodic Fasting (PF) which lasts for 2 or more days followed by the next cycle after 6–7 days (Longo et al. 2016; Vargas et al. 2020) and Time restricted feeding (TRF), where food is taken in a specific time window of 8 h or less (Mattson et al. 2017). Recently, IF has gained popularity due to its various beneficial effects on health (De Cabo et al. 2019). Animals on IF exhibit increased life span, improved glucose metabolism, cardio physiology, neuro-protection and resistance towards cancer (Descamps et al. 2005; Mattson et al. 2005; Varady et al. 2007). Rev-erbα, an important component of the circadian clock, also regulates the expression of genes involved in metabolism and inflammation. It was observed that there is an average 11 fold increase in its levels at the end of the 4th week during 30-days of IF (Mindikoglu et al. 2020). RF schedules elicit a phase shift in molecular and metabolic machinery components of peripheral clocks. Likewise, imposed periods of extended daily fasting, independent of dietary composition and calorie intake have significant metabolic and lifespan benefits (Mitchell et al. 2018).
7.3 Time-Restricted Feeding (TRF)
A feeding regime like Time-restricted feeding (TRF) has shown beneficial effects in animals and is believed to compensate and consolidate the circadian rhythms (Chaix et al. 2014; De Cabo and Mattson 2019). Flies on TRF showed a sustained nocturnal sleep which is about double the total sleep duration of the flies with food ad libitum (Gill et al. 2015). Nocturnal rodents given TRF with food ad libitum during the light phase (normally their rest period) show altered expression of clock genes and clock-controlled genes (CCGs) in the peripheral tissues without affecting SCN (Damiola et al. 2000; Stokkan et al. 2001). A differential adaption to the novel feeding regimens has been observed in peripheral clocks where Kidneys, lungs, or heart adapts to changes slower than the liver. TRF of normal diets improves energy metabolism in humans in short-term studies and contributes to a healthy life span even though calories are not restricted (Sutton et al. 2018; Jamshed et al. 2019). High-fat diets (HFD), representing the western diet model, has been reported to cause circadian dysfunction by disrupting the feeding-fasting rhythms and gene expression rhythms (Hatori and Panda 2015; Potter et al. 2016). A very recent report has shown that an extended HFD and night restricted feeding (NtRF) regimen on senescence-accelerated mouse strain, SAMP8 ameliorates age-related phenotypes. The positive impact of HFD has been manifested till metabolic perturbations kicked in. So, the HFD and chrono-nutritional feeding in combination can be an effective anti-aging strategy (Oike et al. 2020) ensuring a healthy life span.
7.4 Caloric Restriction (CR)
CR has almost similar beneficial effects on health as that mediated through IF and leads to circadian clock plasticity by chromatin remodelling (Nakahata et al. 2007). CR can induce expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, phosphorylation of cAMP response element-binding protein (CREB), dendritic spine density and transcription of BDNF (Whitmore et al. 2000; Eide et al. 2001; Etchegaray et al. 2006). CR not only reduces the risk of age-associated circadian dysfunction and disorders but also significantly impedes aging and enhances longevity (Taormina et al. 2014). When Drosophila was subjected to caloric restriction, expression of several clock genes showed increase in amplitude (Katewa et al. 2015). CR affects significantly the expression of several genes in both central clock-SCN and the peripheral clocklike the liver (Patel et al. 2016). The importance of meal timing by studies on α-MUPA mice have shown that reduced calories alone were not able to sustain rhythms unless feeding was spontaneously timed at night, or the day through a RF protocol. Further, to investigate the involvement of the circadian clock in impacting the metabolic activity and life span via CR, core clock gene knockouts were used (Bmal1 in mice and Per and Timeless (Tim) in Drosophila); circadian clock disruption along with multiple metabolic disorders and increased life span was noted in such experiments (Patel et al. 2016; Katewa et al. 2015). In mammals, CR mediates decline in blood IGF-1 level and the effect was compromised in mice deficient for BMAL1, an important circadian transcriptional factor. With CR, the diurnal activity and sleep pattern dampens in fruit flies as seen in humans with night sleep pattern. An overall diagrammatic representation of effects of different feeding regimens on aging and longevity has been summarised as Fig. 19.2.
A diagrammatic representation of the effect of feeding regimens on aging and longevity through resetting of central and peripheral clock system. Intermittent fasting, caloric restriction, time restricted feeding, and high fat diet with TRF helps in resetting the circadian rhythms in both peripheral and the central clock systems but RF resets the rhythms only in the peripheral clocks
8 Nutritional Epigenetic: Aging and Clock
The nutritional modulation of the circadian clock has been reported to be linked with the epigenetic regulation of various clock genes. The modifications by sirtuins, a class III NAD+ dependent diet-sensitive histone deacetylase enzymes, are of vital importance to genome stability. Sirt1 is believed to be a key epigenetic regulator protecting the mammals from events and consequences that ultimately lead to aging (Hudec et al. 2020). Higher Silent information regulator 2 (Sir2; mammalian Sirt1 equivalent) expression has been reported to extend the lifespan in Drosophila melanogaster and Sirt1 knock-outs die young owing to developmental defects. Single nucleotide polymorphism (SNPs) gene variants for core clock genes have been implicated in age related disorders and individual dietary response in humans. Among the epigenetic mechanisms that control circadian rhythms, microRNAs are the least studied ones compared to SNPs and such studies can be a key to novel chrono-therapeutic interventions (Mico et al. 2016).
9 Conclusion
Dietary interventions are promising and easy-to-adapt strategies for the modulation and prevention of circadian dysfunction and senescence in humans of different ages. Recently, adjustment of caloric intake using different strategies like IF, PF and TRF have emerged as potential strategies towards treatment of metabolic syndromes like cardiovascular diseases, cancer and possibly neurodegenerative diseases etc. and helping in the synchronization of the circadian clock system. RF entrains peripheral clocks suggesting their role via temporal food consumption, whereas, CR and IF appears to synchronize the central pacemaker in the SCN, suggesting their role by involving low calories intake in the entrainment of the central clock system. A direct relationship between feeding time and onset of chronic diseases such as obesity, breast cancer and inflammatory and metabolic disorders, neurological and sleep disorders explains that metabolic state is linked to sensitization in different parts of the brain, especially the hypothalamus and hippocampus, to maintain the coordination between the neuroendocrine system, metabolism, and energy balance. The CTS, thus influences and resets a wide variety of output systems like cellular and physiological systems to perform in a more synchronized manner hence maintaining the robust circadian rhythms using dietary interventions can ensure better tissue and body homeostasis and mediating aging attenuation and promoting longevity.
References
An HS, Lee JY, Choi EB, Jeong EAS, Kim HJ, Park KE, Jin KA, Lee Z, J.E. Koh J.S, (2020) Caloric restriction reverses left ventricular hypertrophy through the regulation of cardiac iron homeostasis in impaired leptin signalling in mice. Sci Rep 10:7176
Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A (2005) Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 146:5631–5636
Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP (2003) Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA 100:6216–6220
Asher G, Sassone-Corsi P (2015) Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161:84–92
Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–328
Barnosky AR, Hoddy KK, Unterman TG, Varady KA (2014) Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res J Lab Clin Med 164:302–311
Bass J, Takahashi JS (2010) Circadian integration of metabolism and energetic. Science 330(6009):1349–1354
Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A et al (2005) The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci 22:2531–2540
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060
Carneiro BTS, Araujo JF (2012) Food entrainment: major and recent findings. Front Behav Neurosci 6:83
Chaix A, Zarrinpar A, Miu P, Panda S (2014) Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20:991–1005
Challet E (2019) The circadian regulation of food intake. Nat Rev Endocrinol 15:393–405
Chaudhari A, Gupta R, Makwana K, Kondratov R (2017) Circadian clocks, diets and aging [JB]. Nutr Healthy Aging 4(2):101–112
Contestabile A, Ciani E (2004) Dietary restriction differentially protects from neurodegeneration in animal models of excitotoxicity. Brain Res 1002:162–166
Damiola F, Le Minli N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14:2950–2961
de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381(26):2541–2551
Descamps O, Riondel J, Ducros V, Roussel AM (2005) Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech Ageing Dev 126:1185–1191
Di Di Francesco A, Germanio C, de Bernier M, Cabo RA (2018) A time to fast. Science 362:770–775
Duez H, Staels B (2008) Rev-erb alpha gives a time cue to metabolism. FEBS Lett 582:19–25
Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485:459–464
Eide EJ, Virshup DM (2001) Casein kinase I: another cog in the circadian clockworks. Chronobiol Int 18:389–398
Erol A (2007) The Functions of PPARs in aging and longevity. PPAR Res 2007:1–10
Etchegaray JP, Yang X, DeBruyne JP, Peters AH, Weaver DR, Jenuwein T, Reppert SM (2006) The polycomb group protein EZH2 is required for mammalian circadian clock function. J Biol Chem 281:21209–21215
Filipski E, Innominato PF, Wu M, Li XM, Iacobelli S, Xian LJ, Levi F (2005) Effects of light and food schedules on the liver and tumor molecular clocks in mice. J Natl Cancer Inst 97:507–517
Flores DEFL, Bettilyon CN, Jia L, Yamazaki S (2016) The running wheel enhances food anticipatory activity: an exploratory study. Front Behav Neurosci 10:143
Froy O (2009) Metabolism and circadian rhythms-implications for obesity. Endocr Rev 31:1–24
Froy O, Miskin R (2010) Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging 2(1):7–27
Gibson EM, Williams WP 3rd, Kriegsfeld LJ (2009) Aging in the circadian system: considerations for health, disease prevention, and longevity. Exp Gerontol 44:51–56
Gill S, Panda S (2015) A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 22(5):789
Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N (1990) Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev 55:69–87
Green CB, Takahashi JS, Bass J (2008) The meter of metabolism. Cell 134:728–742
Grimaldi B, Sassone-Corsi P (2007) Circadian rhythms: metabolic clockwork. Nature 447:386–387
Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH (1997) Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol 133:1–7
López-Lluch G, Del Pozo-Cruz J, Sánchez-Cuesta A, Cortés-Rodríguez AB, Navas P (2019) Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 57:133–140
Gupta NJ, Kumar V, Panda S (2017) A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS ONE 12:e0172852
Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted feeding entrains liver clock without the participation of the suprachiasmatic nucleus. Genes Cells 6:269–278
Hardie DG, Hawley SA, Scott JW (2006) AMP-activated protein kinase–the development of the energy sensor concept. J Physiol 574:7–15
Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (lond) 35:714–727
Hatori M, Panda S (2015) Response of peripheral rhythms to the timing of food intake. Meth Enzymol 2015(552):145–161
Hirao J, Arakawa S, Watanabe K, Ito K, Furukawa T (2006) Effects of restricted feeding on daily fluctuations of hepatic functions including p450 monooxygenase activities in rats. J Biol Chem 281:3165–3171
Hirota T, Fukada Y (2004) Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci 21:359–368
Hofman MA, Swaab DF (2006) Living by the clock: the circadian pacemaker in older people. Ageing Res Rev 5:33–51
Hudec M, Dankova P, Solc R, Bettazova N, Cerna M (2020) Epigenetic regulation of circadian rhythm and its possible role in diabetes mellitus. Int J Mol Sci 21(8):3005
Jagota A (2006) Suprachiasmatic nucleus: the center for circadian timing system in mammals. Proc Indian Natl Sci Acad 71:275–288
Jagota A (2012) Age-induced alterations in biological clock: therapeutic effects of melatonin. In: Brain aging and therapeutic interventions. Springer, Netherlands, pp 111–129
Jagota A, Kalyani D (2008) Daily serotonin rhythms in rat brain during postnatal development and ageing. Biogerontology 9:229–234
Jagota A, Kalyani D (2010) Effect of melatonin on age induced changes in daily serotonin rhythms in suprachiasmatic nucleus of male Wistar rat. Biogerontology 11:299–308
Jagota A, Horacio O, Schwartz WJ (2000) Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci 3(4):372–376
Jagota A, Thummadi NB, Kukkemane K (2019) Circadian regulation of hormesis for health and longevity. In: The science of hormesis in health and longevity. Elsevier, pp 223–233
Jamshed H, Beyl R, Della Manna D, Yang E, Ravussin E, Peterson C (2019) Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 11(6):1234
Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S (2007) Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biol Med 42:665–674
Kalra SP, Bagnasco M, Otukonyong EE, Dube MG, Kalra PS (2003) Rhythmic, reciprocal ghrelin and leptin signalling: new insight in the development of obesity. RegulPept 111:1–11
Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E (2010) Hypothalamic control of energy metabolism via the autonomic nervous system. Ann NY Acad Sci 1212:114–129
Kant AK (2018) Eating patterns of US adults: meals, snacks, and time of eating. Physiol Behav 193:270–278
Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng X, Hall D, Davis S, Nelson CS, Brem RB, Ramanathan A, Sehgal A, Giebultowicz JM, Kapahi P (2015) Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in drosophila. Cell Metab 23(1):143
Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20:1868–1873
Kukkemane K, Jagota A (2020) Therapeutic effects of hydro-alcoholic leaf extract of With aniasomnifera on age-induced changes in daily rhythms of Sirt1, Nrf2 and Rev-erbα in the SCN of male Wistar rats. Biogerontology 21(5):593–607
Lamia KA, Storch KF, Weitz CJ (2008) Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105:15172–15177
Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116:571–580
Lin JD, Liu C, Li S (2008) Integration of energy metabolism and the mammalian clock. Cell Cycle 7:453–457
Longo VD, Panda S (2016) Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 23:1048–1059
Lopez-Minguez J, Gómez-Abellán P, Garaulet M (2019) Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk. Nutrients 11(11):2624
Mahoney CE, Brewer D, Costello MK, Brewer JM, Bittman EL (2010) Lateralization of the central circadian pacemaker output: a test of neural control of peripheral oscillator phase. Am J Physiol 299:R751–R761
Mair W, Dillin A (2008) Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem 77:727–754
Manikonda PK, Jagota A (2012) Melatonin administration differentially affects age-induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat. Biogerontology 13(5):511–524
Manoogian ENC, Panda S (2016) Circadian clock, nutrient quality, and eating pattern tune diurnal rhythms in the mitochondrial proteome. Proc Natl Acad Sci 13(12):3127–3129
Manoogian ENC, Panda S (2017) Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev 39:59–67
Mattam U, Jagota A (2014) Differential role of melatonin in restoration of age-induced alterations in daily rhythms of expression of various clock genes in suprachiasmatic nucleus of male Wistar rats. Biogerentology 15:257–268
Mattson MP, Wan R (2005) Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J NutrBiochem 16:129–137
Mattson MP, Longo VD, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39:46–58
Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D (2017) Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 317:912–924
Micó V, Díez-Ricote L, Daimiel L (2016) Nutrigenetics and nutrimiromics of the circadian system: the time for human health. Int J Mol Sci 17(3):299
Mindikoglu AL, Abdulsada MM, Jain A, Choi JM, Jalal PK, Devaraj S, Mezzari MP, Petrosino JF, Opekun AR, Jung SY (2020) Intermittent fasting from dawn to sunset for 30 consecutive days is associated with anticancer proteomic signature and upregulates key regulatory proteins of glucose and lipid metabolism, circadian clock, DNA repair, cytoskeleton remodelling, immune system and cognitive function in healthy subjects. J Proteomics 217:103645
Mistlberger RE (2006) Circadian rhythms: perturbing a food-entrained clock. Curr Biol 16:R968–R969
Mitchell SJ (2018) Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab 29(1):221–228
Nakahata Y, Grimaldi B, Sahar S, Hirayama J, Sassone-Corsi P (2007) Signalling to the circadian clock: plasticity by chromatin remodelling. CurrOpin Cell Bio 19:230–237
Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P (2008) The NAD+ dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodelling and circadian control. Cell 134:329–340
Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, Mann M, Asher G (2016) Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci USA 113:E1673–E1682
Newman JC, Verdin E (2017) Beta-Hydroxybutyrate: a signalling metabolite. Annu Rev Nutr 37:51–76
Oike H, Ogawa Y, Azami K (2020) Long-term feeding of a high-fat diet ameliorated age-related phenotypes in SAMP8 mice. Nutrients 12(5):1416
Patel SA, Chaudhari A, Gupta R, Velingkaar N, Kondratov RV (2016) Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J 30(4):1634
Potter GD, Cade JE, Grant PJ, Hardie LJ (2016) Nutrition and the circadian system. Br J Nutr 116(3):434–442
Prosser RA, Bergeron HE (2003) Leptin phase-advances the rat suprachiasmatic circadian clock in vitro. Neurosci Lett 336:139–142
Reddy VDK, Jagota A (2014) Effect of restricted feeding on nocturnality and daily leptin rhythms in OVLT in aged male Wistar rats. Biogerontology 15:245–256
Reddy MY, Jagota A (2015) Melatonin has differential effects on age-induced stoichiometric changes in daily chronomics of serotonin metabolism in SCN of male Wistar rats. Biogerontology 16:285–302
Ripperger JA, Schibler U (2006) Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet 38:369–374
Sadacca LA, Lamia KA, DeLemos AS, Blum B, Weitz CJ (2011) An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 54:120–124
Schibler U, Schibler U, Ripperger J, Ripperger J, Sa B, Sa B (2003) Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms 18:250–260
Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O (2012) Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J 26(8):3493
Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G (2012) Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (dordr) 34:917–933
Stephan FK (2002) The “other” circadian system: food as a Zeitgeber. J Biol Rhythms 17(284):292
Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291:490–493
Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM (2018) Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27:1212–1221.e1213
Tahara Y, Shibata S (2013) Chronobiology and nutrition. Neuroscience 253:78–88
Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18:164–179
Taormina G, Mirisola MG (2014) Calorie Restriction in mammals and simple model organisms. BioMed Res Int 10:1155
Thummadi NB, Jagota A (2019) Aging renders desynchronization between clock and immune genes in male Wistar rat kidney: chronobiotic role of curcumin. Biogerontology 20(4):515–532
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045
Varady KA, Hellerstein MK (2007) Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J ClinNutr. 86:7–13
Vieira E, Merino B, Quesada I (2015) Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas. Diabetes Obes Metab 17:106–114
Vinod C, Jagota A (2016) Daily NO rhythms in peripheral clocks in ageing male Wistar rats: protective effects of exogenous melatonin. Biogerontology 17:859–871
Vinod C, Jagota A (2017) Daily Socs1 rhythms alter with ageing differentially in peripheral clocks in male Wistar rats: therapeutic effects of melatonin. Biogerontology 18:333–345
Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, Vitaterna MH, Turek FW, Keshavarzian A (2016) The circadian clock mutation promotes intestinal dysbiosis. Clin Exp Res 40:335–347
Vriend J, Reiter RJ (2014) Melatonin feedback on clock genes : a theory involving the proteosome. J Pineal Res 58:1–11
Whitehead K, Pan M, Masumura K-I, Bonneau R, Baliga NS (2009) Diurnally entrained anticipatory behavior in archaea. PLoS ONE 4(5):e5485
Whitmore D, Cermakian N, Crosio C, Foulkes NS, Pando MP, Travnickova Z, Sassone-Corsi P (2000) A clock work organ. Biol Chem 381:793–800
Wu MW, Li XM, Xian LJ, Levi F (2004) Effects of meal timing on tumor progression in mice. Life Sci 75:1181–1193
Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM (2006) Nuclear receptor expression links the circadian clock to metabolism. Cell 126:801–810
Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH (2009) The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150:2153–2160
Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM (2006) Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147:283–294
Zarrinpar A, Chaix A, Yooseph S, Panda S (2014) Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 20:1006–1017
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548
Acknowledgements
AJ is thankful to Prof. Suresh Rattan and Prof. Gurcharan Kaur for giving this opportunity and sincere patience during preparation of manuscript. The work is supported by DBT (102/IFD/SAN/5407/2011-2012), ICMR (Ref. No. 55/7/2012-/BMS), and UPE II and Grants to AJ. ZAK and SMK are thankful to BBL Fellowships from University of Hyderabad.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
All authors declare they have no conflict of interest.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jagota, A., Khan, Z.A., Khan, M.S. (2021). Diet and Circadian Rhythms: Implications for Aging and Longevity. In: Rattan, S.I.S., Kaur, G. (eds) Nutrition, Food and Diet in Ageing and Longevity. Healthy Ageing and Longevity, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-030-83017-5_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-83017-5_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83016-8
Online ISBN: 978-3-030-83017-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)