Abstract
Circadian rhythms ensure the synchronization of the physiology of cells and tissues in accordance with daily changes in the environment. These rhythms are maintained by transcriptional oscillators located in various organism cells. One of the rhythm sensors for the circadian clock is the intake of nutrients, this synchronizer is especially important in peripheral tissues. With age, the work of both the central and peripheral clock is disturbed. In old age, the amplitude of rhythms decreases and the peaks of expression of clock genes shift. Such changes affect not only the circadian, but also other rhythms. Promising ways to maintain circadian rhythms are a variety of dietary patterns, including both calorie restriction, well known for its ability to prolong the lifespan of laboratory animals, and time-restricted feeding. It is now known that intracellular metabolic sensors are also involved in regulation of the circadian clock. Among these sensors, it should be especially noted AMPK, which coordinates many catabolic and anabolic processes and participates in the implementation of the effect of calorie restriction. It is assumed that non-drug modulation of AMPK activity will not only help fight metabolic disorders, but also maintain circadian rhythms. The review considers the role of AMPK and some other metabolic sensors in the regulation of the circadian clock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
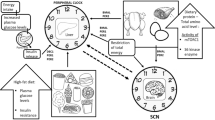
Circadian rhythms help the body adapt to the day/night cycle, providing synchronization of cell and tissue physiology in accordance with diurnal environmental changes. Due to such synchronization, a coherent temporal organismal physiology is established [1, 2]. The circadian clock is based on a complex network of transcriptional oscillators regulated by transcription–translation feedback loops.
The circadian clock mechanism of mammals involves the central and peripheral clocks. The central clock coordinates not only physiology but also behavior in accordance with the light–dark regime; it is localized in the suprachiasmatic nucleus (SCN) of the hypothalamus and synchronized by light stimulus through the retinohypothalamic tract. Peripheral clocks are present in almost all cells and tissues; their main rhythm sensor (Zeitgeber) is the intake of nutrients [3]. Additional rhythm sensors for the circadian clock are temperature, physical activity, and hormonal regulation [4, 5].
At a later age, the functioning of the circadian clock is disrupted: there are shifts in the peaks of clock gene expression, impaired synchronization in peripheral tissues, decay of the rhythm amplitude, etc. [6, 7]. The behavioral and molecular changes developing as a result of such deterioration contribute to further aggravation of age-related disorders [1]. Disorganizations of oscillation of circadian gene expression are observed not only in the experiments with animals and the studies involving humans, but also in the works with cell cultures [3, 8], because even individual cells contain self-sustaining cell-autonomous oscillators.
Nutrition plays an important role in synchronization of at least peripheral clocks (especially in the liver). At the same time, calorie restriction is a proven way to slow down aging. Nutrient sensors affect the signaling pathways that are not only related to aging [9] but also involved in the regulation of circadian rhythms. Among such sensors, we should take particular note of the sensors with a large number of targets: SIRT1, IGF-1, PARP1, FOXO, AMPK, and the mTOR complex. In view of the above, circadian clock modulators and participants of nutrient-sensing signaling cascades capable of activating and deactivating the clock mechanism can potentially be used to slow down aging and to eliminate its negative consequences. AMPK seems to be one of the most interesting sensors as it has a systemic effect and is involved in implementation of the effect of calorie restriction. By phosphorylating multiple targets, it provides the adaptation of both individual cells and whole organism to nutrient deficiencies.
CIRCADIAN RHYTHMS
The major elements of the circadian clock mechanism in mammals—BMAL1 (brain and muscle ARNT-like 1) and CLOCK (circadian locomotor output cycles kaput)—form a heterodimeric complex activating production of the following proteins: Period (PER) 1, 2 and 3 and Cryptochrome (CRY) 1 and 2. Once the PER and CRY concentrations have reached critical values, they form heterotypic complexes and, after migrating into the cell nucleus, inhibit the CLOCK–BMAL1 complex. When PER and CRY concentrations fall, the inhibition of the BMAL1–CLOCK complex is eliminated, followed by initiation of a new transcription cycle, with each cycle taking approximately 24 hours.
In addition to the Per and Cry genes, the CLOCK–BMAL1 complex controls the rhythmic transcription of the so-called clock-controlled genes (CCG) and many other genes [10, 11], providing oscillatory regulation of various biological functions. BMAL1 oscillations are also regulated by other participants. Its expression is activated by the group of retinoic acid-related orphan receptors (ROR) and inhibited by nuclear receptor subfamily 1, group D, member 1 (NR1D1, or REV-ERB-α) and 2 (NR1D2, or REV-ERB-β).
Posttranslational modifications such as phosphorylation and ubiquitination also play an important role in regulation of the molecular circadian clock. For example, casein kinase 1ε (CKIε) regulates the activity of PER–CRY dimers. Recognition of the targets for subsequent proteasomal degradation of PER and CRY is regulated by ubiquitin ligases, e.g., FBXL3 (F-box and leucine-rich repeat protein 3) [12].
AGE-RELATED CIRCADIAN RHYTHM DISORDERS
Deviations in the circadian rhythm are associated with various pathologies, including not only sleep disorders but also eating disorders, inflammation, mental disorders and age-related diseases such as obesity, type 2 diabetes mellitus, and Alzheimer’s disease. For example, social jetlag (discrepancies between the biological circadian clock and the social clock) has been shown to increase the risk of type 2 diabetes and metabolic syndrome [13].
The development of sleep disorders is promoted by the high level of city illumination at night, electronic devices, night and shift work, as well as age-related changes. All these factors disrupt the finely tuned mechanism of circadian rhythms. In different animal species it has been shown that there is a negative relationship between lifespan and deviation of the internal circadian rhythm from the real 24-hour rhythm [6].
Mutations in the Per gene of Drosophila lead to a shorter lifespan, impaired protection against oxidative stress, and high level of neuronal degeneration [14]. The Bmal1 gene deletion in mice as a result of homologous recombination leads to accelerated aging [15]. At the same time, transplantation of the pineal gland from young to old mice [16] and transplantation of fetal SCN cells to old hamsters [17] increase animals’ lifespan.
The patterns of expression of the clock genes in different tissues undergo age-related changes. The level of expression of the Per gene decreases in wild-type D. melanogaster [14]. There is a decrease in the total expression of the Clock and Bmal1 transcripts in the SCN of old mice [18] and golden hamsters [19]. The transcripts of more than 1000 genes with the circadian rhythm in expression, which have been identified in the human prefrontal cortex, are characterized by age-related changes in rhythmicity or exhibit their rhythmicity at a particular age (e.g., transcription of some genes becomes rhythmic at a later age, and the authors believe it to be a compensatory response) [20]. As regards the main circadian genes, the rhythms of PER1, PER2, and CRY1 expression change in people aged over 60 compared to 40-year-old people: CRY1 expression becomes arrhythmic and the peak expression of PER1 and PER2 shows a phase shift to an earlier time; in some cases, the amplitude decreases. Thus, age-related disorders of expression of the circadian rhythm genes develop in central clocks.
AMPK AND ITS ROLE IN METABOLISM, AGING, AND REGULATION OF CIRCADIAN RHYTHMS
AMPK is a serine/threonine protein kinase, which responds to energy deficiency and triggers a wide range of reactions aimed at slowing down anabolic and accelerating catabolic reactions. It is activated when the AMP and ATP level increases and decreases, respectively. The AMPK heterotrimer is composed of a catalytic alpha subunit (α1 or α2) and two regulatory subunits: beta (β1 or β2) and gamma (γ1, γ2 or γ3). There are 12 possible variants of the heterotrimer; it is assumed that specific composition of the subunits allows different complexes to respond to different types of stress stimuli [21]. In the inactive state, the α‑subunit interacts with its own autoinhibitory domain, and its activation requires AMP binding to the gamma-subunit [22]. There are several kinases that can activate AMPK by direct phosphorylation: LKB1 (liver kinase B1), CaMKKβ (Ca2+/calmodulin-dependent protein kinase β), and TAK1 (transforming growth factor β–activated kinase) [23].
AMPK is an important regulator of energy homeostasis at the intersection of different metabolic pathways [22, 24]. It is involved in autophagy and in the effect of calorie restriction; many age-related pathologies are associated with the impairment of its regulatory role. AMPK activation can suppress inflammation and stimulate mitochondrial function, which allows this kinase to be used not only in the treatment of metabolic disorders (obesity, type 2 diabetes, cardiovascular diseases), but also to become a potential tool for the correction of kidney diseases, neuromuscular disorders, and even Alzheimer’s disease [23].
The reciprocal regulation of AMPK and the circadian clock probably plays an important role in the diurnal rhythms of metabolism [25]. AMPK was initially shown to regulate the circadian clock by phosphorylation and CRY degradation [10]. CRYs are blue light receptor proteins, but their function is not confined to photoreception; they can also be magnetoreceptors and photolyases [26]. Lamia et al. [10] report on one more role of CRY, now as a chemical energy sensor, which allows this protein to perform nutrient signal transduction to the circadian clock in peripheral organs of mammals. At the same time, AMPK phosphorylates serines evolutionarily conservative in all cryptochromes, which act as transcriptional repressors and are absent in those functioning as blue light photoreceptors [10, 25].
AMPK can also indirectly destabilize PER2 through phosphorylation of CK1ε [25, 27]. In addition, the composition of subunits comprising the heterotrimeric complex of AMPK varies depending on the time of day, which potentially may also be a way to regulate the diurnal rhythms of metabolism [25].
OTHER PARTICIPANTS OF METABOLIC PROCESSES INVOLVED IN THE REGULATION OF CIRCADIAN RHYTHMS
mTOR
The mTOR complex coordinates nutrient supply with growth and development processes. Different model gerontological objects have shown hyperactivation of this complex in old animals; hence, its inhibition with rapamycin usually increases their lifespan [28, 29]. The activity of mTOR is opposite to that of AMPK; therefore, the inhibition of mTOR with rapamycin or the activation of AMPK with metformin are ones of the most popular life-extending agents in gerontological studies. Both drugs are considered to be the so-called calorie restriction mimetics [30].
The mTOR pathway activity undergoes circadian oscillations [31]. mTOR regulates the proteostasis of BMAL1, affecting its translation, degradation and subcellular localization. Impaired regulation of BMAL1 is one of the hallmarks of activated mTOR. The inhibition of mTORC1 with rapamycin increases the lifespan of Bmal1–/– mice by 50% [32]. Heterozygous mutations in the orthologs of Tsc1/2 (Tuberous sclerosis complex 1/2) in Drosophila neurons or mTOR in mice can result in circadian arrhythmia [33]. In the mathematical model of Sadria and Layton [5], circadian rhythms cause oscillations in the levels of mTORC1 phosphorylation. BMAL1 and PER2 inhibit mTORC1, and phosphorylation of mTORC1 increases the level of BMAL1. According to the model, the phase difference between the phosphorylation of mTORC1 and the synthesis of Bmal1 mRNA is about 9 hours.
SIRT1
Aging is associated with the decrease in the cellular concentration of NAD+ and, as a consequence, with the reduced expression of Sirt1, for which NAD+ is a co-substrate at the transcriptional and translational levels [5, 35]. The NADH level increases with age, leading to a decrease in the NAD+/NADH ratio and in the redox state of total NAD [36].
SIRT1 is related to AMPK activation as it deacetylates LKB1. The Sirt1 knockout in mice contributes to manifestation of premature aging and decreased lifespan, with activity rhythm disturbance similar to those in old wild-type mice [37–39].
SIRT1 deacetylates BMAL1 in the CLOCK–BMAL1 complex and PER2 in the PER2–CRY1 complex [40–43]. Age-related suppression of SIRT1 accounts for the decrease in the amplitude of different rhythms controlled by CLOCK–BMAL1. Resveratrol can potentially compensate for the age-related loss of SIRT1 and restore the suppression of the PER2–CRY1 complex [44]. Interestingly, it has been shown in peripheral blood mononuclear cells that resveratrol either has or has no effect on the AMPK pathway depending on age [45].
The inhibition of AMPK with dorsomorphin (compound C) significantly decreases the expression of SIRT1 and phosphorylated AMPK; at the same time, cardioprotection induced by the combination of melatonin and sitagliptin disappears [46]. These results suggest that melatonin regulating circadian rhythms can promote postischemic cardiac recovery in old diabetic rats by increasing the activity of the AMPK/SIRT1 pathway.
PARP1
PARP1 (poly(ADP-ribose)polymerase 1) is involved in the regulation of different biological processes, including DNA repair, differentiation, proliferation, apoptosis, etc. PARP activity correlates with the age of animals and species-specific lifespan; it also exacerbates some pathological states, inter alia, age-related disorders such as Alzheimer’s disease and type 2 diabetes [47].
PARP1 ribosylates CLOCK when interacting with the CLOCK–BMAL1 complex [48]. PARP1–/– knockout mice demonstrate the changes in gene expression levels in the liver and diurnal rhythms in motor activity [25]. There is supposed to be a functional interaction between PARP1 and SIRT1 as NAD+-dependent proteins aimed at linking metabolic signaling to circadian clock regulation [49, 50]. The relationship between PARP1 and AMPK within the framework of circadian rhythm regulation has yet been poorly studied; however, analysis of the protective effect of biguanides on the cardiovascular system has shown that they exert a favorable effect on the vasculature due to the inhibition of PARP1 activity through its phosphorylation by AMPK [51]. It is probable that a similar mechanism underlies the regulation of circadian rhythms.
CALORIE RESTRICTION AND OTHER DIETARY MODIFICATIONS
Circadian and metabolic pathways are rather closely intertwined [52, 53]. The Per2 deficiency alters lipid metabolism in the liver of mice and stimulates differentiation of fibroblasts into adipocytes. PER2 acts via the nuclear receptor PPARγ playing an important role in adipogenesis [52]. PER3 also has an effect on PPARγ, because the Per3 knockout leads to an increase in the fat mass and a decrease in the muscle mass of mice [54]. The mice with the Per1/Per2 double knockout maintained on a high-fat diet demonstrate abnormalities not only in the circadian rhythm but also in drug metabolism, cell cycle, lipid metabolism, and in the pathways associated with cancer [55]. It should be noted that many of the parameters analyzed in the present work (including the number of differentially expressed circadian genes) were also impaired in wild-type mice fed with a high-fat diet.
Dietary adaptations are important not only for multicellular animals but also for unicellular organisms. For example, the presence of the functional circadian clock has a significant effect on the adaptation of Neurospora crassa to long-term glucose starvation [56].
As mentioned previously, calorie restriction is the proven way to extend the lifespan for quite a number of laboratory animals. In addition to calorie restriction, there is another type of dietary intervention: time-restricted feeding. It has been shown that mice fed with a high-fat diet but only during the period of activity (with calorie intake equal to that of animals fed on the same food ad libitum) demonstrate improvement in the sensitivity to glucose and coordination of movements, the absence of obesity, hyperinsulinemia and inflammation, as well as more stable circadian rhythms [57]. Imposed feeding rhythms with time-restricted feeding increased the ratio of the peak to minimum mRNA levels of circadian clock components in the liver. In addition, the animals under this dietary regimen demonstrated the signs of high AMPK activity. At the same time, the animals fed ad libitum demonstrated the impairment of total and/or diurnal changes in active mTOR and AMPK and attenuated oscillations of circadian clock components (PER1, PER2, CRY1, BMAL1, CLOCK, RORα, REV-ERBα) in the liver.
The feeding of nocturnal animals such as rats and mice only in the daytime when they are inactive results in desynchronization of the peripheral clock, its disconnection from the central clock [3] and, as a consequence, disorders of lipid metabolism and obesity. For example, the daytime feeding of mice induces leptin resistance, increases the peak levels of insulin in plasma, and increases the expression of lipogenic genes (Scd1, Srebp1c, Acc1 and Fasn) compared to the same parameters in animals feeding only at night [58].
It should be noted that the studies of the mechanisms of circadian rhythms and the search of interventions that could be used to correct their dysfunction mostly involve standard laboratory animals living a nocturnal lifestyle. Feeding and experimental manipulations are usually performed in the daytime, which cannot but influence the physiology of studied animals. Despite the fact that laboratory facilities for such studies are specially designed to ensure the specified lighting cycle (i.e., to synchronize the active time for animals with the time of work of the personnel) [59], the results of such experiments still need to be interpreted with caution. For example, behavioral arousal differently modulates the effect of light on SCN in nocturnal and diurnal species [60]. In addition, it is important to take into account the nocturnal chronotype of the most of laboratory mammals not only in the studies devoted to circadian rhythms but also in other experiments, especially in the study of metabolic disorders: diabetes, metabolic syndrome, etc. Thus, many factors should be taken into account when choosing a model for the study of circadian rhythms [61] and metabolism, as well as for the experimental studies of aging [62–64]. First of all, it is necessary to collect more information about the peculiarities of circadian rhythms in diurnal laboratory animals [65].
Different dietary regimens, along with physical activity at certain times of the day, can probably be used to correct deviations in circadian rhythms. In humans, like in animals, eating later in the evening or at night, as well as night shift work, results in circadian clock disruption [66]. At the same time, it has been shown that the timing of physical exercise with regard to chronotype can alleviate disturbances of the circadian rhythm in young men [13]. Probably, intermittent fasting will be more relevant in this case than calorie-restricted diet. Such interventions are now actively investigated in humans. It has been found out, e.g., that intermittent fasting improves the body mass index and arterial blood pressure, reduces the content of prooncogenes and increases the levels of antitumor and antidiabetic factors [67].
CONCLUSIONS
In conclusion, it should be noted that the bidirectional relationship between circadian disorders and metabolic pathologies creates a cyclic interrelation, when rhythm has an effect on metabolic activity and metabolism has an effect on rhythm [68]; on the other hand, bidirectionality suggests that disorders can also be corrected in two ways: either by hormonal effects on the central clock or correction of its function using different light regimens, or by normalization of circadian rhythms using diets, specific nutritional regimens and physical activity. Since almost all participants regulating the circadian clock mechanism have a whole range of targets, we believe that the pharmacological methods of intervention are less promising than physiologic methods. From this perspective, AMPK still seems to be one of the most attractive targets. We suggest that nonpharmacological modulation of AMPK activity may be used not only to correct the rhythm but also to delay the development of age-related pathologies.
REFERENCES
Welz, P.S. and Benitah, S.A., Molecular connections between circadian clocks and aging, J. Mol. Biol., 2020, vol. 432, no. 12, pp. 3661–3679. https://doi.org/10.1016/j.jmb.2019.12.036
Benitah, S.A. and Welz, P.S., Circadian regulation of adult stem cell homeostasis and aging, Cell Stem Cell, 2020, vol. 26, no. 6, pp. 817–831. https://doi.org/10.1016/j.stem.2020.05.002
Dibner, C., Schibler, U., and Albrecht, U., The mammalian circadian timing system: Organization and coordination of central and peripheral clocks, Annu. Rev. Physiol., 2010, vol. 72, pp. 517–549. https://doi.org/10.1146/annurev-physiol-021909-135821
Orozco-Solis, R. and Sassone-Corsi, P., Circadian clock: Linking epigenetics to aging, Opin. Genet. Dev., 2014, vol. 26, pp. 66–72. https://doi.org/10.1016/j.gde.2014.06.003
Sadria, M. and Layton, A.T., Aging affects circadian clock and metabolism and modulates timing of medication, iScience, 2021, vol. 24, no. 4, 102245. https://doi.org/10.1016/j.isci.2021.102245
Hood, S. and Amir, S., The aging clock: Circadian rhythms and later life, J. Clin. Investig., 2017, vol. 127, no. 2, pp. 437–446. https://doi.org/10.1172/JCI90328
Acosta-Rodríguez, V.A., Rijo-Ferreira, F., Green, C.B., and Takahashi, J.S., Importance of circadian timing for aging and longevity, Nat. Commun., 2021, vol. 12, no. 1, 2862. https://doi.org/10.1038/s41467-021-22922-6
Ahmed, R., Reza, H.M., Shinohara, K., and Nakahata, Y., Cellular senescence and its impact on the circadian clock, J. Biochem., 2022, vol. 171, no. 5, pp. 493–500. https://doi.org/10.1093/jb/mvab115
Morgunova, G.V., Shilovsky, G.A., and Khokhlov, A.N., Effect of caloric restriction on aging: Fixing the problems of nutrient sensing in postmitotic cells? Biochemistry (Moscow), 2021, vol. 86, no. 10, pp. 1352–1367. https://doi.org/10.1134/S0006297921100151
Lamia, K.A., Sachdeva, U.M., DiTacchio, L., Williams, E.C., Alvarez, J.G., Egan, D.F., Vasquez, D.S., Juguilon, H., Panda, S., Shaw, R.J., Thompson, C.B., and Evans, R.M., AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation, Science, 2009, vol. 326, no. 5951, pp. 437–440. https://doi.org/10.1126/science.1172156
Trott, A.J. and Menet, J.S., Regulation of circadian clock transcriptional output by CLOCK: BMAL1, PLoS Genet., 2018, vol. 14, no. 1, р. e1007156. https://doi.org/10.1371/journal.pgen.1007156
Gatfield, D. and Schibler, U., Proteasomes keep the circadian clock ticking, Science, 2007, vol. 316, no. 5828, pp. 1135–1136. https://doi.org/10.1126/science.114416
Thomas, J.M., Kern, P.A., Bush, H.M., McQuerry, K.J., Black, W.S., Clasey, J.L., and Pendergast, J.S., Circadian rhythm phase shifts caused by timed exercise vary with chronotype, JCI Insight, 2020, vol. 5, no. 3, e134270. https://doi.org/10.1172/jci.insight.134270
Krishnan, N., Kretzschmar, D., Rakshit, K., Chow, E., and Giebultowicz, J.M., The circadian clock gene period extends healthspan in aging Drosophila melanogaster, Aging (Albany, N. Y.), 2009, vol. 1, no. 11, pp. 937–948. https://doi.org/10.18632/aging.100103
Kondratov, R.V., Kondratova, A.A., Gorbacheva, V.Y., Vykhovanets, O.V., and Antoch, M.P., Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock, Genes Dev., 2006, vol. 20, no. 14, pp. 1868–1873. https://doi.org/10.1101/gad.1432206
Lesnikov, V.A. and Pierpaoli, W., Pineal cross-transplantation (old-to-young and vice versa) as evidence for an endogenous “aging clock,” Ann. N. Y. Acad. Sci., 1994, vol. 719, pp. 456–460. https://doi.org/10.1111/j.1749-6632.1994.tb56850.x
Hurd, M.W. and Ralph, M.R., The significance of circadian organization for longevity in the golden hamster, J. Biol. Rhythms, 1998, vol. 13, no. 5, pp. 430–436. https://doi.org/10.1177/074873098129000255
Bonaconsa, M., Malpeli, G., Montaruli, A., Carandente, F., Grassi-Zucconi, G., and Bentivoglio, M., Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice, Exp. Gerontol., 2014, vol. 55, pp. 70–79. https://doi.org/10.1016/j.exger.2014.03.011
Kolker, D.E., Fukuyama, H., Huang, D.S., Takahashi, J.S., Horton, T.H., and Turek, F.W., Aging alters circadian and light-induced expression of clock genes in golden hamsters, J. Biol. Rhythms, 2003, vol. 18, no. 2, pp. 159–169. https://doi.org/10.1177/0748730403251802
Chen, C.Y., Logan, R.W., Ma, T., Lewis, D.A., Tseng, G.C., Sibille, E., and McClung, C.A., Effects of aging on circadian patterns of gene expression in the human prefrontal cortex, Proc. Natl. Acad. Sci. U.S.A., 2016, vol. 113, no. 1, pp. 206–211. https://doi.org/10.1073/pnas.1508249112
Herzig, S. and Shaw, R.J., AMPK: Guardian of metabolism and mitochondrial homeostasis, Nat. Rev. Mol. Cell Biol., 2018, vol. 19, no. 2, pp. 121–135. https://doi.org/10.1038/nrm.2017.95
Morgunova, G.V. and Klebanov, A.A., Age-related AMP-activated protein kinase alterations: From cellular energetics to longevity, Cell Biochem. Funct., 2019, vol. 37, no. 3, pp. 169–176. https://doi.org/10.1002/cbf.3384
Ge, Y., Zhou, M., Chen, C., Wu, X., and Wang, X., Role of AMPK mediated pathways in autophagy and aging, Biochimie, 2022, vol. 195, pp. 100–113. https://doi.org/10.1016/j.biochi.2021.11.008
Curtis, R., O’Connor, G., and DiStefano, P.S., Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (AAK-2) links multiple aging and metabolism pathways, Aging Cell, 2006, vol. 5, no. 2, pp. 119–126. https://doi.org/10.1111/j.1474-9726.2006.00205.x
Jordan, S.D. and Lamia, K.A., AMPK at the crossroads of circadian clocks and metabolism, Mol. Cell. Endocrinol., 2013, vol. 366, no. 2, pp. 163–169. https://doi.org/10.1016/j.mce.2012.06.017
Fraikin, G.Y., Photosensory and signaling properties of cryptochromes, Moscow Univ. Biol. Sci. Bull., 2022, vol. 77, no. 2, pp. 54–63. https://doi.org/10.3103/S0096392522020031
Sahar, S. and Sassone-Corsi, P., Regulation of metabolism: The circadian clock dictates the time, Trends Endocrinol. Metab., 2012, vol. 23, no. 1, pp. 1–8. https://doi.org/10.1016/j.tem.2011.10.005
Johnson, S.C., Sangesland, M., Kaeberlein, M., and Rabinovitch, P.S., Modulating mTOR in aging and health, in Aging and Health—A Systems Biology Perspective, 2015, vol. 40, pp. 107–127. https://doi.org/10.1159/000364974
Chrienova, Z., Nepovimova, E., and Kuca, K., The role of mTOR in age-related diseases, J. Enzyme Inhib. Med. Chem., 2021, vol. 36, no. 1, pp. 1678–1692. https://doi.org/10.1080/14756366.2021.1955873
Morgunova, G.V., Klebanov, A.A., and Khokhlov, A.N., Autophagy—the way to death or immortality? Activators and inhibitors of autophagy as possible modulators of the aging process, in Aging: Exploring a Complex Phenomenon, Ahmad, Sh.I., Ed., Boca Raton: Taylor & Francis, 2018, pp. 475–485. https://doi.org/10.1201/b21905
Lipton, J.O., Boyle, L.M., Yuan, E.D., Hochstrasser, K.J., Chifamba, F.F., Nathan, A., Tsai, P.T., Davis, F., and Sahin, M., Aberrant proteostasis of BMAL1 underlies circadian abnormalities in a paradigmatic mTOR-opathy, Cell Reports, 2017, vol. 20, no. 4, pp. 868–880. https://doi.org/10.1016/j.celrep.2017.07.008
Khapre, R.V., Kondratova, A.A., Patel, S., Dubrovsky, Y., Wrobel, M., Antoch, M.P., and Kondratov, R.V., BMAL1-dependent regulation of the mTOR signaling pathway delays aging, Aging (Albany, N. Y.), 2014, vol. 6, no. 1, pp. 48–57. https://doi.org/10.18632/aging.100633
Zheng, X. and Sehgal, A., AKT and TOR signaling set the pace of the circadian pacemaker, Curr. Biol., 2010, vol. 20, no. 13, pp. 1203–1208. https://doi.org/10.1016/j.cub.2010.05.027
Cao, R., Robinson, B., Xu, H., Gkogkas, C., Khoutorsky, A., Alain, T., Yanagiya, A., Nevarko, T., Liu, A.C., Amir, S., and Sonenberg, N., Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling, Neuron, 2013, vol. 79, no. 4, pp. 712–724. https://doi.org/10.1016/j.neuron.2013.06.026
Satoh, A., Imai, S.I., and Guarente, L., The brain, sirtuins, and ageing, Nat. Rev. Neurosci., 2017, vol. 18, no. 6, pp. 362–374. https://doi.org/10.1038/nrn.2017.42
Zhu, X.H., Lu, M., Lee, B.Y., Ugurbil, K., and Chen, W., In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences, Proc. Natl. Acad. Sci. U.S.A., 2015, vol. 112, no. 9, pp. 2876–2881. https://doi.org/10.1073/pnas.1417921112
Cohen, D.E., Supinski, A.M., Bonkowski, M.S., Donmez, G., and Guarente, L.P., Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction, Genes Dev., 2009, vol. 23, no. 24, pp. 2812–2817. https://doi.org/10.1101/gad.1839209
Chang, H.C. and Guarente, L., SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging, Cell, 2013, vol. 153, no. 7, pp. 1448–1460. https://doi.org/10.1016/j.cell.2013.05.027
Wang, R.H., Zhao, T., Cui, K., Hu, G., Chen, Q., Chen, W., Wang, X.W., Soto-Gutierrez, A., Zhao, K., and Deng, C.X., Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging, Sci. Rep., 2016, vol. 6, no. 1, р. 28633. https://doi.org/10.1038/srep28633
Levine, D.C., Hong, H., Weidemann, B.J., Ramsey, K.M., Affinati, A.H., Schmidt, M.S., Cedernaes, J., Omura, C., Braun, R., Lee, C., and Brenner, C., NAD+ controls circadian reprogramming through PER2 nuclear translocation to counter aging, Mol. Cell, 2020, vol. 78, no. 5, pp. 835–849. https://doi.org/10.1016/j.molcel.2020.04.010
Ashimori, A., Nakahata, Y., Sato, T., Fukamizu, Y., Matsui, T., Yoshitane, H., Fukada, Y., Shinohara, K., and Bessho, Y., Attenuated SIRT1 activity leads to PER2 cytoplasmic localization and dampens the amplitude of bmal1 promoter-driven circadian oscillation, Front. Neurosci., 2021, vol. 15, р. 647589. https://doi.org/10.3389/fnins.2021.647589
Asher, G., Gatfield, D., Stratmann, M., Reinke, H., Dibner, C., Kreppel, F., Mostoslavsky, R., Alt, F.W., and Schibler, U., SIRT1 regulates circadian clock gene expression through PER2 deacetylation, Cell, 2008, vol. 134, no. 2, pp. 317–328. https://doi.org/10.1016/j.cell.2008.06.050
Shilovsky, G.A., Putyatina, T.S., Morgunova, G.V., Seliverstov, A.V., Ashapkin, V.V., Sorokina, E.V., Markov, A.V., and Skulachev, V.P., A crosstalk between the biorhythms and gatekeepers of longevity: Dual role of glycogen synthase kinase-3, Biochemistry (Moscow), 2021, vol. 86, no. 4, pp. 433–448. https://doi.org/10.1134/S0006297921040052
Cheng, C.K., Luo, J.Y., Lau, C.W., Chen, Z.Y., Tian, X.Y., and Huang, Y., Pharmacological basis and new insights of resveratrol action in the cardiovascular system, Br. J. Pharmacol., 2020, vol. 177, no. 6, pp. 1258–1277. https://doi.org/10.1111/bph.14801
Caldeira, C.A., Santos, M.A., Araújo, G.R., Lara, R.C., Franco, F.N., and Chaves, M.M., Resveratrol: Change of SIRT 1 and AMPK signaling pattern during the aging process, Exp. Gerontol., 2021, vol. 146, р. 111226. https://doi.org/10.1016/j.exger.2021.111226
Song, R., Ren, J., Sun, J., and Li, M., Melatonin postconditioning combined with sitagliptin exerts full cardioprotection in diabetic hearts of aged rats through an AMPK-dependent mechanism, Arch. Biol. Sci., 2021, vol. 73, no. 1, pp. 83–92. https://doi.org/10.2298/ABS210111004S
Shilovsky, G.A., Shram, S.I., Morgunova, G.V., and Khokhlov, A.N., Protein poly (ADP-ribosyl) ation system: Changes in development and aging as well as due to restriction of cell proliferation, Biochemistry (Moscow), 2017, vol. 82, no. 11, pp. 1391–1401. https://doi.org/10.1134/S0006297917110177
Asher, G., Reinke, H., Altmeyer, M., Gutierrez-Arcelus, M., Hottiger, M.O., and Schibler, U., Poly (ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding, Cell, 2010, vol. 142, no. 6, pp. 943–953. https://doi.org/10.1016/j.cell.2010.08.016
Bai, P., Canto, C., Oudart, H., Brunyánszki, A., Cen, Y., Thomas, C., Yamamoto, H., Huber, A., Kiss, B., Houtkooper, R.H., and Schoonjans, K., PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation, Cell Metab., 2011, vol. 13, no. 4, pp. 461–468. https://doi.org/10.1016/j.cmet.2011.03.004
Pang, J., Gong, H., Xi, C., Fan, W., Dai, Y., and Zhang, T.M., Poly (ADP-ribose) polymerase 1 is involved in glucose toxicity through SIRT1 modulation in HepG2 hepatocytes, J. Cell Biochem., 2011, vol. 112, no. 1, pp. 299–306. https://doi.org/10.1002/jcb.22919
Shang, F., Zhang, J., Li, Z., Zhang, J., Yin, Y., Wang, Y., Marin, T.L., Gongol, B., Xiao, H., Zhang, Y.Y., and Chen, Z., Cardiovascular protective effect of metformin and telmisartan: Reduction of PARP1 activity via the AMPK-PARP1 cascade, PLoS One, 2016, vol. 11, no. 3, р. e0151845. https://doi.org/10.1371/journal.pone.0151845
Grimaldi, B., Bellet, M.M., Katada, S., Astarita, G., Hirayama, J., Amin, R.H., Granneman, J.G., Piomelli, D., Leff, T., and Sassone-Corsi, P., PER2 controls lipid metabolism by direct regulation of PPARγ, Cell Metab., 2010, vol. 12, no. 5, pp. 509–520. https://doi.org/10.1016/j.cmet.2010.10.005
Shetty, A., Hsu, J.W., Manka, P.P., and Syn, W.K., Role of the circadian clock in the metabolic syndrome and nonalcoholic fatty liver disease, Dig. Dis. Sci., 2018, vol. 63, pp. 3187–3206. https://doi.org/10.1007/s10620-018-5242-x
Costa, M.J., So, A.Y.L., Kaasik, K., Krueger, K.C., Pillsbury, M.L., Fu, Y.H., Ptacek, L.J., Yamamoto, K.R., and Feldman, B.J., Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate, J. Biol. Chem., 2011, vol. 286, no. 11, pp. 9063–9070. https://doi.org/10.1074/jbc.M110.164558
Bu, Y., Chen, S., Ruan, M., Wu, L., Wang, H., Li, N., Zhao, X., Yu, X., and Liu, Z., Per1/Per2 double knockout transcriptome analysis reveals circadian regulation of hepatic lipid metabolism, Food Sci. Hum. Wellness, 2023, vol. 12, no. 5, pp. 1716–1729. https://doi.org/10.1016/j.fshw.2023.02.034
Szőke, A., Sárkány, O., Schermann, G., Kapuy, O., Diernfellner, A.C., Brunner, M., Gyöngyösi, N., and Káldi, K., Adaptation to glucose starvation is associated with molecular reorganization of the circadian clock in Neurospora crassa, eLife, 2023, vol. 12, р. e79765. https://doi.org/10.7554/eLife.79765
Hatori, M., Vollmers, C., Zarrinpar, A., DiTacchio, L., Bushong, E.A., Gill, S., Leblanc, M., Chaix, A., Joens, M., Fitzpatrick, J.A., and Ellisman, M.H., Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet, Cell Metab., 2012, vol. 15, no. 6, pp. 848–860. https://doi.org/10.1016/j.cmet.2012.04.019
Yasumoto, Y., Hashimoto, C., Nakao, R., Yamazaki, H., Hiroyama, H., Nemoto, T., Yamamoto, S., Sakurai, M., Oike, H., Wada, N., and Yoshida-Noro, C., Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice, Metabolism, 2016, vol. 65, no. 5, pp. 714–727. https://doi.org/10.1016/j.metabol.2016.02.003
Emmer, K.M. and Russart, K.L., Walker, II, W.H., Nelson, R.J., and DeVries, A.C., Effects of light at night on laboratory animals and research outcomes, Behav. Neurosci., 2018, vol. 132, no. 4, pp. 302–314. https://doi.org/10.1037/bne0000252
Jha, P.K., Bouâouda, H., Kalsbeek, A., and Challet, E., Distinct feedback actions of behavioural arousal to the master circadian clock in nocturnal and diurnal mammals, Neurosci. Biobehav. Rev., 2021, vol. 123, pp. 48–60. https://doi.org/10.1016/j.neubiorev.2020.12.011
Kronfeld-Schor, N., Bloch, G., and Schwartz, W.J., Animal clocks: When science meets nature, Proc. Roy. Soc. London, Ser. B, Biol. Sci., 2013, vol. 280, no. 1765, р. 20131354. https://doi.org/10.1098/rspb.2013.1354
Khokhlov, A.N., Klebanov, A.A., and Morgunova, G.V., On choosing control objects in experimental gerontological research, Moscow Univ. Biol. Sci. Bull., 2018, vol. 73, no. 2, pp. 59–62. https://doi.org/10.3103/S0096392518020049
Khokhlov, A.N., Reflections of a pessimistic gerontologist or why we still do not live 1000 years, Moscow Univ. Biol. Sci. Bull., 2021, vol. 76, no. 4, pp. 239–243. https://doi.org/10.3103/S0096392521040040
Morgunova, G.V. and Khokhlov, A.N., Signs of similarities and differences in cellular models of aging: A scoping review, Moscow Univ. Biol. Sci. Bull., 2022, vol. 77, no. 3, pp. 139–146. https://doi.org/10.3103/S0096392522030087
Refinetti, R. and Kenagy, G.J., Diurnally active rodents for laboratory research, Lab. Anim., 2018, vol. 52, no. 6, pp. 577–587. https://doi.org/10.1177/0023677218771720
Mukherji, A., Bailey, S.M., Staels, B., and Baumert, T.F., The circadian clock and liver function in health and disease, J. Hepatol., 2019, vol. 71, no. 1, pp. 200–211. https://doi.org/10.1016/j.jhep.2019.03.020
Mindikoglu, A.L., Abdulsada, M.M., Jain, A., Jalal, P.K., Devaraj, S., Wilhelm, Z.R., Opekun, A.R., and Jung, S.Y., Intermittent fasting from dawn to sunset for four consecutive weeks induces anticancer serum proteome response and improves metabolic syndrome, Sci. Rep., 2020, vol. 10, no. 1, 18341. https://doi.org/10.1038/s41598-020-73767-w
Roenneberg, T. and Merrow, M., Circadian systems and metabolism, J. Biol. Rhythms, 1999, vol. 14, no. 6, pp. 449–459. https://doi.org/10.1177/074873099129001019
Funding
The work was supported by State Assignment for the Moscow State University, part 2 (Fundamental Research, no. 121032300215-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by E. Makeeva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: AMPK, 5'-AMP-activated protein kinase; BMAL1, brain and muscle ARNT-like 1; CaMKKβ, Ca2+/calmodulin-dependent protein kinase kinase β; CCG, clock-controlled genes; CKIε, casein kinase 1ε; CLOCK, circadian locomotor output cycles kaput; CRY, cryptochrome; FBXL3, F-box and leucine-rich repeat protein 3; FOXO, forkhead box protein O; IGF-1, insulin-like growth factor 1; LKB1, liver kinase B1 (serine/threonine kinase 11, STK11); PER, period; NR1D1, nuclear receptor subfamily 1, group D, member 1 (REV-ERB-α); NR1D2, nuclear receptor subfamily 1, group D, member 2 (REV-ERB-β); PARP1, poly(ADP-ribose)polymerase 1; RORα, retinoic acid-related (RAR)-related orphan receptor α; SIRT1, sirtuin (silent mating type information regulation 2 homolog) 1; TAK1, transforming growth factor β-activated kinase; TSC1/2, tuberous sclerosis complex 1/2; mTOR, mammalian target of rapamycin; ULK1, Unc-51 like autophagy activating kinase; SCN, suprachiasmatic nucleus.
Rights and permissions
About this article
Cite this article
Morgunova, G.V., Shilovsky, G.A. & Khokhlov, A.N. Influence of AMPK on the Functioning of the Circadian Clock and Its Possible Role in the Development of Age-Related Metabolic Disorders. Adv Gerontol 13, 54–61 (2023). https://doi.org/10.1134/S207905702460006X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207905702460006X