Abstract
Idiopathic normal pressure hydrocephalus (iNPH) is one of the few reversible causes of cognitive impairment, and many patients can be effectively treated with surgical placement of a ventricular shunt. The clinical diagnosis is based on the classic triad of cognitive decline, gait impairment, and urinary incontinence, combined with MRI evidence of ventricular enlargement. However, the underlying pathophysiological processes that lead to the development of iNPH remain unknown. In addition to MRI, 18F-FDG PET can be used to exclude neurodegenerative diseases that mimic iNPH, including Alzheimer’s disease (AD). Improving outcomes will rest upon developing more specific methods of differentiating iNPH from disease mimics and identifying patients that will best respond to shunt surgery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
Normal pressure hydrocephalus (NPH) is a syndrome that was first described in 1965 by Hakim and Adams [1]. It is characterized by the clinical triad of memory impairment, gait disturbance, and urinary incontinence, accompanied by the presence of normal cerebrospinal fluid (CSF) pressure on lumbar puncture and the radiologic finding of enlarged cerebral ventricles [1].
NPH is now separated into two clinical categories: idiopathic NPH (iNPH) and secondary NPH, when the ventricular enlargement develops as a sequela of a known disease process. Typical conditions that can cause secondary NPH include subarachnoid hemorrhage, infectious meningitis, intracranial surgery, and other inflammatory or neoplastic processes. Surgical ventricular shunting is considered the standard-of-care treatment for many of these patients. However, response to ventricular shunting is variable and can be short-lived, with significant risks including complication rates up to 38% [2, 3].
Etiology
Many theories have been proposed to explain the mechanisms underlying the development of iNPH, although the exact etiology remains unclear. It is likely that iNPH is multifactorial, resulting from impaired CSF dynamics in combination with congenital and vascular etiologies.
The most widely accepted theory is that iNPH results from disordered CSF circulation, comprised of an imbalance among CSF production, circulation, and reabsorption [4]. Over time, CSF volume increases with a compensatory increase in ventricular volume to maintain normal intracranial pressure. Any increased pulse pressure on the ventricular walls can result in a “waterhammer” effect, disturbing the paracentral fibers of the corticospinal tracts and contributing to the gait disturbance described in iNPH [5].
More recently, it has been proposed that alterations in glymphatic pathways can contribute to iNPH. The glymphatic system is a clearance pathway by which CSF flows through the periarterial space into the brain parenchyma, is transported via aquaporin-P4 water channels into the interstitial space, and flows through the perivenous space before draining into the cervical lymphatic system [6]. A defective glymphatic system can result in impaired CSF clearance and buildup of toxic metabolic products, including amyloid-β peptides and tau protein, potentially leading to the development of iNPH and Alzheimer’s disease (AD) [7, 8]. Recent studies using imaging to examine the glymphatic system in iNPH patients found evidence for delayed clearance from the subarachnoid space and entorhinal cortex, as well as ventricular reflux towards the periventricular white matter, which may contribute to the development of dementia in iNPH [5, 7, 9,10,11].
In addition to evidence for altered CSF dynamics and clearance in iNPH, the possibility of a genetic cause of iNPH was proposed by Portenoy et al., who described the occurrence of the syndrome in two siblings [12]. Other studies have reported that more than 10% of patients with iNPH have a large head size, which supports the idea that developmental factors may impact the development of hydrocephalus [13, 14]. More recently, copy number variations in SFMBT1, a gene encoding a protein of the choroid plexus endothelium involved in CSF secretion, have been reported in iNPH patients [15].
Vascular disease has also been reported to alter CSF dynamics and contribute to the development of iNPH [16]. Severity of small vessel disease, especially periventricular white matter changes, have particularly been associated with higher prevalence of iNPH [17] and a less favorable prognosis [18].
Epidemiology and Clinical Presentation
Approximately 10% of the 50 million people worldwide living with dementia suffer from iNPH [11]. iNPH is a disease of older individuals, with prevalence increasing with age; more than 2% of people aged 65 to 79 have the disease, compared to almost 9% of people 80 years and older [19].
Clinical diagnosis is often based on the presence of the classic iNPH triad of symptoms:
-
1.
Gait impairment: Gait dysfunction is typically the initial symptom of iNPH and is characterized by a “magnetic” or apraxic gait, which occurs in the absence of primary sensorimotor deficits, cerebellar dysfunction, or involuntary movements. Rather, it is believed to result from difficulty integrating sensory information about the position of the body and properly executing motor function [20, 21]. However, gait abnormalities occur in 20% of individuals older than the age 75, so it is important to exclude other causes [6, 22].
-
2.
Cognitive impairment and dementia: The cognitive impairment seen with iNPH is commonly described as an executive dysfunction that manifests as slow processing, difficulty with problem solving, and memory deficits with poor retrieval but relatively intact recognition memory [21, 23]. These symptoms can overlap with neurodegenerative disorders, including parkinsonian syndromes, AD, dementia with Lewy bodies, and vascular dementia. Furthermore, many patients with iNPH have comorbid AD and vascular dementia, making diagnosis challenging [24].
-
3.
Urinary urgency and incontinence: Urgency and frequency are the most common urinary symptoms and may occur with or without incontinence [25]. Patients are usually aware of the urinary urge before incontinence occurs. Since bladder symptoms are very common among the elderly, incontinence occurs in 38% of women and 18% of men in this age group [26, 27], other causes should also be considered in patients with suspected iNPH.
Commonly, an unexplained, symmetric gait disturbance is the primary symptom of iNPH. Although cognitive impairment and urinary symptoms are part of the iNPH triad and are frequently present, the complete triad is not required to suspect the disorder [21]. Of note, the remainder of the neurologic examination, besides these three symptoms, is typically normal.
According to the American-European and Japanese NPH evidence-based guidelines for diagnosis and management, patients suspected to have iNPH can be classified into probable, possible, and unlikely categories. Possible iNPH is defined by (a) being older than 60 years old, (b) having one or more symptoms of the clinical triad, (c) MR evidence of ventricular dilatation and a narrow CSF space at the vertex, (d) CSF pressure < 200 mmH2O with a normal CSF cell count and protein level, (e) no other disease that may account for the symptoms, and (f) having had no other previous illnesses that can cause ventricular dilatation. A patient is considered to have probable iNPH if the patient meets the criteria for possible iNPH and has a positive spinal tap test [28, 29].
CSF Tap Test and CSF Biomarkers
The CSF tap test consists of testing gait and cognitive function both before and after the drainage of approximately 40–50 milliliters of CSF via a lumbar puncture. Since the single tap CSF tap test has a low sensitivity, ranging from 26 to 61%, a negative result cannot be used to exclude patients from surgery. If there is a high clinical suspicion for iNPH despite a negative CSF tap test, one alternative diagnostic test is a repeated tap test; this involves performing lumbar punctures on three consecutive days and draining at least 30–40 milliliters of CSF each time. Another alternative would be to perform continuous external lumbar drainage (ELD) for 3–5 days, with a minimum of 150 ml CSF drained daily. ELD has a high positive predictive value of 80–100% and fairly high sensitivity of 50–100%. Since ELD best simulates the effect of definitive shunt surgery, it is considered the most effective test for identifying shunt-responsive cases according to the 2005 International iNPH guidelines [30]. However, ELD also requires hospital admission and is associated with higher complication rates, including meningitis, subdural hematoma, and nerve root inflammation [31, 32].
CSF biomarkers have not yet been proven to be useful in confirming the diagnosis of iNPH. Some studies comparing CSF biomarkers in patients with iNPH and AD have reported low Aβ42 levels in iNPH; however, CSF tau was reported to be both normal and increased [33,34,35,36]. A challenge with using CSF biomarkers in this disease group is that many patients may have concomitant iNPH and AD pathology, resulting in overlap of CSF biomarkers.
Imaging Features
Conventional MRI
Currently, MRI is the imaging method of choice for assessing iNPH and has been incorporated into the international and Japanese diagnostic guidelines [37]. Importantly, iNPH is a communicating hydrocephalus, so any sign of CSF obstruction needs to be excluded.
Three of the cardinal neuroimaging findings of iNPH are as follows:
-
1.
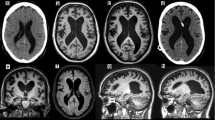
The Evans index (EI) is the most commonly used measure for ventricular enlargement, first described in 1942. It is calculated by taking the ratio of the widest width of the frontal horns of the lateral ventricles and the widest width of the intracranial compartment on an axial image (Fig. 38.1) [38]. International guidelines propose an EI of greater than 0.3 is suggestive of iNPH , although its specificity is questioned [39, 40]. Yamada et al. developed the “z-Evans index” with additional measurements of the frontal horns of the lateral ventricle, resulting in higher accuracy in predicting patient response to a tap test [41].
-
2.
The callosal angle (CA) is defined by two lines tangentially aligned with the medial walls of the lateral ventricular walls on coronal images. iNPH patients commonly have a CA that is less than 90 degrees, and this has been found to be a good predictor of shunt response [42,43,44]. When combined with an EI of greater than 0.3, iNPH can be discriminated from AD with a sensitivity and specificity of 97% and 94%, respectively [45].
-
3.
The MR sign described as “disproportionately enlarged subarachnoid space hydrocephalus” (DESH) is characterized by narrowed sulci in the midline at the high convexity, combined with enlarged Sylvian fissures and ventricular dilation (Fig. 38.1). The evaluation of this sign is performed on a coronal image at the level of the posterior commissure. This sign is highly associated with the clinical triad of iNPH, is not typically seen in the setting of brain atrophy, and is a good predictor of response to shunt surgery [46,47,48,49,50].
Axial (a) and coronal (b) T2-weighted fluid-attenuated inversion recovery MR images showing two commonly used MR features of idiopathic normal pressure hydrocephalus (iNPH). The Evans index (a) can be calculated by dividing the maximum width of the frontal horns of the lateral ventricles (D1) by the maximum width of the intracranial compartment (D2). An Evans’ index of 0.3 or higher is suggestive of ventriculomegaly, as seen in iNPH. The MR sign “disproportionately enlarged subarachnoid space hydrocephalus” (DESH) is show in (b), in which there is narrowing of the sulci at the high convexities, but prominent Sylvian fissures
Volumetric analysis of the hippocampi can also aid in differentiating iNPH from AD, since hippocampal atrophy is not a prominent feature in iNPH (Fig. 38.2) [51]. A few papers have also described an upward bowing of the corpus callosum, although this can be seen in many forms of hydrocephalus and is not specific to iNPH (Fig. 38.3) [52, 53].
Coronal T1-weighted MR image (a) demonstrating preservation of hippocampal volumes (a, curved arrows), which can help differentiate normal pressure hydrocephalus from Alzheimer’s disease. Fused 18F-FDG PET/T1-weighted axial MR image (b) demonstrates cortical hypometabolism in the frontal and parietal lobes (straight arrows). Patterns of hypometabolism in these patients can be heterogeneous
A less commonly used sign is the presence of a prominent CSF flow void, extending through the cerebral aqueduct to the obex of the fourth ventricle [5]. It is believed to be related to the hyperdynamic flow seen in iNPH. During systole, arterial pressure forces blood flow into the brain, putting pressure on already enlarged ventricles and leading to hyperdynamic CSF flow. Because of this increased flow, the walls of the third ventricle, which normally bow inward, become parallel or even bow outward. If these patients are not shunted, they will eventually develop atrophy [5].
CSF Flow Studies with MRI
Newer MR imaging techniques have intrinsic flow compensation, which masks the prominent CSF flow void that has been previously described in iNPH patients [5]. As a result, phase-contrast cine MR imaging has emerged as a promising technique to evaluate CSF flow in iNPH patients and to appropriately select candidates for ventriculoperitoneal shunting [54]. Using this technique, many MR imaging systems can calculate the volume of CSF flowing in a craniocaudad direction during systole and caudocranial during diastole. Aqueductal CSF stroke volume (ACSV) is the commonly used measure for the average volume of CSF passing through the aqueduct of Sylvius during the cardiac cycle. ACSV is increased in iNPH patients and decreases after successful shunting [54,55,56,57]. Bradley et al. found that patients who respond to shunting for iNPH have at least twice the ACSV of healthy elderly patients [5]. Although the reproducibility and reliability of ACSV values for prognosis in iNPH have been questioned [58], [59] phase-contrast MR imaging is often combined with a high-volume CSF tap test or ELD to assess whether or not to perform shunt surgery [5, 60, 61].
Advanced MR Imaging
In the last decade, several studies have explored the utility of advanced imaging techniques, such as diffusion tensor imaging (DTI) and resting state functional MRI (rs-fMRI), in the setting of iNPH. Although some positive correlations have been described in the literature, a clear advantage to using these advanced imaging techniques, either for diagnosis of iNPH or prediction of shunt response, is not evident [55, 62,63,64,65,66,67].
Higher mean diffusivity (MD) and lower fractional anisotropy (FA) in the corpus callosum and subcortical white matter of the bilateral temporal, parietal, and occipital lobes have been reported in iNPH patients, differentiating them from other neurodegenerative diseases. These measures are believed to result from the mechanical force exerted by the enlarged lateral ventricles onto the surrounding tissues and are also reportedly associated with worse gait performance [68,69,70]. Another paper combined the FA values in the internal capsule with the Evans index, achieving a diagnostic specificity of 100% for iNPH [62]. Ivkovic et al. used DTI as a supplementary test to distinguish iNPH from AD, Parkinson’s disease, and Lewy body dementia, achieving a sensitivity of 86% and specificity of 96% [71]. Other papers have combined MD in the superior thalamic radiations with ventricular volumes or alterations in corticospinal tract microstructure to discriminate among iNPH, AD, and age-matched healthy controls [64, 72]. Further validation in larger cohorts and correlation with clinical outcomes are warranted.
Studies using rs-fMRI have shown some promise. For example, reduced interhemispheric, temporal, anterior/posterior cingulate, and precuneus functional connectivity contributed to the accurate classification of iNPH versus controls [73,74,75]. Ogata et al. found that interhemispheric functional connectivity was most relevant to iNPH classification, which suggests that disruption of corpus callosum fibers due to ventricular enlargement may explain the clinical triad of iNPH [76]. However, more validation is needed since the spatial location and direction of the functional connectivity were poorly consistent across studies [75].
Positron Emission Tomography (PET)
18F-FDG-PET may be useful in the evaluation of a patient for iNPH by excluding other neurodegenerative diseases, specifically AD [77]. However, some patients could have iNPH and comorbid AD; in studies in which the brain was biopsied at the time of shunt placement for iNPH, about 30% of patients had concomitant AD pathology [78].
The utility of 18F-FDG-PET as a specific biomarker for iNPH is unclear. The pattern of hypometabolism can be heterogeneous, with many studies reporting hypometabolism globally or in several cortical regions (Figs. 38.2, 38.3, and 38.4) [77, 79]. Recent studies have also suggested that hypometabolism in the basal ganglia and thalami may be diagnostic [80, 81].
PET imaging with an amyloid tracer has also been used in the setting of iNPH. Mostly, this is used to determine whether there is concomitant AD pathology, in the form of beta-amyloid plaques. Some have suggested that shunt surgery may be less effective in individuals with concomitant AD. However, a study of 15 patients who underwent shunt surgery found that 13 benefited from the surgery and 7 of those that benefited had positive amyloid PET scans [82]. As a result, there is no clear evidence for PET imaging for amyloid plaques in this cohort.
Finally, some studies have used 15O-H2O-PET to measure cerebral blood flow in iNPH, finding a global reduction compared to healthy controls. However, studies that evaluated changes in blood flow before and after shunt surgery did not find a significant difference [83,84,85,86,87].
Other Imaging Modalities
Computerized tomographic cisternography is not recommended for clinical examination, due to its low specificity. However, recently, radionuclide cisternography with SPECT/CT has been proposed as an important exam for the evaluation of iNPH [88, 89]. In one study, almost 90% of patients with a positive cisternography study showed clinical improvement after shunt surgery, which suggests that radionuclide cisternography may be superior to measurement of the CA and CSF flow in predicting response to surgical shunting [89].
Brain perfusion SPECT may also be diagnostic in identifying increased midline, high convexity perfusion with decreased Perisylvian fissure perfusion, termed the “convexity apparently hyperperfusion” (CAPPAH) sign [46, 90]. This sign was also confirmed to be useful in a multicenter SPECT study, which demonstrated relatively increased perfusion areas in the high convexity region with severely reduced relative CBF around the corpus callosum and in the Perisylvian region [91].
Treatment
iNPH is one of the few reversible causes of dementia, with treatment relying on efficient surgical diversion of CSF via shunt placement. Up to 10% of patients with dementia may have NPH and therefore may be treatable by shunting. Shunts are usually placed in the lateral ventricle or in the lumbar subarachnoid space and can have one of three different drainage points. The most common drainage site is the peritoneum, known as a ventriculoperitoneal shunt (VPS). Two other types of shunts, ventriculopleural and ventriculoatrial shunts, terminate in the pleural space and the internal jugular vein, respectively. The last type, the lumboperitoneal (LP) shunt, is placed in the lumbar intradural space and drains into the peritoneum. While VPS is the most widely used approach in North America and Europe, LP shunts predominates in Japan and other parts of Asia. Nevertheless, both have been shown to be effective for iNPH treatment [92].
The best response to shunting occurs early in the disease process, when gait disturbance is the primary symptom. In an unblinded randomized controlled trial of iNPH patients who underwent immediate LP shunt versus delayed treatment, only 5% of the delayed group showed clinical improvement at 3 months compared with over 65% of the treated group [93]. Later in the disease process, when dementia is evident, symptoms are less likely to improve with a mean chance of significant improvement of 30–50% [94, 95]. Nevertheless, it has been estimated that only 10% to 20% of patients with iNPH receive appropriate shunting, probably due to difficulties in diagnosing iNPH and the morbidity and mortality associated with shunt surgery [96].
Shunt complications can be significant and include subdural hematomas and effusions, infections, seizures, focal neurologic deficits, shunt malfunction, and death. Twenty percent of patients require additional surgery, and 6% develop permanent neurologic deficits or death [3].
Endoscopic third ventriculostomy (ETV) has been pursued as an alternative approach to shunting in iNPH. However, thus far, outcomes have not been favorable, so ETV is not currently recommended for treatment of iNPH [97].
Nonsurgical pharmacological approaches to managing hydrocephalus have been tried since 1924 using diuretics [98]. Acetazolamide, a carbonic anhydrase inhibitor, has also been studied to manage iNPH. It has been shown to reduce the production of CSF and interstitial edema by inhibiting the carbonic anhydrases present within the choroid plexus and preventing aquaporin-based water conductance via signaling pathways. Ivkovic et al. found improvement in clinical symptoms in a small iNPH cohort, but research is ongoing [96]. Therefore, shunting still remains the main treatment option [98].
Conclusion
iNPH is one of the few reversible causes of dementia in the elderly and can be appropriately treated with shunt surgery, especially if identified early. The precise cause of iNPH remains unclear, although altered CSF dynamics likely play a role. The classic clinical triad consists of gait disturbance, urinary incontinence, and cognitive impairment. A CSF tap test and MRI findings help to confirm the diagnosis. PET imaging can be used to assess the likelihood of a concomitant neurodegenerative disease, such as AD. Ultimately, more research needs to be done to more specifically diagnosis iNPH and better predict who will respond to surgical shunting.
References
Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2(4):307–27.
Halperin JJ, Kurlan R, Schwalb JM, Cusimano MD, Gronseth G, Gloss D. Practice guideline: idiopathic normal pressure hydrocephalus: response to shunting and predictors of response. Neurology. 2015;85(23):2063.
Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166–84; discussion 84-6
Cardoso ER, Piatek D, Del Bigio MR, Stambrook M, Sutherland J. Quantification of abnormal intracranial pressure waves and isotope cisternography for diagnosis of occult communicating hydrocephalus. Surg Neurol. 1989;31(1):20–7.
Bradley WG. CSF flow in the brain in the context of normal pressure hydrocephalus. Am J Neuroradiol. 2015;36(5):831.
Yokota H, Vijayasarathi A, Cekic M, Hirata Y, Linetsky M, Ho M, et al. Diagnostic performance of glymphatic system evaluation using diffusion tensor imaging in idiopathic normal pressure hydrocephalus and mimickers. Curr Gerontol Geriatr Res. 2019;2019:5675014.
Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140(10):2691–705.
Silverberg G, Mayo M, Saul T, Fellmann J, Carvalho J, McGuire D. Continuous CSF drainage in AD: results of a double-blind, randomized, placebo-controlled study. Neurology. 2008;71:202–9.
Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. 2019;39(7):1355–68.
Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight. 2018;3(13):e121537.
Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, et al. Glymphatic system impairment in Alzheimer’s disease and idiopathic Normal pressure hydrocephalus. Trends Mol Med. 2020;26(3):285–95.
Portenoy RK, Berger A, Gross E. Familial occurrence of idiopathic normal-pressure hydrocephalus. Arch Neurol. 1984;41(3):335–7.
Krefft TA, Graff-Radford NR, Lucas JA, Mortimer JA. Normal pressure hydrocephalus and large head size. Alzheimer Dis Assoc Disord. 2004;18(1):35–7.
Wilson RK, Williams MA. Evidence that congenital hydrocephalus is a precursor to idiopathic normal pressure hydrocephalus in only a subset of patients. J Neurol Neurosurg Psychiatry. 2007;78(5):508–11.
Sato H, Takahashi Y, Kimihira L, Iseki C, Kato H, Suzuki Y, et al. A segmental copy number loss of the SFMBT1 gene is a genetic risk for shunt-responsive, idiopathic Normal pressure hydrocephalus (iNPH): a case-control study. PLoS One. 2016;11(11):e0166615.
Graff-Radford NR, Knopman DS, Penman AD, Coker LH, Mosley TH. Do systolic BP and pulse pressure relate to ventricular enlargement? Eur J Neurol. 2013;20(4):720–4.
Jaraj D, Agerskov S, Rabiei K, Marlow T, Jensen C, Guo X, et al. Vascular factors in suspected normal pressure hydrocephalus: a population-based study. Neurology. 2016;86(7):592–9.
Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer HA, et al. Dutch Normal-pressure hydrocephalus study: the role of cerebrovascular disease. J Neurosurg. 1999;90(2):221–6.
Conn HO. Normal pressure hydrocephalus (NPH): more about NPH by a physician who is the patient. Clin Med (Lond). 2011;11(2):162–5.
Stolze H, Kuhtz-Buschbeck JP, Drucke H, Johnk K, Diercks C, Palmie S, et al. Gait analysis in idiopathic normal pressure hydrocephalus--which parameters respond to the CSF tap test? Clin Neurophysiol. 2000;111(9):1678–86.
Williams MA, Malm J. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Continuum (Minneap Minn). 2016;22(2 Dementia):579–99.
Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761–8.
Katzen H, Ravdin LD, Assuras S, Heros R, Kaplitt M, Schwartz TH, et al. Postshunt cognitive and functional improvement in idiopathic normal pressure hydrocephalus. Neurosurgery. 2011;68(2):416–9.
Capone PM, Bertelson JA, Ajtai B. Neuroimaging of normal pressure hydrocephalus and hydrocephalus. Neurol Clin. 2020;38(1):171–83.
Sakakibara R, Kanda T, Sekido T, Uchiyama T, Awa Y, Ito T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507–10.
Anger JT, Saigal CS, Litwin MS, Urologic Diseases of America P. The prevalence of urinary incontinence among community dwelling adult women: results from the national health and nutrition examination survey. J Urol. 2006;175(2):601–4.
Stothers L, Thom D, Calhoun E. Urologic diseases in America project: urinary incontinence in males--demographics and economic burden. J Urol. 2005;173(4):1302–8.
Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4–16; discussion ii-v.
Mori E, Ishikawa M, Kato T, Kazui H, Miyake H, Miyajima M, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo). 2012;52(11):775–809.
Savolainen S, Hurskainen H, Paljarvi L, Alafuzoff I, Vapalahti M. Five-year outcome of normal pressure hydrocephalus with or without a shunt: predictive value of the clinical signs, neuropsychological evaluation and infusion test. Acta Neurochir. 2002;144(6):515–23; discussion 23
Damasceno BP. Neuroimaging in normal pressure hydrocephalus. Dementia Neuropsychol. 2015;9(4):350–5.
Anthony M, Harold FY, Gunes AA, Satoshi S, Osamu T, Takuji Y, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102(6):987–97.
Gloeckner SF, Meyne F, Wagner F, Heinemann U, Krasnianski A, Meissner B, et al. Quantitative analysis of transthyretin, tau and amyloid-beta in patients with dementia. J Alzheimers Dis. 2008;14(1):17–25.
Lins H, Wichart I, Bancher C, Wallesch CW, Jellinger KA, Rosler N. Immunoreactivities of amyloid beta peptide((1-42)) and total tau protein in lumbar cerebrospinal fluid of patients with normal pressure hydrocephalus. J Neural Transm (Vienna). 2004;111(3):273–80.
Kudo T, Mima T, Hashimoto R, Nakao K, Morihara T, Tanimukai H, et al. Tau protein is a potential biological marker for normal pressure hydrocephalus. Psychiatry Clin Neurosci. 2000;54(2):199–202.
Ray B, Reyes PF, Lahiri DK. Biochemical studies in normal pressure hydrocephalus (NPH) patients: change in CSF levels of amyloid precursor protein (APP), amyloid-beta (Abeta) peptide and phospho-tau. J Psychiatr Res. 2011;45(4):539–47.
Agerskov S, Wallin M, Hellström P, Ziegelitz D, Wikkelsö C, Tullberg M. Absence of disproportionately enlarged subarachnoid space hydrocephalus, a sharp callosal angle, or other morphologic MRI markers should not be used to exclude patients with idiopathic normal pressure hydrocephalus from shunt surgery. Am J Neuroradiol. 2019;40(1):74–9.
Evans WA Jr. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatr. 1942;47(6):931–7.
Toma AK, Holl E, Kitchen ND, Watkins LD. Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery. 2011;68(4):939–44.
Park JE, Ju H, Im K, Kwon KY. Revisiting the diagnostic value of Evans’ index: lessons from an unusual case of normal pressure hydrocephalus with Evans’ index less than 0.3. Neurol Sci. 2019;40(12):2637–9.
Yamada S, Ishikawa M, Yamamoto K. Optimal diagnostic indices for idiopathic normal pressure hydrocephalus based on the 3D quantitative volumetric analysis for the cerebral ventricle and subarachnoid space. Am J Neuroradiol. 2015;36(12):2262.
Ishii K, Kanda T, Harada A, Miyamoto N, Kawaguchi T, Shimada K, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol. 2008;18(11):2678–83.
Virhammar J, Laurell K, Cesarini KG, Larsson EM. The callosal angle measured on MRI as a predictor of outcome in idiopathic normal-pressure hydrocephalus. J Neurosurg. 2014;120(1):178–84.
Grahnke K, Jusue-Torres I, Szujewski C, Joyce C, Schneck M, Prabhu VC, et al. The quest for predicting sustained shunt response in normal-pressure hydrocephalus: an analysis of the callosal angle’s utility. World Neurosurg. 2018;115:e717–e22.
Ishii K, Soma T, Shimada K, Oda H, Terashima A, Kawasaki R. Automatic volumetry of the cerebrospinal fluid space in idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord Extra. 2013;3(1):489–96.
Ishii K. Diagnostic imaging of dementia with Lewy bodies, frontotemporal lobar degeneration, and normal pressure hydrocephalus. Jpn J Radiol. 2020;38(1):64–76.
Akiba C, Gyanwali B, Villaraza S, Nakajima M, Miyajima M, Cheng C-Y, et al. The prevalence and clinical associations of disproportionately enlarged subarachnoid space hydrocephalus (DESH), an imaging feature of idiopathic normal pressure hydrocephalus in community and memory clinic based Singaporean cohorts. J Neurol Sci. 2020;408:116510.
Hashimoto M, Ishikawa M, Mori E, Kuwana N. Study of Ioni. Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res. 2010;7:18.
Shinoda N, Hirai O, Hori S, Mikami K, Bando T, Shimo D, et al. Utility of MRI-based disproportionately enlarged subarachnoid space hydrocephalus scoring for predicting prognosis after surgery for idiopathic normal pressure hydrocephalus: clinical research. J Neurosurg. 2017;127(6):1436–42.
Benedetto N, Gambacciani C, Aquila F, Di Carlo DT, Morganti R, Perrini P. A new quantitative method to assess disproportionately enlarged subarachnoid space (DESH) in patients with possible idiopathic normal pressure hydrocephalus: the SILVER index. Clin Neurol Neurosurg. 2017;158:27–32.
Savolainen S, Laakso MP, Paljarvi L, Alafuzoff I, Hurskainen H, Partanen K, et al. MR imaging of the hippocampus in normal pressure hydrocephalus: correlations with cortical Alzheimer’s disease confirmed by pathologic analysis. AJNR Am J Neuroradiol. 2000;21(2):409–14.
Hurley RA, Bradley WG Jr, Latifi HT, Taber KH. Normal pressure hydrocephalus: significance of MRI in a potentially treatable dementia. J Neuropsychiatry Clin Neurosci. 1999;11(3):297–300.
Hofmann E, Becker T, Jackel M, Metzner D, Schneider M, Meixensberger J, et al. The corpus callosum in communicating and noncommunicating hydrocephalus. Neuroradiology. 1995;37(3):212–8.
Nitz WR, Bradley WG Jr, Watanabe AS, Lee RR, Burgoyne B, O’Sullivan RM, et al. Flow dynamics of cerebrospinal fluid: assessment with phase-contrast velocity MR imaging performed with retrospective cardiac gating. Radiology. 1992;183(2):395–405.
Atasoy B, Aralasmak A, Cetinkaya E, Toprak H, Toprak A, Tokdemir S, et al. Normal pressure hydrocephalus: clinical symptoms, cerebrospinal fluid flow metrics and white matter changes. J Comput Assist Tomogr. 2020;44(1):59–64.
Scollato A, Tenenbaum R, Bahl G, Celerini M, Salani B, Di Lorenzo N. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2008;29(1):192–7.
Shanks J, Markenroth Bloch K, Laurell K, Cesarini KG, Fahlström M, Larsson EM, et al. Aqueductal CSF stroke volume is increased in patients with idiopathic Normal pressure hydrocephalus and decreases after shunt surgery. Am J Neuroradiol. 2019;40(3):453.
Kahlon B, Annertz M, Stahlberg F, Rehncrona S. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery. 2007;60(1):124–9; discussion 9-30
Yamada S, Tsuchiya K, Bradley WG, Law M, Winkler ML, Borzage MT, et al. Current and emerging MR imaging techniques for the diagnosis and management of CSF flow disorders: a review of phase-contrast and time-spatial labeling inversion pulse. AJNR Am J Neuroradiol. 2015;36(4):623–30.
Ishikawa M, Hashimoto M, Mori E, Kuwana N, Kazui H. The value of the cerebrospinal fluid tap test for predicting shunt effectiveness in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2012;9(1):1.
Sharma AK, Gaikwad S, Gupta V, Garg A, Mishra NK. Measurement of peak CSF flow velocity at cerebral aqueduct, before and after lumbar CSF drainage, by use of phase-contrast MRI: utility in the management of idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. 2008;110(4):363–8.
Kim MJ, Seo SW, Lee KM, Kim ST, Lee JI, Nam DH, et al. Differential diagnosis of idiopathic normal pressure hydrocephalus from other dementias using diffusion tensor imaging. AJNR Am J Neuroradiol. 2011;32(8):1496–503.
Virhammar J, Ahlgren A, Cesarini KG, Laurell K, Larsson EM. Cerebral perfusion does not increase after shunt surgery for normal pressure hydrocephalus. J Neuroimaging. 2020;30(3):303–7.
Younes K, Hasan KM, Kamali A, McGough CE, Keser Z, Hasan O, et al. Diffusion tensor imaging of the superior thalamic radiation and cerebrospinal fluid distribution in idiopathic normal pressure hydrocephalus. J Neuroimaging. 2019;29(2):242–51.
Kanno S, Abe N, Saito M, Takagi M, Nishio Y, Hayashi A, et al. White matter involvement in idiopathic normal pressure hydrocephalus: a voxel-based diffusion tensor imaging study. J Neurol. 2011;258(11):1949–57.
Hattingen E, Jurcoane A, Melber J, Blasel S, Zanella FE, Neumann-Haefelin T, et al. Diffusion tensor imaging in patients with adult chronic idiopathic hydrocephalus. Neurosurgery. 2010;66(5):917–24.
Grazzini I, Redi F, Sammartano K, Cuneo GL. Diffusion tensor imaging in idiopathic normal pressure hydrocephalus: clinical and CSF flowmetry correlations. Neuroradiol J. 2019;33(1):66–74.
Osuka S, Matsushita A, Yamamoto T, Saotome K, Isobe T, Nagatomo Y, et al. Evaluation of ventriculomegaly using diffusion tensor imaging: correlations with chronic hydrocephalus and atrophy. J Neurosurg. 2010;112(4):832–9.
Hoza D, Vlasák A, Hořínek D, Sameš M, Alfieri A. DTI-MRI biomarkers in the search for normal pressure hydrocephalus aetiology: a review. Neurosurg Rev. 2015;38(2):239–44.
Kang K, Choi W, Yoon U, Lee J-M, Lee H-W. Abnormal white matter integrity in elderly patients with idiopathic normal-pressure hydrocephalus: a tract-based spatial statistics study. Eur Neurol. 2016;75(1–2):96–103.
Ivkovic M, Liu B, Ahmed F, Moore D, Huang C, Raj A. Differential diagnosis of normal pressure hydrocephalus by MRI mean diffusivity histogram analysis. AJNR Am J Neuroradiol. 2013;34:1168–74.
Hattori T, Yuasa T, Aoki S, Sato R, Sawaura H, Mori T. Altered microstructure in corticospinal tract in idiopathic normal pressure hydrocephalus: comparison with Alzheimer disease and Parkinson disease with dementia. AJNR Am J Neuroradiol. 2011;32:1681–7.
Khoo HM, Kishima H, Tani N, Oshino S, Maruo T, Hosomi K, et al. Default mode network connectivity in patients with idiopathic normal pressure hydrocephalus. J Neurosurg. 2016;124(2):350–8.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47.
Griffa A, Van De Ville D, Herrmann FR, Allali G. Neural circuits of idiopathic Normal pressure hydrocephalus: a perspective review of brain connectivity and symptoms meta-analysis. Neurosci Biobehav Rev. 2020;112:452–71.
Ogata Y, Ozaki A, Ota M, Oka Y, Nishida N, Tabu H, et al. Interhemispheric resting-state functional connectivity predicts severity of idiopathic normal pressure hydrocephalus. Front Neurosci. 2017;11:470.
Jagust WJ, Friedland RP, Budinger TF. Positron emission tomography with [18F]fluorodeoxyglucose differentiates normal pressure hydrocephalus from Alzheimer-type dementia. J Neurol Neurosurg Psychiatry. 1985;48(11):1091–6.
Graff-Radford NR, Jones DT. Normal pressure hydrocephalus. Continuum. 2019;25(1):165–86.
Tedeschi E, Hasselbalch SG, Waldemar G, Juhler M, Hogh P, Holm S, et al. Heterogeneous cerebral glucose metabolism in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1995;59(6):608–15.
Townley RA, Botha H, Graff-Radford J, Boeve BF, Petersen RC, Senjem ML, et al. (18)F-FDG PET-CT pattern in idiopathic normal pressure hydrocephalus. Neuroimage Clin. 2018;18:897–902.
Miyazaki K, Hanaoka K, Kaida H, Chiba Y, Ishii K. Changes in cerebral glucose metabolism caused by morphologic features of prodromal idiopathic normal pressure hydrocephalus. EJNMMI Res. 2019;9(1):111.
Rinne JO, Suotunen T, Rummukainen J, Herukka SK, Nerg O, Koivisto AM, et al. [11C]PIB PET is associated with the brain biopsy amyloid-beta load in subjects examined for normal pressure hydrocephalus. J Alzheimers Dis. 2019;67(4):1343–51.
Meltzer C, Cantwell M, Greer P, Ben-Eliezer D, Smith G, Frank G. Does cerebral blood flow decline in healthy aging? A PET study with partial-volume correction. J Nucl Med. 2000;41:1842–8.
Klinge P, Brooks D, Samii A, Weckesser E, van den Hoff J, Fricke H. Correlates of local cerebral blood flow (CBF) in normal pressure hydrocephalus patients before and after shunting—a retrospective analysis of [15O]H2O PET-CBF studies in 65 patients. Clin Neurol Neurosurg. 2008;110:369–75.
Owler B, Pena A, Momjian S, Czosnyka Z, Czosnyka M, Harris N. Changes in cerebral blood flow during cerebrospinal fluid pressure manipulation in patients with normal pressure hydrocephalus: a methodological study. J Cereb Blood Flow Metab. 2004;24:579–87.
Keong NCH, Pena A, Price SJ, Czosnyka M, Czosnyka Z, Pickard JD. Imaging normal pressure hydrocephalus: theories, techniques, and challenges. Neurosurg Focus. 2016;41(3):E11.
Klinge P, Berding G, Brinker T, Knapp W, Samii M. A positron emission tomography study of cerebrovascular reserve before and after shunt surgery in patients with idiopathic chronic hydrocephalus. J Neurosurg. 1999;91:605–9.
Kawaguchi T, Hirata Y, Bundo M, Kondo T, Owaki H, Ito S, et al. Role of computerized tomographic cisternography in idiopathic normal pressure hydrocephalus. Acta Neurochir. 2011;153(10):2041–8; discussion 8
Waitman M, Coutinho A, Nunes R, Trindade M, Zaniboni E, Bastos L, et al. Radionuclide cisternography revisited in the SPECT/CT era: applications in normal pressure hydrocefalus and in the detection of cerebrospinal fistulas in comparison with magnetic resonance imaging techniques. 2019.
Ohmichi T, Kondo M, Itsukage M, Koizumi H, Matsushima S, Kuriyama N, et al. Usefulness of the convexity apparent hyperperfusion sign in 123I-iodoamphetamine brain perfusion SPECT for the diagnosis of idiopathic normal pressure hydrocephalus. J Neurosurg. 2018;130(2):398–405.
Ishii K, Hashimoto M, Hayashida K, Hashikawa K, Chang CC, Nakagawara J, et al. A multicenter brain perfusion SPECT study evaluating idiopathic normal-pressure hydrocephalus on neurological improvement. Dement Geriatr Cogn Disord. 2011;32(1):1–10.
Isaacs AM, Williams MA, Hamilton MG. Current update on treatment strategies for idiopathic normal pressure hydrocephalus. Curr Treat Options Neurol. 2019;21(12):65.
Kazui H, Miyajima M, Mori E, Ishikawa M, Investigators S. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): an open-label randomised trial. Lancet Neurol. 2015;14(6):585–94.
Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol. 2000;247(1):5–14.
Spetzler R. Normal pressure hydrocephalus. Barrow Quart. 2003;19:1.
Ivkovic M, Reiss-Zimmermann M, Katzen H, Preuss M, Kovanlikaya I, Heier L, et al. MRI assessment of the effects of acetazolamide and external lumbar drainage in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2015;12:9.
Halperin JJ, Kurlan R, Schwalb JM, Cusimano MD, Gronseth G, Gloss D. Practice guideline: idiopathic normal pressure hydrocephalus: response to shunting and predictors of response: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(23):2063–71.
Zhang L, Hussain Z, Ren Z. Recent advances in rational diagnosis and treatment of normal pressure hydrocephalus: a critical appraisal on novel diagnostic, therapy monitoring and treatment modalities. Curr Drug Targets. 2019;20(10):1041–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kovanlikaya, I., Chiang, G.C. (2022). Normal Pressure Hydrocephalus. In: Franceschi, A.M., Franceschi, D. (eds) Hybrid PET/MR Neuroimaging. Springer, Cham. https://doi.org/10.1007/978-3-030-82367-2_38
Download citation
DOI: https://doi.org/10.1007/978-3-030-82367-2_38
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82366-5
Online ISBN: 978-3-030-82367-2
eBook Packages: MedicineMedicine (R0)