Abstract
The aim of this study was to characterise the white matter damage involved in idiopathic normal pressure hydrocephalus (INPH) using diffusion tensor imaging (DTI) and the relationship between this damage and clinical presentation. Twenty patients with INPH, 20 patients with Alzheimer’s disease and 20 patients with idiopathic Parkinson’s disease (as disease control groups) were enrolled in this study. Mean diffusivity (MD) and fractional anisotropy (FA) were determined using DTI, and these measures were analysed to compare the INPH group with the control groups and with certain clinical correlates. On average, the supratentorial white matter presented higher MD and lower FA in the INPH group than in the control groups. In the INPH group, the mean hemispheric FA correlated with some of the clinical measures, whereas the mean hemispheric MD did not. On a voxel-based statistical map, white matter involvement with high MD was localised to the periventricular regions, and white matter involvement with low FA was localised to the corpus callosum and the subcortical regions. The total scores on the Frontal Assessment Battery were correlated with the FA in the frontal and parietal subcortical white matter, and an index of gait disturbance was correlated with the FA in the anterior limb of the left internal capsule and under the left supplementary motor area. DTI revealed the presence of white matter involvement in INPH. Whereas white matter regions with high MD were not related to symptom manifestation, those with low FA were related to motor and cognitive dysfunction in INPH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is believed that a disturbance of cerebrospinal fluid (CSF) dynamics is involved in the development of normal pressure hydrocephalus (NPH) [1, 2]. Ventricular dilation, which is an essential neuroradiological and neuropathological indicator of idiopathic NPH (INPH), has been postulated to be caused by reduced CSF absorption by the arachnoid villi or capillaries [3, 4]. Adams has stated that mechanical compression of the neural tracts caused by an expansion in the lateral ventricles produces the typical gait disturbance observed in NPH patients [1]. Regardless of the underlying pathophysiological mechanisms involved in NPH, neuropathological findings are generally consistent in demonstrating the involvement of white matter. Indeed, a variety of pathological characteristics, such as direct mechanical compression of the periventricular white matter, ischaemic demyelination, and infarction, have been observed in the brains of patients with NPH [5–7]. In one neuropathological study, disruption of the ependymal lining, interstitial oedema, axonal degeneration, and gliosis were noted in the periventricular white matter regions of patients who presented with chronic hydrocephalus (including patients with INPH) and were thought to be associated with the development of motor and cognitive dysfunction [8]. Therefore, in the present study, we focused on white matter as the main locus affected by INPH and measured the changes of white matter in vivo using magnetic resonance imaging (MRI).
Diffusion tensor imaging (DTI) is a useful MRI technique that reflects the movement of water molecules within tissues [9, 10]. An increase in the mean diffusivity (MD) of a tissue is associated with increased free water diffusion in the extracellular space of that tissue. The fractional anisotropy (FA) reflects the degree of directionality of intracellular water diffusion and the structural integrity of the white matter [11–13]. Therefore, these DTI indices are both useful tools for evaluating white matter involvement in patients with INPH. Although the involvement of white matter in INPH has previously been studied using DTI [14], the severe morphological changes that occur in the brains of patients with INPH have precluded the use of voxel-based analysis [15]. Nevertheless, a recent study demonstrated microstructural changes in periventricular white matter structures that could be functionally relevant to gait disturbance and indicated a novel possibility for the detection of an association between white matter lesions and clinical symptoms in INPH patients [16]. However, it is doubtful that damage to the pyramidal tract could cause the gait disturbance involved in INPH, which is characterised as broad-based, magnetic, and petit pas gait [17]. In addition, the association between cognitive dysfunction and white matter lesions in INPH patients remains uncertain. In the present study, acquired DTI data were used in a voxel-based analysis that adopted a customised white matter template that reflects morphological aspects of INPH for spatial normalisation, using the technique for both manual and automatic image processing. We included patients with Alzheimer’s disease (AD) and idiopathic Parkinson’s disease (IPD) as disease control groups because the cognitive and gait disturbances that occur in these patients are generally due to gray matter involvement [18, 19]. By comparing INPH patients with patients with AD or with IPD, we attempted to elucidate white matter changes that occur in patients with INPH. We also sought to uncover associations between the occurrence of white matter changes and/or ventricular dilation with a patient’s clinical presentation.
Subjects and methods
This study was conducted in accordance with the Declaration of Helsinki; the protocol was approved by Tohoku University.
Participants
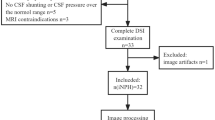
The subjects were patients with INPH, AD, or IPD. All patients were recruited from Tohoku University Hospital and were examined by board-certified neurologists and experienced neuropsychologists. Written informed consent was obtained from each participant and their relatives. The demographic characteristics of the patients in each disease group are shown in Table 1. In addition, we excluded the patients with obvious cerebral vascular lesions in the white matter, as observed by a fluid attenuated inversion recovery (FLAIR) scan, to avoid the possibility that the DTI parameters and clinical symptoms would be affected by a vascular brain disease.
Patients with INPH
Twenty consecutive patients with INPH who fulfilled the criteria for a diagnosis of definite INPH according to the Japanese Clinical Guidelines for Idiopathic Normal Pressure Hydrocephalus were included in the study [20]. The criteria for the diagnosis of definite INPH are as follows: (1) >60 years of age; (2) gait disturbance, dementia, and/or urinary incontinence; (3) ventricular dilation (Evans Index >0.3) with a narrow CSF space in the superior convexity; (4) CSF pressure <200 mmH2O with a normal CSF cell count and normal CSF protein levels; (5) absence of another disease that could account for their symptoms; (6) no previous illness that could have caused ventricular dilation; and (7) improved INPH symptoms after shunt placement.
In the present study, shunt responsiveness was defined as an improvement by one point or more on the idiopathic normal pressure hydrocephalus grading scale (iNPHGS), which is a validated scale for the measurement of INPH symptom severity, within 1 year of shunt placement [21]. To evaluate patients’ motor and cognitive symptoms, the Timed “Up and Go” test (TUG) [22], the Mini-Mental State Examination (MMSE) [23], and the Frontal Assessment Battery (FAB) [24] were administered. We used the FAB, which is a simple and useful tool for assessing the frontal lobe functions of patients, because it has been reported that frontal lobe dysfunction is a characteristic feature of the cognitive impairment that occurs in patients with INPH [25]. In this study, we assessed patients’ symptoms, administered the TUG, MMSE, and FAB, and performed MRI scans prior to performing both a lumbar puncture for CSF removal and the shunt operation to elucidate the clinical and radiological features of pre-operative INPH patients whose symptoms improved after shunt placement.
Patients with AD and IPD
In addition to the 20 INPH patients, 20 patients with AD and 20 patients with IPD were included in this study. The patients with AD fulfilled the criteria for probable AD according to the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA). The patients with IPD fulfilled the diagnostic criteria of the UK Parkinson’s Disease Society Brain Bank. The INPH, AD, and IPD groups did not differ significantly with regard to age, sex, or educational level attained, and there were no significant differences observed between the MMSE scores of the INPH and AD groups (Table 1).
MRI procedure
Three-dimensional spoiled gradient echo imaging (3D-SPGR) and diffusion-weighted imaging (DWI) data were acquired via a single-shot spin echo-type echo planar imaging sequence with a GE Signa 1.5 Tesla MRI unit (General Electric Company, Milwaukee, WI, USA). The imaging parameters used for 3D-SPGR imaging were TR 20 ms, TE 4.1 ms, 1.5 mm thickness/0.0 sp by 108 slices and no intersection gap, FOV 21 × 21 cm, and matrix 256 × 256. The imaging parameters used for the acquisition of the DWI data were TR 15000 ms, TE 82.8 ms, 2.5 mm thickness/0.0 sp by 50 slices and no intersection gap, FOV 23 × 23 cm, and matrix 256 × 256. The DWI data were acquired along 13 gradient directions, with b = 1,000 s/mm2. One volume was acquired without diffusion weighting (b = 0 s/mm2) twice during each run.
MRI data processing
The images of the skull, dura, sinuses, and infratentorial structures that were obtained via 3D-SPGR imaging were stripped manually, and the volumes of the supratentorial intracranial region and the ventricles (the lateral ventricles and the third ventricle) of each subject were measured using ANALYZE 9.0 software (The Biomedical Imaging Resource at Mayo Clinic, Rochester, MN). These measurements were performed by two neurologists who were blinded to the subjects’ clinical data. The infratentorial structures were excluded according to the method of Pfaendner et al. [26]. The VV/STV ratio, which is calculated by dividing the sum of the volume of the lateral ventricles and the volume of the third ventricle by the volume of the supratentorial intracranial region, was calculated as an index of the ventricular dilation of each subject. The inter-observer reproducibility of the VV/ST ratio, as assessed by the intraclass correlation coefficient, was >0.9.
All diffusion images were aligned with the initial b0 image, and we used motion correction and registration software from the FSL software package (http://www.fmrib.ox.ac.uk/fsl) to perform motion correction and eddy current distortion correction [27]. The corrected images for each direction, which were acquired in two scans, were averaged to improve the signal-to-noise ratio, and the MD and FA maps were calculated from the mean diffusion-weighted images for each direction using DTI calculation software from the FSL software package. The initial b0 image and the 3D-SPGR image of each subject were co-registered, and the obtained co-registered parameters were applied to the corresponding MD and FA maps using the SPM5 software package.
To anatomically normalise the MD and FA maps, supratentorial brain segmentation was first performed using the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm/) with individualised masks (created using MRIcron [28] for each subject) fitted to the lateral and third ventricles. Next, we created a mean white matter image from all subjects’ segmented images using the SPM5 realign algorithm. This image was then smoothed using an isotropic Gaussian filter (8 mm, FWHM), and the resultant image served as a white matter template. We used this customised white matter template instead of the standard template because the standard template could not be used to effectively analyse the marked deformations present in the brains of INPH patients. The white matter image of each subject was normalised to the custom white matter template, and the normalised parameters obtained were applied to the co-registered MD and FA maps. The normalised MD and FA maps were subsequently smoothed using an isotropic Gaussian filter (10 mm, FWHM).
Anatomical measures, clinical measures, and hemispheric DTI analyses
The mean MD and FA values of all of the supratentorial white matter (which included all voxels with white matter probability values greater than 0.95 based on the SPM segmentation results) (hemispheric MD and FA) were calculated from the co-registered MD and FA maps for each subject. The VV/STV ratios, hemispheric MD values, and hemispheric FA values of the INPH, AD, and IPD groups were compared by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc analysis. We used Spearman’s rank correlation coefficient to identify possible associations between both the hemispheric MD and FA values and the extent of ventricular dilation (the VV/STV ratios) and the clinical symptoms (MMSE score, FAB score, TUG completion time, and TUG number of steps) exhibited by INPH patients. Statistical significance was defined as p < 0.05. No correction for multiple comparisons was carried out because of the exploratory nature of this study.
Voxel-based analyses of DTI
All voxel-based analyses of MD and FA were performed within the areas of the supratentorial white matter that presented probability values >0.8. The MD and FA analyses were restricted to regions with FA values >0.2. Comparisons of the MD and FA values between the INPH and AD groups and between the INPH and IPD groups were performed using two-sample t tests in SPM5 (p < 0.05, family-wise error correction, minimum cluster size of 40 voxels). In addition, to determine which regions of white matter were most closely associated with the severity of patients’ clinical symptoms, we conducted linear regression analyses in the INPH group using either MD or FA as the dependent variable and with each clinical measure (MMSE score, FAB score, TUG completion time, or TUG number of steps) as the independent variable. Age and sex were included as nuisance variables in each model [29, 30]. For the association analyses, we used a voxel-based threshold of p < 0.005 (uncorrected) for significance and a minimum cluster size of 40 voxels.
Results
Hemispheric DTI and ventricular size
The hemispheric MD values, hemispheric FA values, and VV/STV ratios of each group are shown in Table 2. The mean hemispheric MD value of the patients in the INPH group was significantly higher [F (2, 57) = 11.22, p < 0.001] than those of the patients in the AD and IPD groups, whereas the mean hemispheric FA value of the patients in the INPH group was significantly lower [F (2, 57) = 12.73, p < 0.001] than those of the patients in the AD and IPD groups. The mean VV/STV ratio of the patients in the INPH group was significantly larger than that of the patients in the AD and IPD groups [F (2, 57) = 103.64, p < 0.001]. The hemispheric FA of the INPH group was significantly correlated with the FAB score (r s = 0.54, p < 0.05) and the TUG number of steps (r s = −0.48, p < 0.05). However, there were no correlations observed between the hemispheric FA and the MMSE score or the TUG completion time, and neither the hemispheric MD value nor the VV/STV ratio was significantly correlated with the MMSE score, the FAB score, or either of the TUG indices. We did not observe any significant correlation between the VV/STV ratio and either the hemispheric MD or the hemispheric FA values.
Voxel-based analysis of DTI
The results of the voxel-based group comparisons of the MD values are shown in Fig. 1. The MD values were significantly higher in the periventricular white matter of INPH patients (including the internal capsule, temporal stem, corpus callosum, corona radiata, and subcortical orbitofrontal white matter) than they were in the periventricular white matter of the AD or IPD patients. In contrast, the MD values of the subcortical white matter of the left superior frontal gyrus were significantly lower in INPH patients than in patients in the AD and IPD groups.
White matter regions in which a significantly higher MD was observed in the INPH group than in the AD group (a-1) and in which a significantly lower MD was observed in the INPH group than in the AD group (a-2). White matter regions in which a significantly higher MD was observed in the INPH group than in the IPD group (b-1) and in which a significantly lower MD was observed in the INPH group than in the IPD group (b-2). Coloured bars indicate t values. R right, A anterior, P posterior, RH right hemisphere, LH left hemisphere
Figure 2 shows the results of the voxel-based group comparison of the FA values. The FA values were significantly lower in the corpus callosum, especially in the posterior region and the splenium, and in the subcortical white matter of the bilateral temporal, parietal and occipital lobes [including the superior longitudinal fasciculus (SLF) and the sagittal stratum (SS)] of the INPH patients than in these regions in the AD and IPD patients. In addition, the FA values observed in the anterior limb of the internal capsule were significantly lower in the INPH group than in the IPD group. There were no brain regions in which the INPH group presented a significantly higher mean FA value than either the AD or the IPD group.
The results of the linear regression analyses performed on patients in the INPH group are shown in Fig. 3. The TUG number of steps observed in these patients was significantly and negatively correlated with the FA values of the subcortical white matter in the left supplementary motor area (SMA) and of the left anterior limb of the internal capsule. Furthermore, there was a significant and positive correlation observed between the patients’ FAB scores and FA values in the bilateral periventricular white matter of the frontal lobe (including the superior occipitofrontal fasciculus (SOFF) and the SLF), the genu of the corpus callosum, and the subcortical white matter of the left superior parietal lobule. There were no significant correlations observed between the FA values in any white matter region and the MMSE scores or TUG completion times.
Discussion
In the present study, we found that white matter in the cerebral hemispheres was implicated in INPH. We also found that the white matter involvement in specific regions was correlated with both motor and cognitive symptoms experienced by INPH patients. Before discussing the implications of our findings, it is important to discuss the methodological limitations of our study. Rather than comparing INPH patients with normal, healthy controls, we compared them with disease controls, i.e., patients with either AD or IPD. This might have led to an underestimation of the changes that occur in INPH patients. In AD patients, increased MD values and reduced FA values in the temporoparietal subcortical white matter have been reported [31, 32]. Karagulle et al. [33] observed a significant decrease in the FA values in the medial frontal lobes of IPD patients. However, there is no evidence in the literature to suggest that specific white matter regions exhibit decreased MD values or increased FA values in patients with AD or IPD. Therefore, this suggests that our findings are rather robust.
Increased MD values in periventricular white matter were also found in a previous study in which region-of-interest analysis of periventricular white matter was performed in pre-operative INPH patients [34]. Despite these findings, no significant association was observed between hemispheric MD values and any of the clinical measures that we used. High MD values theoretically reflect an increase in the extracellular water content in the particular region being studied, and the distribution of the areas with increased MD values in INPH patients could suggests the presence of interstitial oedema in periventricular white matter regions. This phenomenon has previously been demonstrated in a neuropathological study of patients with chronic hydrocephalus [8]. The degree of interstitial oedema could be associated with the underlying pathophysiological mechanisms related to white matter involvement in patients with INPH, but it is not directly correlated with the severity of the clinical symptoms of patients with INPH.
In contrast, the MD values observed in the superior convexity of the medial frontal subcortical white matter were lower in the INPH group than in either control group. A low MD value indicates a reduced amount of extracellular space and increased axonal fibre density in the region under study. Kitagaki et al. [35] reported that shunt-responsive INPH presented with neuroradiological features, including an enlarged basal cistern and sylvian fissures and a decreased CSF space in the superior convexity and medial subarachnoid space. Indeed, in a recent clinical study, observation of these features predicted good shunt responsiveness in elderly INPH patients [36]. Ishii et al. [37] demonstrated that the grey matter densities of the para-interhemispheric and frontoparietal cortices at the dorsal convexity were elevated in INPH patients, and they suggested that this could be due to mechanical compression of the brain parenchyma in these regions by enlarged ventricles and sylvian fissures. The white matter in these regions is also likely to be compressed, which could lead to a constriction of extracellular spaces.
The distribution of white matter regions with low FA values in the INPH group was different from that of regions with high MD values, as the areas with low FA values were distributed across the periventricular white matter, predominantly in the posterior cerebrum. The hemispheric FA value was significantly associated with the FAB total score and with the TUG number of steps in INPH patients. Because FA theoretically reflects the structural integrity of the white matter, low FA values imply disintegration of the white matter. This white matter disintegration could lead to motor and cognitive dysfunction. In the present study, the function of the corona radiata, which contains the pyramidal tract and sensory radiation and in which the FA value was preserved despite high MD values, appeared to be maintained in INPH patients, as pyramidal signs and sensory disturbances do not typically develop in INPH patients. Hattingen et al. [16] reported that the FA values observed in the periventricular corticospinal tract in INPH patients were higher than those in healthy controls and were correlated with the severity of gait disturbances. In the present study, we did not detect such effects, despite our use of disease controls. Further studies are needed to understand whether the lesions with high FA values are associated with the clinical symptoms of INPH patients.
In our voxel-based linear regression analyses, gait disturbance, as assessed by the TUG number of steps, was negatively correlated with the subcortical white matter FA value in the left SMA and left anterior limb of the internal capsule. The SMA is thought to play a role in the planning or programming of voluntary movements, including gait. In a single photon emission computed tomography study, the SMA was found to be activated during voluntary walking in normal subjects [38]. Gait apraxia caused by SMA lesions has also been reported [39, 40]. A recent functional MRI study demonstrated that increased activation of the SMA after CSF drainage in INPH patients was significantly correlated with an improvement in sequential motor learning [41]. The anterior limb of the internal capsule, where the thalamofrontal and frontopontine projection fibres run, is also hypothesised to be involved in motor function. Therefore, the results of the present study support the theory that a disconnection between the SMA and subcortical structures causes gait disturbances in INPH patients. In addition, our results demonstrated a left hemispheric dominance related to gait disturbance. In previous functional MRI studies, left SMA-dominance has been reported during unilateral finger movement and bimanual coordination in right-handers [42, 43], although it has not been observed during locomotion. Further studies will be required to investigate the existence of hemispheric dominance in the SMA for locomotion.
The voxel-based linear regression analyses revealed that the FAB score was negatively correlated with the FA values for the frontal subcortical white matter (including the SLF and SOFF), the genu of the corpus callosum, and the superior parietal subcortical white matter. The SLF and SOFF are association fibres that connect the frontal lobe to the parietal, temporal, and occipital lobes. The genu of the corpus callosum is a commissural fibre that projects to the bilateral prefrontal areas, which are thought to be the main centres of executive function [44]. Several functional imaging studies have revealed that the superior parietal lobule is associated with basic attentional processes and that it mediates the fundamental processes of executive function [45]. Thus, the disruption of a number of different interhemispheric and intrahemispheric connections might cause the executive dysfunction that occurs in INPH patients.
The nature of the white matter disintegration that occurs in INPH patients is still unknown. Nevertheless, the finding that some of the clinical symptoms associated with INPH recover after shunt placement implies that the abnormalities observed in the areas of white matter with low FA values should be at least partially reversible. A recent study demonstrated that periventricular white matter lesions observed through MRI and/or CT scans could be reversed by shunt operation in some shunt-responsive INPH patients [46]. In combination with the results of the present study, the neuropathological finding of reversible white matter lesions in INPH patients might be related to interstitial oedema, which is detected by high MD values. Further studies are needed to clarify which regions with low FA values are able to recover after a shunt operation. Our findings concerning the distribution of areas with low FA values suggest that mechanical distortion, extension, and compression of white matter arise from marked deformation of the brain in INPH patients. However, we did not observe any significant correlations between ventricular size and either of the white matter involvement indices or clinical symptoms (as assessed by the MMSE, FAB, and TUG) in INPH patients in this study. Individual differences in the compliance of the brain or other unknown factors might explain this discrepancy.
References
Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH (1965) Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure.a treatable syndrome. N Engl J Med 273:117–126
Hakim S, Adams RD (1965) The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 2:307–327
Dandy WE (1919) Experimental hydrocephalus. Ann Surg 70:129–142
Greitz D (2007) Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst 23:487–489
Akai K, Uchigasaki S, Tanaka U, Komatsu A (1987) Normal pressure hydrocephalus. Neuropathological study. Acta Pathol Jpn 37:97–110
Del Bigio MR (1993) Neuropathological changes caused by hydrocephalus. Acta Neuropathol 85:573–585
Di Rocco C, Di Trapani G, Maira G, Bentivoglio M, Macchi G, Rossi GF (1977) Anatomo-clinical correlations in normotensive hydrocephalus. Reports on three cases. J Neurol Sci 33:437–452
Del Bigio MR, Wilson MJ, Enno T (2003) Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol 53:337–346
Basser PJ, Pierpaoli C (1998) A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med 39:928–934
Le Bihan D, Turner R, Douek P, Patronas N (1992) Diffusion MR imaging: clinical applications. AJR Am J Roentgenol 159:591–599
Chabriat H, Pappata S, Poupon C et al (1999) Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke 30:2637–2643
Hanstock CC, Faden AI, Bendall MR, Vink R (1994) Diffusion-weighted imaging differentiates ischemic tissue from traumatized tissue. Stroke 25:843–848
van Gelderen P, de Vleeschouwer MH, DesPres D, Pekar J, van Zijl PC, Moonen CT (1994) Water diffusion and acute stroke. Magn Reson Med 31:154–163
Assaf Y, Ben-Sira L, Constantini S, Chang LC, Beni-Adani L (2006) Diffusion tensor imaging in hydrocephalus: initial experience. AJNR Am J Neuroradiol 27:1717–1724
Jinkins JR (1991) Clinical manifestations of hydrocephalus caused by impingement of the corpus callosum on the falx: an MR study in 40 patients. AJNR Am J Neuroradiol 12:331–340
Hattingen E, Jurcoane A, Melber J, Blasel S, Zanella FE, Neumann-Haefelin T, Singer OC (2010) Diffusion tensor imaging in patients with adult chronic idiopathic hydrocephalus. Neurosurgery 66:917–924
Stolze H, Kuhtz-Buschbeck JP, Drücke H, Jöhnk K, Diercks C, Palmié S, Mehdorn HM, Illert M, Deuschl G (2000) Gait analysis in idiopathic normal pressure hydrocephalus—which parameters respond to CSF tap test? Clin Neurophysiol 111:1678–1686
Rusinek H, de Leon MJ, George AE et al (1991) Alzheimer disease: measuring loss of cerebral gray matter with MR imaging. Radiology 178:109–114
Summerfield C, Junque C, Tolosa E et al (2005) Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol 62:281–285
Ishikawa M, Hashimoto M, Kuwana N et al (2008) Guidelines for management of idiopathic normal pressure hydrocephalus. Neurol Med Chir 48(Suppl):S1–S23
Kubo Y, Kazui H, Yoshida T et al (2008) Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement Geriatr Cogn Disord 25:37–45
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: a frontal assessment battery at bedside. Neurology 55:1621–1626
Miyoshi N, Kazui H, Ogino A et al (2005) Association between cognitive impairment and gait disturbance in patients with idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 20(2–3):71–76
Pfaendner NH, Reuner G, Pietz J et al (2005) MR imaging-based volumetry in patients with early-treated phenylketonuria. AJNR Am J Neuroradiol 26:1681–1685
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219
Rorden C, Karnath HO, Bonilha L (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19:1081–1088
Kennedy KM, Raz N (2009) Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 47:916–927
Szeszko PR, Vogel J, Ashtari M et al (2003) Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport 14:2469–2473
Fellgiebel A, Wille P, Muller MJ et al (2004) Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord 18:101–108
Medina D, DeToledo-Morrell L, Urresta F et al (2006) White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol Aging 27:663–672
Karagulle Kendi AT, Lehericy S, Luciana M, Ugurbil K, Tuite P (2008) Altered diffusion in the frontal lobe in Parkinson disease. AJNR Am J Neuroradiol 29:501–505
Aygok G, Marmarou A, Fatouros P, Young H (2006) Brain tissue water content in patients with idiopathic normal pressure hydrocephalus. Acta Neurochir Suppl 96:348–351
Kitagaki H, Mori E, Ishii K, Yamaji J, Hirono N, Imamura T (1998) CSF spaces in idiopathic normal pressure hydrocephalus; morphology and volumetry. AJNR Am J Neuroradiol 19:1277–1284
Hashimoto M, Ishikawa M, Mori E, Kuwana N (2010) The study of INPH on neurological improvement (SINPHONI). Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 7:18
Ishii K, Kawaguchi T, Shimada K et al (2008) Voxel-based analysis of gray matter and CSF space in idiopathic normal pressure hydrocephalus. Demen Geriatr Cogn Disord 25:329–335
Fukuyama H, Ouchi Y, Matsuzaki S et al (1997) Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett 228:183–186
lla Sala S, Francescani A, Spinnler H (2002) Gait apraxia after bilateral supplementary motor area lesion. J Neurol Neurosurg Psychiatry 72:77–85
Nadeau SE (2007) Gait apraxia: further clues to localization. Eur Neurol 58:142–145
Lenfeldt N, Larsson A, Nyberg L et al (2008) Idiopathic normal pressure hydrocephalus: increased supplementary motor activity accounts for improvement after CSF drainage. Brain 131:2904–2912
Babiloni C, Carducci F, Del Gratta C et al (2003) Hemispheric asymmetry in human SMA during voluntary simple unilateral movements. An fMRI study. Cortex 39:293–305
Jäncke L, Peters M, Himmelbach M, Nösselt T, Shah J, Steinmetz H (2000) fMRI study of bimanual coordination. Neuropsychologia 38:164–174
Goel V, Grafman J, Tajik J, Gana S, Danto D (1997) A study of the performance of patients with frontal lobe lesions in a financial planning task. Brain 120(Pt 10):1805–1822
Wager TD, Smith EE (2003) Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 3:255–274
Akiguchi I, Ishii M, Watanabe Y et al (2008) Shunt-responsive parkinsonism and reversible white matter lesions in patients with idiopathic NPH. J Neurol 255:1392–1399
Acknowledgments
We thank the patients and their families for their participation in this study. We also thank Takeo Kondo and Kazutomo Nishijima for their constant support. This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas—System study on higher-order brain functions from the MECSST Japan (20020004) and by the Ministry of Health, Labor and Welfare of Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanno, S., Abe, N., Saito, M. et al. White matter involvement in idiopathic normal pressure hydrocephalus: a voxel-based diffusion tensor imaging study. J Neurol 258, 1949–1957 (2011). https://doi.org/10.1007/s00415-011-6038-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6038-5