Abstract
Cancer is a challenging healthcare problem among other health issues worldwide. Although there are several therapies used for cancer treatment, nanoimmunotherapy has attained a lot of considerations for its unique applications and hopeful future. Chemotherapy and radiotherapy are conventional therapeutic methods which are lacking particular targeting and consequent off-target effects. Because of these drawbacks, it has started searching for techniques which could widen the effectiveness of the therapy such as nanoparticles (NPs) which are mandatory. Cancer nanoimmunotherapy for immunology generates a unique and constant response against cancer by provoking the host immune system or suppressing tumour immune evasion. In cancer nanoimmunotherapy, nanoparticles are considered as potential tumour-targeting carriers such as polymers, nanoshells, dendrimers and liposomes. This chapter will highlight the current applications of nanotherapy as well as the potentiality of various NPs in cancer nanoimmunotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cancer is known as the very common deadly illness that affects human beings adversely worldwide. Mutations that occur in the DNA within cells cause cancer. DNA is packaged and folded into a greater number of individual genes, each of which contains a set of instructions informing the cell about the functions that all have to be performed by the cells and also how to grow and divide. If errors occur in the instructions, it could cause the cell to stop its normal function, and that leads a cell to become cancerous. To overcome problems like this, modern therapeutic paths are required because of the complication of cancer as a disease. The usual immunotherapy depends on in vivo immune balance controlled by unfavourable (tumour) and favourable (host) factors. But it is very hard to keep up such linear immune balance. When it comes to nanoimmunotherapy, nanocarriers could produce potential, stable, organized and targeted transmission of drugs for effective treatment and/or stimulating immune reactions. Pharmaceutical nanotechnology also known as cancer nanotechnology or nanomedicine has been giving an efficient quick fix to sort out the barriers of traditional immunotherapy (Li et al., 2014). The main advantage of cancer nanoimmunotherapy is that nanomedicines—therapeutics made up of transporter components usually lesser than 100 nm—had been made for widening the uptaking of chemotherapy substances by carcinoma and for reducing their off-target toxicity. Nanomedicines, such as NPs, gather within tumours via the improved permeation and retention effect, targeting the drug in tumour sites (Irvine & Dane, 2020). Nanoparticle (NP) delivery methods have been formulated to sort out many obstacles to the safe and efficient transfer of nucleic acid therapeutics to immune cells. NPs protect the therapeutic cargo, to evade nuclease degradation and to increase circulation half-life (Whitehead et al., 2014). Nanosystems formed to arrive at immune particles and cells might let the improvement of accesses that would utilize the patient’s immune structure as a further precise tool to fight against cancer (Conniot et al., 2014). In recent days, cancer immunotherapy using NPs have been developed due to their effective role in cancer treatment. The following chapter brings to light an in-depth picture of nanoimmunotherapy that is paramount in cancer treatment. We would address the futuristic technology of artificial intelligence (AI).
Nanoimmunotherapy
Nanoimmunotherapy is mainly designed to develop nanotechnology to sort out the issues occurring in immunotherapy, and its focus is mainly about different types of nanocarrier development to deliver antigens to dendritic cells in a constant, limited and targeted way. We extend to emphasis mechanisms of NPs on tumour therapeutics. Cancer nanomedicine generally targets to advance the direct destroying of tumour cells by developing the delivery of chemotherapeutic drugs to tumours and metastases. Recently, nanomedicine formulations are utilized to increase the potentiality of anticancer immunity with clinically settled immunotherapeutics (Shi & Lammers, 2019). A promising solution to separate with the traditional drug advancement example and direct the delivery of immunotherapeutics is driving their action on target tissues (i.e. tumours and tumour-draining lymph nodes) or cell types, to control the time duration and location of immune modulation. To overcome this, nanomedicine-based proposals, i.e. the formulation of drugs in transporter materials that are lesser than ~100 nm, may increase both the defence and the therapeutic effectiveness of bountiful immunotherapies (Irvine & Dane, 2020). Nanoimmunotherapy is developed in nanotechnology to strengthen immunotherapy, which combines the advancement of nanocarriers to deliver antibodies on targeted tumour cells (passive immunotherapy) and of antigens to dendritic cells to induce immune reactions towards the disease (Li et al., 2013a, b).
Mechanisms of Nanoparticle Therapeutics
Nanoparticle therapeutics are customarily fragmented makeup of therapeutic bodies like nucleic acids, proteins, mini-molecule drugs, peptides and elements that gather with the therapeutic entities, such as lipids and polymers to form NPs. Those NPs could have increased anticancer properties correlated with the therapeutic bodies they contain (Davis et al., 2010). Targeted NPs have the following features that differentiate those NPs from other therapeutic approaches for cancer. (i) NPs can transport a huge payload of drug material and also save them from depravity. For instance, a 70-nm nanoparticle can have relatively 2000 short interfering RNA (siRNA) molecules (Bartlett & Davis, 2007). (ii) The NPs are adequately abundant to consist of various targeting ligands that would let on multivalent attaching to cell-surface receptors (Hong et al., 2007). (iii) NPs are big enough to shelter various kinds of drug molecules. Numerous therapeutic mediations can be applied together with a nanoparticle in a controlled way. (iv) The discharge kinetics of drug molecules from NPs could be modified to meet the mode of action. For instance, topoisomerase I inhibitors such as the camptothecin-based chemotherapeutic drugs are reversible binders of the enzyme. So, the mechanism of action for camptothecin-based drugs on the topoisomerase I enzyme recommends enhanced strength with extended exposure to the drug (Pommier, 2004). (iv) NPs can have the capability of bypass multidrug resistance processes that associate cell-surface protein pumps (e.g. glycoprotein P), as they go into cells through endocytosis. The physicochemical properties of the NPs could determine the stage of complexity for its function, whether it is organic or inorganic. For example, reacting nanoparticle’s surface area with thiols could promptly functionalize gold NPs, whereas organic polymers need more plan of actions so that side chains 475 are reactive prior to the nanoparticle synthesis (Shi et al., 2009). Generally, it may control the mixing of these traits by nanoparticle construction that could reduce the adverse side effects of anticancer medicines while increasing potency, and results obtained by clinical studies are proposed that this potential is opening to be understood.
Nanomedicinebased drug formulations had formerly been generated for changing the pharmacokinetics and toxicity outlines of chemotherapy promoters and to develop their aggregation in cancer-affected cells. The capability of focusing on drugs could be within cancer cells or the tumours.
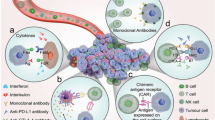
The tumour microenvironment (TME) is applicable of improving immunotherapy. Nevertheless, nanomaterials also let on new mechanisms of action for immunotherapy promoters, including the potentiality to show ligands to the immune cells, drive intracellular transfer of cell-impermeable mixture and restrict the drug-releasing time and/or activation. Mechanism of nanoparticles delivery has been described in Fig. 6.1.
Developing Immunogenic Tumour Cell Death
An anti-tumour immune response could be promoted by tumour cell death which is stated as immunogenic cell death (ICD). Nanomedicine formulations are a potential method to develop ICD since the cytotoxic agents could get targeted in tumour cells. Also, nanomaterials would be made to precisely interact with outer energy sources, letting on amplification of ICD provoked by therapies called radiotherapy and magnetic hyperthermia (Duan et al., 2019). Nanomedicines could be utilized as radio enhancers that straight away combine with ionizing radiation to improve ICD (Rancoule et al., 2016). By exchanging magnetic fields to induce paramagnetic iron oxide NPs within the tumour macro-environment, localized hyperthermia could be understood. Tumour ICD can be evoked by localized hyperthermia, whereas CD8+ T-cell-mediated immune response can also be promoted by the same in preclinical models of glioma, colon adenocarcinoma and melanoma (Toraya-Brown et al., 2016; Yanase et al., 1998). After getting positive results from these preclinical studies, it was clinically studied for the utilization of magnetic NPs to induce tumour hyperthermia. Since iron NPs studied comparatively very less or non-toxic and are acceptable to functionalize with targeting molecules, they have the potentiality to be successful. The experiment was done on encapsulation of oxaliplatin in the same nanoparticle-granted mixed ICD-promoting reactions like photodynamic therapy, chemotherapy and provoked regression of irradiated primary tumours and non-irradiated secondary tumours in mouse models of colorectal cancer (He et al., 2016).
Ligand Presentation to Immune Cells
The advancement of ligand-targeted NPs to remove solid tumours is expressed in advance to have a great impact on human wellness. The choice of a targeting ligand is mainly instructed by the receptors existing on the target cells. Clustering ligands on NPs contain a major profit of strengthening the affinity of receptor binding. This renowned phenomenon can increase ligand affinity by various orders of magnitude because of the simultaneous occupation of receptor-binding sites on the cell surface. Targeted NPs will drive the path to decrease the lethal side effects of general cancer treatments and to reduce the number of deaths related to cancer worldwide (Duskey & Rice, 2014). Ligands of NPs connect with cell-surface receptors which let on the gathering of high intracellular NP concentrations by receptor-mediated endocytosis. For example, cyclo-arginine-glycine-glutamic acid (cRGD) is a peptide that attaches to integrin receptors expressed on the surfaces of various kinds of tumours (Ahmad et al., 2019). Polymeric micelles for siRNA delivery consist of a cRGD ligand over the micellar surface that especially identifies tumour cells and increases their intracellular ability (Christie et al., 2012). PK2 (FCE28069), an HPMA-polymer-Gly-Phe-Leu-Gly-doxorubicin conjugate which has sugar galactosamine, was the pioneer nanoparticle targeted at the ligand to arrive at the clinic. The galactose-based ligand is used against the asialoglycoprotein receptor (ASGPR), and that reacts on hepatocytes. Hence, it is believed that its high expression is maintained on primary liver cancer cells (Seymour et al., 2002).
Nano-delivery Systems for Cancer Immunotherapy
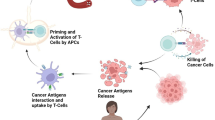
Nanocarriers encompass multipurpose composition, tunable size and morphology, and surface functions. Immuno-nanomedicine is one of the advanced techniques that have been utilized in different ways in cancer therapy. Nanocarriers could be classified into inorganic NPs, polymeric NPs, lipid-based nanovesicles, DNA nanostructures, biomimetic and naturally derived particles based on their composition. Nanosystem-based identification of specific tumour neoantigens is a promising field (Wang et al., 2018a, b). Since nanomaterials have several significant characteristics such as high effectiveness for drug loading, low drug loss ratio and high stability of avoiding body clearance, they act as effective drug delivery carriers (Shi et al., 2011). Further, we discussed in detail about antigenic peptide delivery systems and monoclonal antibody (mAb) delivery systems. Nano-delivery using various systems has been shown in Fig. 6.2.
Antigenic Peptide Delivery Systems
Although several nanostructures exist, peptide self-gathered nanostructures have gained more consideration for anticancer drug delivery and become a promising platform to treat cancer. The peptide has the potentiality of self-assembling into several various types of nanostructures like NPs, nanotubes, nanovesicles and nanofibers that form hydrogels (Yu et al., 2015). These hydrogels with injectable features could also be utilized directly to contact with the tumour sites for enhancing the potency of tumour treatment (Yishay-Safranchik et al., 2014). Gold nanorods inserted dipeptide microspheres and stacked with the anticancer agent, doxorubicin (DOX), have been chosen as a smart drug delivery stage for natural, steady and pulsatile drug release. Outcomes of the experiments revealed the ability to attain a sustained and on-demand DOX discharge from the microspheres by using the laser exposure timing (Erdogan et al., 2016). NPs’ surface chemistry can be designed to target tumour-derived protein antigens. The NPs which are designed to capture the antigen further improve the exposure between the antigens to APC. Researchers showed the utilization of NPs that capture antigens (AC-NPs) increases the abscopal effect, a phenomenon by which local radiotherapy provokes a systematic response of immune cells and also the reversion of metastatic lesions. Radiation would generate pro-inflammatory proteins and enhance the liability of immune cells to tumour-specific antigens once the cancer cells are induced to death (Barker et al., 2015). A study showed the natural properties of antigen clubbing over NPs (adsorption versus encapsulation) and the surfactants (poly(vinyl alcohol) (PVA) or PF127) disturbed the DC activation, and it has been revealed that antigen-adsorbed NPs promote the MHC II on DCs in a highly expressed way, whereas antigen-encapsulated NPs promote the maximum expression of MHC I. It was concluded that antigen-encapsulated NPs promoted the response of antigen-specific T-cell (Zupančič et al., 2017). Delivering peptide antigens fixed to NPs provides various benefits. NPs are capable of protecting the peptides from enzyme peptidases, during prolonged transportation of peptide circulation and delivery. Virus-like distribution in NPs helps them get identified and captured by antigen-presenting cells, driving to a huger gathering of antigens in lymphoid tissues. Antigens and immune adjuvants could be simultaneously co-delivered by NPs to prevent immune tolerance (Kuai et al., 2017).
Polymeric Systems
Delivery of antigens or antibodies towards either cancer cells or dendritic cells (active immunotherapy or passive immunotherapy) to provoke the immune system would happen efficiently by the polymeric NPs because of their composition, convenient particle size and particular intelligent characteristics (Li et al., 2013b). Poly(lactic-co-glycolic acid) (PLGA) is one of the most successfully used biodegradable polymers that was approved by the FDA. Various types of therapeutics had been encapsulated in PLGA NPs for their potential use in the field of the pharmaceutical industry. Tumour lysate, OVA and antigenic peptides are loaded in PLGA particles to evoke T-cell responses after intradermal injection (Cruz et al., 2014; Mueller et al., 2012; Zhang et al., 2011). A pH-responsive amphiphilic polymeric micelle has been fabricated by the group of researchers in order to dual delivery of OVA antigen and CpG adjuvant at the same time (Wilson et al., 2013). The responses of anti-tumours can rebuild after depleting MDSCs (myeloid-derived suppressor cells) with nanomaterials. MDSCs are valuable types of immunosuppressive cells, which have been found in several types of cancers such as gastrointestinal cancer, breast cancer, hepatocellular carcinoma and lung cancer (Parker et al., 2015). Poly(ethylene glycol)-poly(propylene sulphide) (PEG-PSS) polymer micelles are loaded with 6-thioguanine (MCTG) to deplete MDSCs in tumour-bearing mice and increase T-cell-mediated anti-tumour responses (Jeanbart et al., 2015). Recently, endosome-disrupting polymersomes have been utilized by Wilson and his colleagues for intracellular delivery of interferon gene stimulator (STING) agonist in which the natural formation does not overpass the cell membrane. Therapy with these polymersomes made better the anticancer immunity as well as the efficiency of checkpoint blockade therapy substantially (Shae et al., 2019). Rowan, Figdor and their fellow workers engineered synthetic APCs made on poly(isocyano peptide) altering with three to five anti-CD3 antibodies/150–200 nm of the polymer chain by which the expression of CD69 (early T-cell activation marker) has been induced and also the IFN-γ production has been promoted (Mandal et al., 2013). Gao and associates used NPs based on pH-sensitive PEG-polymethacrylate polymers for the efficient delivery of antigens to APCs in lymph nodes. NPs loaded with antigen evoked forceful vaccination than free antigens incorporated with conventional adjuvants (e.g. polyinosinic:polycytidylic acid (i.e. poly(I:C))), likely by stimulating the pathway of STING (Luo et al., 2017).
Liposomes
Nanocapsules, liposomes, micelles, nanoemulsions and solid lipid NPs are generally known lipid-based NPs that are administered by different directions such as oral, topical and parenteral (Dong & Mumper, 2010). A liposome is the most known NPs that are accepted medicine for cancer treatment (Qu et al., 2014). Since liposomes have the ability to raise the targeting and reduce the elimination and harmful adverse effects of chemotherapeutic agents, they are promising targets and delivery materials of the chemotherapy (Mandal et al., 2017). The study was described that doxorubicin (DOX) loaded with PEGylated egg phosphatidylcholine-cholesterol liposomes containing ~100 nm passively assembled in the tumour vessels of a multidrug-resistant breast cancer xenograft model, expressing a phenomenal anti-tumour effect, where the free DOX fails to deliver any detectable therapeutic reaction (Kibria et al., 2016). Mitoxantrone (MTO), anthracenedione relevant to anthracyclines, was encapsulated in PEGylated liposomes, and these MTO-encapsulated liposomes reduced the toxicity that let on the highest MTO dose administration of maximum MTO dose administration to treat breast carcinoma on mice (Pedrosa et al., 2015). A new nanocarrier of emulsion liposomes having perfluoropentane nanodroplet inside the aqueous interior of a dipalmitoylphosphatidylcholine liposome, along with the anticancer drug DOX, has been explained. Studies carried out in vitro resulted in liposomes showing an effective release of DOX over the application of less-intensity ultrasound at 20 kHz, 1.0 MHz and 3.0 MHz. This new drug delivery process ensures the effective delivery of DOX, and comparatively they are capable of minimizing the adverse effects of cardiotoxicity produced by DOX than old stealth liposomes (Lin et al., 2014). Liposome particles either with encapsulated cytokines (IL-15, IL-21) or drugs (glycogen synthase kinase-3 β inhibitor TWS119) were conjoined on the living T-cell surface through thiol-reactive maleimide head groups over the surface of lipid bilayer particles. These surface-coated NPs are not harmful to their carrier T-cells which have not interfered with intrinsic cell action or migration patterns. The function of these carrier cells extensively improved with the utilization of very few drug doses, and that was not effective enough while using alone old systemic routes. After crossing the endothelial barrier, 83% (± 3%) of their original NP cargo was still physically attached to the carrier CD8+ T-cells (Stephan et al., 2010).
Exosomes
Exosomes delivered as resourceful drug tools have gained attention because of their internal skill of shuttling proteins, lipids and genes among cells and their native affinity towards target cells. Salient properties of exosomes, such as the size of nanoscope, less immunogenicity, great biocompatibility, encapsulation of several cargoes and the strength to defeat biological blockades, differentiate them from other nanocarriers (Zhang et al., 2019). Exosomes show an effective drug delivery because of their satisfactory biodistribution, biocompatibility and low immunogenicity. Exosomes contain better permeability and could pass the utmost biological membranes. Inspired by natural exosomes, researchers developed exosomes mimicking nanocarriers for siRNA delivery. A good yield of nano-sized vesicles, called exosome-mimics (EMs), by extruding non-tumourigenic epithelial MCF-10A cells through filters with various pore sizes was obtained (Yang et al., 2016). After encapsulated in exosome-based nanocarriers, protein/peptide drugs can obtain improved pharmacokinetic properties, increased bioavailability and the potentiality to reach and penetrate targeting tissues (Sterzenbach et al., 2017). Apart from native exosomes, exosomes using particular ligands could be made and designed in vitro to spot tumour cells effectively. For example, αv integrin-specific iRGD peptide provides exosomes which were utilized to supply doxorubicin and strengthened the anti-tumour efficacy in αv integrin positive breast cancer cells in vivo compared to free drug group (Tian et al., 2014). Different therapeutic cargoes such as anti-cancer drugs and cancer gene suppressors could be packed with exosomes in order to destroy cancer cells efficiently. Notably, exosomes give their therapeutic cargoes straight away to the cellular compartment with the capability of mediating cell-to-cell communication (Li et al., 2018; Turturici et al., 2014). Doxorubicin (DOX), paclitaxel (PTX), celastrol and curcumin are chemotherapeutic drugs that have been found to encapsulate into exosomes. Diverse explorations have proved that the drug-loaded exosomes are capable of increasing the effectiveness of chemotherapy (Hadla et al., 2016; Aqil et al., 2016). Exosomes have the feature of showing improved stability of blood which allows them to move far inside the body under both physiological and pathological conditions. Additionally, exosomes are having a hydrophilic core, which makes them as host water-soluble drugs (Jiang & Gao, 2017). Because of the advancements on tumour treatment, exosomes are used in cancer diagnosis, immunotherapy and drug delivery vehicles (Li et al., 2018).
Monoclonal Antibody (mAb) Delivery Systems
Cancer cell-specific treatment became possible with the improvement of a technique to develop monoclonal antibodies (mAbs) in year 1975 (Köhler & Milstein, 1975). Nanomaterials facilitated the supply of bioactive monoclonal antibodies (mAbs) and drugs to tumours which encompass the great advantage as they are well-known to improve permeability and retention (EPR) effect as well. The combination of leaky tumour vasculature and poor tumour drainage through the lymphatics provides a better advantage for nanoconjugates (Torchilin, 2005; Hofheinz et al., 2005). For the first time in year 1982, Levy and fellow workers used mAbs to cure human malignancy (Miller et al., 1982). It has not been done until 1986, and then the US Food and Drug Administration (FDA) approved the first monoclonal antibody [Orthoclone OKT3). The FDA approved the first humanized monoclonal antibody in 1997 against CD25 to treat multiple sclerosis in adults. Russia approved the first cancer vaccine Oncophage in 2008. Sipuleucel-T (Provenge) was approved by the FDA in 2010 as therapeutic cancer to treat prostate cancer (Waldmann, 2003; Parish, 2003). Schneck’s team examined the synergy between PLGA-based antigen-presenting cells and anti-PD1 monoclonal antibody (mAb). This particular combination promoted the higher-level secretion of IFN-γ in vitro and delayed tumour growth in vivo with long survival (Kosmides et al., 2017). In many cases, anti-CD28 mAbs are alone, not able to work, and their usage has to be followed by antigen-dependent T-cell receptor (TCR)-interfered signals to activate T-cells. 4-1BB which is also called as CD137 is possible to identify on T-cells, natural killer cells, DCs, mast cells and even sometimes endothelial cells of metastatic tumours (Vinay & Kwon, 2012). Application of anti-4-1BB in this receptor induces signalling pathways that drive to the strengthened expression of anti-apoptotic genes. As like 4-1BB, OX40 is another type of the TNF receptor superfamily, and anti-OX40 mAbs are efficient to stimulate CD4+ and CD8+ T-cells (Aspeslagh et al., 2016). Adjuvants, cytokines, and mAbs all perform as immunotherapeutic agents that would benefit from the improved transport given by nano-delivery. Table 6.1 shows the different types of nanoparticle delivery in cancer immunotherapy.
Nucleic Acid-Based Delivery Systems
The extreme need for a vector that is able to perform efficiently in transporting and supplying nucleic acid (NA) therapeutics towards the target cells has prompted intense research. NA delivery methods could be of endogenous (viral vectors) or exogenous (natural and synthetic delivery materials) origin (Yin et al., 2014; Xiao et al., 2019). Nanomaterials play important roles in the delivery system of siRNA, and nanomaterial-mediated siRNA delivery in cancer immunotherapy is one of the major directions for future clinical cancer therapy. siRNA is known as a double-stranded RNA that contains the length of 19–21 nucleotides and has been broadly checked for potential cancer therapy in animal models. Nano-sized non-viral carriers like liposomes, polyethyleneimine (PEI), polypeptides, chitosan, inorganic NPs, etc. have been promoted as promising vehicles in the process of nucleic acid delivery (Mei et al. 2019). RNAi consists of post-transcriptional gene silencing mediated by endogenously produced mini (19–25 base pairs) oligoribonucleotides with the potency of degrading a target RNA specifically and selectively, thus repressing translation of an encoded protein (Whitehead et al., 2009). NA therapeutics have been considered as effective applicants for cancer treatment, including immunotherapy (Opalinska & Gewirtz, 2002). NA therapeutics are a broad category of DNA or RNA; they are plasmids, mRNA, ASO, siRNA, miRNA, small-activating RNA (saRNA), aptamers, gene-editing gRNA as well as immunomodulatory DNA/RNA. NA therapeutics are multifunctionalities ranging from gene expression alteration (up- or downregulating) to immune response modulation (Pastor et al., 2018; Kleinman et al., 2008; Ishikawa & Barber, 2008). siRNA is responsible for gene regulation, whereas ASO is responsible for regulating gene expression after transcription and silence-targeted genes further regulating intracellular signalling pathway which plays a role in cancer progression (Dahlman et al., 2014). NA immune stimulants such as unmethylated cytosine-guanine deoxynucleotides (CpG), poly I:C, 5′-triphosphate RNA as well as di-cyclic nucleotides that active stimulator interferon genes (STING) stimulate anticancer immune activation (Barber, 2015; Vollmer & Krieg, 2009; Kyi et al., 2018). mRNA therapeutics and plasmid DNA (pDNA) can be made to express proteins or peptides of interest, such as antigens or cancer immunotherapeutic proteins. Genome editing-related nucleic acids such as gRNA are currently started using to edit target genes accurately which could modulate gene expression for cancer immunotherapy (Gilboa et al., 2015; Yin et al., 2017). The ability of mRNA to express essentially any proteins and peptides for a longer duration on nuclear localization for gene expression makes mRNA therapeutics of tremendous potential for versatile applications, including cancer immunotherapy (Sahin et al., 2014). mRNA can now be manufactured in vitro at large scales at a low cost. Particularly, mRNA can be reproducibly synthesized by in vitro transcription (IVT) using DNA templates, a T7, a T3 or an Sp6 phage RNA polymerase (Pardi et al., 2018). These technology developments have altogether provided RNA therapeutics as a very powerful platform for cancer immunotherapy by multipurpose approaches like ex vivo mRNA transfer for therapeutic adoptive cell engineering and using cancer-specific antigen-encoding mRNA as tumour therapeutic vaccines (Sahin et al., 2014). siRNA is a dsRNA that consists of 21–23 nucleotides. siRNA guides RNA-induced silencing complexes to bind to the specific sequence of mRNA and subsequently degrades it. Given that some genes are highly expressed in many diseases including cancer, siRNA can be used as a therapeutic agent to silence them (Agrawal et al., 2003). Guillermo et al. showed that hMCP1 siRNA-DOPC NPs suppress tumour growth and decrease the infiltration of CD68+ and F4/80+ macrophage cells in tumour samples obtained from mice models that are under day-to-day restraint stress (Armaiz-Pena et al., 2015). Arvizo et al. delivered MICU1 siRNA/positively charged AuNPs to human ovarian cancer cell lines (OVCAR5, OV167 and OV202). The decreased expression of Bcl-2 simultaneously raises in the range of cytosolic [Ca2]cyto leading to the activation of the mitochondrial pathway of apoptosis. An experiment shows MICU1 as a novel regulator, which prevents apoptosis in tumour cells (Arvizo et al., 2013). EZH2 is a histone-lysine N-methyltransferase enzyme and is functional in some cell processes. It tends to be increased in some tumour cells. EZH2 suppresses the expression of vasohibin-1 with antiangiogenic properties. Gharpure et al. established that siRNA coated with CS NPs, along with docetaxel against EZH2, reduces angiogenesis and tumour mass in HeyA8 and SKOV-3ip1 orthotopic mouse models (Gharpure et al., 2014). In a study, Lingegowda et al. used a siRNA targeting the platinum resistance genes ATP7A and ATP7B in ovarian carcinoma. For in vivo delivery, they utilized neutral nanoliposome DOPC with incorporated siRNA to decrease the expression of ATP7B in 48 h. Tumour shrinkage, cancer cell apoptosis and proliferation reductions have been reported (Mangala et al., 2009). Hatakeyama et al. delivered CTGF (a key factor in hypothermia resistance) siRNA-DOPC nanoliposome to xenograft HTRSKOV-3 and HeyA8 mice. And then PEG-CuS NPs were intravenously injected. Due to CTGF underexpression and hyperthermia, tumour burden was decreased in the HeyA8 model. Besides, local hyperthermia and CTGF silencing led to decreased metastasis rate and tumour burden in HTR SKOV-3 tumours (Hatakeyama et al., 2016). Clinical trials involving lipoplexes containing RNA oligonucleotides are at the starting blocks within the Mutanome Engineered Nanomaterials 2016, 6, 131 15 of 22RNA Immuno-Therapy (MERIT) project, an initiative that has got research financial support from the European Union, coordinated by BioNTech AG. CLs to shape RNA lipoplexes, namely, MERIT-Lipo (ClinicalTrials.gov Identifier: NCT02410733), has been selected for the clinical trial entitled “Evaluation the safety and tolerability of i.v. administration of a cancer vaccine in patients with advanced melanoma (Lipo-MERIT)”. The cationic liposomes of the Lipo-MERIT vaccine entail four naked ribonucleic acid (RNA)-drug products (DPs) such as RBL001.1, RBL002.2, RBL003.1 and RBL004.1. These are the ability to induce antigen-specific CD8+ and CD4+ T-cell responses in contradiction of designated malignant melanoma-associated antigens. The corresponding investigation under the clinical phase I trial (Campani et al., 2016). Anti-tumour immune responses are elevated by adjuvants. NA-based adjuvants include CpG-oligodeoxynucleotide (CpG-ODN) (Wang et al., 2016, 2018a, b; Kadiyala et al., 2019), polyinosinic:polycytidylic acid (poly I:C) (Yang et al., 2016; Zhang et al., 2019) and cyclic guanosine monophosphate-adenosine monophosphate (cGMP). These can induce pattern recognition receptors (PRR) and consequently activate the immune response (Shae et al., 2019; Cheng et al., 2018; Wilson et al., 2018).
Obstacles and Future Perspective
NPs have been revealing a huge promise in cancer immunotherapy. However, it should be noted that the obstacles of this technology meet the stability of nano-dimensions at the target site under different physiological circumstances, protein corona formation and accumulation. Therefore, it should be discussed the fundamental to incorporate systematic investigations on nanomaterials with biological systems, to finalize the nano-formulation in the nano-treatment. Further, we would give a glimpse of futuristic technology like artificial intelligence in cancer nanoimmunotherapy. Nanotechnology with its special features can multiply cancer immunotherapy considering the present barriers and technical complications. The growth of nanotechnology, more specifically NPs, grants a novel paradigm for cancer immunotherapy. For instance, we could say that few researchers have obtained PD-1-expressing cellular NPs utilizing genetically designed cells to give immunological molecules of smaller sizes. New concepts for personalized immunotherapy may attain by these strategies. Although the targeting capability of NPs is confined by the controllability, they can play an exclusive role in targeted delivery for cancer immunotherapy. The main obstacle in all types of therapy depends on the time, dose and patient-specific at any point of treatment. To sort out these issues, the development towards nanomedicine-mediated co-delivery of multiple treatments has created the potentiality of collaborating artificial intelligence (AI) along with nanomedicine for maintaining the optimization of combinatorial nanotherapy. AI-facilitated paths that essentially account for things like drug targeting, ratiometric delivery and other features are activated by nanotechnology-mediated delivery, and also a dynamic patient reaction to treatment would need exceptional levels of actionability during drug administration. This necessity shows the chances for the field of artificial intelligence (AI) (Zarrinpar et al., 2016). Nanomaterials encompass an extraordinary function in targeted delivery to provide effective cancer immunotherapy, though their targeting capacity is narrowed by the controllability of nanomaterials. Hence, the use of nanomaterials relies on the advancement of analysis and characterization techniques, as well as the constant updating of clinical data.
Conclusions
The advent of nanotechnological development in cancer immunotherapy exhibited the prominent results to overcome the obstacles of transferring water-soluble drugs in hydrophobic lipid particles, improving the target-specific activity and delivering rapid phagocytosis by immune cells. Futuristic technology of artificial intelligence would pave the improvement in the selection and advance the process in nanoimmune cancer therapy. To date, the significance of nanomaterials on cancer patients and clinical transformations is insufficient. Despite the clinical evolution of nanomaterials which still has a lot of objections, challenges and questions, the improvement of nanotechnology, clinical research and the form and fabrication of nanomaterials will largely support the evolution of safe and powerful cancer immunotherapeutics. The interfusion and prolongation of nanotechnology, as well as the cancer immunotherapy, will move ahead in the future world.
References
Agrawal, N., Dasaradhi, P. V. N., Mohmmed, A., Malhotra, P., Bhatnagar, R. K., & Mukherjee, S. K. (2003). RNA interference: Biology, mechanism, and applications. Microbiology and Molecular Biology Reviews, 67(4), 657–685.
Ahmad, K., Lee, E. J., Shaikh, S., Kumar, A., Rao, K. M., Park, S. Y., Jin, J. O., Han, S. S., & Choi, I. (2019). Targeting integrins for cancer management using nanotherapeutic approaches: Recent advances and challenges. In Seminars in Cancer Biology. Academic Press.
Aqil, F., Kausar, H., Agrawal, A. K., Jeyabalan, J., Kyakulaga, A. H., Munagala, R., & Gupta, R. (2016). Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Experimental and Molecular Pathology, 101(1), 12–21.
Armaiz-Pena, G. N., Gonzalez-Villasana, V., Nagaraja, A. S., Rodriguez-Aguayo, C., Sadaoui, N. C., Stone, R. L., Matsuo, K., Dalton, H. J., Previs, R. A., Jennings, N. B., & Dorniak, P. (2015). Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget, 6(6), 4266.
Arvizo, R. R., Moyano, D. F., Saha, S., Thompson, M. A., Bhattacharya, R., Rotello, V. M., Prakash, Y. S., & Mukherjee, P. (2013). Probing novel roles of the mitochondrial uniporter in ovarian cancer cells using NPs. Journal of Biological Chemistry, 288(24), 17610–17618.
Asadujjaman, M., Cho, K. H., Jang, D. J., Kim, J. E., & Jee, J. P. (2020). Nanotechnology in the arena of cancer immunotherapy. Archives of Pharmacal Research, 43(1), 58–79.
Aspeslagh, S., Postel-Vinay, S., Rusakiewicz, S., Soria, J. C., Zitvogel, L., & Marabelle, A. (2016). Rationale for anti-OX40 cancer immunotherapy. European Journal of Cancer, 52, 50–66.
Barber, G. N. (2015). STING: Infection, inflammation and cancer. Nature Reviews Immunology, 15(12), 760–770.
Barker, H. E., Paget, J. T., Khan, A. A., & Harrington, K. J. (2015). The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nature Reviews Cancer, 15(7), 409–425.
Bartlett, D. W., & Davis, M. E. (2007). Physicochemical and biological characterization of targeted, nucleic acid-containing NPs. Bioconjugate Chemistry, 18(2), 456–468.
Campani, V., Salzano, G., Lusa, S., & De Rosa, G. (2016). Lipid nanovectors to deliver RNA oligonucleotides in cancer. Nanomaterials, 6(7), 131.
Cerqueira, B. B. S., Lasham, A., Shelling, A. N., & Al-Kassas, R. (2015). Nanoparticle therapeutics: Technologies and methods for overcoming cancer. European Journal of Pharmaceutics and Biopharmaceutics, 97, 140–151.
Cheng, N., Watkins-Schulz, R., Junkins, R. D., David, C. N., Johnson, B. M., Montgomery, S. A., Peine, K. J., Darr, D. B., Yuan, H., McKinnon, K. P., & Liu, Q. (2018). A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1–insensitive models of triple-negative breast cancer. JCI Insight, 3(22), e120638.
Christie, R. J., Matsumoto, Y., Miyata, K., Nomoto, T., Fukushima, S., Osada, K., Halnaut, J., Pittella, F., Kim, H. J., Nishiyama, N., & Kataoka, K. (2012). Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano, 6(6), 5174–5189.
Conniot, J., Silva, J. M., Fernandes, J. G., Silva, L. C., Gaspar, R., Brocchini, S., Florindo, H. F., & Barata, T. S. (2014). Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Frontiers in Chemistry, 2, 105.
Cruz, L. J., Rosalia, R. A., Kleinovink, J. W., Rueda, F., Löwik, C. W., & Ossendorp, F. (2014). Targeting NPs to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: A comparative study. Journal of Controlled Release, 192, 209–218.
Dahlman, J. E., Barnes, C., Khan, O. F., Thiriot, A., Jhunjunwala, S., Shaw, T. E., Xing, Y., Sager, H. B., Sahay, G., Speciner, L., & Bader, A. (2014). In vivo endothelial siRNA delivery using polymeric NPs with low molecular weight. Nature Nanotechnology, 9(8), 648.
Davis, M. E., Chen, Z., & Shin, D. M. (2010). Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience and technology: A collection of reviews from nature journals (pp. 239–250).
Dong, X., & Mumper, R. J. (2010). Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine, 5(4), 597–615.
Duan, X., Chan, C., & Lin, W. (2019). Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. AngewandteChemie International Edition, 58(3), 670–680.
Duskey, J. T., & Rice, K. G. (2014). Nanoparticle ligand presentation for targeting solid tumors. AAPS Pharmscitech, 15(5), 1345–1354.
Erdogan, H., Yilmaz, M., Babur, E., Duman, M., Aydin, H. M., & Demirel, G. (2016). Fabrication of plasmonicnanorod-embedded dipeptide microspheres via the freeze-quenching method for near-infrared laser-triggered drug-delivery applications. Biomacromolecules, 17(5), 1788–1794.
Gharpure, K. M., Chu, K. S., Bowerman, C. J., Miyake, T., Pradeep, S., Mangala, S. L., Han, H. D., Rupaimoole, R., Armaiz-Pena, G. N., Rahhal, T. B., & Wu, S. Y. (2014). Metronomic docetaxel in PRINT NPs and EZH2 silencing have synergistic antitumor effect in ovarian cancer. Molecular Cancer Therapeutics, 13(7), 1750–1757.
Gilboa, E., Berezhnoy, A., & Schrand, B. (2015). Reducing toxicity of immune therapy using aptamer-targeted drug delivery. Cancer Immunology Research, 3(11), 1195–1200.
Hadla, M., Palazzolo, S., Corona, G., Caligiuri, I., Canzonieri, V., Toffoli, G., & Rizzolio, F. (2016). Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine, 11(18), 2431–2441.
Hatakeyama, H., Wu, S. Y., Lyons, Y. A., Pradeep, S., Wang, W., Huang, Q., Court, K. A., Liu, T., Nie, S., Rodriguez-Aguayo, C., & Shen, F. (2016). Role of CTGF in sensitivity to hyperthermia in ovarian and uterine cancers. Cell Reports, 17(6), 1621–1631.
He, C., Duan, X., Guo, N., Chan, C., Poon, C., Weichselbaum, R. R., & Lin, W. (2016). Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nature Communications, 7(1), 1–12.
Hofheinz, R. D., Gnad-Vogt, S. U., Beyer, U., & Hochhaus, A. (2005). Liposomal encapsulated anti-cancer drugs. Anti-Cancer Drugs, 16(7), 691–707.
Hong, S., Leroueil, P. R., Majoros, I. J., Orr, B. G., Baker, J. R., Jr., & Holl, M. M. B. (2007). The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chemistry & Biology, 14(1), 107–115.
Inman, S. (2015). FDA approves second-line MM-398 regimen, for metastatic pancreatic cancer. In OncLive.
Irvine, D. J., & Dane, E. L. (2020). Enhancing cancer immunotherapy with nanomedicine. Nature Reviews Immunology, 20(5), 321–334.
Ishikawa, H., & Barber, G. N. (2008). STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature, 455(7213), 674–678.
Jeanbart, L., Kourtis, I. C., Van Der Vlies, A. J., Swartz, M. A., & Hubbell, J. A. (2015). 6-Thioguanine-loaded polymeric micelles deplete myeloid-derived suppressor cells and enhance the efficacy of T cell immunotherapy in tumor-bearing mice. Cancer Immunology, Immunotherapy, 64(8), 1033–1046.
Jiang, X. C., & Gao, J. Q. (2017). Exosomes as novel bio-carriers for gene and drug delivery. International Journal of Pharmaceutics, 521(1–2), 167–175.
Kadiyala, P., Li, D., Nuñez, F. M., Altshuler, D., Doherty, R., Kuai, R., Yu, M., Kamran, N., Edwards, M., Moon, J. J., & Lowenstein, P. R. (2019). High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano, 13(2), 1365–1384.
Kaneshiro, T. L., & Lu, Z. R. (2009). Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials, 30(29), 5660–5666.
Kibria, G., Hatakeyama, H., Sato, Y., & Harashima, H. (2016). Anti-tumor effect via passive anti-angiogenesis of PEGylated liposomes encapsulating doxorubicin in drug resistant tumors. International Journal of Pharmaceutics, 509(1–2), 178–187.
Kleinman, M. E., Yamada, K., Takeda, A., Chandrasekaran, V., Nozaki, M., Baffi, J. Z., Albuquerque, R. J., Yamasaki, S., Itaya, M., Pan, Y., & Appukuttan, B. (2008). Sequence-and target-independent angiogenesis suppression by siRNA via TLR3. Nature, 452(7187), 591–597.
Köhler, G., & Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256(5517), 495–497.
Kosmides, A. K., Meyer, R. A., Hickey, J. W., Aje, K., Cheung, K. N., Green, J. J., & Schneck, J. P. (2017). Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials, 118, 16–26.
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A., & Moon, J. J. (2017). Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Materials, 16(4), 489–496.
Kyi, C., Roudko, V., Sabado, R., Saenger, Y., Loging, W., Mandeli, J., Thin, T. H., Lehrer, D., Donovan, M., Posner, M., & Misiukiewicz, K. (2018). Therapeutic immune modulation against solid cancers with intratumoral poly-ICLC: A pilot trial. Clinical Cancer Research, 24(20), 4937–4948.
Li, W., Feng, S. S., & Guo, Y. (2013a). Polymeric nanoparticulates for cancer immunotherapy. Nanomedicine, 8(5), 679–682.
Li, W., Zhang, L., Zhang, G., Wei, H., Zhao, M., Li, H., Guo, S., Gao, J., Kou, G., Li, B., & Dai, J. (2013b). The finely regulating well-defined functional polymeric nanocarriers for anti-tumour immunotherapy. Mini Reviews in Medicinal Chemistry, 13(5), 643–652.
Li, W., Wei, H., Li, H., Gao, J., Feng, S. S., & Guo, Y. (2014). Cancer nanoimmunotherapy using advanced pharmaceutical nanotechnology. Nanomedicine, 9(16), 2587–2605.
Li, X., Wang, Y., Wang, Q., Liu, Y., Bao, W., & Wu, S. (2018). Exosomes in cancer: Small transporters with big functions. Cancer Letters, 435, 55–65.
Lin, C. Y., Javadi, M., Belnap, D. M., Barrow, J. R., & Pitt, W. G. (2014). Ultrasound sensitive eLiposomes containing doxorubicin for drug targeting therapy. Nanomedicine: Nanotechnology, Biology and Medicine, 10(1), 67–76.
Luo, M., Wang, H., Wang, Z., Cai, H., Lu, Z., Li, Y., Du, M., Huang, G., Wang, C., Chen, X., & Porembka, M. R. (2017). A STING-activating nanovaccine for cancer immunotherapy. Nature Nanotechnology, 12(7), 648.
Mandal, S., Eksteen-Akeroyd, Z. H., Jacobs, M. J., Hammink, R., Koepf, M., Lambeck, A. J., van Hest, J. C., Wilson, C. J., Blank, K., Figdor, C. G., & Rowan, A. E. (2013). Therapeutic nanoworms: Towards novel synthetic dendritic cells for immunotherapy. Chemical Science, 4(11), 4168–4174.
Mandal, A., Bisht, R., Rupenthal, I. D., & Mitra, A. K. (2017). Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. Journal of Controlled Release, 248, 96–116.
Mangala, L. S., Zuzel, V., Schmandt, R., Leshane, E. S., Halder, J. B., Armaiz-Pena, G. N., Spannuth, W. A., Tanaka, T., Shahzad, M. M., Lin, Y. G., & Nick, A. M. (2009). Therapeutic targeting of ATP7B in ovarian carcinoma. Clinical Cancer Research, 15(11), 3770–3780
Mei, Y., Wang, R., Jiang, W., Bo, Y., Zhang, T., Yu, J., ... & Ma, W. (2009). Recent progress in nanomaterials for nucleic acid delivery in cancer immunotherapy. Biomaterials science, 7(7), 2640–2651.
Miller, R. A., Maloney, D. G., Warnke, R., & Levy, R. (1982). Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. New England Journal of Medicine, 306(9), 517–522.
Mueller, M., Reichardt, W., Koerner, J., & Groettrup, M. (2012). Coencapsulation of tumor lysate and CpG-ODN in PLGA-microspheres enables successful immunotherapy of prostate carcinoma in TRAMP mice. Journal of Controlled Release, 162(1), 159–166.
Opalinska, J. B., & Gewirtz, A. M. (2002). Nucleic-acid therapeutics: Basic principles and recent applications. Nature Reviews Drug Discovery, 1(7), 503–514.
Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines—a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261.
Parish, C. R. (2003). Cancer immunotherapy: The past, the present and the future. Immunology and Cell Biology, 81(2), 106–113.
Parker, K. H., Beury, D. W., & Ostrand-Rosenberg, S. (2015). Myeloid-derived suppressor cells: Critical cells driving immune suppression in the tumor microenvironment. In Advances in cancer research (Vol. 128, pp. 95–139). Academic Press.
Pastor, F., Berraondo, P., Etxeberria, I., Frederick, J., Sahin, U., Gilboa, E., & Melero, I. (2018). An RNA toolbox for cancer immunotherapy. Nature Reviews Drug Discovery, 17(10), 751–767.
Pedrosa, L. R. C., van Tellingen, O., Soullié, T., Seynhaeve, A. L., Eggermont, A. M., Ten Hagen, T. L., Verheij, M., & Koning, G. A. (2015). Plasma membrane targeting by short chain sphingolipids inserted in liposomes improves anti-tumor activity of mitoxantrone in an orthotopic breast carcinoma xenograft model. European Journal of Pharmaceutics and Biopharmaceutics, 94, 207–219.
Pommier, Y. (2004). Camptothecins and topoisomerase I: A foot in the door. Targeting the genome beyond topoisomerase I with camptothecins and novel anticancer drugs: Importance of DNA replication, repair and cell cycle checkpoints. Current Medicinal Chemistry-Anti-Cancer Agents, 4(5), 429–434.
Qu, M. H., Zeng, R. F., Fang, S., Dai, Q. S., Li, H. P., & Long, J. T. (2014). Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. International Journal of Pharmaceutics, 474(1–2), 112–122.
Rancoule, C., Magné, N., Vallard, A., Guy, J. B., Rodriguez-Lafrasse, C., Deutsch, E., & Chargari, C. (2016). NPs in radiation oncology: From bench-side to bedside. Cancer Letters, 375(2), 256–262.
Sahin, U., Karikó, K., & Türeci, Ö. (2014). mRNA-based therapeutics—Developing a new class of drugs. Nature Reviews Drug Discovery, 13(10), 759–780.
Seymour, L. W., Ferry, D. R., Anderson, D., Hesslewood, S., Julyan, P. J., Poyner, R., Doran, J., Young, A. M., Burtles, S., & Kerr, D. J. (2002). Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. Journal of Clinical Oncology, 20(6), 1668–1676.
Shae, D., Becker, K. W., Christov, P., Yun, D. S., Lytton-Jean, A. K., Sevimli, S., Ascano, M., Kelley, M., Johnson, D. B., Balko, J. M., & Wilson, J. T. (2019). Endosomolyticpolymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nature Nanotechnology, 14(3), 269–278.
Shi, Y., & Lammers, T. (2019). Combining nanomedicine and immunotherapy. Accounts of Chemical Research, 52(6), 1543–1554.
Shi, M., Lu, J., & Shoichet, M. S. (2009). Organic nanoscale drug carriers coupled with ligands for targeted drug delivery in cancer. Journal of Materials Chemistry, 19(31), 5485–5498.
Shi, J., Xiao, Z., Kamaly, N., & Farokhzad, O. C. (2011). Self-assembled targeted nanoparticles: Evolution of technologies and bench to bedside translation. Accounts of Chemical Research, 44(10), 1123–1134.
Smith, A. D. (2013). Big moment for nanotech: Oncology therapeutics poised for a leap. In OncLive.
Stephan, M. T., Moon, J. J., Um, S. H., Bershteyn, A., & Irvine, D. J. (2010). Therapeutic cell engineering with surface-conjugated synthetic NPs. Nature Medicine, 16(9), 1035–1041.
Sterzenbach, U., Putz, U., Low, L. H., Silke, J., Tan, S. S., & Howitt, J. (2017). Engineered exosomes as vehicles for biologically active proteins. Molecular Therapy, 25(6), 1269–1278.
Tekade, R. K., Dutta, T., Gajbhiye, V., & Jain, N. K. (2009). Exploring dendrimer towards dual drug delivery: pH responsive simultaneous drug-release kinetics. Journal of Microencapsulation, 26(4), 287–296.
Tian, Y., Li, S., Song, J., Ji, T., Zhu, M., Anderson, G. J., Wei, J., & Nie, G. (2014). A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials, 35(7), 2383–2390.
Toraya-Brown, S., Sheen, M. R., Zhang, P., Chen, L., Baird, J. R., Demidenko, E., Turk, M. J., Hoopes, P. J., Conejo-Garcia, J. R., & Fiering, S. (2016). Local hyperthermia treatment of tumors induces CD8+ T cell-mediated resistance against distal and secondary tumors. In Handbook of immunological properties of engineered nanomaterials: Volume 3: Engineered nanomaterials and the immune cell function (pp. 309–347).
Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145–160.
Turturici, G., Tinnirello, R., Sconzo, G., & Geraci, F. (2014). Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. American Journal of Physiology-Cell Physiology, 306(7), C621–C633.
Vinay, D. S., & Kwon, B. S. (2012). Immunotherapy of cancer with 4-1BB. Molecular Cancer Therapeutics, 11(5), 1062–1070.
Vollmer, J., & Krieg, A. M. (2009). Immunotherapeutic applications of CpGoligodeoxynucleotide TLR9 agonists. Advanced Drug Delivery Reviews, 61(3), 195–204.
Waldmann, T. A. (2003). Immunotherapy: Past, present and future. Nature Medicine, 9(3), 269–277.
Wang, C., Sun, W., Wright, G., Wang, A. Z., & Gu, Z. (2016). Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and anti-PD1 antibody. Advanced Materials, 28(40), 8912–8920.
Wang, K., Wen, S., He, L., Li, A., Li, Y., Dong, H., Li, W., Ren, T., Shi, D., & Li, Y. (2018a). “Minimalist” nanovaccine constituted from near whole antigen for cancer immunotherapy. ACS Nano, 12(7), 6398–6409.
Wang, Z., Liu, W., Shi, J., Chen, N., & Fan, C. (2018b). Nanoscale delivery systems for cancer immunotherapy. Materials Horizons, 5(3), 344–362.
Whitehead, K. A., Langer, R., & Anderson, D. G. (2009). Knocking down barriers: Advances in siRNA delivery. Nature Reviews Drug Discovery, 8(2), 129–138.
Whitehead, K. A., Dorkin, J. R., Vegas, A. J., Chang, P. H., Veiseh, O., Matthews, J., Fenton, O. S., Zhang, Y., Olejnik, K. T., Yesilyurt, V., & Chen, D. (2014). Degradable lipid NPs with predictable in vivo siRNA delivery activity. Nature Communications, 5(1), 1–10.
Wilson, J. T., Keller, S., Manganiello, M. J., Cheng, C., Lee, C. C., Opara, C., Convertine, A., & Stayton, P. S. (2013). pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano, 7(5), 3912–3925.
Wilson, D. R., Sen, R., Sunshine, J. C., Pardoll, D. M., Green, J. J., & Kim, Y. J. (2018). Biodegradable STING agonist NPs for enhanced cancer immunotherapy. Nanomedicine: Nanotechnology, Biology and Medicine, 14(2), 237–246.
Xiao, Y., Shi, K., Qu, Y., Chu, B., & Qian, Z. (2019). Engineering NPs for targeted delivery of nucleic acid therapeutics in tumor. Molecular Therapy-Methods & Clinical. Development, 12, 1–18.
Yanase, M., Shinkai, M., Honda, H., Wakabayashi, T., Yoshida, J., & Kobayashi, T. (1998). Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. Japanese Journal of Cancer Research, 89(7), 775–782.
Yang, Z., Xie, J., Zhu, J., Kang, C., Chiang, C., Wang, X., Wang, X., Kuang, T., Chen, F., Chen, Z., & Zhang, A. (2016). Functional exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo evaluation. Journal of Controlled Release, 243, 160–171.
Yin, H., Kanasty, R. L., Eltoukhy, A. A., Vegas, A. J., Dorkin, J. R., & Anderson, D. G. (2014). Non-viral vectors for gene-based therapy. Nature Reviews Genetics, 15(8), 541–555.
Yin, H., Kauffman, K. J., & Anderson, D. G. (2017). Delivery technologies for genome editing. Nature Reviews Drug Discovery, 16(6), 387.
Yishay-Safranchik, E., Golan, M., & David, A. (2014). Controlled release of doxorubicin and Smac-derived pro-apoptotic peptide from self-assembled KLD-based peptide hydrogels. Polymers for Advanced Technologies, 25(5), 539–544.
Yu, Z., Xu, Q., Dong, C., Lee, S. S., Gao, L., Li, Y., D'Ortenzio, M., & Wu, J. (2015). Self-assembling peptide nanofibrous hydrogel as a versatile drug delivery platform. Current Pharmaceutical Design, 21(29), 4342–4354.
Zarrinpar, A., Lee, D. K., Silva, A., Datta, N., Kee, T., Eriksen, C., Weigle, K., Agopian, V., Kaldas, F., Farmer, D., & Wang, S. E. (2016). Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Science Translational Medicine, 8(333), 333–349.
Zhang, Z., Tongchusak, S., Mizukami, Y., Kang, Y. J., Ioji, T., Touma, M., Reinhold, B., Keskin, D. B., Reinherz, E. L., & Sasada, T. (2011). Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials, 32(14), 3666–3678.
Zhang, M., Zang, X., Wang, M., Li, Z., Qiao, M., Hu, H., & Chen, D. (2019). Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: Recent advances and challenges. Journal of Materials Chemistry B, 7(15), 2421–2433.
Zupančič, E., Curato, C., Paisana, M., Rodrigues, C., Porat, Z., Viana, A. S., Afonso, C. A., Pinto, J., Gaspar, R., Moreira, J. N., & Satchi-Fainaro, R. (2017). Rational design of NPs towards targeting antigen-presenting cells and improved T cell priming. Journal of Controlled Release, 258, 182–195.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Veluchamy, C., Kamaraj, SK., Thirumurugan, R., Sánchez-Cárdenas, M., Sánchez-Olmos, L.A. (2021). Cancer Nanoimmunotherapy: Recent Advances and New Opportunities. In: Saravanan, M., Barabadi, H. (eds) Cancer Nanotheranostics. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-76263-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-76263-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76262-9
Online ISBN: 978-3-030-76263-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)