Abstract
With the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) acute promyelocytic leukemia (APL) has become from a detrimental to one of the most curable malignant diseases in humans. In particular, the chemotherapy-free combination regimen with ATO/ATRA has been proven to be advantageous compared to chemotherapy-based treatments in de novo APL and has become standard first-line therapy in non-high-risk APL patients. We here discuss the diagnostic approach, treatment as well as management of complications of patients with APL.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Acute promyelocytic leukemia (APL), characterized by the balanced translocation t(15;17)(q22;q12) resulting in the fusion transcript PML-RARA, is a rare entity of acute myeloid leukemia (AML), accounting for roughly 5–8% of AML patients (Swerdlow et al. 2017). According to the prior French-American-British (FAB) classification, APL was designated as “M3 leukemia”(Bennett et al. 1985) and is now assigned to the World Health Organization (WHO) defined type of AML with recurrent cytogenetic abnormalities, “acute promyelocytic leukemia with t(15;17)(q22;q12), (PML-RARA) and variants” (Swerdlow et al. 2017). According to the current WHO classification, patients with specific cytogenetic and molecular genetic abnormalities such as t(15;17)(q22;q12)/PML-RARA are classified as AML independently of the percentage of blast cells in the bone marrow and peripheral blood (Swerdlow et al. 2017).
Detection of the PML-RARA fusion is carried out by conventional cytogenetics including fluorescence in situ hybridization (FISH) and/or reverse transcriptase polymerase chain reaction (RT-PCR). Alternative fusion partners are the zinc finger gene (PLZF), the nucleophosmin gene (NPM), the nuclear mitotic apparatus (NUMA) or the STAT5b gene (Grimwade et al. 2000). These fusion partners are therapeutically relevant, since the alternative fusion partner involving the PLZF gene (t(11;17)(q23;q21)) is not sensitive to all-trans retinoic acid (ATRA) (Redner 2002).
8.2 Diagnostic Work-Up
The vast majority of APL patients mostly display a characteristic abnormal hypergranulation of blast cells and or promyelocytes (Fig. 8.1a) (Swerdlow et al. 2017). Thus, rapid morphological evaluation of peripheral blood as well as bone marrow is mandatory if an APL is suspected. The nuclei of the cells vary in shape and size, being often bilobulated and kidney-shaped (Fig. 8.1b). The cytoplasm of the cells is completely filled with dense and partially condensed granulation. In some cells the cytoplasm is filled with dust granulation. Cells with characteristic bundles of Auer rods are found in the bone marrow or in the peripheral blood, the so-called Faggot cells. The M3 variant (M3v), however, contains fewer cells with hypergranulations or bundles of Auer rods.
(a) Promyelocytes with characteristic Auer rods/Fagott cells in the cytoplasm. Faggot cells are cells normally found in the hypergranular form of APL (FAB-M3). The promyelocytes have numerous Auer rods in the cytoplasm which gives the appearance of a bundle of sticks. (b) Bilobulated and kidney-shaped blast cells which are characteristic for the microgranular variant (FAB M3v) of APL
Hypergranulated promyelocytes strongly react with POX, SSB, and chloroacetate esterase. The expression of CD33, CD117, and absence of HLA-DR and CD34 on the surface of APL blasts is characteristic of the disease (Table 8.1). The t(15;17) translocation and the respective PML-RARA fusion transcript are diagnostically conclusive and represent definitive hallmarks of APL diagnosis (Swerdlow et al. 2017). The molecular analysis for the detection of PML-RARA is carried out by either RT-PCR or by FISH. Whereas both methods are used as a fast and highly sensitive verification of the initial diagnosis, only RT-qPCR is sensitive enough for the measurement of measurable residual disease (MRD) in the course of APL therapy. The results of several independent studies have shown that RT-qPCR positivity for PML-RARA transcripts during morphological remission within consolidation cycles is a predictive factor for an early hematological recurrence, whereas RT-qPCR negativity in the bone marrow is usually associated with long-term survival and cure after therapy (also in patients with relapse) (Burnett et al. 1999; Mandelli et al. 1997; Schnittger et al. 2003; Cicconi et al. 2018).
8.2.1 Diagnostic Examination Schedule
Morphological analyses of bone marrow and peripheral blood are recommended at the following time points:
-
at initial diagnosis
-
after induction
-
prior to the second and following consolidation therapy
-
after the last consolidation therapy
-
quarterly during maintenance therapy in high-risk patients
-
after therapy quarterly during a 3 years follow-up from start of therapy
-
At suspected relapse
Cytogenetic and immunophenotypic analyses should be performed at diagnosis and in case of relapse.
Molecular analyses with RT-qPCR for evaluation of MRD are recommended according to the risk-status at diagnosis as indicated in Table 8.2.
8.3 Treatment
APL must be classified as an emergency with immediate initiation of treatment with all-rans retinoic acid (ATRA) 45 mg/m2/day as well as supportive therapy. Even when APL is only suspected based on clinical and morphological findings, therapy must be started immediately before a genetic diagnosis is available due to the potential lethal complications and the potential for cure. Prior to therapy, bone marrow and blood diagnostics are essential. Treatment with ATRA has revolutionizedimproved therapeutic success in APL, providing the prime example of molecularly targeted treatment (Huang et al. 1988; Tallman et al. 1997). ATRA causes differentiation of abnormal promyelocytes to mature neutrophils in vitro and in vivo. Complete remissions (CR) were achieved with single-agent ATRA in up to 80–90% of newly diagnosed and relapsed APL patients (Huang et al. 1988; Tallman et al. 1997; Ablain and de The 2011; Castaigne et al. 1990; Chen et al. 1991; Chomienne et al. 1990). Additionally, treatment with ATRA abrogates the disturbed coagulation cascade. However, the accelerated differentiation to mature neutrophils often induces a rapid WBC increase. In fact, in 15–20% of patients, the so-called “differentiation-syndrome” (DS) occurs, consisting of, for example, weight gain, respiratory distress, unexplained fever, interstitial pulmonary infiltrates, pleural or pericardial effusions with or without leukocytosis. This syndrome is associated with a high mortality rate (Fenaux et al. 1992; Frankel et al. 1992, 1994; Warrell et al. 1994); specific treatment to overcome DS is discussed in Chap. 4.
Unfortunately, remissions after single agent treatment with ATRA in most of the patients were not sustained (Huang et al. 1988; Tallman et al. 1997; Ablain and de The 2011; Castaigne et al. 1990; Chen et al. 1991; Chomienne et al. 1990). These findings led to the concurrent use of ATRA with chemotherapy (CTX; either an anthracycline plus cytarabine or an anthracycline alone) as the standard of care for induction in newly diagnosed APL (Coombs et al. 2015). More recently, the combination of arsenic trioxide (ATO) with ATRA has been shown to be a very effective CTX-free treatment strategy in de novo, low-/intermediate-risk (low-/intermediate-risk: WBC ≤ 10.0 × 109/l; high-risk: WBC > 10.0 × 109/l) APL (Estey et al. 2006).
In addition, published data of a large multicenter phase 3 randomized trial on the direct comparison of ATO/ATRA vs ATRA in combination with idarubicin (AIDA) or mitoxantrone in adult patients with de novo, non-high-risk APL showed very promising results in favor of ATO/ATRA, with a 2-year event-free survival (EFS) rate of 97 vs 86% (P = 0.02) (Lo-Coco et al. 2013). Within this trial, early mortality as well as hematological toxicities were significantly lower in patients treated with ATO/ATRA as compared to AIDA. Particularly, the cumulative incidence of relapse (CIR) after 50 months was only 1.9% after ATO/ATRA as compared to 13.9% after CTX + ATRA (Platzbecker et al. 2016). Moreover, none of the patients treated with ATO/ATRA developed a therapy-related myeloid neoplasm as compared to two patients in the CTX/ATRA arm (Platzbecker et al. 2016). Another publication of the Medical Research Council supports these results, with a 4-year EFS rate of 91% after ATO/ATRA as compared to 70% after CTX/ATRA (P = 0.002) (Burnett et al. 2015). However, the regimen with ATO/ATRA was associated with a higher frequency of grade 3 or 4 hepatic toxicity as compared to CTX/ATRA (44% vs 3%; P < 0.001). In all cases, the toxic effects resolved with temporary discontinuation of ATO and/or ATRA (Platzbecker et al. 2016). Taken together, the CTX-free regimen with ATO/ATRA has become standard first-line therapy in non-high-risk de novo APL. Figure 8.2 and Table 8.3 give an overview of the treatment schedule and dosages. We recommend the following approach:
-
prophylaxis of differentiation syndrome with prednisone 0.5 mg/kg/day p.o. from day 1 of ATO application to the end of induction therapy as well as hydroxyurea (see Chap. 4, Sect. 4.3) if WBC count raises up to >10 × 109/L)
-
bone marrow evaluation on day 28
-
induction therapy should be terminated on the basis of morphological criteria (if CR or CRi is reached on day 28)
-
in case CR or CRi is not achieved by day 28, ATO/ATRA therapy should be continued up to max. day 60 until terminal differentiation is reached; this should be accompanied by serial bone marrow assessments to definitively demonstrate CR
-
cytogenetic and molecular assessment at the end of induction therapy has no value in case of CR. Molecular responses should be assessed after consolidation only
ATO/ATRA-based induction therapy is followed by 4 courses of ATO/ATRA-based consolidation. Start of consolidation cycles is considered after hematological recovery with neutrophils ≥1.0 × 109/L and platelets ≥100 × 109/L. In case of morphological CR and hematological recovery, consolidation therapy should be started within 4 weeks after documented CR. Each course of therapy should be initiated at hematological recovery from the previous course. The PCR status after the end of consolidation is an important stratification parameter for the subsequent therapy. However, it needs to be mentioned that the rate of molecular remission was 100% after ATO/ATRA in the pivotal study (Platzbecker et al. 2016).
During all consolidation cycles (Swerdlow et al. 2017; Bennett et al. 1985; Grimwade et al. 2000; Redner 2002) the following diagnostics are recommended:
-
bone marrow samples should be collected after full hematological recovery prior to the start of the second, third, and fourth consolidation cycle as well as after the last consolidation cycle and should be tested for morphology and by RT-qPCR for assessment of molecular remission
-
patients without molecular remission at the end of all consolidation cycles are very rare cases (<1%) and will be considered molecularly resistant and should be offered conventional chemotherapy (e.g., AIDA) followed by an autologous or allogeneic hematopoietic stem cell transplantation
In countries where ATO is not yet available AIDA-based CTX is still the standard.
8.3.1 Dose Modifications
In case of non-hematological toxicities (grade 3/4 toxicities according to CTCAE Version 4.03) of ATO and ATRA (e.g., QT prolongation, differentiation syndrome, hepatotoxicity, pseudotumor cerebri) dose modifications according to Table 8.4 are recommended. As soon as the symptoms and the patients’ clinical conditions improve, treatment with ATRA and/or ATO should be resumed at 50% of the previous dose during the first 7 days after the disappearance of the symptoms. Thereafter, in the absence of worsening of the previous toxicity, ATRA and/or ATO should be resumed at full dosage. In case of the reappearance of symptoms, ATRA and ATO should be reduced to the previous dosage.
8.4 Supportive Measures and Management of Complications
8.4.1 Treatment of Coagulopathy
APL is typically associated with frequently life-threatening hemorrhagic diathesis, which is attributed to a disseminated intravascular coagulation-like coagulopathy (Swerdlow et al. 2017; Tallman and Kwaan 1992; Sanz and Montesinos 2010). The pathogenesis of hemorrhagic complications in patients with APL is complex, often triggered by higher white blood cell (WBC) counts and includes factors of blood coagulation and fibrinolysis such as severe hypofibrinogenemia, increased levels of fibrin degradation products, or D-dimers combined with a prolonged prothrombin or activated partial thromboplastin time as well as thrombocytopenia (Mantha et al. 2016). The hemorrhagic diathesis is one of the main causes of early death (ED) in APL patients (Sanz and Montesinos 2010; Mantha et al. 2016). Release and exposure of tissue factor and annexin II by the leukemic blasts are triggering these processes. Thus, the absolute WBC count, reflecting the absolute leukemic mass, seems to correlate with the severity of bleeding complications (Mantha et al. 2016).
Before the ATRA era, the risk of early hemorrhagic death for newly diagnosed patients with APL was up to 20% and decreased to 5–10% after introduction of ATRA in 1988 (Rodeghiero et al. 1990). Therefore, current guidelines advise to start ATRA as soon as the diagnosis of APL is suspected to treat and prevent hemorrhagic complications (Sanz et al. 2009). However, it must be noted that the therapy with ATRA can result in a reversion of the clotting disorder into a thrombophilic constellation with thromboembolic events. The benefit of heparin, tranexamic acid, or other anticoagulant or anti-fibrinolytic therapy to attenuate the thrombohemorrhagic risk remains questionable. In a historical comparison of the LPA99 with the LPA96 trials, the use of tranexamic acid had no impact on decreasing the hemorrhagic mortality (Sanz and Montesinos 2010). Additionally, the role of factor VIIa or prothrombotic complex concentrates for treating life-threatening hemorrhages in APL remains uncertain. Although there are case reports in which the use of recombinant factor VIIa was effective for the treatment of life-threatening hemorrhage in patients with APL (Zver et al. 2004; Alimoghaddam et al. 2006), theoretically these agents may enhance the thrombotic risk (Mantha et al. 2016; Rodeghiero et al. 1990). Therefore, the prophylactic use of anticoagulant, antifibrinolytic, or procoagulant agents should be restricted to clinical trials. Finally, any invasive procedures, including the insertion of central intravenous catheters as well as other procedures (e.g., bronchoscopy or lumbar puncture), should be avoided until coagulopathy has resolved (Sanz et al. 2009). Supportive therapy to counteract the coagulopathy should be initiated in parallel to APL-specific treatment. This includes the application of fibrinogen as well as platelet transfusions to maintain fibrinogen concentration above 100 mg/dL and platelet count as high as possible (>50 × 109/L) but at least above 30 × 109/L, respectively (Sanz et al. 2009). In case of unavailability of pure fibrinogen preparation, a substitution with fresh frozen plasma is indicated.
Only limited data exist about the effect on hemorrhagic risk by the addition of ATO to induction therapy. There was no case of early hemorrhagic death in the ATO/ATRA-arm for patients with low-/intermediate-risk (pretreatment WBC ≤ 10 × 109/L) disease within the APL0406 trial (Lo-Coco et al. 2013). Nevertheless, in high-risk patients, CTX (preferably idarubicin) in combination with ATRA should be initiated as early as possible to terminate the perilous bleeding cascade.
8.4.2 Therapy of Differentiation Syndrome
DS is a complication during induction caused by the differentiating effects of ATRA and/or ATO on leukemic blasts, which can be fatal if not treated (Sanz and Montesinos 2014). Symptoms may include unexplained fever, dyspnea, acute respiratory distress, interstitial pulmonary infiltrates, pleural or pericardial effusions, weight gain or peripheral edema, hypotension, and renal, hepatic, or multi-organ dysfunction. Leukocytosis frequently but not always accompanies DS and often precedes its clinical manifestations (Montesinos et al. 2009a).
If DS is suspected, 10 mg dexamethasone twice daily intravenously, concomitant diuretic therapy and hemodynamic monitoring should immediately be initiated until resolution of signs and symptoms. Temporary discontinuation of ATRA and/or ATO may be required in cases of severe DS (Sanz et al. 2009). Early transfer of patients to an intermediate care unit for improved monitoring of vital signs should be considered. As soon as the patients’ clinical condition improves, the symptoms have disappeared and the WBC count is sustainably lowered to <10 × 109/L, the APL treatment with ATRA and/or ATO can be resumed at 50% of the previous dose during the first 7 days. ATRA and/or ATO might be resumed at full dosage in the absence of worsening of the previous toxicity. In case of reappearance of the previous symptoms, ATRA and ATO should be reduced to the previous dosage. The evidence for the use of corticosteroids as a prophylactic approach to prevent DS, however, is limited. Within the APL2000 trial, the DS-related death rate decreased from 5.7 to 3.9% in high-risk patients after the prophylactic use of dexamethasone as compared to the earlier APL93 trial, in which prophylactic dexamethasone was not used (Sanz and Montesinos 2010; Kelaidi et al. 2009). Within the APL0406 trial, prednisone was given prophylactically at a dose of 0.5 mg/kg/day from day 1 until the end of induction therapy (Lo-Coco et al. 2013). DS developed in 19% in the ATO/ATRA group and in 16% in the CTX/ATRA group, but was fatal in only 2.5% assigned to CTX/ATRA (Lo-Coco et al. 2013). Based on these results, we recommend prednisone prophylaxis as done in the APL0406 trial.
8.4.3 Treatment of Leukocytosis During Induction
Leukocytosis commonly occurs, either at initial presentation or during therapy in patients treated with ATRA and/or ATO induction as a result of DS. Thus, in low-risk APL, hydroxyurea 500 mg once/daily for WBC between 10 and 20 × 109/L, 500 mg twice/daily for WBC between 21 and 50 × 109/L, and 1.000 mg twice daily above 50 × 109/L should be used in case of leukocytosis and should be continued at a given dose to keep the WBC count <10 × 109/L and subsequently tapered (Table 8.5) (Lo-Coco et al. 2013). Additionally, APL cells are sensitive to therapy with anthracyclines. Thus, treatment with anthracyclines such as idarubicin should be considered as early as possible during induction therapy of high-risk patients. ATO/ATRA in combination with idarubicin was used up-front within the phase 2 APML4 trial, in part to prevent hyperleukocytosis and DS (Iland et al. 2012). In this trial, no deaths from DS occurred. Furthermore, gemtuzumab ozogamicin (GO) was successfully used within the AML17 trial in high-risk patients to control leukocytosis (Burnett et al. 2015).
In contrast, leukapheresis has no role in upfront treatment, and may even be harmful in high-risk patients with leukocytosis, because this procedure may exacerbate the coagulopathy and was associated with a high risk of death (Vahdat et al. 1994).
8.4.4 QT Prolongation Associated with ATO
Treatment with ATO is associated with electrolyte abnormalities and prolongation of the QT interval corrected for the heart rate (QTc), which can lead to ventricular tachycardia with fatal outcome (Barbey et al. 2003; Unnikrishnan et al. 2004). Prolongation of the QTc interval occurred in 12 of 77 (16%) patients in the ATO/ATRA group within the APL0406 trial and was severe (QTc ≥ 501 ms) in one patient. Therefore, close monitoring of the electrocardiogram and electrolytes is necessary during treatment with ATO. Particularly, magnesium and potassium levels should always be kept within the upper-normal range. Concomitant therapy with drugs that are known to prolong the QTc interval should be discontinued. In patients with an absolute QTc interval > 500 ms, ATO should be discontinued, ideally together with any QTc prolonging medication, and electrolytes should be repleted. The time between discontinuing ATO and normalization of the QTc interval may take several days. Once QTc is normalized, ATO should be continued at 0.075 mg/kg (50%) for the first 7 days, and, if no further prolongation occurs, ATO should be escalated to 0.11 mg/kg for a second week. Thereafter, if no prolongation occurs, ATO could be continued at full dose (Lo-Coco et al. 2013).
8.4.5 Pseudotumor cerebri with ATRA Therapy
A “pseudotumor cerebri,” manifesting with headaches, nausea, vomiting, and blurred vision, may occur during ATRA therapy, particularly in younger patients. It is recommended to discontinue ATRA treatment temporarily and to administer pain killers. As soon as the symptoms and the patients’ clinical conditions improve, the treatment with ATRA should be resumed at 50% of the previous dose during the first 7 days. In the absence of worsening of the previous toxicity, ATRA should be resumed at full dosage thereafter.
8.4.6 Long-Term Toxicities with ATO/ATRA
The up-front use of ATO/ATRA is anticipated to reduce the long-term toxicities associated with anthracycline therapy. However, studies indicate that potential long-term complications exist. In one long-term follow-up study among 265 newly diagnosed APL patients treated with ATO/ATRA between 2001 and 2012, with a median follow-up of 83 months, higher rates of grade 1 liver dysfunction (15% vs 2%) and hepatic steatosis (43% vs 18%) were seen as compared to healthy controls (Zhu et al. 2016). Breast cancer developed in one patient 3 years after termination of ATO. Eight patients developed hyper-, or hypopigmentation, or hyperkeratosis/hyperplasia. All skin lesions occurred during maintenance therapy or within 6 months after treatment, and patients recovered within 2 to 18 months (Zhu et al. 2016). However, the common signs of chronic arseniasis, such as cardiovascular events, chronic renal insufficiency, diabetes, or neurological dysfunction, were not observed (Zhu et al. 2016). In some cases, peripheral neuropathy has been reported during and after treatment with ATO (Kühn et al. 2016; Shigeno et al. 2005). Symptoms are usually mild and reversible following discontinuation of treatment, but may be severe and irreversible in patients with coexistence of thiamine deficiency (Kühn et al. 2016).
Further evidence suggests a high frequency of varicella zoster virus (VZV) reactivation after ATO-based treatment. In a publication by Yamakura et al. VZV reactivation occurred in seven (46.7%) of 15 patients after ATO-based treatment as compared to none in ten patients treated with conventional CTX. All patients responded promptly to treatment with acyclovir or valacyclovir and did not develop postherpetic neuralgia. Thus, we recommend the prophylactic use of acyclovir or valacyclovir throughout ATO-based therapy (Yamakura et al. 2014).
Very recently, Norsworthy et al. reported data of 124 adult APL patients from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute who were diagnosed with APL between 2006 and 2015 (Norsworthy et al. 2019). The authors performed an exploratory population-based analysis of secondary malignancies in patients treated with or without ATO. This exploratory analysis revealed a higher incidence of second malignancies in APL patients treated with ATO, although the risk was not significantly increased compared to patients who received other APL therapies (9.9% vs 6.0% at 24 months, P = 0.24). Despite that, survival outcomes appeared better after ATO-based therapy (Norsworthy et al. 2019). However, the analyses were limited by a small sample size, short follow-up, potential selection and immortal time bias, and unaccounted for differences between comparator groups.
Based on this limited data, no firm conclusions can be drawn regarding the occurrence of comorbidities and organ toxicities. However, we suggest routine follow-up to monitor for and manage cardiovascular risk factors. Finally, age-appropriate cancer screening should be emphasized in all patients after completion of APL therapy.
8.5 Treatment of High-Risk APL
Patients with high-risk APL account for roughly 30% of patients. After induction treatment with AIDA, subsequent risk-guided consolidation cycles have shown to equalize the risk of relapse between both APL risk groups based on initial WBC counts (Sanz et al. 2000, 2004a). Due to its success in de novo non-high-risk APL (Lo-Coco et al. 2013), ATO/ATRA has also been explored as front-line use in high-risk APL. However, phase 2 studies have demonstrated lower CR rates with single agent ATO ± ATRA as compared to classical AIDA-based induction regimens in high-risk patients (Estey et al. 2006; Sanz et al. 2000, 2004a; Ghavamzadeh et al. 2011; Mathews et al. 2006; Ravandi et al. 2009).
Recently, Abaza et al. published outcome data on 187 APL patients, including 54 with high-risk APL (Abaza et al. 2017). In an attempt to improve outcomes in high-risk patients, they added GO (n = 45) or idarubicin (n = 7) to ATO/ATRA. Albeit results were drawn from a small cohort, 5-year overall survival (OS) were not significantly different between the two treatment arms (84% vs 100%; P = 0.45) and are in-line reported by others (Estey et al. 2006; Ravandi et al. 2009). Similar results were reported by Burnett et al. on the phase-3 AML17 trial comparing ATO/ATRA with CTX/ATRA in newly diagnosed patients with APL (Burnett et al. 2015). High-risk patients treated with ATO/ATRA received one initial dose of GO (6 mg/m2). The 4-year EFS-rate was 91% after ATO/ATRA/GO as compared to 70% in the CTX/ATRA group. Furthermore, the cumulative incidence of morphological and molecular relapses were reduced from 18% and 27% in the CTX/ATRA group to 1% and 0% in the ATO/ATRA/GO group (Burnett et al. 2015). Currently, the European randomized intergroup study “APOLLO” investigates idarubicin 12 mg/m2 on days 1 and 3 in addition to oral ATRA 45 mg/m2 twice daily on days 1–28 and ATO 0.15 mg/kg/day intravenously on days 5–28 followed by four cycles of ATO/ATRA consolidation therapy as compared to the standard CTX/ATRA approach (ClinicalTrails.gov identifier: NCT02688140).
In patients with high-risk APL, treatment with idarubicin + ATRA should be started as soon as possible. After achieving a hematological CR, three consolidation cycles of ATRA plus either idarubicin/cytarabine (course 1 and 3) or plus mitoxantrone (course 2) are intended (Norsworthy et al. 2019). This approach is also supported by published data combining intensive CTX according to the 7 + 3 scheme and ATRA (Lengfelder et al. 2009). Moreover, a positive impact of adding ATO to consolidation regimens was reported for all risk groups of APL in the C9710 trial (Powell et al. 2010). The efficacy of ATO as consolidation therapy was recently confirmed by Lou et al., who reported that treatment with ATO as post-remission therapy significantly improved long-term outcome as compared to standard CTX (Lou et al. 2014).
Thus, ATO as consolidation therapy in high-risk patients could be considered, although currently not authority approved.
8.6 Maintenance Therapy
8.6.1 Maintenance in Patients with High-Risk APL
The clinical benefit of maintenance therapy particularly in patients with negative MRD is still discussed controversial due to adverse side effects (AEs) including cytopenia and/or increase of the liver values. In the European APL-93 study, triple-agent maintenance therapy with ATRA, 6-mercaptopurine (6-MP) and methotrexate (MTX) resulted in a lower recurrence rate, particularly in patients with high-risk (Fenaux et al. 1999). However, this study did not differentiate between patients according to the MRD status after consolidation. Several other publications also demonstrated that an ATRA-based maintenance is needed after consolidation to ameliorate survival (Tallman et al. 1997, 2002; Kantarjian et al. 1987; Adès et al. 2010). In contrast, patients randomized to maintenance therapy with 6-MP and MTX in the LAP 0389 study did not have better outcomes than those randomized to observation, which is in line with recently published results (Avvisati et al. 2002, 2011; Asou et al. 2007). It is currently unclear, if maintenance therapy further enhances the risk for secondary malignancies, including therapy-related myeloid neoplasm. Within the recently published long-term follow-up data of the LPA96&99 as well as LPA2005 trials, 24 patients (11%) developed a secondary neoplasm in CR within a median time of 51 months (range, 6–112 months; 11 solid tumors and 7 therapy-related myeloid neoplasms within the LPA96&99 trials; 3 solid tumors and 3 therapy-related myeloid neoplasms within the LPA2005 trial, respectively) (Martínez-Cuadrón et al. 2018). Twenty-one patients died because of the secondary neoplasm. Cumulative incidence of secondary neoplasms at 5 and 10 years was 8% and 16%, respectively. However, the authors stated that no predictive factors for this event were found (Martínez-Cuadrón et al. 2018).
Taken together, maintenance therapy may still play a role in patients with high-risk receiving CTX/ATRA while its omission in the setting of ATRA and ATO is currently under investigation.
8.6.2 ATO as Maintenance Therapy
Treatment with oral ATO was shown to be well absorbed and to achieve a bioavailability of up to 95% of an equivalent dose of intravenous ATO (Kumana et al. 2002). Since slow oral absorption results in lower peak plasma arsenic levels compared with intravenous ATO, the oral formulation is associated with minimal prolongation of the QT interval (Siu et al. 2006; Kwong et al. 2001). Thus, a home-based treatment without the need of daily hospital visits and monitoring for QT prolongation or cardiac arrhythmias seems to be feasible.
Recently, Au et al. have published 10-year follow-up data on outcome after oral ATO-based maintenance therapy (Au et al. 2011). Seventy-six APL patients in first CR after induction and consolidation with daunorubicin/cytarabine received oral maintenance therapy with ATO ± ATRA or ATO/ATRA/ascorbic acid, given for 2 weeks every 2 months for 2 years. Prolonged oral ATO maintenance was feasible and safe and resulted in 3-year leukemia-free and OS of 87.7% and 90.6%, respectively (Au et al. 2011).
Taken together, maintenance treatment has been mainly used in CTX/ATRA regimen. Based on the results of the APL0406 trial, it seems that using the CTX-free regimen in low-risk APL, no maintenance was needed (Lo-Coco et al. 2013). In contrast, in high-risk APL treated with CTX/ATRA, maintenance might still play a role, particularly in MRD positive patients. Thus, maintenance therapy is included in the majority of protocols based on CTX/ATRA and, so far, still recommended for high-risk patients after an AIDA-based therapy in the absence of toxicities.
8.6.3 Treatment of Elderly Patients
Although it is generally noted that APL seems to be rather uncommon in elderly patients (Sanz et al. 2009), its true incidence in this age cohort is unclear, particularly in patients beyond the age of 70 years. According to a population-based report from the Swedish adult acute leukemia registry, the proportion of patients with APL decreased significantly with age from 17% in patients younger than 30 years to 0.9% in patients 80 years and older (Lehmann et al. 2011), In addition, since comorbidities are more common in elderly patients, these patients are less likely to be admitted to a hematological department. More importantly, ED rate after ATRA ± anthracycline-based induction therapy was 60% in patients above the age of 80 years as compared to 18.8% in patients aged 50–59 years. ED was associated with poor performance status, explaining the high rate in very elderly patients (Lehmann et al. 2011). A previous report on 104 elderly (median age, 68 years; range, 60–83 years) patients showed that older patients could be successfully treated using ATRA plus anthracycline for induction and consolidation (Sanz et al. 2004b). Patients who were MRD negative at the end of consolidation received oral 6-mercaptopurine (50 mg/m2/day), intramuscular methotrexate (15 mg/m2/week), and ATRA (45 mg/m2/day for 15 days every 3 months) over 2 years as maintenance therapy. Overall, outcome was favorable with an ED-rate of 15%, CR-rate of 84%, a 6-year CIR of 8.5%, and disease-free survival (DFS) of 79%, respectively (Sanz et al. 2004b). However, the CR-rate was lower in patients older than 70 years as compared to patients aged 60–70 years (74% vs 89%) (Sanz et al. 2004b). These results had recently been confirmed by Martinez-Cuadrón et al. comparing the long-term outcome of older patients (median age, 67 years) with de novo APL treated within the LPA2005 vs LPA96&99 trials (Martínez-Cuadrón et al. 2018). The LPA2005 trial, which was based on an age- and risk-adapted therapy with reduced post-consolidation CTX, resulted in a higher 5-year DFS (87% vs 69%; P = 0.04) and 5-year OS (74% vs 60%; P = 0.06) as compared to the LPA96&99 trials (Martínez-Cuadrón et al. 2018).
However, contrary results were published recently by Lengfelder et al. who reported on 91 newly diagnosed APL patients (median age, 67 years) registered by the German AML Cooperative Group between 1994 and 2011 (Lengfelder et al. 2013). Overall, 75% of the patients were treated on clinical trials, but the 25% non-eligibility rate was remarkably high, attributable to multimorbidity and low performance status. Fifty-six patients received induction therapy with ATRA and 6-thioguanine, cytarabine, daunorubicin (TAD), and consolidation and maintenance therapy. Treatment intensification with a second induction cycle (high dose cytarabine and mitoxantrone, (HAM)) was optional (n = 14). The 7-year OS, EFS and relapse-free survival (RFS) were 45%, 40%, and 48%, respectively. In patients treated with TAD-HAM induction, 7-year RFS was superior (83%; P = 0.006) compared to TAD only, and no relapse was observed. Thus, intensified induction therapy seemed to be highly effective, but was restricted to a selection of those patients, who could be treated intensively, since elderly patients have a higher vulnerability to treatment toxicity (Lengfelder et al. 2013). Sanz et al. noted that 6 of 25 (24%) patients ≥70 years died in remission (Sanz et al. 2004b), while Ades et al. reported that 19% of patients ≥60 years died due to complications of myelosuppression during consolidation with daunorubicin/cytarabine (Ades et al. 2005). Therefore, a higher vulnerability to treatment toxicities in older patients may result in a higher treatment-related mortality.
Regarding the distribution of risk-category according to WBC count at diagnosis, published data are again contradictory (Sanz et al. 2004b; Lengfelder et al. 2013). Sanz et al. reported that older patients seem to be more likely to present with non-high-risk APL as compared to their younger counterparts (37% vs 18%), which in part may account for the low relapse rate observed in their publication (Sanz et al. 2004b). In contrast, Lengfelder et al. reported on 31% (n = 28/91) of patients with high-risk APL (Lengfelder et al. 2013).
Regarding outcome after ATO/ATRA in elderly patients, data are scarce also since age limit in the pivotal APL0406 trial was 70 years and only a very low number of patients above 60 years were included (Lo-Coco et al. 2013). On the other side, there is no evidence to assume that the biology of non-high-risk APL in the elderly might be different as compared to younger APL patients. Zhang et al. reported on 33 de novo APL patients with a median age of 65 years (range, 60–79 years) treated with single-agent ATO for remission induction and consolidation therapy (Zhang et al. 2013). The CR-rate was 88% and the ED-rate 12%. The 10-year CIR-, OS-, and DFS-rates were 10.3%, 69.3%, and 64.8%, respectively. Overall, monotherapy with ATO was well tolerated with leukocytosis (64%) being the most common adverse event during induction, whereas non-hematological adverse events were all manageable and reversible. In addition, non-relapse mortality (NRM) was only 6.9% after monotherapy with ATO due to noninfectious diseases (Zhang et al. 2013) as compared to 10–18.6% despite reduced intensities of CTX in older patients, mainly due to infection (Ades et al. 2005; Mandelli et al. 2003; Disperati et al. 2007). None of the patients treated with ATO developed a secondary malignancy with the exception of one patient who had longstanding hepatitis B virus infection and hepatic cirrhosis, and died of liver cancer 117 months after achievement of CR (Zhang et al. 2013). Very recently, we have evaluated the outcome of 433 elderly patients (median age, 73.4 years) treated either with CTX/ATRA or ATO-based therapy (Kayser et al. 2020). CR was achieved in 92% after therapy with ATO/ATRA and in 82% after CTX/ATRA; induction death rates were 8% and 18%, respectively. CIR was significantly lower after ATO/ATRA ± CTX as compared to CTX/ATRA (P < 0.001) (Kayser et al. 2020). High (>10 × 109/L) WBC counts at diagnosis were associated with higher CIR (P < 0.001) as compared to lower WBC in the CTX/ATRA group, but not in the ATO/ATRA ± CTX group (P = 0.48). Thus, it seems reasonable to offer ATO/ATRA ± CTX as first line treatment to older patients irrespective of the risk-group.
8.6.4 Treatment of APL During Pregnancy
The occurrence of APL during pregnancy seems to be rather rare with limited evidence-based information available limited to small retrospective series and case reports. Most reliable data are therefore only available of national and international cancer registry databases (Sanz et al. 2015). Miguel Sanz on behalf of the PETHEMA study group has reported so far on the largest cohort of 14 (0.8%) pregnant women of overall 1.744 APL patients, who had been registered in their database between 1996 and 2012 (Sanz et al. 2015). Besides supportive therapy, the initiation of effective APL treatment to stop coagulopathy is of utmost importance. Table 8.6 provides an overview of fetal and maternal outcome after treatment of pregnant APL patients.
8.6.4.1 Treatment Options During the First Trimester
Overall, therapeutic options are extremely limited during the first or early second trimester in terms of successful outcome of the fetus. Isotretinoin (a compound comparable to ATRA) has been shown to be teratogenic, leading to a range of severe craniofacial, cardiac, and central nervous system abnormalities as well as increased rate of abortions (Lammer et al. 1985; Rosa 1983; Lynberg et al. 1990; Chalmers 1992). In a systematic review by Verma and colleagues of 71 APL patients diagnosed during pregnancy, 23% were diagnosed with APL in the first trimester and 69% of those were treated with ATRA (Verma et al. 2016). Abortion rate, either spontaneously or therapeutically induced, was very high (90%) during the first trimester. Moreover, women in the first trimester were more likely to experience obstetric and fetal complications as compared to the subsequent trimesters (Verma et al. 2016). Therefore, ATRA should not be offered to pregnant APL patients during the first trimester, particularly during organogenesis (~8–10 weeks following conception) given the teratogenic potential of ATRA (Lammer et al. 1985; Rosa 1983; Lynberg et al. 1990; Chalmers 1992). Cytarabine and/or anthracyclines are known to increase the risk of spontaneous abortions or cause major malformations by up to 20% (Lishner et al. 2016; Caligiuri 1992; Yang and Hladnik 2009; Williams and Schilsky 2000; Amant 2012).
Thus, the option of therapeutic abortion has to be discussed with the patient, in particular during the first trimester. In cases, in which an abortion is no option, treatment with an anthracycline should be given and combined with ATRA in early second trimester. Since idarubicin is more lipophilic and may therefore be associated with an increased placental transfer and possible fetotoxicity (Reynoso and Huerta 1994; Achtari and Hohlfeld 2000), daunorubicin 60 mg/m2 should be used for a maximum of three consecutive days. The addition of cytarabine 100–200 mg/m2 days 1–7 should be considered during induction and consolidation (Adès et al. 2006), particularly in patients with high-risk APL. CTX alone, however, increases the risk of hemorrhage due to the release of pro-coagulants and plasminogen activators from malignant cells (Sanz and Montesinos 2010).
Moreover, early labor or cesarean section has to be considered the best option as soon as the fetus can be delivered at a viable stage. In addition, CTX with an anthracycline in combination with ATRA or ATO/ATRA (non-high-risk APL) should be given as soon as possible after delivery.
8.6.4.2 Treatment Options During the Second or Third Trimester
CTX/ATRA after the beginning of the second trimester results in a more successful outcome for the unborn as compared to therapy in the first trimester, since the risk of fetal malformations reduces with advanced stage of pregnancy (Sanz et al. 2015; Claahsen et al. 1998; Consoli et al. 2004; Giagounidis et al. 2000). A high CR-rate of 92% had been reported in 11 of 12 pregnant APL patients treated with AIDA-based induction therapy; one woman died 2 weeks after start of induction therapy due to a DS. All women proceeded to consolidation and maintenance therapy and were reported to be in an ongoing CR after a median follow-up time of 83 months (Verma et al. 2016). In addition, the rate of fetal complications was comparable between the ATRA as compared to the non-ATRA group. Similarly, receipt of consolidation therapy in the study population was not associated with obstetric or fetal complications (Verma et al. 2016). Moreover, CTX rather increases the risk of abortion, prematurity, low birth weight, neonatal neutropenia, and sepsis, than to cause congenital malformations (Culligan et al. 2007).
Potentially, ATRA could be given as single agent therapy with the addition of an anthracycline after delivery. In case presentations, equivalent remission rates of ATRA as compared to CTX/ATRA have been observed (Fadilah et al. 2001; Harrison et al. 1994; Stentoft et al. 1994; Lipovsky et al. 1996). However, in pregnancies with a gestation of at least 20 weeks, there is still a risk of major malformations with ATRA monotherapy (Lammer et al. 1985). Additionally, ATRA monotherapy increases the risk of DS and possible ATRA resistance (Fenaux et al. 1999). Thus, the PML-RARA transcript needs to be monitored carefully by quantitative reverse-transcriptase polymerase chain-reaction (RT-qPCR); rise of the PML-RARA transcript potentially indicates the need to introduce CTX (Culligan et al. 2007).
As a result, ATRA monotherapy seems to be a valid option during the second or third trimester and low/intermediate-risk APL. However, molecular remission should be monitored carefully by RT-qPCR. Alternatively, in spite of the limited clinical experience, ATRA in combination with an anthracycline, particularly daunorubicin, seem reasonably safe during the second or third trimester of pregnancy.
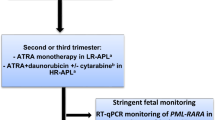
We recommend a combination of CTX/ATRA for high-risk patients, and where RT-qPCR monitoring for PML-RARA is not feasible. Figure 8.3 shows the suggested approach to APL during pregnancy.
Suggested algorithm for management of pregnancy in acute promyelocytic leukemia. APL acute promyelocytic leukemia, ATO arsenic trioxide, ATRA all-trans retinoic acid, HR high-risk, LR low-risk, PML promyelocytic leukemia, RARA retinoic acid receptor alpha, RT-qPCR quantitative reverse-transcriptase polymerase chain-reaction, WBC white blood count. aAddition of cytarabine in high-risk APL; bRisk categorization based on WBC at diagnosis (low-/intermediate-risk: WBC ≤ 10.0 × 109/L; high-risk: WBC >10.0 × 109/L)
In addition, stringent fetal monitoring, with particular emphasis on cardiac function, is recommended for patients receiving ATRA during pregnancy because some cases of reversible fetal arrhythmias have been reported (Culligan et al. 2007; Siu et al. 2002; Terada et al. 1997).
ATO has been shown to be embryotoxic and to induce teratogenicity in animal studies (Holson et al. 2000). Therefore, ATO cannot be recommended throughout pregnancy. Similarly, GO is not justifiable for use in pregnancy (Culligan et al. 2007).
Finally, men and women of childbearing potential should use effective contraception, and breastfeeding must be discontinued during CTX or treatment with ATO.
8.6.5 Treatment of Extramedullary Relapse
Relapse at extramedullary sites was reported to occur in 3–5% of patients after CTX/ATRA, particularly within the CNS (Tallman 2007). Predictive factors for an extramedullary relapse may include the development of an ATRA syndrome (Ko et al. 1999), the predominance of the PML-RARA breakpoint cluster region isoform 3 (de Botton et al. 2006) and high-risk APL (de Botton et al. 2006; Breccia et al. 2003; Montesinos et al. 2009b). Montesinos et al. have evaluated the incidence of CNS recurrence on a large group of 739 patients between 1996 and 2005 treated on the LPA96 and LPA99 PETHEMA trials (Montesinos et al. 2009b). No CNS prophylaxis was given in either protocol. Overall, CNS relapse was documented in 11 patients and the 5-year CIR within the CNS was 1.7% (Montesinos et al. 2009b). Of note, patients with high-risk had a CIR of 5.5% as compared to 0% and 0.8% in low- or intermediate-risk patients, respectively. Another independent risk factor was CNS hemorrhage during induction therapy (5-year CIR 18.7%, P = 0.006) (Montesinos et al. 2009b).
However, the strategy of an up-front CNS prophylaxis in high-risk patients is still a matter of debate. For low-risk patients, in whom the risk of CNS relapse is extremely low, there is a general consensus to avoid CNS prophylaxis (Sanz et al. 2019). Nevertheless, the possibility of CNS disease should be considered in any relapsed patient, particularly in those with neurological symptoms.
Data on the ability of ATO to cross the blood-brain barrier are derived from single case descriptions are fairly contradictory. Knipp et al. reported on a 42-year-old APL patient who developed a hematological relapse 1 year after AIDA-based therapy (Knipp et al. 2007). Since this patient had previously experienced an ATRA syndrome, he received ATO 10 mg daily for 30 days plus intrathecal therapy (40 mg cytarabine, 40 mg prednisone, and 15 mg MTX three times weekly for a total of nine treatments). In addition, his neuroaxis was irradiated with 30 Gy. Measurement of ATO in the cerebrospinal fluid (CSF) revealed a low CSF concentration of 0.11 μmol/L, representing only about 14% of blood levels. The authors concluded that ATO seems to cross the blood-CSF barrier when administered intravenously, but the concentration in CSF is probably not sufficient for treatment of meningeal leukemia (Knipp et al. 2007). Au et al. reported on a patient who relapsed 9 months after induction and consolidation therapy with ATRA, daunorubicin, and cytarabine (Au et al. 2000). Since reinduction with ATRA and cytarabine (four doses of 3 g/m2) failed, he was treated with ATO at 10 mg/day. Eight months after achievement of a second CR, the patient experienced a second hematological relapse with involvement of the CNS. Despite urgent radiotherapy, the patient died of massive CNS bleeding 2 days later (Au et al. 2000). Hence, treatment with ATO seemed not sufficient to prevent CNS relapse. Contrary, Helwig et al. reported on a patient who was diagnosed with relapsed APL involving the CNS (Helwig et al. 2007). Treatment with ATO led to morphological changes in CNS cellularity consistent with the induction of a DS. Since ATO could be identified in the CNS, the authors concluded that the drug can cross the blood-brain barrier and could be used for treatment of extramedullary APL (Helwig et al. 2007).
Since the existing data are rather limited as well as contradictory, we recommend using triple intrathecal therapy with MTX, corticosteroids, and cytarabine until complete clearance of blasts in the CSF in case of a confirmed CNS relapse/involvement, followed by 6 to 10 more space out intrathecal therapies as consolidation therapy. Since a CNS relapse is almost invariably accompanied by a hematological or molecular relapse in the marrow, systemic therapy should also be given (Sanz et al. 2009).
References
Abaza Y, Kantarjian HM, Garcia-Manero G, Estey E, Borthakur G, Jabbour E et al (2017) Long-term outcome of acute promyelocytic leukemia treated with all-transretinoic acid, arsenic trioxide, and gemtuzumab. Blood 129:1275–1283
Ablain J, de The H (2011) Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood 117:5795–5802
Achtari C, Hohlfeld P (2000) Cardiotoxic transplacental effect of idarubicin administered during the second trimester of pregnancy. Am J Obstet Gynecol 183:511–512
Ades L, Chevret S, De Botton S, Thomas X, Dombret H, Beve B et al (2005) Outcome of acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy in elderly patients: the European group experience. Leukemia 19:230–233
Adès L, Chevret S, Raffoux E, de Botton S, Guerci A, Pigneux A et al (2006) Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol 24:5703–5710
Adès L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S et al (2010) Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood 115:1690–1696
Alimoghaddam K, Ghavamzadeh A, Jahani M (2006) Use of NovoSevenR for arsenic trioxide-induced bleeding in PML. Am J Hematol 81:720
Amant F (2012) Safety of chemotherapy in pregnancy. Clin Adv Hematol Oncol 10:258–259
Asou N, Kishimoto Y, Kiyoi H, Okada M, Kawai Y, Tsuzuki M et al (2007) A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood 110:59–66
Au WY, Ma SK, Ooi C, Liang R, Kwong YL (2000) Unusual manifestations of acute leukemia. J Clin Oncol 18:3435–3437
Au WY, Kumana CR, Lee HK, Lin SY, Liu H, Yeung DY et al (2011) Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood 118:6535–6543
Avvisati G, Petti MC, Lo-Coco F, Vegna ML, Amadori S, Baccarani M et al (2002) Induction therapy with idarubicin alone significantly influences event-free survival duration in patients with newly diagnosed hypergranular acute promyelocytic leukemia: final results of the GIMEMA randomized study LAP 0389 with 7 years of minimal follow-up. Blood 100:3141–3146
Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M et al (2011) AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood 117:4716–4725
Barbey JT, Pezzullo JC, Soignet SL (2003) Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol 21:3609–3615
Bennett JM, Catovsky D, Daniel MT et al (1985) Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med 103(4):620–625
Breccia M, Carmosino I, Diverio D, De Santis S, De Propris MS, Romano A et al (2003) Early detection of meningeal localization in acute promyelocytic leukaemia patients with high presenting leucocyte count. Br J Haematol 120:266–270
Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH (1999) Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the randomized MRC trial. Blood 93:4131–4143
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S et al (2015) Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol 16:1295–1305
Caligiuri MA (1992) Leukemia and pregnancy: treatment and outcome. Adv Oncol 8:10–17
Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P et al (1990) All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I Clinical results. Blood 76:1704–1709
Chalmers RJ (1992) Retinoid therapy: a real hazard for the developing embryo. Br J Obstet Gynaecol 99:276–278
Chen ZX, Xue YQ, Zhang R, Tao RF, Xia XM, Li C et al (1991) A clinical and experimental study on all-trans retinoic acid-treated acute promyelocytic leukemia patients. Blood 78:1413–1419
Chomienne C, Ballerini P, Balitrand N, Daniel MT, Fenaux P, Castaigne S et al (1990) All-trans retinoic acid in acute promyelocytic leukemias. II. In vitro studies: structure-function relationship. Blood 76:1710–1717
Cicconi L, Fenaux P, Kantarjian H, Tallman M, Sanz MA, Lo-Coco F (2018) Molecular remission as a therapeutic objective in acute promyelocytic leukemia. Leukemia 32(8):1671–1678
Claahsen HL, Semmekrot BA, van Dongen PW, Mattijssen V (1998) Successful foetal outcome after exposure to idarubicin and cytosine-arabinoside during the second trimester of pregnancy-a case report. Am J Perinatol 15:295–297
Consoli U, Figuera A, Milone G, Meli CR, Guido G, Indelicato F et al (2004) Acute promyelocytic leukaemia during pregnancy: report of 3 cases. Int J Hematol 79:31–36
Coombs CC, Tavakkoli M, Tallman MS (2015) Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J 5:e304
Culligan DJ, Merriman L, Kell J, Parker J, Jovanovic JV, Smith N et al (2007) The management of acute promyelocytic leukaemia presenting during pregnancy. Clin Leukaemia 1:183–191
de Botton S, Sanz MA, Chevret S, Dombret H, Martin G, Thomas X et al (2006) Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia 20:35–41
Disperati P, Minden MD, Gupta V, Schimmer AD, Schuh AC, Yee KW et al (2007) Acute promyelocytic leukemia in patients aged 70 years and over—a single center experience of unselected patients. Leuk Lymphoma 48:1654–1658
Estey E, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D et al (2006) Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 107:3469–3473
Fadilah SA, Hatta AZ, Keng CS, Jamil MA, Singh S (2001) Successful treatment of acute promyelocytic leukemia in pregnancy with all-trans retinoic acid. Leukemia 15:1665–1666
Fenaux P, Castaigne S, Chomienne C, Dombret H, Degos L (1992) All trans-retinoic acid treatment for patients with acute promyelocytic leukemia. Leukemia 6:64–66
Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E et al (1999) A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood 94:1192–1200
Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP (1992) The retinoic acid syndrome in acute promyelocytic leukemia. Ann Intern Med 117:292–296
Frankel SR, Eardley A, Heller G et al (1994) All-trans-retinoic acid for acute promyelocytic leukemia—results of the New-York study. Ann Intern Med 120:278–286
Ghavamzadeh A, Alimoghaddam K, Rostami S, Ghaffari SH, Jahani M, Iravani M et al (2011) Phase II study of single agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol 29:2753–2757
Giagounidis AA, Beckmann MW, Giagounidis AS, Aivado M, Emde T, Germing U et al (2000) Acute promyelocytic leukemia and pregnancy. Eur J Haematol 64:267–271
Grimwade D, Biondi A, Mozziconacci MJ, Hagemeijer A, Berger R, Neat M et al (2000) Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): results of the European Working Party. Groupe Français de Cytogénétique Hématologique, Groupe de Français d’Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-concerted action “molecular cytogenetic diagnosis in haematological malignancies”. Blood 96(4):1297–1308
Harrison P, Chipping P, Fothergill GA (1994) Successful use of all-trans retinoic acid in acute promyelocytic leukaemia presenting during the second trimester of pregnancy. Br J Haematol 86:681–682
Helwig A, Klemm M, Schüttig R, Röllig C, Wassilew N, Ehninger G et al (2007) Arsenic-induced APL differentiation in cerebrospinal fluid. Leuk Res 31:703–705
Holson JF, Stump DG, Clevidence KJ, Knapp JF, Farr CH (2000) Evaluation of the prenatal developmental toxicity of orally administered arsenic trioxide in rats. Food Chem Toxicol 38:459–466
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L et al (1988) Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 72:567–572
Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M et al (2012) All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 120:1570–1580
Kantarjian HM, Keating MJ, Walters RS, Smith TL, McCredie KB, Freireich EJ (1987) Role of maintenance chemotherapy in acute promyelocytic leukemia. Cancer 59:1258–1263
Kayser S, Rahmé R, Martínez-Cuadrón D, Ghiaur G, Thomas X, Sobas M et al (2020) Outcome of older (≥70 years) APL patients frontline treated with or without arsenic trioxide-an International Collaborative Study. Leukemia 34(9):2333–2341. https://doi.org/10.1038/s41375-020-0758-4. [Epub ahead of print]
Kelaidi C, Chevret S, De Botton S, Raffoux E, Guerci A, Thomas X et al (2009) Improved outcome of acute promyelocytic leukemia with high WBC counts over the last 15 years: the European APL Group experience. J Clin Oncol 27:2668–2676
Knipp S, Gattermann N, Schapira M, Käferstein H, Germing U (2007) Arsenic in the cerebrospinal fluid of a patient receiving arsenic trioxide for relapsed acute promyelocytic leukemia with CNS involvement. Leuk Res 31:1585–1597
Ko BS, Tang JL, Chen YC, Yao M, Wang CH, Shen MC et al (1999) Extramedullary relapse after all-trans retinoic acid treatment in acute promyelocytic leukemia-the occurrence of retinoic acid syndrome is a risk factor. Leukemia 13:1406–1408
Kühn M, Sammartin K, Nabergoj M, Vianello F (2016) Severe acute axonal neuropathy following treatment with arsenic trioxide for acute promyelocytic leukemia: a case report. Mediterr J Hematol Infect Dis 8:e2016023
Kumana CR, Au WY, Lee NS, Kou M, Mak RW, Lam CW et al (2002) Systemic availability of arsenic from oral arsenic-trioxide used to treat patients with hematological malignancies. Eur J Clin Pharmacol 58:521–526
Kwong YL, Au WY, Chim CS, Pang A, Suen C, Liang R (2001) Arsenic trioxide- and idarubicin-induced remissions in relapsed acute promyelocytic leukaemia: clinicopathological and molecular features of a pilot study. Am J Hematol 66:274–279
Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT et al (1985) Retinoic acid embryopathy. N Engl J Med 313:837–841
Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Möllgård L et al (2011) Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia 25:1128–1134
Lengfelder E, Haferlach C, Saussele S, Haferlach T, Schultheis B, Schnittger S et al (2009) High dose ara-C in the treatment of newly diagnosed acute promyelocytic leukemia: long-term results of the German AMLCG. Leukemia 23:2248–2258
Lengfelder E, Hanfstein B, Haferlach C, Braess J, Krug U, Spiekermann K et al (2013) Outcome of elderly patients with acute promyelocytic leukemia: results of the German Acute Myeloid Leukemia Cooperative Group. Ann Hematol 92:41–52
Lipovsky MM, Biesma DH, Christiaens GC, Petersen EJ (1996) Successful treatment of acute promyelocytic leukaemia with all-trans-retinoic-acid during late pregnancy. Br J Haematol 94:699–701
Lishner M, Avivi I, Apperley JF, Dierickx D, Evens AM, Fumagalli M et al (2016) Hematologic malignancies in pregnancy: management guidelines from an international consensus meeting. J Clin Oncol 34:501–508
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S et al (2013) Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369:111–121
Lou Y, Qian W, Meng H, Mai W, Tong H, Tong Y et al (2014) Long-term efficacy of low-dose all-trans retinoic acid plus minimal chemotherapy induction followed by the addition of intravenous arsenic trioxide post-remission therapy in newly diagnosed acute promyelocytic leukaemia. Hematol Oncol 32:40–46
Lynberg MC, Khoury MJ, Lammer EJ, Waller KO, Cordero JF, Erickson JD (1990) Sensitivity, specificity, and positive predictive value of multiple malformations in isotretinoin embryopathy surveillance. Teratology 42:513–519
Mandelli F, Diverio D, Avvisati G et al (1997) Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and Idarubicin (AIDA) therapy. Blood 90:1014–1021
Mandelli F, Latagliata R, Fazi P, Rodeghiero F, Leoni F et al (2003) Treatment of elderly patients (> or =60 years) with newly diagnosed acute promyelocytic leukemia. Results of the Italian multicenter group GIMEMA with ATRA and idarubicin (AIDA) protocols. Leukemia 17:1085–1090
Mantha S, Tallman MS, Soff GA (2016) What’s new in the pathogenesis of the coagulopathy in acute promyelocytic leukemia? Curr Opin Hematol 23:121–126
Martínez-Cuadrón D, Montesinos P, Vellenga E, Bernal T, Salamero O, Holowiecka A et al (2018) Long-term outcome of older patients with newly diagnosed de novo acute promyelocytic leukemia treated with ATRA plus anthracycline-based therapy. Leukemia 32:21–29
Mathews V, George B, Lakshmi KM, Viswabandya A, Bajel A, Balasubramanian P et al (2006) Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood 107:2627–3262
Montesinos P, Bergua JM, Vellenga E, Rayón C, Parody R, de la Serna J et al (2009a) Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood 113:775–783
Montesinos P, Díaz-Mediavilla J, Debén G, Prates V, Tormo M, Rubio V et al (2009b) Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica 94:1242–1249
Norsworthy KJ, Bird T, Avagyan A, Li Y, Akhtar S, Liao J et al (2019) Second cancers in adults with acute promyelocytic leukemia (APL) treated with or without arsenic trioxide (ATO): a SEER-medicare analysis. Blood 134:3497. (abstract)
Platzbecker U, Avvisati G, Cicconi L, Thiede C, Paoloni F, Vignetti M et al (2016) Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol 35:605–612
Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM et al (2010) Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 116:3751–3757
Ravandi F, Estey E, Jones D, Faderl S, O’Brien S, Fiorentino J et al (2009) Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol 27:504–510
Redner RL (2002) Variations on a theme: the alternate translocations in APL. Leukemia 16(10):1927–1932
Reynoso EE, Huerta F (1994) Acute leukaemia and pregnancy-fatal foetal outcome after exposure to idarubicin during the second trimester. Acta Oncol 33:709–710
Rodeghiero F, Avvisati G, Castaman G, Barbui T, Mandelli F (1990) Early deaths and anti-hemorrhagic treatments in acute promyelocytic leukemia: a GIMEMA retrospective study in 268 consecutive patients. Blood 75:2112–2117
Rosa FW (1983) Teratogenicity of isotretinoin. Lancet 2:513
Sanz MA, Montesinos P (2010) Open issues on bleeding and thrombosis in acute promyelocytic leukemia. Thromb Res 125(Suppl 2):S51–S54
Sanz MA, Montesinos P (2014) How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood 123:2777–2782
Sanz MA, Lo-Coco F, Martín G, Avvisati G, Rayón C, Barbui T et al (2000) Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood 96:1247–1253
Sanz MA, Martin G, Gonzalez M, Leon A, Rayon C, Rivas C et al (2004a) Risk-adapted treatment of acute promyelocytic leukemia with alltrans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood 103:1237–1243
Sanz MA, Vellenga E, Rayón C, Díaz-Mediavilla J, Rivas C, Amutio E et al (2004b) All-trans retinoic acid and anthracycline monochemotherapy for the treatment of elderly patients with acute promyelocytic leukemia. Blood 104:3490–3493
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH et al (2009) Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 113:1875–1891
Sanz MA, Montesinos P, Casale MF, Díaz-Mediavilla J, Jiménez S, Fernández I et al (2015) Maternal and fetal outcomes in pregnant women with acute promyelocytic leukemia. Ann Hematol 94:1357–1361
Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T et al (2019) Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood 133(15):1630–1643
Schnittger S, Weisser M, Schoch C, Hiddemann W, Haferlach T, Kern W (2003) New score predicting for prognosis in PML-RARA+, AML1-ETO+, or CBFBMYH11+ acute myeloid leukemia based on quantification of fusion transcripts. Blood 102(8):2746–2755
Shigeno K, Naito K, Sahara N, Kobayashi M, Nakamura S, Fujisawa S et al (2005) Arsenic trioxide therapy in relapsed or refractory Japanese patients with acute promyelocytic leukemia: updated outcomes of the phase II study and postremission therapies. Int J Hematol 82:224–229
Siu BL, Alonzo MR, Vargo TA, Fenrich AL (2002) Transient dilated cardiomyopathy in a newborn exposed to idarubicin and all-trans-retinoic acid (ATRA) early in the second trimester of pregnancy. Int J Gynecol Cancer 12:399–402
Siu CW, Au WY, Yung C, Kumana CR, Lau CP, Kwong YL et al (2006) Effects of oral arsenic trioxide therapy on QT intervals in patients with acute promyelocytic leukemia: implications for long-term cardiac safety. Blood 108:103–106
Stentoft J, Nielsen JL, Hvidman LE (1994) All-trans retinoic acid in acute promyelocytic leukemia in late pregnancy. Leukemia 8:1585–1588
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al (2017) WHO classification of tumours of haematopoietic and lymphoid tissues, revised 4th edn. WHO Press, Geneva
Tallman MS (2007) Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol 20:57–65
Tallman MS, Kwaan HC (1992) Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood 79:543–553
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A et al (1997) All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 337:1021–1028
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Woods WG et al (2002) All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood 100:4298–4302
Terada Y, Shindo T, Endoh A, Watanabe M, Fukaya T, Yajima A (1997) Foetal arrhythmia during treatment of pregnancy-associated acute promyelocytic leukaemia with all-trans retinoic acid and favorable outcome. Leukemia 11:454–455
Unnikrishnan D, Dutcher JP, Garl S, Varshneya N, Lucariello R et al (2004) Cardiac monitoring of patients receiving arsenic trioxide therapy. Br J Haematol 124:610–617
Vahdat L, Maslak P, Miller WH Jr, Eardley A, Heller G, Scheinberg DA et al (1994) Early mortality and the retinoic acid syndrome in acute promyelocytic leukemia: impact of leukocytosis, low-dose chemotherapy, PMN/RAR-alpha isoform, and CD13 expression in patients treated with all-trans retinoic acid. Blood 84:3843–3849
Verma V, Giri S, Manandhar S, Pathak R, Bhatt VR (2016) Acute promyelocytic leukemia during pregnancy: a systematic analysis of outcome. Leuk Lymphoma 57:616–622
Warrell RP, Maslak P, Eardley A et al (1994) Treatment of acute promyelocytic leukemia with all-trans-retinoic acid—an update of the New-York experience. Leukemia 8:929–933
Williams SF, Schilsky RL (2000) Antineoplastic drugs administered during pregnancy. Semin Oncol 27:618–622
Yamakura M, Tsuda K, Ugai T, Sugihara H, Nisihida Y, Takeuchi M et al (2014) High frequency of varicella zoster virus reactivation associated with the use of arsenic trioxide in patients with acute promyelocytic leukemia. Acta Haematol 131(2):76–77
Yang D, Hladnik L (2009) Treatment of acute promyelocytic leukaemia during pregnancy. Pharmacotherapy 29(6):709–724
Zhang Y, Zhang Z, Li J, Li L, Han X, Han L et al (2013) Long-term efficacy and safety of arsenic trioxide for first-line treatment of elderly patients with newly diagnosed acute promyelocytic leukemia. Cancer 119:115–125
Zhu H, Hu J, Chen L, Zhou W, Li X, Wang L et al (2016) The 12-year follow-up of survival, chronic adverse effects, and retention of arsenic in patients with acute promyelocytic leukemia. Blood 128:1525–1528
Zver S, Andoljsek D, Cernelc P (2004) Effective treatment of life-threatening bleeding with recombinant activated factor VII in a patient with acute promyelocytic leukaemia. Eur J Haematol 72:455–456
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kayser, S., Platzbecker, U. (2021). Management of Acute Promyelocytic Leukemia. In: Röllig, C., Ossenkoppele, G.J. (eds) Acute Myeloid Leukemia . Hematologic Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-030-72676-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-72676-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72675-1
Online ISBN: 978-3-030-72676-8
eBook Packages: MedicineMedicine (R0)