Abstract
Acute promyelocytic leukemia (APL) is a rare form of childhood leukemia, but cure rates for pediatric APL have dramatically improved due to the availability of disease-specific (targeted) treatments. Traditional cytotoxic chemotherapy is now combined with all-trans retinoic acid (ATRA) and, more recently, arsenic trioxide (ATO) to result in relapse rates lower than other subtypes of acute myeloid leukemia. APL, however, is unique among the subtypes of pediatric leukemia for its increased risk of early death which may result from coagulopathy or differentiation syndrome. White blood cell count at diagnosis has been utilized to distinguish two risk groups (standard and high risk) on recent pediatric clinical trials to allow risk-adapted therapy. Current research efforts focus on the incorporation of ATO into treatment regimens and further defining risk factors for early death and relapse.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Acute promyelocytic leukemia (APL) is rare in children, but it is now one of the most curable forms of acute leukemia. Both children and adults with APL have shared in the great success of advances in treatment of this disease. While new cancer therapies in children frequently wait for safety demonstration in adult populations, current standard treatments for children with APL now include all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). These treatments have demonstrated tolerability and excellent activity in children. Further advances in treatment of children with APL will come from regimens proven in adult patients, and currently under investigation in pediatric patients, that remove or reduce traditional chemotherapy through repeated cycles of ATRA and ATO.
Epidemiology
While leukemia is the most common type of cancer in children, acute myeloid leukemia (AML) accounts for only 15% of childhood leukemia and acute promyelocytic leukemia accounts for approximately 10–15% of AML. Thus due to its rarity, larger cooperative group or multinational trials are required to study this disease. There does appear to be some variance in prevalence among different geographic areas. Some parts of Central and South America as well as Mediterranean countries have reported that APL comprises over 20% of AML cases in those regions [1,2,3]. It is unclear whether this is due to genetic factors among different ethnic groups or if environmental exposures play a role. In children, APL most often occur de novo without an identified predisposing cause. APL can more rarely occur as a secondary cancer following prior chemotherapy, and such cases have been reported in pediatric patients [4, 5].
Clinical Characteristics
Diagnosis
Children presenting with signs and symptoms concerning for leukemia are often first identified as potential cases of APL by the unique appearance of the blasts either in peripheral blood or bone marrow. APL most often has a characteristic morphology with hypergranular promyelocytes which may contain Auer rods. This comprises the M3 morphologic category in the French-American-British (FAB) classification of AML. It is important to note that non-APL AML cases may also have Auer rods, and thus this should not be used as the sole diagnostic criteria. There is also a microgranular variant of APL designated M3v which may be more difficult for clinicians to initially distinguish from other AML cases due to the lack of the more classic APL granules and Auer rods. In children with APL, the M3v microgranular variant accounts for a minority of cases (up to 30%) but occurs at higher rates than among adults with APL [6, 7]. Flow cytometry markers are useful in diagnosis, but confirmation of an APL diagnosis is made by cytogenetic analysis including fluorescence in situ hybridization (FISH) and chromosome analysis. The 2008 WHO classification of AML includes APL due to t(15;17) as a distinct diagnosis under the category of AML with recurrent genetic changes. Recent pediatric cooperative group studies have further required confirmation of PML-RARA transcript by reverse transcriptase PCR (RT-PCR). This carries the advantage of high specificity for PML-RARA including cryptic lesions (those not demonstrable by standard chromosome analysis) and establishes the breakpoint-specific transcript for monitoring for persistent MRD or for surveillance to detect relapse. While t(15;17) accounts for most cases of APL, there are rare variant fusion gene partners [8]. Due to their rarity, the prevalence of these variants has not been adequately described in a pediatric population.
CNS Disease
Due to coagulopathy risk, lumbar puncture at diagnosis is not standard in the initial evaluation of APL. Thus, the true incidence of CNS disease in pediatric APL is difficult to estimate. However, there have been evaluations of the incidence of CNS relapse in pediatric APL. A review of trials including children with standard-risk APL found isolated CNS relapse in less than 1% of patients [9]. This is similar to data from adult patients with APL such as the LPA96 and LPA99 trials conducted by the Spanish PETHEMA group which demonstrated a 1.1% 3-year incidence of CNS relapse [10].
Risk Grouping
WBC at initial diagnosis is the most validated risk marker in both adult and pediatric APL. Pediatric trials have used Sanz modified criteria to identify high-risk patients as those with WBC ≥ 10,000/μL. Patients with WBC < 10,000/μL are considered standard risk (including Sanz low and intermediate risk). The AIDA 0493 trial demonstrated that pediatric patients with high-risk disease had a significantly worse EFS compared to those with standard-risk disease [11]. In the Children’s Oncology Group (COG) Study AAML0631 and the European I-BFM study ICC-APL 01, patients with high-risk disease were treated with an extra cycle of consolidation compared to those with standard-risk disease. Early induction deaths due to differentiation syndrome and coagulopathy occur more frequently in patients with high-risk disease. The risk of relapse has also been higher in patients with high-risk disease, although recent data from the COG AAML0631 study suggests that with arsenic trioxide (ATO) consolidation, the risk of relapse is not significantly different between standard- and high-risk APL [personal communication, John Gregory, MD].
There are some studies suggesting that worse outcomes may be seen in other subsets of patients including those with M3v, certain immunophenotypes, or FLT3 mutant [6, 12]. These factors, however, are associated with higher WBC, and thus it has been unclear whether any of these represent independent risk criteria. Further, there is limited data on these risk factors specific to pediatric patients. A COG study did demonstrate an increased risk of early death in pediatric patients with APL who had mutations of the FLT3 gene [13].
The effect of age on APL outcomes within the pediatric population has been studied and there have been disparate results. Data from two consecutive trials conducted by the European APL group showed similar outcomes for children <13 years old compared to adolescents. Among children, however, those less than 5 years old had a higher risk of relapse compared to older children. For patients <5 years old (N = 12), the relapse rate was 52%, and for patients 5–12 years old (N = 16), the relapse rate was 17.6% [7]. In contrast, the COG analyzed pediatric patients treated on the North American Intergroup Trial CALGB 9710 and found no difference in outcomes between young children <5 years old (N = 16), older children 5–12 years old (N = 25), and adolescents 13–18 years old (N = 45) [14].
Disease Complications
Differentiation Syndrome
Differentiation syndrome is a constellation of symptoms including weight gain, pulmonary edema with respiratory distress, pleural and pericardial effusions, hypotension, and renal failure. This was initially described as “retinoic acid syndrome ” or “ATRA syndrome ” as it most often occurs following initiation of treatment with ATRA during induction therapy prior to achievement of remission [15]. Differentiation syndrome can occur without ATRA treatment due to effects of the APL blasts alone. Standard treatment includes use of steroids (most commonly dexamethasone) and holding doses of ATRA. Differentiation syndrome is associated with higher WBC including both high WBC at diagnosis and also high WBC (hyperleukocytosis) that develops due to the differentiating effect of ATRA and/or ATO treatment.
Children treated on the first North American Intergroup study (INT0129) were randomized to induction with ATRA 45 mg/m2/day divided BID (N = 27) or chemotherapy with daunorubicin and cytarabine (N = 26). Per protocol, differentiation syndrome was managed with dexamethasone and temporary cessation of ATRA. Differentiation syndrome occurred in 19% of children receiving ATRA, and one patient died of this toxicity during induction [16]. In the Italian GIMEMA-AIEOP AIDA 0493 trial, children with APL were treated in induction with ATRA 25 mg/m2/day divided BID and idarubicin. Differentiation syndrome was managed similar to INT0129 with dexamethasone and holding of ATRA. Among 107 patients, only three had differentiation syndrome, and no deaths were attributed to this toxicity [11]. It is difficult to know whether the lower rate of differentiation syndrome seen on AIDA0493 compared to INT0129 might be due to the lower ATRA dose, the use of idarubicin or other confounders including patient characteristics such as WBC.
Rates of differentiation syndrome in children with APL have been reported from several other multi-institutional studies (Table 14.1). Pediatric patients with APL from France, Belgium, Switzerland, Spain, and Germany treated on APL93 and APL 2000 all received ATRA at 45 mg/m2/day divided BID during induction and randomizations (assigned by initial WBC) included addition of daunorubicin with or without cytarabine. Differentiation syndrome was managed with dexamethasone. Differentiation syndrome occurred in 16% of 26 children and in 26.7% of 58 adolescents treated on these trials, and there were no deaths due to differentiation syndrome [7]. The second North American Intergroup study C9710 induction therapy included ATRA 45 mg/m2/day divided BID along with cytarabine and daunorubicin. Among 83 patients <18 years old treated on this study, the rate of differentiation syndrome was 37%, but there were no deaths due to differentiation syndrome. On the COG AAML0631 trial, patients received ATRA 25 mg/m2/day divided BID along with idarubicin. Differentiation syndrome was managed with dexamethasone. Among 101 patients, differentiation syndrome occurred in 20% of patients and resulted in two deaths (personal communication, John Gregory, MD).
All the studies above utilized dexamethasone to treat differentiation syndrome once symptoms arose. More recently, some studies including predominantly adult APL patients have utilized prophylactic treatment with steroids to prevent differentiation syndrome. There are multiple variations in these strategies including choice of steroid (oral prednisone, IV methylprednisolone, or dexamethasone), the duration of prophylactic treatment, and the target population (selected based on WBC versus all patients). Since death due to differentiation syndrome occurs in <5% of patients, it has been impossible to conclude from these studies whether steroid prophylaxis impacts death rate due to differentiation syndrome [17].
Coagulopathy
In comparison to other types of leukemia, APL is associated with a unique propensity toward coagulopathy. This complication is associated with fatal bleeding and thrombotic events that occur early in the course, either at presentation or during induction therapy. The risk of coagulopathy is directly correlated with WBC, and patients with high-risk APL (WBC ≥ 10,000 at diagnosis) more commonly experience this deadly complication. An analysis of pediatric patients treated on the North American intergroup trial C9710 demonstrated that presence of a FLT3 mutation was associated with increased risk of early death due to coagulopathy. Patients with elevated WBC and FLT3 mutation had a 47% induction death rate compared to no deaths among patients with elevated WBC and no FLT3 mutation [13]. Other clinical characteristics including laboratory results of prothrombin time, platelets, and D-dimer have been used in computation of bleeding risk based upon the International Society on Thrombosis and Haemostasis (ISTH) DIC scoring system, and a report on adult APL patients suggested that a score ≥ 6 was correlated with risk of fatal coagulopathy events [18]. An analysis of pediatric APL patients treated on the COG AAML0631 also demonstrated that a ISTH DIC score ≥ 6 was significantly correlated with risk of both fatal and significant but nonfatal bleeding and thrombotic events [19].
Supportive care recommendations on pediatric APL trials have included correction of abnormal prothrombin time (PT), abnormal partial thromboplastin time (PTT), thrombocytopenia, and hypofibrinogenemia with aggressive blood product support. Specific thresholds for platelet support have varied but most often include maintenance of platelets above 50,000 during the initial risk period of coagulopathy (1–2 weeks). The role of fibrinolytic therapy has not been well studied in pediatric patients, but data in adult patients does not support their routine use [20,21,22,23]. Recent studies of recombinant thrombomodulin (rTM) suggest this is a very promising new therapy for coagulopathy arising from various etiologies including APL [24, 25]. Use of rTM remains investigational, and it is currently only approved for use in Japan. Further there has only been a few case reports of pediatric patients receiving rTM for coagulopathy due to leukemia [26, 27].
Treatment
ATRA Plus Chemotherapy Regimens
Prior to the discovery of ATRA as an effective APL treatment, pediatric APL was treated similarly to other types of AML with chemotherapy including combination of cytarabine and anthracyclines. Combination therapy with ATRA and chemotherapy still required high cumulative doses of anthracyclines to achieve high cure rates for this subtype of AML. The Italian AIDA0493 regimen demonstrated long-term survival near 90%, but therapy included 80 mg/m2 of idarubicin and 50 mg/m2 of mitoxantrone which is approximately 600 mg/m2 daunorubicin equivalents (assuming a 5:1 conversion ratio for idarubicin:daunorubicin and a 4:1 conversion ratio for mitoxantrone:daunorubicin as used in the Children’s Oncology Group, Long-Term Follow-up Guidelines, Version 4.0, Oct 2013, www.survivorshipguidleins.org) [11]. On the LPA96/99 trials, the PETHEMA group used dose intensification of anthracycline plus ATRA (without any cytarabine) to achieve an overall survival of 87%, but this therapy used a very high cumulative anthracycline dose of 600–735 mg/m2 [28]. Treatment with anthracyclines places patients at significant risk for cardiac toxicity [29, 30]. The risk increases with higher cumulative doses (especially over 300 mg/m2 daunorubicin equivalents), and the risk is higher when children are exposed at a young age [31]. There were 26 children and 58 adolescents on the European APL93 and APL2000 trials in which the treatments included a total of 495 mg/m2 of daunorubicin. Three cases of severe cardiac toxicity occurred in these young patients including one patient with fatal heart failure while on treatment, one patient with heart failure occurring 6 years after therapy, and one patient with heart failure requiring heart transplant occurring after treatment for relapsed APL [7]. In the North American Intergroup C9710 trial, 56 children were treated with 400 mg/m2 of daunorubicin, and there were two deaths due to cardiac toxicity (personal communication, James Feusner, MD) [32].
High-dose cytarabine consolidation has been reported to reduce relapse risk in APL when studied in cohorts including predominantly adult patients [33, 34]. A direct comparison of treatment with and without high cytarabine has not been studied in a larger group of pediatric APL patients. The AIDA0493 trial and AML-BFM 93/98/2004 series both included high-dose cytarabine treatment of pediatric patients and reported 82–89% overall survival at 10 years. The CI relapse at 5 years was 14% on the AML-BFM series and 19% on the ADIA0493 trial. In contrast, the C9710 trial did not include high-dose cytarabine and reported a relapse risk of 36% at 5 years [32].
Following discovery of ATRA as an effective medication to induce APL remission, the efficacy of this targeted therapy was evaluated in children treated on the first North American Intergroup trial (INT0129). Patients randomized to receive ATRA during induction, maintenance, or both had a superior 5-year disease-free survival of 48% compared to 0% for patients not receiving ATRA [16]. In addition to reducing relapse risk, treatment with ATRA during induction has resulted in reduction of early deaths in children with APL [35]. The rates of induction death on pediatric APL trials including ATRA in induction range from 3–8% (Table 14.2) [7, 11, 28, 32, 36, 37]. An analysis of multiple pediatric hospitals in the United States including 163 pediatric patients presenting with APL and treated with ATRA found a 7.4% early death rate. Resource utilization during the first week of treatment included vasopressors, steroids, and diuretics used in approximately 11%, 40%, and 50% of patients, respectively. Pediatric APL patients required significantly more blood product support (platelets, fresh frozen plasma, and cryoprecipitate) compared to non-APL AML patients treated during the same period [38].
A particular side effect of ATRA called pseudotumor cerebri (PTC) involves increased intracranial pressure causing headache and blurry vision. Children and adolescents treated with ATRA have higher rates of PTC compared to adults. Decreased doses of ATRA, however, result in lower rates of PTC. Thus, a number of pediatric trials (including the most recent cooperative group trials of the COG and I-BFM) have now adopted the standard pediatric dose of ATRA as 25 mg/m2/day divided BID. The COG AAML0631 trial required sites to report detailed information on PTC during each course of therapy. With an ATRA dose of 25 mg/m2/day, the incidence of PTC was ≤6% during each cycle (personal communication, John Gregory, MD).
Arsenic Trioxide
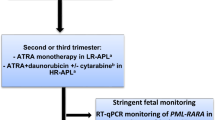
Arsenic trioxide (ATO) was initially used as a highly effective salvage therapy for relapsed APL. The North American Intergroup C9710 trial randomized patient’s ≥15 years old to therapy with or without two cycles (5 weeks each) of ATO consolidation. Adult patients receiving ATO had significantly improved outcomes [39]. Among adolescents 15–18 years old (N = 21), 12 patients received ATO with 92% EFS and 0% relapse, while 9 patients received standard consolidation without ATO with 56% EFS and 44% relapse risk (personal communication, Jim Feusner, MD). In the COG AAML0631 trial, children received 10 weeks of ATO (given as two courses of 5 weeks each) similar to the C9710 ATO consolidation cycles. This trial demonstrated excellent outcomes with 3-year EFS and OS >90% and a low relapse risk of 4%. The European ICC APL 01 included chemotherapy but without ATO consolidation and the 3-year EFS was lower at 83% due to increased relapses. Overall survival on AIDA 0493 (no ATO), ICC APL 01 (no ATO but reduced anthracycline), and AAML0631 (ATO consolidation and reduced anthracycline) all demonstrate that pediatric APL is a highly curable disease with even relapsed patients having a good salvage rate using ATO therapy, but high-risk APL patients do have a worse survival than standard-risk APL patients due to increased incidence of early death events (Fig. 14.1).
ATO has also been given as monotherapy for newly diagnosed APL in trials conducted in Iran and India. These studies, including both children and adults, reported 5-year OS rates of 64–74% [40, 41]. Combination therapy of ATO and ATRA has been more effective achieving excellent results for adult patients with standard-risk disease on the Italian-German APL0406 trial [42]. Based upon those results, trials for pediatric APL are currently active in the COG (AAML1331) and the I-BFM (ICC APL 02) which include an ATO/ATRA regimen with the addition of either idarubicin (COG) or gemtuzumab ozogamicin (I-BFM) during induction therapy for high-risk APL patients.
Similar to ATRA, ATO can induce differentiation of APL blasts, and thus differentiation and hyperleukocytosis may occur during ATO therapy. ATO is also known to prolong the QT interval with risk for cardiac dysrhythmia. In the COG AAML0631 trial, children received 10 weeks of ATO (given as two courses of 5 weeks each), and QT interval prolongation was monitored closely on the trial. Twenty-four patients had prolonged QT interval during their ATO cycles, but all were Grade 1 or 2, were transient, and did not require dose adjustment [43].
Maintenance Therapy
The Italian AIDA 0493 trial included four arms for maintenance randomization. Patients could receive no maintenance (observation arm), oral chemotherapy including mercaptopurine and methotrexate, ATRA alone, or a combination of both ATRA and oral chemotherapy. The first two arms (without ATRA) were closed early, but an analysis of the ATRA arms in pediatric patients showed a superior disease-free survival in the combination ATRA plus chemotherapy arm versus ATRA alone (77% vs. 42%, P = 0.018) [11]. An evaluation with long-term follow-up of adult patients treated on AIDA0493, however, showed no significant difference in survival between the four maintenance arms [44]. The North American Intergroup C9710 study began with a maintenance randomization to ATRA alone versus observation. Only three pediatric patients were randomized before the maintenance randomization was amended to ATRA alone versus ATRA plus oral chemotherapy with mercaptopurine and methotrexate. There was a nonsignificant trend toward lower EFS in the ATRA only maintenance compared to ATRA plus chemotherapy maintenance (41% vs. 72%, P = 0.12) (personal communication, James Feusner, MD).
The benefit of maintenance therapy for patients who receive ATO consolidation is uncertain. Among adult patients enrolled on C9710, there was no difference in survival for the initial randomization to observation versus ATRA, and patients who received ATO consolidation had similar survival in both arms of the amended randomization to ATRA alone versus ATRA plus oral chemotherapy maintenance [45, 46]. Adult patients with standard-risk APL treated with ATO/ATRA on APL0406 had excellent outcomes without maintenance therapy [42]. The current COG AAML1331 and I-BFM ICC APL 02 trials include ATO/ATRA regimens without maintenance therapy and will thus determine if ATO treatment can allow maintenance-free regimens in pediatric patients with APL.
Novel Therapies
APL blasts have robust expression of CD33 , and thus the anti-CD33 immunoconjugate gemtuzumab ozogamicin (GO) has been an agent of great interest in APL treatment. Following initial expedited approval by the FDA, this medication was later voluntarily withdrawn due to failure to achieve superiority in a confirmatory study. With these supply challenges, there has been limited experience with GO in pediatric APL. However, the current I-BFM ICC APL 02 is studying the efficacy of GO in conjunction with ATO/ATRA for treatment of children with high risk APL.
Disease Response
Reverse transcriptase PCR (RT-PCR) is a very sensitive assay to detect very low levels of residual PML-RARA transcript. Quantified RT-PCR (RQ-PCR) allows standardization to a housekeeping gene and ensures adequate RNA quality. Failure to enter molecular remission (persistent of PCR detectable PML-RARA transcripts) at the end of consolidation prior to maintenance therapy is correlated with high risk of relapse [47, 48]. In the COG study AAML0631, which included ATO consolidation, all patients tested PCR negative at end of consolidation (personal communication, John Gregory, MD). In the I-BFM ICC APL 01 trial (which did not include ATO consolidation) 4% of patients failed to enter molecular remission at end of consolidation, and these patients were eligible for treatment with ATO salvage therapy [37].
PCR monitoring is often employed during remission to monitor for disease recurrence. It is preferable to detect molecular relapse rather than awaiting an overt hematologic relapse in order to minimize the risk of coagulopathy or differentiation syndrome [49]. Molecular relapse as detected by PCR will invariably progress to hematologic relapse if left untreated [50, 51].
RQ-PCR testing should be performed on bone marrow samples every 3 months. With the assay’s sensitivity of 1 in 104 cells in bone marrow and the kinetics of relapse disease progression at approximately 1.1 log fold increase in RQ-PCR transcript per month, testing every 3 months generally allows detection before frank hematologic disease [52]. However, in pediatric patients, bone marrow evaluations are frequently done with sedation. Thus, risks of these sedated procedures must be balanced with the benefit of such monitoring particularly as patients are treated with ATO consolidation, and the relapse risk is expected to be very low.
Management of APL Relapse
Incidence of Relapse and Risk Factors
In pediatric patients with APL, despite the improvement in survival rate after modern combination regimens including ATRA and anthracycline-based first-line therapy, 17–27% of children have been reported to relapse [11, 7, 16, 34, 36, 37]. Retrospective studies involving both adults and children have clearly demonstrated that high WBC count (≥10 × 109/L) at diagnosis and persistence of PML/RARA positivity after first-line consolidation phase are associated with increased risk of disease recurrence [11, 53]. In adults, complex karyotype, expression of CD56 , and FLT3 mutation are other poor prognostic factors with ATRA and chemotherapy regimens [12, 54]. In APL, most relapses occur within 3 years from consolidation treatment, but rare very late relapses (>36 months from diagnosis) have also been described (incidence 4–6%). The majority of relapses occur in the bone marrow as hematological or molecular relapse. Hematological relapse is defined as the reappearance of >5% leukemic promyelocytes; molecular relapse is conventionally defined as two consecutive PML/RARA RT-PCR positive tests in marrow samples collected 2 weeks apart after previous negative results. Rarely, other sites such as CNS, the skin and the testis (extramedullary relapse) can be involved at disease recurrence [55, 56]. Interestingly, several reports in adults and children have suggested that the external auditory canal and the skin at sites of vascular access may be unique sites of extramedullary APL relapse and are mostly associated with concomitant bone marrow molecular relapse [57,58,59].
In APL, early identification of disease recurrence and preemptive therapy at the time of molecular relapse have clearly demonstrated benefits including better tolerated treatment, reduced hospitalization time, and decreased incidence of reinduction deaths [60,61,62]. Although MRD monitoring by sequential RT-PCR measurement of PML/RARA can predict overt relapse, some hematological relapses occur in patients, both adult and children, with previous PCR negativity. Recently, the quantitative RQ-PCR assays offer the possibility of measuring the kinetics of PML/RARA transcript and better monitoring for minimal residual disease (MRD) [63, 64]. This increases the opportunity to deliver early salvage treatment and consequently improve the outcome of patients who present with only MRD positive disease compared with those treated at the time of hematological relapse.
Reinduction Salvage Therapy
Current literature on the treatment of relapsed APL comes predominantly from adult studies and is mainly available for patients relapsing after ATRA and chemotherapy. During the past decades, the widely adopted strategy for the treatment of APL relapse included ATRA and cytotoxic chemotherapy as salvage reinduction therapy with autologous or allogeneic transplantation for consolidation after second or greater remission. These regimens applied in adults and only in small series of children induced high second remission rates (CR2) but had low cure rates. They were often associated with severe toxicity leading to fatal outcome or to a considerable rate of contraindications against subsequent stem cell transplantation [65, 66]. In particular, the high cumulative doses of anthracycline delivered in the front-line therapy could result in significant cardiac toxicity for which pediatric patients are at increased risk [30, 31]. Other drugs such as GO have also been successfully tested in relapsed APL. A number of preliminary reports have highlighted the sensitivity of APL to GO given alone or in combination with other agents. GO, as single agent, has demonstrated strong activity for the treatment of 16 patients with APL who had relapsed at the molecular level [67]. Quantitative RQ-PCR studies showed that responding patients experienced a dramatic decline of the PML/RARA transcript after the first GO dose. At high doses, GO can favor the occurrence of the hepatic sinusoidal obstructive syndrome (SOS); lower doses of GO have been employed in patients at high risk of complications, and comparable results have been reported with reduced treatment toxicity [68, 69].
Currently, the salvage strategy for relapsed APL includes the administration of ATO-based reinduction. The first experience with ATO in relapsed APL was reported by a group from China, and several subsequent studies, mainly in adults, demonstrated that approximately 80–90% of patients who relapse following initial ATRA and chemotherapy can achieve a CR2 with limited toxicity from ATO therapy [70]. Some reports suggest that remission duration is longer when ATO is combined with either ATRA or chemotherapy; a synergistic effect between ATO and ATRA accelerates the differentiation and apoptosis of abnormal promyelocytes. For this reason, the current adult guidelines support ATO ± ATRA as salvage therapy for relapsed APL. In 2008, a European registry of relapsed APL was established by the European LeukemiaNet; data of the outcome were available for 155 patients (141 adults and 14 children) treated, in first relapse, with ATO, after front-line therapy with ATRA and chemotherapy [59]. The results confirmed the efficacy of ATO in reinduction remission, with 91% CR2. The rate of molecular CR, after induction and consolidation therapy, did not differ significantly in patients with hematological or molecular relapse. However, induction deaths occurred only in patients with hematological relapse.
In pediatric APL patients, ATO-containing salvage therapy has been only sporadically described, but reports demonstrate that ATO as a single agent, or in combination with ATRA, can induce CR2 in 85% of relapse/refractory childhood APL and result in long-term molecular remission [71,72,73]. Based on reports in limited pediatric series and adult data, ATO has become the current preferred pediatric salvage treatment. In this age group, the formulation of oral ATO is particularly interesting; oral ATO in association with ATRA has demonstrated to be equally effective and better manageable [66]. Limited data are available on the efficacy of ATO treatment in relapsed patients who had prior ATO-containing therapy. These reports suggest that ATO-based reinduction regimens remain effective despite prior ATO therapy with a CR2 rate of approximately 80% [74,75,76].
Consolidation Salvage Therapy
Despite high remission rate with ATO ± ATRA in relapsed cases of APL, second or subsequent relapses are observed in a high proportion of these cases. Thus, post-remission treatment is very important to prolong remission and achieve a long-term cure. Hematopoietic stem cell transplant (HSCT) represents a widely adopted strategy as part of salvage therapy in relapsed APL. However, the optimal strategy for post-remission therapy remains controversial. Options for consolidation include repeated courses of ATO ± ATRA, conventional chemotherapy (with or without ATO), or HSCT. In retrospective studies, mostly performed during ATRA + chemotherapy era, autologous (auto)-HSCT showed a trend toward better outcomes compared with allogeneic (allo)-HSCT or consolidation chemotherapy [77, 78]. Allo-HSCT decreased the relapse risk, but this advantage was outweighed by higher treatment-related mortality compared with auto-HSCT and other treatments. However, the major limitation of these studies is their retrospective nature and missing data; in particular, the pre-transplant PML/RARA status was often lacking in these reports. More recently, the registry study of the European LeukemiaNet has clearly demonstrated the role of allogeneic HSCT as consolidation therapy for patients with relapse not achieving a molecular CR and suggested autologous HSCT as a suitable option for patients in molecular CR2 [59]. In this report, that included the largest cohort of APL patients in first relapse treated with ATO, the results of univariable and multivariable analyses demonstrated that first CR duration <18 months and persistent PCR positivity after consolidation are poor prognostic factors on overall survival (OS) and leukemia-free survival (LFS). Other studies have confirmed the unfavorable impact of first CR duration and failure to achieve molecular CR on OS after relapse. In the more recent studies including the administration of ATO-based reinduction, the long-term survival for those patients who received previous ATO-based regimens was inferior compared to those never treated with ATO (ATO-naïve) [74]. Prior ATO treatment has shown to be independently associated with worse relapse-free survival (RFS) [74].
Recommendations in Pediatric Age
In an attempt to develop therapy guidelines for children with relapsed APL, pediatric APL experts including members of the North American Children’s Oncology Group (COG) and the International Berlin-Frankfurt-Munster Study Group (I-BFM SG), have recently published treatment recommendations that are based upon informative literature and personal experience with relapsed APL [79]. Prognostic factors such as time to relapse <18 months from diagnosis, prior ATO therapy, and failure to achieve a second molecular remission were used to predict the risk of further relapse and consequently to guide the salvage treatment. In summary, ATO-naïve children with late (>18 months from diagnosis) or very late relapse (≥36 months) can be reinduced with ATO-ATRA plus GO followed by ATO consolidation without HSCT if they demonstrate molecular remission after four salvage cycles. ATO-naïve children with early relapse or children with prior ATO exposure and early or late relapse who demonstrate molecular remission after four cycles can be consolidated with auto-HSCT. In selected children who are ATO- and GO-naïve with early relapse who rapidly reach molecular remission after four cycles, or not suitable for auto-HSCT, consolidation with ATO-based therapy could represent a reasonable alternative. Children with primary/refractory APL, those with previous ATO exposure and early relapse, those with ≥ second relapse, or those with persistence of PML/RARA after four cycles should be considered for consolidation with allo-HSCT. Relapsed APL patients, however, are a heterogeneous population and these schemas may require modifications based on individual patient characteristics as well as the resources that are available to the treating physician [79].
Very Late Relapse
Only sporadic reports of patients with very late APL relapses (>36 months from diagnosis) have been published [80, 81]. Late relapse seems to occur in less than 5% of APL patients. In these cases, bone marrow involvement is frequently associated with relapse in extramedullary sites as well. Most of the patients present at late relapse with the same immunophenotypic, cytogenetic, and molecular pattern as at diagnosis suggest that the relapse is due to reemergence of the initial disease clone. There are no systematic trials evaluating treatment of late relapses in adults and children. Given the long period of disease-free survival (DFS) , drug resistance is unlikely in these patients. However, for patients who previously received intensive chemotherapy, the risk of cumulative toxicity must be weighed as a contraindication for the reemployment of these drugs in the salvage schemas. It has been reported that patients with late relapse can be salvaged with regimens similar to those used at initial diagnosis with CR2 achieved in a majority of patients [82]. As previously reported, in the recent years, ATO ± ATRA demonstrated high efficacy and low toxicity for the treatment of APL relapses; although very limited data are available on the use of ATO in very late relapse in children, the use of this agent should be considered. While the role of ATO in remission reinduction is now well established, the best consolidation therapy for patients with late relapses, is still controversial. The utility of transplant can be questioned for patients relapsing after very prolonged first CR since ATRA-ATO salvage alone might be curative. Though limited to a small number of patients, a prolonged molecular CR2 with ATO-based salvage therapy has been described in the literature. Eight out of nine Italian APL patients were salvaged with prolonged ATO-ATRA therapy without transplant procedures [83]; an 8-year-old boy with bone marrow relapse occurring at 7 years from initial diagnosis achieved a durable molecular CR2 with prolonged ATO as single treatment [72]. As previously reported, GO has also been demonstrated to be safe, tolerable, and particularly active in APL patients with molecular relapse. Thus, GO is an appealing therapeutic option for patients with very late relapse. A very late relapse occurring after more than 15 years of molecular remission has been recently described in a pediatric patient previously treated with ATRA + chemotherapy. In this case, molecular relapse was also associated with extramedullary involvement of the left mastoid [84]. The patient was rescued with an ATO-based protocol including GO without HSCT consolidation. Combinations of these new drugs, in repeated consolidation courses, together with PML/RARA quantitative monitoring may be used to avoid HSCT in patients with late relapse achieving a molecular CR2.
Extramedullary Relapse
Extramedullary relapse is an uncommon complication of APL occurring in about 3–5% of patients. Several factors that increase the risk of extramedullary relapse have been identified including WBC count at diagnosis (≥10 × 109/L), expression of CD56, bcr3 isoform, and FLT3 gene mutation. The most common site of extramedullary relapse is the CNS, and it is often accompanied by disease in the bone marrow [85]. The PETHEMA group has also identified elevated serum dehydrogenase (LDH) levels and previous CNS hemorrhage during induction as risk factors for subsequent CNS relapse [10]. The best management of such patients is still controversial. The European LeukemiaNet has recommended treating CNS relapse with intrathecal chemotherapy together with systemic therapy that should include drugs with high CNS penetrance [59]. In these patients, high-dose cytarabine has been used successfully. In patients who achieve CNS remission and molecular CR2, consolidation with auto- or allo-HSCT should be considered. It has also been shown that ATO and its metabolites are capable of crossing the blood-brain barrier and may be beneficial as a therapeutic agent for CNS disease. However, the concentration of ATO in cerebral spinal fluid (CSF) is probably not adequate to treat meningeal leukemia alone, and further studies are necessary to identify the exact role of ATO treatment in patients with CNS relapse [86]. APL extramedullary relapse can involve other sites such as the skin, the testis, or the external auditory canal. Infiltration of the ear is exceedingly infrequent in other types of leukemia, and the anatomical and biological reasons underlying this particular APL localization are unknown. Some authors suggested a role of ATRA during initial therapy as a predisposing factor. ATRA has been shown to influence the expression of adhesion molecules on leukemic cells; this could explain the pathophysiology of extramedullary involvement in APL patients treated with this agent. However, APL relapse in the ear was also observed before the advent of ATRA. Patients with extramedullary relapse frequently also have bone marrow molecular recurrence.
ATO accumulates well in epidermal tissue, and thus it could represent a therapeutic choice in cutaneous relapses. In patients with external auditory canal relapse, ATO ± ATRA demonstrated high efficacy and low toxicity [86]. These observations suggest that ATO is reasonable as single agent or in combination with ATRA, for the treatment of non-CNS extramedullary relapse. Local radiotherapy has also been used in extramedullary relapse with mixed results [87, 88]. The optimal therapeutic approach for these patients is still unknown especially for those with isolated and/or very late extramedullary relapse. Management of these patients should be individualized.
References
Gomez SM, Schuttenberg V, Armendariz H, Alba L, Martinez M, Fynn A, et al. Childhood acute leukemia: a single institution experience in La Plata, Argentina. Med Pediatr Oncol. 2001;36(3):383–5.
Malta Corea A, Pacheco Espinoza C, Cantu Rajnoldi A, Conter V, Lietti G, Masera G, et al. Childhood acute promyelocytic leukemia in Nicaragua. Ann Oncol. 1993;4(10):892–4.
Maule MM, Dama E, Mosso ML, Magnani C, Pastore G, Merletti F, et al. High incidence of acute promyelocytic leukemia in children in northwest Italy, 1980–2003: a report from the Childhood Cancer Registry of Piedmont. Leukemia. 2008;22(2):439–41.
Ogami A, Morimoto A, Hibi S, Todo S, Sugimoto T, Mori K, et al. Secondary acute promyelocytic leukemia following chemotherapy for non-Hodgkin’s lymphoma in a child. J Pediatr Hematol Oncol. 2004;26(7):427–30.
Lopez-Andrew JA, Ferris J, Verdeguer A, Esquembre C, Senent ML, Castel V. Secondary acute promyelocytic leukemia in a child treated with epipodophyllotoxins. Am J Pediatr Hematol Oncol. 1994;16(4):384–6.
Guglielmi C, Martelli MP, Diverio D, Fenu S, Vegna ML, Cantu-Rajnoldi A, et al. Immunophenotype of adult and childhood acute promyelocytic leukaemia: correlation with morphology, type of PML gene breakpoint and clinical outcome. A cooperative Italian study on 196 cases. Br J Haematol. 1998;102(4):1035–41.
Bally C, Fadlallah J, Leverger G, Bertrand Y, Robert A, Baruchel A, et al. Outcome of acute promyelocytic leukemia (APL) in children and adolescents: an analysis in two consecutive trials of the European APL Group. J Clin Oncol. 2012;30(14):1641–6.
Grimwade D, Biondi A, Mozziconacci MJ, Hagemeijer A, Berger R, Neat M, et al. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): results of the European Working Party. Groupe Francais de Cytogenetique Hematologique, Groupe de Francais d’Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action “Molecular Cytogenetic Diagnosis in Haematological Malignancies”. Blood. 2000;96(4):1297–308.
Chow J, Feusner J. Isolated central nervous system recurrence of acute promyelocytic leukemia in children. Pediatr Blood Cancer. 2009;52(1):11–3.
Montesinos P, Diaz-Mediavilla J, Deben G, Prates V, Tormo M, Rubio V, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica. 2009;94(9):1242–9.
Testi AM, Biondi A, Lo Coco F, Moleti ML, Giona F, Vignetti M, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106(2):447–53.
Montesinos P, Rayon C, Vellenga E, Brunet S, Gonzalez J, Gonzalez M, et al. Clinical significance of CD56 expression in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline-based regimens. Blood. 2011;117(6):1799–805.
Kutny MA, Moser BK, Laumann K, Feusner JH, Gamis A, Gregory J, et al. FLT3 mutation status is a predictor of early death in pediatric acute promyelocytic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59(4):662–7.
Kutny MA, Geyer S, Laumann KM, Gregory J, Willman CL, Stock W, et al. Effect of young age on outcomes in pediatric acute promyelocytic leukemia (APL): North American Intergroup Study CALGB 9710 (Alliance). Blood. 2014;124(21):2301.
Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP Jr. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117(4):292–6.
Gregory J, Kim H, Alonzo T, Gerbing R, Woods W, Weinstein H, et al. Treatment of children with acute promyelocytic leukemia: results of the first North American Intergroup trial INT0129. Pediatr Blood Cancer. 2009;53(6):1005–10.
Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123(18):2777–82.
Mitrovic M, Suvajdzic N, Bogdanovic A, Kurtovic NK, Sretenovic A, Elezovic I, et al. International Society of Thrombosis and Hemostasis Scoring System for disseminated intravascular coagulation >/= 6: a new predictor of hemorrhagic early death in acute promyelocytic leukemia. Med Oncol. 2013;30(1):478.
Kutny MA, Rajpurkar M, Alonzo TA, Gerbing RB, Gamis AS, Feusner JH, et al. Evaluation of ISTH DIC score to predict significant bleeding and thrombosis events in pediatric acute promyelocytic leukemia; a report from the Children’s Oncology Group AAML0631 Trial. Blood. 2014;124(21):3669.
Rodeghiero F, Avvisati G, Castaman G, Barbui T, Mandelli F. Early deaths and anti-hemorrhagic treatments in acute promyelocytic leukemia. A GIMEMA retrospective study in 268 consecutive patients. Blood. 1990;75(11):2112–7.
Hashimoto S, Koike T, Tatewaki W, Seki Y, Sato N, Azegami T, et al. Fatal thromboembolism in acute promyelocytic leukemia during all-trans retinoic acid therapy combined with antifibrinolytic therapy for prophylaxis of hemorrhage. Leukemia. 1994;8(7):1113–5.
Sanz MA, Martin G, Gonzalez M, Leon A, Rayon C, Rivas C, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–43.
Brown JE, Olujohungbe A, Chang J, Ryder WD, Morganstern GR, Chopra R, et al. All-trans retinoic acid (ATRA) and tranexamic acid: a potentially fatal combination in acute promyelocytic leukaemia. Br J Haematol. 2000;110(4):1010–2.
Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5(1):31–41.
Kawano N, Kuriyama T, Yoshida S, Yamashita K, Ochiai H, Nakazaki S, et al. Clinical features and treatment outcomes of six patients with disseminated intravascular coagulation resulting from acute promyelocytic leukemia and treated with recombinant human soluble thrombomodulin at a single institution. Intern Med. 2013;52(1):55–62.
Ogawa E, Yagasaki H, Kato M, Shichino H, Chin M, Mugishima H. Successful treatment of disseminated intravascular coagulation in a child with acute myelogenous leukaemia using recombinant thrombomodulin. Br J Haematol. 2010;149(6):911–2.
Saito A, Okamoto Y, Seki Y, Matsunaga M, Nakagawa S, Kodama Y, et al. DIC Complicating APL Successfully Treated With Recombinant Thrombomodulin Alfa. J Pediatr Hematol Oncol. 2016;38(6):e189–90.
Ortega JJ, Madero L, Martin G, Verdeguer A, Garcia P, Parody R, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol. 2005;23(30):7632–40.
Aldouri MA, Lopes ME, Yacoub M, Mitchell AG, Fox K, Evans TR, et al. Cardiac transplantation for doxorubicin-induced cardiomyopathy in acute myeloid leukaemia. Br J Haematol. 1990;74(4):541.
Thomas X, Le QH, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol. 2002;81(9):504–7.
Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121(2):e387–96.
Feusner JG, Gregory J, Moser B, Hars V, Willman C, Powell B, Larson R. Dose-intensified daunorubicin induction and consolidation plus combined modality maintenance therapy for children with newly diagnosed acute promyelocytic leukemia (APL): North American Intergroup Study C9710. J Clin Oncol. 2010;28(15s):abstr 9510.
Ades L, Chevret S, Raffoux E, de Botton S, Guerci A, Pigneux A, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24(36):5703–10.
Sanz MA, Montesinos P, Rayon C, Holowiecka A, de la Serna J, Milone G, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–46.
Mann G, Reinhardt D, Ritter J, Hermann J, Schmitt K, Gadner H, et al. Treatment with all-trans retinoic acid in acute promyelocytic leukemia reduces early deaths in children. Ann Hematol. 2001;80(7):417–22.
Creutzig U, Zimmermann M, Dworzak M, Urban C, Henze G, Kremens B, et al. Favourable outcome of patients with childhood acute promyelocytic leukaemia after treatment with reduced cumulative anthracycline doses. Br J Haematol. 2010;149(3):399–409.
Testi AM, Pession A, Grimwade D, Diverio D, Ragu C, Elitzur S, et al. Risk-group stratified and minimal residual disease (MRD)-guided treatment with extended ATRA and reduced-anthracycline chemotherapy in childhood acute promyelocytic leukemia (APL): results from ICC APL Study 01. Blood. 2015;126(23):563.
Fisher BT, Singh S, Huang YS, Li Y, Gregory J, Walker D, et al. Induction mortality, ATRA administration, and resource utilization in a nationally representative cohort of children with acute promyelocytic leukemia in the United States from 1999 to 2009. Pediatr Blood Cancer. 2014;61(1):68–73.
Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–7.
Mathews V, George B, Chendamarai E, Lakshmi KM, Desire S, Balasubramanian P, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28(24):3866–71.
Ghavamzadeh A, Alimoghaddam K, Rostami S, Ghaffari SH, Jahani M, Iravani M, et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29(20):2753–7.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21.
Kutny MA, Alonzo TA, Gerbing RB, Wang Y-C, Fu C, Meshinchi S, et al. Arsenic trioxide is a well tolerated consolidation regimen in children with newly diagnosed acute promyelocytic leukemia; toxicity results of the Children’s Oncology Group AAML0631 Trial. Blood. 2014;124(21):985.
Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117(18):4716–25.
Powell BL, Moser BK, Stock W, Gallagher RE, Willman CL, Stone RM, et al. Adding mercaptopurine and methotrexate to alternate week ATRA maintenance therapy does not improve the outcome for adults with acute promyelocytic leukemia (APL) in first remission: results from North American Leukemia Intergroup Trial C9710. ASH Annual Meeting Abstracts. Blood. 2011;118(21):258.
Coutre SE, Othus M, Powell B, Willman CL, Stock W, Paietta E, et al. Arsenic trioxide during consolidation for patients with previously untreated low/intermediate risk acute promyelocytic leukaemia may eliminate the need for maintenance therapy. Br J Haematol. 2014;165(4):497–503.
Grimwade D, Lo Coco F. Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia. 2002;16(10):1959–73.
Lo Coco F, Diverio D, Falini B, Biondi A, Nervi C, Pelicci PG. Genetic diagnosis and molecular monitoring in the management of acute promyelocytic leukemia. Blood. 1999;94(1):12–22.
Esteve J, Escoda L, Martin G, Rubio V, Diaz-Mediavilla J, Gonzalez M, et al. Outcome of patients with acute promyelocytic leukemia failing to front-line treatment with all-trans retinoic acid and anthracycline-based chemotherapy (PETHEMA protocols LPA96 and LPA99): benefit of an early intervention. Leukemia. 2007;21(3):446–52.
Breccia M, Diverio D, Noguera NI, Visani G, Santoro A, Locatelli F, et al. Clinico-biological features and outcome of acute promyelocytic leukemia patients with persistent polymerase chain reaction-detectable disease after the AIDA front-line induction and consolidation therapy. Haematologica. 2004;89(1):29–33.
Diverio D, Rossi V, Avvisati G, De Santis S, Pistilli A, Pane F, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter “AIDA” trial. GIMEMA-AIEOP Multicenter “AIDA” Trial. Blood. 1998;92(3):784–9.
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27(22):3650–8.
Tallman M, Douer D, Gore S, Powell BL, Ravandi F, Rowe J, et al. Treatment of patients with acute promyelocytic leukemia: a consensus statement on risk-adapted approaches to therapy. Clin Lymphoma Myeloma Leuk. 2010;10(Suppl 3):S122–6.
Cicconi L, Divona M, Ciardi C, Ottone T, Ferrantini A, Lavorgna S, et al. PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30(10):1987–92.
Kaspers G, Gibson B, Grimwade D, pession A, Smith O, Testi AM. Central nervous system involvement in relapsed acute promyelocytic leukemia. Pediatr Blood Cancer. 2009;53(2):235–6.
Vega-Ruiz A, Faderl S, Estrov Z, Pierce S, Cortes J, Kantarjian H, Ravandi F. Incidence of extramedullary disease in patients with acute promyelocytic leukemia: a single-institution experience. Int J Hematol. 2009;89(4):489–96.
Albano F, Specchia G. Extramedullary disease in acute promyelocytic leukemia: two-in-one disease. Mediterr J Hematol Infect Dis. 2011;3(1).
Igarashi K, Hori T, Yamamoto M, Inazawa N, Noguchi H, Suzuki N. Extramedullary relapse in RARA rearrangement-negative acute promyelocytic leukemiasuccessfully treated in combination with chemotherapy, local radiotherapy, and cord blood transplantation. J Pediatr Hematol Oncol. 2015;37(4).
Lengfelder E, Lo-Coco F, Ades L, Montesinos P, Grimwade D, Kishore B, et al. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet. Leukemia. 2015;29(5):1084–91.
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, et al. Therapy of molecular relapse in acute promyelocytic leukemia. Blood. 1999;94(7):2225–9.
Mandelli F, Avvisati G, Lo Coco F. Advances in the understanding and management of acute promyelocytic leukemia. Rev Clin Exp Hematol. 2002;6(1):60–71.
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91.
De Angelis F, Breccia M. Molecular monitoring as a path to cure acute promyelocytic leukemia. Rare Cancers Ther. 2015;3:119–32.
Zhang L, Cao Z, Zou Y, Ruan M, Li Q, Wang J, Zhu X. Quantification of PML/RARa transcript after induction predicts outcome in children with acute promyelocytic leukemia. Int J Hematol. 2012;95(5):500–8.
Thomas X, Dombret H, Cordonnier C, Pigneux A, Gardin C, Guerci A, et al. Treatment of relapsing acute promyelocytic leukemia by all-trans retinoic acid therapy followed by timed sequential chemotherapy and stem cell transplantation. Leukemia. 2000;14:1006–13.
Castagnola C, Lunghi M, Corso A, Tajana M, Zappasodi P, Dabusti M, et al. Management of acute promyelocytic leukemia relapse in the ATRA era. Haematologica. 1998;83:714–7.
Lo Coco F, Cimino G, Breccia M, Noguera NI, Diverio D, Finolezzi E, et al. Gentuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood. 2004;104(7):1995–9.
Lo Coco F, Latagliata R, Breccia M. Management of acute promyelocytic leukemia in the elderly. Mediterr J Hematol Infect Dis. 2013;5(1).
Aplenc R, Alonzo TA, Gerbing RB, Lange BJ, Hurwitz CA, Wells RJ, et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:2390–3295.
Yanada M, Tsuzuki M, Fujita H, Fujimaki K, Fujisawa S, Sunami K, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood. 2013;121(16):3095–102.
Fox E, Razzouk BI, Widemann BC, Xiao S, O’Brien M, Goodspeed W, et al. Phase I trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008;111:566–73.
Ebinger M, Schwarze CP, Feuchtinger T, Scheel-Walter HG, Lang P, Hildenbrand S, et al. Long-term remission after first-line single-agent treatment with arsenic trioxide of relapsed acute promyelocytic leukemia in an 8-year-old boy. Pediatr Hematol Oncol. 2011;28:334–7.
Au WY, Li CK, Lee V, Yuen HL, Yau J, Chan GC, et al. Oral arsenic trioxide for relapsed acute promyelocytic leukemia in pediatric patients. Pediatr Blood Cancer. 2012;58(4):630–2.
Lou Y, Suo S, Tong Y, Tong H, Qian W, Meng H, et al. Outcomes and prognostic factors of first relapsed acute promyelocytic leukemia patients undergoing salvage therapy with intravenous arsenic trioxide and chemotherapy. Ann Hematol. 2014;93(6):941–8.
Thirugnanam R, George B, Chendamarai E, Lakshmi KM, Balasubramanian P, Viswabandya A, et al. Comparison of clinical outcomes of patients with relapsed acute promyelocytic leukemia induced with arsenic trioxide and consolidated with either an autologous stem cell transplant or an arsenic trioxide-based regimen. Biol Blood Marrow Transplant. 2009;15(11):1479–84.
Au WY, Chim CS, Lie AK, Liang R, Kwong YL. Combined arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukemia recurring from previous relapses successfully treated using arsenic trioxide. Br J Haematol. 2002;117(1):130–2.
Ganzel C, Mathews V, Alimoghaddam K, Ghavamzadeh A, Kuk D, Devlin S, et al. Autologous transplant remains the preferred therapy for relapsed APL in CR2. Bone Marrow Transplant. 2016;51(9):1180–3.
De Botton S, Fawaz A, Chevret S, Dombret H, Thomas X, Sanz M, et al. Autologous and allogeneic stem-cell transplantation as salvage treatment of acute promyelocytic leukemia initially treated with all-trans-retinoic acid: a retrospective analysis of the European acute promyelocytic leukemia group. J Clin Oncol. 2005;23:120–6.
Abla O, Kutny MA, Testi AM, Feusner JH, Creutzig U, Gregory J Jr, et al. Management of relapsed and refractory childhood acute promyelocytic leukemia: recommendations from an international expert panel. Br J Haematol. 2016;175:588–601.
Au WY, Chan GC, Chim CS, Shek TW, Ooi GC, Ho WK, et al. Unusual sites of involvement by hematologic malignancies. Case 3. External auditory canal tumor: a rare chloroma in acute promyelocytic leukemia with a complete response to arsenic trioxide. J Clin Oncol. 2001;19:3993–5.
Cicconi L, Divona M, Ciardi C, Ottone T, Ferrantini A, Lavorgna S, et al. PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30(10):1987–92.
Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL group experience. Blood. 2010;115:1690–6.
Breccia M, Cicconi L, Minotti C, Latagliata R, Gianni L, Lo Coco F. Efficacy of prolonged therapy with combined arsenic trioxide and ATRA for relapse of acute promyelocytic leukemia. Haematologica. 2011;96:1390–1.
Testi AM, Moleti ML, Canichella M, Mohamed S, Diverio D, De Propris MS, et al. Very late relapse in a patient with acute promyelocytic leukemia (APL) rescued with a chemotherapy-free protocol. Leuk Lymphoma. 2016;23:1–3. [Epub ahead of print].
Sahin DG, Gunduz E, Akay OM, Gulbas Z. Central nervous system relapse in a patient with acute promyelocytic leukaemia: does the risk stratification matter? BMJ Case Rep. 2013;6:2013.
Au WY, Tam S, Fong BM, Kwong YL. Determinants of cerebrospinal fluid arsenic concentration in patients with acute promyelocytic leukemia on oral arsenic trioxide therapy. Blood. 2008;112(9):3587–90.
He Z, Tao S, Deng Y, Chen Y, Song L, Ding B, et al. Extramedullary relapse in lumbar spine of patient with acute promyelocytic leukemia after remission for 16 years: a case report and literature review. Int J Clin Exp Med. 2015;8(12):22430–4.
Kai T, Kimura H, Shiga Y, Ogawa K, Sato H, Maruyama Y, et al. Recurrent extramedullary relapse of acute promyelocytic leukemia after allogeneic stem cell transplantation: successful treatment by arsenic trioxide in combination with local radiotherapy. Int J Hematol. 2006;83(4):337–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kutny, M.A., Testi, A.M. (2018). APL in Children. In: Abla, O., Lo Coco, F., Sanz, M. (eds) Acute Promyelocytic Leukemia . Springer, Cham. https://doi.org/10.1007/978-3-319-64257-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-64257-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-64256-7
Online ISBN: 978-3-319-64257-4
eBook Packages: MedicineMedicine (R0)