Abstract
The evolving treatment of acute promyelocytic leukemia (APL) represents one of the greatest success stories in modern medicine. Detailed understanding of the molecular pathogenesis of APL has provided a mechanistic basis for the successful clinical use of all-trans retinoic acid (ATRA) as well as arsenic trioxide (ATO) in targeting the PML/RARa fusion protein, which is pivotal in the pathogenesis of the disease. Although the advent of molecularly targeted therapy with ATRA and ATO has revolutionized the treatment of APL, the addition of conventional cytotoxic chemotherapy including anthracyclines still plays an important role, particularly in high-risk APL (white blood cell count >10,000/μL). For patients with high-risk APL who are able to tolerate cytotoxic chemotherapy, regimens used for treatment induction and consolidation include anthracyclines such as daunorubicin or idarubicin and in some treatment regimens also the pyrimidine analog cytarabine. In order to achieve the highest rates of cure, these chemotherapy agents are used in combination with ATRA ± ATO in high-risk APL. This chapter will focus on the role of first-line chemotherapy agents in combination with ATRA for APL. While clinicians may be familiar with the routine administration of conventional chemotherapy, vigilant attention must also be paid to prevent early death from specific complications in APL patients, including coagulopathy and differentiation syndrome.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- ATRA
- Anthracycline-based therapy
- Cytarabine
- Bone marrow evaluation
- Consolidation regimens
- Maintenance therapy
- Prophylactic intrathecal chemotherapy
- CNS relapse
- Thrombocytopenia
- Differentiation syndrome
- Prophylactic steroids
Introduction
The Role of Early ATRA
Patients with APL usually present with cytopenias with or without leukocytosis. Life-threatening coagulopathy also serves as one of the most concerning presentations of APL. In a patient with suspected acute leukemia, the presence of coagulopathy should prompt rapid evaluation of the peripheral smear for the possibility of APL. Even as this chapter focuses on chemotherapy, the paramount role of ATRA in APL must always be emphasized. Should APL be suspected, early treatment with ATRA must be initiated as soon as possible to induce APL blast differentiation and reverse or avert the development of the life-threatening coagulopathy. More than resistant disease, early death almost always from hemorrhage now represents the most important limitation to cure in APL [1,2,3,4]. Clinicians should not wait for the diagnostic confirmation of APL to initiate ATRA, as prompt administration of ATRA is likely critical to reduce the rate of early death [5].
Risk Stratification by White Blood Cell Count (WBC): Chemotherapy as a Component of Therapy for High-Risk APL
At diagnosis, patients with APL can be risk stratified for relapse into high-risk and low-risk disease, based on the presenting white blood cell count (WBC) [6,7,8]. Patients with high WBC (>10,000/μL) are considered to have high-risk disease, while patients with lower WBC (<10,000/μL) are considered to have low-risk disease. APL patients were previously risk stratified by WBC as well as platelet count into high-, intermediate-, and low-risk disease [7]. However, more recent data suggest that outcomes are similar in low- and intermediate-risk groups with contemporary therapies, thereby eliminating platelet count from risk stratification and enabling APL patients to be risk stratified only by WBC into high- and low-risk groups [8] (Fig. 8.1). Beyond blood counts, other factors including age greater than 60 years, male sex, and renal insufficiency with creatinine greater than 1.4 have been shown to be predictive of poor prognosis, largely due to death during induction [6,7,8]. Although data are mixed, some studies also suggest that APL patients with internal tandem duplication (ITD) of the FMS-like tyrosine kinase 3 gene (FLT3 -ITD) may have an inferior prognosis, particularly patients treated with combined ATRA + idarubicin (AIDA) regimens [9,10,11]. Emerging data suggest this negative prognostic impact of FLT3-ITD in APL may be abrogated by the combined use of ATRA plus ATO [12]. However, at this time, despite the various prognostic factors noted above, only the presenting WBC is used to select the optimal choice of therapy.
Relapse-free survival by risk stratification in GIMEMA and PETHEMA trials. Kaplan-Meier product-limit estimate of relapse-free survival in the GIMEMA and PETHEMA trials according to risk groups defined by the predictive model. Figure is adapted from Sanz MA., et al., Blood 2000 [7]
The management of low-risk APL patients (WBC < 10,000/mcL) which accounts for approximately 75% of patients is addressed in other chapters. Notably, for patients with low-risk APL, recent studies have shown at least equivalent and apparently superior outcomes for ATRA plus ATO vs. ATRA plus chemotherapy [13, 14]. Therefore, in the modern management of APL, chemotherapy is generally not a component of standard therapy for low-risk disease. As this chapter highlights the role of first-line chemotherapy in APL, the remaining discussion focuses predominantly on patients with high-risk APL who would warrant combined chemotherapy + ATRA. Chemotherapy + ATRA also remains an option for low-risk patients unable to tolerate ATO. A detailed discussion of ATO-containing regimens will be provided in Chap. 9.
Tolerance of Anthracycline-Based Therapy
For patients with high-risk APL already started on treatment with ATRA, one of the first decision points is whether the patient can tolerate anthracycline-based chemotherapy. Given the potential cardiotoxicity of anthracyclines, cardiac evaluation with an echocardiogram or multiple-gated acquisition (MUGA) scan should be considered prior to anthracycline-based chemotherapy [8]. Delayed cardiomyopathy is a rare consequence of anthracycline-related toxicity in long-term disease-free survivors of APL [15]. Risk factors for anthracycline cardiotoxicity include cumulative anthracycline dose, rate of anthracycline administration, age, obesity, sex (with females at greater risk), and pre-existing cardiac risk factors [16].
Evolution of Chemotherapy and ATRA Regimens for APL
The current standard of care for newly diagnosed patients with high-risk APL remains ATRA- and anthracycline-based chemotherapy with or without ATO [17]. Prior to the introduction of ATRA, APL was treated with standard AML induction chemotherapy including anthracycline- and cytarabine-based chemotherapy. Anthracyclines have excellent activity as single agents in APL [18, 19]. One possible explanation for the high sensitivity of APL cells to anthracyclines involves reduced expression of the multidrug resistance (MDR1) gene product P-glycoprotein in APL in comparison to other leukemias [20, 21]. Even without the inclusion of modern therapies such as ATRA and arsenic, the majority of APL patients treated with anthracycline-based chemotherapy achieved complete remission (CR), with remission rates of 70–80% [22, 23]. However, with chemotherapy alone, early death from coagulopathy as well as relapse remained significant clinical barriers to cure for most APL patients [22,23,24]. Chemotherapy alone is also unlikely to lead to long-term cure in the absence of additional consolidation or maintenance therapy. In the North American Intergroup study I0129 (ECOG E2491), 5-year disease-free survival was 16% for APL patients randomized to induction and consolidation chemotherapy followed by observation without ATRA [25].
Therefore, the introduction of ATRA provided a powerful new tool in the treatment armamentarium for APL. ATRA targets the PML/RARa fusion protein, inducing differentiation of leukemic promyelocytes into mature cells [26,27,28]. During the 1980s and 1990s, single-agent ATRA was shown to have remarkable activity, with CR rates of 85% in APL [29].
The European APL91 trial and the North American Intergroup study I0129 (ECOG E2491) established that APL patients treated with ATRA had improved outcomes over those treated with chemotherapy alone [25, 30,31,32]. In the European APL91 trial, patients with newly diagnosed APL randomized to ATRA followed by chemotherapy had an improved survival and reduced relapse rate compared to patients randomized to chemotherapy alone [30, 31]. In the larger North American Intergroup study, single-agent ATRA was compared with daunorubicin and cytarabine in 401 patients with previously untreated APL. In this study, single-agent ATRA was shown to have equivalent rates of CR (70%) as induction chemotherapy with daunorubicin and cytarabine (73%) but markedly improved disease-free (69% vs. 29%) and overall survival (69% vs. 45%) at 5 years [25, 32]. These studies provided justification for the standard inclusion of ATRA in the treatment of APL. However, resistance and relapse are not uncommon for patients treated with single-agent ATRA, particularly if ATRA is only given as induction. The North American Intergroup study demonstrated a durable benefit for ATRA in both induction and maintenance therapy, as patients randomized to ATRA for both induction and maintenance had a 5-year DFS of 74%, in comparison to 55% for patients who received ATRA followed by observation. However, 35% of patients with high-risk APL failed to achieve a CR with ATRA alone, suggesting that there may be a role for additional chemotherapy for these high-risk patients [25]. In addition, differentiation syndrome remains a problem with ATRA monotherapy [33]. Multiple studies therefore tested combination therapies including ATRA and concurrent or sequential chemotherapy.
ATRA + Chemotherapy
Several large cooperative group studies demonstrated excellent outcomes with ATRA-based induction in combination with anthracycline-based chemotherapy, with greater than 90% of patients achieving CR [34, 35]. Although some of these patients relapsed with induction therapy alone, cure rates were increased to greater than 80% with the use of ATRA-based induction followed by consolidation with ATRA + anthracycline or cytarabine + anthracycline [34,35,36].
The European APL 93 trial demonstrated the superiority of concurrent ATRA + chemotherapy over sequential ATRA followed by chemotherapy. Chemotherapy in this study consisted of daunorubicin 60 mg/m2/day for 3 days and cytarabine 200 mg/m2/day for 7 days, starting on day 3 of ATRA for the concurrent group or after ATRA-induced CR for the sequential group. Chemotherapy was also added early to ATRA for increases in WBC. In this study of 413 newly diagnosed APL patients, the relapse rate at 2 years was 6% in the concurrent ATRA + chemotherapy group vs. 16% in the sequential ATRA followed by chemotherapy group [37].
The Italian GIMEMA 93 trial established the efficacy of the AIDA regimen (combined ATRA + idarubicin), consisting of induction chemotherapy with ATRA in combination with four 12 mg/m2 doses of idarubicin given intravenously on days 2, 4, 6, and 8. With this regimen, 95% of patients achieved a hematologic remission [38, 39]. Patients then received three consolidation combination polychemotherapy regimens, with excellent event-free survival (EFS) of 79% at 2 years. The AIDA regimen provided the basis for further risk-adapted approaches that are still listed in NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) as appropriate first-line therapy for patients with high-risk APL [8, 35] (Fig. 8.2).
Contemporary regimens for induction and consolidation therapy in high-risk APL. Highly effective induction and consolidation regimens supported by consensus guidelines for treatment of high-risk APL. ATRA, all-trans retinoic acid. Figure adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.3.2017. © 2017 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available. [8, 35, 41, 44, 49]. ATRA all-trans retinoic acid
The Spanish PETHEMA LPA 96 trial modified the AIDA regimen to reduce toxicity by omitting etoposide and cytarabine from consolidation [40]. In the PETHEMA LPA 96 trial, 51% of patients became PCR negative for PML-RARA after induction, and 93% were PCR negative after induction and consolidation. With this modified AIDA regimen, rates of 2-year OS were 82%, suggesting that cytarabine and etoposide may not be necessary for most APL patients.
Multivariate analysis of the GIMEMA 93 and PETHEMA LPA 96 trials demonstrated that the initial WBC and platelet counts in newly diagnosed APL patients provided robust independent prognostic value for patients who received AIDA-based therapies [7]. This analysis provided further evidence that the omission of non-intercalating drugs such as cytarabine and etoposide did not lead to inferior outcomes for most patients. However, APL patients with presenting WBC >10,000/mcL had inferior RFS with AIDA induction, providing justification for risk-adapted approaches based on WBC (Fig. 8.1). In the PETHEMA LPA99 risk-adapted study by Sanz and colleagues, all patients received AIDA induction followed by consolidation chemotherapy, with ATRA added to consolidation cycles 1 and 3 in all but low-risk patients (WBC < 10,000/mcL and platelets > 40,000/mcL) [6]. The LPA99 study demonstrated that ATRA in consolidation therapy significantly reduced rates of relapse from 20.1 to 8.7%. This benefit of ATRA in consolidation was most notable in intermediate-risk patients, where relapse rates decreased from 14 to 2.5% [6].
Role of Cytarabine in High-Risk APL
Given the excellent outcomes of the modified AIDA regimen which eliminated cytarabine, the role of cytarabine in APL induction and consolidation chemotherapy remains controversial. Comparison of the French APL 2000 trial and the PETHEMA LPA 99 trials provides some insight into the role of cytarabine [41, 42]. Both the APL 2000 and the PETHEMA LPA 99 trials used ATRA in combination with chemotherapy for induction. The European APL 2000 trial combined ATRA with 7 + 3 (cytarabine 200 mg/m2/day × 7 days and daunorubicin 60 mg/m2/day × 3 days), while the PETHEMA LPA 99 study used an AIDA induction regimen. APL 2000 and PETHEMA LPA 99 also provided different consolidation approaches that were risk stratified based on the presenting WBC. No PETHEMA LPA 99 patients received cytarabine. However, all high-risk APL 2000 patients received cytarabine during induction as well as consolidation in combination with ATRA and daunorubicin. For high-risk patients, rates of CR (95.1% vs. 83.6%, P = 0.018) and 3-year OS (91.5% vs. 80.8%, P = 0.026) were significantly higher in the cytarabine-containing APL 2000 vs. LPA 99 trial. In an initial analysis of 104 high-risk patients in the LPA 99 trial, there was also a trend toward a lower 3-year incidence of relapse in the APL 2000 trial (9.9% in APL 2000 vs. 18.5% in LPA 99, P = 0.12), further suggesting a potential role for cytarabine in high-risk patients. In the final analysis of a total of 140 high-risk patients in the cytarabine-free LPA 99 trial, the 3-year cumulative incidence of relapse (CIR) was even higher at 26% [35]. In contrast, for patients with WBC < 10,000/mcL, CR rates and 3-year OS were similar, but rates of relapse were higher in the APL 2000 trial vs. the LPA 99 trial (14.3% vs. 4.2%), indicating that this non-cytarabine containing AIDA-based regimen is appropriate for low-risk patients. Based on these data, the APL 2000 induction and consolidation regimen using ATRA + chemotherapy including both daunorubicin and cytarabine represents one standard approach for treatment of high-risk APL [8, 41] (Fig. 8.2).
Although cytarabine may be reasonably excluded from AIDA-based therapy for low-risk APL, recent data indicate that cytarabine should not be excluded from regimens using daunorubicin [43]. Longer-term follow-up from the APL 2000 trial demonstrated unacceptably high rates of relapse for patients treated without cytarabine. Even in low-risk APL patients with WBC < 10,000/mcL, those who received daunorubicin without cytarabine had a 7-year CIR of 28.6% vs. 12.9% for those who received daunorubicin and cytarabine. This study therefore suggests that if daunorubicin is used as the anthracycline for APL induction and consolidation, cytarabine may not be dispensable for patients in any risk group [43].
Cytarabine has also been studied in combination with ATRA and idarubicin during consolidation therapy following AIDA-based induction for high-risk APL patients younger than 60 in the PETHEMA LPA 2005 trial [35]. The LPA 2005 trial followed the excellent results of the risk-adapted PETHEMA LPA 99 trial and was designed with the goal of further improving outcomes for younger high-risk APL patients. In the LPA 2005 trial, for high-risk patients younger than 60 years, cytarabine was added to the combination of ATRA and idarubicin during cycles 1 and 3 of consolidation therapy. Low- and intermediate-risk patients received a reduced course of mitoxantrone for the second consolidation course and did not receive cytarabine. For high-risk patients in the LPA 2005 trial, the 3-year relapse rate was 11%, significantly lower (P = 0.03) than the 3-year relapse rate of 26% in the LPA 99 trial. The 3-year DFS rates were 92% for the entire LPA 2005 study, with excellent 3-year DFS of 82% for high-risk patients. Therefore, the LPA2005 trial regimen using AIDA-based induction followed by ATRA + idarubicin + cytarabine for cycles 1 and 3 of consolidation and ATRA + mitoxantrone for cycle 2 of consolidation now represents another standard approach for treatment of high-risk APL [8, 35]. The APL 2000 and LPA 2005 regimens have never been directly compared, and they both represent highly effective approaches for induction and consolidation therapy for high-risk APL patients (Fig. 8.2).
In the modern era, following the demonstration of arsenic trioxide (ATO) as the most active single agent in APL, multiple studies have also tested combinations of ATRA, ATO, and chemotherapy. In particular, the APML4 regimen including combination ATRA + ATO + idarubicin for induction represents a highly efficacious option for treatment of high-risk APL patients [8, 44,45,46]. Please see Chaps. 9 and 10 for details regarding the use of ATO with and without ATRA and chemotherapy.
Bone Marrow Evaluation After Induction Therapy
The timing of bone marrow evaluation after induction therapy for APL differs from evaluation in the setting of other subtypes of AML. With modern regimens, much of the efficacy of APL induction therapy results from prolonged ATRA-induced differentiation of APL promyelocytes, in addition to the more rapid cytotoxic effects of chemotherapy and the induction of apoptosis caused by ATO [26,27,28, 47, 48]. Therefore, an initial bone marrow evaluation on day 14 is too early to adequately evaluate the effects of APL induction therapy. Contemporary consensus guidelines recommend marrow evaluation in APL after recovery of blood counts, often 4–6 weeks after induction therapy. While cytogenetic evaluation may no longer detect the t(15;17) translocation after modern induction therapy, PCR for molecular detection of PML-RARa may remain positive. Therefore, additional therapy for APL in the form of consolidation is required before assessment of molecular remission [8].
Consolidation Regimens
Following the use of induction therapy, the goal of additional consolidation therapy is to eliminate residual APL cells to achieve a durable molecular remission and prevent relapse [8, 17]. As noted above, various trials including the North American Intergroup study E2491 have shown that outcomes for APL patients who underwent induction therapy but failed to undergo further therapy had higher rates of relapse than patients who received further therapy with consolidation or maintenance [25].
For high-risk APL patients, chemotherapy is included in consolidation regimens for all modern standard therapies with the exception of the APML4 trial, which uses ATRA and ATO during consolidation and includes chemotherapy only during induction and maintenance [44, 45]. Anthracyclines , such as daunorubicin and idarubicin, and the related anthraquinone, mitoxantrone represent key components of consolidation for contemporary regimens including the Intergroup C9710, APL 2000, and PETHEMA LPA 2005 trials [35, 41, 49] (Fig. 8.2). As these consolidation regimens may include high cumulative doses of cardiotoxic medications, repeat cardiac evaluation is important prior to initiating each cycle of consolidation chemotherapy containing anthracyclines or mitoxantrone [8]. The pyrimidine analog cytarabine is also a component of consolidation therapy in the APL 2000 and LPA 2005 trials, and clinicians following these protocols may need to dose adjust for age or renal dysfunction [35, 41].
Differentiation or apoptosis-inducing therapies such as ATRA ± ATO also serve as critically important components of contemporary consolidation regimens for high-risk APL. As described above, the inclusion of these agents during consolidation is based upon multiple studies showing improvements in DFS and OS with the use of ATRA and ATO [6, 35, 41, 44, 45, 49]. For example, the LPA99 study demonstrated that adding ATRA to consolidation therapy significantly reduced rates of relapse [6]. This finding was confirmed by the GIMEMA AIDA-2000 trial, which demonstrated that the inclusion of ATRA in consolidation particularly improved outcomes for high-risk patients [36]. The Intergroup C9710 study demonstrated that adding two cycles of ATO consolidation prior to two cycles of ATRA + daunorubicin consolidation improved outcomes including DFS and OS across APL risk groups [49]. Even in the ATRA and ATO era, chemotherapy likely plays an important role for patients with high-risk APL, as evidenced by the poor outcomes for high-risk APL patients with ATRA or ATO monotherapy [32, 50].
Contemporary protocols therefore still include chemotherapy during consolidation or maintenance for patients with high-risk APL. Both the Intergroup C9710 and PETHEMA LPA 2005 protocols use ATRA in combination with chemotherapy during consolidation therapy for high-risk APL, while the APML4 trial uses ATRA + ATO without chemotherapy for consolidation and reintroduces low-dose chemotherapy + ATRA during maintenance (Figs. 8.2 and 8.3) [44, 45, 49]. Despite the omission of chemotherapy during consolidation, the APML4 trial using only ATRA + ATO consolidation has excellent 5-year OS rates of 87% and DFS rates of 95% for high-risk patients [44, 45] (Table 8.1). The European APL 2000 study uses only chemotherapy for consolidation, but these cycles of consolidation chemotherapy are sandwiched between ATRA + chemotherapy during induction and maintenance [41].
Relapse-free survival by risk stratification in APML4. Kaplan-Meier curves for relapse-free survival following achievement of documented hematologic complete remission (HCR) in the APML4 trial, stratified by Sanz risk groups. Figure from Collins M, Di Iulio J, Beresford J. Australasian Leukaemia & Lymphoma Group APML4 Statistical Report, June 2013, courtesy of H Iland (APML4 principal investigator) [46]
In choosing consolidation and maintenance therapies following induction for high-risk disease, clinicians should follow the specific consolidation and maintenance regimen for the protocol used for induction therapy [8]. With the participation of hundreds of APL patients in numerous clinical trials as described above, treatment outcomes have continued to improve over the last several decades, transforming APL into a largely curable disease. However, realization of this success for individual patients depends upon rigorous adherence to established protocols. Although the outstanding outcomes of protocols such as APML4 might tempt clinicians to use ATRA + ATO consolidation following any induction regimen, mixing and matching regimens should be strongly discouraged outside of the context of a clinical trial. One exceptional circumstance involves consolidation therapy for a high-risk APL patient with cardiotoxicity or for whom an anthracycline is otherwise contraindicated. In that setting, consensus guidelines suggest the use of consolidation with combination ATRA and ATO as recently described for low-risk APL [8, 13].
Maintenance Therapy
The goal of maintenance therapy is to maintain molecular remission, decrease rates of relapse, and ideally to increase rates of cure. Given the high efficacy of treatment strategies in the modern era of ATRA + ATO, the role of maintenance therapy in contemporary APL treatment is controversial and may not be necessary for patients who achieve a molecular CR [51,52,53]. In addition, the use of chemotherapy in maintenance has the potential to harm some patients who might already be cured with induction and consolidation therapy [17]. Maintenance is therefore no longer a component of therapy for low-risk APL patients treated with ATRA + ATO induction and consolidation [13]. However, based upon evidence from previous clinical trials discussed below, the use of maintenance therapy still represents the standard of care for patients with high-risk APL [8, 17].
Agents used in maintenance regimens for APL include ATRA, the folate antimetabolite methotrexate (MTX), and the purine antagonist 6-mercaptopurine (6-MP). The North American Intergroup E2491 study showed superior 5-year DFS with maintenance ATRA over observation (61% vs. 36%, P < 0.0001) [25, 32]. The European APL93 trial tested four different maintenance strategies: no maintenance, intermittent ATRA, continuous chemotherapy with 6-MP and MTX, and combination ATRA + 6-MP + MTX [34, 37]. In this study, the 10-year CIR was significantly decreased from 43.2% to 33%, 23.4%, and 13.4%, respectively, with the regimens above, with the lowest incidence of relapse seen with ATRA in combination with chemotherapy (P < 0.001). The greatest benefit of maintenance therapy was seen in patients with WBC > 5000/μL, with a decrease in CIR from 68.4% to 53.1%, 32.8%, and 20.6% (P < 0.001). However, this decrease in relapse from maintenance therapy came at the price of increased toxicity, particularly in older patients, with a marked 21.7% 10-year cumulative incidence of death in CR for patients older than 65 years, primarily from myelosuppression [34, 37].
A recent Cochrane review conducted a meta-analysis to evaluate the role of maintenance therapy for APL in CR1 [51]. In this meta-analysis of ten randomized controlled trials in APL, maintenance therapy had no statistically significant effect on OS but did improve DFS. Studies including the Japanese APL 97 study and the AIDA 0493 trial have suggested that there are no long-term benefits to maintenance therapy [54, 55]. The SWOG 0521 trial randomized low-risk patients in molecular CR after standard induction and consolidation including ATO to maintenance ATRA + 6-MP + MTX vs. observation. Although enrollment was stopped early because of slow accrual, no relapses were seen in the 68 patients randomized to either arm with a median follow-up of 36 months. This study therefore suggests that if an intensive post-remission consolidation regimen including ATO is used to achieve molecular CR, further maintenance may not be necessary for low-risk patients [52]. However, the best long-term outcomes for high-risk patients have all been achieved using protocols that use maintenance therapy, including the APL 2000, LPA 2005, APML4, and Intergroup C9710 [35, 41, 44, 45, 49]. Improvements in outcomes for high-risk APL patients over time are likely due to a variety of factors, but combined ATRA + chemotherapy maintenance has been suggested as a factor leading to reduced rates of relapse in European clinical trials [56].
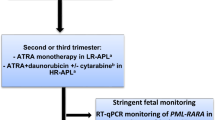
Following the completion of consolidation therapy, APL patients should be assessed for molecular remission using RT-PCR on bone marrow samples. Patients who are PCR positive and remain so on a repeat bone marrow PCR in 2–4 weeks should be treated as relapsed APL [8] (see Chap. 13). High-risk APL patients who are PCR negative following consolidation should be treated with maintenance therapy per the initial treatment protocol (Fig. 8.4). The importance of not mixing and matching treatment regimens applies to maintenance strategies as well as consolidation as discussed above, although most modern maintenance approaches are nearly identical. In contemporary treatment of APL, the APL 2000 and PETHEMA LPA 2005 protocols include the same regimen of maintenance as the LPA 99 trial: 2 years of 6-mercaptopurine (50 mg/m2/day), methotrexate (15 mg/m2/week), and intermittent ATRA (45 mg/m2/day) for 15 days every 3 months [6, 35, 41]. The APML4 trial also uses a nearly identical regimen for 2 years of maintenance therapy starting 3–4 weeks following the end of consolidation cycle 2 [44, 45]. The North American Intergroup C9710 attempted to evaluate the role of maintenance ATRA vs. ATRA + chemotherapy but was underpowered to detect a difference. For those patients who received ATRA + chemotherapy maintenance, the regimen was a similar combination of ATRA, MTX, and 6-MP, although only given for 1 year [49] (Fig. 8.4). As it is advisable to follow an established protocol from induction through consolidation and maintenance, foregoing maintenance therapy for high-risk APL patients should not be undertaken outside of a clinical trial.
CNS Relapse and the Role of Prophylactic Intrathecal Chemotherapy
The role of prophylactic intrathecal (IT) chemotherapy to prevent CNS relapse remains controversial. However, several lines of evidence suggest that prophylactic IT chemotherapy may be important for preventing CNS relapse, particularly in high-risk APL patients. Although ATRA and ATO cross the blood-brain barrier, CSF concentrations may vary significantly from patient to patient and may not be adequate for significant antileukemic activity [57]. As treatment of systemic disease improved over time with the use of ATRA, relapsed CNS disease was increasingly reported in APL patients who presented with high WBC [58]. In the European experience, trials with prophylactic IT chemotherapy for high-risk APL patients demonstrated decreased rates of relapse. Patients with high-risk APL had a 4% incidence of CNS relapse in the APL 93 trial, while the APL 2000 trial had no CNS relapses at 5 years [56]. This decreased rate of relapse may have been due to the use of five doses of IT chemotherapy in high-risk APL patients in APL 2000, as well as the use of higher doses of cytarabine during consolidation [56]. Longer-term 7-year follow-up from the APL 2000 trial did reveal one CNS relapse, although this occurred in a patient with low-risk APL treated without cytarabine and without prophylactic IT chemotherapy [43]. For patients with high-risk APL, lumbar puncture should therefore be considered at count recovery prior to consolidation therapy as the CNS can serve as a sanctuary site for residual APL cells [8, 58]. Consensus guidelines support the use of four to six doses of IT chemotherapy with MTX or liposomal cytarabine combined with corticosteroids to be given during consolidation for patients with high-risk APL [8].
Aggressive Supportive Care for Thrombocytopenia in the Setting of Chemotherapy and Coagulopathy
Life-threatening coagulopathy represents a potentially catastrophic complication of APL and is discussed in detail elsewhere (see Chap. 5). With the advent of remarkably effective therapies in the modern era of treatment for APL, early death from coagulopathy has emerged as the single most important barrier to cure [1,2,3,4, 59,60,61,62]. The prompt use of ATRA is likely critical to prevent early death from hemorrhage [59, 61, 63]. From the perspective of induction chemotherapy for high-risk APL, cytotoxic chemotherapy including anthracyclines and cytarabine has the potential to exacerbate thrombocytopenia. In addition to vigilant monitoring of coagulation parameters and repletion of fibrinogen with cryoprecipitate, meticulous monitoring of the CBC and frequent transfusions of platelets are often needed to prevent death from hemorrhage. Platelet counts should be maintained above 30,000–50,000/μL and fibrinogen above 100–150 mg/dL [5].
Differentiation Syndrome and the Role of Prophylactic Steroids and Early Chemotherapy for High-Risk APL
Differentiation syndrome represents a unique complication of APL therapy (see Chap. 17). Upon treatment of APL blasts with ATRA, the block to lineage differentiation induced by PML/RARα is reversed, and the resulting surge of differentiated myeloid cells can result in pleural and pericardial effusions, pulmonary infiltrates, dyspnea, hypotension, and renal failure [64, 65]. Prophylactic steroids with either prednisone or dexamethasone are recommended for patients with high WBC count to prevent differentiation syndrome [8, 66]. As the risk of differentiation syndrome is increased in patients with high WBC [67], early introduction of chemotherapy is recommended for high-risk disease. For APL patients with WBC > 10,000/μL, some expert guidelines recommend initiation of chemotherapy as early as day 1 within a few hours of the first dose of ATRA, both to reduce the risk of differentiation syndrome and to achieve better control of coagulopathy [5, 64].
Alternate Role of Chemotherapy: Patients Unable to Tolerate Arsenic Trioxide
Current standard treatment for APL utilizes chemotherapy mostly in the setting of high-risk disease. However, unusual circumstances may also warrant the use of chemotherapy in low-risk APL to increase rates of cure. Rarely, a low-risk APL patient on ATRA + ATO may experience a complication from ATO such as pancreatitis or a prolonged QT interval leading to significant arrhythmia. ATO is commonly associated with QT interval prolongation (24–32%), but clinically significant arrhythmias are rare and can generally be avoided with appropriate precautions including careful monitoring and electrolyte repletion [68]. In cases of unusual complications precluding further ATO, switching to a non-ATO chemotherapy-based approach such as APL 2000 or LPA 2005 is reasonable, as combined ATRA + chemotherapy improves rates of cure over ATRA alone [25, 32].
Conclusion
Despite the development of highly effective disease-directed agents such as ATRA and ATO, cytotoxic chemotherapy still plays an important role in contemporary treatment of APL. Although recent data demonstrate that chemotherapy is not required for patients with low-risk APL [13, 69, 70], chemotherapy remains an important component of curative therapy for high-risk disease. Several ATRA + chemotherapy combination approaches are appropriate for standard induction therapy, and ATRA + ATO + idarubicin induction results in particularly excellent outcomes [35, 41, 44, 45, 49]. Early ATRA and aggressive supportive care remain critical for preventing early death from coagulopathy and hemorrhage [5, 59, 61, 63]. Early chemotherapy following ATRA is also important to reduce the risk of differentiation syndrome and for controlling coagulopathy in high-risk patients [5, 64]. Prophylactic steroids are recommended to reduce the risk of differentiation syndrome in this patient population [8, 66].
Various agents are used for consolidation and maintenance therapies, including ATRA, ATO, anthracyclines, and cytarabine during consolidation, as well as ATRA, 6-MP, and MTX for maintenance. However, mixing and matching induction, consolidation, and maintenance regimens should be strongly discouraged. Following induction, consolidation and maintenance therapy for patients with high-risk APL should be given according to the initial protocol. Although controversial, prophylactic intrathecal chemotherapy during consolidation is advisable to prevent CNS relapse in high-risk APL patients [8]. Some APL patients experience long-term complications including therapy-related myeloid neoplasms as well as cardiomyopathy [15, 70]. Although APL has been transformed over the last several decades into a largely curable disease, future studies are needed to reduce rates of early death and increase rates of cure and to minimize the use of chemotherapy where possible.
References
Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Mollgard L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25(7):1128–34.
Paulson K, Serebrin A, Lambert P, Bergeron J, Everett J, Kew A, et al. Acute promyelocytic leukaemia is characterized by stable incidence and improved survival that is restricted to patients managed in leukaemia referral centres: a pan-Canadian epidemiological study. Br J Haematol. 2014;166(5):660–6.
Rahme R, Thomas X, Recher C, Vey N, Delaunay J, Deconinck E, et al. Early death in acute promyelocytic leukemia (APL) in French centers: a multicenter study in 399 patients. Leukemia. 2014;28(12):2422–4.
Altman JK, Rademaker A, Cull E, Weitner BB, Ofran Y, Rosenblat TL, et al. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res. 2013;37(9):1004–9.
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91.
Sanz MA, Martin G, Gonzalez M, Leon A, Rayon C, Rivas C, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–43.
Sanz MA, Lo Coco F, Martin G, Avvisati G, Rayon C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–53.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.3.2017. © National Comprehensive Cancer Network, Inc. 2017. All rights reserved. Accessed August 21, 2017. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [Internet].
Molica M, Breccia M. FLT3-ITD in acute promyelocytic leukemia: clinical distinct profile but still controversial prognosis. Leuk Res. 2015;39(4):397–9.
Breccia M, Loglisci G, Loglisci MG, Ricci R, Diverio D, Latagliata R, et al. FLT3-ITD confers poor prognosis in patients with acute promyelocytic leukemia treated with AIDA protocols: long-term follow-up analysis. Haematologica. 2013;98(12):e161–3.
Schnittger S, Bacher U, Haferlach C, Kern W, Alpermann T, Haferlach T. Clinical impact of FLT3 mutation load in acute promyelocytic leukemia with t(15;17)/PML-RARA. Haematologica. 2011;96(12):1799–807.
Cicconi L, Divona M, Ciardi C, Ottone T, Ferrantini A, Lavorgna S, et al. PML-RARalpha kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30(10):1987–92.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21.
Lo-Coco F, Orlando SM, Platzbecker U. Treatment of acute promyelocytic leukemia. N Engl J Med. 2013;369(15):1472.
Thomas X, Le QH, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol. 2002;81(9):504–7.
Raj S, Franco VI, Lipshultz SE. Anthracycline-induced cardiotoxicity: a review of pathophysiology, diagnosis, and treatment. Curr Treat Options Cardiovasc Med. 2014;16(6):315.
Watts JM, Tallman MS. Acute promyelocytic leukemia: what is the new standard of care? Blood Rev. 2014;28(5):205–12.
Avvisati G, Mandelli F, Petti MC, Vegna ML, Spadea A, Liso V, et al. Idarubicin (4-demethoxydaunorubicin) as single agent for remission induction of previously untreated acute promyelocytic leukemia: a pilot study of the Italian cooperative group GIMEMA. Eur J Haematol. 1990;44(4):257–60.
Bernard J, Weil M, Boiron M, Jacquillat C, Flandrin G, Gemon MF. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973;41(4):489–96.
Paietta E, Andersen J, Racevskis J, Gallagher R, Bennett J, Yunis J, et al. Significantly lower P-glycoprotein expression in acute promyelocytic leukemia than in other types of acute myeloid leukemia: immunological, molecular and functional analyses. Leukemia. 1994;8(6):968–73.
Michieli M, Damiani D, Ermacora A, Geromin A, Michelutti A, Masolini P, et al. P-glycoprotein (PGP), lung resistance-related protein (LRP) and multidrug resistance-associated protein (MRP) expression in acute promyelocytic leukaemia. Br J Haematol. 2000;108(4):703–9.
Cunningham I, Gee TS, Reich LM, Kempin SJ, Naval AN, Clarkson BD. Acute promyelocytic leukemia: treatment results during a decade at Memorial Hospital. Blood. 1989;73(5):1116–22.
Head DR, Kopecky KJ, Willman C, Appelbaum FR. Treatment outcome with chemotherapy in acute promyelocytic leukemia: the Southwest Oncology Group (SWOG) experience. Leukemia. 1994;8(Suppl 2):S38–41.
Rodeghiero F, Avvisati G, Castaman G, Barbui T, Mandelli F. Early deaths and anti-hemorrhagic treatments in acute promyelocytic leukemia. A GIMEMA retrospective study in 268 consecutive patients. Blood. 1990;75(11):2112–7.
Tallman MS. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100(13):4298–302.
Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, et al. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990;76(9):1704–9.
Chomienne C, Ballerini P, Balitrand N, Daniel MT, Fenaux P, Castaigne S, et al. All-trans retinoic acid in acute promyelocytic leukemias. II. In vitro studies: structure-function relationship. Blood. 1990;76(9):1710–7.
Warrell RP Jr, Frankel SR, Miller WH Jr, Scheinberg DA, Itri LM, Hittelman WN, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N Engl J Med. 1991;324(20):1385–93.
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72(2):567–72.
Fenaux P, Le Deley MC, Castaigne S, Archimbaud E, Chomienne C, Link H, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82(11):3241–9.
Fenaux P, Chastang C, Chomienne C, Degos L. Tretinoin with chemotherapy in newly diagnosed acute promyelocytic leukaemia. European APL Group. Lancet. 1994;343(8904):1033.
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021–8.
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95(1):90–5.
Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115(9):1690–6.
Sanz MA, Montesinos P, Rayon C, Holowiecka A, de la Serna J, Milone G, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–46.
Lo-Coco F, Avvisati G, Vignetti M, Breccia M, Gallo E, Rambaldi A, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–9.
Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94(4):1192–200.
Mandelli F, Diverio D, Avvisati G, Luciano A, Barbui T, Bernasconi C, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90(3):1014–21.
Avvisati G, Lo Coco F, Diverio D, Falda M, Ferrara F, Lazzarino M, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) pilot study. Blood. 1996;88(4):1390–8.
Sanz MA, Martin G, Rayon C, Esteve J, Gonzalez M, Diaz-Mediavilla J, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94(9):3015–21.
Ades L, Sanz MA, Chevret S, Montesinos P, Chevallier P, Raffoux E, et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL): a comparison of French-Belgian-Swiss and PETHEMA results. Blood. 2008;111(3):1078–84.
Ades L, Chevret S, Raffoux E, de Botton S, Guerci A, Pigneux A, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24(36):5703–10.
Ades L, Chevret S, Raffoux E, Guerci-Bresler A, Pigneux A, Vey N, et al. Long-term follow-up of European APL 2000 trial, evaluating the role of cytarabine combined with ATRA and Daunorubicin in the treatment of nonelderly APL patients. Am J Hematol. 2013;88(7):556–9.
Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570–80; quiz 752.
Iland HJ, Collins M, Bradstock K, Supple SG, Catalano A, Hertzberg M, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial. Lancet Haematol. 2015;2(9):e357–66.
Collins M, Di Iulio J, Beresford J. Australasian Leukaemia & Lymphoma Group APML4 Statistical Report. 2013.
Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, et al. Arsenic degrades PML or PML–RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10(5):547–55.
Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, et al. Arsenic trioxide controls the fate of the PML-RAR oncoprotein by directly binding PML. Science. 2010;328(5975):240–3.
Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–7.
Mathews V, George B, Chendamarai E, Lakshmi KM, Desire S, Balasubramanian P, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28(24):3866–71.
Muchtar E, Vidal L, Ram R, Gafter-Gvili A, Shpilberg O, Raanani P. The role of maintenance therapy in acute promyelocytic leukemia in the first complete remission. Cochrane Database Syst Rev. 2013;(3):CD009594.
Coutre SE, Othus M, Powell B, Willman CL, Stock W, Paietta E, et al. Arsenic trioxide during consolidation for patients with previously untreated low/intermediate risk acute promyelocytic leukaemia may eliminate the need for maintenance therapy. Br J Haematol. 2014;165(4):497–503.
Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J. 2015;5:e304.
Asou N, Kishimoto Y, Kiyoi H, Okada M, Kawai Y, Tsuzuki M, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RAR transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110(1):59–66.
Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117(18):4716–25.
Kelaidi C, Chevret S, De Botton S, Raffoux E, Guerci A, Thomas X, et al. Improved outcome of acute promyelocytic leukemia with high WBC counts over the last 15 years: the European APL Group Experience. J Clin Oncol. 2009;27(16):2668–76.
Au WY, Tam S, Fong BM, Kwong YL. Determinants of cerebrospinal fluid arsenic concentration in patients with acute promyelocytic leukemia on oral arsenic trioxide therapy. Blood. 2008;112(9):3587–90.
Breccia M, Carmosino I, Diverio D, De Santis S, De Propris MS, Romano A, et al. Early detection of meningeal localization in acute promyelocytic leukaemia patients with high presenting leucocyte count. Br J Haematol. 2003;120(2):266–70.
Breccia M, Lo CF. Thrombo-hemorrhagic deaths in acute promyelocytic leukemia. Thromb Res. 2014;133(Suppl 2):S112–6.
McClellan JS, Kohrt HE, Coutre S, Gotlib JR, Majeti R, Alizadeh AA, et al. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012;97(1):133–6.
Tallman M, Lo-Coco F, Kwaan H, Sanz M, Gore S. Clinical roundtable monograph. Early death in patients with acute promyelocytic leukemia. Clin Adv Hematol Oncol. 2011;9(2):1–16.
Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118(5):1248–54.
Mantha S, Tallman MS, Soff GA. What’s new in the pathogenesis of the coagulopathy in acute promyelocytic leukemia? Curr Opin Hematol. 2016;23(2):121–6.
Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123(18):2777–82.
Montesinos P, Bergua JM, Vellenga E, Rayon C, Parody R, de la Serna J, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113(4):775–83.
Wiley JS, Firkin FC. Reduction of pulmonary toxicity by prednisolone prophylaxis during all-trans retinoic acid treatment of acute promyelocytic leukemia. Australian Leukaemia Study Group. Leukemia. 1995;9(5):774–8.
Camacho LH, Soignet SL, Chanel S, Ho R, Heller G, Scheinberg DA, et al. Leukocytosis and the retinoic acid syndrome in patients with acute promyelocytic leukemia treated with arsenic trioxide. J Clin Oncol. 2000;18(13):2620–5.
Roboz GJ, Ritchie EK, Carlin RF, Samuel M, Gale L, Provenzano-Gober JL, et al. Prevalence, management, and clinical consequences of QT interval prolongation during treatment with arsenic trioxide. J Clin Oncol. 2014;32(33):3723–8.
Lo-Coco F, Cicconi L, Breccia M. Current standard treatment of adult acute promyelocytic leukaemia. Br J Haematol. 2016;172(6):841–54.
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–305.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Goldberg, A.D., Tallman, M.S. (2018). First-Line Therapy for APL: Chemotherapy-Based Approach. In: Abla, O., Lo Coco, F., Sanz, M. (eds) Acute Promyelocytic Leukemia . Springer, Cham. https://doi.org/10.1007/978-3-319-64257-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-64257-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-64256-7
Online ISBN: 978-3-319-64257-4
eBook Packages: MedicineMedicine (R0)