Abstract
Peptide receptor radionuclide therapy (PRRT) has emerged as a promising therapeutic tool to confront neoplasms of neuroendocrine character both primaries and secondaries. Particularly, locoregional therapeutic schemes have been developed focused on the management of liver metastases. Among these novel therapeutic procedures, intra-arterial infusions of high doses of 111In-Octreotide were performed in our institution for more than a decade covering the whole spectrum of liver metastases both in early and in advanced cases. This chapter prescribes the Auger electron efficacy in paraganglioma primaries and liver-metastasized cases, as well.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
Paragangliomas are rare, non-epithelial NETs [1], which derive from paraganglial system, and are classified as (a) sympathetic, almost always producing catecholamines, and (b) parasympathetic, usually not releasing catecholamines [2].
They are metastatic in about 10% of cases. Paragangliomas are known to express high levels of somatostatin receptors (sst), especially subtype sst2. However, according to Saveanu et al. [3], Reubi et al. [4], and Binderup et al. [5], some paragangliomas have cytoplasmic localization of sst2 receptor rather than a membrane, and it has been suggested that this might account for not only the failure of sst2 agonists in controlling catecholamine secretion and tumor proliferation but also failure of 111In-Octreotide imaging to detect some of them. 111In-Octreotide shows a modest sst2 binding, having a good sensitivity (up to 90%) for head and neck paragangliomas [6] and according to Charrier et al. [7] a mediocre sensitivity as low as 20% as far as the abdominal ones. Nowadays, for their diagnosis, a new generation of somatostatin analogs with superior sst2 binding affinity has been developed for use with PET/CT imaging such as 68Ga-DOTATOC, 68Ga-DOTANOC, and 68Ga-DOTATATE [8, 9] (Table 16.1).
According to WHO, paragangliomas are classified into two groups, based on their clinical and biological behavior: those arising from the parasympathetic system, primary located in the head and neck and less frequently in the thorax and pelvis, and those from the sympathetic one (Table 16.2).
16.1.1 Parasympathetic (Head and Neck) Paragangliomas
Accounting approximately a 20% of all paragangliomas [10,11,12] are generally nonfunctioning subcategorized according to their anatomical sites of origin as carotid body paragangliomas, jugulotympanic paragangliomas (Fig. 16.1), vagal paragangliomas, and laryngeal paragangliomas [13]. As a whole, less than 5% of head and neck paragangliomas metastasize. Hereditary cases of head and neck paragangliomas could be multiple and occur in association with sympathetic ones. The germline mutation, most commonly noted in one of the succinate dehydrogenase genes (SDHx), can be screened by immunohistochemical staining for SDHB protein. Paragangliomas associated with SDHB mutations have a high risk of metastasis. Thus, even in the absence of family history, genetic testing should be recommended for at least the most common genes in all patients, depending on local resources [14].
16.1.2 Sympathetic Paragangliomas
Approximately 85% of sympathetic paragangliomas arise below the diaphragm. Sympathetic paraganglioma could be found in retroperitoneum around the adrenal/renal area, around the organ of Zuckerkandl or in the urinary bladder [15]. The other sympathetic paragangliomas are noted in the thorax, heart, and other locations [16,17,18]. Sympathetic paragangliomas are more likely to be functioning when compared to head and neck paragangliomas [19]. Patients with sympathetic paragangliomas usually have elevated norepinephrine only or both norepinephrine and dopamine. Sympathetic paragangliomas have high risk of metastases and even higher (even up to 50%) in those with SDHB mutation [20].
16.1.3 Treatment Stratification
Although most paragangliomas are benign, factors such as genetic background, tumor size, tumor location, and high methoxy-tyramine levels are associated with higher rate of metastatic disease. Their proximity to cranial nerves and vasculature may result in considerable morbidity due to compression or infiltration of the adjacent structures, necessitating balanced decisions between a wait-and-see policy and active treatment [21]. Surgery is the only curative treatment. Treatment options for patients with metastatic disease are limited. Paragangliomas have a strong genetic background, with at least one-third of all cases linked with germline mutations in 11 susceptibility genes. As genetic testing becomes more widely available, the diagnosis assessment of paragangliomas will be made earlier due to routine screening of at-risk patients. As a result, a multidisciplinary approach for the management of paragangliomas is required to ensure a consistent and optimal level of care and treatment (Table 16.3).
For both sympathetic and parasympathetic PGLs, surgery is the treatment of choice, in cases where it can be performed. Further options of therapeutic schemes include systematic treatment with agents as gemcitabine, cisplatin or sunitinib, and radiotherapy (external-beam radiotherapy or stereotactic surgery). However, surgery and radiotherapy can cause severe side effects: the former hemostatic complications and nerve damage, particularly when tumor is in tight proximity with cranial nerves, and the latter vascular complications and peripheral nerve damage as well. In the international library, limited clinical data are available focused on radiopeptide schemes as treating option to confront paragangliomas. By the present, we describe and discuss the challenges of treating these rare tumors with 111In-octreotide in high doses, using established protocols, performed in our institution for other NET histotypes.
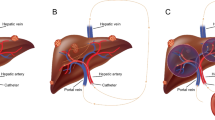
We report on the effectiveness of PRRT in three paragaglioma patients [one with surgically excised primary in the lower part of the sigmoid colon associated with liver metastases and two inoperable cases (one at the petrous portion of the right temporal bone of the skull and the second situated in the lower half of the mediastinum)], verified by Octreo-Scan® (Figs. 16.1 and 16.2) and biochemically and radiologically confirmed. The dose per session administered monthly to each patient ranged from 4.070 to 5.920 GBq. Repetitions for the inoperable cases did not exceed the nine sessions and for liver secondaries the twelve, with treatment intervals of 5–8 weeks. Patients with inoperable PGLs were infused intravenously, after centesis of the dorsal vein hand system or the antecubital vein, whereas for the liver secondaries, 111In-Octreotide was infused intra-arterially, after catheterization of the hepatic artery. Absorbed doses delivered to the primaries, to the liver metastases, kidneys, and red marrow were calculated according to OLINDA/EXM program, and response assessment was classified, based on RECIST criteria 1.1. CT/MRI scans were performed before, during, and after the end of treatment and monthly ultrasound images for the follow-up of the liver lesions.
16.2 111In-Octreotide Treatment Results
Liver metastatic load: None of the three treated patients resulted in complete response; partial response was assessed in one, whereas disease stabilization in two. Petrous bone and mesothorax primary neoplasms load: The aforementioned two cases resulted in initially disease stabilization for a short term, whereas on the progress of the therapy, they did not respond at all and died within 26–29 months after the initialization of the therapy due to aggravation and complications of the tumors (Table 16.4). According to the therapy response results, the 24-month PFS ratio was found to be 2/3 (66.6%) while the 24-month OS was 3/3 (100%) (Fig. 16.3). On CT, the mesothorax and petrous bone tumor masses progressed dramatically, whereas the liver metastases showed a mean target diameter shrinkage ranging from 33 to 45%. Grade I to II erythro-, leuko-, and thrombocytopenia occurred in all three cases. Dosimetric calculations for both the intra-arterial and intravenous infusions are tabulated in Table 16.5. Remarkably, an over than threefold higher tumor absorbed dose in favor of the intra-arterial one was noticed.
16.3 Discussion
This single-center analysis provides results including the respective progression-free survival and overall survival rates of PRRT in three patients with advanced paragangliomas of different primary origins after failing standard treatment. Despite the tiny patient number (n = 3), the treatment results forward the feature of the persistent anti-proliferative activity of 111In-Octreotide Auger and internal conversion electron emission in this specific NET rare entity at an advanced stage. However, this sort of electron emission achieves a rather insignificant disease control in the case of inoperable mediastinum and petrous paraganglioma patients, intravenously treated, a short-term stabilization, and a median progression-free survival (PFS) of 19–25 months, thus rendering 111In-Octrerotide not promising for an objective outcome in that context of lacking established treatment alternatives. On the contrary, concerning the intra-arterially treated hepatic metastases originating from the surgically excised colorectal paraganglioma, a long-term partial response was achieved with a PFS of 108 months.
According to the worldwide ergography, few studies report on the effect of PRRT in the management of patients suffering from metastatic or unresectable paragangliomas and no one on the response and efficacy of Auger and internal conversion indium’s electron emission. As in the majority of NETs, surgery consists the treatment of choice to confront this rare neuroendocrine histotype; however, even after radical operation, patients lurk the risk of recurrence and the development of metastases, even after many years. Fortunately, the low toxicity of the neoplasm and the usually settled long-term follow-up create good conditions for an efficient disease control. However, the real challenge for clinicians is the treatment of inoperable or metastatic tumors. Furthermore, the functioning cases, the elevated risk for severe cardiovascular disease, and the risk of malignancy add additional doctors’ dilemmas [22,23,24,25,26,27].
We studied the PRRT results after 111In-Octreotide administration in three patients suffering from paragangliomas. Comparing the internal references of several experteer reports on PRRT (Table 16.6), not with 111In-octreotide (an Auger and internal conversion emitter) but with 90Y-DOTATATE/DOTATOC or 177Lu-DOTATATE (β-emitters), an objective response was observed in one of three (33.3%) patients of the treated cases, with a median PFS of 25 and median OS of 29 months. The outcome of this three-patient cohort gives a disease stabilization in two patients and a partial response in one. In 2006, in a 177Lu-DOTATATE study by van Essen et al. [28], including 12 PGL patients during a median of 13 (range 4–30) months follow-up, an ORR of 16.7% and a SD of 58.3% were reported. PD was 25%, whereas PFS and OS were not tabulated. In a 90Y DOTATOC study by Imhof et al. [29], in a cohort of 28 paragangliomas, a median OS of 82 months was reported; ORR and PFS were not clearly discriminated. The same year, Zovato et al. [30] achieved in a cohort of four paragangliomas, treated with 177Lu DOTATATE, a median PFS in a range of 15–25 months with a 50% ORR; median OS was not referred. Cecchin et al. [31] in a paraganglioma case study reported a PR (100%) with a median PFS of 16 months; median OS was not referred. Pinato et al. [32], in a study of five paraganglioma-treated cases implementing 177Lu DOTATATE, reported a median PFS and a mean OS of 17 and 53 months, respectively; median OS was not achieved. In a paraganglioma case study of Ashwathanarayana et al., in 2017 [33], a 100% objective response was observed with a mean PFS of about 5 months and a mean OS of 11 months. In a study of Kong et al. [34], 20 patients with paragangliomas, treated with 177Lu-DOTATATE, reached a median PFS of 39 months, whereas the median OS was not reached. The authors reported a PR in 19 (70%) patients and a SD in 7 (26%). In a study by Nastos et al. [35] in 15 paraganglioma cases treated with 131I-mIBG as well as with 177Lu DOTATATE, the median PFS was 20.6 and 38.5 months, respectively. The authors achieved a median OS of 41.8 months for 131I-mIBG and 60.8 months for 177Lu DOTATATE; ORR was not clearly discriminated and tabulated. In a recent prospective study of Garske-Román et al. [36] on 11 rectal NETs, median PFS and OS were 34 (17–35) and 50 months, respectively, in 8 out of 11 patients, in whom the absorbed dose to the kidneys reached 23 Gy; in the rest three, in whom it did not, PFS and OS were 12 (3–12) and 12 (11–33) months accordingly. Yavad et al. [37] evaluated in a recent study the role of combined capecitabine and 177Lu-DOTATATE in malignant PGL patients. He observed in a cohort of 25 cases a 28% objective response with a median PFS of 32 months; median OS was not referred. Vyakaranam et al. [38] in a recent study in 13 paraganglioma patients, treated with 177Lu-DOTATATE, reported a PR in 1 out of 13 (7.7%) patients, a median OS of 37.3 months, and a median PFS of 21.6 months.
Finally, in a prospective open-label, single-center, phase II study of 13 patients with unresectable advanced paraganglioma treated with 90Y DOTATATE by Kolasinska-Cwikła et al. [39], a median PFS of 35 and a median OS of 68 months were observed.
In our three patients’ cohort, the equivocal and almost expected disappointing response to 111In-Octreotide intravenous infusions in PGL primaries was also predicted and expected during each PRRT cycle by scintigraphy, including both whole-body scans and SPECT/CT. Furthermore, the visual rating (score) of the tumor radiotracer uptake ranges between II and III, never reaching the visual score IV of the hepatic secondaries.
16.4 Conclusion
In unresectable metastatic liver lesions positive for somatostatin receptors, originated from PGLs, repeated, intra-arterial high doses of 111In-Octreotide resulted in a partial response in all the affected liver lesions. As far as both primaries, i.e., that of the inoperable petrous bone and the other of the mesothorax, after a short-term disease stabilization, progressed dramatically. The dosimetric calculations proved the poor absorbed dose, predicting the disappointing results. Systematic PRRT in primary PGLs with intravenously injected 111In-Octreotide does not seem to have clinical effects in PGLs due to the over 20 mm tumor size and has no meaning to perform. On the contrary, liver secondaries not exceeding the 20 mm in diameter, intra-arterially treated, show the same successful results observed in GEP-NET histotype (Chap. 7).
References
Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86.
Hayashi T, Mete O. Head and neck paragangliomas: what does the pathologist need to know? Diagn Histopathol. 2014;20:316–25.
Saveanu A, Muresan M, de Micco C, et al. Expression of somatostatin receptors, dopamine D2 receptors, noradrenaline transporters, and vesicular monoamine transporters in 52 pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2011;18:287–300.
Reubi JC, Waser B, Liu Q, Laissue JA, et al. Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: membranous versus intracellular location. J Clin Endocrinol Metab. 2008;85:3882–91.
Binderup T, Knigge U, Mellon Mogensen A, et al. Quantitative gene expression of somatostatin receptors and noradrenaline transporter underlying scintigraphic results in patients with neuroendocrine tumors. Neuroendocrinology. 2008;87:223–32.
Koopmans KP, Jager PL, Kema IP, et al. 111In-octreotide is superior to 123I-metaiodobenzylgianidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med. 2008;49:1232–7.
Charrier N, Deveze A, Fakhry N, et al. Comparison of [111In] pentetreotide-SPECT and [18F]FDOPA-PET in the localization of extra-adrenal paragangliomas: the case for a patient-tailored use of nuclear imaging modalities. Clin Endocrinol. 2011;74:21–9.
Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–82.
Wild D, Schmitt JS, Ginj M, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003;30:1338–47.
Sajid MS, Hamilton G, Baker DM, Joint Vascular Research Group. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007;34:127–30.
Chan JKC, Kimura N, Capella C, et al. Chapter 10: Paraganglion tumours. In: El-Nigger AK, Chan JKC, Grandis JF, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. 4th ed. Lyon: IARC; 2017.
Blumenfeld J, Cohen N, Anwar M, et al. Hypertension and a tumor of the glomus jugular region. Evidence for epinephrine biosynthesis. Am J Hypertens. 1993;6:382–7.
Piccini V, Rapizzi E, Bacca A, et al. Head and neck paragangliomas: genetic spectrum and clinical variability in 79 consecutive patients. Endocr Relat Cancer. 2012;19:149–55.
Lam KY, Lo CY, Wat NMS, et al. The clinicopathological features and importance of p53, Rb and mdm2 expression in pheochromocytomas and paragangliomas. J Clin Pathol. 2001;54:443–8.
Lam KY, Chan ACL. Paraganglioma of urinary bladder: an immunohistochemical study and report of an unusual association with intestinal carcinoid. Aust N Z J Surg. 1993;63:740–5.
Garg A, Mishra D, Bansal M, et al. Right atrial paraganglioma: an extremely rare primary cardiac neoplasm mimicking myxoma. J Cardiovasc Ultrasound. 2016;24:334–6.
Michałowska I, Ćwikła J, Prejbisz A, et al. Mediastinal paragangliomas related to SDHx gene mutations. Kardiochir Torakochirurgia Pol. 2016;13:276–82.
Soomro NH, Zahid AB, Zafar AA. Non-functional paraganglioma of the mediastinum. J Pak Med Assoc. 2016;66:609–11.
Blanchet EM, Martucci V, Pacak K. Pheochromocytoma and paraganglioma: current functional and future molecular imaging. Front Oncol. 2012;1:58.
Assadipour Y, Sadowski SM, Alimchandani M, et al. SDHB mutation status and tumor size but not tumor grade are important predictors of clinical outcome in pheochromocytoma and abdominal paraganglioma. Surgery. 2017;161:230–9.
Coresmitt EP, Romijn JA. Clinical management of paragangliomas. Eur J Endocrinol. 2014;171(6):232–43.
Berends AMA, Buitenwerf E, de Krijger R, et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: a nationwide study and systematic review. Eur J Intern Med. 2018;51:68–73.
Lenders JW, Duh QY, Eisenhofer G. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42.
Jimenez C, Rohren E, Habra MA, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15:356–71.
Fishbein L, Orlowski R, Cohen D. Pheochromocytoma/paraganglioma; review of perioperative management of blood pressure and update on genetic mutations associated with pheochromocytoma. J Clin Hypertens (Greenwich). 2013;15:428–34.
Elston MS, Meyer-Rochow GY, et al. Increased SSTR2A and SSTR3 expression in succinate dehydrogenase-deficient pheochromocytomas and paragangliomas. Hum Pathol. 2015;46:390–6.
Lenders JWM, Eisenhofer G. Update on modern management of pheochromocytoma and paraganglioma. Endocrinol Metab. 2017;32:152–61.
van Essen M, Krenning EP, Kooij PP, et al. Effects of therapy with [177Lu-DOTA0, Tyr3]-octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47(10):1599–606.
Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90YDOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416–23.
Zovato S, Kumanova A, Demattè S, et al. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Horm Metab Res. 2012;44(5):411–4.
Cecchin D, Schiavi F, Fanti S, et al. Peptide receptor radionuclide therapy in a case of multiple spinal canal and cranial paragangliomas. J Clin Oncol. 2011;29(7):e171–4.
Pinato DJ, Black JR, Ramaswami R, et al. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol. 2016;33(5):47.
Ashwathanarayana AG, Biswal CK, Sood A, et al. Imaging guided use of combined 177Lu-DOTATATE and capecitabine therapy in metastatic mediastinal paraganglioma. J Nucl Med Technol. 2017;45(4):314–6.
Kong G, Grozinsky-Glasberg S, Hofman MS, et al. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab. 2017;102(9):3278–87.
Nastos K, Cheung VT, Toumpanakis C, et al. Peptide Receptor Radionuclide Treatment and (131)I-MIBG in the management of patients with metastatic/progressive pheochromocytomas and paragangliomas. J Surg Oncol. 2017;115(4):425–34.
Garske-Román U, Sandström M, Fröss Baron K, et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45(6):970–88.
Yadav MP, Ballal S, Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res. 2019;9:13. https://doi.org/10.1186/s13550-019-0484-y.
Vyakaranam AR, Crona J, Norlén O. Favorable outcome in patients with pheochromocytoma and paraganglioma treated with 177Lu-DOTATATE. Cancers. 2019;11(7):909. https://doi.org/10.3390/cancers11070909.
Kolasinska-Cwikła A, Peczkowska M, Cwikła JB. A clinical efficacy of PRRT in patients with advanced, nonresectable, paraganglioma-pheochromocytoma, related to SDHx gene mutation. J Clin Med. 2019;8:952. https://doi.org/10.3390/jcm8070952www.mdpi.com/journal/jcm.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Limouris, G.S., Krylov, V., Dolgushin, M.B., Zafeirakis, A.G. (2021). 111In-Octreotide Infusions for the Treatment of Paraganglioma. In: Limouris, G.S. (eds) Liver Intra-arterial PRRT with 111In-Octreotide. Springer, Cham. https://doi.org/10.1007/978-3-030-70773-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-70773-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70772-9
Online ISBN: 978-3-030-70773-6
eBook Packages: MedicineMedicine (R0)