Abstract
Electrochemical Micromachining (EMM) is a non-conventional technique that has the potential to provide excellent accuracy due to its ionic dissolution nature. The technique is evolving rapidly with continuous research works and emerging as a frontline technology in the micro-fabrication domain. The dominant input factors of electrochemical machining (ECM) process become very sensitive at micro-domain and parametric optimization is inevitable for enhanced performance. New researches are required to enhance every aspect of the processing system that includes electric power, electrolyte, feed mechanism, gap control etc. The aim of the current work is to produce micro-holes on SS-304 sheet using tungsten tool and citric acid electrolyte through EMM process. An EMM device fabricated for experimentation purpose with pulse generator was utilized in this research. The operating factors preferred for study are voltage, current, pulse-on-time & electrolyte concentration. Taguchi’s L9 design for trials and Grey Relational Analysis (GRA) for multi response optimization were employed. The optimum set of parameters obtained through GRA included 12 V,1.2 A, 15 ms of pulse-on-time period and 20 g/l of solution concentration. The most influential factor observed was pulse-on-time. Though machining was slow with citric acid as electrolyte, holes produced were of good accuracy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Electrochemical micromachining
- SS304
- Citric acid solution
- Grey relational analysis
- Machining rate
- Overcut
1 Introduction
Electrochemical micromachining is emerging as one of the capable techniques among the non-conventional machining methods that are preferred for its accuracy, surface finish along with other favorable outcomes. EMM is considered as a complex process because the parameters that are not significant at macro level become very sensitive at micro level [1]. The effect of scale on electrochemical machining (ECM) applied in the micro level was investigated by Mouliprasanth et al. and stated that operating parameters require down scaling for similarity between macro and micro ECM [2]. .As the process requires research for numerous combination of materials and other input variations, this technique is under constant investigation to overcome the challenges and improve its capability. The electrochemical machining has been brought into micromachining domain because of the continuous works of the researchers world-wide. Reducing the inter-electrode-gap (IEG) without distracting the performance of EMM was a great challenge encountered by the researchers. By employing ultra-short pulses, the machining gap was considerably reduced by Schuster et al. to realize the potential utilization of EMM in the micro-fabrication domain. The research used a mixed electrolyte of CuSO4 + HClO4 to machine copper [3]. Also the EMM experimental setups are being developed by different organizations, laboratories, educational institutions and individual researchers all over the world to enhance the capability and refine the various sub-systems and components involved in the process. An EMM setup with pulsed power was designed in which experiments were carried out successfully with precision by Bhattacharyya et al. The IEG was reduced to the lowest possible micrometer range to achieve better accuracy. The inter electrode gap was monitored by detecting the value of current at the gap through a sensor. The experiments also revealed the impact of various input parameters on performance parameters and emphasized the need for optimization of process parameters [4]. Mithu et al. designed and developed an EMM setup having three co-ordinate movements of tool and automation of tool-feed system with computer monitoring [5]. To avoid deposition of sludge on micro-tool, it was made to vibrate during machining by Ghoshal et al. and obtained improved performance [6].
Micro tools with different shapes and aspect ratios have been experimented in EMM to find their influence on the performance [1]. Thanigaivelan et al. conducted EMM experiments with micro tools having different tip shapes to investigate their effect on the process and found the conical shaped tools to provide better performance [7]. To avoid stray machining and as well the taper effect on the workpieces having higher depths, the side surface of the tool requires insulation [8, 9]. The surface quality of the work materials have been improved by using masks in the EMM process [10]. Kumar et al. have utilized reusable masked tool to produce micro square pattern on SS-304 material through EMM [11]. An electrolyte flushing technique for continuous flushing of passive layer and sludge from the electrode interface was utilized for improving the circularity of the micro-hole fabricated through EMM by Pooranachandran et al. [12]. A sensor arrangement was used to maintain constant flow velocity in the electrode gap to cause improvement in the performance of the EMM process by Geethapriyan [13]. Apart from metals, materials such as alloys and electrically conducting composites of different types have also been machined for micro-features using EMM and the input parameters optimized [14, 15].
Using appropriate electrolyte for a particular materials is very important in EMM process. It is the electrolyte which carries the ionic charges and causes dissolution of the anode work material. With every experimental research, electrolytes for different materials are identified and data base made available. Aluminium metal matrix composites have been machined through EMM using NaNO3 (sodium nitrate) and NaCl (sodium chloride), considered as neutral solutions to investigate the variation in performance by Hackert et al. [16]. For machining very hard alloys, a small fraction of acid is added in the neutral electrolytes which otherwise becomes very difficult to machine through EMM process [17]. Some alloy compositions having multiple elements require mixed electrolytes for machining through EMM. A composite solution containing NaNO3 and NaCl was used for machining Cr12Ni9Mo4Cu2 and the input parameters were optimized by Tang et al. [18]. A material called ‘Gamma-Titanium—Aluminium-Intermetallic’ used in engine applications in aero industry which has poor machinability was machined through EMM using NaCl solution successfully by Liu et al. [19]. Eco-friendly electrolytic solution like citric acid solution has also been attempted to machine steel under EMM and found to give excellent performance by Ryu [20]. Even pure water as electrolyte was used with an ion-exchange catalyst to machine stainless steel work-material under EMM process successfully by Huaiqian et al. [21]. The performance of the EMM process is affected mainly by the contaminants. The various contaminants such as conductive/non-conductive particles, chemical by-products, sludge, gas bubbles that hinder the machining gap and lower the performance of the process were investigated by Schulze et al. [22].
Due to complex nature and interaction between input factors, EMM process requires multi-objective optimization to get optimal parametric combination to maximize the performance. Researchers have been applying many different optimization techniques and their combinations to enhance the predictions. In the optimization of micro-EDM process variables for SS-316L material, the response function modeled through RSM was utilized in GA (genetic algorithm) to get optimal set for maximum MRR (material removal rate) and minimum TWR (tool wear rate) by Suresh et al. [23]. In the ECM of die-tool-steel (containing high carbon & high chromium), copper nano-particles mixed NaNO3 solution (nano-fluid) was utilized as electrolyte to reduce spike formation. Also MOGA (multiobjective genetic-algorithm) was applied for achieving enhanced MRR and surface quality through optimization of voltage and tool-feed levels by Sathiyamoorthy et al. [24]. The ECM process parameters such as voltage and electrolyte feeding rate were optimized in the machining of AISI-202 austenitic SS material to attain improved MRR and surface texture by making use of RSM [25]. Researchers have also optimized EMM operating parameters using Taguchi technique, GRA (Grey relational analysis) and other techniques [26, 27]. The current research involves electrochemical micromachining of SS304 steel work-material using citric acid to generate micro-holes and multi objective optimization of the input parameters through GRA.
2 Materials and Experimental Details
The details of experimental setup used and the materials used for experimentation along with input parameters are discussed below.
2.1 Experimental Device for EMM Process
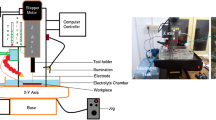
An experimental setup built in-house was utilized to perform electrochemical micromachining experiments. The experimental arrangement is presented in Fig. 1. The setup is an assembly of all the essential sub-units required for EMM operation like mechanical support elements, micro-tool feeding system with stepper motor and lead-screw provision, micro-controller kit to monitor tool-feed and a pulse rectifier unit to supply pulsed power. It is a single axis machine with only vertical movement of the tool.
2.2 Input Factors Used for the Experiments
The work material used in this experimentation was SS-304 sheet of 45 mm × 43 mm × 200 µm size. The tool was made of tungsten with a diameter of 380 µm. The lateral surface of the tool was insulated to enable localized machining. Citric acid (C6H8O7) solution which is considered as an environment friendly electrolyte was used for machining. Citric acid is normally extracted from citrus fruits (lime, lemon etc.), vegetables and plants and this organic compound belongs to the group of carboxylic acids. It is applied for metal cleaning purposes and has the potential to prevent precipitates formed during machining that are insoluble in nature and cause hindrance to the process [20]. Organic acids are generally considered as weak electrolytes with less conducting capabilities [28].
The input parameters selected for study were supply voltage (Volts), current (Ampere), pulse on-time (in milliseconds: ms) and electrolyte concentration (gram/litre: g/l). The frequency at 50 Hz and the electrode gap at 40 µm were kept as constant values. The machining rate (MR) which reveals the speed of the process and the overcut (OC) which shows the accuracy of the generated micro-hole dimensionally were taken to assess the performance. The micrograph of the micro tool is presented in Fig. 2.
2.2.1 Estimation of Performance Characteristics
The estimation of some important characteristics in EMM process are given below.
where Pulse period is equal to ‘Pulse on-time + Pulse off-time’
For example 50 Hz denotes 50 pulses/s which means 1/50 = 20 ms duration for each pulse. Now a duty cycle of 1:1 indicates 10 ms pulse on-time and 10 ms pulse-off-time
In pulse on-time period, the machining occurs while the pulse- off-period is utilized for flushing the by-products and other contaminants from the electrode gap by the passing electrolyte.
Preliminary trials were performed to decide the initial level of selected input parameters. When the voltage level was kept below 8 v, no dissolution of the workpiece was observed for a long time. Hence 8 V served as initial base value and other factors were set accordingly. The experimental design followed was Taguchi’s L9 orthogonal array with four factors, each at three levels. The input parameters were optimized using GRA multi-objective optimization technique.
3 Optimization by GRA
The GRA technique was applied for optimization of EMM process in which the required outcome of each performance parameter may vary as higher machining rate and lower overcut are preferred. GRA could convert a process having multiple objectives into a process of single base objective that gives an optimal solution to satisfy all the objectives. The technique uses a sequences of mathematical expressions to reduce the multi-objectives into common relational grade for comparing and finding the solution [29].
The input factors, their levels used in the L9 design and the outcomes attained are presented in Table 1.
3.1 Different Stages of GRA
Data processing or Normalization is the first step in this method in which the different units of the performance characteristics are made comparable by converting them to fit into a range of values between 0 and 1. Thus the original sequence of values is now converted into a comparable sequence by normalizing the values.
In accordance with targeted value designed to obtain, the expression for normalization varies as follows.
To obtain the characteristic of ‘larger the better’, the following expression is applied.
where \( x_{i}^{o} (k) \) is originally existing sequence, \( x_{i}^{\text{ * }} (k) \) is sequence obtained after the data underwent preprocessing, \( \hbox{max} \,x_{i}^{o} (k) \) implies the largest value of \( x_{i}^{o} (k) \), and \( \hbox{min} \,x_{i}^{o} (k) \) implies the smallest value of \( x_{i}^{o} (k) \).
For “the lower-the better”, the normalization expression is:
Grey relational coefficient (GRC) is found out next. This value expresses the correlation between normalized quality characteristics of ideal and actual values. It is given by:
where \( x_{i}^{o} \) is the ideal results of normalization obtained for the ith characteristics and \( \zeta \) is the distinctive coefficient, defined within the range of \( 0\,{ \le }\,\zeta \,{ \ge }\,1 \).
GRG (Grey relational grade): To reduce the number of GRCs requiring comparison, the sequences are converted into its mean values which are referred as GRGs. The GRGs are obtained by:
Here, αj is the GRG for jth trial and N provides the number of response parameters.
3.2 GRA Results
The results obtained at various stages of GRA process for the EMM performance characteristics like Machining rate (higher-the–better) and Overcut (lower-the-better) are summarized below in Table 2. The table includes S/N ratio values, normalized values, GRC values and the GRG values obtained through the mathematical equations at different stages of the GRA calculations.
The Table 2 provides grey relational grade for all the nine EMM experiments conducted to generate micro-hole on the sheet of SS-304 utilizing tungsten tool and solution of citric acid. The grey relational grade with highest value is considered to be nearer to the ideal value. The table shows experiment number 2 as having the highest grade of 0.848 that gives the best multiple performance characteristics. The parametric combination includes 8 V (Volts), 1.2A (Ampere), 15 ms (‘Pulse-on-time’ period) and 20 g/l (concentration of electrolyte).
3.2.1 Analysis of Response Table for Means
The response table for means reveals the influence of each input factor on the process. The response tale for means is depicted below in Table 3.
The response table values clearly reveal that pulse on-time as the most significant factor that is followed by electrolyte concentration, supply current and supply voltage in that order for their influence on the electrochemical micromachining process of SS-304 by citric acid solution.
3.2.2 Analysis of Response Plot of GRG
The average response table as well as the average response graph could provide the optimal levels of each input factor of the EMM process. The mean response plot of the GRG is presented in Fig. 3. It can graphically show the significance of each parameter on the basis of mean values. With reference to the response-table and the response-plot, the optimal set could be formed as V3−A2−T–on2−EC2
.
The response graphs of GRG presents the corresponding variation of the response while each parameter’s level changes from 1 to 3. Based on response graph for GRG revealed in Fig. 3, the optimum combination of machining parameters are found to be: The voltage at level-3 (12 V), current at level-2 (1.2A), pulse-on-time at level-2 (15 ms) and concentration of electrolyte at level-2 (20 g/l).
4 Discussion
The observations made on the process of generating micro-holes on SS-304 material through electrochemical micromachining by using tungsten tool and citric acid as electrolyte have been discussed below.
4.1 Optimal Parametric Combination
The performance parameters evaluated in the process of making micro-holes by EMM are machining rate and overcut. The requirement is that of higher machining rate and lower overcut. The Grey relational analysis technique used for multi-objective optimization has produced an optimal combination comprising a voltage of 12 V, supply current of 1.2 A, pulse on-time period of 15 ms and an electrolytic concentration of 20 g/l among the different input combinations used in the experiments.
An analysis of the above combination shows that the voltage at highest level (level 3) would account for the higher machining speed of the process whereas the middle level inputs of current, pulse on-time and electrolytic concentration (all at level 2) contribute for the moderate speed of the process as well as reduction in overcut. Past literatures on EMM process have revealed that increase in applied voltage increases machining rate but reduces the accuracy. This is because, tendency for stray machining increases at higher voltages resulting in overcut. Using a weak electrolyte like citric acid also reduces the machining speed which leads to improved process accuracy [28].
4.2 How the Input Factors Affect the EMM Process?
Bhattacharyya et al. investigated the effect of various input parameters on the performance of EMM process during the investigation to generate micro-holes on copper sheet. The report states that the input factors affect the process in such a way that improved accuracy can be achieved only along with moderate machining speed. Such an input combination include voltage at higher level, moderate level of pulse on-time (to provide enough pulse off-time to flush the sludge from the inter electrode gap) and the electrolyte at lower concentration level [30].
Higher voltage normally increases the machining rate as it causes increase in current, current density (current/area) and electric field (voltage/unit length) which increase the dissolution rate at the machining gap. But if it exceeds optimum level, tendency for the evolution of H2 at the cathode tool increases and also these gas bubbles may get trapped in the machining gap as a result of which current density of the anode workpiece is lowered and electrolyte resistivity is increased thereby reducing the machining rate [31].
Normally the machining rate is increased for increased electrolyte concentration as more conducting ions are available for dislodging the ions from the anode work material as per the investigation of Ayyappan et al. in the machining of an alloy steel with a mixed electrolyte [32]. But there is an optimum limit up to which this increase in machining rate can happen after which the ions tend to clog the electrode gap and disrupt the machining. Ghoshal et al. employed H2SO4 (sulphuric acid) solution to produce micro-channels on SS-304 material and observed that electrolyte concentration of lower level as well as higher level, both result in stray machining. The study revealed that only moderate level of electrolyte concentration could serve as optimum level [33]. With higher level of electrolyte concentration, more ions are released in the solution and their mobility may be curtailed due to attraction between ions. If more ions are available in the electrolyte solution, as charge carriers their chaotic distribution around the inter electrode gap cause non-uniform electric field resulting in stray dissolution.
For rise in pulse on-time, the machining rate also gets raised. Increase in duty-cycle (i.e. pulse on-time) simultaneously decreases pulse off-time. Hence, if the pulse-off-time period is insufficient to eliminate the reaction products from the electrode gap, it may lead to short-circuit between the anode (workpiece) and the cathode (tool) causing micro sparks that produce overcut. Again if the pulse on-period is longer, the electrolyte solution at the gap may get heated up causing sparks and stray cut.
4.3 Micro-hole Images
The micrographs of the micro-holes machined on SS-304 sheet using citric acid solution are shown in Figs. 4 and 5. The micrographs show moderate quality of circular holes, the circumference of which have slightly rough edges. The usage of citric acid as electrolyte has resulted in the reduction of overcut considerably. Citric acid being a weak electrolyte provides a very slow machining rate compared to other inorganic electrolytes. However it produces good quality holes with less stray cut.
5 Conclusions
An experimental EMM setup was utilized to generate micro-holes on a sheet of SS-304, using a tool made of tungsten material and citric acid as electrolyte. Taguchi’s design of L9 was applied to conduct the experiments and the input parameters were optimized through GRA multi-objective optimization technique. The important conclusions are noted down.
-
Citric acid which is an environment friendly electrolyte can be successfully utilized in the EMM process for micromachining.
-
The machining speed of citric acid is slow due to its weak acidic nature. However it improves the accuracy of the drilled holes.
-
Among the input parameters studied, ‘pulse-on-time’ was found to be the most significant factor followed by electrolyte concentration, supply current and supply voltage in that order for their influence on the electrochemical micromachining process of SS-304 by citric acid solution.
-
The optimum combination of machining parameters found through GRA is: The voltage at level-3 (12 V), current at level-2 (1.2A), pulse-on-time at level-2 (15 ms) and concentration of electrolyte solution at level-2 (20 g/l) for maximizing the machining rate and minimizing the overcut.
References
Bhattacharyya, B. (2015). Electrochemical micromachining for nanofabrication, MEMS and nanotechnology. UK: William Andrew Publications.
Mouliprasanth, B., & Hariharan, P. (2020). Scaling approach towards electrochemical micromachining: A method to evaluate similarity. The International Journal of Advanced Manufacturing Technology. (Springer-Verlag London Ltd., part of Springer Nature). https://doi.org/10.1007/s00170-020-05604-3.
Schuster, R., Kirchner, V., Allongue, P., & Ertl, G. (2000). Electrochemical micromachining. Science, 289, 98–101.
Bhattacharya, B., Doloi, B., & Sridhar, P. S. (2001). Electrochemical micro-machining: New possibilities for micro-manufacturing. Journal of Materials Processing Technology, 113, 301–305.
Mithu, M. A. H., Fantoni, G., & Ciampi, J. (2011). A step towards the in process monitoring for electrochemical microdrilling. International Journal of Advanced Manufacturing Technology, 57, 969–982. https://doi.org/10.1007/s00170-011-3355-x.
Ghoshal, B., & Bhattacharyya, B. (2013). Influence of vibration on micro-tool fabrication by electrochemical machining. International Journal of Machine Tools and Manufacture, 64, 49–59.
Thanigaivelan, R., & Arunachalam, R. M. (2010). Experimental study on the influence of tool electrode tip shape on electrochemical micromachining of 304 Stainless steel. Materials and Manufacturing Processes, 25, 1181–1185.
Rathod, V., Doloi, B., & Bhattacharyya, B. (2014). Sidewall insulation of microtool for electrochemical micromachining to enhance the machining accuracy. Materials and Manufacturing Processes, 29(3), 305–313. https://doi.org/10.1080/10426914.2013.864407.
Jain, V. K., Kalia, S., Sidpara, A., & Kulkarni, V. N. (2012). Fabrication of micro-features and micro-tools using electrochemical micromachining. The International Journal of Advanced Manufacturing Technology, 61(9–12), 1175–1183.
Wang, Q., Xiao, J., & Li, Y. (2011). Experimental study on the through-mask electrochemical micromachining (EMM) Process. Advanced Materials Research, 189–193, 69–692. (Trans Tech Publications, Switzerland).
Kumar, S., & Bhattacharyya, B. (2018). Electrochemical micromachining of micro square pattern using reusable masked tool. Materials and manufacturing processes. Taylor & Francis. https://doi.org/10.1080/10426914.2018.1532582.
Pooranachandran, K., Deepak, J., Hariharan, P. & Mouliprasanth, B. (2019). Effect of flushing on electrochemical micromachining of copper and inconel 718 alloy. Advances in Manufacturing Processes, Lecture Notes, in Mechanical Engineering. Springer Nature Singapore Pte Ltd. https://doi.org/10.1007/978-981-13-1724-8_6.
Geethapriyan, T., Manoj Samson, R, Thavamani, J., Arun Raj, A. C., & Pulagam, B. R. (2019). Experimental investigation of electrochemical micro-machining process parameters on stainless steel 316 using sodium chloride electrolyte. Advances in Manufacturing Processes, Lecture Notes, in Mechanical Engineering. Springer Nature Singapore Pte Ltd. https://doi.org/10.1007/978-981-13-1724-8_6.
Subburam, V., Ramesh, S., Mohan Kumar, P. N., & Srinivasan, A. (2018). Performance optimization of electrochemical micromachining of micro-holes on inconel 625 alloy. International Journal of Precision Technology, 8(1), 66–84.
Ramesh, S., & Subburam, V. (2019). Electrochemical micromachining of aluminium alloy composite. Advances in Manufacturing Technology, Lecture notes in Mechanical Engineering, 309–317. https://doi.org/10.1007/978-981-13-6374-0_36.
Hackert-Oschatzchen, M., Lehnert, N., Martin, A., & Schubert, A. (2016). Jet-electrochemical machining of particle reinforced aluminum matrix composites with different neutral electrolytes. IOP Conference Series: Materials Science and Engineering, 118, 012036.
Subburam, V., Ramesh, S., Arunachalam, R. M., & Thanigaivelan, R. (2013). Effect of acidified electrolyte on the performance of electrochemical micromachining, in Proceedings of the Second International Conference on Advances in Materials Processing and Characterisation (AMPC 2013), vol. II, pp. 799–806.
Tang, L., & Yang, S. (2013). Experimental investigation on the electrochemical machining of 00Cr12Ni9Mo4Cu2 material and multi-objective parameters optimization. International Journal of Advanced Manufacturing Technology, 67, 2909–2916.
Liu, J., Zhu, D., Zhao, L., & Xu, Z. (2015). Experimental investigation on electrochemical machining of γ-TiAl intermetallic, 15th machining innovations conference for aerospace industry. Procedia CIRP, 35, 20–24.
Ryu, S. H. (2009). Micro fabrication by electrochemical process in citric acid electrolyte. Journal of Materials Processing Technology, 209, 2831–2837. https://doi.org/10.1016/j.jmatprotec.2008.06.044.
Huaiqian, B., Jiawen, X., & Ying, L. (2008). Aviation-oriented micromachining technology—micro-ECM in pure water. Chinese Journal of Aeronautics, 21, 455–461.
Schulze, H. P., & Schatzing, W. (2013). Influences of different contaminations on the electro-erosive and the electrochemical micro-machining, The Seventeenth CIRP Conference on Electro Physical and Chemical Machining (ISEM), Procedia CIRP, vol. 6, pp. 58–63.
Suresh, P., Venkatesan, R., Sekar, T., Elango, N., & Sathiyamoorthy, V. (2014). Optimization of intervening variables in micro EDM of SS316L using a genetic a algorithm and response-surface methodology, strojniski vestnik. Journal of Mechanical Engineering, 60(10), 656–664. https://doi.org/10.5545/sv-jme.2014.1665.
Sathiyamoorthy, V., Sekar, T., & Elango, N. (2015). Optimization of processing parameters in ECM of die tool steel using nanofluid by multiobjective genetic algorithm, Article ID895696. https://doi.org/10.1155/2015/895696.
Sathiyamoorthy, V., Sekar, T., Suresh, P., Vijayan, R., & Elango, N. (2015). optimization of processing parameters in electrochemical machining of AISI 202 using response surface methodology. Journal of Engineering Science and Technology, 10(6), 780–789.
Krishnan, R., Duraisamy, S., Palanisamy, P., & Veeramani, A. (2018). Optimization of the machining parameters in the electrochemical micro-machining of nickel. Materials and technology, 52(3), 253–258.
Wang, M., Shang, Y., He, K., Xuefeng, X., & Chen, G. (2019). Optimization of nozzle inclination and process parameters in air-shielding electrochemical micromachining. Micromachines, 10, 846. https://doi.org/10.3390/mi10120846.
Leese, R., & Ivanov, A. (2018). Electrochemical micromachining: Review of factors affecting the process applicability in micro-manufacturing. Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture, 232(2) 195–207. (sagepub.co.uk/journalsPermissions.nav). https://doi.org/10.1177/0954405416640172.
Deng, J. (1989). Introduction to grey theory. Journal of Grey Systems, 1(1), 1–2.
Bhattacharyya, B., Munda, J., & Malapati, M. (2004). Advancement in electrochemical micro-machining. International Journal of Machine Tools and Manufacture, 44(15), 1577–1589.
Bilgi, D. S., Jain, V. K., Shekhar, R., et al. (2007). Hole quality and inter electrode gap dynamics during pulse current electrochemical deep hole drilling. The International Journal of Advanced Manufacturing Technology, 34(1–2), 79–95.
Ayyappan, S., & Sivakumar, K. (2015). Investigation of electrochemical machining characteristics of 20MnCr5 alloy steel using potassium dichromate mixed aqueous NaCl electrolyte and optimization of process parameters. Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture, 229, 1984–1996.
Ghoshal, B., & Bhattacharyya, B. (2013). Micro electrochemical sinking and milling method for generation of micro features. Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture, 227(11), 1651–1663.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Subburam, V., Ramesh, S., Freitas, L.I. (2021). Optimization and Effect Analysis of Sustainable Micro Electrochemical Machining Using Organic Electrolyte. In: Palanikumar, K., Natarajan, E., Sengottuvelu, R., Davim, J.P. (eds) Futuristic Trends in Intelligent Manufacturing. Materials Forming, Machining and Tribology. Springer, Cham. https://doi.org/10.1007/978-3-030-70009-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-70009-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70008-9
Online ISBN: 978-3-030-70009-6
eBook Packages: EngineeringEngineering (R0)