Abstract
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility, for which the insulin sensitizer metformin has been used therapeutically. It has been shown that curcumin also exhibits insulin-sensitizing properties. Given that metformin acts as an ovulation inducing agent and both curcumin and metformin can reduce insulin resistance, the aim of the current study was to evaluate the effect of metformin with and without curcumin nanomicelles in the treatment of women with polycystic ovary syndrome. This clinical trial was conducted on 100 women with PCOS, diagnosed according to the Rotterdam criteria, who were sequentially recruited and randomly divided into two groups (n = 50 each). Group 1 received 500 mg metformin three times daily and group 2 received 80 mg/day capsule of curcumin nanomicelle and 500 mg metformin three times a day for 3 months. After collecting fasting blood samples, biochemical parameters including triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol, plasma glucose, alanine amino transferase (ALT) and aspartate aminotransferase (AST) were evaluated based on enzymatic methods. Hormonal parameters were assessed using immunoassay kits. Insulin resistance (HOMA-IR) and insulin-sensitivity check index (QUICKI) were also assessed. After treatment, fasting insulin, HOMA-IR, and total testosterone in group 2 were significantly lower than those in group 1 (p < 0.05). Post-treatment LDL-C levels in groups 1 and 2 were 117.9 ± 24 and 91.12 ± 19.46 mg/dL, respectively (p < 0.01). In addition, HDL-C levels were increased with curcumin (group 1: 38.1 ± 4.36 mg/dL; group 2: 44.12 ± 7.3 mg/dL, p < 0.05). Total cholesterol was decreased with curcumin level (group 1: 207.9 ± 39.84 mg/dL; group 2; 159.7 ± 48.43 mg/dL, p < 0.05), with a decrease in triglycerides levels (group 1: 141.6 ± 9.57; group 2: 97.5 ± 8.8 mg/dL, p < 0.01). This study showed that curcumin has a synergistic effect with metformin in the improvement of insulin resistance and lipid profile in patients with PCOS. Therefore, the combined use of metformin and curcumin may have therapeutic utility in patients with PCOS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous and complex disease [1, 2] characterized by chronic anovulation, menstrual irregularity and hyperandrogenism [1], with associated infertility and metabolic disturbances including insulin resistance, glucose intolerance, hypertension, obesity [3], and dyslipidemia [1] in women of reproductive age. PCOS is a polygenic disorder influenced by genetic susceptibility and environmental risk factors. Among environmental parameters, lifestyle aspects including sedentary behavior and improper diet contribute to the pathogenesis of PCOS [3, 4].
Metformin is the most commonly used drug in the treatment of type 2 diabetes that improves the sensitivity of peripheral tissues to insulin, prevents hepatic gluconeogenesis and decreases oxidative stress. Moreover, the use of this drug decreases androgen levels and increases the levels of sex hormone binding globulin (SHBG) [1]. In addition, it improves metabolic syndrome in patients with PCOS. Therefore, the use of metformin in women with PCOS is common. However, it is associated with the side effects of abdominal pain, nausea, diarrhea, anorexia, flatulence and bloating [1].
Curcumin is an extracted polyphenol from turmeric, which is safe and endowed with numerous biological effects [5,6,7,8,9,10,11,12]. Anti-inflammatory, anti-obesity and anti-diabetic properties of curcumin are reported in some studies [13]. Curcumin exerts anti-inflammatory effects by modulating cytokines and chemokines [14,15,16], and it shows antioxidant activities by inhibiting reactive oxygen species and inducing an antioxidant response [17]. In addition, favorable effects of curcumin on cancers may be due to direct anti-inflammatory and anti-oxidative effects as well as its ability to modulate the immune system. Recently, some studies have reported that curcumin through reducing luteinizing hormone (LH) can induce ovulation and modulate ovarian responsiveness [18]. The effect of curcumin in diabetes is through decreasing death of pancreatic islet beta cells, preventing insulin resistance and improving beta cell function. Current research on curcumin has concentrated on nanocarrier delivery systems including nanostructured lipid carrier and nanoparticles to enhance aqueous stability, solubility and bioavailability [19]. Therefore, it seems that nanocarrier and nanomicelles can be considered as promising delivery systems for curcumin [14].
Given that insulin resistance plays an important role in the pathogenesis of PCOS [20], we hypothesized that insulin resistance would be reduced to a greater degree with nanomicelle curcumin plus metformin in comparison to metformin alone in the treatment of women with PCOS.
9.2 Materials and Methods
9.2.1 Sample Selection
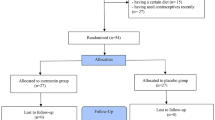
This clinical trial study was conducted on 100 women with PCOS presented sequentially to the Shahid Sadoughi Hospital- Yazd- Iran from March 2017 to March 2018 and were randomized either into one or the other clinical trial arms . All patients fulfilled the Rotterdam criteria for diagnosis with 2 out of 3 features of oligomenorrhea/amenorrhoea, clinical or biochemical hyperandrogenism (Ferriman-Gallwey score >8; free androgen index >4 respectively), and polycystic ovaries on transvaginal ultrasound (≥ 12 antral follicles in at least one ovary or ovarian volume of ≥10 cm3) [21]. Study participants had no concurrent illness, were not on any medication for the preceding 9 months and were not planning to conceive. Non-classical 21-hydroxylase deficiency, hyperprolactinaemia, Cushing’s disease and androgen-secreting tumors were excluded by appropriate tests. Written informed consent was given by each participant and the study was approved by ethics committee (IR.SSU.MEDICINE.REC.1396.195) of Shahid Sadoughi University. The study was recorded in the Iranian Registry of Clinical Trial system with number IRCT20090422001836N11. The patient flowchart in shown in Fig. 9.1.
9.2.2 Classification of Patients
Patients were randomly divided into two groups (n = 50), using a random number table. The first group received 500 mg metformin three times daily and the second group received 80 mg/day capsule of curcumin nanomicelle (Exir Nano Sina Co, Tehran, Iran) and 500 mg metformin three times daily. In each group, treatment was undertaken for 12 weeks.
9.2.3 Analysis of Biochemical and Hormonal Parameters
Blood samples were taken from patients after 8 h fasting. After collecting blood samples, serum was separated by centrifugation at 1000 x g for 1 min and frozen at −20 °C for subsequent analyses. Biochemical parameters including fast blood sugar, cholesterol, low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase (AAT) were assessed based on an enzymatic method (Pars Azmoon kit, Tehran, Iran) using a BT-3000 PLUS analyzer. Fasting serum insulin was evaluated by an enzyme-linked immunoadsorbent assay (ELISA) kit (Insulin AccuLite CLIA Kit, Monobind Inc., Lake Forest, CA, USA). TSH was measured based on immunoassay method using an ELISA kit (Padtan Gostar kit, Iran) and Prolactin (PRL) was measured using a PRL assay kit (Padyab Teb kit, Tehran, Iran). Dehydroepiandrosterone (DHEAS) was also measured using an ELISA method (DEMEDITEC Diagnostics GmbH, Kiel, Germany). An ELISA kit was used for measuring serum level of LH and testosterone according to Pidgin Teb kit protocol, and follicle stimulating hormone (FSH) was measured according to the Padyab kit protocol. The homeostatic model assessment (HOMA) for quantify insulin resistance (IR) was calculated using the formula:
The quantitative insulin-sensitivity check index (QUICKI) was assessed based on following formula:
Data including weight, body mass index (BMI), age and gender were extracted from the medical records.
9.2.4 Statistical Analysis
Sample size was calculated using a statistical power of 80%, an α value of 0.05, and a difference in means of insulin levels between intervention and control groups (11μU/mL) [22]. Data trends were visually evaluated for each androgen and non-parametric tests were applied on data that violated the assumptions of normality when analyzed using the Kolmogorov-Smirnov Test. Significance was defined at α = 0.05. All analyses were conducted using IBM-SPSS version 17.0.
9.3 Results
In this study, the age range of patients was 18–40 years old . The mean ages of patients in the two groups did not differ (28.8 ± 2.44 and 29.2 ± 2 years old, respectively). Comparison of biochemical parameters in two groups before treatment is shown in Table 9.1. This revealed no significant difference between the two groups for any of the biochemical parameters before treatment. Table 9.2 shows the between group effect of metformin and metformin/curcumin on biochemical parameters after 3 months treatment. This shows that significant differences were seen between two groups for fasting insulin, HOMA-IR, LDL, HDL, total cholesterol, triglycerides and testosterone (p < 0.05).
Table 9.3 shows the within group comparison of biochemical parameters before and after treatment with metformin alone or curcumin with metformin. Significant differences were seen before and after treatment with metformin, in terms of fasting glucose, fasting insulin, HOMA-IR, QUICKI, fasting glucose/insulin (G/I) ratio, ALT, AST, testosterone, LH and the LH/FSH ratio (p < 0.05). In addition, significant differences were seen before and after treatment of metformin/curcumin, regarding fasting glucose, fasting insulin, HOMA-IR, QUICKI, fasting G/I ratio, cholesterol, triglyceride, LDL, HDL, total testosterone, LH, LH/FSH ratio, AST and ALT (p < 0.05).
9.4 Discussion
This study showed a significant reduction in fasting insulin and HOMA-IR after metformin plus curcumin treatment in women with PCOS. Aguilar et al. evaluated the effect of curcumin supplementation on insulin sensitivity and observed that curcumin consumption decreased insulin resistance and improved glucose tolerance [23], which is consistent with our findings. Additionally, Rahimi et al. observed decreased fasting plasma glucose following the administration of nano-curcumin in diabetic patients [24]. Ameli et al. reported that curcumin improves insulin secretion via reducing plasma glucose in diabetic rats [25]. Another study showed that curcumin improved glucose metabolism by activation of adenosine monophosphate (AMP) kinase in the liver and induced glucose transporter-4 (GLUT-4) expression leading to increased peripheral glucose uptake [26]. Furthermore, it was revealed that curcumin reduced glucose levels and increased insulin secretion through peroxisome proliferator-activated receptor (PPAR)-γ activation [27, 28], explaining the hypoglycemic effects of this natural agent.
In our study, curcumin therapy increased FSH levels and decreased LH, DHEAS, and testosterone concentrations in patients with PCOS. Mohammadi et al. found increased levels of FSH and decreased LH and testosterone concentrations following curcumin treatment in a model of PCOS [1], which is consistent with our results. These effects may be because curcumin inhibits tumor necrosis factor (TNF)-α, interleukin (IL)-6, and C-reactive protein expression, improving ovulation and the corpus luteum [1]. This suggests that this polyphenol could improve the reproductive endocrine function and induce follicular development. However, this study was not designed to look at this specifically. Nabiuni et al. demonstrated that curcumin treatment resulted in improvement of PCOS symptoms and initiation of ovulation via antioxidant and anti-inflammatory effects [18]. Other studies have shown that curcumin induces apoptosis, inhibits pituitary tumor cell proliferation, and reduces the production and release of LH [29].
Yan et al. found that curcumin treatment mitigates oxidative stress, apoptosis, and ovarian injury through regulation of nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathways in mice with induced ovarian failure [30]. Consistent with this, Melekoglu et al. observed that curcumin administration ameliorated ovarian failure by decreasing oxidative stress markers, FSH and LH levels, and improving histophatological parameters [31], suggesting a protective effect of this natural compound against ovarian failure. It is important to note that although the intervention period of our study was too short to demonstrate the regularization of menstrual cycle, the benefits of curcumin on PCOS symptoms and hormonal levels may reflect an amelioration in menstrual and ovulatory cycles. Therefore, longer clinical trials are needed in order to corroborate the potential clinical impact of curcumin on menstrual disorders and endocrine dysfunction.
Beneficial effects of curcumin plus metformin were seen on lipid parameters by a reduction in total cholesterol, LDL, triglycerides, and increased HDL levels. In accordance with our findings, others have reported a significant decrease in total cholesterol, LDL, and triglyceride concentrations after curcumin therapy [32, 38]. These lipid-lowering effects of curcumin could be explained by a number of possible mechanisms, such as increased activity of lipoprotein lipase and fatty acid β-oxidation, and inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase, fatty acid synthase, and acyl coenzyme A cholesterol acyltransferase [33]. Moreover, a previous meta-analysis revealed that curcumin supplementation significantly decreased plasma concentrations of triglycerides and increased HDL levels [34], which is also consistent with our study. This potential cardioprotective action of curcumin could be explained through an increase in lipoprotein lipase activity and hydrolysis of triglyceride-rich lipoproteins, causing reduced circulating triglyceride levels [35]. Also, curcumin may ameliorate HDL function by regulating apolipoprotein-AI, cholesteryl ester transfer protein (CETP), lecithin–cholesterol acyltransferase (LCAT), serum paraoxonase/arylesterase 1 (PON1), and myeloperoxidase (MPO) activities [36]. It is well-known that atherogenic dyslipidemia, characterized by elevated triglycerides and low HDL concentrations, is a central therapeutic target in patients with cardiovascular disease due to the high residual risk of cardiovascular events and microvascular complications [37]. Because curcumin showed a combined effect by raising HDL levels and decreasing triglycerides, this natural compound might be considered as an additional therapeutic option in the treatment of atherosclerotic cardiovascular disease.
Limitations of this study include that the treatment period was short to assess menstrual frequency and ovulatory cycles. However, 3 months were sufficient to found significant effects of curcumin administration on metabolic parameters. Secondly, although our study was not blinded and did not use a placebo, randomization would have minimized this potential source of bias.
The main strength of our study was the sample size which conferred adequate statistical power to support the efficacy of curcumin treatment in PCOS.
9.5 Conclusions
This study suggests that the nanomicellar curcumin exerts a synergistic effect with metformin in the improvement of insulin resistance and the lipid profile in patients with PCOS. Therefore, combined use of metformin and nanomicelle curcumin may be a promising alternative therapy for the treatment of PCOS. However, the clinical benefits of curcumin on menstrual disorders remain to be determined in clinical trials of longer duration.
References
Mohammadi S, Kayedpoor P, Karimzadeh-Bardei (2017) The effect of Curcumin on TNF-α, IL-6 and CRP expression in a model of polycystic ovary syndrome as an inflammation state. J Reprod Infertil 18(4):352–360
Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A (2005) Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab 90(5):2571–2579
Spritzer P, Marchesan L, Betânia R, Santos B, Felipe V, Cureau (2009) Prevalence and characteristics of polycystic ovary syndrome in brazilian women: protocol for a nation-wide case–control study. BMJ Open 9(10):e029191. https://doi.org/10.1136/bmjopen-2019-029191
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS et al (2016) Polycystic ovary syndrome. Nat Rev Dis Primers 2:16057. https://doi.org/10.1038/nrdp.2016.57
Aggarwal BB, Sundaram C, Malani N, Ichikawa (2007) Curcumin: the indian solid gold. Adv Exp Med Biol 595:1–75
Soleimani V, Sahebkar A, Hosseinzadeh H (2018) Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother Res 32(6):985–995.
Momtazi AA, Derosa G, Maffioli P, Banach M, Sahebkar (2016) A role of micrornas in the therapeutic effects of curcumin in non-cancer diseases. Mol Diagn Ther 20(4):335–345
Hassanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar, A (2020) Curcumin: an inflammasome silencer. Pharmacol Res 159:104921. https://doi.org/10.1016/j.phrs.2020.104921
Ghandadi M, Sahebkar A (2017) Curcumin: An effective inhibitor of interleukin-6. Curr Pharm Des 23(6):921–931.
Sadeghian M, Rahmani S, Jamialahmadi T, Johnston TP, Sahebkar A (2021) The effect of oral curcumin supplementation on health-related quality of life: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord 278:627–636. https://doi.org/10.1016/j.jad.2020.09.091
Iranshahi M, Sahebkar A, Hosseini ST, Takasaki M, Konoshima T, Tokuda H (2010) Cancer chemopreventive activity of diversin from ferula diversivittata in vitro and in vivo. Phytomedicine 17(3–4):269–273
Teymouri M, Pirro M, Johnston TP, Sahebkar A (2017) Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: a review of chemistry, cellular, molecular, and preclinical features. Biofactors 43(3):331–346
Ejaz A, Wu D, Kwan P, Meydani M (2009) Curcumin inhibits adipogenesis in 3t3-l1 adipocytes and angiogenesis and obesity in c57/bl mice. J Nutr 139(5):919–925
Yadav R, Jee B, Awasthi SK (2015) Curcumin suppresses the production of pro-inflammatory cytokine interleukin-18 in lipopolysaccharide stimulated murine macrophage-like cells. Indian J Clin Biochem 30(1):109–112
Karimian MS, Pirro M, Majeed M, Sahebkar A (2017) Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev 33:55–63
Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A (2019) Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr 59(1):89–101
Maheshwaria RK, Singha AK, Gaddipatia J, Srimal RC (2006) Multiple biological activities of curcumin: a short review. Life Sci 78(18):2081–2087
Nabiuni M, Mohammadi S, Kayedpoor P, Karimzadeh (2015) The effect of curcumin on the estradiol valerate-induced polycystic ovary in rats. Feyz 18(6):515–523
Li M, Xin M, Guo C, Lin G, Wu X (2017) New nanomicelle curcumin formulation for ocular delivery: improved stability, solubility, and ocular anti-inflammatory treatment. Drug Dev Ind Pharm 43(11):1846–1857
Lashan R (2010) Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab 1(3):117–128
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M et al (2000) Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab 85(1):139–146
Aguilar C (2019) Effect of oral supplementation with curcumin on insulin sensitivity in subjects with prediabetes. https://www.smartpatients.com/trials/NCT03917784
Rahimi H, Mohammadpour AH, Dastani M, Jaafari MR, Abnous K, Ghayour Mobarhan M et al (2016) The effect of nano-curcumin on hba1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed 6(5):567–577
Ameli H, Moini-Zangani T, Masoudnia F, Sabetkasaei M (2015) The comparison of curcumin’s effect with or without metformin on blood glucose levels in diabetic rats. Pejouhandeh 19(6):312–319
Jiménez-Osorio AS, Monroy A, Alavez S (2016) Curcumin and insulin resistance-molecular targets and clinical evidences. Biofactors 42(6):561–580
Kim HS, Hwang YC, Koo SH, Park KS, Lee MS, Kim KW et al (2013) PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor gpr40 in pancreatic β-cells. PLoS One 8(1):e50128. https://doi.org/10.1371/journal.pone.0050128
Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M et al (2005) Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem 53(4):959–963
Miller M, Chen S, Woodliff J, Kansra S (2008) Curcumin (diferuloylmethane) inhibits cell proliferation, induces apoptosis, and decreases hormone levels and secretion in pituitary tumor cells. Endocrinology 149(8):4158–4167
Yan Z, Dai Y, Fu H, Zheng Y, Bao D, Yin Y et al (2018) Curcumin exerts a protective effect against premature ovarian failure in mice. J Mol Endocrinol 60(3):261–271
Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N (2018) Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res 11(1):33. https://doi.org/10.1186/s13048-018-0409-9
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A (2016) Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol 68(3):223–229
Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W et al (2012) Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One 7(1):e28784. https://doi.org/10.1371/journal.pone.0028784
Simental-Mendía LE, Pirro M, Gotto AM Jr, Banach M, Atkin SL, Majeed M et al (2019) Lipid-modifying activity of curcuminoids: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 59(7):1178–1187
Na LX, LiY PHZ, Zhou XL, Sun DJ, Meng M et al (2013) Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res 57(9):1569–1577
Ganjali S, Blesso C, Banach M, Pirro M, Majeed M, Sahebkar A (2017) Effects of curcumin on HDL functionality. Pharmacol Res 119:208–218
Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R et al (2008) The residual risk reduction initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol 102(10 Suppl):1K–34K. https://doi.org/10.1016/S0002-9149(08)01833-X
Panahi Y, Ahmadi Y, Teymouri M, Johnston TP, Sahebkar A (2018) Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol 233(1):141–152.
Conflict of Interests
None.
Funding
Iran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sohrevardi, S.M. et al. (2021). Therapeutic Effect of Curcumin in Women with Polycystic Ovary Syndrome Receiving Metformin: A Randomized Controlled Trial. In: Barreto, G.E., Sahebkar, A. (eds) Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health. Advances in Experimental Medicine and Biology, vol 1308. Springer, Cham. https://doi.org/10.1007/978-3-030-64872-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-64872-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64871-8
Online ISBN: 978-3-030-64872-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)