Abstract
Polycystic ovary syndrome (PCOS) is a heterogeneous condition that affects women of reproductive age. It is associated with menstrual disturbances, hyperandrogenism, and polycystic ovaries. In addition to this, it results in altered anthropometric parameters, insulin resistance, and subsequently untoward cardio-metabolic sequelae. Therapeutic approaches that target weight loss and improve insulin sensitivity are used to address the metabolic complications in PCOS. Curcumin is a phytochemical which exhibits anti-inflammatory and anti-oxidant properties, and therefore, its use in PCOS has been a subject of substantial interest and research. The aim of this study was to synthesize the existing evidence on the effects of curcumin on glycaemic and lipid parameters in PCOS. We searched PubMed, Embase, Web of Science, Scopus, and ClinicalTrials databases from inception to June 07, 2021. Only randomized controlled trials (RCT) presenting sufficient data on glycemic and lipid parameters in patients with PCOS at baseline and the end of the follow-up period in each group were included. Meta-analysis of five RCTs showed a significant reduction on fasting glucose (WMD: − 3.68 mg/dL, 95% CI: − 5.11, − 2.25, p < 0.00001, I2 = 18%), insulin levels (WMD: − 1.72 µUI/mL, 95% CI: − 2.53, -0.92, p < 0001, I2 = 41%), and HOMA-IR index (WMD: − 0.94, 95% CI: − 1.73, − 0.16, p = 0.02, I2 = 90%) after curcumin therapy. None of the lipid indices were significantly altered by curcumin. Curcumin administration in PCOS resulted in significant improvement in glycaemic parameters; however, no significant changes were seen in lipid parameters with its use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with a prevalence of up to 13% [1] that may rise further subject to the diagnostic criteria used [2]. Although the etiology of PCOS is not clear, endogenous hormonal disparity and genetic, environmental factors are likely to raise androgen and insulin levels resulting in PCOS [3,4,5]. Clinical and biochemical features of hyperandrogenism, menstrual disturbances, and polycystic appearance of the ovaries are the characteristic features of PCOS [6]. Further, it leads to adverse metabolic sequelae, including impaired glucose tolerance, gestational diabetes, type 2 diabetes (T2D), dyslipidemias, and obesity, all of which confer a cardiovascular risk [7,8,9]. In clinical practice, the Rotterdam criteria [10, 11] is widely used to diagnose PCOS. A majority of the women with PCOS are overweight [12], and a weight loss of up to 5% can reinstate ovulation and increase insulin sensitivity [13]. Therefore, pharmacological and non-pharmacological strategies facilitating weight loss form a vital component for the management of PCOS [14]. Furthermore, specific abnormalities, including menstrual irregularities, manifestations of hyperandrogenism, and infertility, can be addressed with targeted therapies in a patient-centered approach [15].
Curcumin, also known as diferuloylmethane, is a biologically active polyphenol derived from turmeric [16]. By targeting a variety of signaling molecules and acting at the cellular level, it has been shown to be beneficial in metabolic syndrome and inflammatory conditions [17,18,19,20,21,22,23]. curcumin has been shown to exhibit anti-diabetic effects [24], reduce serum cholesterol [25] and oxidative stress [26]. These beneficial effects of curcumin administration have drawn considerable interest for its use in alleviating untoward metabolic complications in patients with PCOS.
Although some studies suggest a favorable outcome of curcumin supplementation in PCOS over the years, there are significant gaps in the available evidence, and the therapeutic effects of curcumin in PCOS remain inconclusive. This systematic review and meta-analysis of RCTs aim to evaluate and analyze the available evidence for the effectiveness of curcumin in improving glycaemic and lipid parameters in PCOS, which will help guide clinical practice.
Methods
Search Strategy
This study was conducted following the PRISMA guidelines [27]. Scopus, PubMed, Embase, Web of Science, and ClinicalTrials databases were searched using the following search terms in titles and abstracts (also in combination with MESH terms): (curcumin OR cur-cuminoid OR curcuminoids OR Curcuma OR “Curcuma longa”) AND (glucose OR insulin OR insulin resistance OR insulin sensitivity OR HbA1c OR hemoglobin A1c OR glycated hemoglobin OR glycosylated hemoglobin OR cholesterol OR “low-density lipoprotein” OR LDL OR LDL-C OR LDL-cholesterol OR “high-density lipoprotein” OR HDL-cholesterol OR HDL-C OR triglyceride OR hyperlipidemia OR hyperlipidemic OR dyslipidemia OR dyslipidemic OR lipid OR lipoprotein). The wild-card term “*” was used to increase the sensitivity of the search strategy. The literature was searched from inception to June 07, 2021.
Study Selection
Clinical trials were included if they met the following inclusion criteria: (1) randomized controlled trials with either parallel or cross-over design, (2) evaluating the impact of curcumin on either glycemic or lipid markers, and (3) presenting sufficient data on glycemic and lipid parameters at baseline and the end of follow-up in each group or providing the net change values. Exclusion criteria were (1) non-randomized trials, (2) uncontrolled trials, (3) observational studies, and (4) lack of sufficient information of glycemic and lipid markers at baseline or follow-up (or net change). Two independent authors selected the studies and any disagreements were discussed and resolved with another author.
Data Extraction
The following data were extracted from eligible studies: (1) name of the first author, (2) publication year, (3) study design, (4) dose of curcumin, (5) treatment duration, (6) number of participants, (7) patients characteristics, and (8) levels of glycemic and lipid parameters.
Quality Assessment
The bias evaluation of the clinical trials was performed using the Cochrane criteria [28]. The blinding of participants, sequence generation, allocation concealment, incomplete outcome data, personnel and outcome assessors, selective outcome reporting, and other sources of bias were the items used for quality assessment.
Quantitative Data Synthesis
Meta-analysis was performed using the Review Manager statistical software version 5.3. A random-effects model and the generic inverse variance weighting method were used for meta-analysis. Mean and SD values were estimated when the outcome measures were reported in median and interquartile range (or 95% confidence interval [CI]) [29]. If the standard error of the mean (SEM) was provided, SD was calculated. Effect sizes were expressed as weighted mean difference (WMD) and 95% CI. Sensitivity analysis was performed using the leave-one-out method to assess the influence of each study on the overall effect size [30, 31].
Results
Study Selection Process
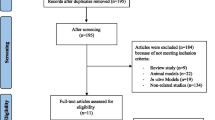
A total of 181 published studies were identified after a systematic databases search, and 169 were excluded since they did not meet the criteria for inclusion. Next, 12 articles were carefully reviewed in full-text, and 6 were excluded for being non-randomized clinical trials. Finally, 6 RCTs were included (Fig. 1).
Characteristics of the Randomized Controlled Trials
Data were pooled from 5 RCTs, including 296 subjects: 148 and 148 individuals in the treatment and control groups. Included studies were published between 2019 and 2021 [32,33,34,35,36]. The treatment duration ranged from 5 to 12 weeks. All selected clinical trials had a parallel design. All studies included enrolled women with PCOS (Table 1).
Quality of Bias Assessment of Clinical Trials
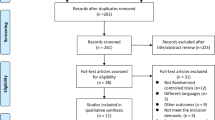
Only one study showed insufficient information about sequence generation [32]. Additionally, three clinical trials lacked the information for allocation concealment [32, 34, 36]. According to the blinding of participants, personnel, and outcome assessment, two studies had a high risk of bias for these parameters. Finally, all included clinical trials exhibited a low risk of bias for incomplete outcome data and selective outcome reporting (Fig. 2).
Effects of Curcumin on Glycemic Parameters
Meta-analysis of 5 RCTs showed a significant reduction on fasting glucose (WMD: − 3.68 mg/dL, 95% CI: − 5.11, − 2.25, p < 0.00001, I2 = 18%), insulin levels (WMD: − 1.72 µUI/mL, 95% CI: − 2.53, − 0.92, p < 0001, I2 = 41%), and HOMA-IR index (WMD: − 0.94, 95% CI: − 1.73, − 0.16, p = 0.02, I2 = 90%) after curcumin therapy (Fig. 3). Additionally, the sensitivity analysis was robust for fasting glucose and insulin levels; however, the effect size for HOMA-IR index was sensitive to two studies (Table 2) [33, 36].
Effects of Curcumin on Lipid Profile
The meta-analysis of the pooled data revealed that curcumin treatment has no significant changes in circulating concentrations of TC (WMD: − 14.19 mg/dL, 95% CI: − 28.97, 0.59, p = 0.06, I2 = 66%), LDL-C (WMD: − 8.21 mg/dL, 95% CI: − 22.90, 6.47, p = 0.27, I2 = 79%), HDL-C (WMD: − 4.01 mg/dL, 95% CI: − 1.71, 9.73, p = 0.17, I2 = 86%), and triglycerides (WMD: − 9.79 mg/dL, 95% CI: − 32.44, 12.85, p = 0.40, I2 = 83%) (Fig. 4). The effects of curcumin on LDL-C and HDL-C were robust in the sensitivity analysis, while the estimated effect sizes for triglycerides and TC were sensitive to one study each (Table 3).
Discussion
To our knowledge, this is the first systematic review that summarizes and reports up-to-date evidence regarding the impact of curcumin administration on glycaemic and lipid parameters in patients with PCOS. The meta-analysis of 5 RCTs showed a significant reduction in fasting plasma glucose, insulin levels, and HOMA-IR index after curcumin therapy. Interestingly, however, no significant changes in circulating concentrations of TC, LDL-C, HDL-C, and triglycerides were seen. Four RCTs compared varying doses of curcumin vs. placebo [32,33,34,35] whereas one of them compared the effect of curcumin + metformin vs. metformin alone [36]. Besides, in one of these studies, all patients were matched by metformin treatment [35]; however, three RCTs did not clarify the use of this drug [32,33,34]. It will be interesting to assess the impact of curcumin on glycaemic parameters when used as an adjunct to metformin therapy compared to its use alone in PCOS. However, this will require well-designed future studies. We report no significant effect on lipid parameters with curcumin use in PCOS, which was rather surprising as several previous studies have suggested that it improves serum lipid concentrations in humans [37, 38] and animal models [25, 39]. However, these human clinical studies were not conducted in subjects with PCOS. Particularly, patients with PCOS often exhibit dyslipidemia such as elevated levels of total cholesterol, LDL-C, and triglycerides as well as reduced concentrations of HDL-C [40]; however, it is noteworthy that some of the studies included in our meta-analysis were characterized by lipid levels within the normal range, which could explain the lack of effect of curcumin administration. In this regard, a previous meta-analysis of lipid levels in PCOS indicated a wide variability of LDL-C values among the evaluated studies, which may be affected by several factors such as ethnicity, severity of PCOS, diet, and body weight [41]. Also, although triglyceride levels were higher in women with PCOS, only 3 out of 30 included studies showed values greater than 150 mg/dl; this finding could be related to age or steroid production which can partially prevent elevated triglycerides [41]. Additionally, it has been suggested that the short treatment period following curcumin supplementation (2 months) is insufficient to affect the lipid parameters [42]. This was a comprehensive and systematic search of relevant databases that only included RCTs with either a parallel or cross-over design, while uncontrolled, non-randomized, and observational studies were excluded to reduce the risk of bias. Some limitations of this meta-analysis include a paucity of well-designed RCTs reporting these outcomes. Well-designed clinical research is required for an in-depth analysis of the beneficial effects of curcumin in PCOS. Furthermore, in this meta-analysis, only trials reported in the English language were included, and therefore, several clinical trials in foreign languages may not have been retrieved. Assessing such trials requires sophisticated translation, which is challenging, and could also affect the methodology of this review. In addition to this, only fully published trials were eligible, and publication bias was not performed. All the included trials were of a smaller sample size, and the statistical power used to calculate sample size and detect the meaningful differences between the groups were not reported. All the trials were of short duration and reported baseline and immediate post-intervention data. Therefore, the long-term effects of curcumin on glycaemic and lipid parameters in PCOS women are not studied extensively.
Based on our findings, it is clear that there is a lack of robust clinical trials assessing the effect of curcumin on metabolic parameters in PCOS. Therefore, further clinical trials with robust and standardized designs are needed to enable informed clinical practice in this domain.
Conclusion
This systematic review and meta-analysis of 5 RCTs shows that curcumin alone or in combination with metformin, regardless of the dose and duration of administration, results in significant improvement in fasting plasma glucose, insulin, and HOMA-IR. However, a similar effect was not observed in lipid parameters as there was no significant change in serum TC, triglycerides, LDL-c, and HDL-c with curcumin use. The results of this review are encouraging as they support curcumin supplementation in PCOS by showing that its use improves glycaemic parameters and, therefore, will help manage metabolic sequelae in PCOS. However, further large-scale, well-designed clinical trials over a longer duration are required to underpin the effectiveness of curcumin in PCOS conclusively.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Strowitzki T. Advanced diagnosis of polycystic ovary syndrome-new prediction models with standard parameters. Fertil Steril. 2021;115(1):92–3. https://doi.org/10.1016/j.fertnstert.2020.09.031.
March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–51.
Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1):41. https://doi.org/10.1186/1741-7015-8-41.
Legro RS, Strauss JF III. Molecular progress in infertility: polycystic ovary syndrome. Fertil Steril. 2002;78(3):569–76.
Doi SA, Al-Zaid M, Towers P, Scott C, Al-Shoumer KA. Ovarian steroids modulate neuroendocrine dysfunction in polycystic ovary syndrome. J Endocrinol Invest. 2005;28(1):882–92.
Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. Pharm Ther. 2013;38(6):336.
Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50(1):205–25.
Garruti G, Depalo R, Vita MG, Lorusso F, Giampetruzzi F, Damato AB, Giorgino F. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online. 2009;19(4):552–63.
Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Position statement: glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab. 2007;92(12):4546–56.
Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004. 19 (1):41-47. https://doi.org/10.1093/humrep/deh098
Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004. 81 (1):19-25. https://doi.org/10.1016/j.fertnstert.2003.10.004
Domecq JP, Prutsky G, Mullan RJ, Hazem A, Sundaresh V, Elamin MB, Phung OJ, Wang A, Hoeger K, Pasquali R. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(12):4655–63.
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97. https://doi.org/10.1016/s0140-6736(07)61345-2.
Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20.
Lua ACY, How CH, King TFJ. Managing polycystic ovary syndrome in primary care. Singapore Med J. 2018;59(11):567–71. https://doi.org/10.11622/smedj.2018135.
Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1/A):363–98.
Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59.
Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendía LE, Majeed M, Sahebkar A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed Pharmacother. 2016;82:578–82.
Ghandadi M, Sahebkar A. Curcumin: An effective inhibitor of interleukin-6. Curr Pharm Des. 2017;23(6):921–31. https://doi.org/10.2174/1381612822666161006151605.
Ghasemi F, Shafiee M, Banikazemi Z, Pourhanifeh MH, Khanbabaei H, Shamshirian A, Amiri Moghadam S, ArefNezhad R, Sahebkar A, Avan A, Mirzaei H. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol Res Prac. 2019;215(10):152556. https://doi.org/10.1016/j.prp.2019.152556.
Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE, Majeed M, Sahebkar A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Research. 2018;68(7):403–9. https://doi.org/10.1055/s-0044-101752.
Mohajeri M, Bianconi V, Ávila-Rodriguez MF, Barreto GE, Jamialahmadi T, Pirro M, Sahebkar A. Curcumin: a phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacol Res. 2020;156:104765. https://doi.org/10.1016/j.phrs.2020.104765.
Sadeghian M, Rahmani S, Jamialahmadi T, Johnston TP, Sahebkar A. The effect of oral curcumin supplementation on health-related quality of life: A systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2021;278:627–36. https://doi.org/10.1016/j.jad.2020.09.091.
Wojcik M, Krawczyk M, Wojcik P, Cypryk K, Wozniak LA. Molecular mechanisms underlying curcumin-mediated therapeutic effects in type 2 diabetes and cancer. Oxid Med Cell Longev. 2018;2018:9698258. https://doi.org/10.1155/2018/9698258.
Shin SK, Ha TY, McGregor RA, Choi MS. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol Nutr Food Res. 2011;55(12):1829–40.
Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, Tanbakooei S, Shidfar F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020;14(2):77–82.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Higgins JPT GS, eds. Cochrane Handbook for Systematic Reviews of Interventions. , vol Version 5.0.2. London. 2009
Hozo SPDB, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. https://doi.org/10.1186/1471-2288-5-13.
Sahebkar ACA, Simental-Mendía LE, Aggarwal BB, Gupta SC. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;107:234–42. https://doi.org/10.1016/j.phrs.2016.03.026.
Serban CSA, Ursoniu S, Mikhailidis DP, Rizzo M, Lip GY, Kees Hovingh G, Kastelein JJ, Kalinowski L, Rysz J, Banach M. A systematic review and meta-analysis of the effect of statins on plasma asymmetric dimethylarginine concentrations. Sci Rep. 2015;5:9902. https://doi.org/10.1038/srep09902.
Asan SABM, Eren B, Karaca E. The effects of curcumin supplementation added to diet on anthropometric and biochemical status in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. Progr Nutr. 2020;22(4):e2020089. https://doi.org/10.23751/pn.v22i4.10460.
Heshmati JMA, Sepidarkish M, Morvaridzadeh M, Salehi M, Palmowski A, Mojtahedi MF, Shidfar F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine. 2021;80:153395. https://doi.org/10.1016/j.phymed.2020.
Jamilian MFF, Kavossian E, Aghadavod E, Shafabakhsh R, Hoseini A, Asemi Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN. 2020;36:128–33. https://doi.org/10.1016/j.clnesp.2020.01.005.
Sohaei SAR, Tarrahi MJ, Ghasemi-Tehrani H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement Ther Med. 2019;47:102201. https://doi.org/10.1016/j.ctim.2019.
Sohrevardi SMHB, Azarpazhooh MR, Teymourzadeh M, Simental-Mendía LE, Atkin SL, Sahebkar A, Karimi-Zarchi M. Therapeutic Effect of Curcumin in Women with Polycystic Ovary Syndrome Receiving Metformin: A Randomized Controlled Trial. Adv Exp Med Biol. 2021;1308:109–17. https://doi.org/10.1007/978-3-030-64872-5_9.
Qin S, Huang L, Gong J, Shen S, Huang J, Ren H, Hu H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr J. 2017;16(1):68. https://doi.org/10.1186/s12937-017-0293-y.
Yang YS, Su YF, Yang HW, Lee YH, Chou JI, Ueng KC. Lipid-lowering effects of curcumin in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Phytother Res. 2014;28(12):1770–7. https://doi.org/10.1002/ptr.5197.
Hasan ST, Zingg JM, Kwan P, Noble T, Smith D, Meydani M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis. 2014;232(1):40–51. https://doi.org/10.1016/j.atherosclerosis.2013.10.016.
Legro RSKA, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607–13. https://doi.org/10.1016/s0002-9343(01)00948-2.
Wild RARM, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95(3):1073–9. https://doi.org/10.1016/j.fertnstert.2010.12.027.
LE Simental-Mendía PM, Gotto AM Jr, Banach M, Atkin SL, Majeed M, Sahebkar A. Lipid-modifying activity of curcuminoids: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;59(7):1178–87. https://doi.org/10.1080/10408398.2017.1396201.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
Muhammed Majeed is the founder of Sabinsa Corporation and Sami Labs Ltd. Other authors have no conflicting interests to disclose.
Rights and permissions
About this article
Cite this article
Simental-Mendía, L.E., Shah, N., Sathyapalan, T. et al. Effect of Curcumin on Glycaemic and Lipid Parameters in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Reprod. Sci. 29, 3124–3133 (2022). https://doi.org/10.1007/s43032-021-00761-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00761-6