Abstract
Sleep and epilepsy have an intimate relationship. Some epilepsy syndromes are characterized by seizures occurring mostly or exclusively during sleep or on awakening. Many studies have reported autonomic changes in epileptic patients both during seizures and in the interictal state. Sudden unexpected death in epilepsy (SUDEP) is related to the concurrence of a number of predisposing and precipitating factors. Specifically, autonomic dysfunction has been identified as one of the major pathogenetic mechanisms of this fatal event. Moreover, SUDEP is considered primarily a sleep-related phenomenon and evidence suggests that the autonomic changes observed in epileptic patients are mainly evident during nocturnal sleep. In this chapter, we illustrate the clinical aspects of different sleep-related seizures, reporting the available data regarding modification of the cardiac autonomic modulation in each type of these syndromes. Furthermore, we discuss the new findings about SUDEP, focusing on the role of sleep and the autonomic nervous system (ANS) dysfunction in its pathogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sleep-related epilepsy
- Sleep-related hypermotor epilepsy
- Benign epilepsy with centrotemporal spikes
- Panayiotopoulos syndrome
- Juvenile myoclonus epilepsy
- West syndrome
- Lennox–Gastaut syndrome
- Electrical status epilepticus during slow wave sleep
- Sudden unexpected death in epilepsy
Sleep-Related Epilepsy
The strong relationship between sleep and epilepsy has long been documented, but it is not completely understood because of the multiple aspects involved and the possible bidirectional influences. Sleep may favor seizure occurrence and in some epilepsy syndromes seizures occur mostly or exclusively during sleep or on awakening (sleep-related epilepsies [SRE]). Sleep deprivation aggravates epilepsy due to decreased seizure threshold; in addiction, different sleep disorders may represent relevant factors in seizure control. Conversely, epilepsy may alter sleep structure, both directly through seizures and epileptiform activity and indirectly through antiepileptic drug’s (AED) side effects.
Evidence reported an alteration of cardiac autonomic control (CAC) in patients with epilepsy [1]. The study of the autonomic function in epilepsy is important for several reasons. For instance, the majority of patients with epilepsy display altered interictal heart rate variability (HRV) with a shift in the autonomic balance toward an increase of the sympathetic tone. Moreover, different types of seizures are associated with a rapid change in autonomic balance, probably depending on the activation of brain regions involved in the autonomic modulation. Finally, autonomic dysfunction has been identified as one of the major pathogenetic mechanisms of sudden unexpected death in epilepsy (SUDEP) [2]. Although it is well known that the autonomic balance oscillates during sleep, only few studies have examined HRV in sleep and wakefulness in epilepsy patients.
In this chapter, we present an overview of the clinical aspects of SRE, reporting the available data regarding the modification of the cardiac autonomic modulation in each type of SRE. Finally, we will update the new findings about SUDEP, focusing on the role of sleep and the autonomic nervous system (ANS) dysfunction in its pathogenesis.
Seizures Occurring Predominantly or Exclusively During Sleep Associated with ANS Dysfunction
Sleep-Related Hypermotor Epilepsy
Sleep-related hypermotor epilepsy (SHE)—formerly known as nocturnal frontal lobe epilepsy —was first described in 1981 by Lugaresi and Cirignotta in five patients with sleep-related stereotyped episodes characterized by bizarre movements or dystonic/tonic posturing of the limbs [3]. Considering the absence of electroencephalographic (EEG) abnormalities, the complex motor features of the manifestations and their sleep-related occurrence, the authors initially considered the episodes as “unusual motor disorder of sleep,”, coining the term of “hypnogenic paroxysmal dystonia,”, modified 5 years later to “nocturnal paroxysmal dystonia” (NPD) [4]. Clinicians debated for several years about the epileptic or nonepileptic nature of NPD. Subsequently, different studies conducted in patients with frontal lobe seizures undergoing neurosurgical treatment for drug-resistant epilepsy led to a better understanding of the physiopathological mechanism based on these manifestations, suggesting their epileptic origin. Therefore, the term “nocturnal frontal lobe epilepsy” was adopted [5,6,7].
In 2014, a consensus conference including experts in epilepsy and sleep medicine was convened in Bologna, with the aim of defining the electroclinical features and the diagnostic criteria of the disorder [8] (for diagnostic criteria see Table 19.1). One of the major outcomes of the consensus conference was the need to change nomenclature; the proposed new name was “sleep-related hypermotor epilepsy,” reflecting evidence that the attacks are associated with sleep rather than time of day, seizures can have an extrafrontal origin and the motor features of the seizures are specific to this entity.
Epidemiology
SHE is not a homogeneous disease as familial, idiopathic, sporadic, cryptogenetic or symptomatic forms exist [9,10,11]. The nonlesional cases of SHE seem to be predominant in the familial form [12]. This epileptic syndrome is characterized by onset during infancy or childhood with persistence in adulthood [13]. SHE is reported to be a rare disorder but epidemiologic data are scanty. In 1999, Provini et al. showed that SHE represented the diagnosis in 13% of patients referred to their tertiary center for a video-polysomnographic evaluation [10]. More recently, a population-based retrospective cohort study conducted in the northeast of Italy showed that SHE is a rare epileptic condition with a crude prevalence of 1.8 per 100,000 residents [12]. The majority of SHE patients show a positive response to antiepileptic drugs, especially carbamazepine. However, 30% of cases are resistant to carbamazepine and other antiepileptic drugs [10].
Genetic Forms of Sleep-Related Hypermotor Epilepsy
The first genetic form of SHE was described by Scheffer et al. in 1994 in a large Australian family with autosomal-dominant inherited SHE (ADSHE) associated with a mutation of the CHRNA4 (neuronal acetylcholine receptor subunit alpha-4) gene coding for the alpha4 subunit of the neuronal nicotinic acetylcholine receptor (nAChR) [9]. Subsequently, further mutations in genes (CHRNA2, neuronal acetylcholine receptor subunit alpha-2, and CHRNB2, neuronal acetylcholine receptor subunit beta-2) coding for other nAChR subunits (alpha2 and beta2) [14, 15] were found to be implicated in ADSHE with phenotype usually indistinguishable [16].
More recently, additional mutations in genes not coding for the cholinergic receptor were discovered in ADSHE. Specifically, mutations of the KCNT1 gene (coding for a sodium-activated potassium channel subunit 1) have been associated with a severe form of ADSHE accompanying intellectual disabilities, regression, and behavioral and psychiatric symptoms [17]. Of note, the same gene has been found to be mutated also in a rare early infantile epileptic encephalopathy with poor prognosis, “epilepsy with migrating focal seizures of infancy” [18, 19].
In some families with ADSHE and in rare sporadic SHE cases mutations have been found in DEPDC5 (DEP domain containing 5) and NPRL (nitrogen permease regulator-like protein) 2 and 3, proteins coding for the mTORC1 (mammalian target of rapamycin complex 1)-regulating GATOR1 (Gap Activity TOward Rags 1) complex, implicated in the cell growth regulation [20,21,22]. Of note, DEPDC5 has been associated with a variety of familial epilepsies, including familial focal epilepsy with variable foci, familial temporal lobe epilepsy, epileptic spasms, and epilepsies associated with cortical dysplasia [23,24,25].
Recently, mutation in the CABP4 gene, encoding the neuronal Ca2+-binding protein 4 (CaBP4), was found in a Chinese family with 11 individuals diagnosed with ADSHE [26]. Finally, Peres et al. described a case of SHE with peri-ictal hypotension associated with syntaxin-1B gene mutation [27].
Symptomatic Forms
Studies conducted in patients with drug-resistant epilepsy showed that the most frequent etiological substrate of SHE is represented by type II focal cortical dysplasia (type II FCD) [28]. Notably, although type II FCD is more frequently located within the frontal lobe, evidence demonstrated that regardless of its anatomical localization, type II FCD (especially the type IIb) increases the risk of sleep-related epilepsy [29]. This correlation could be probably ascribed to the particular firing pattern of FCD IIb during non-REM sleep, characterized by the periodic occurrence of fast discharge frequently evolving into a seizure.
Clinical Features
From a clinical point of view, patients with familial forms of SHE do not show a clear distinction from sporadic NFLE cases, except for certain ADSHE mutations frequently associated with specific additional neurological or psychiatric symptoms. Seizure frequency is usually high and sometimes diminishes during adulthood. Almost 30–40% of SHE patients experience occasional seizures during wakefulness [10, 30]. Of note, subjective seizure manifestations, uncommon during the night, are more frequently reported by patients during diurnal events [10, 31].
SHE patients frequently exhibit different sleep-related motor manifestation of increasing complexity and duration during a single night [10, 11, 32, 33]. These include (a) minor events, consisting of short-lasting (2–4 s) stereotyped movements involving the limbs, the axial musculature, and/or the head; (b) paroxysmal arousals (PA), characterized by frequent and recurring abrupt brief arousals lasting about 5–10 s, accompanied by stereotyped movements (trunk and head elevation) often associated with vocalization and frightened expression; and (c) major attacks. Moreover, a minority of patients may show ictal ambulatory behavior (wandering behavior) often associated with frightened expression and fear, called “epileptic nocturnal wanderings.”.
It has been postulated that the increasing complexity of the ictal motor manifestation is strictly related to a different duration and propagation of the epileptic discharge. This hypothesis was confirmed by studies conducted in drug resistant SHE patients with seizure arising from supplementary motor area, studied with intracerebral implanted electrodes during the presurgical evaluation [33, 34]. However, more recent evidence has demonstrated that minor motor events, whose occurrence is highly related to the presence of intracerebrally recorded epileptic discharges, may not be differentiated from physiological movements [35]. In fact, other studies by means of stereo-EEG investigations in drug-resistant SHE patients showed that periodic epileptic discharges, not detectable on scalp EEG, can increase arousal fluctuations and in turn increase and modulate the occurrence of physiological movements or different kinds of sleep disturbances such as periodic leg movements, sleep-talking, and bruxism [34, 36]. On the other hand, the resulting increased arousal instability might facilitate the production of epileptic discharges in a bi-directionally influenced system [37].
Considering major attacks, early studies focusing on seizure semiology in SHE have highlighted the heterogeneous features of the sleep-related manifestations [9, 10]. A recent retrospective study on 135 patients with drug-resistant SHE patients identified peculiar ictal semiologic patterns in this population of patients [31]. In particular, seizures were classified according to four semiology patterns (SPs): SP1, characterized mainly by early elementary motor signs, which included early clonic signs, asymmetric tonic postures or an asymmetric facial contraction; SP2, consisting of unnatural hypermotor movements, that is, nonintegrated or anarchic gestural hypermotor movements with axial tonic postures or symmetric facial contractions; SP3, characterized by integrated hypermotor movements, which included hyperkinetic behaviors (pedaling, kicking, rocking), distal stereotypies, or manipulation/utilization movements in the absence of a clear goal-directed purpose; and SP4, including gestural behaviors with high emotional content, such as integrated gestural behaviors of fear, fight or flight behavior, frightened facial expression or autonomic signs. Within the frontal lobe, the occurrence of a peculiar SP was strictly related to the location of the seizure onset zone (SOZ) with an anteroposterior distribution of the SOZ in relation to the SP: SP1 was mainly placed in the vicinity of the precentral sulcus (posterior mesial or posterior lateral surface of the frontal lobe) whereas SP4 was located predominantly near the frontopolar and orbitofrontal regions; SOZ in SP2 and SP3 was more scattered but followed the same anteroposterior tendency.
Sleep-Related Hypermotor Epilepsy: Always a Frontal Onset?
Hypermotor (hyperkinetic or dystonic) seizures , sleep-related or not, have been considered for a long time the hallmark of frontal lobe seizures (hence the name NFLE). However, over the past decade many case reports and case series have challenged this idea, demonstrating that other sites of seizure onset may trigger tonic/dystonic or hyperkinetic behaviors. Studies by means of Stereo-EEG investigations documented an extrafrontal origin in up to 30% of cases of drug-resistant SHE [28]. The majority of the described extrafrontal SHE cases displayed a temporal or an insular-opercular onset although cases of parietal lobe and even occipital lobe onset have been reported [31, 38,39,40,41]. Distinguishing a frontal from an extrafrontal form of SHE may be challenging. In the above-mentioned study [31], it has been shown that extrafrontal drug-resistant SHE exhibited the same SPs described for frontal SHE, but certain SPs were more likely or unlikely to occur in some extrafrontal SHE subgroups. In particular, seizures characterized by gestural behaviors with high emotional content (SP4) were frequently observed in temporal SHE, while they were absent in operculo-insular and parietal SHE, suggesting that the presence of SP4 in SHE could be strongly related to a SOZ involving a network between the ventromedial prefrontal cortex and the anterior temporal region. As far as the early nonmotor manifestations, the study [32] showed that some nonmotor features may provide useful localizing and/or lateralizing information. For instance, auditory and visual manifestations were observed only in extrafrontal SHE. As expected, visual symptoms were associated with posterior cortex SOZ while auditory symptoms were found in both temporal and operculoinsular SOZ. Conversely, cephalic symptoms were observed only in frontal SHE while a focal sensory onset was observed in both frontal and extrafrontal (parietal and operculoinsular) SHE. The characteristic of the sensory manifestation was sometimes very helpful to localize the SOZ. For example, insular patients frequently experienced pain or a choking sensation at the beginning of their seizures. Conversely, patients with parietal SOZ reported more often vertigo or falling sensation. Finally, epigastric and autonomic features were present in all but the posterior subgroup, while emotional manifestations were observed only in frontal and temporal SHE.
Another recent work on drug resistant SHE patients with different location of the SOZ found that the mean duration of electrographic seizures and clinically observable ictal manifestations were significantly shorter in frontal SHE compared to extrafrontal SHE. Moreover, the mean latency between the first video-detectable movement (e.g., eye opening or a minor motor event) and the onset of hypermotor manifestations was also shorter in frontal SHE [42].
Electroencephalographic Features
The interictal and ictal scalp electroencephalographic features of SHE patients are often uninformative, especially in cases in which SOZ is located in deep brain regions [9, 10, 43]. However, prolonged video-EEG recordings can sometimes be useful in characterizing the EEG abnormalities in selected subgroups of patients undergoing presurgical investigation. In the study of Gibbs et al. [32], it has been shown that the majority of drug resistant patients (82%) with both frontal or extrafrontal SHE exhibited interictal abnormalities during scalp EEG recordings (routine and video-EEG). However, this percentage decreased when the localizing value of EEG based on postoperative outcome was assessed (48%), except in temporal SHE where 93% of interictal abnormalities correctly localized to the epileptogenic temporal lobe [31].
Treatment
About two thirds of SHE/ADSHE patients benefit from carbamazepine administration [9, 10]. More recently, studies conducted in small population of SHE patients demonstrated the efficacy of other antiepileptic drugs administered as single or add-on therapy, such as oxcarbazepine, topiramate, acetazolamide, lacosamide, and fenofibrate [44]. Finally, different studies demonstrated the high efficacy of epilepsy surgery in selected patients with drug resistant SHE [45].
SHE and Autonomic System
Ictal and interictal abnormalities in autonomic control of cardiac frequency have been reported in different population of patients with focal and generalized epilepsies. For instance, faster interictal heart rates and reduced parasympathetic drive have been reported in patients with frontal lobe epilepsy [46]. However, studies analyzing modifications of cardiac autonomic control (CAC) specifically in patients with SHE are scant.
From a physiological point of view, sleep is characterized by oscillatory changes of CAC, with a predominant sympathetic modulation during REM sleep and, in contrast, a shift toward a vagal predominance during NREM sleep [47]. Furthermore, NREM sleep does not represent a stable phenomenon but it is punctuated by the occurrence of “arousals,” consisting of transient episodes of cortical and autonomic activation. Notably , some studies demonstrated that autonomic modification can precede cortical/EEG activation during an arousal event [47]. Specifically, the physiological arousal response seems to follow a hierarchic pattern, starting with an early autonomic activation followed by different phasic EEG changes and ending in delayed cortical activation for stimuli of greater intensity [48].
As discussed above, a bidirectional association between arousal fluctuations and SHE has been observed [37], and higher level of arousal activity during NREM sleep has been described in SHE patients [49]. Calandra et al. [50] conducted a study to investigate whether signs of autonomic activation precede onset of seizure motor manifestations. Analysis of HRV showed a shift of sympathetic/parasympathetic cardiac control toward a sympathetic predominance in the 10 s immediately preceding seizure onset, while changes in HR were evident only 1 s before seizure onset. Moreover, to clarify the nature (epileptic or physiologic) of the CAC changes preceding the seizures, they also investigated time-dependent variations in HR and HRV related to physiological cortical arousals associated with motor activity (phases of transitory activation [PAT]). Notably, patterns of CAC variation before the motor onset of PAT reproduced the features of the autonomic activation observed before seizures. Therefore, authors hypothesized that the sympathetic activation observed before the onset of seizure motor manifestations may reflect the autonomic preparation that preceded the cortical and behavioral response to spontaneous arousal and not the direct effect of the discharge involving cortical areas of the CAC.
More recently, a study investigated interictal CAC modification in SHE patients. In particular, authors analyzed HRV modification during the cyclic alternating pattern (CAP) in patients with SHE with respect to controls [51]. SHE patients showed a significant increase in LF/HF (low frequency/high frequency) and LF (low frequency) power with respect to healthy subjects only around A1 phases. Conversely, the CAC control during A2 and A3 phases was similar in the two populations. This seems to suggest that A1 phases in SHE patients may be characterized by a shift in the sympatho-vagal balance toward a more sympathetically mediated control of heart rate. Authors hypothesized that this autonomic behavior could be related to a higher arousal activity described in SHE patients [49].
Benign Epilepsy with Centrotemporal Spikes (BECTS)
Benign epilepsy with centrotemporal spikes is the most frequent type of idiopathic age-dependent childhood epilepsy (8–20% of childhood epilepsy) [52]. The onset is usually between 3 and 13 years and remission is frequently before 16 years of age. Seizures are mainly characterized by hemi lateral clonic deviation of the mouth and tongue often associated with sialorrhea. Seizure frequency is usually low and up to 70% occur during NREM sleep, typically just after falling asleep or before awakening. EEG is characterized by the interictal presence of high-voltage centrotemporal spikes and sharp waves with amplitude and frequency increment during NREM sleep when they often become synchronous or asynchronous bilaterally. Even if this kind of epilepsy is often described as “benign” with good prognosis and no cognitive impairment, it is known that mild cognitive impairment may be present when associated with a marked increment of interictal discharges during sleep [53]. A recent systematic review and metanalysis comprising 1237 [off therapy) children with BECTS and 1137 healthy controls showed significantly lower scores predominantly in the cognitive domain, regarding long-term storage and retrieval [54]. Cognitive impairment both in language and academic performance has also been observed [55]. According to other studies, cognitive disturbances seem to be associated with the presence of bilateral discharges [56]. The observed cognitive dysfunction somehow fills the gap between the apparently benign BECTS and the atypical variants that can proceed to severe epileptic encephalopathies phenotypes as Landau–Kleffner syndrome and electrical status epilepticus in sleep (ESES), supporting the concept of a clinical spectrum of BECTS [52]. Genetic disposition has been observed; about one-fourth of patients present one or more relatives with seizures and recently, GRIN2A, PRRT2, SRPXA, and ELP4 pathogenic variants were detected in some children with BECTS [57, 58]. Treatment (usually valproic acid, carbamazepine, levetiracetam, etc.) is indicated only when seizures are frequent or interfere significantly with daily life.
The analysis of HRV in 50 patients with BECTS compared with 50 controls revealed that during wakefulness BECTS patients exhibited an increased vagal and reduced sympathetic tone expressed by a higher HF and a lower LF/HF index than typically developing controls [59]. Thus, the study revealed a negative relationship between both seizure load as well as frequency of interictal sleep EEG abnormalities, and parasympathetic drive levels. Authors hypothesized that higher parasympathetic activation could be associated with a more effective seizure control and that failure of these adaptive processes might result in more difficult controlling seizures.
Panayiotopoulos Syndrome
Panayiotopoulos syndrome , also known as early onset childhood occipital epilepsy, is another common type of age-related focal epilepsy (estimated prevalence of 13% in children aged 3–6 years) [60]. Onset is usually between 1 and 11 years, frequently before 6 years of age. Remission occurs typically also without treatment within 1–2 years of the onset [60]. Seizures are generally characterized by nausea and vomiting often associated with a various combination of autonomic symptoms such as pallor, flushing, thermoregulatory alterations, mydriasis, urinary incontinence, and cardiorespiratory irregularities. Visual symptoms may be rarely present at onset and facial clonic manifestations, and loss of consciousness may follow. Evolution into secondary generalized seizures is common, and duration longer than 30 min may occur. Seizure frequency is usually extremely low, with a majority of patients reporting only one to five seizures in their lifetime [61]. About two-thirds of seizures occur during sleep, soon after falling asleep, or in the early hours of the morning [62, 63]. Interictal EEG is characterized by focal or multifocal spikes with variable localization, frequently involving occipital areas and with an increase during NREM sleep, even if less intense than in BECTS. A robust correlation of interictal epileptiform discharges (IEDs) with sleep spindles in this and other age-related epilepsies was specifically observed [64]. Occipital interictal spikes may be “fixation off” sensitive (meaning precipitated by the suppression of visual fixation) and brief diffuse discharges of small spikes and slow waves are occasionally observed [62]. Ictal EEG usually starts before clinical symptoms onset; it is usually characterized by theta waves mixed with small spikes and fast rhythms that may start variably (unilateral or bilateral) and spreading to other brain areas. Autonomic symptoms are not associated with a specific localization of ictal EEG abnormalities [65]. The genetic background is unknown. Rectal diazepam should be administered for prolonged seizures. Treatment should be prescribed only in patients with frequent and prolonged seizures; valproic acid and carbamazepine are the most frequently used drugs.
Juvenile Myoclonus Epilepsy
Juvenile myoclonus epilepsy (JME) is also known as Janz syndrome. The prevalence of JME has been estimated to be 5–10% of all epilepsies [66]. Onset is typical during adolescence, even if it can seldom occur before 10 years of age or in early adulthood. Seizures are characterized by the presence of brief, spontaneous, bilateral, arrhythmic, irregular, single, or repetitive myoclonic jerks repeatedly occurring on or shortly after awakening, without loss of consciousness. Myoclonic jerks usually present in brief clusters with upper limbs predominant involvement, and according to a consensus meeting in 2011 their presence is an obligatory requisite for JME diagnosis [67]. Sleep deprivation and forced awakening during sleep are known as triggers [68]. Generalized tonic-clonic seizures (GTCs), absences, perioral myoclonia, and praxis induced seizures may also occur. In particular , generalized tonic-clonic seizures are often subsequent to a cluster of myoclonic jerks, frequently intense and associated with prolonged postictal asthenia. Absences are rare (prevalence 10–38%), short, with partial consciousness impairment, and often are detected only during prolonged EEG recording [68]. Perioral and praxis-induced seizures are often precipitated by reading, speaking, and other neuropsychological activation [69]. Photosensitivity could be also present. Interictal EEG shows normal background activity and sleep pattern; widespread polyspikes and diffuse spike and waves at 2.3–3.5 Hz increased during sleep onset and awakening are typical. Myoclonic jerks usually are associated with rapid 5–20 polyspikes of increasing amplitude mainly localized in the frontal areas, followed or preceded by high amplitude slow waves [70]. Sleep disturbances have been often observed in JME patients, such as daytime sleepiness, disturbed night sleep, despite adequate medications and good seizure control [71]. A recent study observed the absence of expected apnea mediated HRV changes, including long-term HRV changes during sleep in JME patients compared with healthy controls, suggesting an impairment of reflex baroceptor activation and autonomic nervous system in these patients [72]. JME is not usually self-limiting, although the response to pharmacological treatment is satisfactory in most patients [61, 67]. Most used and efficacious drugs are valproic acid, topiramate, and levetiracetam. Lamotrigine may aggravate myoclonic jerks, carbamazepine, oxcarbazepine, gabapentin, phenytoin, and vigabatrin may worsen absences, GTCs (generalized tonic-clonic seizures), and myoclonic seizures and should be avoided [73].
West Syndrome
West syndrome is characterized by infantile spasms and hypsarrhythmia on EEG. Onset is usually between 3 and 12 months of life, even if earlier or later start is not uncommon. The estimated incidence is 2.5–10/10.000 newborns and the prevalence is around 1–2/10.000 children at the age of 10 years with onset within 1 year of age in 90% of cases [74]. The spasms usually manifest shortly before awakening as brief, synchronous flexor or extensor movement of head, trunk, and limbs and occur in clusters. Sometimes spasms may be very subtle presenting as a series of tonic contractions or focal jerks or even isolated eye deviation and facial grimacing. EEG is characterized by high amplitude pathognomonic hypsarrhythmic pattern, more prominent during early NREM sleep and in some cases occurring only during sleep [75, 76]. The etiology is the most important prognostic factor, but also the lack of physiological sleep patterns and the severity of hypsarrhythmia play a role for the developmental outcome [77, 78]. In particular, previous studies observed, similarly to ESES/CSWS, significant impairment of overnight slow wave sleep and alterations of NREM slow oscillations (with steeper slow waves] [79]. There is increasing evidence of NREM sleep slow wave activity playing an important role in synaptic homeostasis, synaptic plasticity regulation, and brain reorganization [80,81,82]. Slow wave sleep alteration may be a further causative factor for cognitive impairment in children with West syndrome. Possible etiologies are several including genetic, metabolic, hypoxic, ischemic, malformation, or other brain damages. Treatment should be started as soon as possible, and the effect frequently monitored by sleep EEG recordings. According to international recommendations, the most effective drugs are vigabatrin, adrenocorticotropic hormone (ACTH), and large doses of oral steroids (prednisolone) [83,84,85].
A few studies analyzed HRV in children with epileptic spasms. In particular, Moller et al. found an initial and transient reduction of HRV at the time of onset of West syndrome, probably related to the presence of hypsarrhythmia [86]. Moreover, while two studies reported a sympathetic dominance in patients with epileptic spasms [86, 87], one did not find significant differences other than lower heart rate in slow wave sleep [88]. A fourth study analyzing HRV during NREM sleep found an increased orthosympathetic component in patients with West syndrome with respect to control group [89]. Finally, ACTH treatment seems to induce a shift toward recovery of parasympathetic function [86, 87, 89].
Lennox–Gastaut Syndrome
Lennox–Gastaut syndrome is a severe developmental and epileptic encephalopathy characterized by the presence of drug-resistant tonic, tonic-clonic, myoclonic and atypical absence seizures and intellectual disability. It is a rare disorder, representing 1–5% of all epilepsies and 3–10% of childhood epilepsies with an annual estimated incidence of 0.2–2.8/10,000 births in European countries [90]. The typical age of onset is between 2 and 8 years, and it can appear as apparently cryptogenic or symptomatic of various etiologies such as pre- or perinatal ischemic or hypoxic brain damages, infections, tumors, and malformations. It is often preceded by a West syndrome or focal seizures [90]. Tonic seizures typically occur during sleep, although they can be subtle and detectable only with video-EEG polysomnographic recordings. Interictal EEG usually shows diffuse slow spike-wave complexes (<3 Hz) increasing during NREM sleep and fast rhythmic bursts (10 Hz) during sleep; ictal EEG typically presents diffuse fast 10–20/s activity often preceded by attenuation of the background activity [91]. The long-term outcome is poor and it is associated with high mortality and severe morbidity [92]. Several drugs are available for the treatment of Lennox–Gastaut syndrome and have been validated through randomized, controlled trials. However, at present, there is no evidence to suggest that any one drug is more efficacious than another [93]. Nonpharmacological treatment (e.g., ketogenic diet, epilepsy surgery or vagus nerve stimulation) has also been tried with variable results and may be considered after failure of two-to-three drugs [93].
Koenig et al. reported a case of a patient affected by Lennox–Gastaut syndrome with a severe impairment of HRV at baseline, which improved after implantation of a vagus nerve stimulator [94].
Electrical Status Epilepticus During Slow Sleep (ESES)
ESES , also known as continuous spike-wave during slow wave sleep (CSWS) , is a developmental epileptic encephalopathy with seizure onset between 2 months and 12 years [95]. Seizures are frequent and types are various with focal motor or generalized seizures occurring during sleep, atypical absences, and atonic seizures during the awake state. Tonic seizures are not observed, allowing differential diagnosis with Lennox–Gastaut syndrome. EEG is characterized by continuous diffuse spike-waves during slow waves sleep, which is usually noted after seizure onset. Spike-waves index (SWI) is a parameter frequently used to assess ESES severity and it is usually ranged between 85% and 100% during all night NREM sleep stages [95, 96]. Global cognitive regression, either acute or insidious, is typical some months after seizure onset , generally associated with behavioral disturbances [97]. As in West syndrome, an impairment in physiological decrease of slow waves during sleep has been observed. The impairment is directly correlated with the amount of SWI during sleep. The evolution of seizures is usually benign, but neuropsychological disorders and behavioral disturbances may be pronounced, irrespective of previous cognitive functions and development [63]. The severity of neuropsychological impairment correlates with duration of CSWS EEG pattern and the topography of the spike-waves is associated with the type of cognitive deficit [98]. Half of the patients present brain malformation or lesions, in particular pre or perinatal thalamic lesions have been reported in these patients [97, 99]. Two epileptic syndromes, BECTS and Landau–Kleefner, show similar characteristic to ESES syndrome; both are characterized by a specific increase of spike-waves during sleep. These three syndromes have been indeed associated with the same genes pathogenic variants GRIN2A, PRRT2, SRPXA, and ELP4 and the hypothesis is that they constitute an electro-clinical spectrum with a continuum between these disorders [100, 101]. Treatment options are various comprising steroids, benzodiazepines, levetiracetam, ethosuximide, valproic acid, ketogenic diet, but there are no controlled trials establishing the efficacy of different antiepileptic drugs and successful treatment is often difficult to achieve. The primary goal of treatment is to prevent cognitive deterioration or to improve neuropsychological impairment that are directly associated with CSWS duration and aggressive approach including steroid use should be considered [102].
Sudden Unexpected Death in Epilepsy (SUDEP)
Definitions
Epileptic patients have a higher probability to die prematurely with respect to the general population. Indeed, the risk of sudden unexpected death is considered to be 24–40 times higher in patients with epilepsy compared to people not affected [103]. Sudden unexpected death in epilepsy (SUDEP) has been defined as “sudden, unexpected, witnessed or unwitnessed, non-traumatic, and non-drowning death that occurs in benign circumstances in an individual with epilepsy, with or without evidence for a seizure, and excludes documented status epilepticus, in which postmortem examination does not reveal a cause of death” [104]. In the 1990s, two complementary definitions were published [105, 106] and since then, these two definitions have been used in most SUDEP studies, but often with variations. For this reason, in 2012 Nashef et al. proposed a unified SUDEP definition and classification to resolve current ambiguities and to retrieve cases that would not have been further studied if the previous definitions were used [104] (for details regarding the definitions see Table 19.2).
Epidemiology
Incidence of SUDEP varies depending on several factors, such as the modality of assessment of the causes of death, selection criteria, different definitions of SUDEP, and documentation requirements. In general, the risk of SUDEP is estimated in two types of studies: general community-based populations and clinical cohorts (for a review see Thurman et al. [107]). Considering community-based studies including all age groups, estimates ranged from 0.33 to 1.35 cases of SUDEP annually per 1000 people with epilepsy. Nine clinic-based studies, mainly representing people with treatment-resistant epilepsy, yielded higher estimates of SUDEP occurrence, ranging from 1.2 to 6.3 cases of SUDEP annually per 1000 individuals. In 2017, the American Academy of Neurology (AAN) and American Epilepsy Society (AES) published a clinical practice parameter guideline on SUDEP risk factors and incidence [108]. They found that the SUDEP risk in children with epilepsy is low (0.22/1000 patient-years) and increases in adults (1.2/1000 patient-years). However, this finding was challenged by two recent studies conducted with a more comprehensive study design employing multiple data sources to screen for SUDEP cases. In particular, Keller et al. [109] reported the overall incidence of pediatric SUDEP as 1.17 per 1000 person-years in the province of Ontario, Canada while Sveinsson et al. [110], using linked records from the Swedish National Patient Registry and National Cause of Death Registry, reported the incidence of SUDEP to be similar across all age groups: 1.1 per 1000 person-years in children <16 years and in adults 1.13 (age 16–50 years) and 1.29 (age > 50 years), respectively.
Studies analyzing the frequency of SUDEP in patients with sleep-related epilepsy are rare. In 2015, Mostacci et al. conducted a retrospective study showing an incidence of SUDEP in SHE patients similar to that observed in the general epilepsy population (0.36 per 1000 person-years) [13]. Authors hypothesized that the low prevalence of SUDEP could be probably related to the low occurrence of generalized tonic-clonic seizures in people with SHE. Considering that recent findings demonstrated that SOZ in SHE can be located also outside the frontal lobe, it is possible that SHE patients with an insular onset are at higher risk of SUDEP. Indeed, it has been shown that seizures originating from the insula may be accompanied by more evident autonomic dysfunctions.
Three boys with a diagnosis of BECTS were identified among 189 cases reported in the North American SUDEP Registry [111]. The three patients spanned the spectrum of BECTS severity: one had only a few seizures, one had more than 30 focal motor seizures, and one had four witnessed generalized tonic-clonic seizures and approximately 30 suspected generalized tonic-clonic seizures. None of the patients was prescribed antiseizure drugs, either owing to physician recommendation or mutual decision by the physician and parents. All three patients were found dead in circumstances typical of SUDEP.
A population study was conducted in Finland to analyze long-term survival and mortality among 207 patients treated for West syndrome in three tertiary care hospitals [112]. Etiology at onset was symptomatic in 87% patients and cryptogenic in 13%; six of the latter 26 patients later turned out to be symptomatic. During follow-up, 10 of 207 patients had died of SUDEP, representing the most common epilepsy-related cause of death. The mean age at SUDEP was 27.4 years. All but one of the SUDEP cases had intellectual disability and all but one had an underlying neurologic disorder.
Risk Factors for SUDEP
The identification of risk factors is essential to support clinicians to recognize individuals at risk and to identify how SUDEP risk can be mitigated and prevented, but also potentially help understand the underlying pathophysiology of SUDEP. Studies analyzing SUDEP risk factors are different with regard to the populations studied, methodology, and study design. The above cited AAN/AES guidelines [108] considered the presence and frequency of generalized tonic-clonic seizures (GTCs) as the most important risk factors for SUDEP (OR 10). Other factors associated with higher SUDEP risk were not being seizure free for 1–5 years (OR 4.7) and not adding an antiepileptic drug (AED) when patients are medically refractory (OR 6); the presence of nocturnal supervision was found to reduce SUDEP risk (OR 0.4), as did the use of a nocturnal listening device (OR 0.1).
Previous studies reported that specific AEDs (i.e., lamotrigine or carbamazepine) were associated with heightened SUDEP risk; however, these findings have been challenged from the results of recent studies. Therefore, the AAN/AES concluded that the evidence was low or low/conflicting for specific AED use and polytherapy, respectively, for SUDEP risk [108]. Conversely, optimization of AED therapy may reduce SUDEP risk: hence, in individuals with refractory epilepsy, the risk of SUDEP seems to be increased when an AED is not added to the treatment regimen.
Different scales are in development to help identify persons at risk. DeGiorgio et al. [113] reported the first SUDEP Inventory (the SUDEP-7), which was drawn from a single large cohort study [114]. The SUDEP-7 has been proposed to quantify SUDEP risk in living people with epilepsy based upon five factors: frequency of generalized tonic–clonic seizures, monthly seizure frequency, number of antiepileptic drugs in use, duration of epilepsy, and intellectual disability. Each risk factor is assigned a point score based on its respective odds ratio for SUDEP, with a possible score of 0–12. The revised SUDEP-7 (rSUDEP-7) combines generalized tonic-clonic seizures and seizure frequency measures to reduce score inflation [115] (Table 19.3).
Genetics of SUDEP
Recently, several pathogenic alterations in different genes have been reported to increase SUDEP risk through multiple pathophysiological mechanisms. Specific genetic and epilepsy syndromes are also associated with increased risk of SUDEP. Patients with Dravet syndrome (DS), a genetic epileptic encephalopathy in which a SCN1A loss-of-function mutation is found in 80% of cases, are at increased risk of SUDEP and premature mortality [116]. Analogously, individuals with mutations in SCN8A, associated with early-infantile encephalopathy, may also be at increased risk of SUDEP although a recent review of all-cause mortality in SCN8A did not confirm this association [117]. Moreover, patients with isodicentric chromosome 15 syndrome —especially those with severe neurological dysfunction —have higher risk of SUDEP. Several other genes have been found to be associated with SUDEP such as, SCN2A, SCN5A, KCNA1, KCNQ1, KCNH2, DEPDC5 (for a detailed review see [118]). Notably, some of these ion channel mutations are co-expressed in the brain and heart and predispose to both epileptic seizures and cardiac arrhythmias.
SUDEP and Sleep
Prince John the youngest child of King George V of England died having SUDEP during sleep. At that time, the royal doctor, Alan Reeve Manby wrote: “His Royal Highness Prince John, who has since infancy suffered from epileptic fits, which have lately become more frequent and severe, passed away in his sleep following an attack this afternoon at Sandringham.” This is one of the first witnessed SUDEP case that happened during sleep. Subsequently, different studies demonstrated an association between SUDEP and night time or sleep [119, 120]. For instance, Lamberts et al. observed that 62% of SUDEP cases happened between midnight and noon and that 58% of SUDEP cases were sleep-related [121].Similarly, a recent systematic review and meta-analysis [122] demonstrated that SUDEP is significantly associated with sleep as compared to wakefulness (69.3% of cases occurred during sleep and 30.7% occurred during wakefulness).
All these data suggest that sleep exert a fundamental role as a risk factor that can act in a particular circumstance with a peri-ictal coincidence of several precipitating factors. Moreover, other than the function of sleep as a possible precipitating factor for SUDEP, recent evidence suggests that sleep-epilepsy interaction could also exert a long-standing effect leading to an increase of SUDEP susceptibility. Different studies showed that ANS factors affecting cardiac or respiratory functions play a pivotal role in the SUDEP pathogenesis. However, evidence on how they might be related to one another or to an epileptic seizure, eventually leading to sudden death, especially during sleep, are not conclusive [2, 119].
Longstanding Effects of Sleep and Epilepsy Interaction: Implications for SUDEP
Different studies reported autonomic changes in epileptic patients, especially during sleep. Interictal HRV abnormalities have been described in various populations of both focal (especially temporal] and generalized epilepsy [1]. These HRV variations seem to be more pronounced during night time, suggesting that this period might be more vulnerable to impaired ANS heart control. Moreover, HRV modifications in epileptic patients improve after epilepsy surgery; in particular, patients with a poor surgical outcome show a more pronounced HRV impairment than those individuals with a good outcome [123]. These findings suggest that the ANS control could be mainly related to seizure reduction.
It is not completely clarified if HRV modifications reported in epileptic patients during sleep are related to a direct effect of interictal discharges or to their indirect effect on arousal instability. Indeed, as discussed above for SHE, periodic epileptic discharges can increase arousal fluctuations, inducing in turn a chronic sympathetic over activation. Moreover, among drug-resistant patients, a further vulnerability factor is represented by the repeated effects of seizures on the cardiovascular system that might also result in damage to the nervous network regulating excitation of the cardiac tissue. Indeed, sudden cardiac death (SCD) is more frequent in patients with underlying cardiac structural abnormalities. However, although several studies demonstrated postmortem myocardial injuries in SUDEP subjects [124], to date no evidence exists to support this possibility.
Some studies correlate specific AEDs use with greater degrees of autonomic dysfunction. For instance, carbamazepine can slow cardiac conduction, reduce HRV and, rarely, induce atrio-ventricular (AV) conduction block in elderly patients [125]. Moreover, the side effects of some AEDs such as weight gain or hyperhomocysteinemiae may in turn cause an indirect dysfunction of the cardiovascular system [119]. Different studies analyzed the effects of weaning AEDs in adults, including the rapid tapering that typically occurs during epilepsy monitoring unit admissions with conflicting results. While two studies demonstrated that rapid AEDs reduction led to acute decreases in HRV, the third study found instead no significant differences [1]. Another study in seizure-free outpatients with epilepsy on drug monotherapy demonstrated that slow withdrawal of AEDs appeared to improve HRV [126]. In summary, there is not enough evidence of a negative impact of AEDs on sleep-related autonomic changes; conversely, achieving complete seizure control represents one of the main tools to reduce SUDEP risk. Moreover, AEDs may show beneficial effects not only on the control of neuronal excitability but also on stabilization of sleep structure, with a consequent reduction of sleep arousals and in turn an indirect positive improvement of ANS functions.
Although different studies analyzed HRV in epileptic living patients, evaluating HRV parameters in relation to SUDEP risk factors and prospective data on HRV from SUDEP patients are scanty. In 2009, Surges et al. did not find significant differences in interictal HRV measures on seven patients with SUDEP with respect to a control group [127]. In a recent work, Myers et al. analyzed HRV data in wakefulness and sleep from 80 patients with drug-resistant epilepsy, half of whom had sodium channel mutations. They found that patients with sodium channel mutations who died due to SUDEP showed more extreme HRV derangements with low HRV in wakefulness and extremely high or very low values of sleep/awake HRV ratios [1].
Seizures During Sleep: Precipitating Factors for SUDEP
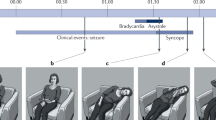
Studies evaluating the circumstances of death suggest that the most common scenario for SUDEP is a convulsive seizure occurring during sleep especially in prone position. The MORTEMUS study represents the largest study to date describing cardiorespiratory function at the time of death in epilepsy [128]. In this study, seven of the 10 cases for which sleep state could be determined occurred during sleep (one during REM, one during stage 1, two in stage 2, and three in sleep stages 3 or 4). Moreover, all SUDEP death happened after a convulsive seizure. In summary, the triggering seizure was followed by a short period of normal or increased heart and respiratory rates, after which a combination of central apnea, severe bradycardia, or transient asystole occurred concomitant with postictal generalized EEG suppression (PGES), typically after 1–3 min. This cardiorespiratory collapse was terminal in a third of patients. In the other patients, this was followed by transient restoration of cardiac function associated with abnormal and possibly ineffective respiration, probably aggravated by the prone position. Respiration then progressively worsened until terminal apnea, which occurred before terminal asystole in all cases. These data suggest that the highly abnormal cardiorespiratory patterns seen in all these cases could have been induced by a catastrophic autonomic dysfunction consequent to a severe brainstem problem.
Although a severe cardiorespiratory dysfunction induced by a GTCS represents the most common pathogenetic mechanism in SUDEP, rare cases of ventricular tachyarrhythmia in near-SUDEP or SUDEP patients were described. For instance, Espinosa et al. reported a case of near-SUDEP in a 51-year-old woman with refractory temporal lobe epilepsy. During video-EEG, a right temporal focal seizure occurred out of sleep, followed by a secondary generalization, which triggered ventricular tachycardia, requiring placement of an implantable cardiac defibrillator [129].
Studies conducted in animal models showed that both interictal and ictal epileptic activity may affect cardiac rhythm. Moreover, different kind of cardiac arrhythmias (i.e., severe tachyarrhtythmias, bradycardia, or asystole) have been shown not only in GTCS but also in focal seizures, especially originating from the temporal lobes, the insular cortex, or the limbic system. A study conducted with long-term ECG recording via implantable loop recorders in patients with refractory epilepsy reported the occurrence of at least one episode of ictal bradycardia or asystole in 21% of patients [130]. A retrospective analysis of multiday ECG data obtained during video-EEG recording in a population of drug resistant patients found that patients who subsequently died having SUDEP had greater increases in heart rate during seizures as compared with a clinically similar group of patients with refractory epilepsy. These changes in heart rate were particularly evident during sleep-onset seizures in the SUDEP group [131].
Different studies investigated the role of the respiratory system in the pathophysiology of SUDEP. Indeed, ictal respiratory impairment, often closely linked to cardiac dysfunction, is a prominent feature in witnessed SUDEP cases as demonstrated by the MORTEMUS study [128]. Since 1950s, Kaada and Jasper demonstrated that electrical stimulation of different brain regions such as human insular cortex, ventromedial prefrontal cortex, hippocampus, and amygdala produced an autonomic dysfunction which may cause respiratory arrest in rare cases [132]. It has been shown that ictal desaturations and ictal central apnea strongly correlate with temporal lobe epilepsy. In particular, intracerebral recordings in drug-resistant epileptic patients revealed that ictal apnea is related to the spread of a temporal onset seizure to the contralateral temporal lobe rather than the seizure onset itself [133]. More recently, another study with stereo EEG in patients with medically intractable epilepsy found that central apnea and O2 desaturation occurred when seizures spread to the amygdala. Moreover, in the same patient localized electrical stimulation of the amygdala reproduced the same respiratory dysfunction [134]. Severe and prolonged increases in end-tidal CO2 (ETCO2) and modification of regional cerebral oxygen saturation are also reported to occur with seizures. Indeed, a study conducted with video-EEG telemetry with recording of respiratory data revealed that one-third of patients with focal epilepsy had seizures with ETCO2 elevation above 50 mm Hg. Of note, ictal/postictal ETCO2 increase above baseline was recorded for a mean duration of 424 s [133]. Moreover, studies by means of near-infrared spectroscopy reported decreased regional cerebral oxygenation with ictal onset [135].
In the majority of SUDEP cases, the victim is found in the prone position [136]. It is generally agreed that ending a convulsive seizure in the prone position may increase the risk of SUDEP. The most plausible explanation for this is that this position might increase the risk of an obstruction of the nose and mouth due to pressure against the bed clothing and alter ventilation by reducing vital capacity and tidal volume [137].
Serotonergic neurons seem to be implicated in the basic neurobiological mechanisms for SUDEP. Indeed, serotonergic neurotransmission modulates different brain functions, such as breathing, sleep-wake transitions and circadian rhythmicity. Serotonin levels are highest during wakefulness, reduced during NREM sleep, and almost absent during REM sleep [138]. Stable breathing requires serotonergic neurotransmission [139]. During sleep, increase in serotonergic neurotransmission is essential for the arousal response to inspired CO2. An ictal epileptic discharge induces a suppression of serotonergic neurotransmission during both ictal and postictal period [140]. Therefore, a disruption of normal serotonergic arousal mechanisms may alter the normal arousal response to CO2 in the postictal period during sleep, thus favoring death. In DBA/2 mice which lack several 5 HT receptor proteins in the brainstem, provoked audiogenic seizures leading to death due to respiratory arrest, which can be prevented with oxygenation. It is noteworthy that this seizure-related death is reduced by use of selective serotonin reuptake inhibitors (SSRI) [141]. An animal study conducted with kainic acid to induce recurring seizures, showed that seizure activity caused increased firing of the recurrent laryngeal nerve resulting in laryngospasm and airway occlusion, with a consequent obstructive apnea followed by ST segment elevation, bradycardia, and death [142].
An electrophysiological phenomenon which can be observed following seizures is PGES. This consists in the absence of detectable scalp EEG activity at <10 microvolts amplitude, typically following a primary or secondarily GTCS. In rare cases of SUDEP, a PGES started before the occurrence of any fatal cardiac or respiratory arrest [143]. A primary cause of this unclear phenomenon could be an alteration of cerebral blood flow autoregulation that induce a sudden drop of cerebral perfusion and a subsequent cessation of electrical activity. In epileptic patients, an overactivity of the sympathetic drive is reported to induce an impairment of interictal cerebrovascular autoregulation [144]. During NREM sleep, a reduction of cerebral blood flow and cerebrovascular response to hypercapnia and especially to hypoxia occurs, thus suggesting that during this sleep stage cerebral circulation may be particularly vulnerable [145].
Sleep Disorders Comorbidities in Epileptic Patients: Implications for SUDEP
Patients suffering from nocturnal epilepsy and, in general, from epilepsy can also suffer from primary sleep disorders. These can manifest themselves independently from epilepsy in the same patient but can negatively influence the epileptic disorder itself; conversely, the expression of sleep disorders can be negatively influenced by the epileptic condition. The most frequent sleep disorder in epilepsy patients is represented by obstructive sleep apnea (OSA). The frequency of comorbid OSA in various epilepsy cohorts has been estimated to range between approximately 10% and 30%. Recently, McCarter et al. demonstrated that drug resistant epilepsy patients are at an increased risk of sleep disordered breathing, particularly OSA [146]. Moreover, they also found a possible association between OSA and SUDEP risk. Indeed, OSA has been identified as an independent risk factor for sudden cardiac death [147]. The autonomic dysfunction and lower resting oxygen saturation observed in OSA patients might increase a patient’s vulnerability to SUDEP. In particular, recurrent apnea/hypopnea episodes may lead to an increase in sympathetic tone and hypoxemia that, in turn, increase myocardial oxygen demand, resulting in cardiac ischemia and potentially fatal dysrhythmias. Moreover, OSA could further increase the QTc interval in vulnerable refractory epilepsy patients who may already have underlying prolonged QTc due to seizures or AEDs side effects [148]. Finally, OSA could induce a further impairment of HRV, worsening a poor pre-existing underlying autonomic function in patients with epilepsy that, in turn, could favor the development of tachydysrhythmias and sudden death [149]. The increased SUDEP risk in epileptic patients with OSA may be also mediated by a respiratory dysfunction. A chronic hypercapnia with a decreased ventilatory response to higher carbon dioxide levels has been described in patients with OSA [150]. Therefore, the above-mentioned dysfunctional serotonin transmission induced by seizures together with the blunted respiratory response to hypercapnia caused by chronic OSA may further reduce the respiratory drive following postictal apnea, increasing the risk of SUDEP. Finally, OSA could induce a sleep fragmentation and a consequent sleep deprivation [151], lowering the seizure threshold, and increasing the risk for refractory seizures and, in turn, the susceptibility for SUDEP.
Prevention of SUDEP
The primary purpose of understanding the incidence, risk factors, and biomarkers for SUDEP is to help identify individuals at risk and to determine how risk can be mitigated and SUDEP prevented. As discussed above, the most important risk factor for SUDEP is the presence of GTCS. The American Academy of Neurology and American Epilepsy Society recommend that clinicians inform their patients about the SUDEP risk [108]. Different survey studies revealed that epilepsy patients and family members prefer to know about the risk factors of SUDEP during the early phase of management [152]. However, recent evidence showed that only a small minority of neurologists counsel all of their patients about SUDEP [153]. A meta-analysis conducted by Ryvlin et al. found that an adjunctive AED treatment might reduce the SUDEP risk by seven times in drug resistant epilepsy patients [154]. In case of refractory epilepsy, other treatments should be evaluated, such as epilepsy surgery, vagal nerve stimulation (VNS), responsive neurostimulation, and the ketogenic diet.
Considering the role of sleep as risk factor for SUDEP, nocturnal supervision and nocturnal listening devices have been proposed as preventive measures for SUDEP. In accordance with the AAN/AES guideline, “for persons with frequent GTCS and nocturnal seizures, clinicians may advise selected patients and families, if permitted by their individualized epilepsy type and psychosocial circumstances, to use nocturnal supervision or other nocturnal precautions, such as the use of a remote listening device, to reduce SUDEP risk” [108].
Moreover, a growing number of automated devices to help detect seizures are now available in the market. There are two categories of devices: EEG-based systems (i.e., video EEG, ambulatory EEG, neurostimulation devices, VNS) and non-EEG systems, which employ several different technologies, such as accelerometry, electrodermal activity or skin resistance/conductance, near-infrared spectroscopy, electrocardiogram, electromyogram, and video monitoring [155]. However, to date, studies evaluating their efficacy are scanty, frequently conducted on small population of patients and by the same team developing the devices.
Although evidence regarding prone position as a risk factor for SUDEP is discordant, different simple preventive measures have been suggested, such as positioning or the use of antisuffocation pillow. Moreover, using early intervention, such as stimulating and turning the patient to the lateral position and suction with or without supplemental oxygen administration could help to reduce the duration of peri-ictal hypoxemia and seizure [156].
Finally, Bateman et al. found that SSRIs are associated with reduced severity of ictal hypoxemia in patients with medically refractory partial seizures [157]; however, further studies are needed to determine if SSRIs can reduce SUDEP risk.
In summary, epilepsy patients often display ANS dysfunction during interictal states that can be related to genetic, medication, and other factors. Seizures may induce acute cardiac and respiratory dysfunction. Indeed, during seizures a sinus tachycardia frequently occurs but also asystole and malignant tachyarrhythmias have been observed. Seizures can also trigger an acute respiratory dysfunction, such as central ictal and obstructive apnea related to laryngospasm. Thus, recent data suggest that the underlying autonomic dysfunction in epilepsy patients might predispose them to a sudden fatal cardio-respiratory dysfunction during a seizure, resulting in SUDEP.
References
Myers KA, Sivathamboo S, Perucca P. Heart rate variability measurement in epilepsy: how can we move from research to clinical practice? Epilepsia. 2018;59(12):2169–78.
Barot N, Nei M. Autonomic aspects of sudden unexpected death in epilepsy (SUDEP). Clin Auton Res. 2019;29(2):151–60.
Lugaresi E, Cirignotta F. Hypnogenic paroxysmal dystonia: epileptic seizure or a new syndrome? Sleep. 1981;4(2):129–38.
Lugaresi E, Cirignotta F, Montagna P. Nocturnal paroxysmal dystonia. J Neurol Neurosurg Psychiatry. 1986;49(4):375–80.
Tharp BR. Orbital frontial seizures. An unique electroencephalographic and clinical syndrome. Epilepsia. 1972;13(5):627–42.
Williamson PD, Spencer DD, Spencer SS, Novelly RA, Mattson RH. Complex partial seizures of frontal lobe origin. Ann Neurol. 1985;18(4):497–504.
Waterman K, Purves SJ, Kosaka B, Strauss E, Wada JA. An epileptic syndrome caused by mesial frontal lobe seizure foci. Neurology. 1987;37(4):577–82.
Tinuper P, Bisulli F, Cross JH, Hesdorffer D, Kahane P, Nobili L, et al. Definition and diagnostic criteria of sleep-related hypermotor epilepsy. Neurology. 2016;86(19):1834–42.
Scheffer IE, Bhatia KP, Lopes-Cendes I, Fish DR, Marsden CD, Andermann F, et al. Autosomal dominant frontal epilepsy misdiagnosed as sleep disorder. Lancet Lond Engl. 1994;343(8896):515–7.
Provini F, Plazzi G, Tinuper P, Vandi S, Lugaresi E, Montagna P. Nocturnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(6):1017–31.
Nobili L, Francione S, Mai R, Cardinale F, Castana L, Tassi L, et al. Surgical treatment of drug-resistant nocturnal frontal lobe epilepsy. Brain J Neurol. 2007;130(Pt 2):561–73.
Vignatelli L, Bisulli F, Giovannini G, Licchetta L, Naldi I, Mostacci B, et al. Prevalence of nocturnal frontal lobe epilepsy in the adult population of Bologna and Modena, Emilia-Romagna region, Italy. Sleep. 2015;38(3):479–85.
Mostacci B, Bisulli F, Vignatelli L, Licchetta L, Di Vito L, Rinaldi C, et al. Incidence of sudden unexpected death in nocturnal frontal lobe epilepsy: a cohort study. Sleep Med. 2015;16(2):232–6.
Aridon P, Marini C, Di Resta C, Brilli E, De Fusco M, Politi F, et al. Increased sensitivity of the neuronal nicotinic receptor alpha 2 subunit causes familial epilepsy with nocturnal wandering and ictal fear. Am J Hum Genet. 2006;79(2):342–50.
De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, et al. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet. 2000;26(3):275–6.
McLellan A, Phillips HA, Rittey C, Kirkpatrick M, Mulley JC, Goudie D, et al. Phenotypic comparison of two Scottish families with mutations in different genes causing autosomal dominant nocturnal frontal lobe epilepsy. Epilepsia. 2003;44(4):613–7.
Heron SE, Smith KR, Bahlo M, Nobili L, Kahana E, Licchetta L, et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 2012;44(11):1188–90.
Barcia G, Fleming MR, Deligniere A, Gazula V-R, Brown MR, Langouet M, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44(11):1255–9.
Cataldi M, Nobili L, Zara F, Combi R, Prato G, Giacomini T, et al. Migrating focal seizures in autosomal dominant sleep-related hypermotor epilepsy with KCNT1 mutation. Seizure. 2019;67:57–60.
Ricos MG, Hodgson BL, Pippucci T, Saidin A, Ong YS, Heron SE, et al. Mutations in the mammalian target of rapamycin pathway regulators NPRL2 and NPRL3 cause focal epilepsy. Ann Neurol. 2016;79(1):120–31.
Korenke G-C, Eggert M, Thiele H, Nürnberg P, Sander T, Steinlein OK. Nocturnal frontal lobe epilepsy caused by a mutation in the GATOR1 complex gene NPRL3. Epilepsia. 2016;57(3):e60–3.
Ishida S, Picard F, Rudolf G, Noé E, Achaz G, Thomas P, et al. Mutations of DEPDC5 cause autosomal dominant focal epilepsies. Nat Genet. 2013;45(5):552–5.
Dibbens LM, de Vries B, Donatello S, Heron SE, Hodgson BL, Chintawar S, et al. Mutations in DEPDC5 cause familial focal epilepsy with variable foci. Nat Genet. 2013;45(5):546–51.
Baulac S, Ishida S, Marsan E, Miquel C, Biraben A, Nguyen DK, et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77(4):675–83.
Carvill GL, Crompton DE, Regan BM, McMahon JM, Saykally J, Zemel M, et al. Epileptic spasms are a feature of DEPDC5 mTORopathy. Neurol Genet. 2015;1(2):e17.
Chen Z-H, Wang C, Zhuo M-Q, Zhai Q-X, Chen Q, Guo Y-X, et al. Exome sequencing identified a novel missense mutation c.464G>A (p.G155D) in Ca2+−binding protein 4 (CABP4) in a Chinese pedigree with autosomal dominant nocturnal frontal lobe epilepsy. Oncotarget. 2017;8(45):78940–7.
Peres J, Antunes F, Zonjy B, Mitchell AL, Lhatoo SD. Sleep-related hypermotor epilepsy and peri-ictal hypotension in a patient with syntaxin-1B mutation. Epileptic Disord. 2018;20(5):413–7.
Proserpio P, Cossu M, Francione S, Gozzo F, Lo Russo G, Mai R, et al. Epileptic motor behaviors during sleep: Anatomo-electro-clinical features. Sleep Med. 2011;12:S33–8.
Nobili L, Cardinale F, Magliola U, Cicolin A, Didato G, Bramerio M, et al. Taylor’s focal cortical dysplasia increases the risk of sleep-related epilepsy. Epilepsia. 2009;50(12):2599–604.
Licchetta L, Bisulli F, Vignatelli L, Zenesini C, Di Vito L, Mostacci B, et al. Sleep-related hypermotor epilepsy: long-term outcome in a large cohort. Neurology. 2017;88(1):70–7.
Gibbs SA, Proserpio P, Francione S, Mai R, Cardinale F, Sartori I, et al. Clinical features of sleep-related hypermotor epilepsy in relation to the seizure-onset zone: a review of 135 surgically treated cases. Epilepsia. 2019;60(4):707–17.
Oldani A, Zucconi M, Ferini-Strambi L, Bizzozero D, Smirne S. Autosomal dominant nocturnal frontal lobe epilepsy: electroclinical picture. Epilepsia. 1996;37(10):964–76.
Nobili L, Francione S, Mai R, Tassi L, Cardinale F, Castana L, et al. Nocturnal frontal lobe epilepsy: intracerebral recordings of paroxysmal motor attacks with increasing complexity. Sleep. 2003;26(7):883–6.
Nobili L, Sartori I, Terzaghi M, Tassi L, Mai R, Francione S, et al. Intracerebral recordings of minor motor events, paroxysmal arousals and major seizures in nocturnal frontal lobe epilepsy. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2005;26(Suppl 3):S215–9.
Terzaghi M, Sartori I, Mai R, Tassi L, Francione S, Cardinale F, et al. Sleep-related minor motor events in nocturnal frontal lobe epilepsy. Epilepsia. 2007;48(2):335–41.
Nobili L, Sartori I, Terzaghi M, Stefano F, Mai R, Tassi L, et al. Relationship of epileptic discharges to arousal instability and periodic leg movements in a case of nocturnal frontal lobe epilepsy: a stereo-EEG study. Sleep. 2006;29(5):701–4.
Gibbs SA, Proserpio P, Terzaghi M, Pigorini A, Sarasso S, Lo Russo G, et al. Sleep-related epileptic behaviors and non-REM-related parasomnias: insights from stereo-EEG. Sleep Med Rev. 2016;25:4–20.
Proserpio P, Cossu M, Francione S, Tassi L, Mai R, Didato G, et al. Insular-opercular seizures manifesting with sleep-related paroxysmal motor behaviors: a stereo-EEG study: nocturnal insular-opercular seizures. Epilepsia. 2011;52(10):1781–91.
Mai R, Sartori I, Francione S, Tassi L, Castana L, Cardinale F, et al. Sleep-related hyperkinetic seizures: always a frontal onset? Neurol Sci. 2005;26(S3):s220–4.
Ryvlin P, Minotti L, Demarquay G, Hirsch E, Arzimanoglou A, Hoffman D, et al. Nocturnal hypermotor seizures, suggesting frontal lobe epilepsy, can originate in the insula. Epilepsia. 2006;47(4):755–65.
Rheims S, Ryvlin P, Scherer C, Minotti L, Hoffmann D, Guenot M, et al. Analysis of clinical patterns and underlying epileptogenic zones of hypermotor seizures. Epilepsia. 2008;49(12):2030–40.
Gibbs SA, Proserpio P, Francione S, Mai R, Cossu M, Tassi L, et al. Seizure duration and latency of hypermotor manifestations distinguish frontal from extrafrontal onset in sleep-related hypermotor epilepsy. Epilepsia. 2018;59(9):e130–4.
Nobili L, Francione S, Mai R, Cardinale F, Castana L, Tassi L, et al. Surgical treatment of drug-resistant nocturnal frontal lobe epilepsy. Brain. 2007;130(2):561–73.
Menghi V, Bisulli F, Tinuper P, Nobili L. Sleep-related hypermotor epilepsy: prevalence, impact and management strategies. Nat Sci Sleep. 2018;10:317–26.
Losurdo A, Proserpio P, Cardinale F, Gozzo F, Tassi L, Mai R, et al. Drug-resistant focal sleep related epilepsy: results and predictors of surgical outcome. Epilepsy Res. 2014;108(5):953–62.
Harnod T, Yang CCH, Hsin Y-L, Wang P-J, Shieh K-R, Kuo TBJ. Heart rate variability in patients with frontal lobe epilepsy. Seizure. 2009;18(1):21–5.
Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N. Heart rate variability in normal and pathological sleep. Front Physiol. 2013;4:294.
Halász P, Terzano M, Parrino L, Bódizs R. The nature of arousal in sleep. J Sleep Res. 2004;13(1):1–23.
Halász P, Kelemen A, Szűcs A. Physiopathogenetic interrelationship between nocturnal frontal lobe epilepsy and nrem arousal parasomnias. Epilepsy Res Treat. 2012;2012:1–8.
Calandra-Buonaura G, Toschi N, Provini F, Corazza I, Bisulli F, Barletta G, et al. Physiologic autonomic arousal heralds motor manifestations of seizures in nocturnal frontal lobe epilepsy: implications for pathophysiology. Sleep Med. 2012;13(3):252–62.
Dorantes G, Méndez M, Alba A, Gonzaáez JS, Parrino L, Milioli G. Heart rate variability in cyclic alternating pattern during sleep in healthy and nocturnal front lobe epilepsy patients. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:5944–7.
Pal DK, Ferrie C, Addis L, Akiyama T, Capovilla G, Caraballo R, et al. Idiopathic focal epilepsies: the “lost tribe”. Epileptic Disord Int Epilepsy J Videotape. 2016;18(3):252–88.
Baglietto MG, Battaglia FM, Nobili L, Tortorelli S, De Negri E, Calevo MG, et al. Neuropsychological disorders related to interictal epileptic discharges during sleep in benign epilepsy of childhood with centrotemporal or Rolandic spikes. Dev Med Child Neurol. 2001;43(6):407–12.
Wickens S, Bowden SC, D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: a systematic review and meta-analysis. Epilepsia. 2017;58(10):1673–85.
Neumann H, Helmke F, Thiels C, Polster T, Selzer LM, Daseking M, et al. [Cognitive development in children with benign rolandic epilepsy of childhood with centrotemporal spikes – results of a current systematic database search]. Fortschr Neurol Psychiatr. 2016;84(10):617–632. (Article in German).
Chan S, Pressler R, Boyd SG, Baldeweg T, Cross JH. Does sleep benefit memory consolidation in children with focal epilepsy? Epilepsia. 2017;58(3):456–66.
Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet. 2013;45(9):1067–72.
Dimassi S, Labalme A, Lesca G, Rudolf G, Bruneau N, Hirsch E, et al. A subset of genomic alterations detected in rolandic epilepsies contains candidate or known epilepsy genes including GRIN2A and PRRT2. Epilepsia. 2014;55(2):370–8.
Seri S, Di Lorenzo G, Pisano T, Pinci M, Brazzo D, Betteridge H, et al. Interictal autonomic abnormalities in idiopathic Rolandic epilepsy. Epilepsy Behav. 2012;24(2):241–5.
Panayiotopoulos CP. Benign childhood epilepsy with occipital paroxysms: a 15-year prospective study. Ann Neurol. 1989;26(1):51–6.
Koutroumanidis M. Panayiotopoulos syndrome: an important electroclinical example of benign childhood system epilepsy. Epilepsia. 2007;48(6):1044–53.
Caraballo R, Cersósimo R, Fejerman N. Panayiotopoulos syndrome: a prospective study of 192 patients. Epilepsia. 2007;48(6):1054–61.
Schmitt B. Sleep and epilepsy syndromes. Neuropediatrics. 2015;46(3):171–80.
Beelke M, Nobili L, Baglietto MG, De Carli F, Robert A, De Negri E, et al. Relationship of sigma activity to sleep interictal epileptic discharges: a study in children affected by benign epilepsy with occipital paroxysms. Epilepsy Res. 2000;40(2–3):179–86.
Specchio N, Trivisano M, Claps D, Battaglia D, Fusco L, Vigevano F. Documentation of autonomic seizures and autonomic status epilepticus with ictal EEG in Panayiotopoulos syndrome. Epilepsy Behav. 2010;19(3):383–93.
Camfield CS, Striano P, Camfield PR. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S15–7.
Kasteleijn-Nolst Trenité DGA, Schmitz B, Janz D, Delgado-Escueta AV, Thomas P, Hirsch E, et al. Consensus on diagnosis and management of JME: from founder’s observations to current trends. Epilepsy Behav. 2013;28(Suppl 1):S87–90.
Genton P, Thomas P, Kasteleijn-Nolst Trenité DGA, Medina MT, Salas-Puig J. Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S8–14.
Mayer TA, Schroeder F, May TW, Wolf PT. Perioral reflex myoclonias: a controlled study in patients with JME and focal epilepsies. Epilepsia. 2006;47(6):1059–67.
Serafini A, Rubboli G, Gigli GL, Koutroumanidis M, Gelisse P. Neurophysiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S30–9.
Krishnan P, Sinha S, Taly AB, Ramachandraiah CT, Rao S, Satishchandra P. Sleep disturbances in juvenile myoclonic epilepsy: a sleep questionnaire-based study. Epilepsy Behav. 2012;23(3):305–9.
Nayak C, Sinha S, Nagappa M, Thennarasu K, Taly AB. Lack of heart rate variability during apnea in patients with juvenile myoclonic epilepsy (JME). Sleep Breath Schlaf Atm. 2015;19(4):1175–83.
Glauser TA, Loddenkemper T. Management of childhood epilepsy. Contin Minneap Minn. 2013;19(3 Epilepsy):656–81.
Pavone P, Striano P, Falsaperla R, Pavone L, Ruggieri M. Infantile spasms syndrome, west syndrome and related phenotypes: what we know in 2013. Brain Dev. 2014;36(9):739–51.
Guerrini R, Pellacani S. Benign childhood focal epilepsies. Epilepsia. 2012;53(Suppl 4):9–18.
Watanabe K, Miura K, Natsume J, Hayakawa F, Furune S, Okumura A. Epilepsies of neonatal onset: seizure type and evolution. Dev Med Child Neurol. 1999;41(5):318–22.
Rating D, Seidel U, Grimm B, Hanefeld F. The prognostic value of EEG patterns in epilepsies with infantile spasms. Brain Dev. 1987;9(4):361–4.
Kramer U, Sue WC, Mikati MA. Hypsarrhythmia: frequency of variant patterns and correlation with etiology and outcome. Neurology. 1997;48(1):197–203.
Fattinger S, Schmitt B, Bölsterli Heinzle BK, Critelli H, Jenni OG, Huber R. Impaired slow wave sleep downscaling in patients with infantile spasms. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2015;19(2):134–42.
Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27(4):628–39.
Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33(4):475–80.
Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Mölle M, Born J. Sleep selectively enhances memory expected to be of future relevance. J Neurosci Off J Soc Neurosci. 2011;31(5):1563–9.
Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev. 2013;6:CD001770.
O’Callaghan FJK, Edwards SW, Alber FD, Cortina Borja M, Hancock E, Johnson AL, et al. Vigabatrin with hormonal treatment versus hormonal treatment alone (ICISS) for infantile spasms: 18-month outcomes of an open-label, randomised controlled trial. Lancet Child Adolesc Health. 2018;2(10):715–25.
Go CY, Mackay MT, Weiss SK, Stephens D, Adams-Webber T, Ashwal S, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78(24):1974–80.
Møller MM, Høgenhaven H, Uldall P, Ballegaard M. Heart rate variability in infants with west syndrome. Seizure. 2015;27:10–5.
Gencpinar P, Kocabas A, Duman Ö, Dündar NO, Haspolat S, Kardelen F. Cardiac autonomic dysfunction in patients with infantile spasm and the effect of adrenocorticotropic hormone treatment. J Child Neurol. 2016;31(2):134–7.
Jansen K, Vandeput S, Van Huffel S, Lagae L. Cardiac autonomic dysfunction in west syndrome. Epilepsy Res. 2012;102(3):167–72.
Hattori A, Hayano J, Fujimoto S, Ando N, Mizuno K, Kamei M, et al. Cardiac vagal activation by adrenocorticotropic hormone treatment in infants with west syndrome. Tohoku J Exp Med. 2007;211(2):133–9.
van Rijckevorsel K. Treatment of Lennox-Gastaut syndrome: overview and recent findings. Neuropsychiatr Dis Treat. 2008;4(6):1001–19.
Arzimanoglou A, French J, Blume WT, Cross JH, Ernst J-P, Feucht M, et al. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009;8(1):82–93.
Crumrine PK. Lennox-Gastaut syndrome. J Child Neurol. 2002;17(Suppl 1):S70–5.
Borrelli S, El Tahry R. Therapeutic approach to Lennox-Gastaut syndrome: a systematic review. Acta Neurol Belg. 2019;119(3):315–24.
Koenig SA, Longin E, Bell N, Reinhard J, Gerstner T. Vagus nerve stimulation improves severely impaired heart rate variability in a patient with Lennox-Gastaut-syndrome. Seizure. 2008;17(5):469–72.
Tassinari CA, Cantalupo G, Rios-Pohl L, Giustina ED, Rubboli G. Encephalopathy with status epilepticus during slow sleep: “the Penelope syndrome”. Epilepsia. 2009;50(Suppl 7):4–8.
Aeby A, Poznanski N, Verheulpen D, Wetzburger C, Van Bogaert P. Levetiracetam efficacy in epileptic syndromes with continuous spikes and waves during slow sleep: experience in 12 cases. Epilepsia. 2005;46(12):1937–42.
Galanopoulou AS, Bojko A, Lado F, Moshé SL. The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev. 2000;22(5):279–95.
Halász P, Bódizs R, Ujma PP, Fabó D, Szűcs A. Strong relationship between NREM sleep, epilepsy and plastic functions – a conceptual review on the neurophysiology background. Epilepsy Res. 2019;150:95–105.
Guzzetta F, Battaglia D, Veredice C, Donvito V, Pane M, Lettori D, et al. Early thalamic injury associated with epilepsy and continuous spike-wave during slow sleep. Epilepsia. 2005;46(6):889–900.
De Negri M. Electrical status epilepticus during sleep (ESES). Different clinical syndromes: towards a unifying view? Brain Dev. 1997;19(7):447–51.
Lesca G, Rudolf G, Bruneau N, Lozovaya N, Labalme A, Boutry-Kryza N, et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat Genet. 2013;45(9):1061–6.
Veggiotti P, Pera MC, Teutonico F, Brazzo D, Balottin U, Tassinari CA. Therapy of encephalopathy with status epilepticus during sleep (ESES/CSWS syndrome): an update. Epileptic Disord Int Epilepsy J Videotape. 2012;14(1):1–11.
Cockerell OC, Johnson AL, Sander JW, Hart YM, Goodridge DM, Shorvon SD. Mortality from epilepsy: results from a prospective population-based study. Lancet Lond Engl. 1994;344(8927):918–21.
Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy: unifying the definitions of SUDEP. Epilepsia. 2012;53(2):227–33.
Annegers JF. United States perspective on definitions and classifications. Epilepsia. 1997;38(11 Suppl):S9–12.
Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia. 1997;38(11 Suppl):S6–8.
Thurman DJ, Logroscino G, Beghi E, Hauser WA, Hesdorffer DC, Newton CR, et al. The burden of premature mortality of epilepsy in high-income countries: a systematic review from the mortality task force of the international league against epilepsy. Epilepsia. 2017;58(1):17–26.
Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674–80.
Keller AE, Whitney R, Li S-A, Pollanen MS, Donner EJ. Incidence of sudden unexpected death in epilepsy in children is similar to adults. Neurology. 2018;91(2):e107–11.
Sveinsson O, Andersson T, Carlsson S, Tomson T. The incidence of SUDEP: a nationwide population-based cohort study. Neurology. 2017;89(2):170–7.
Doumlele K, Friedman D, Buchhalter J, Donner EJ, Louik J, Devinsky O. Sudden unexpected death in epilepsy among patients with benign childhood epilepsy with centrotemporal spikes. JAMA Neurol. 2017;74(6):645–9.
Sillanpää M, Riikonen R, Saarinen MM, Schmidt D. Long-term mortality of patients with west syndrome. Epilepsia Open. 2016;1(1–2):61–6.
DeGiorgio CM, Miller P, Meymandi S, Chin A, Epps J, Gordon S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 2010;19(1):78–81.
Walczak TS, Leppik IE, D’Amelio M, Rarick J, So E, Ahman P, et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology. 2001;56(4):519–25.
Novak JL, Miller PR, Markovic D, Meymandi SK, DeGiorgio CM. Risk assessment for sudden death in epilepsy: the SUDEP-7 Inventory. Front Neurol. 2015;6:252.
Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: a review. Epilepsy Behav. 2016;64(Pt A):69–74.
Johannesen KM, Gardella E, Scheffer I, Howell K, Smith DM, Helbig I, et al. Early mortality in SCN8A-related epilepsies. Epilepsy Res. 2018;143:79–81.
Coll M, Oliva A, Grassi S, Brugada R, Campuzano O. Update on the genetic basis of sudden unexpected death in epilepsy. Int J Mol Sci. 2019;20(8):1979.
Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, Tassinari CA. Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med Rev. 2011;15(4):237–46.
Purnell BS, Thijs RD, Buchanan GF. Dead in the night: sleep-wake and time-of-day influences on sudden unexpected death in epilepsy. Front Neurol. 2018;9:1079.
Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia. 2012;53(2):253–7.
Ali A, Wu S, Issa NP, Rose S, Towle VL, Warnke P, et al. Association of sleep with sudden unexpected death in epilepsy. Epilepsy Behav. 2017;76:1–6.
Persson H, Kumlien E, Ericson M, Tomson T. Preoperative heart rate variability in relation to surgery outcome in refractory epilepsy. Neurology. 2005;65(7):1021–5.
Natelson BH, Suarez RV, Terrence CF, Turizo R. Patients with epilepsy who die suddenly have cardiac disease. Arch Neurol. 1998;55(6):857–60.
Tomson T, Kennebäck G. Arrhythmia, heart rate variability, and antiepileptic drugs. Epilepsia. 1997;38(11 Suppl):S48–51.
Lossius MI, Erikssen JE, Mowinckel P, Gulbrandsen P, Gjerstad L. Changes in autonomic cardiac control in patients with epilepsy after discontinuation of antiepileptic drugs: a randomized controlled withdrawal study. Eur J Neurol. 2007;14(9):1022–8.
Surges R, Henneberger C, Adjei P, Scott CA, Sander JW, Walker MC. Do alterations in inter-ictal heart rate variability predict sudden unexpected death in epilepsy? Epilepsy Res. 2009;87(2–3):277–80.
Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966–77.
Espinosa PS, Lee JW, Tedrow UB, Bromfield EB, Dworetzky BA. Sudden unexpected near death in epilepsy: malignant arrhythmia from a partial seizure. Neurology. 2009;72(19):1702–3.
Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet Lond Engl. 2004;364(9452):2212–9.
Nei M, Ho RT, Abou-Khalil BW, Drislane FW, Liporace J, Romeo A, et al. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia. 2004;45(4):338–45.
Kaada BR, Jasper H. Respiratory responses to stimulation of temporal pole, insula, and hippocampal and limbic gyri in man. AMA Arch Neurol Psychiatry. 1952;68(5):609–19.
Seyal M, Bateman LM. Ictal apnea linked to contralateral spread of temporal lobe seizures: intracranial EEG recordings in refractory temporal lobe epilepsy. Epilepsia. 2009;50(12):2557–62.
Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci. 2015;35(28):10281–9.
Seyal M. Frontal hemodynamic changes precede EEG onset of temporal lobe seizures. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2014;125(3):442–8.
Kloster R, Engelskjøn T. Sudden unexpected death in epilepsy (SUDEP): a clinical perspective and a search for risk factors. J Neurol Neurosurg Psychiatry. 1999;67(4):439–44.
Reay DT, Howard JD, Fligner CL, Ward RJ. Effects of positional restraint on oxygen saturation and heart rate following exercise. Am J Forensic Med Pathol. 1988;9(1):16–8.
Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6(1):55–69.
Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9(12):542–8.
Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100(4):857–73.
Uteshev VV, Tupal S, Mhaskar Y, Faingold CL. Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest. Epilepsy Res. 2010;88(2–3):183–8.
Nakase K, Kollmar R, Lazar J, Arjomandi H, Sundaram K, Silverman J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res. 2016;128:126–39.
McLean BN, Wimalaratna S. Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatry. 2007;78(12):1395–7.
Dütsch M, Devinsky O, Doyle W, Marthol H, Hilz MJ. Cerebral autoregulation improves in epilepsy patients after temporal lobe surgery. J Neurol. 2004;251(10):1190–7.
Corfield DR, Meadows GE. Control of cerebral blood flow during sleep and the effects of hypoxia. Adv Exp Med Biol. 2006;588:65–73.
McCarter AR, Timm PC, Shepard PW, Sandness DJ, Luu T, McCarter SJ, et al. Obstructive sleep apnea in refractory epilepsy: a pilot study investigating frequency, clinical features, and association with risk of sudden unexpected death in epilepsy. Epilepsia. 2018;59(10):1973–81.
Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–6.
Lamberts RJ, Blom MT, Novy J, Belluzzo M, Seldenrijk A, Penninx BW, et al. Increased prevalence of ECG markers for sudden cardiac arrest in refractory epilepsy. J Neurol Neurosurg Psychiatry. 2015;86(3):309–13.
Roche F, Xuong ANT, Court-Fortune I, Costes F, Pichot V, Duverney D, et al. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol PACE. 2003;26(3):669–77.
Yuan H, Pinto SJ, Huang J, McDonough JM, Ward MB, Lee YN, et al. Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. Sleep. 2012;35(9):1257–67.
Malow BA. Sleep deprivation and epilepsy. Epilepsy Curr. 2004;4(5):193–5.
RamachandranNair R, Jack SM. SUDEP: what do adult patients want to know? Epilepsy Behav. 2016;64(Pt A):195–9.
Strzelczyk A, Zschebek G, Bauer S, Baumgartner C, Grond M, Hermsen A, et al. Predictors of and attitudes toward counseling about SUDEP and other epilepsy risk factors among Austrian, German, and Swiss neurologists and neuropediatricians. Epilepsia. 2016;57(4):612–20.
Ryvlin P, Cucherat M, Rheims S. Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomised trials. Lancet Neurol. 2011;10(11):961–8.
Zhao X, Lhatoo SD. Seizure detection: do current devices work? And when can they be useful? Curr Neurol Neurosci Rep. 2018;18(7):40.
Seyal M, Bateman LM, Li C-S. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2013;54(2):377–82.
Bateman LM, Li C-S, Lin T-C, Seyal M. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51(10):2211–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Proserpio, P., Giacomini, T., Agostoni, E.C., Nobili, L. (2021). Sleep-Related Epilepsy, Dysautonomia, and Sudden Nocturnal Death. In: Chokroverty, S., Cortelli, P. (eds) Autonomic Nervous System and Sleep. Springer, Cham. https://doi.org/10.1007/978-3-030-62263-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-62263-3_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62262-6
Online ISBN: 978-3-030-62263-3
eBook Packages: MedicineMedicine (R0)