Abstract
Purpose
Apneas occurring during sleep may precipitate autonomic instability in epilepsy patients making them susceptible to sudden death (SUDEP). Literature on heart rate variability (HRV) during apnea among patients with juvenile myoclonic epilepsy (JME) is sparse. The aim was to characterize the HRV during the peri-apneic/hypopneic period in patients with JME.
Methods
Overnight polysomnography of 25 patients with JME (M/F = 14:11; mean age, 21.28 ± 4.34 years) and 25 gender-matched healthy controls (M/F = 11:14; mean age, 23.32 ± 3.68 years) were analyzed. In both patients and controls, the time domain, frequency domain, and nonlinear HRV indices were analyzed for two minutes before and after apnea/hypopnea termination and compared using paired t test (p ≤ 0.05). Additionally, the changes in HRV parameters in the peri-apnea/hypopnea period were compared between the two groups using independent samples t test (p ≤ 0.05).
Results
In controls, there was a significant decrease of mean RR interval (p = 0.029) and a significant increase of standard deviation of RR interval (SDNN; p = 0.046) in the post-apneic/hypopneic period as compared with the pre-apneic/hypopneic period. Analysis using nonlinear measures showed a significant increase in the long-term HRV (p = 0.042) in the post-apnea period, but a comparable short-term HRV (p = 0.266). Conversely, in JME, all the HRV parameters, including nonlinear measures were comparable in the pre- and post-apneic/hypopneic period. Finally, comparison of the changes in HRV parameters in the peri-apnea/hypopnea period in patients with JME and healthy controls showed significant differences in SDNN (p = 0.026) and long-term HRV (p = 0.018).
Conclusions
This study showed that there was a lack of apnea-mediated HRV changes, including long-term HRV changes in patients with JME. This might suggest an alteration in reflex baroreceptor activation in patients with JME, which might explain the vulnerability for SUDEP in patients with epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep is an actively generated process, carefully regulated by hormonal influences (i.e., melatonin and orexin), circadian variations, and several other factors, including a significant contribution from the autonomic nervous system (ANS). The ANS has been shown to play an important role in the modulation of cardiovascular functions during sleep onset and transition to different sleep stages. A significant reduction of sympathetic modulation during transition from wake to nonrapid eye movement (NREM) sleep, and conversely, a possible sympathetic predominance during transition from NREM to rapid eye movement (REM) sleep has been demonstrated in several studies [1–3]. Most sleep disorders such as obstructive sleep apnea and insomnia are associated with autonomic disturbances, which are likely to contribute to the cardiovascular comorbidity seen in these conditions [4].
Seizures are often accompanied by autonomic fluctuations [5, 6], probably attributed to the spread of epileptiform activity to the limbic or hypothalamic regions [7]. Moreover, autonomic dysregulation causing cardiac or respiratory dysfunctions or both has been implicated to play an important role in the pathogenesis of sudden unexpected death in epilepsy patients (SUDEP), which frequently occurs during sleep [8]. Although, epilepsy-related mortality and SUDEP have been recognized, its patho-physiology remains to be ascertained. Various risk factors have been reported such as drug-resistant epilepsy, frequent generalized seizures, lesional epilepsy, old age, polytherapy, etc. [9]. The underlying mechanisms vary from being unknown to ictal/post-ictal arrhythmia, dysautonomia, pulmonary edema, and central apnea [9–11]. It might be postulated that apneas during sleep may precipitate autonomic instability in selected patients with epilepsy making them susceptible to sudden death. Juvenile myoclonic epilepsy (JME) is the most common and well-defined idiopathic generalized epilepsy (IGE) and recognized as an epileptic syndrome since 1989 [12–14] assessed sleep among 50 patients with JME and 50 healthy controls. They found significant sleep disturbances characterized by excessive daytime sleepiness and disturbed night sleep, despite adequate medications and good seizure control. Patients with JME exhibit seizures/myoclonic jerks during the sleep–wake transition period sometimes while on medications.
Heart rate variability (HRV) analysis is a noninvasive and reliable measure of autonomic function, which has been widely used to study autonomic disturbances in various disease conditions. Chouchou et al. [15] had studied cardiac sympathetic modulation in response to apneas/hypopneas using HRV analysis in subjects with obstructive sleep apnea. There is lack of such data in patients with JME, and such analytical studies might provide valuable insights and one could hypothesize to carry out similar studies in patients with drug resistant epilepsy. The aim of this study was to characterize the HRV during the peri-apneic/hypopneic period in patients with JME and compare with matched healthy individuals during the overnight polysomnographic (PSG) recording.
Materials and methods
This cross-sectional, hospital-based, case-control study was conducted at a tertiary neurology center in south India. We have a large database of patients with JME at our center, who have fulfilled the criteria laid down by the ILAE commission on classification and terminology (1989) for the diagnosis of JME [16], as well as data of healthy controls, all of which had been collected between March 2010 and December 2013. We randomly selected 35 patients with JME above the age of 12 years from this database. The only anti-epileptic medication being consumed by patients at the time of entry into the study was sodium valproic acid (SVA) at weight-adjusted doses. Blood levels of valproate were not measured. Exclusion criteria included: (a) Use of medications known to affect sleep other than SVA, (b) drug or substance abuse, (c) abnormal brain imaging, (d) symptoms of primary sleep and/or autonomic nervous system disorders, and (e) co-existing medical or surgical disorder known to affect sleep or autonomic function. Thirty-five healthy controls were also randomly selected from our database, which comprised friends/unrelated volunteers (n = 22) of the patients from similar educational and socio-economic status and medical personnel (doctors = 7; medical technologists = 6) of the hospital on routine day duties (9:00 a.m. to 4:30 p.m.) were recruited, who were age (p = 0.08) and gender (p = 0.39) matched with the patients. Thirty-five healthy controls were also randomly selected from our database, which comprised friends/unrelated volunteers (n = 22) of the patients from similar educational and socio-economic status and medical personnel (doctors = 7; medical technologists = 6) of the hospital on routine day duties (9:00 a.m. to 4:30 p.m.). Control individuals were matched for both age (p = 0.08) and gender (p = 0.39). Controls were not related to patients and also did not have family history of headache or any other medical or neurological illness. The Institute Ethical Committee (IEC) approved the study, and all patients and controls gave written informed consent to participate in the study.
Clinical and sleep questionnaire-based evaluation
All patients underwent a structured evaluation, including a detailed clinical, family and treatment history, and a thorough neurological examination. All patients underwent 16-channel digital electroencephalogram (EEG) recording using the international 10–20 system of electrode placement, brain imaging (magnetic resonance imaging (MRI)), and other investigations as indicated. Validated sleep questionnaires namely Epworth sleepiness scale (ESS) to assess daytime somnolence [17] and Pittsburgh sleep quality index (PSQI) to assess night-time sleep quality [18], were administered to all subjects at the time of study entry. The subjects were also screened for underlying sleep disorders using the NIMHANS comprehensive sleep disorders questionnaire (NCSDQ) [19]. The subjects were instructed to follow their regular sleep schedule for a week prior to the sleep study and refrain from alcohol and caffeine at least 1 day prior to the PSG recording. Menstrual phase was not controlled for in female subjects.
Polysomnographic recordings and analysis
Overnight PSG recording was done (Sleepscan Vision Collection Software, version 7.11.01, Biologic Systems Corp, IL, USA) after obtaining a written informed consent from all participants using standard protocols. The parameters recorded included (i) an eight-channel EEG using bi-hemispheric referential montage (F7-A2, C3-A2, T3-A2, O1-A2 and F8-A1, C4-A1, T4-A1, O2-A1): sensitivity of 7 μV/mm, low pass filter at 0.3 Hz, high pass filter at 35 Hz; (ii) two-channel electro-oculogram (EOG): for eye movements, sensitivity of 10 μV/mm, low pass filter, 0.3 Hz, high-pass filter 35 Hz; (iii) electromyogram (EMG) from the sub-mentalis and right tibialis anterior muscle: sensitivity of 7 and 20 μV/mm, respectively, low pass filter of 0.3 Hz, high pass filter of 100 Hz; (iv) electrocardiogram (EKG): sensitivity of 20 μV/mm, low pass filter at 0.5 Hz, high pass filter and at 35 Hz; (v) body position monitor; and (vi) respiratory events: oro-nasal airflow (sensitivity of 7 μV/mm, low pass filter at 0.5 Hz, high pass filter at 15 Hz), chest and abdominal wall movements (sensitivity of 10 μV/mm, low pass filter at 0.5 Hz, high pass filter at 15 Hz), snore (sensitivity of 2 μV/mm, low pass filter at 0.3 Hz, high pass filter at 70 Hz), and arterial oxygen saturation (sensitivity of 7 μV/mm, high pass at filter 70 Hz). All channels were sampled at 256 Hz; electrode impedance was kept less than 5000 ohms and a notch filter of 50 Hz was applied to remove noise artifact caused by electrical power lines. The subjects were allowed to fall asleep spontaneously and the recording was continued until their spontaneous awakening in the morning. All subjects reported a comfortable, undisturbed and refreshing sleep.
Sleep was scored visually in 30-s epochs using standard criteria [20]. The conventional PSG parameters studied included total time in bed (TIB; min), total sleep time (TST; min), sleep efficiency (%), time spent in various sleep stages (min), number of arousals occurring during the entire period of sleep, as well as during each of the nonrapid eye movement (NREM) and rapid eye movement (REM) sleep stages. The overnight mean blood oxygen saturation (mean SaO2) and overnight minimum blood oxygen saturation (min SaO2) were assessed. Apneas were analyzed in both patients and controls, during the entire sleep period, as well as during the NREM and REM sleep stages. The number and type of apneas were classified. Careful visual analysis of all the EKG segments corresponding with the peri-apnea period was done to look for skipped/premature beats and obvious artifacts. Premature beats were defined as heartbeats occurring within 20 % of the interval measured between the two preceding QRS complexes. Any apneas occurring in association with premature beats were excluded from the analysis. Also, apneas that were lying in close proximity with each other, i.e., within 4 min of the peri-apneic/hypopneic period (2 min before and after the apnea termination), were excluded in order to avoid overlap and ambiguity in analysis. Twenty-five patients and 25 healthy controls having at least 5 apneas/hypopneas occurring throughout the overnight PSG recording, and that satisfy the above criteria for selection of apneas/hypopneas, were enrolled in the study. The inclusion of patients and controls with at least five analyzable apneas/hypopneas in the over-night PSG recording was merely to make sure that sufficient segments of apneas/hypopneas were available for analysis. However, none of the healthy controls or patients had an apnea-hypopnea Index (AHI) of ≥5.
HRV analysis
Digital EKG recordings were converted into European data format + (EDF+) and exported into Kubios HRV software version 2.1 (University of Eastern Finland, Kuopio, Finland) [21], for time domain, frequency domain and nonlinear analysis of HRV at a sampling rate of 256 Hz. The HRV measures were analyzed for two minute artifact free segments [22] before and after apnea/hypopnea termination (peri-apneic/hypopneic period) in both controls and patients with JME. The mean heart rate (HR), mean of RR interval (mean RR), standard deviation of R–R intervals (SDNN), square root of the mean squared differences between successive RR intervals (RMSSD), number of successive RR interval pairs that differ more than 50 ms (NN50), and the NN50 divided by the total number of RR intervals (pNN50) were computed as time domain measures. Autoregressive spectral estimation was used for HRV analysis in the frequency domain. The power in the HR spectrum between 0.003 and 0.40 Hz was defined as total power (TP), and this power was divided into three frequency bands: very low frequency (VLF; 0.003–0.04 Hz), low frequency (LF; 0.04–0.15 Hz), and high frequency (HF; 0.15–0.4 Hz). VLF, LF, and HF components of TP were taken into account. VLF was evaluated although this component does not have a well-defined physiological explanation [22]. The HF was regarded as a measure of solely parasympathetic activity. The LF was considered to be a measure of mainly sympathetic activity that was modulated by the influence of the parasympathetic system. To minimize the effects of TP on the absolute values of VLF, LF, and HF, relative values of these components were measured and defined as a proportion (percentage) of the total power. Furthermore, the absolute values of LF and HF were also represented in normalized units (n.u.), which is the relative value of each power component (i.e., LF and HF) in proportion to the total power minus the VLF component [23]. LFnu and HFnu were calculated using the equations:
The LF/HF ratio (ratio of low frequency by high frequency) was used as an index of sympatho-vagal balance to establish the contribution of parasympathetic activity to LF spectral power. Nonlinear measures were also analyzed using standard deviation 1 (SD1) and standard deviation 2 (SD2) of Poincare plot, which are representative of short-term and long-term HRV, respectively.
Data analysis he data was incorporated into a digital spreadsheet and analyzed using Statistical Package for the Social Sciences (SPSS) version 15. Chi-square test was employed to compare qualitative parameters and independent t test for quantitative parameters. HRV parameters were compared before and after apnea/hypopnea termination using paired t–test (i ≤ 0.05) in both controls and patients with JME. The changes in HRV parameters during the peri-apneic/hypopneic termination period in all apneas/hypopneas were compared among controls and patients using independent samples t test (p ≤ 0.05). Furthermore, the changes in HRV parameters between different types of respiratory events including central apneas, obstructive apneas, mixed apneas and hypopneas were also similarly compared. Finally, Pearson correlation test examined the correlation between peri-apneic/hypopneic changes in HRV parameters and SaO2, taking into account only apneic/hypopneic events from each patient and healthy control.
Results
Twenty-five patients with JME (mean age, 21.28 ± 4.34 years (range, 15–30); (M/F = 14:11)), and 25 age (p = 0.079) and gender (p = 0.396- matched healthy controls (mean age, 23.32 ± 3.68 years (range, 18–31); (M/F = 11:14)) were enrolled in the study.
Clinical profile
The mean age at onset of seizures was 15.43 ± 3.8 years, while the mean age at diagnosis was 21 ± 5.1 years with a mean delay in diagnosis being 5.56 ± 5.8 years. Sixteen (64 %) patients had onset of myoclonus in puberty. The first seizure type was generalized tonic clonic seizures (GTCS) in 19 (76 %) and myoclonus in 6 (24 %). The mean ESS scores in healthy controls and patients with JME were 4.7 ± 2.76 and 6.27 ± 4.42, respectively, which were comparable (p = 0.1385). The mean PSQI in healthy controls and patients with JME were 2.66 ± 1.21 and 6.63 ± 5.27 respectively, showing poor sleep quality in patients with JME (p = 0.0006). None of the patients with JME were observed to have any myoclonic jerks or generalized seizures during the sleep recordings. The apnea/hypopnea index (AHI) was significantly higher in patients with JME as compared with the controls (p = 0.0133), along with NREM AHI (p = 0.024) and REM AHI (p = 0.036). However, the apnea index (AI) and hypopnea index (HI), as well as, the mean SaO2 and minimum SaO2 were comparable between the two groups. The polysomnographic parameters in normal healthy controls and in patients with JME are depicted in Table 1.
HRV analyses
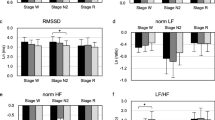
In controls, there was a decreased mean RR (p = 0.029) in the post-apneic/hypopneic period. There was also an increased SDNN (p = 0.046) in the post-apneic/hypopneic period as compared with the pre-apneic/hypopneic period. Analysis using nonlinear measures showed an increased SD2 (p = 0.042) in the post-apneic/hypopneic period, but a comparable SD1 (p = 0.266). However, the frequency domain measures including LFnu (p = 0.886), HFnu (p = 0.725), and low-frequency/high-frequency ratio (LF/HF ratio; p = 0.969) did not show any significant changes in HRV from pre- to post-apneic/hypopneic period. Conversely, in JME patients, all the HRV parameters, including time domain, frequency domain, and nonlinear indices were comparable in the pre- and post-apneic/hypopneic period. The HRV parameters before and after apnea termination in healthy controls and patients with JME are depicted in Table 2.
Comparison of changes in HRV parameters in the peri-apnea/hypopnea period between healthy controls and patients with JME showed differences in SDNN (p = 0.026) and SD2 (p = 0.018). The comparison of changes in HRV parameters in the peri-apnea/hypopnea period between patients with JME and healthy controls are depicted in Table 3. Similarly, comparison of HRV changes during the peri-mixed apnea period also showed differences in SDNN (p = 0.047) and SD2 (p = 0.038). In contrast, comparison of HRV changes during the peri-obstructive apnea period; peri-central apnea period and peri-hypopnea period showed that there were differences in LF (p = 0.002), NN50 (p = 0.022) and SD1 (p = 0.034), and NN50 (p = 0.023), respectively, between patients with JME and healthy controls. The comparison of changes in HRV parameters for individual respiratory events including central apneas, obstructive apneas, mixed apneas, and hypopneas between patients with JME and healthy controls are depicted in Table 4.
Lastly, there was a significantly greater difference (p = 0.030) in the mean change in SaO2 in the JME group (0.717 ± 0.466) and compared with the controls (0.395 ± 0.545). However, Pearson correlation indicated that the peri-apneic/hypopneic differences in HRV parameters and SaO2 did not correlate with each other in the two study groups. The correlation between the mean peri-apneic/hypopneic differences in SaO2 and SD1 has been depicted using a scatter diagram for both control (Fig. 1a) and JME groups (Fig. 1b).
Discussion
Sinus arrhythmia, which is the cyclic change in heart rate during respiration, is mediated by cardio-respiratory coupling at the level of the brainstem. This physiologic HRV is indicative of cardiac health, whereas its absence/ reduction is considered as a marker of disease and aging process [24]. Apneas occurring during sleep can precipitate autonomic nervous system instability in predisposed individuals. HRV analysis is a powerful tool providing semi-quantitative information about the relationship between cardiovascular sympathetic and parasympathetic modulations in several physiological and patho-physiological conditions [25]. An alteration of HRV during sleep has been observed in infants at risk for sudden infant death syndrome (SIDS) [26]. Analysis of spectral measures of frequency domain in patients with epilepsy has shown an increased sympathetic tone in association with a decreased parasympathetic tone, which might explain the mechanism underlying SUDEP in selected cases of epilepsy. It has also been suggested that the major determinants of suppressed SDNN are multiple AEDs and >10 years of epilepsy. Owing to these observations relating sleep-related apneas with SUDEP, it was felt that analysis of HRV during the peri-apneic/hypopneic period in patients with JME might provide important clues regarding the possible autonomic disturbances in this patient group, predisposing them to sudden death.
In this study, we found that there was absence of expected apnea mediated HRV changes in patients with JME, evidenced by a lack of alteration in time domain, frequency domain, and nonlinear indices in the post-apneic/hypopneic period as compared with the pre-apneic/hypopneic period. This was in contrast to a characteristic change in time-domain (SDNN) and long-term (SD2) HRV indices in healthy controls, in the post-apneic/hypopneic period as compared with the pre-apneic/hypopneic period. Furthermore, we also found that there were significant differences in the peri-apnea/hypopnea HRV changes, including SDNN and long-term HRV between patients with JME and healthy controls. Similar differences were also observed for mixed apneas, independently. Contrastingly, comparison of HRV changes during the peri-obstructive apnea period, peri-central apnea period and peri-hypopnea period showed differences in LF, NN50 and SD1, and NN50, respectively, between patients with JME and healthy controls. We also found that the peri-apneic/hypopneic change in SaO2 was significantly higher in the JME group as compared to healthy controls. However, there was no significant correlation between the peri-apenic/hypopneic change in SaO2 and the peri-apneic/hypopneic change in HRV parameters in both JME and controls suggesting that SaO2 and HRV vary independently of each other in the peri-apneic/hypopneic period in the two study groups.
Several studies in patients with localization-related epilepsy, especially temporal lobe epilepsies (both medically refractory and well controlled) have shown significant circadian variations of HRV, with a prominent decrease of HRV during nighttime, suggesting that this period might be vulnerable to autonomic instability [27, 28]. Studies evaluating the presence of a sleep-related HRV as a risk factor for SUDEP are lacking, nevertheless many reports show autonomic changes in epileptic patients during sleep. Patients suffering from epilepsy are also known to have a fragmented sleep architecture associated with increased arousal indices [29, 30]. Cortical arousals are often precipitated by sleep intrusions like apneas and periodic limb movements and are associated with periodic surges of sympathetic activity causing alteration of neuro-vegetative functions like heart rate and blood pressure. Moreover, NREM sleep in patients with epilepsy shows an increase in the cyclical variation in EEG amplitude and frequency and alteration in sleep microstructure, which has been widely studied as the cyclic alternating pattern (CAP). This is known to be representative of cortical arousal instability, with cyclical alteration from a period of “greater arousal (CAP A)” to “lesser arousal (CAP B)” [31, 32]. Recurrent interictal epileptiform discharges in patients with poorly controlled epilepsy may be responsible for these repeated arousal fluctuations [30, 33, 34] and chronic sympathetic over-activity during sleep. This can lead to long-term hemodynamic alterations and ischemic cardiac insult, resulting in myocardial tissue damage, along with disruption of cardiac autonomic innervation. This may be supported by several studies, which demonstrated post-mortem myocardial injuries in SUDEP subjects [35–37].
Other studies have also investigated the role of the respiratory system in the pathophysiology of SUDEP. Seizure episodes are known to induce respiratory impairment in patients, usually manifesting as central apneas or hypopneas, rather than obstructive or mixed apneas [38, 39]. Irrespective of the type of respiratory event, all of them are invariably associated with a fall in oxygen saturation (SaO2) causing some degree of hypoxemia and hypercapnia [39]. Recurrent nocturnal hypoxemia is suspected to be an important risk factor for cardiac disease in obstructive sleep apnea/hypopnea syndrome (OSAHS) patients and has been shown to strongly influence cardiac sympathetic modulation during apneas/hypopnea [15], predisposing to turbulences in cardiac electrophysiology [40, 41]. Furthermore, apneas may also precipitate rapid, sympatho-parasympathetic autonomic co-activation, usually giving rise to reflex bradycardia and hypertension [42].
The findings noted in this study substantiates some of the previous observations of the role of autonomic dysregulation as a mechanism by which apneas during sleep might predispose patients with epilepsy to SUDEP. However, our study did not show significant changes in frequency domain parameters of HRV in healthy controls. This suggests that there was no specific sympathetic/parasympathetic predominance in the peri-apneic/hypopneic period among controls. Moreover, the healthy controls showed predominant peri-apneic/hypopneic HRV changes in long-term HRV, but not in short-term HRV. Further studies using a larger sample size and in patients with drug-resistant localization-related epilepsy may further confirm these findings.
References
Berlad II, Shlitner A, Ben-Haim S, Lavie P (1993) Power spectrum analysis and heart rate variability in Stage 4 and REM sleep: evidence for state-specific changes in autonomic dominance. J Sleep Res 2(2):88–90
Baharav A, Kotagal S, Gibbons V, Rubin BK, Pratt G, Karin J, Akselrod S (1995) Fluctuations in autonomic nervous activity during sleep displayed by power spectrum analysis of heart rate variability. Neurology 45(6):1183–1187
Versace F, Mozzato M, De Min Tona G, Cavallero C, Stegagno L (2003) Heart rate variability during sleep as a function of the sleep cycle. Biol Psychol 63(2):149–162
Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N (2013) Heart rate variability in normal and pathological sleep. Front Physiol 4:294. doi:10.3389/fphys.2013.00294
Van Buren JM (1958) Some autonomic concomitants of ictal automatism; a study of temporal lobe attacks. Brain J Neurol 81(4):505–528
Van Buren JM, Ajmone-Marsan C (1960) A correlation of autonomic and EEG components in temporal lobe epilepsy. Arch Neurol 3:683–703
Wannamaker BB (1985) Autonomic nervous system and epilepsy. Epilepsia 26(Suppl 1):S31–S39
Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, Tassinari CA (2011) Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med Rev 15(4):237–246. doi:10.1016/j.smrv.2010.07.006
Shorvon S, Tomson T (2011) Sudden unexpected death in epilepsy. Lancet 378(9808):2028–2038. doi:10.1016/S0140-6736(11)60176-1
Gleb P, Tolstykh JEC (2013) Potential mechanisms of sudden unexpected death in epilepsy. Epilepsy Behav 26:410–414
Milroy CM (2011) Sudden unexpected death in epilepsy in childhood. Forensic Sci Med Pathol 7(4):336–340. doi:10.1007/s12024-011-9245-6
Janz D (1997) The idiopathic generalized epilepsies of adolescence with childhood and juvenile age of onset. Epilepsia 38(1):4–11
Panayiotopoulos CP, Obeid T, Tahan AR (1994) Juvenile myoclonic epilepsy: a 5-year prospective study. Epilepsia 35(2):285–296
Krishnan P, Sinha S, Taly A, Ramachandraiah C, Rao S, Satishchandra P (2012) Sleep disturbances in juvenile myoclonic epilepsy: a sleep questionnaire-based study. Epilepsy Behav 23(3):305–309. doi:10.1016/j.yebeh.2011.12.018
Chouchou F, Pichot V, Barthelemy JC, Bastuji H, Roche F (2014) Cardiac sympathetic modulation in response to apneas/hypopneas through heart rate variability analysis. PLoS ONE 9(1):e86434. doi:10.1371/journal.pone.0086434
Cocatot ILAE (1989) Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 30(4):389–399
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6):540–545
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
Panda S, Basavaraju S, Taly A (2007) Insight into prevalence of sleep related disorders among healthy Indians. Ann Indian Acad Neurol 10:24
Iber C, Ancoli-Israel S, Chesson A, Quan S (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester
Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA (2014) Kubios HRV—heart rate variability analysis software. Comput Methods Prog Biomed 113(1):210–220. doi:10.1016/j.cmpb.2013.07.024
Task force of European Society of Cardiology and North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93(5):1043–1065
Piccirillo G, Magri D, Naso C, di Carlo S, Mois EA, De Laurentis T, Torrini A, Matera S, Nocco M (2004) Factors influencing heart rate variability power spectral analysis during controlled breathing in patients with chronic heart failure or hypertension and in healthy normotensive subjects. Clin Sci 107(2):183–190. doi:10.1042/CS20030401
Verrier RL, Harper R, Hobson JA (2000) Cardiovascular physiology: central and autonomic regulation. In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine, 5th edn. WB Saunders Company, Philadelphia, pp 179–192
Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR, Iellamo F (2009) Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev 33(2):71–80. doi:10.1016/j.neubiorev.2008.07.006
Spassov L, Curzi-Dascalova L, Clairambault J, Kauffmann F, Eiselt M, Medigue C, Peirano P (1994) Heart rate and heart rate variability during sleep in small-for-gestational age newborns. Pediatr Res 35(4 Pt 1):500–505
Ronkainen E, Ansakorpi H, Huikuri HV, Myllyla VV, Isojarvi JI, Korpelainen JT (2005) Suppressed circadian heart rate dynamics in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 76(10):1382–1386. doi:10.1136/jnnp.2004.053777
Persson H, Kumlien E, Ericson M, Tomson T (2007) Circadian variation in heart-rate variability in localization-related epilepsy. Epilepsia 48(5):917–922. doi:10.1111/j.1528-1167.2006.00961.x
Mendez M, Radtke RA (2001) Interactions between sleep and epilepsy. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 18(2):106–127
Parrino L, Halasz P, Tassinari C, Terzano M (2006) CAP, epilepsy and motor events during sleep: the unifying role of arousal. Sleep Med Rev 10(4):267–285. doi:10.1016/j.smrv.2005.12.004
Guilleminault C, Stoohs R (1995) Arousal, increased respiratory efforts, blood pressure and obstructive sleep apnoea. J Sleep Res 4(S1):117–124
Terzano MG, Parrino L (2000) Origin and significance of the cyclic alternating pattern (CAP). Review article. Sleep Med Rev 4(1):101–123. doi:10.1053/smrv.1999.0083
Nobili L, Sartori I, Terzaghi M, Stefano F, Mai R, Tassi L, Parrino L, Cossu M, Lo Russo G (2006) Relationship of epileptic discharges to arousal instability and periodic leg movements in a case of nocturnal frontal lobe epilepsy: a stereo-EEG study. Sleep 29(5):701–704
Terzaghi M, Sartori I, Mai R, Tassi L, Francione S, Cardinale F, Castana L, Cossu M, LoRusso G, Manni R, Nobili L (2008) Coupling of minor motor events and epileptiform discharges with arousal fluctuations in NFLE. Epilepsia 49(4):670–676. doi:10.1111/j.1528-1167.2007.01419.x
Nei M, Ho RT, Abou-Khalil BW, Drislane FW, Liporace J, Romeo A, Sperling MR (2004) EEG and ECG in sudden unexplained death in epilepsy. Epilepsia 45(4):338–345. doi:10.1111/j.0013-9580.2004.05503.x
Falconer B, Rajs J (1976) Post-mortem findings of cardiac lesions in epileptics: a preliminary report. Forensic Sci 8(1):63–71
Natelson BH, Suarez RV, Terrence CF, Turizo R (1998) Patients with epilepsy who die suddenly have cardiac disease. Arch Neurol 55(6):857–860
Bateman LM, Li CS, Seyal M (2008) Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain J Neurol 131(Pt 12):3239–3245. doi:10.1093/brain/awn277
Walker F, Fish DR (1997) Recording respiratory parameters in patients with epilepsy. Epilepsia 38(11 Suppl):S41–S42. doi:10.1111/j.1528-1157.1997.tb06126.x
Kiely DG, Cargill RI, Lipworth BJ (1996) Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest 109(5):1215–1221
Roche F, Reynaud C, Pichot V, Duverney D, Costes F, Garet M, Gaspoz JM, Barthelemy JC (2003) Effect of acute hypoxia on QT rate dependence and corrected QT interval in healthy subjects. Am J Cardiol 91(7):916–919
Lindholm P, Nordh J, Linnarsson D (2002) Role of hypoxemia for the cardiovascular responses to apnea during exercise. Am J Physiol Regul Integr Comp Physiol 283(5):R1227–R1235. doi:10.1152/ajpregu.00036.2002
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nayak, C., Sinha, S., Nagappa, M. et al. Lack of heart rate variability during apnea in patients with juvenile myoclonic epilepsy (JME). Sleep Breath 19, 1175–1183 (2015). https://doi.org/10.1007/s11325-015-1133-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-015-1133-y