Abstract
Kidney transplantation is the treatment of choice for end-stage renal disease, allowing a better quality of life and a greater survival than dialysis. Recent innovations in transplant surgery and the use of novel immunosuppressive drugs have significantly improved graft outcomes; however part of the transplanted graft still develops acute and chronic dysfunction due to infective, toxic, vascular, and/or immune-mediated damage. Although ultrasonography represents the imaging technique of choice for native kidney diseases, its diagnostic role is not fully understood in case of parenchymal and vascular complications of the graft. Color Doppler ultrasound has demonstrated low diagnostic accuracy in the detection of acute parenchymal complications, whereas its specificity is very important in case of stenosis of the transplanted renal artery, pseudoaneurysms, arteriovenous fistulas, and thrombosis. For chronic complications, a meticulous follow-up through measurements of resistance indices repeated over time seems to be more effective than single determinations in improving renal graft outcomes. Modern techniques, including tissue pulsatility index, maximal fractional area, and contrast-enhanced ultrasound (CEUS), are promising tools that will help increase the diagnostic power of ultrasound in case of both parenchymal and vascular complications of the kidney allograft.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Kidney transplantation, providing a better quality of life and long-term outcome compared to maintenance dialysis, represents an essential option for the replacement of kidney function, although the burden of graft loss remains very high [1].
Careful clinical monitoring and appropriate imaging diagnosis can play an important role in renal transplant patients. Different imaging tools are now available in routine clinical practice. Ultrasonography, both in B-mode and with Doppler ultrasound, is the most frequent first-line imaging approach in the posttransplant period [2].
However, its role and usefulness in case of parenchymal complications of the graft are not fully clarified [3]. Some patients still develop several complications, which might cause the loss of the graft or its dysfunction, as reported in Table 16.1. In Table 16.2 the list of common indications for kidney transplant imaging is reported.
In this chapter, we analyze the use and clinical relevance of imaging techniques in most common parenchymal graft complications, including:
-
(a)
Acute or immediate complications, such as acute tubular necrosis, drug toxicity, acute graft pyelonephritis, and hyper-acute rejection
-
(b)
Early complications, such as acute rejection, calcineurin inhibitor toxicity, and infectious acute graft pyelonephritis
-
(c)
Chronic complications, such as chronic allograft nephropathy [4]
2 Methods for the Study of Renal Transplant
The ultrasonographic study of the v kidney is easy to perform given the superficial position of the graft. Convex probes with frequencies ranging from 3.5 to 5.0 MHz allow obtaining longitudinal, transversal, and oblique scans. In some cases, a linear probe with frequencies ranging from 6.0 to 10 MHz can be used (Fig. 16.1). To minimize technical failures, it is worthwhile standardizing the working methodology, starting the test in B-mode and identifying the site, form, and position of the graft. The morphological and echo-structural parameters observed at the scale of Gray’s test (rapid volumetric increase over 20%, parenchymal hypoechogenicity, presence of a hyperechogenous shade between the echoes of the renal sinus and the walls of the renal pelvis, reduction in volume, and thinning of the parenchyma) might provide a large amount of information concerning a possible damage of the organ. Once the B-mode test is completed, B-flow, color Doppler imaging is fundamental for a rapid and clear assessment of arterial and venous perfusion of the organ. The extension of the box color must be limited, to improve the Doppler analysis capacity and the frame rate (FR). Pulse repetition frequency (PRF) should be set at values of 1.0–1.5 kHz and wall filter at values of 100 Hz and the color gain should be regulated as well, to optimize the image without aliasing and to avoid color diffusion to the perivascular tissues (color bleeding). Finally, the spectral analysis module should be activated with the positioning of the “sample volume” in the lumen of the interlobular artery and the recording of the velocity/time curve (V/t), which will allow obtaining the resistance index (RI) and the pulsatility index (PI).
2.1 Computed Tomography
CT may be complementary in the evaluation of renal transplant complications. If the patient’s renal function allows, intravenous contrast-enhanced CT angiography (CTA) may be performed to noninvasively confirm a diagnosis of renal artery stenosis if, for example, PSVs are elevated at ultrasound.
Contrast-enhanced imaging can also be used to characterize indeterminate focal lesions detected at ultrasound as cystic or solid. Additionally, intravenous contrast-enhanced CT with excretory phase imaging (acquired 5–20 min after intravenous contrast material administration) can be used to confirm a urine leak or urinoma. Administration of intravenous contrast material should be avoided in individuals with impaired renal function because of the risk of developing contrast-induced nephropathy. Noncontrast CT is helpful to evaluate the full extent of a perinephric fluid collection and its relationship to surrounding structures if portions of the collection are obscured by bowel gas at ultrasound. Also, noncontrast CT is more accurate than ultrasound for the diagnosis of renal and ureteral stones, especially for small stones.
2.2 MR Imaging
MR imaging also plays a complementary role in the evaluation of renal transplant complications. Magnetic resonance angiography (MRA) can be used to noninvasively confirm renal artery stenosis.
Contrast-enhanced MR imaging can also characterize focal renal lesions as cystic or solid. Additionally, excretory phase imaging can be performed to diagnose a urine leak or urinoma.
Administration of gadolinium-based contrast agents in individuals with impaired renal function has been associated with the development of nephrogenic systemic fibrosis, a potentially fatal fibrosing condition. Contrast agents associated with the greatest number of nephrogenic systemic fibrosis cases are gadodiamide (Omniscan™), gadopentetate dimeglumine (Magnevist™), and gadoversetamide (OptiMARK™) and should not be administered to individuals with impaired renal function. Other gadolinium-based contrast agents are associated with few if any unconfounded cases of nephrogenic systemic fibrosis, and understanding of their potential role in the evaluation of individuals with impaired renal function is evolving.
2.3 Nuclear Medicine
Nuclear medicine studies may be used to evaluate transplant perfusion or to collect system injury following renal transplantation. Technetium-99 m (99mTc)-labeled pharmaceuticals are typically used, either 99mTc-mercaptoacetyl triglycine or 99mTc-diethylene triamine. The radiopharmaceutical is injected intravenously and sequential images are acquired to evaluate the vascular phase (flow phase), parenchymal phase (functional phase), and washout phase (excretory phase). Nuclear medicine studies are an option for patients with contraindications to iodinated and gadolinium-based intravenous contrast agents.
3 Ultrasound Evaluation of Normal Renal Transplant
The healthy transplanted kidney has ultrasound features comparable to the healthy native one; however, a more detailed two-dimensional image is apparent as the transplant is usually located more superficially, and thus higher frequency transducers can be utilized. The reniform outline and central echo complex, resulting from the collecting system and the renal vasculature together with any sinus fat, are well depicted, while the distinction between the renal cortex and the relative echo-poor medulla is usually apparent (Fig. 16.2). The size is similar to native kidneys, but after 2 months, the normal transplant kidney usually becomes hypertrophic and can elongate by 2–3 cm [5]. The collecting system of a well-functioning transplant is often slightly dilated, presumably because of a combination of an increased production of urine (since it is working as the sole kidney) and the loss of ureter’s tonicity from denervation. However, in the unobstructed transplant, the filling should be minor and confined to the renal pelvis, while filling of the infundibula or the calyces is suspicious of significant outflow obstruction. In color Doppler imaging, arterial and venous flow should extend to within a few millimeters of the capsule throughout all renal segments, although this depends on the depth of the transplant kidney, the transducer frequency, and the sensitivity of the color Doppler settings. Occasionally flow will appear to be decreased or absent in deeper portions of the kidney, which should prompt evaluation with B-flow or power Doppler, as it is more sensitive than color Doppler (Fig. 16.3). Spectral Doppler interrogation from the segmental and interlobar vessels shows the normal fast systolic upstroke with a subsequent slow decay in diastole, with forward flow being maintained until the next cardiac cycle. Thus, RI values of 0.8 or lower are expected, although the clinical context should be evaluated as well. The main renal artery is usually readily demonstrated but is often much more tortuous than that of the native kidneys because of the way the transplant is relocated into the iliac fossa after the anastomoses have been performed (Fig. 16.4). Therefore, the straight portions of the artery as optimum locations for Doppler measurements are those that tend to lie parallel to the skin and thus subtend the worst beam-to-vessel angles. The branch and main renal veins are easily accessed for Doppler studies.
(a) Normal transplant kidney on grayscale ultrasound demonstrates good contrast resolution between cortex and medulla. (b) Slight dilatation of the collecting system and pigtail catheter (arrow) is noted. (c) Normal renal artery and vein of the transplant kidney on color Doppler ultrasound. (d) Normal homogeneous blood flow throughout the transplant kidney on color Doppler ultrasound. Interlobar, arcuate, and peripheral cortical branches are illustrated. (e) Normal intrarenal artery waveform on spectral Doppler ultrasound shows a brisk systolic upstroke and high diastolic flow. Resistive index is normal (RI = 0.55). (f) Normal waveform of the renal artery on spectral Doppler ultrasound
4 Parenchymal Complications
4.1 Immediate Graft Parenchymal Complications
“Immediate” graft parenchymal complications are all the adverse events occurring within the first week since the surgical procedure.
Immediate parenchymal complications are often responsible for delayed graft function and are associated with a higher rate of hospitalization and a worse graft survival both in the short and in the long period. These complications are mostly represented by hyper-acute rejection, acute tubular necrosis (ATN), calcineurin inhibitor toxicity, and acute pyelonephritis.
4.1.1 Hyper-Acute Rejection
Hyper-acute rejection has become a very rare event caused by the presence of preformed antibodies against HLA, ABO, or other alloantibody-to-donor endothelial surface antigens in the recipient, responsible for an immediate graft loss [6].
The diagnosis is made very early, within minutes or hours of transplantation in a recipient, often even in the theater, during the surgical intervention. The kidney usually stops functioning within minutes after transplantation and appears flaccid and blue-cyanotic, due to the thrombotic and inflammatory processes, with a graft loss rate above 60%.
Therefore, B-mode US in hyper-acute rejection management has a very limited usefulness due to the extremely quick necrosis process which involves the graft. Anyway, the sonographic features are nonspecific and like those observed in case of acute rejection and ATN; CDUS evaluation may show the complete absence of intrarenal blood flow [7].
4.1.2 Acute Tubular Necrosis (ATN)
ATN is one of the most frequent causes of delayed graft function (DGF) and is detected with different levels of severity in 20–60% of deceased donor renal transplantation biopsies.
It is the most common cause of early graft dysfunction observed during the first 48 h posttransplantation and is related to the reversible ischemic injury involving tubular renal cells in the pretransplant period. The main risk factors for acute tubular necrosis include deceased (vs. living) donor renal transplantation, donor hypotension, and prolonged warm and cold ischemia period.
The grayscale imaging feature of ATN is extremely variable and aspecific. Besides size variations, such as graft enlargement, decreased echogenicity and/or scattered heterogeneous areas of variable echogenicity with a loss of corticomedullary differentiation can be observed. Parenchymal edema and sometimes a complete compression of the renal sinus may also be seen. However, graft swelling or enlargement can also be caused by other conditions, including a normal early postoperative state, as well as delayed graft function, cellular or humoral rejection, acute CNI toxicity, and obstruction. Moreover, these findings may be observed to be totally normal as well or totally indistinguishable from an acute rejection. CDUS evaluation shows that a renal resistive index (RRI) is always greater than 0.80 (Fig. 16.5).
4.2 Early Graft Parenchymal Complications
4.2.1 Acute Rejection
Acute rejection is a major cause of graft dysfunction affecting more than 30% of transplanted kidneys. Most episodes of acute rejection occur between 48 h and 6 months after surgery. Despite a dramatic reduction in the incidence and severity of acute rejection due to the introduction of new immunosuppressive protocols in the past three decades, some kidneys do not regain function and even among patients who recover, acute rejection can have a negative impact on long-term graft survival. Clinical manifestations are unspecific and may include malaise and oliguria. The traditional symptoms, such as graft pain and tenderness, and fever occasionally, are now often absent, so that the clinical onset can also be completely silent. Laboratory findings mostly include increased serum creatinine, proteinuria, and sometimes pyuria.
US B-mode may be helpful mainly for excluding other causes of acute kidney injury, because in most cases the findings are similar to those observable in other acute parenchymal nephropathies (Fig. 16.6):
-
Increased volume of the graft due to inflammatory edema (very frequent but nonspecific)
-
Enlarged and hypoechogenous pyramids, due to medullary edema (early but nonspecific)
-
Thickened and hyperechogenous cortex (rarely hypoechogenous cortex and increased medullar thickness, with consequent loss of the corticomedullary differentiation)
-
Edema of the collecting system and focal parenchymal hypoechogenicity, due to focal infarctions
-
Perigraft anechogenous collections due to necrosis and/or hemorrhages
-
Increased parenchymal thickness with obliteration of the hyperechogenous pyelic sinus
Acute rejection: (a) sagittal ultrasonography shows an enlarged, globular shaped allograft with loss of renal sinus fat; (b) the study of the renal parenchyma, at the color Doppler with a linear probe, shows significant increase, poor vascularization, and RI = 1.00. The v/t curves show absence of the diastolic phase. (c) Acute rejection: B-mode linear probe shows inhomogeneity of the renal parenchyma. Kidney biopsy shows acute cell rejection
With the color Doppler study it is possible to find out elevated RRI, an unspecific feature detectable also in ureteral obstruction, ATN, renal vein occlusion, pyelonephritis, and calcineurin inhibitor toxicity [8].
In patients receiving a kidney transplant, graft RRI is poorly correlated with the donor parameters such as age or allograft function while it is strictly connected with host-related factors such as pulse pressure (PP), intima-media thickness (IMT), and ankle brachial index (ABI).
In a study involving 321 renal allograft recipients, Naesens et al. confirmed that RRI was not related to graft function or intrarenal histologic disease pattern, while, being strictly connected with the recipient age, it could represent a survival indicator [9, 10].
In conclusion, there are no specific ultrasonographic signs of acute rejection of the graft, even though the presence of these patterns associated with a rapid worsening of renal function may help in the initial differential diagnosis of acute graft dysfunction.
Unfortunately, a definitive discrimination of ATN and AR by Doppler ultrasound is not possible [11].
Recently, contrast-enhanced ultrasound (CEUS) has been indicated as a new promising noninvasive imaging tool that may also be helpful in the early diagnosis of kidney transplant complications, such as delayed graft function (DGF) and acute allograft rejection.
Differently from conventional US techniques, CEUS allows a more precise study of graft microvascularization providing also quantitative information about transplant perfusion [12].
Schwenger et al. in 2006 tested the efficacy of real-time quantitative determination of arterial arrival of a US contrast medium in kidney transplantation demonstrating a higher accuracy of CEUS compared to CDUS in the estimation of graft perfusion [12].
Recently, Grzelak et al. showed that CEUS was a better tool in the identification of acute rejection than conventional techniques. Authors demonstrated, in patients with normal graft function, the regular inflow of contrast medium in all graft regions. Conversely, subjects with DGF showed delayed inflow of the contrast medium; moreover the time of inflow to the regions of interest significantly differed between ATN and acute rejection [13].
Therefore, this led to the suggestion that with the help of CEUS-derived parameters of the quantitative assessment, it could be possible to distinguish ATN from AR [14]. However, the ability of CEUS in differentiating between ATN, AR, and calcineurin inhibitor toxicity has not been investigated. Consequently, in the context of acute graft dysfunctions, performing a biopsy remains mandatory to obtain a definitive diagnosis.
4.2.2 Drug Toxicity
CNIs, such as tacrolimus and cyclosporine, are used as maintenance immunosuppressive therapy in almost all renal transplant recipients. Unfortunately, these compounds are also potentially nephrotoxic and a frequent cause of acute and chronic graft dysfunction.
Typically, CNI toxicity occurs during the first 3 months after transplantation when the therapeutic target is higher and may induce an impairment of renal function, which is mostly reversible after reducing the dose. However, when the CNI toxicity is severe and/or persisting, a chronic, progressive, and irreversible renal disease can result. Rarely, thrombotic microangiopathy leading to acute graft loss may be induced by CNI treatment in susceptible patients.
The standard US exam in CNI injury does not disclose specific features. Graft swelling, increased or decreased renal echogenicity, and loss of corticomedullary differentiation are usually observed at the common B-mode examination. At CDUS only a nonspecific RRI increase can be revealed. Long-term CNI toxicity is not generally differentiable from chronic allograft rejection, in which US may show small-sized graft with thinner cortical layer or normal findings, according to the degree of renal insufficiency.
Consequently, a biopsy may be required for the differential diagnosis with other acute or chronic causes of graft dysfunction.
4.2.3 Acute Graft Pyelonephritis (AGPN)
Acute graft pyelonephritis (AGPN) can complicate any phase of posttransplantation period. A recent analysis demonstrates that transplant recipients have a 72-fold higher risk of first-time hospitalization for pyelonephritis compared to matched population controls [15].
According to the literature [16], the diagnosis of AGPN should include (1) positive microbiology or imaging (urinary dipstick positive for nitrite and/or leukocyte esterase and/or a positive urine culture and/or ultrasound marks indicating pyelonephritis) and/or (2) positive laboratory test results (leukocyte count ≥12x 109 cells/L and/or C-reactive protein (CRP) ≥80 mg/L and/or temperature >37.8 °C) and/or (3) positive clinical signs (one or more of the following: graft-pain/flank pain, chills, clinical symptoms consistent with cystitis (dysuria, urge, suprapubic pain)). The variable incidence is around 20%. Important risk factors for this complication include immunosuppressive regimens, invasive urological procedures such as bladder catheterism or ureteral stenting (Fig. 16.7), and posttransplantation urethral bladder reflux (Fig. 16.8).
The early differential diagnosis is essential, because AGPN episodes increase the risk of scarring, acute rejection, and worsening of renal function. Imaging is necessary in AGPN both to evaluate the presence and extent of complications such as renal and perirenal lesions and to detect potentially treatable causes [17].
The basal B-mode evaluation does not allow high accuracy (11–40% sensitivity and 50% specificity) in detecting renal parenchymal lesions. Standard US is more helpful in the presence of renal abscess or hydronephrosis, since these changes are usually characterized by inflammation-induced graft swelling, thickener sinus, and reduced corticomedullary differentiation.
Occasionally, parenchymal infections may display as focal acute pyelonephritis, which are characterized by hypo- or anechogenous and poorly defined areas with faded borders at US.
Posterior parietal reinforcement might be present as an expression of nonsuppurative paracellular inflammation related to interstitial edema and/or hemorrhages. CDUS has a higher sensitivity (38–63%) in detecting parenchymal abnormalities in case of AGPN. Power Doppler has been shown to have a higher sensitivity (41%) and specificity (89%), when compared with CDUS (Fig. 16.9).
Computed tomography (CT) is the gold standard for the diagnosis of AGPN, with an excellent performance in recognizing underlying obstructive causes such as calculi or anatomic alterations due to perirenal collections. On the other hand, CT imaging is highly expensive and implies a high-dose radiation and contrast media nephrotoxicity risk. Magnetic resonance imaging (MRI-DWI) is increasingly used in patients with posttransplant creatinine increase and may overcome CT limits.
The advent of newer US contrast agents helps to better detect areas of poor renal parenchymal perfusion. Contrast-enhanced US (CEUS) is a toxic-free, quick, and feasible procedure that can improve the detection of low flow by improving the signal-to-noise ratio, and can improve the sensitivity of US for AGPN (Fig. 16.10). For example, the presence at CEUS test of a triangular hypoechoic, hypoperfused area in the medulla indicates ischemic parenchymal lesions and is helpful for distinguishing between APN and a simple pyelitis. Acute rejection-induced hypoperfusion areas show a “diffuse pattern,” easily distinguishable by the “triangular shape” of the focal hypoperfused areas characteristic of AGPN.
Renal abscess. (a) Image A is a grayscale sagittal image through the transplant kidney that shows an exophytic multi-septated abscess (calipers). (b) A color Doppler image of the same area shows peripheral vascularity without any significant internal flow. (c) An axial CT scan image through the pelvis shows transplant kidney (short arrows) with the abscess protruding anteriorly (long arrows). (d) Image D shows a drain placed in the abscess (courtesy of Prof. Ishrad)
In a recent analysis Granata et al. demonstrated the utility of CEUS examination in the diagnosis of AGPN. CEUS resulted to be an excellent tool in the workup of complicated acute pyelonephritis, so that it might be considered as the imaging technique of choice in the evaluation and follow-up for these patients [18,19,20,21].
4.2.4 Viral Infections: BK and Cytomegalovirus Nephropathy
BK (polyomavirus) and cytomegalovirus are viral infections that can lead to allograft dysfunction due to interstitial nephritis, glomerulopathy, or cytokine release. Additionally, adenoviral infections of the renal allograft are a rare cause of allograft dysfunction.
BK virus nephropathy occurs in up to 5–10% of kidney transplants, usually within the first 12–18 months of transplantation. The confirmatory diagnosis of BKV-associated nephropathy (BKVAN) is made by the discovery of viruria, viremia by PCR analysis, and tubule-interstitial changes at graft histology [22].
Information from diagnostic imaging is mostly not specific and of limited utility in the diagnosis of BKVAN. An uncommon exception is represented by the renal obstruction caused by the ureteral involvement of the virus. Moreover, other authors have described as specific some other findings using CT and ultrasound in transplanted patients affected by BKVAN [23].
More recently, Dugo et al. have described typical CDUS patterns (Fig. 16.11) observed in 17 consecutive patients affected by BKVAN [24].
They used high-frequency sonography with a linear array transducer (56 mm linear probe, bandwidth 5–12 MHz) and reported the presence of characteristic localized hypoechoic areas from the papilla to the renal cortex alternating with normal echoic areas (zebralike pattern), without any vascular abnormality. Should these findings be confirmed in a larger population, CDUS could be proposed as a more specific imaging technique for the diagnosis of BKVAN (Fig. 16.12).
4.3 Late Graft Parenchymal Complications
4.3.1 Recurrent Primary Disease and “De Novo” Renal Disease
Primary glomerular disease recurrence must be considered as a possible and unpredictable cause of graft function impairment. Recurrence risk depends upon the specific glomerular disease. Glomerular diseases with the higher recurrence rate include primary focal segmental glomerulosclerosis (FSGS), primary membranous nephropathy, membranoproliferative glomerulonephritis, complement-mediated HUS, and C3 glomerulopathy. Late recurrence (i.e., after more than 1 year posttransplant) has been described for many diseases, including FSGS, membranoproliferative glomerulonephritis, immunoglobulin A (IgA) nephropathy, and diabetic nephropathy. In all these conditions, imaging alone has a limited diagnostic role and graft biopsy remains mandatory for the precise diagnosis [25].
4.3.2 Chronic Renal Rejection
The most common cause of graft failure after the first year is the so-called chronic renal allograft nephropathy (CAN). CAN is a clinicopathological entity that the new revised Banff 2005 classification system redefined as “interstitial fibrosis and tubular atrophy (IF/TA), without evidence of any specific etiology.”
CAN is characterized by a progressive decline of the renal function, often accompanied by arterial hypertension and proteinuria without manifestations of nephrotoxicity or acute rejection.
The diagnosis of CAN is not easily made, since the only alteration is represented by a progressive and slow increase of serum creatinine.
The differential diagnosis of CAN implies distinguishing among the many underlying conditions that cause progressive allograft dysfunction and/or are associated with similar histologic findings such as primary disease recurrence, transplanted renal artery stenosis, calcineurin inhibitor nephrotoxicity, BKVAN, rejection relapses, and occasionally obstructive nephropathies.
CDUS usefulness is limited to differentiate parenchymal chronic complications from vascular, surgical, and/or urological causes of graft dysfunction. Moreover, it guides the decision to perform graft histological examination. However, allograft biopsy remains the gold standard and is necessary in most cases to exclude potentially treatable causes of chronic rejection, such as chronic CIN.
In chronic rejection biopsies, glomerulosclerosis, tubular atrophy, interstitial fibrosis, and severe vascular involvement are usually observed. These changes are similar to those seen in native renal biopsies from patients with chronic kidney disease (CKD) and the US appearance is not different, showing:
-
Reduced graft length/volume
-
Reduced graft cortical thickness
-
Increased graft cortical echogenicity
-
Poor visibility of the renal pyramids and the renal sinus
-
Marginal irregularities
-
Papillary calcifications
On occasion, the chronic process can lead to the formation of lymphatic accumulation under renal capsular surface (subcapsular lymphocele) due to the occlusion of the intrarenal lymphatic collectors. US will show a thin subcapsular hypoechogenous band/strip.
At the color Doppler examination, reduced graft vascularity and interlobar RRI increase can be observed in most patients (Fig. 16.13).
A role for high RRI was described in the early 2000s as an early, strong negative predictor of long-term graft survival [26].
However, more recent data from Naesens et al. did not support such a relation between RRI and graft function or intrarenal histologic disease pattern. In fact, these authors showed in a 321 transplanted patients’ cohort that RRI was strictly connected with the recipient and not to the donor age, reflecting the characteristics of the host and representing a recipient survival indicator [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].
A method to quantify the pixels of color of the power Doppler in the region of interest (ROI) has been recently described by Nankivell et al. [28].
According to this technique, parenchymal vascularization can be quantified in relation to the maximum fractional area (MFA) at the color Doppler and the distance between the most peripheral color pixels and the renal capsule.
This method has a proven excellent reproducibility and diagnostic accuracy. The distance between the peripheral color pixels and the renal capsule showed a 91% sensitivity in the diagnosis of CAN and reached 94% when combined with MFA. Scholbach et al. have suggested the use of tissue Doppler for the diagnosis of CAN to detect the tissue pulsatility index (PI: calculated as the ratio between the differences between systolic mean and diastolic mean in the ROI and the mean velocity), to quantify graft perfusion [29].
Recently, novel ultrasonic technologies have been applied in the evaluation and diagnosis of kidney allograft status, such as contrast-enhanced ultrasonography (CEUS) and tissue elasticity quantification using acoustic radiation force impulse (ARFI) technology.
The quantitative information about microvascular perfusion of the renal allograft can be evaluated by CEUS, which can offer an improved diagnostic accuracy compared with CDUS for the detection of CAN. Moreover, the measure of graft perfusion parameters such as US RRI, pulsatility index, and renal blood flow estimated by CEUS is significantly more accurate compared to conventional CDUS [12]. Schwenger et al., in a small cohort of 26 transplanted subjects, demonstrated that quantitative determination of renal blood flow provided by CEUS has a significantly higher diagnostic power for the detection of graft dysfunction due to CAN compared with conventional color Doppler resistance indices. Nevertheless, those data should be confirmed in larger trials. In addition, the CEUS accuracy in the differential diagnosis between different causes of late graft dysfunctions has not yet been investigated [30].
Another novel tool in the evaluation of CAN is US-based elastography (elasticity) by the acoustic radiation force impulse method (ARFI). Parenchymal stiffness can be measured by this transient elastography technique and seems to correlate with histologic measures of interstitial fibrosis (Fig. 16.14).
Initially, encouraging results of ARFI efficacy in the quantification of liver fibrosis have led to the hypothesis of its potential effectiveness in detecting parenchymal fibrosis in kidney transplant as well. Accordingly, tissue elasticity measured by ARFI has been explored by Stock et al. as a new promising noninvasive technique for the assessment of renal allograft fibrosis. A moderate correlation between mean ARFI values and the grade of fibrosis and the BANFF category was found.
More recently, Lee et al. showed that there was no significant correlation between shear wave velocity (SWV) measured by ARFI and IF/TA or renal dysfunction. Tissue elasticity parameters were related to the time after transplantation and were influenced by graft hemodynamics.
Further long-term longitudinal assessments are necessary to confirm these results [31].
4.3.3 Neoplasm (Posttransplant Lymphoproliferative Disorder)
Renal transplant patients have a generally increased risk of neoplasm related primarily to chronic immunosuppression. Most neoplasms are skin cancers and lymphomas. Patients are also at increased risk for renal cell carcinoma, although most of these are in the native kidneys in patients with acquired cystic disease from hemodialysis. Ultrasonography may be used to screen for renal neoplasms in both the transplant and native kidneys, although marked atrophy and increased echogenicity in the native kidneys may decrease the ability to detect small tumors with ultrasonography [32].
Posttransplantation lymphoproliferative disorder (PTLD) is a general term for a variety of lymphoid disorders ranging from lymphoid hyperplasia to frank lymphoma. Most cases of PTLD are B-cell lymphocyte proliferations related to Epstein-Barr virus, although the heterogeneity of these disorders suggests a variety of mechanisms that predispose to lymphoproliferation.
PTLD after solid organ transplantation usually occurs within the first year; however, the median time of presentation for renal transplant patients is 5 years, as a result of less aggressive immunosuppressive therapy. The most common site for PTLD in renal transplant patients is the allograft, followed by extranodal involvement, with particular predilection for the gastrointestinal tract and liver. Typical patterns of kidney involvement include a heterogeneous hilar mass and multiple parenchymal masses. Although one may suspect PTLD based on sonographically detected renal or gastrointestinal abnormalities, the high frequency of multisystem involvement makes positron-emission tomography combined with CT the preferred imaging modality.
5 Vascular Complications
Recently, the improvement of immunosuppressive regimens has led to a progressive reduction in both early and long-term graft loss due to acute or chronic rejection. Accordingly, the relevance of other causes of premature kidney failure such as drug toxicity and metabolic consequences, infections, primary disease recurrence, and vascular complications has progressively increased [33].
Vascular complications after renal transplantation usually include renal artery stenosis and thrombosis, transplant renal vein thrombosis, biopsy-induced vascular injuries such as pseudoaneurysm, and intrarenal arteriovenous fistula formation [34] (Table 16.3).
Kidney transplant vascular complications are a life-threatening, relatively rare, event, accounting for approximately 10% of all posttransplant abnormalities. However, their diagnosis is extremely important because of the potential for rapid graft loss and poor prognosis. On the other hand, repair of vascular defects is mostly possible by angioplasty or surgical procedures, making graft conservation achievable [35].
Reducing the incidence of vascular complications depends to a great extent on a prompt and precise diagnosis. Ultrasound and color Doppler exam (CDUS) is frequently the first-line investigation for both suspected native renal tract and renal transplant vascular complications. It is a noninvasive, inexpensive, rapid, and safe tool with a high diagnostic performance when performed by an experienced operator [36, 37].
Other imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI), may be complementary and are often used as solving tools when US results are doubtful. Finally, angiographic methods remain the gold standard in the diagnosis and treatment of graft vascular abnormalities, even though its indication must be strictly based on a favorable risk/benefit ratio assessed by the nephrologist.
New promising ultrasound-based technologies have been developed such as contrast-enhanced sonography (CEUS). CEUS is a radiation and toxin-free, reproducible, and very rapid US imaging modality that showed to enhance the diagnostic performance of CDUS in many different settings avoiding the limitation and potentially dangerous effect of other radiological techniques. Therefore, it is reasonable to suppose a future widespread application also in transplant medicine [38, 39].
6 Transplanted Renal Artery Stenosis (TRAS)
6.1 Epidemiology
Renal artery stenosis (TRAS) is the most frequent vascular complication in renal transplant, accounting for 75% of all graft vascular defects. It is important to detect this process because it represents a potentially reversible cause of refractory hypertension and graft loss. The reported incidence ranges between 1 and 23%, even though its epidemiology substantially differs depending on the diagnostic approach and the time elapsed from the transplant. An incidence of 12.4% has been registered when transplanted patients undergo a planned Doppler examination during the first year of follow-up [40]. However in more recent and powered trials the incidence did not exceed 1–3% [41]. TRAS is more frequently diagnosed between 3 months and 2 years from the surgical procedure; however it can equally occur anytime. Discrepancies in the reported prevalence might also be explained by a not-well-established criterion for defining a hemodynamically significant TRAS. Factors associated with an increased risk of TRAS are the following: cadaveric kidney transplant, atherosclerosis in donor vessels, cytomegalovirus infection, younger donor age, and extended criteria donors [42].
6.2 Clinical Manifestations and Pathogenesis
Based on the anatomic localization of the stenosis we can distinguish three main types of TRAS: proximal due to recipient atherosclerotic arterial disease, anastomotic, and distal in the donor renal artery. However, considering the extension of the stenosis it can be differentiated into localized, diffuse, and multiple. In about half of all TRAS cases, the stenosis is localized and anastomotic and iatrogenic, being mainly caused by scarring related to the surgical procedure during explantation, clamping, and/or anastomosis of the vessel with the iliac artery axis [43]. Less commonly, multiple segments of the artery (or even the entire vessel) are stenotic. In these cases, the stenosis is generally the result of catheter-related trauma to the intima during the phase of cold ischemia, but it may also be caused by torsion and/or kinking occurred after surgical implantation [44]. Both anastomotic and distal donor stenosis may also be caused by rejection. An immunological mechanism has also been proposed, particularly in diffuse and multiple stenoses. In most cases, TRAS is asymptomatic, but in some patients it can result in resistant hypertension and/or graft dysfunction in the absence of rejection, ureteric obstruction, or infection, because of severe renal hypoperfusion. TRAS is the underlying cause of 5% of all posttransplant hypertension [45].
The pathophysiology of hypertension occurring in the setting of TRAS is comparable to the renovascular hypertension in the “one-kidney, one-clip” experimental models. However, since, differently from Goldblatt’s models, the transplanted kidney is denervated, in this case the stenosis-induced kidney ischemia does not directly trigger the sympathetic response.
Nevertheless, as in non-transplanted patients with bilateral RAS or solitary kidney stenosis, the renin-angiotensin-aldosterone system (RAAS) is activated in TRAS. This leads to sodium and fluid retention and, in rare cases, patients may develop edema, congestive heart failure, or recurrent bouts of pulmonary edema. Moreover, in transplanted patients, a sudden, not-justified kidney function reduction after the administration of antiangiotensin drugs should be considered as suggestive of TRAS. An association with erythrocytosis has also been reported. Physical examination is mostly silent and a vascular bruit in the iliac fossa can often be present, but is not specific for significant stenosis [46]. Limited evidence regarding the progression showed that in most patients TRAS remains stable over time and can be treated medically. Occasionally a spontaneous regression has also been observed. There are reports of favorable long-term follow-up of hemodynamically significant stenosis treated by percutaneous angioplasty (PTA) [47]. On the other hand, the USRDS registry reported an adjusted hazard ratio for death and graft loss of 2.84 (95% CI 1.70–4.72) in transplant recipients with TRAS compared with those without TRAS [41].
6.3 Diagnosis
Although angiography remains the gold standard for the final diagnosis of TRAS, color Doppler examination, as performed by an experienced operator, plays an essential role in the screening, diagnosis, and follow-up. Other imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI), have a complementary role. New ultrasound-based techniques as contrast-enhanced ultrasound (CEUS) have already demonstrated to improve the diagnostic performance of color Doppler ultrasound examination (CDUS) examination, but are still under evaluation in transplanted kidney [39, 48].
6.4 Color Doppler Ultrasound (CDUS)
The CDUS examination, being performed at the bedside, is a noninvasive, highly sensible, specific, and inexpensive tool in the management of TRAS [49] (Fig. 16.15). It has progressively replaced renal scintigraphy because of its superior performance (87–94% sensitivity; 86–100% specificity) [50].
CDUS allows assessing both the localization and hemodynamic significance of TRAS. Moreover, Doppler study is ideal to estimate the result of revascularization and helps in grading the stenosis during a long-term follow-up. The main disadvantages are that this investigation is operator dependent and time consuming [39]. As in the native kidney, CDUS evaluation criteria of RAS are based on direct findings at the level of the stenosis (proximal criteria), or on flow changes induced in the renal vasculature induced by the stenosis (distal criteria).
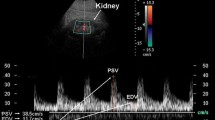
Differently from native kidney, in TRAS it is more difficult to set a peak systolic velocity (PSV) cutoff as a direct diagnostic criterion. Furthermore, the more frequent presence of transplant renal artery tortuosity and/or multiple renal arteries leads to incorrect insonation angles and reduces the accuracy of PSV assessment, thus representing a significant source of technical errors in CDUS evaluation. For the same reason, the direct visualization of color artifacts, such as aliasing at the site of the stenosis and presence of turbulence at color evaluation, may not be a sign of significant TRAS [49]. If possible, the operator should consult the surgery report to know whether multiple artery or anastomosis problems had been observed.
Elevated PSV of the RA is not necessarily indicative of TRAS. In fact, PSV as measured in a hypertrophic, well-functioning transplanted kidney may rise beyond 250–300 cm/s longwise the artery even in the absence of TRAS (Fig. 16.16). On the other hand, in case of chronic graft dysfunction with reduced organ volume, a focal PSV of 180–200 cm/s may be suggestive of significant stenosis, particularly when the other segments of the artery show markedly lower PSVs (40–50 cm/s) [51].
Therefore, an isolated acceleration of the flow that is 2.5 times higher than the pre- or post-stenotic velocity (e.g., PSV 250–270 vs. 80–120 cm/s) might represent a direct criterion for the diagnosis of TRAS. Concerns about a specific PVS limit have been deepened by a recent retrospective analysis of 844 transplanted kidneys performed by Robinson K. et al., in which almost 30% of renal transplants showed a PVS over 250 cm/s within the first 9 months and 19% had velocities over 250 cm/s also after the first-year posttransplant without any sign of graft dysfunction [51] (Table 16.4).
Eventually, some authors have pointed out that the PSV threshold for stenosis may also depend on the type of anastomosis, differing between end-to-side and end-to-end graft anastomosis, but this observation has not been confirmed in all studies [52]. Anyhow, in transplanted patients, when a high PSV is not associated with other clinical findings, a closer CDUS follow-up is advisable before immediate invasive intervention [53]. A recently proposed direct TRAS criterium is the ratio of PSV in the renal artery to the adjacent external iliac artery (RAA-to-EIA) with a threshold of 1.8 or greater, but its diagnostic and prognostic value is still poorly defined [54]. Looking at distal criteria, while the evaluation of the resistive index (RI) at CDUS may be very useful for the diagnosis (or recognizing) of RAS in native kidneys and predicting of the response to revascularization, in TRAS it has a less confirmed value. Initially, a high RI (<0.80 or higher) measured at least 3 months after transplantation had been found associated with both allograft failure and death with a functioning graft [55]. More recently, it has been demonstrated that RI reflects the recipient vascular status, but not the vasculature integrity of the graft [10, 27]. In addition, the waveform and resistive indexes of the transplanted kidney cannot be compared with the contralateral kidney, making more uncertain the interpretation of the results. A class of other distal parameters has been evaluated in RAS studies. The intrarenal parameters are measured from the intraparenchymal arteries which are often easier to evaluate than the main arteries [27, 53].
In native kidneys with significant renal artery stenosis, waveforms from the intraparenchymal arteries have been reported to be dampened [27].
This dampening has been evaluated quantitatively by measuring the prolonged rise time in early systole or qualitatively by assessing waveform shapes. Acceleration time (AT) alone is a validated high-sensitivity indirect index in the diagnosis of TRAS. In a small study performed by Gottlieb et al. a 0.10-s cutoff or a subjective assessment of dampening of the waveforms had a 95% accuracy in the detection of a hemodynamically significant TRAS, compared to a 62% obtained using the standard PVS threshold of 2.0 re/s as the sole criterion (Table 16.3).
CDUS is also particularly useful in the assessment of the effectiveness of perfusion restoration after revascularization procedures. When RI increases to the normal range, it invariably indicates restored kidney perfusion achieved by effective revascularization [56]. Doppler examination is essential for monitoring the TRAS during the posttreatment follow-up, allowing early diagnosis of recurrence, while MRA and spiral CTA are less suitable for assessing restenosis because of artifacts caused by the stent material [56]. Accordingly, CDUS follow-up to assess for restenosis may be warranted in patients after stent placement for TRAS, even in the absence of clinical signs of restenosis. Finally, because the number of patients affected by peripheral artery disease (older age and diabetes) that undergo kidney transplant is increasing, the possibility of a concomitant iliac artery disease that mimics TRAS (pseudo-TRAS) should always be considered [57].
Lesions upstream to the transplant anastomosis, reducing blood flow, may cause transplant hypoperfusion with signs and symptoms resembling TRAS. Also, these lesions may be detected by CDUS examination [57].
In summary, there is a reasonable level of consensus on a PVS above 250–300 cm/s as the limit to predict significant TRAS, in clinically suspicious patients, with an acceptable grade of sensitivity and specificity. In contrast to RAS in the native kidneys, indirect signs are less sensible and less accepted as valid diagnostic criteria in TRAS. In fact, an AT below 0.10 s is the only accepted distal criterion in this setting.
6.4.1 New Renal Ultrasound-Based Imaging: Contrast-Enhanced Ultrasound (CEUS)
In the evaluation of TRAS, contrast-enhanced ultrasound may be complementary to the standard CDUS examination. To date, few papers have tested the use of CEUS in TRAS, so that the role of this technique is still ill defined. Quantitative and qualitative CEUS examination seems to be a promising, quick, and noninvasive assay of graft perfusion, providing a real-time imaging of renal vasculature and perfusion [14, 40].
Longer time of contrast agent inflow indicates the presence of stenosis, and the rate of contrast agent inflow positively correlates with the severity of arterial stenosis on cross-sectional imaging.
Since PSV measurements require an angle of interrogation parallel to the vessel, which is not always obtainable by Doppler ultrasound, CEUS does not require identification of the renal artery, nor is it angle dependent. Furthermore, ultrasound examination during microbubble infusion can be used to quantify total organ and regional nutrient blood flow to the kidney [15, 58].
6.5 Nuclear Medicine Imaging (NMI)
NMI or isotope renography imaging of the allograft is a less invasive, non-nephrotoxic tool to evaluate graft function and perfusion, particularly in the early postoperative period. It may represent a complementary procedure for TRAS assessment when performed both at basal and after renin-angiotensin system inhibition [16]. The optimal radiopharmaceutical agent in individuals with normal renal function is still undefined. However, 99mTc-mercaptoacetyltriglycine (MAG-3) and 99mTc-diethylenetriaminepen-triacetic acid (DTPA) are the most commonly used in clinical practice. Given its higher extraction rate, 99mTc-MAG3 is to be preferred over 99mTc-DTPA in patients with impaired kidney function [59].
Nevertheless, though in the past NMI was considered in the diagnostic algorithms of TRAS, it is now less applied because of its low specificity (almost 67%) compared to CDUS and MRI. For years, isotope renography (basal or after renin-angiotensin system stimulation) has been the most popular noninvasive screening procedure for TRAS. However, despite relatively good sensitivity (75%), the procedure is seriously limited by its poor specificity (67%).
6.6 CT and MRI Angiography
Computed tomography can provide excellent imaging by a three-dimensional reconstruction of graft vessels; however, it still requires the administration of nephrotoxic contrast agents, as in subtraction angiography [60]. Therefore, when clinical and less invasive instrumental features are strongly suggestive of hemodynamically significant stenosis, it is preferable to directly perform angiography that, in the case a TRAS is detected, allows to treat the stenotic vessel by angioplasty with or without stent placing (PTCA). Magnetic resonance imaging (MRI) can be safely used to diagnose or confirm TRAS and can be considered as a noninvasive alternative to CT angiography.
Gadolinium-based contrast agents are not nephrotoxic; nevertheless, they are not indicated when eGFR is below 30 mL/min due to the debated risk of nephrogenic systemic fibrosis.
Moreover, the opinions about MRI application in TRAS are contrasting, because some of them have reported that MRI angiography well correlates with digital subtraction angiography results, while others reported that, as compared with CDUS and digital subtraction angiography, MRI angiography may cause a conspicuous number of false positives (75%) due to artifacts. Other limitations may be related to the presence of clips on the anastomosis [52, 61].
6.7 Arteriography
Selective arteriography provides the precise diagnosis of RAS both in native and transplanted kidneys. Although its execution needs some relatively large amounts of potentially nephrotoxic radiocontrast medium, it is invasive and burdened by various complications such as thromboembolism, pseudoaneurysms, traumatic arteriovenous fistulas, and groin hematomas occurring in 10% of cases. Moreover, it can induce graft function loss with a reported incidence of 9% [62]. Therefore, angiographic techniques are not used as a screening tool, but are recommended in patients in whom a strong suspect TRAS has been previously elicited by other noninvasive screening tests or in patients particularly in those with a good expectancy of successful revascularization. Indeed, arteriography allows an immediate correction by transluminal angioplasty followed by balloon angioplasty and stent placement. Carbon dioxide images produce contrast columns like those with iodinated contrast media, giving images of the inflow iliac artery, artery anastomosis, and transplant renal artery with a resolution comparable to that of classical arteriography [62].
7 Renal Artery Thrombosis (RAT)
7.1 Epidemiology
The complete occlusion of the renal transplant artery is a rare and poor-prognosis vascular complication. Mainly, it occurs in the early postoperative period, even though very few papers reported cases detected after the first month, especially in rejected kidney or in a kidney with high-grade TRAS39. The incidence peak is observed in the first 48 h after surgery [63]. In the literature, the reported incidence of thrombotic complications in case series is about 0.5–6% and is more frequent in recipients of cadaveric donor kidneys and in pediatric age which may occur in 35% of transplanted kidneys, as reported by the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) [64]. Recognized risk factors are the following: hypovolemia, atherosclerosis of the donor vessels, technical errors, administration of the monoclonal antibody such as OKT3 (plus high-dose methylprednisolone), antiphospholipid antibodies, high-dose steroids especially as premedication, a too long cold ischemia time, and delayed graft function recovery. A clinical history of renal or extrarenal venous thrombosis, diabetic nephropathy, and peri- and early postoperative poor hemodynamic status is significantly associated with an increased risk of RAT and renal vein occlusions.
Transplant centers should consider prevention strategies in at-risk population such as patients with thrombophilic states or coagulation abnormalities, children, patients with a previous history of vascular thrombosis, and patients who lost a first kidney transplant because of graft thrombosis if not related to technical errors.
7.2 Clinical Manifestations and Pathogenesis
Usually, graft arterial thrombosis is characterized by sudden anuria and graft tenderness and/or severe pain over the graft region. Thrombocytopenia can be caused by platelet accumulation and consumption after thrombus formation. In the suspicion of RAT an urgent investigation is mandatory because it may result in immediate graft loss without a prompt correction. On occasion, segmental infarctions may be completely silent.
7.3 Diagnosis
CDUS has a 100% sensitivity and specificity in the detection of RAT and it typically leads to the complete absence of arterial flow within the kidney, from the point of occlusion along the vessel to distal arterial branches and with the complete absence of venous flow [65]. An occlusion that involves a segmental artery producing a segmental infarction occurs with the absence of arteriovenous flow only in the affected segment. Power Doppler imaging can be an excellent tool in these cases, because of its capacity to identify low-flow vessels, even though sometimes an arteriography proof is required [65]. Renal vascular thrombosis may be confirmed by supplementary imaging modalities including angiography, scintigraphy, CT, and MRI. As in TRAS, CEUS may be applied in the detection of RAT. It may provide an immediate diagnosis, without recourse to other potentially nephrotoxic agents. In addition, with CEUS clinicians can directly obtain additional information not available from other modalities about the microcirculation [38].
8 Renal Vein Thrombosis (TTRV)
8.1 Epidemiology, Clinical Manifestations, and Pathogenesis
Transplanted renal vein thrombosis (TTRV), affecting between 0.4% and 6% of all renal allografts, is an infrequent vascular complication, but it commonly causes early graft loss [66].
Even though in most cases the pathogenesis is undefined, TTRV is frequently the consequence of a technical problem with the vein or the extension of a lower-limb venous occlusion [66]. Similarly, to deep vein thrombosis (TVP), TTRV risk factors are coagulation disorders such as factor V Leiden mutation, metabolic diseases, high hematocrit, renal impairment, nephrotic syndrome, rejection, infections, and calcineurin inhibitor treatment. In this regard, USRDS data reported in transplanted subjects an increased incidence of TVP episodes (2.9 episodes/1000 person-years) compared to normal population [67]. The clinical presentation is comparable to RAT displaying as a sudden drop in urine output combined with graft tenderness and enlargement, swelling, and proteinuria, resulting in an immediate graft dysfunction. It may represent a life-threatening condition leading to a dramatic graft rupture and hemorrhage [68]. Moreover, only a small number of reported cases were successfully treated by surgical procedure or by less invasive intra-arterial injections of antifibrinolytic agents, such as recombinant tissue plasminogen activator or urokinase.
8.2 Diagnosis
8.2.1 Ultrasound-Based Diagnosis (CDUS and CEUS)
CDUS is an easy and accurate technique in TTVR diagnosis. The most common CDUS feature in the presence of complete TTRV is the absence of the venous color signal associated with a scarce or absent vessel’s compressibility. Less frequently, the occlusion may be incomplete [67]. In case of total graft’s vein occlusion, the Doppler trace shows a typical “reverse” diastolic flow, with the wave flow below the baseline and a peculiar bidirectional flow in the parenchymal arteries (Fig. 16.17). The intrarenal arteries’ reverse flow is a 100% specific evidence of TTRV and an immediate indication for sudden surgical transplanted kidney revision regardless of any further diagnostic confirmation.
Renal vein thrombosis. (a) Doppler US of an intrarenal arcuate artery shows reversal of the diastolic flow below the baseline; (b) color Doppler image through the renal hilum shows two renal arteries (A) with no flow in the central renal vein (V) consistent with renal vein thrombosis (courtesy of Prof. Ishrad)
In addition, the color imaging of the hilar region may display a complete lack in venous flow.
Other B-mode combined, but less specific, signs are graft enlargement with augmented parenchymal thickening, decreased echogenicity, and reduced or absent corticomedullary differentiation [67]. An incomplete occlusion and/or graft renal vein stenosis is a very uncommon event. External factors such as perinephric fluid collection (hematomas, lymphocele, urinomas) and perivascular fibrosis may cause renal vein narrowing by compression (Fig. 16.18). The CDUS and B-mode findings are less suggestive in the context of incomplete vein occlusions. Usually, the most accepted diagnostic criterion for a partial TTRV is a 3–4-fold rise in the PSV between the stenotic and prestenotic segments [69, 70]. CEUS has been demonstrated to be an excellent tool in assessing venous patency in renal transplants. It allows quantifying cortical perfusion and microcirculation. Recently, more specific thrombus-targeting microbubble media have also been tested in animal models aiming at improving the efficiency and precision in the detection of thrombi.
8.2.2 Arteriography
Eventually, an angiographic (or other investigations) confirmation may be needed if CDUS findings are highly suggestive of partial and treatable TTRV or when clinical evidences are strongly suspicious of stenosis, despite ambiguous imaging results.
9 Arteriovenous Fistulas and Pseudoaneurysms
9.1 Epidemiology, Clinical Manifestations, and Pathogenesis
AVFs are unusual both native and transplanted kidney vascular complications, accounting for 1–2% of all vascular lesions [71]. They are a well-known kidney biopsy consequence and, for this reason, a certain incidence is inevitable, especially in per-protocol transplanted kidney biopsies. In animal studies, the incidence after biopsy is close to 50%; however, in human angiographic studies the incidence in native kidneys is almost 15–16%. Isolated case of post-ureterorenoscopy lithotripsy and congenital AVFs and infection-related pseudoaneurysms have been described in literature.
During kidney biopsy, the concurrent puncture and damage of an artery and vein walls, producing a vessel junction, may result in an AVF. On the other hand, pseudoaneurysms can develop when the damage is limited to the arterial wall. Both lesions are generally small, asymptomatic, and likely to resolve spontaneously. Even though sometimes AVFs may be more extended, inducing a renal perfusion reduction and leading to graft ischemia, indeed they may be a concerning source of intragraft hemorrhages. Therefore, only large AVFs deserve to be invasively treated.
Pseudoaneurysms are always clinically silent; nevertheless they are at high risk of bleeding; consequently, the treatment is mandatory and not related to the lesion’s size [72]. Selective embolization is the treatment of choice with an 80% rate of good outcome.
9.2 Diagnosis
9.2.1 Ultrasound-Based Diagnosis (CDUS and CEUS)
We can appreciate AVFs at the B-mode examination only when they display a huge extension. On the other hand, they can be steadily visualized at color mode as localized areas with both arterial and venous flow with the characteristic appearance of a color mosaic pattern. Moreover, this feature allows to discriminate high-flow AVFs, presenting an accelerate flow even though increasing the PRF until only the anomalous focus. This maneuver usually produces diagnostic results. Spectral analysis may show increased systo-diastolic flow around the interest area with normal or reduced resistive and pulsatility indexes compared to nearby vessels, whereas Doppler waveform from draining vein shows higher flow velocities and an arterialized pattern occurs in around 30% of all cases (Fig. 16.19).
(a) Color Doppler ultrasound shows large-caliber interlobar artery and vein. The resistance index of the nutrient artery is 0.50 (vs. approximately 0.80 in other regions of the graft). The venous spectrum reflects markedly pulsatile flow that cannot be found in other interlobar veins. Post-biopsy arteriovenous fistula (arrow); (b) Doppler shows alternating flow
Pseudoaneurysms display at B-mode as anechoic circular areas, similarly to cystic lesions, while at the color Doppler analysis, they exhibit a turbulence and “to-and-fro” flow comparable to that seen in pseudoaneurysms involving the arterial anastomosis and distal tract (Figs. 16.20 and 16.21). Even in the suspect of AVFs and pseudoaneurysms, echocontrast exam may be one of the best suitable diagnostic investigations. As previously mentioned, CEUS provides a 30-fold more accurate renal circulation. Furthermore, it may be very useful to detect parenchymal perfusion impairment that leads to kidney function decline in large AVFs. This may help to identify and discriminate AVFs that urgently need to be treated [73]. CEUS seems to be a viable diagnostic option even in pseudoaneurysm characterization and follow-up with high sensibility and specificity [74]. It would appear in few described cases that echocontrast exam improved the identification of the lesion’s margins and, consequently, a more accurate size estimation [75]. Accordingly, CEUS might be used in the monitoring of coil embolization effectiveness; indeed contrast sonography has been demonstrated to be helpful in the study of small bleeding focus, refining the diagnosis of kidney parenchyma hemorrhages.
9.2.2 Arteriography
Arteriography is not justified when AVF is clearly asymptomatic and a prompt treatment is not required. However, when necessary, arteriography transcatheter embolization is a safe and effective minimally invasive technique to treat biopsy-related vascular injuries in renal transplants.
10 Rare Vascular Complications
Torsion of the vascular pedicle of the transplanted kidney is extremely rare. It is caused by intraperitoneal placement of the graft (which occurs during combined renal-pancreatic transplants). Because of its increased mobility, the kidney may rotate around the vascular pedicle, causing vascular occlusion that will lead to parenchymal necrosis and graft loss if not identified promptly. The clinical presentation varies: it may resemble acute rejection or renal vein thrombosis. Ultrasonography can facilitate the diagnosis by documenting a change in the orientation of the kidney, so that the hilum is anterior rather than posterior. Color Doppler ultrasound findings are variable with low diagnostic accuracy. Dissection of the iliac artery and the renal artery are extremely rare events, which are caused by dissection of the aorta and present the same features on CDUS.
Torsion is determined by the rotation of the allograft around its vascular pedicle. This may completely compromise kidney transplant vascular perfusion leading to immediate graft loss if not timely recognized. Data in literature are very scarce and this complication has been described in only 24 cases. It is more frequent in intraperitoneal graft placement, which usually occurs during combined renal-pancreatic transplants [76].
Grafts that are intraperitoneally located are more likely to rotate around the vascular pedicle due to their increased mobility [77].
Similarly, to TTRV the clinical presentation is not specific and symptoms may include nausea, vomiting, abdominal pain, and signs of acute kidney injury such as decreased urine output with sudden kidney function impairment [77]. Renal allograft torsion requires a rapid surgical detorsion to avoid allograft loss. For these reasons, imaging diagnosis plays an essential role in the detection of these events.
10.1 Diagnosis
10.1.1 Ultrasound-Based Diagnosis (CDUS and CEUS)
As the first step, US B-mode evaluation can document a different orientation of the kidney compared to previous exams (the hilum may exhibit an anterior rather than posterior position). CDUS findings are inconsistent and in this setting the diagnostic value is very low. Main features are the following: augmented renal resistive index and high PVS at the main renal artery anastomosis. At the beginning, when the torsion is not complete, it may only result in venous involvement [78].
Then, as the twisting progresses, the arterial implication evolves from a decreased resistive index until the complete lack of blood flow on color Doppler, eventually showing also a reverse diastolic flow. Afterwards, the torsion may include the ureter; consequently the graft may also display enlargement and hydronephrosis at standard B-mode [78].
CEUS, providing a better vascular visualization, may allow to better appreciate the vascular kinking and/or torsion [78]. As in RAT, the presence of ischemia or impending necrosis may be demonstrated by a delayed or absent enhancement of the corticomedullary area and nephrographic phases of contrast [78].
10.1.2 CT and MRI Angiography
The change in axis of the allograft can be more precisely documented on cross-sectional imaging such as CT and MRI, even though obtaining more accurate imaging should not delay surgical treatment, and since patients with pedicle torsion have a severe renal impairment the use of iodinate contrast agents or gadolinium is not recommendable. Recently, iron-based contrast agents have been investigated in transplanted patients [79].
11 Conclusions
Both grayscale B-mode and color Doppler ultrasound are portable inexpensive first-step tools in the management of graft parenchymal and vascular complications. These methods provide a quick differential diagnosis with other causes of graft failure such as surgical complications, hydronephrosis, and early large perinephric collections. CDUS and calculation of the RI (the peak systolic velocity minus the end diastolic velocity divided by the peak systolic velocity) can confirm suspicions of allograft dysfunction and might be a useful tool in biopsy indication and subsequent transplant follow-up.
New advanced techniques such as CEUS might overcome standard CDUS limitations in the next future providing unique functional data in grafts, with promising results in terms of monitoring graft function, optimizing antirejection therapy, and thus minimizing the risk of nephrotoxicity. Additional, well-designed studies are needed to further validate their role in these settings.
References
Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378–83.
O’Neill WC, Baumgarten DA. Ultrasonography in renal transplantation. Am J Kidney Dis. 2002;39:663–78.
Granata A, Di Nicolò PP, Scarfia VR, Insalaco M, Lentini P, Veroux M, Fatuzzo P, Fiorini F. Renal transplantation parenchymal complications: what Doppler ultrasound can and cannot do. J Ultrasound. 2015;18(2):109–16.
Granata A, Clementi S, Clementi A, Di Pietro F, Scarfia VR, Insalaco M, Aucella F, Prencipe M, Figuera M, Fiorini F, Sicurezza E. Parenchymal complications of the transplanted kidney: the role of color-Doppler imaging. G Ital Nefrol. 2012;29(S57):S90–8.
Lockhart ME, Wells CG, Morgan DE, Fineberg NS, Robbin ML. Reversed diastolic flow in the renal transplant: perioperative implications versus transplants older than 1 month. AJR Am J Roentgenol. 2008;190(3):650–5.
Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18(4):1046–56.
Sharfuddin A. Renal relevant radiology: imaging in kidney transplantation. CJASN. 2014;9(2):416–29.
Fischer T, Mehrsai A, Salem S, Ahmadi H, Baradaran N, Tahe-rimahmoudi M, Nikoobakht MR, Rezaeidanesh M, Mansoori D, Pourmand G. Role of resistive index measurement in diagnosis of acute rejection episodes following successful kidney transplantation. Transplant Proc. 2009;41(7):2805–7.
Heine GH, Gerhart MK, Ulrich C, Kohler H, Girndt M. Renal Doppler resistance indices are associated with systemic atherosclerosis in kidney transplant recipients. Kidney Int. 2005;68:878–85.
Naesens M, Heylen L, Lerut E, Claes K, De Wever L, Claus F, et al. Intrarenal resistive index after renal transplantation. N Engl J Med. 2013;369:1797–806.
Dupont PJ, Dooldeniya M, Cook T, Warrens AN. Role of duplex Doppler sonography in diagnosis of acute allograft dysfunction-time to stop measuring the resistive index? Transpl Int. 2003;16:648–52.
Schwenger V, Hinkel UP, Nahm AM, Morath C, Zeier M. Real-time contrast-enhanced sonography in renal transplant recipients. Clin Transplant. 2006;20(Suppl 17):51–4.
Grzelak P, Szymczyk K, Strzelczyk J, Kurnatowska I, Sapieha M, Nowicki M, Stefanczyk L. Perfusion of kidney graft pyramids and cortex in contrast-enhanced ultrasonography in the determination of the cause of delayed graft function. Ann Transplant. 2011;16:48–53.
Girometti R, Stocca T, Serena E, Granata A, Bertolotto M. Impact of contrast-enhanced ultrasound in patients with renal function impairment. World J Radiol. 2017;9(1):10–6.
Graversen ME, Dalgaard LS, Jensen-Fangel S, Jespersen B, Østergaard L, Søgaard OS. Risk and outcome of pyelonephritis among renal transplant recipients. BMC Infect Dis. 2016;16:264.
Brown ED, Chen MYM, Wolfman NT, et al. Complications of renal transplantation: evaluation with US and radionuclide imaging. Radiographics. 2000;20:607–22.
Pelle G, Vimont S, Levy PP, Hertig A, Ouali N, Chassin C, Arlet G, Rondeau E, Vandewalle A. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am J Transplant. 2007;7:899–907.
Granata A, Andrulli S, Fiorini F, Basile A, Logias F, Figuera M, Sicurezza E, Gallieni M, Fiore CE. Diagnosis of acute pyelonephritis by contrast-enhanced ultrasonography in kidney transplant patients. Nephrol Dial Transplant. 2011;26(2):715–20.
Granata A, Floccari F, Insalaco M, Clementi A, Di Lullo L, D'Amelio A, Zamboli P, Logias F, Romano M, Fiorini F. Ultrasound assessment in renal infections. G Ital Nefrol. 2012;29(Suppl 57):S47–57.
McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol. 2012;67(9):909–22.
Fontanilla T, Minaya J, Cortés C, Hernando CG, Arangüena RP, Arriaga J, Carmona MS, Alcolado A. Acute complicated pyelonephritis: contrast-enhanced ultrasound. Abdom Imaging. 2012;37(4):639–46.
Chang CY, Gangji A, Chorneyko K, Kapoor A. Urological manifestations of BK polyomavirus in renal transplant recipients. Can J Urol. 2005;12:2829–36.
Tourret J, Mercadal L, Penna RR, Brocheriou I, Barrou B. Visualizing BK virus nephropathy. NDT Plus. 2010;3(2):185–6.
Dugo M, Mangino M, Meola M, Petrucci I, Valente ML, Laurino L, Stella M, Mastrosimone S, Brunello A, Virgilio B, Rizzolo M, Maresca MC. Ultrasound findings of BK polyomavirus-associated nephropathy in renal transplant patients. J Nephrol. 2017;30(3):449–53.
Allen PJ, Chadban SJ, Craig JC, Lim WH, Allen RDM, Clayton PA, Teixeira-Pinto A, Wong G. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017;92(2):461–9.
Radermarcher J, Mengel M, Ellis S, Stuht S, Hiss M, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115–24.
Di Nicolò P, Granata A. Renal resistive index: not only kidney. Clin Exp Nephrol. 2017;21(3):359–66.
Nankivell BJ, Chapman JR, Gruenewald SM. Detection of chronic allograft nephropathy by quantitative Doppler imaging. Transplantation. 2002;74(1):90–6.
Scholbach T, Girelli E, Scholbach J. Tissue pulsatility index: a new parameter to evaluate renal transplant perfusion. Transplantation. 2006;81:751–5.
Harvey CJ, Sidhu PS. Bachmann Nielsen M contrast-enhanced ultrasound in renal transplants: applications and future directions. Ultraschall Med. 2013;34(4):319–21.
Lee J, Oh YT, Joo DJ, Ma BG, Lee AL, Lee JG, Song SH, Kim SU, Jung DC, Chung YE, Kim YS. Acoustic radiation force impulse measurement in renal transplantation: a prospective, longitudinal study with protocol biopsies. Medicine (Baltimore). 2015;94(39):e1590.
Lentini P, Granata A, Ciancio A, Gallieni M. Regular ultrasound examination of transplanted kidneys allows early diagnosis of renal cell carcinoma and conservative nephron sparing surgery. Arch Ital Urol Androl. 2011;83(4):195–9.
Ghazanfar A, Tavakoli A, Augustine N. Management of transplant artery stenosis and its impact on long-term allograft survival: a single-centre experience. Nephrol Dial Transpl. 2011;26:336–43.
Osman Y, Shokeir A, Ali-el-Dein B, Tantawy M, Wafa EW, et al. Vascular complications after live donor renal transplantation: study of risk factors and effects on graft and patients survival. J Urol. 2003;169:859–62.
Estrada CC, Musani M, Darras F, Suh H, Abate MT, Mani A, Nord EP. 5 years experience with drug eluting and bare metal stents as primary intervention in transplant renal artery stenosis. Transplant Direct. 2017;3(2):e128.
Rodgers SK, Sereni CP, Horrow MM. Ultrasonographic evaluation of the renal transplant. Radiol Clin N Am. 2014;52(6):1307–24.
Granata A, Floccari F, Lentini P, Vittoria S, Di Pietro F, Zamboli P, et al. Vascular complications following kidney transplant: the role of color-Doppler imaging. G Ital Nefrol. 2012;29(S57):S99–S105.
Granata A, Zanoli L, Insalaco M, Valentino M, Pavlica P, Di Nicolò PP, Scuderi M, Fiorini F, Fatuzzo P, Bertolotto M. Contrast-enhanced ultrasound (CEUS) in nephrology: has the time come for its widespread use? Clin Exp Nephrol. 2015;19(4):606–15.
Granata A, Floccari F, Logias F, Insalaco M, Di Lullo L, Andrulli S, Zamboli P, Ricciardi B, Fiorini F. Contrast enhanced ultrasound in renal diseases. G Ital Nefrol. 2012;29(Suppl 57):S25–35.
Weir MR, Salzberg DJ. Management of hypertension in the transplant patient. J Am Soc Hypertens. 2011;5:425–32.
Hurst FP, Abbott KC, Neff RT, et al. Incidence, predictors and outcomes of transplant renal artery stenosis after kidney transplantation: analysis of USRDS. Am J Nephrol. 2009;30(5):459–67.
Pararajasingam R, Riad H, Chalmers, Etemadi J, Rahbar K, Haghighi AN, Bagheri N, Falaknazi K, Ardalan MR, Ghabili K, Shoja MM. Renal artery stenosis in kidney transplants: assessment of the risk factors. Vasc Health Risk Manag. 2011;7:503–7.
Chen W, Kayler LK, Zand MS, Muttana R, Chernyak V, DeBoccardo GO. Transplant renal artery stenosis: clinical manifestations, diagnosis and therapy. Clin Kidney J. 2015;8(1):71–8.
Willicombe M, Sandhu B, Brookes P, Gedroyc W, Hakim N, Hamady M, Hill P, McLean AG, Moser S, Papalois V, Tait P, Wilcock M, Taube D. Postanastomotic transplant renal artery stenosis: association with de novo class II donor-specific antibodies. Am J Transplant. 2014;14(1):133–43.
Weir MR, Burgess ED, Cooper JE, Fenves AZ, Goldsmith D, McKay D, Mehrotra A, Mitsnefes MM, Sica DA, Taler SJ. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015;26(6):1248–60.
Roberts JP, Ascher NL, Fryd DS, et al. Transplant renal artery stenosis. Transplantation. 1989;48:580–3.
Ngo AT, Markar SR, De Lijster MS, Duncan N, Taube D, Hamady MS. A systematic review of outcomes following percutaneous Transluminal angioplasty and stenting in the treatment of transplant renal artery stenosis. Cardiovasc Intervent Radiol. 2015;38(6):1573–88.
Zhang X, Wang H, Liu S, Yan J, Liu X, Xu D, Tian C. Three-Dimensional Computed Tomography Reconstruction in Transplant Renal Artery Stenosis. Exp Clin Transplant. 2017;
Granata A, Fiorini F, Andrulli S, Logias F, Gallieni M, Romano G, Sicurezza E, Fiore CE. Doppler ultrasound and renal artery stenosis: an overview. J Ultrasound. 2009;12(4):133–43.
Erley CM, Duda SH, Wakat JP, et al. Noninvasive procedures for diagnosis of renovascular hypertension in renal transplant recipients—a prospective analysis. Transplantation. 1992;54:863–7.
Robinson KA, Kriegshauser JS, Dahiya N, Young SW, Czaplicki CD, Patel MD. Detection of transplant renal artery stenosis: determining normal velocities at the renal artery anastomosis. Abdom Radiol (NY). 2017;42(1):254–9.
Gao J, Li JC, Xiao MS, Ng A, Trost D, Goldstein M, et al. Color duplex sonography in severe transplant renal artery ste- nosis: a comparison of end-to-end and end-to-side arterial anastomoses. Clin Imaging. 2009;33:116–22.
Siskind E, Lombardi P, Blum M, Tyrell R, Villa M, Kuncewitch M, Olsen EM, Alex A, Lumermann L, Bhaskaran MC, Jhaveri KD, Sachdeva M, Calderon K, Greben C, Putterman D, Gandras E, Caplin D, D’Agostino C, Pellerito J, Coppa G, Molmenti EP. Significance of elevated transplant renal artery velocities in the postoperative renal transplant patient. Clin Transplant. 2013;27:E157–60.
de Morais RH, Muglia VF, Mamere AE, et al. Duplex Doppler sonography of transplant renal artery stenosis. J Clin Ultrasound. 2003;31(3):135–41.
Radermacher J, Mengel M, Ellis S, Stuht S, Hiss M, Schwarz A, Eisenberger U, Burg M, Luft FC, Gwinner W, Haller H. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115–24.
Bruno S, Ferrari S, Remuzzi G, Ruggenenti P. Doppler ultrasonography in posttransplant renal artery stenosis: a reliable tool for assessing effectiveness of revascularization? Transplantation. 2003;76(1):147–53.
Aslam S, Salifu MO, Ghali H, Markell MS, Friedman EA. Common iliac artery stenosis presenting as renal allograft dysfunction in two diabetic recipients. Transplantation. 2001;71:814–7.
Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–40.
Fananapazir G, Bashir MR, Corwin MT, Lamba R, Vu CT, Troppmann C. Comparison of ferumoxytol-enhanced MRA with conventional angiography for assessment of severity of transplant renal artery stenosis. J Magn Reson Imaging. 2017;45(3):779–85.
Sun IO, Hong YA, Kim HG, Park HS, Choi SR, Chung BH, Chun HJ, Choi BS, Park CW, Kim YS, Yang CW. Clinical usefulness of 3-dimensional computerized tomographic renal angiography to detect transplant renal artery stenosis. Transplant Proc. 2012;44(3):691–3.
Gaddikeri S, Mitsumori L, Vaidya S, Hippe DS, Bhargava P, Dighe MK. Comparing the diagnostic accuracy of contrast-enhanced computed tomographic angiography and gadolinium-enhanced magnetic resonance angiography for the assessment of hemodynamically significant transplant renal artery stenosis. Curr Probl Diagn Radiol. 2014;43(4):162–8.
Spinosa DJ, Isaacs RB, Matsumoto AH, Angle JF, Hagspiel KD, Leung DA. Angiographic evaluation and treatment of transplant renal artery stenosis. Curr Opin Urol. 2001;11:197–205.
Wu CK, Leu JG, Wei CC, Hsieh SC. Acute thrombosis of a transplanted renal artery after gastric ulcer bleeding in a patient with a long-term well-functioning renal allograft: a case report and literature review. Medicine (Baltimore). 2016;95(30):e4301.
McDonald RA, Smith JM, Stablein D, Harmon WE. Pretransplant peritoneal dialysis and graft thrombosis following pediatric kidney transplantation: a NAPRTCS report. Pediatr Transplant. 2003;7:204.
Cosgrove DO, Chan KE. Renal transplants: what ultrasound can and cannot do. Ultrasound Q. 2008;24(2):77–87.
Ponticelli C, Moia M, Montagnino G. Renal allograft thrombosis. Nephrol Dial Transplant. 2009;24(5):1388–93.
Drudi FM, Cascone F, Pretagostini R, Ricci P, Trippa F, Righi A, et al. Role of color Doppler US in the evaluation of renal transplant. Radiol Med. 2001;101:243–50.
Giustacchini P, Pisanti F, Citterio F, et al. Renal vein thrombosis after renal transplantation: an important cause of graft loss. Transplant Proc. 2002;34:2126–7.
Schwenger V, Hinkel UP, Nahm AM, et al. Color doppler ultrasonography in the diagnostic evaluation of renal allografts. Nephron Clin Pract. 2006;104:c107–12.
Gao J, Ng A, Shih G, et al. Intrarenal color duplex ultrasonography: a window to vascular complications of renal transplants, J Ultrasound Med , 2007, 26 (pg. 1403–1418).
Middleton WD, Kellman GM, Melson GL, Madrazo BL. Postbiopsy renal transplant arteriovenous fistulas: color Doppler US characteristics. Radiology. 1989;171(1):253–7.
Antonopoulos IM, Yamacake KG, Tiseo BC, Carnevale FC, Z E Jr, Nahas WC. Renal pseudoaneurysm after core-needle biopsy of renal allograft successfully managed with superselective embolization. Int Braz J Urol. 2016;42(1):165–7.
Siracusano S, Bertolotto M, Ciciliato S, Valentino M, Liguori G, Visalli F. The current role of contrast-enhanced ultrasound (CEUS) imaging in the evaluation of renal pathology. World J Urol. 2011;29(5):633–8.
Durkin N, Deganello A, Sellars ME, Sidhu PS, Davenport M, Makin E. Post-traumatic liver and splenic pseudoaneurysms in children: diagnosis, management, and follow-up screening using contrast enhanced ultrasound (CEUS). J Pediatr Surg. 2016;51(2):289–92.
Helck A, Hoffmann RT, Sommer WH, Notohamiprodjo M, Albyaa H, Reiser M, Clevert DA. Diagnosis, therapy monitoring and follow up of renal artery pseudoaneurysm with contrast-enhanced ultrasound in three cases. Clin Hemorheol Microcirc. 2010;46(2–3):127–37.
Ozmen M, Bilgic I, Ziraman I, Koc M. Torsion of extraperitoneally transplanted kidney: an unusual complication. Exp Clin Transplant. 2013;11(2):186–90.
Sofue K, Vikraman DS, Jaffe TA, Chaubal GN, Bashir MR. Graft kidney torsion after simultaneous kidney-pancreas transplant: report of 2 cases and literature review. J Comput Assist Tomogr. 2015;39(4):506–9.
Dewan R, Dasyam AK, Tan H, Furlan A. Renal Allograft Torsion: US and CT Imaging Findings of a Rare Posttransplant Complication. Case Rep Radiol. 2016;2016:4273780.
Sebastià C, Quiroga S, Boyé R, Cantarell C, Fernandez-Planas M, Alvarez A. Helical CT in renal transplantation: normal findings and early and late complications. Radiographics. 2001;21(5):1103–17.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dugo, M., Maccarrone, R., Campo, I., Pesce, F., Di Lullo, L., Granata, A. (2021). Transplanted Kidney. In: Granata, A., Bertolotto, M. (eds) Imaging in Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-030-60794-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-60794-4_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-60792-0
Online ISBN: 978-3-030-60794-4
eBook Packages: MedicineMedicine (R0)