Abstract
Cardiac hypertrophy is an adaptive response of the heart to hemodynamic overload that is initially designed to maintain cardiac functioning, but prolonged hypertrophy becomes detrimental and results in cardiac dysfunction and heart failure (HF). Population studies have indicated that men and women are different in their risk and etiology in developing HF. In fact, female sex hormones are believed to have a cardioprotective role during premenopause. Although cardiac remodeling in experimental volume overload has also been shown to occur in males, there is still a paucity of information regarding sex differences in the pattern of ventricular remodeling and contractile function in response to volume overload. Therefore, this article discusses the sex differences in changes of ventricular dimensions and contractile function in cardiac hypertrophy and HF due to volume overload. Furthermore, it describes cardiomyocyte Ca2+-handling properties that could potentially contribute to sex differences in cardiac remodeling and contractile function. The influence of sex hormones that are present during these processes is also highlighted. Since sex differences in the development of cardiac hypertrophy have been reported to occur in pressure overload and myocardial infarction, some of these changes are also described for a comparative perspective. Understanding the sex differences in the pathophysiology of heart disease may form the foundations for the development of new approaches for sex-specific treatment and prevention of HF.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Globally cardiovascular disease (CVD) is the leading cause of death, however; significant differences exist between males and females in the development of CVD [1,2,3,4,5,6,7,8,9,10,11,12]. Premenopausal women have a reduced risk of CVD, in fact, the incidence and severity of CVD increases markedly after menopause [13]. Women tend to develop heart disease 10–15 years later than men, pointing to the existence of specialized mechanisms in females that attenuate their risk of developing heart disease [14]. Females have also been reported to be less susceptible to ischemic insult and have improved survival following myocardial ischemic-reperfusion injury [15, 16].
The incidence of heart failure (HF) due to elevated blood pressure and diabetes is greater in women than in men [17]. However, as compared to men, the development of HF in women is more benign and is predominantly characterized by preserved systolic function and diastolic dysfunction [17]. Interestingly, older women tend to be more hypertensive and are less likely to present with coronary heart disease [18]. Postmenopausal women with diabetes are at a 3- to 6-fold higher risk of a myocardial infarction (MI), in comparison to a 2- to 4-fold risk of MI in men [19]. Women seem to be better protected and show a later onset of cardiac decompensation than men with HF [20]. In terms of survival in older adults with HF, women fare significantly better than men [21]. It is interesting to note that since 1984, more women die of HF each year, and yet more men are diagnosed with HF [22].
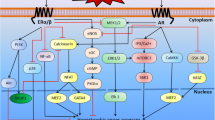
In women, an increased left ventricle (LV) mass or degree of hypertrophy has been suggested to be a stronger predictor of mortality than traditional measures of LVsize and function [23]. Thus, changes in the size, shape, structure, and function (so-called ventricular remodeling or cardiac remodeling) of the heart, may occur in a sex-dependent manner. Accordingly, it is planned to discuss sex differences in the changes in LVdimensions and contractile function in cardiac hypertrophy and heart failure due to volume overload as well as to describe the potential of cardiomyocyte Ca2+-handling properties that may contribute to sex differences in cardiac remodeling and contractile function (Fig. 6.1). Also, the role of sex hormones in these processes will be highlighted. Reference to cardiac remodeling and cardiac function in response to pressure overload and MI will also be made for the purpose of comparison.
General Characteristics of Cardiac Remodeling and Function in Hypertrophy and Heart Failure
Geometrical and structural alterations in the heart are the hallmarks of cardiac remodeling. Such cardiac changes occur in a range of different cardiac pathophysiological conditions including hypertrophic cardiomyopathy, dilated cardiomyopathy, diabetic cardiomyopathy, ischemic cardiomyopathy as well as hypertensive cardiomyopathy, which can progressively lead to HF. With an excessive workload or sustained hemodynamic overload, ventricular myocytes grow in response to a complex cascade of events [24] such as mechanical stress, ischemia, and the activation of multiple intracellular signaling pathways by neurohormones. In the initial stage, cardiac hypertrophy may be seen as a beneficial response to normalize LV wall stress and preserve normal cardiac contractile function, whereas the prolongation of hypertrophy leads to HF and sudden death [25,26,27]. This transition of cardiac hypertrophy to HF is generated by the occurrence of marked ventricular dilatation once the myocardial hypertrophic response is exhausted [27]. The progressive deterioration of LV function is an established characteristic of HF in men; however, it appears that a different pattern of ventricular remodeling occurs in women, which is designed to preserve cardiac contractility.
Sex Differences in Cardiac Remodeling in Hypertrophy and Heart Failure Due to Volume Overload
Experimental and clinical studies have revealed sex differences in myocardial remodeling in aging, pressure overload, volume overload and MI. The outcome of the remodeling processes are that women more often present HF with preserved systolic function, but exhibit an increased risk for acute low output syndrome [28]. Experimental studies have also shown that males experience 10-times higher mortality due to MI than females [14]. Female rats showed a different pattern of LV remodeling than males with less increase in the thickness of the noninfarcted portions of LV than males, but comparable LV cavity enlargement and systolic dysfunction due to MI [17, 18]. Although, we have earlier reviewed the mechanisms of sex differences in cardiac dysfunction due to heart disease [29,30,31], there is still a relative paucity of information regarding cardiac remodeling in hypertrophy and heart failure due to volume overload. Some studies have been conducted to examine differences in male and female rats during both the hypertrophic and failing stages following induction of volume overload. Gardner et al. [32] have identified that after 8 wks post AV fistula, mortality was tenfold less in female rats as compared to male rats. Although females had increased LV and right ventricle (RV) weights, these animals showed no signs of CHF whereas the males had increased lung weight, marked ventricular dilatation and increased compliance thus indicating the presence of HF. It should be pointed out that this study was carried out at 8 wks post fistula and the LV volume and function studies were carried out under in vitro conditions.
We have also observed sex differences in the cardiac remodeling process in volume overload induced by an AV-shunt [33]. Both males and females developed significant cardiac hypertrophy. Furthermore, echocardiographic data revealed increases in both diastolic and systolic left ventricle internal diameters (LVID) in male rats, whereas females showed no significant change in these parameters. It seems that the principle difference between males and females at this time point is that females show an increase in LV posterior wall thickness (PWT) indicating that concentric hypertrophic remodeling has taken place (Fig. 6.2). On the other hand, in males it appears that eccentric cardiac hypertrophy leading to ventricular dilatation occurs. Our study also examined remodeling at 16 wks post-AV shunt. In this regard, significant cardiac hypertrophy was seen to remain 16 wks post AV shunt in both males and female rats (Fig. 6.3). Such sex differences in cardiac remodeling have been shown in other models of heart failure, including pressure overload [34], MI [35], and spontaneously hypertension [36]. For example, it was shown that following pressure overload; males were unable to maintain concentric hypertrophy and transitioned to HF much faster than their female counterparts who were able to maintain a concentrically remodeled heart, therefore delaying the transition to HF [34]. It is interesting to note that in aortic stenosis, women tolerate pressure overload with less concentric remodeling and myocardial fibrosis, but are more likely to develop symptoms, which may be related to higher wall stress and filling pressures in women [37]. Experimentally, female rats also demonstrated a different pattern of LV remodeling than males following MI, the LV noninfarcted region was not as thick in females as it was in male animals. Although both males and females exhibited similar infarct size, females were able to preserve LVdiastolic filling capacity [35].

Data are taken from our paper—Dent MR, Tappia PS, Dhalla NS. J Card Fail 16: 439–449, 2010. Erratum in: J Card Fail. 17:179, 2011 [33]. LVIDs, systolic left ventricular internal diameter; LVIDd, diastolic left ventricular internal diameter; PWTs, systolic posterior wall thickness; PWTd, diastolic posterior wall thickness; *P < 0.05 versus sham values
Echocardiographic analysis of male and female rats 4 weeks post-AV shunt for inducing cardiac hypertrophy due to volume overload.

Data are taken from our paper—Dent MR, Tappia PS, Dhalla NS. J Card Fail 16: 439–449, 2010. Erratum in: J Card Fail. 17:179, 2011 [33]. LVIDs, systolic left ventricular internal diameter; LVIDd, diastolic left ventricular internal diameter; PWTs, systolic posterior wall thickness; PWTd, diastolic posterior wall thickness; *P < 0.05 versus sham values.
Echocardiographic analysis of female and male sham and arteriovenous shunt groups at 16 weeks.
It appears that the major sex difference that occurs in many of models of HF is that females are able to develop cardiac hypertrophy in such a way that they maintained cardiac function and delay the transition to HF. It has been suggested by Grossman et al. [38] that increased LVEDP is the trigger for developing eccentric hypertrophy or inappropriate hypertrophy that is unable to maintain cardiac function. However, females subjected to volume overload were able to increase LV wall thickness, thereby developing concentric hypertrophy to compensate for the increased workload and maintain relatively normal cardiac function.
The development of cardiac hypertrophy in response to volume overload subsequent to chronic aortic valve regurgitation (AVR) in both male and female rats has also been evaluated [39]. In this regard, AVR-inducd volume overload resulted in comparable increases in LV dilation and heart weight mass in both male and female rats, as compared to respective sham controls. However, the relative LV wall thickness as measured by the ratio between wall thickness to end-diastolic diameter, was diminished in male rats, but remained unchanged in females as compared to respective sham controls. Treatment of the experimental animals with the angiotensin II receptor blocker (ARB), valsartan, was observed to reverse LV thickening in female rats only, with no effect on LV dilation in both sexes. In addition, valsartan treatment normalized left atrial mass and E-wave slope, but only in female AVR rats [39].
Earlier we have reported differences in basal hemodynamic parameters between male and female rats [33]. In this regard, it was observed that both the rate of contraction (+dP/dt) and rate of relaxation (-dP/dt) were significantly lower in the female control rats as compared to the male counterparts at 4 (Fig. 6.4) and 16 wks (Fig. 6.5) time points, except –dP/dt at 4 wks. While LVEDP was seen to be higher at 4 wks, no changes in baseline left ventricular systolic pressure (LVSP) and left ventricular end-diastolic pressure (LVEDP) values were seen at 16 wks in both female and male rats (Figs. 6.4 and 6.5). On the other hand, hemodynamic assessment at the same time points i.e. at 4 and 16 wks post-AV shunt revealed that LVEDP, unlike other parameters, was significantly increased only in males at 4 wks post-AV shunt (Fig. 6.4). However, at 16 wks post-AV shunt, cardiac function was significantly depressed in males as evidenced by decreases in + dP/dt and -dP/dt as well as a significant increase in LVEDP; these parameters were unaltered in the 16 wk post-AV shunt female group (Fig. 6.5). No significant differences in heart rate (beats/min) were observed in both male and female rats at 4 wks and 16 wks post-AV shunt as compared to respective sham control values. In this study, an echocardiographic assessment of normal and post-AV male and female hearts was also conducted. In this regard, at 4 wks post-AV shunt (hypertrophy stage), an increase in the cardiac output, but decrease in the fractional shortening were seen in male rats (Fig. 6.4). At 16 wks following AV shunt (HF stage), while cardiac output was increased, a decrease in fractional shortening were seen in male rats. On the other hand, a small, but significant increase in the cardiac output, was observed at 16 wks post-AV shunt, but no changes occurred in fractional shortening (Fig. 6.5).

Values are taken from our paper—Dent MR, Tappia PS, Dhalla NS. J Card Fail 16: 439–449, 2010. Erratum in: J Card Fail. 17:179, 2011 [33]. Sham, age-matched controls; + dP/dt, rate of contraction; -dP/dt, rate of relaxation. LVEDP, LVend-diastolic pressure; LVSP, LVsystolic pressure. * P < 0.05 versus sham-operated values
Hemodynamic changes in male and female rats at 4 weeks post AV shunt.

Values are taken from our paper—Dent MR, Tappia PS, Dhalla NS. J Card Fail 16: 439–449, 2010. Erratum in: J Card Fail. 17:179, 2011 [33]. Sham, age-matched controls; + dp/dt, rate of contraction; -dP/dt, rate of relaxation. LVEDP, LVend-diastolic pressure; LVSP, LVsystolic pressure. * P < 0.05 versus sham-operated control values
Hemodynamic changes in male and female rats at 16 weeks post AV shunt.
Role of Estrogen in Cardiac Hypertrophy and Heart Failure
Since there is an elevated risk of CVD in postmenopausal women, the role of female sex hormones has generally been considered to be a major factor in providing protection against CVD in premenopausal women. Observations from several investigations such as Women's Health Initiative Studies, Cochrane Review Studies, Early Versus Late Intervention Trial with Estradiol Study, and Kronos Early Estrogen Prevention Study have indicated that hormone replacement therapy (HRT) may exert some beneficial action in women. However, it was also observed that the beneficial action occurs in those women that were under 60 yrs of age, and if HRT was started within 10 years of menopause. On the other hand, there was no benefit to be found in women who were of more than 60 years, and in particular if their HRT was initiated at more than 10 years of menopause [40].
Estrogen has been thought to have a cardioprotective effect. However, the recent outcomes of the Heart and Estrogen Replacement Study (HERS) and The WHI have indicated that estrogen or a combination estrogen-progestin therapy increased the coronary heart disease events and therefore physicians were advised to use hormone replacement therapy strictly for menopausal symptom relief [41]. Another publication reported on the CV data from two arms of WHI and concluded that the age of initiation of therapy may play a role on the effect on coronary heart disease outcomes. It appears that younger women who are closer to menopause have a lower event rate than women where therapy was initiated over the age of 60 [42]. In different rat models of CHF estrogen has been suggested to play a major role in the underlying mechanisms for the gender differences in the cardiac remodeling process. For example, in the in vitro study by Brower et al. [43] it was found that ovariectomy lead to the development of eccentric hypertrophy and low contractility measurements as opposed to concentric hypertrophy and maintained contractility in the control females. In addition, the prevention of adverse cardiac remodeling in volume overload induced by aortocaval fistula in the female rat has been suggested to be the result of an estrogen-altered mast cell phenotype and/or prevention of mast cell activation [44].
Subsequent studies conducted by this group showed that the administration of phytoestrogens added to the female advantage against volume overload [45] and the estrogen treatment prevented pulmonary edema and clearly attenuated LV hypertrophy and dilatation but did not maintain contractility [46]. In contrast, Drolet et al. [47], observed that in an aortic regurgitation model, female rats had increased cardiac remodeling than males, which imposed a greater workload on their hearts; it was concluded that hormone status did not have any implication on the remodeling process [47]. Following MI, it was shown that 17β-estradiol increased LV and cardiomyocyte hypertrophy [48] and prevented deterioration of cardiac function [49]. In contrast, 17β-estradiol attenuated hypertrophy following pressure overload [48, 50]. These authors concluded that the effect of estrogen on cardiac hypertrophy might be dependent on the initial stimulus [49]. Interestingly, we have shown that the administration of estrogen in ovariectomized female rats following the induction of volume overload induces a higher degree of hypertrophy than that seen in the intact control female rats. Echocardiographic analysis has also revealed that the treatment of the AV shunt ovariectomized female rat with estrogen results in attenuation in the ventricular dilatation, as well as increased the wall thickness. These findings indicate that estrogen may be implicated in the signaling mechanism that initiate concentric hypertrophy as opposed to inappropriate eccentric hypertrophy.
To investigate the role of estrogen in cardiac function in heart failure, we have earlier conducted experiments in sham-operated as well as ovariectomized animals with or without 17-β estradiol treatment. Figure 6.6 shows that in contrast to intact female rats, AV shunt produced significant depressions in + dP/dt, -dP/dt and LVSP and a marked increase in LVEDP in ovariectomized rats; these alterations were either fully or partially prevented by estrogen treatment. Although ovariectomy reduced LVEDP, unlike +dP/dt, -dP/dt and LVSP, this change was not affected by estrogen treatment (Fig. 6.6). Furthermore, echocardiographic assessment of cardiac performance of females and ovariectomized female rats treated with or without estrogen, revealed a preservation of fractional shortening and cardiac output subsequent to induction of volume overload by an AV shunt [33]. Since cardiac function as represented by ±dP/dt was maintained and a partial attenuation of the increase in the LVEDP was observed upon administration of estrogen to ovariectomized animals, estrogen likely plays an important role in the cardiac remodeling observed in females subjected to volume overload. Indeed, alterations in intraventricular dimensions of intact female rats and ovariectomized females treated with or without estrogen, in sham and AV animals can be prevented by estrogen treatment [33].

Data are taken from our paper—Dent MR, Tappia PS, Dhalla NS. J Card Fail 16: 439–449, 2010. Erratum in: J Card Fail. 17:179, 2011 [33]. Sham, age-matched controls; + dP/dt, rate of contraction; -dP/dt, rate of relaxation. LVEDP, LVend-diastolic pressure; LVSP, LVsystolic pressure; OVX, ovariectomized. * P < 0.05 versus sham-operated control values
Hemodynamic changes in female, ovariectomized treated with or without estrogen rats at 16 weeks post AV shunt.
Interestingly, sex differences in LV function at different stages of LV hypertrophy due to pressure overload induced by the banding of the abdominal aorta have also been reported [51]. In early stage of LV hypertrophy, increased LV mass index, heart weight-to-tibial length, cardiomyocyte diameter, concentric LV geometry, and moderate interstitial fibrosis were detected in both male and female rats subjected to aortic banding. These changes were associated with impaired relaxation, increased contractility, and preserved ventricular-arterial coupling in both sexes. However, the late stage was associated with eccentric remodeling, increased fibrosis, and enhanced chamber stiffness in male rats only. Furthermore, augmented contractility declined in male rats, but not in the female animals. It was concluded that contractile augmentation, preserved ventricular-arterial coupling, and improved myocardial compliance in female rats contribute to sex differences in LV function during the progression of pressure overload-induced LV hypertrophy [51].
Sex Differences in Cardiomyocyte Ca2+-handling Proteins
Contractile abnormalities in HF have been linked to ventricular remodeling and progressive defects in cardiomyocyte Ca2+-handling as well as changes in myofibrillar sensitivity to Ca2+ [52,53,54,55,56]. Sex differences have been reported to exist in cardiac contractility [57], as well as the inotropic responses to Ca2+ [58], myocardial Ca2+-channel density [59] and the cardiac response to adrenergic stimulation [57, 60]. Thus, it has been postulated that sex hormones may have a role to play for changes in cardiac remodeling in males and females under a variety of different cardiac pathologies including volume overload and pressure overload [33, 45, 61,62,63,64]. Since the sarcoplasmic reticulum (SR) and sarcolemma (SL) are intimately involved in the Ca2+ handling and cardiac contractility, differences in abnormalities in SR and SL functions would be seen to contribute to sex differences in the development of cardiac dysfunction. Furthermore, alterations in the sensitivity of myofibrils (MF) to Ca2+ in male and female hearts would also be a factor in determining the sex difference.
The functionality of sex hormones has been examined in ovariectomized female rats as well as castrated male rats. Treatment of the ovariectomized female rats with estrogen eliminated defective cardiac function as well as alterations in SR Ca2+-pump, Ca2+-release channels (RyR2), and SL Na+-Ca2+-exchange activities [65, 66]. While ovariectomy in female rats and castration in male rats have both been reported to produce a shift in myosin enzymes and decrease cardiac contractile function, treatment with estrogen and testosterone was demonstrated to attenuate these changes [67]. It is also pointed out that castration in male rats has also been reported to depress SL Na+-Ca2+-exchanger and L-type Ca2+-channels mRNA levels, with partial normalization upon testosterone treatment [68,69,70].
It should be noted that 17-β estradiol and estrogen receptors have a sex-specific role in mitochondrial function and Ca2+ ion channel activity in the heart [71] as well as for sustaining cardiac contractile function. These observations provide further evidence that sex hormones are an important factor in determining differences in the regulation of cardiac function in male and females. The relation between sex and contractile properties at the actin-myosin level in patients with chronic volume overload due to mitral regurgitation (leakage of blood backwards through the mitral valve during each contraction from LV) has recently been examined [72]. This study demonstrated that female fibers from patients exposed to chronic volume overload developed higher force values at a given Ca2+ concentration compared to fibers from male patients. The Ca2+ sensitivity among the male and female patients was significantly different, and it was suggested that males have higher Ca2+ sensitivity and might compensate for lower force values at maximal Ca2+ concentrations by a higher affinity for this cation. Thus, female patients with mitral regurgitation would appear to work in a more energy efficient manner [72].
It should be mentioned that MI represents the major form of HF and a large amount of the experimental data have been obtained by employing male hearts. Consequently, the effects of female sex hormones in modifying MI-induced changes in Ca2+-cycling proteins need to be examined in females with and without ovariectomy. On the other hand, the deleterious effects of testosterone and castration in the MI-induced changes in Ca2+-cycling proteins should also be examined. It is possible that a divergent pattern of sex differences in the Ca2+-cycling proteins may exist depending on the etiology of HF. In this regard, some of our work on MI-induced HF has indicated that changes in the SR Ca2+-cycling proteins are more marked as compared to the changes at the level of the SL and myofibrils.
It is interesting to note that temporal sex differences in Ca2+-signaling have been reported in pressure overload-induced LV hypertrophy [73]. An initial hypertrophy of the LV due to pressure overload-induced by thoracic aortic constriction was seen in female mice and was associated with a concomitant down-regulation of SERCA2a, CaMKII activation, and GSK3β inactivation. Although both male and females showed systolic dysfunction, which may be associated with the down-regulation of RyR2, only males exhibited preserved diastolic LV function. Thus, HF caused by different etiology may exert different types of changes for Ca2+-handling in a sex-dependent manner. These possibilities, in volume overload-induced cardiac hypertrophy and heart failure, therefore warrant further investigation. In addition, since MI is the most prevalent cause of HF when compared to other etiologies such as pressure overload, volume overload, valvular defects and other type of cardiomyopathies [74,75,76], sex-differences in MI-induced HF should also be further examined.
Conclusions
It is evident that echocardiographic and hemodynamic studies have revealed significant sex differences in cardiac remodeling and contractile function due to volume overload. Females undergo concentric remodeling and maintain an appropriate level of cardiac hypertrophy that is sufficient to delay the transition to HF. In contrast, males develop eccentric hypertrophy and transition to HF. Estrogen plays a key role in cardiac remodeling following the induction of volume overload. While the mechanisms involved in sex differences in cardiac remodeling and contractile function are still a matter for extensive research, understanding sex differences in the pathophysiology of heart disease may help in developing novel sex-specific interventions for the management and prevention of HF.
References
Zhang Y, Liu B, Zhao R et al (2020) The influence of sex on cardiac physiology and cardiovascular diseases. J Cardiovasc Transl Res 13:3–13
Lundberg G, Walsh MN, Mehta LS (2019) Sex specific differences in risk factors for development of heart failure in women. Heart Fail Clin 15:1–8
Mentzer G, Hsich EM (2019) Heart failure with reduced ejection fraction in women: epidemiology, outcomes, and treatment. Heart Fail Clin 15:19–27
Madan N, Itchhaporia D, Albert CM et al (2019) Atrial fibrillation and heart failure in women. Heart Fail Clin 15:55–64
Kessler EL, Rivaud MR, Vos MA, van Veen TAB (2019) Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol Sex Differ 10:7
Stanhewicz AE, Wenner MM, Stachenfeld NS (2018) Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315:H1569–H1588
Marra AM, Salzano A, Arcopinto M et al (2018) The impact of gender in cardiovascular medicine: lessons from the gender/sex-issue in heart failure. Monaldi Arch Chest Dis 88:998
Cauwenberghs N, Kuznetsova T (2018) Sex-specific differences in cardiac maladaptation to hypertension and arterial stiffening. Kardiol Pol 76:1303–1311
Kerkhof PLM, Peace RA, Macfarlane PW (2018) Sex- and Age-related reference values in cardiology, with annotations and guidelines for interpretation. Adv Exp Med Biol 1065:677–706
Savarese G, D’Amario (2018) Sex differences in heart failure. Adv Exp Med Biol 1065:529–544
Shufelt CL, Pacheco C, Tweet MS, Miller VM (2018) Sex-specific physiology and cardiovascular disease. Adv Exp Med Biol 1065:433–454
Kerkhof PLM, Peace RA, Heyndrickx GR et al (2018) Heart function analysis in cardiac patients with focus on sex-specific aspects. Adv Exp Med Biol 1065:361–377
Schonfelder G (2005) The biological impact of estrogens on gender differences in congestive heart failure. Cardiovasc Res 67:573–574
Rossouw JE (2002) Hormones, genetic factors, and gender differences in cardiovascular disease. Cardiovasc Res 53:550–557
Bae S, Zhang L (2005) Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther 315:1125–1135
Wang M, Wang Y, Weil B et al (2009) Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol 296:R972–R978
Stromberg A, Martensson J (2003) Gender differences in patients with heart failure. Eur J Cardiovasc Nurs 2:7–18
Rosengren A, Hauptman P (2008) Women, men and heart failure: a review. Heart Fail Monit 6:34–40
Regitz-Zagrosek V, Lehmkuhl E (2005) Heart failure and its treatment in women: role of hypertension, diabetes and estrogen. Herz 30:356–367
Guerra S, Leri A, Wang X et al (1999) Myocyte death in the failing human heart is gender dependent. Circ Res 85:856–866
Parashar S, Katz R, Smith NL et al (2009) Race, gender, and mortality in adults > or = 65 years of age with incident heart failure (from the Cardiovascular Health Study). Am J Cardiol 103:1120–1127
Sobhani K, Nieves Castro DK, Fu Q et al (2018) Sex differences in ischemic heart disease and heart failure biomarkers. Biol Sex Differ 9:43
Mejhert M, Kahan T, Edner M, Persson HE (2008) Sex differences in systolic heart failure in the elderly: the prognostic importance of left ventricular mass in women. J Womens Health 17:373–381
Eizema K, Van Heugton H, Bezstarosti K et al (2000) SERCA2 and ANF promoter-activity studies in hypertrophic cardiomyocytes using liposome-, gene gun-, and adenovirus-mediated gene transfer. In: Takeda N, Nagano M, Dhalla NS (eds) The hypertrophied heart. Kluwer Academic Publishers, Boston, pp 52–66
Sakurai K, Sugawara H, Watanabe T, et al (2000) Ca2+ transients, contractility, and inotropic responses in rabbit volume-overloaded cardiomyocytes. In: Takeda N, Nagano M, Dhalla NS (eds) The hypertrophied heart. Kluwer Academic Publishers, Boston, pp 67–81
Van Bilsen M, Chien KR (1993) Growth and hypertrophy of the heart: towards an understanding of cardiac specific and inducible gene expression. Cardiovasc Res 27:1140–1149
Takano H, Zou Y, Akazawa H et al (2003) Ca2+-dependent signaling pathways through calcineurin and Ca2+/calmodulin-dependent protein kinase in development of cardiac hypertrophy. In: Dhalla NS, Hryshko L, Kardami E, Singal PK (eds) Signal transduction and cardiac hypertrophy. Kluwer Academic Publishers, Boston, pp 85–103
Piro M, della Bona R, Abbate A et al (2010) Sex-related differences in myocardial remodeling. J Am Coll Cardiol 55:1057–1065
Dhalla NS, Dent MR, Elimban V, Zieroth S (2006) Mechanisms of gender differences in cardiac dysfunction due to heart disease. In: Kimchi A (ed) Proceedings of the16th world congress on heart disease. International Academy of Cardiology, Monduzzi Editore S.p.A-Medimond Inc. Bologna, pp 239–245
Dent MR, Elimban V, Malik A et al (2013) Functional adaptation during the development of cardiac hypertrophy and heart failure in females. In: Ostadal B, Dhalla NS (eds) Cardiac adaptation: molecular mechanisms. Springer Science+Business Media, LLC, New York, pp 201–212
Dhalla NS, Malik A, Zieroth S, Tappia PS (2013) Role of gender in Ca2+ cycling and cardiac remodeling due to heart failure. In: Jugdutt BI, Dhalla NS (eds) Cardiac remodeling - molecular mechanisms. Springer Science+Business Media, LLC, New York
Gardner JD, Brower GL, Janicki JS (2002) Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail 8:101–107
Dent MR, Tappia PS, Dhalla NS (2010) Gender Differences in cardiac dysfunction and remodeling due to volume overload. J Card Fail 16: 439–449; Erratum in: J Card Fail. 17:179, 2011
Douglas PS, Katz SE, Weinberg EO et al (1998) Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol 32:1118–1125
Litwin SE, Katz SE, Litwin CM et al (1999) Gender differences in postinfarction left ventricular remodeling. Cardiology 91:173–183
Tamura T, Said S, Gerdes AM (1999) Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension 33:676–680
Singh A, Chan DCS, Greenwood JP et al (2019) Symptom onset in aortic stenosis: relation to sex differences in left ventricular remodeling. JACC Cardiovasc Imaging 12:96–105
Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in human left ventricle. J Clin Invest 56:56–64
Walsh-Wilkinson E, Drolet MC, Le Houillier C et al (2019) Sex differences in the response to angiotensin II receptor blockade in a rat model of eccentric cardiac hypertrophy. PeerJ 7:e7461
Pabbidi MR, Kuppusamy M, Didion SP et al (2018) Sex differences in the vascular function and related mechanisms: role of 17β-estradiol. Am J Physiol Heart Circ Physiol 315:H1499–H1518
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Rossouw JE, Prentice RL, Manson JE et al (2007) Postemenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477
Brower GL, Gardner JD, Janicki JS (2003) Gender mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol Cell Biochem 251:89–95
Lu H, Meléndez GC, Levick SP, Janicki JS (2012) Prevention of adverse cardiac remodeling to volume overload in female rats is the result of an estrogen-altered mast cell phenotype. Am J Physiol Heart Circ Physiol 302:H811–H817
Gardner JD, Brower GL, Janicki JS (2005) Effects of dietary phytoestrogens on cardiac remodeling secondary to chronic volume overload in female rats. J Appl Physiol 99:1378–1383
Gardner JD, Brower GL, Voloshenyuk TG, Janicki JS (2008) Cardioprotection in female rats subjected to volume overload: synergistic interaction of estrogen and phytoestrogens. Am J Physiol Heart Circ Physiol 294:H198–H204
Drolet MC, Lachance D, Plante E et al (2006) Gender-related differences in left ventricular remodeling in chronic severe aortic valve regurgitation in rats. J Heart Valve Dis 15:345–351
Patten RD, Pourati I, Aronovitz MJ et al (2008) 17 Beta-estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail 14:245–253
Cavasin MA, Sankey SS, Yu AL et al (2003) Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol 284:H1560–H1569
Van Eickels M, Grohe C, Cleutjens JP et al (2001) 17β-estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104:1419–1423
Ruppert M, Korkmaz-icöz S, Loganathan S et al (2018) Pressure-volume analysis reveals characteristic sex-related differences in cardiac function in a rat model of aortic banding-induced myocardial hypertrophy. Am J Physiol Heart Circ Physiol 315:H502–H511
Dhalla NS, Das PK, Sharma GP (1978) Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol 10:363–385
Dhalla NS, Afzal N, Beamish RE et al (1993) Pathophysiology of cardiac dysfunction in congestive heart failure. Can J Cardiol 9:873–887
Dhalla NS, Liu X, Panagia V, Takeda N (1998) Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40:239–247
Dhalla NS, Saini HK, Tappia PS et al (2007) Potential role and mechanisms of subcellular remodeling in cardiac dysfunction due to ischemic heart disease. J Cardiovasc Med 8:238–250
Dhalla NS, Rangi S, Babick AP et al (2012) cardiac remodeling and subcellular defects in heart failure due to myocardial infarction and aging. Heart Fail Rev 17:671–681
Capasso JM, Remily RM, Smith RH, Sonnenblick EH (1983) Sex differences in myocardial contractility in the rat. Basic Res Cardiol 78:156–171
Wang SN, Wyeth RP, Kennedy RH (1998) Effects of gender on the sensitivity of rat cardiac muscle to extracellular Ca2+ Eur J Pharmacol 361:73–77
Ishii K, Kano T, Ando J (1988) Sex differences in 3H nitrendipine binding and effects of sex steroid hormones in rat cardiac and cerebral membranes. Jpn J Pharmacol 46:117–125
Schwertz DW, Vizgirda V, Solaro RJ et al (1999) Sexual dimorphism in rat left atrial function and response to adrenergic stimulation. Mol Cell Biochem 200:143–153
Gardner JD, Murray DB, Voloshenyuk TG et al (2010) Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol. 298:H497–H504
Wankerl M, Böhm M, Morani I et al (1990) Calcium sensitivity and myosin light chain pattern of atrial and ventricular skinned cardiac fibers from patients with various kinds of cardiac disease. J Moll Cell Cardiol 22:1425–1438
Ayaz O, Howlett SE (2015) Testosterone modulates cardiac contraction and calcium homeostasis: cellular and molecular mechanisms. Biol Sex Differ 6:9
Dent MR, Tappia PS, Dhalla NS (2010) Gender differences in apoptotic signaling in heart failure due to volume overload. Apoptosis 15:499–510
Bupha-Intr T, Wattanapermpool J (2006) Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 291:H1101–H1108
Kravtsov GM, Kam KW, Liu J et al (2007) Altered Ca2+ handling by ryanodine receptor and Na+-Ca2+ exchange in the heart from ovariectomized rats: role of protein kinase A. Am J Physiol Cell Physiol 292:C1625–C1635
Scheuer J, Malhotra A, Schaible TF et al (1987) Effects of gonadectomy and hormonal replacement on rat hearts. Circ Res 61:12–19
Golden KL, Marsh JD, Jaing Y (2002) Castration reduces mRNA levels for calcium regulatory proteins in rat heart. Endocrine 19:339–344
Golden KL, Marsh JD, Jaing Y et al (2003) Gonadectomy of adult male rats reduces contractility of isolated cardiac myocytes. Am J Physiol Endocrinol Metab 285:E449–E453
Golden KL, Marsh JD, Jaing Y (2004) Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Endocrine 36:197–202
Mahmoodzadeh S, Dworatzek E (2019) The role of 17β-estradiol and estrogen receptors in regulation of Ca2+ channels and mitochondrial function in cardiomyocytes. Front Endocrinol 10:310
Bening C, Hamouda K, Leyh R (2016) Sex differences in volume overload in skinned fibers. BMC Cardiovasc Disord 16:197
Prévilon M, Pezet M, Semprez F et al (s2011) FKBP12.6 mice display temporal gender differences in cardiac Ca2+-signaling phenotype upon chronic pressure overload. Can J Physiol Pharmacol 89:769–782
Müller AL, Dhalla NS (2012) Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev 17:395–409
Yousef ZR, Redwood SR, Marber MS (2000) Postinfarction left ventricular remodeling: where are the theries and trials leading us? Heart 83:76–80
Duhamel TA, Dhalla NS (2007) New insights into the causes of heart failure. Drug Discov Today Dis Mech 4:175–184
Acknowledgments
Infrastructural support was provided by the St. Boniface Hospital Research Foundation. Dr. Nusier is a Visiting Professor from Jordan University of Science and Technology, School of Medicine, Department of Physiology and Biochemistry, Jordan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tappia, P.S., Shah, A.K., Nusier, M., Dhalla, N.S. (2020). Sex Differences in Contractile Function in Cardiac Hypertrophy and Heart Failure Subsequent to Volume Overload. In: Ostadal, B., Dhalla, N.S. (eds) Sex Differences in Heart Disease. Advances in Biochemistry in Health and Disease, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-030-58677-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-58677-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58676-8
Online ISBN: 978-3-030-58677-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)