Abstract
Cardiac function is best described by investigating the pressure-volume relationships. This information permits description in terms of the ventricular volume regulation graph (VRG), estimation of systolic elastance, evaluation of lusitropic properties, and assessment of ventriculo-arterial coupling. Current techniques yield noninvasive determination of cardiac compartmental volumes, along with systolic/diastolic arterial pressure, while ventricular end-diastolic pressure can be inferred from an echocardiography-based surrogate measure. Ventricular volume is known to vary with age, as well as to be affected by intrinsic cardiac disease and abnormalities of the vascular system. Moreover, 35 years ago it has been shown in healthy adults that left ventricular volume is significantly smaller in women compared to men. This important observation has serious implications for several metrics which are routinely used in clinical practice, e.g., ejection fraction. The remarkable difference between ventricular size in men and women is also a powerful starting point for the study of aging and the investigation of interventions such as exercise. In this review we evaluate sex-specific characteristics of the VRG and the implications for various cardiac patient populations, during basal conditions and intervention such as exercise.
 Repairing a diseased heart. Art work by Piet Michiels, Leuven, Belgium
Repairing a diseased heart. Art work by Piet Michiels, Leuven, Belgium
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Volume regulation graph
- Ventricular function
- Ejection fraction
- Strain analysis
- Torsion
- Remodeling
- Ventricular geometry
- Cardiophysiology
- Exercise and cardiac pump
- Ventricular mass
- Aging and the heart
- Cardiac dimensions
- Right ventricle
- LAVI
- Sex-specific analysis
- Heart failure
- Diabetes mellitus
- Review
Die Figur ist etwas geändert worden,
da die in der früheren Arbeit S.9 gegebene Berechnung
eine kleine Berichtiging erfahren musste.
Otto Frank, in: Die Grundform des arteriellen Pulses. Z. Biol. 37 (1898) 483–526
Introduction
Thousands of publications are devoted to the measurement of the size of the heart which determination is found to be clinically important [12]. More specifically the volume of each of the four compartments has been estimated. Measurement techniques have evolved from the use of beeswax [19] to X-ray, MRI, and 3D echocardiography. The rationale for such efforts is clear: the heart is a pump, and to characterize the performance of any pump, both the pressure levels and volume displacements need to be known. While a technical pump is defined by certain “specifications”, the biological pump displays an enormous range of plasticity, mainly depending on age and history (in terms of training state and pathologic events). Interestingly, significant differences in size have been observed in adults, both for healthy hearts [5, 54] and in patients with some type of cardiac (co)morbidity, as in heart failure (HF) [28]. Data collected by Kuznetsova et al. [38] are summarized in Table 23.1, illustrating typical differences between healthy men and women, notably regarding average ventricular volume at end-systole, indexed to body surface area (BSA).
Studying 1155 individuals without known coronary or structural heart disease, and free from hypertension and diabetes mellitus (DM) , while using cardiac computed tomography, Gebhard et al. [14] found that left ventricular (LV) remodeling is a process which continues throughout life. Ejection fraction (EF) in females was significantly higher than in males but mostly at ages above 70 years. The findings about changing LV shape are confirmed, e.g., in a longitudinal community study which suggests that dynamic changes in LV geometric pattern over time are common. Higher blood pressure and greater body mass index (BMI) were found to be modifiable factors associated with the development of abnormal LV geometry, and such progression portends an adverse prognosis [39].
Cardiac dimension differences vary with aging , while among sexes these disparities are larger than those observed for arterial pressure and heart rate differences, both in healthy individuals (Table 23.1 and Fig. 23.1) and cardiac patients . Therefore, a dimensional comparison is attractive to pursue, and we primarily concentrate on the comparative study of ventricular size and related metrics in men and women.
Associations among age, measurement of left ventricular (LV) mechanics, or torsion in healthy elderly of both sexes. Graded reduction of longitudinal systolic shortening, including lower longitudinal strain (LS), lower mitral annulus relaxation velocity E′, and modest increase of circumferential fiber shortening (GCS) were shown by age quintiles, which were accompanied by larger LV torsion indices. All strain data and torsion to circumferential ratio are negative and presented as absolute values. Linear trend of P for all <0.05. TDI tissue Doppler imaging. (From Hung et al. [21]. Reproduced with permission)
LV remodeling may be intrinsically related to the senescence process, and in part driven by age-related comorbidities, such as long-standing hypertension and increased vascular stiffness. Initially they lead to asymptomatic alterations in diastolic function (i.e., LV stiffness) and longitudinal systolic function (as reflected by strain). It has been postulated that myocardial twist may increase as a response to the loss of longitudinal function that occurs with aging and that this compensation may help preserve EF [21]. Furthermore, these alterations have been noted to differ between men and women because a greater degree of cardiac torsion has been observed in women [22]. Alterations in LV structure also may occur with cardiac disease. In particular the development of HF is accompanied by LV remodeling .
Three-dimensional echocardiography (3DE) currently allows a comprehensive characterization of cardiac kinematics, enabling simultaneous assessment of various myocardial systolic components and torsion mechanics using speckle tracking. Using these methods, Hung et al. [21] investigated sex-related differences in cardiac mechanics in an elderly population without known HF, including 1105 participants. For both the left atrium (LA) and the LV, end-diastolic volume (EDV), end-systolic volume (ESV), LV stroke volume (SV), and EF were measured by the biplane modified Simpson method using apical 4- and 2-chamber views. The LV mass was assessed from LV linear dimensions and indexed to BSA. Right ventricular (RV) function was measured by fractional area change expressed as percentage of changes in cavity area from apical 4-chamber view. LV twist and torsion (twist per length, degree/cm or °/cm) were also estimated. Less negative global longitudinal strain (GLS) and global circumferential strain (GCS) indicate a functional decline in global myocardial deformation assessment, with more positive twist/torsion values representing better torque mechanics. The torsion to circumferential ratio (TCR) was also calculated. Figure 23.1 shows the results for five age groups, stratified for males and females, as well as for combined data. Women have smaller LV cavity size, greater concentricity, though demonstrated greater global longitudinal strain, global circumferential strain, and torsion than men (all P < 0.05). Overall, older age was associated with smaller LV EDV and ESV, lower SV, greater wall thicknesses, and larger LV mass index, resulting in greater LV concentricity in terms of higher mass to volume ratio. Increasing age was also associated with larger BSA-indexed LA volume, though unchanged RV area, RV fractional area change, or global LV EF. In general, women showed significantly smaller LV EDV and SV and higher EF than men (all P < 0.001). Conversely, men had greater LV wall thickness and larger LV mass index than women (both P < 0.001). Thus, in this asymptomatic, senescent population, a distinct, sex-specific pattern of cardiac remodeling was observed, while a significant increase of torsion was more pronounced in women [21].
Ventricular Volume Regulation in Children
During childhood and adolescence, the size of the heart in healthy individuals mainly relates to body mass (BM) or BSA . After indexation for BM or BSA, there is no real difference between boys and girls when LV dimensions are compared. Absence of a distinction is also observed for systolic and diastolic blood pressure in children up to 16 years [7]. Figure 23.2 illustrates the volume regulation graph (VRG) for boys and girls (from neonates to 16 years), both healthy and those with cardiac disease. In these children the regression lines are not different for the two sexes. The usefulness of the VRG and the implications have been explained elsewhere [34].
Left ventricular end-systolic volume index (ESVi) and end-diastolic volume index (EDVi) in children (26 healthy and 13 with “intrinsic” myocardial disease). Age ranges from 1 month to 16 years. Average ESVi and EF are not significantly (P > 0.15) different for boys and girls (in contrast to findings in adults). (Data from Graham et al. [17])
Comparing Volumetric Characteristics of Adult Males and Females
Volume-related data are best represented in a diagram which relates ESV(i) to EDV(i) [4]. This choice applies to all four cardiac compartments [27], both in healthy individuals and patients with cardiac disease. An example regarding the LV in patients with anginal complaints is shown in Fig. 23.3. Data were obtained by gated SPECT. The linear regression lines for males and females are almost superimposed. However, average values for EF are significantly (P < 0.0001) higher in women.
Left ventricular (LV) volume regulation in males and females, showing the higher average ejection fraction in women. Distribution of end-systolic (ESVi) and end-diastolic (EDVi) volumes for 276 patients evaluated for chest pain and dyspnea, as determined by gated SPECT, presented in the LV volume domain. Regression lines for males and females are similar, but their average (ave) values (yellow triangle and circle, respectively) are significantly different, resulting in a substantially higher (P < 0.0001) average value for ejection fraction (EF) in females (see solid lines with arrowhead). (Data described by Peace et al. [44])
The Heart and Other Pumps
Ziman [56] posed the question: Which came first: science or technology? The latter term implies “knowing how to do.” We address this question in relation to the development of a pumping system, including the biological muscular pump known as the heart. It must be added that other biological pumps do exist: spiders, for example, use no muscles to extend their legs, sticking them out hydraulically instead, with pressures which may exceed 450 mmHg [52]. The physician Willem Kolff devoted most of his life to the development of an artificial kidney, extending his work to the development of an artificial heart [15]. An alternative route is based on assist devices [37]. Interestingly, Moscato et al. [43] were able to construct the ESV vs EDV relationship (i.e., the VRG representation), yielding high correlations in acute animal experiments using cardiac assist devices in sheep. Going back to Ziman’s question, we may ask if the concept of a cardiac pump promoted similar technical inventions, if mechanical insights helped to understand the biological pump, or rather if both ideas matured in parallel. Further details on technical and biological pumps are summarized elsewhere [35].
Ejection Fraction and Ventricular Volume
An earlier variant of the metric EF was introduced around 1955 to quantify indicator dilution curves . During steady-state conditions, it was observed that the washout fraction of tracer material (injected during diastole) which remained in the ventricle was rather constant [20]. This information yields residual volume (as it was called at that time), which is the same as our present end-systolic volume (ESV) . The fraction was referred to as residual fraction and equals (1-EF). Subsequently, the EF gained popularity because of its ease of use and the impression that it reflects efficiency of forward pumping. The relationship between EF and ESV has intrigued investigators. Using a linear description, it was found that the correlation between EF and ESV is significantly less in patients using beta-blockers when compared to those not using this pharmaceutical [23]. Next, the relationship was described more accurately by using a universal nonlinear analytical description [23]. Effects of age and sex on the relationship between EF and ESV were studied applying a logarithmic approximation [44], which has been shown to work remarkably well [32]. The initial linear EF vs ESV approach [23] was further analyzed [51] to derive a performance index, while a few investigators have employed the slope as an indicator of survival by comparing various groups of cardiac patients [26, 31]. It must be emphasized that EF also depends on the imaging method employed to measure volumes [53].
Stroke Volume (SV), Heart Rate (HR), and Ejection Fraction (EF)
SV equals EDV minus ESV. HR multiplied by SV yields CO; both components are shown in Fig. 23.4 in the case of heart failure patients, where EF is reflected by the size of the bubbles . Low values for EF tend to be associated with low levels of SV, but a wide variation can be seen. Although SV is (mathematically seen) an important component of EF, there is no clear correlation between SV and EF. Interestingly, in general there is no significant correlation between EF and EDV either [32]. However, EF is strongly associated with ESV, as we will see later (Fig. 23.7).
Cardiac output index is obtained by multiplying stroke volume index (SVi) and heart rate (HR). This diagram also shows ejection fraction (EF) as reflected by the bubble size in 179 heart failure patients. Patients with low EF values tend to have low SVi and high HR. In this study group, the female and male patients have values for SVi and HR which are not significantly different, as described elsewhere [28]
Left Ventricular Dimensions During Singleton Pregnancy
Pregnancy offers a physiological model to evaluate the adaptation of the healthy heart of the mother to transient preload and afterload changes [47]. Ideally, the performance and remodeling of the LV should be described during the uncomplicated singleton pregnancy while also explicitly considering shape changes. We used published data [16] and constructed a diagram showing average LV volume for five points in time during gestation in 34 women and one additional measurement at 48 weeks (Fig. 23.5). Remarkably, EF remains rather constant, while changes of EDV were most pronounced. Further details on hemodynamic adaptation can be found in Fu [13].
Average values (N = 34) for end-diastolic volume (EDV) and end-systolic volume (ESV) for the left ventricle (LV) in healthy women with uncomplicated singleton pregnancy (Based on data published by Geva et al. [16])
Ventricular-Arterial Coupling and Ejection Fraction
In an attempt to describe the efficiency of energy transfer from the ventricular pump to the adjacent vascular bed, a formalism has been introduced based on the elastance concept [26]. Ventricular end-systolic elastance (Emax) is derived from the pressure-volume loop [34], while effective arterial elastance (Ea) is defined by the ratio of end-systolic pressure and SV. Since Emax is the tangent to successive loops under constant conditions of contractility, this tangent has a slope and an intercept, assuming linearity which is reasonable when considering a sufficiently small range [34]. If this intercept is chosen along the abscissa, it is termed Vo (i.e., the volume where the Emax line intersects with the theoretical point where pressure would be zero). Hemodynamic coupling (k) between ventricle and afterload is then defined by the ratio Emax/Ea. Since pressure cancels out, k = SV/(ESV−Vo). Figure 23.6 shows k versus EF based on the classical study by Grossman et al. [18], covering the complete spectrum of EF values. Two scenarios are depicted: one with the “real” values for Vo and the other assuming that for simplicity Vo can be neglected. In the latter case, the connection between k and EF is nearly perfect, meaning that both metrics are telling the same story. Surprisingly, the outcome is not much different if the actual value of Vo is considered, yielding a high correlation even for a not very sophisticated exponential approximation. In conclusion, the coupling index has not much more informational value than whatever may be revealed in the EF.
Arterial-ventricular (AV) coupling is strongly related to ejection fraction (EF) , simply based on their definitions. Data from patients described by Grossman et al. [18] are shown, to illustrate the effect of neglecting the volume intercept (Vo) which refers to the end-systolic pressure-volume relationship. Clearly, the coupling index is tightly connected to EF if the real value of Vo is omitted (circles) and even if this simplification is not considered (squares)
The Right Ventricle
Holt [20] was the first investigator to report values for ESV and EDV as measured in the RV, using a dilution method in dogs. Using data on both LV and RV as collected by Rominger et al. [46], we created a diagram showing EF versus ESV for both compartments (Fig. 23.7). Note that the range for ESV is much smaller for the RV , as expected. The two logarithmic regression lines almost coincide for the groups considered which span the spectrum from healthy volunteers to severe heart disease. In contrast, we found two distinct regression curves for LV and RV in post-Fallot repair patients [30], likely expressing consequences of the transformation following surgical intervention. Using data from Maffessanti et al. [41], we found for the (age group-based) average data available that the regression lines in the VRG representation were very close for men and women, with women having a significantly (P = 0.01) higher EF, similar as we have observed elsewhere for the LV [32]. This data set will be discussed later in more detail when describing age dependency.
Ejection fraction (EF ) versus end-systolic volume index (ESVi ) for left ventricle (LV) and right ventricle (RV), measured by fast cine MRI . The study included 52 healthy volunteers and 325 cardiac patients subdivided into 22 diagnostic groups without attention to possible sex differences (Based on diagnostic-group averaged data (with specified sex ratios of individual groups), as published by Rominger et al. [46]. Note that group sizes are unequal, ranging from 4 to 52 individuals)
In 18 patients with sepsis, while employing thermodilution to estimate RV size , Dhainaut et al. [9] found that SV is poorly correlated with EDV (R = 0.32) during military antishock trouser (MAST) inflation to manipulate preload. However, they found a high correlation (R = 0.93) for ESV vs EDV, which is in support of our VRG approach.
Left Atrium
The left atrium (LA) has an important reservoir function and may during late (ventricular) diastole actively assist LV filling (by the so-called atrial kick ). The same study by Graham et al. [17] showing LV volumes in children (Fig. 23.2) also estimated LA size. Figure 23.8 illustrates the findings for the healthy subgroup (N = 20). Although the regression lines seem to differ, the numbers of participants involved are too limited for a meaningful statistical comparison of boys and girls. However, there is no significant sex-based difference between average minimum and maximum volumes, and this observation was also the case when the cardiac patients were included (not shown). These findings in children contrast to findings often seen in healthy adults. For example, Yu et al. [55] found in women a greater (P < 0.001) LA volume index (LAVI) than in their male counterparts in the risk factor (according to their CHA2DS2-VASc scores) ≥2 group.
Left atrium (LA) minimum volume index versus maximum volume index in 20 healthy children (9 girls). Age ranges from 3 months to 16 years. Average minimum and maximum (BSA indexed) volumes are not significantly different for boys and girls. (Data from Graham et al. [17])
In an editorial Di Tullio and Homma [10] note that LA minimum volume is strongly associated with LA EF in the entire population studied (1142 healthy elderly). Furthermore, LA minimum volume is the strongest correlate of LA EF in every diagnostic subgroup they analyzed. This finding is to be expected, as it is essentially based on a mathematical triviality, which applies to all four cardiac compartments. A proof based on Monte Carlo simulation (i.e., employing random numbers) has been published elsewhere [29].
Right Atrium
Relatively few studies concern the right atrium (RA) , and among those the majority concentrates on diameter or area. Actual RA volume (mean value and 95% confidence interval) was reported in healthy adults free of hypertension and obesity as determined by using 64-multidetector row computed tomography [40], yielding reference values. Figure 23.9 shows in a heterogeneous group of adult cardiac patients the MRI-based RA diameters, along with LA diameters and LV volumes (scaled so as to visually cover the diameter ranges for the atria) and stratified for sex.
Right atrial (RA), left atrial (LA ), and left ventricular (LV) data shown in a combined volume regulation graph , where LV volumes are scaled so as to visually display a similar space as the atrial diameters (Data refer to 51 adult cardiac patients, including 33 males (M) and 18 females (F) investigated by cardiac MRI at VU University Medical Center, Amsterdam, the Netherlands)
Ventricular Dimensions Change with Aging
There has been some debate about the variation of LV EF in men and women during the aging process. An inevitable problem resides in the fact that physiological aging is difficult to delineate and separate from subclinical deterioration. In a gated SPECT study, Peace et al. [44] found in 127 patients with suspected coronary artery disease (CAD) for the LV that ESVi slightly decreases with age, while EF remained unchanged. A study in 442 dogs (280 male) with various types of cardiac disease [25] revealed that fractional shortening (FS), based on echocardiographically determined LV diameters, did not change with age (range a few months up to 15 years), while the slope in the diameter domain equivalent of the VRG tends to increase with age , when three groups are considered (with cutoff at 2 and 6 years).
Relatively few investigators have extended their studies to include the RV. Age-related findings obtained for RV ESV and EDV estimated by 3D echo in healthy adult men and women are illustrated in Fig. 23.10, based on data collected by Maffessanti et al. [41].
Right ventricular (RV) end-systolic volume (ESV) and end-diastolic volume (EDV) as a function of age groups in healthy adults, estimated by 3D echo. Note that volumes are not indexed for body surface area. Ejection fraction was rather independent of age (not shown). Average values for six age groups with range 18–90 years (N = 507); first group <30 and last group >70 years. Thus, also in the RV, a larger EF (64.0 vs 61.7 %) is found in healthy women. (Based on data from Maffessanti et al. [41])
Another investigation compared LV volumes in both sexes for three age groups (Fig. 23.11) and confirmed larger ESVi and EDVi in males (N = 43), compared to females (N = 59) based on a gated SPECT study concerning individuals with a low likelihood of CAD [8]. The different volumes as observed in both sexes have implications for the values of EF, as illustrated in the VRG, where trajectories for constant EF values of 60% and 70% are shown. In this study average EF increased with aging , notably in women.
The volume regulation graph for the left ventricle (healthy individuals N = 102), with group averages referring to ages < 45 and > 65 years and intermediate ages, stratified for sex. The lines with arrowhead indicate direction of increasing age for each sex. Note that the highest ejection fraction (EF) occurs in the youngest group of women. This value is considerably higher compared to the equivalent point in males. (Data from De Bondt et al. [8])
The VRG During Exercise in Men and Women with Heart Disease
Apart from polypill considerations [36], it is now generally accepted that age-dependent appropriate levels and types of exercise constitute a natural and powerful means to maintain health. The impact of physical activity on the functioning and adaptability of the heart has been studied in humans and animal models . Data published by Rerych et al. [45] permit the analysis of effects of exercise in healthy volunteers (N = 30) and individuals with ischemic heart disease (N = 30), 10 with single coronary artery disease and 20 patients with multiple-vessel coronary disease. Radionuclide angiocardiograms were performed at rest and during exercise, with all individuals studied in the erect posture on a bicycle ergometer. In the controls, the mean HR doubled and the cardiac output tripled during exercise. Their findings indicate that patients with ischemic myocardial disease respond to the stress of exercise by cardiac dilatation to maintain or increase SV at increased HR. Figure 23.12 illustrates the findings in terms of the EF vs ESVi relationship, subdivided for sex, and shows that the inverse nonlinear relationship applies to all groups, both at rest and during exercise. Only at higher ESVi values the curves seem to diverge for men and women. However, in that region (where ESVi > 50 mL/m2), there are no data points for women, suggesting that the larger ESVi values often seen in men are responsible for this difference.
Ejection fraction (EF) as determined by end-systolic volume index (ESVi) during rest and exercise in patients with coronary artery disease (N = 30) and healthy controls (N = 30). Regression curves show an excellent correlation between EF and ESVi and appear in the higher ESVi region to be elevated for women (N = 16), both at rest and during exercise, but this finding is not supported by presence of actual data points. Instead, the regression curves for ESVi < 50 mL/m2 suggest a similar pattern for men and women, indicating on average comparable EF values at identical ESVi. (Data from Rerych et al. [45])
Cardiac Function in Patients with Diabetes Mellitus
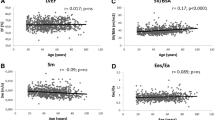
The heart progressively remodels over the life course, and the impact of DM has been investigated. Over a 16-year period in 4062 Framingham Heart Study participants (mean age 45 years, 54% women; 11,485 person-observations), LV wall thickness, LV systolic and diastolic dimensions, and FS were studied in relation to age, sex, body mass index, blood pressure, smoking, and DM. With advancing age, LV dimensions decreased, whereas FS and LV wall thickness increased concomitantly. Female sex, greater blood pressure load, and presence of DM were found to attenuate the remodeling pattern , suggesting a mechanism for the preponderance of women with hypertension and individuals with DM among patients with diastolic HF [6]. We investigated LV volume in men and women with DM and found that average EF is significantly higher in women (Fig. 23.13), as also observed in healthy individuals [5] and in patients [44] with suspected CAD.
Volume regulation graph for cardiac patients (N = 180) with diabetes mellitus (DM), stratified for males (M) and females (F). The regression lines for M and F (N = 69) almost coincide, but average ESVi and EDVi are significantly (P = 0.032) smaller in females. Although higher in women, no significant difference for ejection fraction (EF) was found. (Data from patients as described elsewhere [35])
The VRG and Heart Failure
Heart failure (HF) can be manifest as different phenotypes [26], which are readily discerned in the VRG representation based on the selected cutoff value for EF. Figure 23.14 illustrates the three most common types with reduced (r), midrange (m), and preserved (p) values for EF, respectively. They correspond to precisely defined areas which can clearly be identified in the LV volume domain [27]. In the past a borderline zone was introduced for EF values between 40% and 50%, where frequent transitions between HFpEF and HFrEF (in both directions) might occur. Currently, this zone is recognized as a separate phenotype, called midrange . However, since average EF is significantly higher in healthy adult women [5] and in cardiac patients [44] and is likely also the case in HF [28], establishing the same cutoff values for men and women is inappropriate, and thus the HF phenotypes should require separate analysis. Several studies have proposed to define a cutoff level at 55% for women with HFpEF, as reviewed elsewhere [27] and as also proposed by the Cardiac Review and Evaluation Committee reviewing trastuzumab (see below). Moreover, there are strong indications to not rely only on a vague metric such as EF, which has limited meaning as it is just a dimensionless ratio. In fact, many decades ago the potential weakness of EF has been emphasized [2, 24]. Another obvious shortcoming of the current guidelines resides in the application of plain linear borders between HF phenotypes as seen on the VRG presentation (Fig. 23.14), as suggested by the committee(s) responsible for formulating such rules, without justification or any thoughts on reconsideration. The application of machine learning could be of substantial importance to resolve the distinctions between HF groups, as shown elsewhere when addressing these issues [1].
In the volume domain, the three proposed phenotypes of heart failure (HF) can be easily recognized. The HFrEF type is confined to the upper area between the black identity line and the green line indicating that EF < 40%, whereas the HFpEF syndrome is located in the lower triangular region (yellow area). The gray wedge-shaped area is reserved for midrange HF. As a consequence of this separation, we find that the resulting regression lines are clearly different for the three groups, and thus their volume regulation differs, i.e., in HFpEF it is more “Starling-like,” implying that SVi clearly increases as filling volume (EDVi) becomes larger. The fact that average EF for women is higher for most patient groups may imply that the cutoff levels for women are higher
Need for Sex-Specific EF Cutoff in Phenotyping and Treatment Considerations
There are concerns about using EF as a marker in studies of cardiotoxicity . HF may be induced by chemotherapy or treatments for breast cancer with radiation to the chest wall area [49, 50]. Therefore, it is important to predict and detect the development of cardiac dysfunction in these patients, e.g., by using imaging techniques and the determination of biomarkers. Cardiotoxicity, as defined by the guidelines of the Cardiac Review and Evaluation Committee reviewing trastuzumab, refers to a reduction of the EF of ≥5% to <55% with symptoms of HF or an asymptomatic reduction of the EF of ≥10% to <55% [48]. Other EF-based criteria have been applied, as reviewed elsewhere [42]. In one report, assessment of the EF fails to detect subtle alterations in LV function due to cardiotoxicity associated with chemotherapeutic regimens (such as anthracyclines and trastuzumab) administered to women with breast cancer [48]. Also, in that study parameters of diastolic function (i.e., mitral valve A and E wave filling velocities) and N-terminal pro-B-type natriuretic peptide did not predict cardiotoxicity. However, cardiac troponin plasma concentrations and longitudinal strain were found to predict the development of cardiotoxicity in those patients [48]. Furthermore, Bergamini et al. [3] found that a cutoff of 58 mL/m2 for EDVi (rather than EF changes) may act as an early indicator of trastuzumab-related cardiotoxicity in human epidermal growth factor receptor II (HER2+) breast cancer patients. These observations highlight the current confusion about the metric EF. While traditional HF (i.e., with reduced EF) is often defined as EF < 50%, it appears that the threshold for women is higher [27] and that EF is not always a sensitive predictor [48]. The metric EF apparently cannot be reliably used as a single marker of cardiotoxicity, as cardiac damage seems to be more complicated than what the EF ratio can capture.
Apart from the discussion about sex-specific cutoff values for EF, there is also increasing attention for sex-specific treatment of cardiovascular diseases. The goal of the Multi-Ethnic Study of Atherosclerosis (MESA) cohort was to examine correlates and progression of subclinical cardiovascular disease. This means that by design the MESA cohort included participants with conditions known as risk factors for cardiovascular disease, such as diabetes and hypertension. In patients with hypertension, cardiac myocyte sarcomeres are added in parallel, causing myocardial wall thickness to increase out of proportion to the volume of the LV cavity. Thus, a relative decrease in LV volume might be explained by an increase in LV mass. However, it was observed that in women, LV volume decreased with age even though there was no corresponding increase in LV mass. This finding suggests that some other mechanism may be diminishing LV volume in women or that the causal relationship between mass and volume in women may be different than that in men. One possible hypothesis is that these results reflect sex-specific variations in diffuse myocardial fibrosis, which may inhibit LV volume changes during remodeling . In the MESA cohort, there is evidence that diffuse myocardial fibrosis is greater in women. However, the fibrosis is associated with a greater age-related increase in men. The sex-specific LV mass and volume differences observed in the MESA study may also signal the need for sex-specific treatment approaches for the adverse effects of myocardial remodeling , such as in HF. In summary, in a cohort of individuals who were free of clinical cardiovascular disease at baseline, a longitudinal LV mass increase in men and a slight decrease in women are observed, while EDV decreased and mass-to-volume ratio increases with aging for both men and women. The longitudinal trends are congruent with previous cross-sectional observations in this cohort. These results suggest sex-specific differences in age-related cardiac remodeling [11].
Conclusions
Cardiac function can in detail be described by pressure-volume relationships. A reduced approach, not explicitly including information on pressure, employs the VRG description , which has been shown to be applicable to all four cardiac compartments. Examples have been presented regarding the LV in healthy children and those with myocardial disease (Fig. 23.2), with for the linear regression coefficients no difference between boys and girls. Similar regression results were found for the LV in adults with anginal complaints , indicating that average EF in women is higher (P < 0.0001) than in men (Fig. 23.3), and also for cardiac patients with DM (Fig. 23.13), but now without a sex-related difference in EF. The VRG concept was extended to LA and RA diameters (Fig. 23.9), yielding similar linear relationships for a heterogeneous group of cardiac patients . Interestingly, a high correlation was found for the inverse relationship between EF and ESVi in a combination of healthy individuals and cardiac patients (Fig. 23.7) for both LV and RV. This finding indicates that EF is closely connected to the actual value of ESVi, which observation is further supported by analysis of CAD patients at rest and during exercise (Fig. 23.12). The traditional subdivision of HF in three phenotypes (Fig. 23.14) becomes readily recognizable in the VRG representation and illustrates that the current HF paradigm may be based on an oversimplification, especially if EF cutoff values are considered identical for both sexes. ESVi seems to play a key role in various types of analysis, and, interestingly, this metric also refers to the largest percentwise difference between (healthy) men and women (Table 23.1). Not only is ESVi the primary determinant of EF but also of the ventriculo-arterial coupling index (k), which fact readily explains the almost trivial connection between k and EF (Fig. 23.7).
In retrospect, it is amazing to learn about the seemingly parallel developmental patterns regarding insight and design of mechanical and biological pump systems. Originating in Italy, the epicenter moved to England while also attracting engineers from France and Germany. During the past decades, both lines came to a synthesis with the introduction of artificial hearts and cardiac assist devices. The technical product is not yet as reliable and energy-efficient as the average biological pump and also requires maintenance. Despite the sophisticated measurement of pressure, volume, Doppler-derived velocities, strain (rate) , and torsion , it is evident that our current understanding of the natural pump is far behind the technical insight concerning the simple technical imitations created thus far. Failure of a mechanical system is relatively easy to detect, and spare parts are usually available. In sharp contrast, in cases of a failing heart, we often must admit that defining the problem is not always precise and occasionally still a riddle.
References
Alonso-Betanzos A, Bolón-Canedo V, Heyndrickx GR, Kerkhof PLM. Exploring guidelines for classification of major heart failure subtypes by using machine learning. Clin MedInsights Cardiol. 2015;9(Suppl 1):57–71.
Altschule MD. Limited usefulness of the so-called ejection fraction measurement in clinical practice. Chest. 1986;90:134–5.
Bergamini C, Torelli F, Ghiselli L, Rossi A, Trevisani L, Vinco G, et al. Left ventricular end-diastolic volume as early indicator of trastuzumab-related cardiotoxicity in HER2+ breast cancer patients: results from a single-center retrospective study. Minerva Cardioangiol. 2017;65:278–87.
Beringer JY, Kerkhof PLM. A unifying representation of ventricular volumetric indexes. IEEE Trans BME. 1998;45:365–71.
Buonanno C, Arbustini E, Rossi L, Dander B, Vassanelli C, Paris B, Poppi A. Left ventricular function in men and women. Another difference between sexes. Eur Heart J. 1982;3:525–8.
Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–8. https://doi.org/10.1161/CIRCULATIONAHA.110.937821.
Dallaire F, Sarkola T. Growth of cardiovascular structures from the fetus to the young adult. In: Kerkhof PLM, Miller VM, editors. Sex-specific analysis of cardiovascular function. Cham: Springer; 2018; p. 347–60.
De Bondt P, Van de Wiele C, De Sutter J, De Winter F, De Backer G, Dierckx RA. Age- and gender-specific differences in left ventricular cardiac function and volumes determined by gated SPET. Eur J Nucl Med. 2001;28:620–4.
Dhainaut JF, Pinsky MR, Nouria S, Slomka F, Brunet F. Right ventricular function in human sepsis. Chest. 1997;112:1043–9.
Di Tullio MR, Homma S. Left atrial morphology and function: The other side of cardiovascular risk. Circ Cardiovasc Imaging. 2016;9:e004494. https://doi.org/10.1161/CIRCIMAGING.116.004494.
Eng J, McClelland RL, Gomes AS, Hundley WG, Cheng S, Wu CO, Carr JJ, Shea S, Bluemke DA, Lima JA. Adverse left ventricular remodeling and age assessed with cardiac MR imaging: The multi-ethnic study of atherosclerosis. Radiology. 2016;278:714–22. https://doi.org/10.1148/radiol.2015150982.
Ford LE. Heart size. Circ Res. 1976;39:297–303. https://doi.org/10.1161/01.RES.39.3.297.
Fu Q. Hemodynamic and electrocardiographic aspects of uncomplicated singleton pregnancy. In: Kerkhof PLM, Miller VM, editors. Sex-specific analysis of cardiovascular function. Cham: Springer; 2018; p. 413–31.
Gebhard C, Buechel RR, Stähli BE, Gransar H, Achenbach S, Berman DS, et al. Impact of age and sex on left ventricular function determined by coronary computed tomographic angiography: results from the prospective multicentre CONFIRM study. Eur Heart J Imag. 2017;18:990–1000.
Gedeon A. Science and technology in medicine. New York: Springer; 2006.
Geva T, Mauer MB, Striker L, Kirshon B, Pivarnik JM. Effects of physiologic load of pregnancy on left ventricular contractility and remodeling. Am Heart J. 1997;133:53–9.
Graham TP Jr, Jarmakani MM, Canent RV Jr, Capp MP, Spach MS. Characterization of left heart volumes and mass in normal children and in infants with intrinsic myocardial disease. Circulation. 1968;38:826–37.
Grossman W, Braunwald E, Mann T, McLaurin LP, Green LH. Contractile state of the left ventricle in man as evaluated from end-systolic pressure-volume relations. Circulation. 1977;56:845–52.
Hales S. Statical essays. London: Innys & Co; 1733.
Holt JP, Allensworth J. Estimation of the residual volume of the right ventricle of the dog's heart. Circ Res.1957;5:323–6.
Hung CL, Gonçalves A, Shah AM, Cheng S, Kitzman D, Solomon SD. Age- and sex-related influences on left ventricular mechanics in elderly individuals free of prevalent heart failure: The ARIC Study. Circ Cardiovasc Imaging. 2017;10. pii: e004510. doi:https://doi.org/10.1161/CIRCIMAGING.116.004510.
Kaku K, Takeuchi M, Tsang W, Takigiku K, Yasukochi S, Patel AR, Mor-Avi V, Lang RM, Otsuji Y. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27(1):55–64.
Kerkhof PLM, Baan J, Buis B, Arntzenius AC. Relations between ejection fraction and ventricular volume, and their alteration by chronic beta-blockade. Br Heart J. 1981;46:17–22.
Kerkhof PLM. Importance of end-systolic volume for the evaluation of cardiac pump performance. In: Chazov EI, Smirnov VN, Oganov RG, editors. Cardiology, an international perspective. New York: Plenum Press; 1984. p. 1339–52.
Kerkhof PL, Roos A, ter Haar G, Kocsis S, Pijnenburg HL, Stokhof AA. Age variance of left ventricular diameters in dogs with cardiac disease. J Gerontol A Biol Sci Med Sci. 1998;53:B25–31.
Kerkhof PLM, Kresh JY, Li JK-J, Heyndrickx GR. Left ventricular volume regulation in heart failure with preserved ejection fraction. Physiol Rep. 2013;1:e00007.
Kerkhof PLM. Characterizing heart failure in the ventricular volume domain. Clin Med Insights Cardiol. 2015;9(S1):11–31.
Kerkhof PLM, Heyndrickx GR, Li JJ-K. Hemodynamic determinants and ventriculo-arterial coupling are sex-associated in heart failure patients. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:3286–9. https://doi.org/10.1109/EMBC.2016.7591430.
Kerkhof PLM, Yoo BW, Merillon JP, Peace RA, Handly N. Monte Carlo method applied to the evaluation of the relationship between ejection fraction and its constituent components. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:1295–8. https://doi.org/10.1109/EMBC.2017.8037069.
Kerkhof PLM, Yoo BW, van de Ven PM, Handly N. Sex-specific aspects of left and right ventricular volume regulation in patients following tetralogy of Fallot repair. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:1303–6. https://doi.org/10.1109/EMBC.2017.8037071.
Kerkhof PL, Peace RA, Handly P. Importance of (measuring) the end-systolic volume index in predicting survival. Heart. 2018;104:180. https://doi.org/10.1136/heartjnl-2017-312051.
Kerkhof PL, van de Ven PM, Yoo BW, Peace RA, Heyndrickx GR, Handly N. Ejection fraction as related to basic components in the left and right ventricular volume domains. Int J Cardiol. 2018;255:105–10.
Kerkhof PLM, Kuznetsova T, Kresh JY, Handly N. Cardiophysiology illustrated by comparing ventricular volumes in healthy adult males and females. In: Kerkhof PLM, Miller VM, editors. Sex-specific analysis of cardiovascular function. Cham: Springer; 2018; p. 123–38.
Kerkhof PLM, Kuznetsova T, Ali R, Handly N. Left ventricular volume analysis as a basic tool to describe cardiac function. Adv Physiol Educ. 2018;42(1):130–9. https://doi.org/10.1152/advan.00140.2017.
Kerkhof PLM, Peace RA, Macfarlane PW. Sex- and age-related reference values in cardiology, with annotations and guidelines for interpretation. In: Kerkhof PLM, Miller VM, editors. Sex-specific analysis of cardiovascular function. Cham: Springer; 2018; p. 677–706.
Kerkhof PLM, Osto E. Women and men in the history of Western cardiology: some notes on their position as patients, role as investigational subjects, and impact as professionals. In: Kerkhof PLM, Miller VM, editors. Sex-specific analysis of cardiovascular function. Cham: Springer; 2018; p. 1–30.
Kresh JY, Kerkhof PL, Goldman SM, Brockman SK. Heart-mechanical assist device interaction. ASAIO Trans. 1986;32:437–43.
Kuznetsova T, Haddad F, Tikhonoff V, et al. Impact and pitfalls of scaling of left ventricular and atrial structure in population-based studies. J Hypertens. 2016;34:1186–94.
Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S, Benjamin EJ, Vasan RS. The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC Cardiovasc Imaging. 2014;7:870–8. https://doi.org/10.1016/j.jcmg.2014.05.008.
Lin FY, Devereux RB, Roman MJ, et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1:782–6. https://doi.org/10.1016/j.jcmg.2008.04.015.
Maffessanti F, Muraru D, Esposito R, Gripari P, Ermacora D, Santoro C, Tamborini G, Galderisi M, Pepi M, Badano LP. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging. 2013;6:700–10.
Martín M, Esteva FJ, Alba E, Khandheria B, Pérez-Isla L, García-Sáenz JA, Márquez A, Sengupta P, Zamorano J. Minimizing cardiotoxicity while optimizing treatment efficacy with trastuzumab: review and expert recommendations. Oncologist. 2009;14:1–11. https://doi.org/10.1634/theoncologist.2008-0137.
Moscato F, Vollkron M, Bergmeister H, Wieselthaler G, Leonard E, Schima H. Left ventricular pressure-volume loop analysis during continuous cardiac assist in acute animal trials. Artif Organs. 2007;31:369–76.
Peace RA, Adams PC, Lloyd JJ. Effect of sex, age, and weight on ejection fraction and end-systolic volume reference limits in gated myocardial perfusion SPECT. J Nucl Cardiol. 2008;15:86–93.
Rerych SK, Scholz PM, Newman GE, Sabiston DC Jr, Jones RH. Cardiac function at rest and during exercise in normals and in patients with coronary heart disease: evaluation by radionuclide angiocardiography. Ann Surg. 1978;187:449–64.
Rominger MB, Bachmann GF, Pabst W, Rau WS. Right ventricular volumes and ejection fraction with fast cine MR imaging in breath-hold technique: applicability, normal values from 52 volunteers, and evaluation of 325 adult cardiac patients. J Magn Reson Imaging. 1999;10:908–18.
Savu O, Jurcuţ R, Giuşcă S, van Mieghem T, Gussi I, Popescu BA, Ginghină C, Rademakers F, Deprest J, Voigt JU. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging. 2012;5:289–97.
Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–80. https://doi.org/10.1016/j.amjcard.2011.01.006.
Shufelt C, Pacheco C, Tweet MS, Miller VM. Sex-specific physiology and cardiovascular disease. In: Kerkhof PLM, Miller VM, editors. Sex-specific analysis of cardiovascular function. Cham: Springer; 2018; p. 433–54.
Tadic M, Cuspidi C, Vasic D, Kerkhof PLM. Cardiovascular implications of diabetes, metabolic syndrome, thyroid disease and cardio-oncology in women. In: Kerkhof PLM, Miller VM, editors. Sex-specific analysis of cardiovascular function. Cham: Springer; 2018; p. 471–88.
Thormann J, Kramer W, Kindler M, Neuss H, Bahawar H, Schlepper M. Analysis of the efficacy of the new cardiotonic agent TA-064. Am Heart J. 1985;110:426–38.
Vogel S. Vital circuits – on pumps, pipes, and the workings of circulatory systems. New York: Oxford University Press; 1992.
Wood PW, Choy JB, Nanda NC, Becher H. Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography. 2014;31:87–100.
Yamada AT, Campos Neto Gde C, Soares J Jr, Giorgi MC, Araújo F, Meneghetti JC, Mansur AJ. Gender differences in ventricular volumes and left ventricle ejection fraction estimated by myocardial perfusion imaging: comparison of Quantitative Gated SPECT (QGS) and Segami software programs. Arq Bras Cardiol. 2007;88:285–90.
Yu HT, Lee JS, Kim TH, Uhm JS, Joung B, Hong GR, Lee MH, Shim CY, Pak HN. Advanced left atrial remodeling and appendage contractile dysfunction in women than in men among the patients with atrial fibrillation: Potential mechanism for stroke. J Am Heart Assoc. 2016;5(7). pii: e003361. doi:https://doi.org/10.1161/JAHA.116.003361.
Ziman J. The force of knowledge; the scientific dimension of society. Cambridge: Cambridge University Press; 1976.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kerkhof, P.L.M., Peace, R.A., Heyndrickx, G.R., Meijboom, L.J., Sprengers, R.W., Handly, N. (2018). Heart Function Analysis in Cardiac Patients with Focus on Sex-Specific Aspects. In: Kerkhof, P., Miller, V. (eds) Sex-Specific Analysis of Cardiovascular Function. Advances in Experimental Medicine and Biology, vol 1065. Springer, Cham. https://doi.org/10.1007/978-3-319-77932-4_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-77932-4_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77931-7
Online ISBN: 978-3-319-77932-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)