Abstract
The emerging role of the endocannabinoid system (ECS) in the control of symptoms and disease progression in multiple sclerosis (MS) has been highlighted by recent studies. MS is a chronic, immune-mediated, and demyelinating disorder of the central nervous system with no cure so far. It is widely reported that cannabinoids might be used to control MS symptoms and that they also might exert neuroprotective effects and slow down disease progression. The aim of this chapter is to give an overview of the main endogenous and synthetic cannabinoids used for the symptomatic amelioration of MS and their beneficial outcomes, providing new possible perspectives for the treatment of this disease.
This chapter is based in great parts on the Open Access article: Medicines (Basel) 2018, 5(3): 91. doi: 10.3390/medicines5030091.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple sclerosis
- Endocannabinoid system
- Cannabinoid receptors
- Monoacylglycerol lipase (MAGL) inhibitors
- Fatty acid amide hydrolase (FAAH) inhibitors
- Arachidonoylethanolamine (AEA) reuptake inhibitors
8.1 Introduction
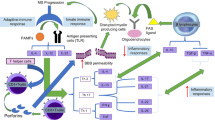
Multiple sclerosis (MS) is an important neurological disease that affects the central nervous system (CNS). It is the most common neurological disorder in young adults and affects approximately 2.3 million people worldwide (Browne et al. 2014). MS is more common in women than in men (Koch-Henriksen and Sørensen 2010; Koch-Henriksen et al. 2018), with a prevalence ratio of 3:1 (Dunn et al. 2015b; Dunn et al. 2015a). Regarding its etiology, it is now widely accepted that genetic and environmental factors may contribute to the onset and development of the disease (Hafler et al. 2007; Huynh and Casaccia 2013). MS is a chronic inflammatory immune-mediated condition characterized by demyelination of the axons in the CNS. It gradually leads to progressive neurodegeneration that damages CNS myelin, leading to neuronal dysfunction and a broad spectrum of neurological symptoms that depend upon the site where lesions have occurred in the brain and spinal cord. The symptoms of MS include spasticity, sensory alterations, weakness, painful spasms, bladder dysfunction, tremor, ataxia, optic neuritis, fatigue, and dysphagia (Compston 2008).
Molecular mechanisms of MS progression remain unclear. However, the observed hallmarks are considered as a consequence of three synergistically mechanisms: inflammation, demyelination, and axonal damage. Recent evidence indicates that MS is primarily a neurodegenerative disease that starts in the brain and then develops because of inflammation (Lassmann et al. 2012). This hypothesis has led to two models of MS immune-pathogenesis: the “inside-out” and “outside-in.” In the first model, a dysfunction of brain cells causes the immune response that destroys myelin and leads to blood-brain barrier (BBB) breakdown. In the second model, a dysfunction of the periphery leads to BBB damage, myelin disruption, and axonal death (Stys et al. 2012). The subsequent high presence of lymphocytes in the CNS and the activation of innate immune cells (dendritic cell, macrophages, and microglia) play key roles in MS pathogenesis. The activation of autoimmune cells, resident microglia, astrocytes, and macrophages, results in an immunological storm that involves abundant secretion of reactive species, cytokines, chemokines, autoantibody production, and enhanced excitotoxicity. There is a continuing activation of resident microglia and astrocytes producing pro-inflammatory mediators that potentiate the neuroinflammatory response. This results in oligodendrocytes and axonal damage, and ultimately in demyelination, synaptic alteration, and neuronal loss (Compston 2008; Dutta and Trapp 2011; Calabrese et al. 2015; Mahad et al. 2015). In the early phases of MS, the oligodendrocytes generate new myelin, and this remyelination is one of the reasons why symptoms decrease or temporarily disappear in relapsing-remitting MS (RR-MS) (Peferoen et al. 2014), which is the most common form of MS (approximately 85–90% of all cases) (Compston 2008) and it is typified by unpredictable relapses with full recovery or with sequelae. However, the myelin sheaths are not completely rebuilt by oligodendrocytes, and repeated attacks lead to damage in the axons where scar-like plaques build up with subsequent axonal loss (Cambron et al. 2012), associated with the characteristic symptoms of MS (Polman et al. 2011).
Over the last 15 years, a great amount of preclinical studies has demonstrated that compounds targeting the endocannabinoid system (ECS) exert anti-inflammatory properties, neuroprotective and immunomodulatory effects (Chiurchiù et al. 2015b), allowing them to alleviate symptoms and to limit progressive neurodegeneration in animal models of MS (Chiurchiù et al. 2018).
Cannabinoids exert neuroprotective effects acting at multiple molecular sites that are in all key cellular elements for the control of neuronal survival (e.g., neurons, astrocytes, resting and reactive microglia, and oligodendrocytes) and also in key brain structures (e.g., BBB) (Fernández-Ruiz et al. 2015). These effects are due to activation of two G protein-coupled receptors, the type-1 (CB1R) and type-2 (CB2R) cannabinoid receptors.
CB1R is widely expressed within the CNS (cortical neurons and interneurons, oligodendrocytes, and astrocytes) and also in several leukocytes infiltrating the brain (Galve-Roperh et al. 2013). Initially, CB2R has been restricted exclusively to immune cells (macrophages, mast cells, and B and T lymphocytes) and immune organs (spleen, thymus, and lymph nodes) (Howlett et al. 2002). However, some evidence showed the expression of CB2R in microglia of the CNS (Klegeris et al. 2003), and more recently, it has been also reported to be expressed in brainstem neurons and astrocytes upon cellular activation by an insult or inflammation (Chiurchiù et al. 2015b; Atwood and Mackie 2010; Chiurchiù et al. 2015a).
The multiplicity of action of cannabinoids allows reducing the excitotoxicity by acting through neuronal CB1R, as well as the toxic influence of reactive microgliosis by acting through microglial CB2R, or enhancing the trophic and metabolic support to neurons by acting through astroglial CB1R and/or CB2R. In particular, the activation of CB1R provides neuroprotection regulating glutamate homeostasis (Docagne et al. 2007). In fact, it is well known that glutamate is a key mediator in neuronal and oligodendrocyte damage in MS (Musella et al. 2014), and CB1R agonists exert direct neuroprotective effects by limiting glutamate release and the excitotoxic damage characteristic of several neurodegenerative disorders (Fernández-Ruiz et al. 2010).
Furthermore, the protective effects of CB2R activation in microglial cells upon inflammatory-induced CNS damage have been demonstrated in preclinical models of MS (Fernández-Ruiz et al. 2010). Microglia may be in two activated states: M1 and M2. The classical M1 state is characterized by release of pro-inflammatory factors, i.e., interleukins (IL-1beta, IL-18, and IL-6), prostanoids, and inducible nitric oxide synthase (NOS2)-derived NO. On the other hand, the neuroprotective M2 state, known as “alternative activation”, is associated with the release of anti-inflammatory factors, such as IL-10, IL-4, and NGF (Orihuela et al. 2016). Microglia has a functional endocannabinoid signaling system, composed of cannabinoid receptors and the complete machinery for the synthesis and degradation of endocannabinoids. The expression of cannabinoid receptors, mainly CB2R, and the production of endocannabinoids have been related to the activation profile of these cells (Mecha et al. 2016).
In preclinical studies, the beneficial effects of cannabinoids have been reported in different animal models of MS that are highly useful for studying different aspects of inflammation, demyelination, remyelination, and neurodegeneration in the CNS (Lassmann and Bradl 2017). Anyway, so far, none of the available models is able to cover the entire spectrum of clinical, immunological, and pathological features of the disease. For this reason, in order to have the complexity of MS fully replicated, multiple models should be used. The animal models more used are: – Experimental Autoimmune Encephalomyelitis (EAE), which is a useful animal model of MS since many of the pathologies observed in the CNS of mice with EAE show strong similarity to those found in the CNS of MS patients (McCarthy et al. 2012); – chronic relapsing experimental allergic encephalomyelitis (CREAE) is an animal model, which also presents relapsing-remitting paralytic episodes and tremor and spasticity of limb muscles during postrelapse remission strongly similar to those of MS (Heremans et al. 1996); – Theiler’s murine encephalomyelitis virus (TMEV), which is an animal model characterized by inflammation and demyelination similar to those described in MS patients (Lassmann and Bradl 2017).
8.2 Medicinal Cannabinoids
The significant interest for introducing cannabinoid-based medicines into clinical practice for the treatment of MS substantially derived from anecdotal reports of MS patients that experienced symptomatic relief after recreational use of cannabis (i.e., smoking) (Clark et al. 2004). These pieces of evidence have indeed stimulated scientific research regarding the use of cannabinoids in this therapeutic field. Therefore, the efficacy of various cannabinoid preparations in symptomatic treatment of MS and other neurological disorders has been evaluated in several clinical studies in human patients (Fife et al. 2015). The medicinal grade cannabinoids that are licensed for MS treatment change from country to country; off-label use also varies widely (Fife et al. 2015; Johnson 2013; Gloss and Maa 2015).
The four licensed cannabinoid-based medicines currently available are Marinol®, Cesamet®, Sativex®, and Epidiolex®. The active principle of Marinol® is dronabinol, i.e., synthetic Δ9-tetrahydrocannabinol (Δ9-THC); Cesamet® is based on nabilone (a synthetic analog of Δ9-THC); nabiximols, a standardized ~1:1 (w/w) mix of Δ9-THC and cannabidiol (CBD), both extracted from Cannabis sativa, is the active principle of Sativex®; Epidiolex®, whose active principle is CBD, has been very recently approved for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients of 2 years of age and older.
Other preparations based on natural cannabinoids contain essentially various quantitative ratios of Δ9-THC and CBD, i.e., Bedrocan® (22% Δ9-THC and < 1% CBD from Cannabis sativa), Bedrobinol® (13.5% Δ9-THC and < 1% CBD from Cannabis sativa), Bediol® (6.5% Δ9-THC and 8% CBD from Cannabis sativa), Bedica® (14% Δ9-THC and < 1% CBD from Cannabis indica), Bedrolite® (<1% Δ9-THC and 9% CBD from Cannabis sativa), and Bedropuur® (20%–24% Δ9-THC and < 1% CBD from Cannabis indica).
The structures of the above-mentioned natural and synthetic cannabinoids that have been studied for the treatment of MS are shown in Table 8.1.
In the following paragraphs of this part, an overview of the current findings about dronabinol, nabilone, and nabiximols in the treatment of MS will be provided.
8.2.1 Dronabinol
The synthetic pure isomer (−)-trans-Δ9-tetrahydrocannabinol (the main THC isomer found in the cannabis plant) is officially called “dronabinol”. Its original indication was the treatment of chemotherapy-induced nausea and vomiting (CINV). Subsequently, its use has been extended to anorexia associated with weight loss in patients affected with AIDS. These indications are still retained.
Soft gelatin capsules with a range of three dosages (2.5, 5, and 10 mg) are the current pharmaceutical form of this drug.
Efficacy and safety of dronabinol in treating MS symptoms has been specifically evaluated in 10 clinical studies published between 1981 and 2013 (Killestein et al. 2002; Zajicek et al. 2003; Clifford 1983; Killestein et al. 2003; Svendsen et al. 2004; Zajicek et al. 2005; Petro and Ellenberger 1981; Ungerleider et al. 1987; Freeman et al. 2006; Zajicek et al. 2013); almost all of these studies are reported in 10 reviews published between 2003 and 2016 (Shakespeare et al. 2003; Whiting et al. 2015; Ben Amar 2006; Zhornitsky and Potvin 2012; Koppel et al. 2014; Karst et al. 2010; Jawahar et al. 2013; Mills et al. 2007; Andrzejewski et al. 2016; Wang et al. 2008) that have been included in a systematic review of reviews on the basis of eligibility criteria of methodological quality (AMSTAR Tool: A Measurement Tool to Assess systematic Reviews) (Nielsen et al. 2018). In particular, with the aim of providing an overview of the current findings about dronabinol, eight key clinical outcomes in MS have been considered: disability/disease progression, pain, spasticity, bladder function, ataxia/tremor, sleep, quality of life, and adverse effects (Nielsen et al. 2018). Table 8.1 shows a summary of clinical evidence for dronabinol.
Regarding the pain related to MS, positive results were found (Zajicek et al. 2003; Svendsen et al. 2004; Zajicek et al. 2005; Petro and Ellenberger 1981). In the assessment of ataxia and tremor, substantially no change was evidenced (Killestein et al. 2002; Zajicek et al. 2003; Clifford 1983); the same for disability and disease progression (Killestein et al. 2002; Zajicek et al. 2003; Clifford 1983; Killestein et al. 2003; Zajicek et al. 2013). Indeed, in some cases, negative effects have been detected (Killestein et al. 2002). In a noteworthy CUPID study (Zajicek et al. 2013), a large amount of data concerning treatment with dronabinol has been provided, showing that it has no overall effect on the progression of MS. Mixed findings, although mostly positive, were highlighted regarding the quality of sleep (Zajicek et al. 2003; Zajicek et al. 2005). For the rest of the clinical outcomes (spasticity (Killestein et al. 2002; Zajicek et al. 2003; Zajicek et al. 2005; Petro and Ellenberger 1981; Ungerleider et al. 1987), bladder function (Zajicek et al. 2003; Freeman et al. 2006), and quality of life (Killestein et al. 2002; Zajicek et al. 2003)), mixed findings were also reported. The main adverse effects reported for dronabinol are dizziness, euphoria, dry mouth, fatigue, and drowsiness (Killestein et al. 2002; Zajicek et al. 2003; Clifford 1983; Svendsen et al. 2004; Zajicek et al. 2005; Petro and Ellenberger 1981; Ungerleider et al. 1987; Freeman et al. 2006); however, these effects have been described more frequently as mild to moderate. With the exception of patients with pre-existing cognitive disfunctions, cognitive impairment associated with the use of dronabinol did not seem to be relevant (Killestein et al. 2002; Zajicek et al. 2003; Svendsen et al. 2004; Zajicek et al. 2005; Freeman et al. 2006).
On the basis of the above-mentioned results, the only significant clinical evidence regarding dronabinol relates to its ability to relieve pain associated with MS, while quite inconsistent conclusions can be made for the other clinical outcomes.
8.2.2 Nabilone
Nabilone is a synthetic dibenzopyran-9-one analog of Δ9-THC (Table 8.1), available as a racemic mixture of (S,S)-(+)- and (R,R)-(−)-isomers. In 1985, it was originally licensed for the treatment of CINV in patients not responding to conventional antiemetic therapies. The use of nabilone for this therapeutic application has been partially supplanted by the development of serotonin 5-HT3 receptor antagonists.
The current pharmaceutical form of nabilone consists of capsules in strengths of 0.25, 0.5, and 1 mg. The effectiveness of nabilone in the treatment of neuropathic, chronic and cancer pain, and spasticity related to MS has recently been addressed. However, there has been a minimal amount of research on its use beyond its license, over the last two decades. In fact, it has been specifically evaluated for its effectiveness and safety in treating MS symptoms in only three clinical studies published between 1995 and 2015 (Martyn et al. 1995; Wissel et al. 2006; Turcotte et al. 2015). These studies are reported in four reviews published between 2006 and 2015 (Whiting et al. 2015; Ben Amar 2006; Koppel et al. 2014; Karst et al. 2010) included in a systematic review of reviews on the basis of eligibility criteria of methodological quality (AMSTAR Tool) (Nielsen et al. 2018). As mentioned above, eight MS clinical outcomes have been considered: disability/disease progression, pain, spasticity, bladder function, ataxia/tremor, sleep, quality of life, and adverse effects (Nielsen et al. 2018). A summary of clinical evidence about nabilone is shown in Table 8.1.
Regarding pain (Martyn et al. 1995), spasticity (Martyn et al. 1995; Wissel et al. 2006), and bladder dysfunction (Martyn et al. 1995) related to MS, positive effects due to nabilone were found. Mixed findings (although mostly positive) emerged in the evaluation of quality of life (Martyn et al. 1995; Turcotte et al. 2015): one study reported a significant improvement in objective rating of general health status (Martyn et al. 1995); another study, in which nabilone was evaluated as an adjunctive drug to gabapentin, reported an improvement in patient global impression of change, but no statistically significant difference in “VAS impact” between nabilone and placebo groups (Turcotte et al. 2015) (“VAS impact” refers to influence of pain on patient’s daily activities, recorded using a visual analog scale). Currently, there are no studies about the effect of nabilone on sleep quality of MS patients and on disability/disease progression. Moderate sedation, dizziness, and moderate weakness in the legs are the main adverse effects reported for nabilone (Martyn et al. 1995; Wissel et al. 2006).
It can be concluded that concerning three clinical outcomes related to MS, i.e., pain, spasticity, and bladder problems, there is positive evidence for nabilone.
8.2.3 Nabiximols
First approved in Canada in 2005 for the treatment of neuropathic pain associated with MS and suddenly approved (2007) in the same country as adjunctive analgesic treatment of advanced cancer pain, nabiximols is a specific extract from cloned plants of Cannabis sativa consisting of an approximate 1:1 fixed ratio of Δ9-THC and CBD (Russo and Guy 2006).
Several European countries approved the drug in the following years, and today it is available in about 20 countries worldwide for the treatment of MS-related moderate to severe spasticity in patients not responsive to other antispasticity therapies.
Nabiximols was developed in response to widespread anecdotal reports about the usefulness of cannabis for treating various symptoms related to MS. The introduction of nonpsychoactive phytocannabinoid CBD in the drug essentially aims to reduce side effects of Δ9-THC.
Sativex® is the trade name of the drug based on nabiximols; it is a pharmaceutical product standardized in composition, formulation, and dosage. It is formulated as an oro-mucosal spray containing 27 mg of Δ9-THC and 25 mg of CBD/1.0 mL, in an aromatized water–ethanol solution. Each spray (or “puff”) delivers 0.1 mL of solution, which corresponds to 2.7 mg of Δ9-THC and 2.5 mg of CBD. Sativex® is available as 5.5 mL spray bottles (maximum 48 sprays) or as 10 mL spray bottles (maximum 90 sprays).
Being the absorption after an oro-mucosal administration slower with respect to inhalation, the high plasma levels that occur when cannabis is smoked or vaporized are avoided. The oro-mucosal administration is more rapid and consistent than the oral administration (Novotna et al. 2011), allowing a more direct access to blood vessels through the mucosa and, as a consequence, a more rapid plateau of plasma concentration, without the problems related to the oral route (Giacoppo et al. 2017). Furthermore, the delivery system in such a formulation is very simple for the patients, allowing them to self-manage a convenient and accurate titration of dosage.
The effectiveness and safety of nabiximols in treating MS symptoms has been widely evaluated in 11 clinical studies published between 2004 and 2014 (Wade et al. 2004; Collin et al. 2010; Centonze et al. 2009; Rog et al. 2005; Wade et al. 2006; Conte et al. 2009; Rog et al. 2007; Langford et al. 2013; Collin et al. 2007; Leocani et al. 2014; Kavia et al. 2010); these studies are reported in 11 reviews published between 2006 and 2017 (Whiting et al. 2015; Ben Amar 2006; Koppel et al. 2014; Karst et al. 2010; Jawahar et al. 2013; Mills et al. 2007; Andrzejewski et al. 2016; Wang et al. 2008; Giacoppo et al. 2017; Lakhan and Rowland 2009; Keating 2017). Most of these reviews have been included in a systematic review of reviews on the basis of eligibility criteria of methodological quality (AMSTAR Tool) (Nielsen et al. 2018). The eight main clinical outcomes related to MS that have been considered are the same reported in the above paragraphs, i.e., disability/disease progression, pain, spasticity, bladder function, ataxia/tremor, sleep, quality of life, and adverse effects (Nielsen et al. 2018). Table 8.1 shows a summary of clinical evidence about nabiximols.
Most of the results support the use of nabiximols for MS-related pain. In fact, a significant reduction in numeric rating scales (NRS) and visual analogic scales (VAS) score was highlighted in the treated group with respect to placebo group in many randomized controlled trials (RCT) (Wade et al. 2004; Collin et al. 2010; Centonze et al. 2009; Rog et al. 2005; Wade et al. 2006; Conte et al. 2009; Rog et al. 2007; Langford et al. 2013). The effectiveness of nabiximols in the treatment of spasticity associated with MS is highlighted in some clinical trials, in particular regarding the patient’s subjective evaluation scales (NRS) (Wade et al. 2004; Collin et al. 2010; Collin et al. 2007; Leocani et al. 2014); the data concerning the objective evaluation scales (Ashworth scale (AS) and modified Ashworth scale (MAS)), although in favor of the nabiximols, are not statistically significant in some cases (Collin et al. 2010; Collin et al. 2007). On the other hand, no change in spasticity was found in some studies (Centonze et al. 2009; Conte et al. 2009). The usefulness of nabiximols in ameliorating overall bladder symptoms related to MS is controversial, given the contradictory evidence emerged in diverse studies (Wade et al. 2004; Kavia et al. 2010). Nevertheless, this drug seems to be effective in reducing the number of bladder voids per day (Kavia et al. 2010). While nabiximols has been shown to improve subjective quality of sleep (Wade et al. 2004), no statistically significant positive change in tremor and ataxia associated with MS has been demonstrated to date (Wade et al. 2004; Collin et al. 2010). Mixed findings are in general reported about the quality of life; however, in some cases, a significant average number of MS patients reported an improvement of the global impression of change following the treatment with nabiximols (Wade et al. 2004; Rog et al. 2005; Langford et al. 2013; Collin et al. 2007). The main adverse effects associated with nabiximols are drowsiness, dizziness, headache, fatigue, impaired balance, and disturbance in attention (Wade et al. 2004; Collin et al. 2010; Centonze et al. 2009; Rog et al. 2005; Wade et al. 2006; Conte et al. 2009; Rog et al. 2007; Collin et al. 2007). These effects are referred to as mild to moderate, and generally well tolerated. Clinical studies about nabiximols have not shown any significant change in parameters that can be referred to disability and disease progression; these include the Barthel index of activity of daily living (ADL) and walking time (10 mt) (Wade et al. 2004; Collin et al. 2010).
These extensive clinical evidences indicate overall that pain, spasticity and quality of sleep in MS patients are the indications for which nabiximols could represent a valid therapeutic option, and that in general the incidence of adverse effects (not serious and well tolerated) is quite low.
8.3 Endocannabinoid System Modulators
8.3.1 CB1R and CB2R Ligands
One of the first studies of cannabinoids’ effect in animal models of MS was reported by Lyman et al. in 1989 (Lyman et al. 1989), who showed the effects of daily administration of Δ9-THC, an active component of marijuana with partial CB1R agonist activity and limited effects on CB2R, on EAE progression in rats. Indeed, the development of EAE was ameliorated and the examination of central nervous system tissue revealed a marked reduction of inflammation in the Δ9-THC-treated animals with respect to control animals (Lyman et al. 1989) indicating that Δ9-THC was able to inhibit both clinical and histologic EAE. Subsequently, Wirguin et al. (Wirguin et al. 1994) reported the activity of Δ8-THC (Table 8.2) on EAE. This phytocannabinoid is more stable and less psychotropic than Δ9-THC and it binds CB1Rs with high affinity. Δ8-THC was shown to significantly reduce the incidence and severity of neurological manifestations of EAE. This compound was considered a prodrug, indeed the inhibition of the prostanoid production by action of an active metabolite of Δ8-THC, formed from the first-pass metabolism in the liver, was proposed as potential mechanisms of action. This hypothesis was supported by the evidence that the beneficial influence of Δ8-THC only occurred on oral administration and not with parenteral injection (Wirguin et al. 1994).
Several studies were developed regarding the role of endogenous and synthetic cannabinoids in CREAE animal model. Baker et al. evidenced that the CBR agonists R(+)-WIN 55,212–2 (Table 8.2), Δ9-THC, methanandamide (Table 8.2) and JWH-133 (Table 2) were able to ameliorate both tremor and spasticity in CREAE mice (Baker et al. 2000). In particular, a role of CB1Rs in controlling tremor and of both cannabinoid receptors in the development of spasticity was suggested (Baker et al. 2000). In the same work was reported that the endocannabinoid palmitoylethanolamide (PEA) (Table 8.2) caused a transient inhibition of spasticity (Baker et al. 2000). However, more recently, it was demonstrated that co-administration of PEA with CBD in EAE was not as active as treatment with each compound alone, indicating that these nonpsychoactive cannabinoids could have antagonistic interactions in EAE (Rahimi et al. 2015).
Further research showed that in TMEV-infected mice, WIN 55,212–2 (Table 8.2), arachidonyl-2-chloroethylamide (ACEA), a selective CB1R agonist (Table 8.2), and JWH-015, a weak selective CB2R agonist (Table 8.2), were able to improve motor function, to promote the remyelination, and to reduce microglial activation and the number of CD4+ infiltrated T cells (Arevalo-Martin et al. 2003). Further studies reported that WIN 55,212–2 restored self-tolerance to a myelin self-antigen while ameliorating the disease in a long-term manner. The therapeutic effect of WIN 55,212–2 correlated with a decrease in the activation of CD4+CD25+Foxp3− T cells and an increase in regulatory CD4+CD25+Foxp3+ T cells in the CNS, along with alterations in the cytokine and chemokine milieu. These findings demonstrated for the first time that the suppression of autoimmune responses to myelin antigens underlies the therapeutic effect of CBR agonists in the treatment of MS (Arevalo-Martin et al. 2003).
Recent studies were focused on the development and study of CB2R selective agonists as the best therapeutic approach for the treatment of MS, thanks to their lack of central side effects usually associated with a CB1R modulation.
First of all, the resorcinol derivative O-1966 (Table 8.2) was shown to significantly improve motor function in the chronic EAE model, at a concentration of 1 mg kg-1 and to reduce rolling and adhesion of endogenous leukocytes (Zhang et al. 2009). Moreover, the 1,4-dihydro-6-methylindeno[1,2-c]pyrazole derivative, Gp-1a (Table 8.2), was demonstrated to be able to reduce clinical scores and ameliorate the recovery in EAE mice presenting a long-term reduction in demyelination and axonal loss. Two different mechanisms were proposed for this compound, indeed at first, it was able to affect Th1/Th17 differentiation in peripheral immune organs and subsequently it affects pathogenic T cell accumulation in the CNS and reduces the expression of chemokine and adhesion molecules in the CNS (Kong et al. 2014).
Furthermore, in 2015, Han et al. reported that a new quinoline-2,4(1H,3H)-dione derivative, compound 21 (Table 8.2), with selective CB2R agonist activity, significantly reduced the clinical scores and symptoms of the EAE mice model, by remarkably decreasing leukocyte infiltration in the spinal cord and demyelination in white matter (Han et al. 2015).
In the same year, Fu et al. (Fu and Taylor 2015) showed that intrathecal administration of JWH-133 (Table 8.2), a selective CB2R agonist, in EAE mice, dose dependently reduced both mechanical and cold hypersensitivity without any signs of ataxia or sedation. The co-administration of JWH-133 with a selective CB2R antagonist dose dependently attenuated the inhibitory effects of JWH-133. These data suggested that the selective targeting of spinal CB2Rs reduced signs of neuropathic pain in EAE mice without any side effects (Fu and Taylor 2015).
During the following year, chromenopyrazole nucleus was identified as the promising scaffold to obtain CBR ligands (Morales et al. 2016). Structural modifications have been studied in order to achieve CB2R selectivity and the structural changes led to the synthesis of chromenoisoxazole derivative PM-226 (Table 8.2) as selective CB2R agonist. This compound dampened neuroinflammation in the TMEV mouse model by reducing microglial activation to levels close to those of the control group (Morales et al. 2016). This decrease in the microglia activation determined a reduction of inflammatory events and an improvement of the neurological status of treated animals (Mecha et al. 2013).
In 2017, Ying Shi et al. reported the identification of new potent and selective indole-based CB2R agonists (Shi et al. 2017) and one of them, compound 57 (Table 8.2), was selected to be studied in a EAE mouse model. This compound significantly showed to be able to alleviate the clinical symptoms and to protect the murine central nervous system from immune damage. Furthermore, histological examination of spinal cords demonstrated a significant reduction of leukocyte infiltration and the extent of demyelination (Shi et al. 2017).
Very recently, Navarrete et al. (Navarrete et al. 2018) provided evidence that VCE-004.8 (Table 8.2), an amino-quinone derivative of CBD, is a promising small molecule with multitarget activity, being a PPAR and CB2R agonist with potent anti-inflammatory activity. VCE-004.8 showed immunomodulatory activity in EAE and TMEV mice models, inhibiting several inflammatory chemokines, chemokines receptors, and cytokines that play a key role in the pathogenesis of MS. In addition, VCE-004.8 inhibited the expression of adhesion molecules such as VCAM and ICAM-1. Remarkable is the finding that VCE-004.8 strongly induced the expression of the hypoxia-inducible factor (HIF), which can have a beneficial role in MS by modulating the immune response and favoring neuroprotection and axonal regeneration (Navarrete et al. 2018).
The sesquiterpene β-caryophyllene (BCP) (Table 8.2) is a CB2R-selective agonist already reported in the literature for its anti-inflammatory and analgesic effects in mouse models of inflammatory and neuropathic pain (Sharma et al. 2016). Very recently, it is reported that BCP is able to attenuate disease progression by reducing mechanical hyperalgesia, inflammation, and pain in the EAE mouse model (Alberti et al. 2017). When BCP was co-administered with a selective CB2R antagonist, the effects were reversed, demonstrating that BCP action was CB2R-mediated (Alberti et al. 2017).
8.3.2 Inhibitors of Metabolic Enzymes of Endocannabinoids
An alternative approach to modulate ECS consists in the blocking of the metabolic enzymes of the two main endocannabinoids (ECs), 2-AG and AEA. This is an interesting therapeutic strategy, as enhancing EC levels is expected to preserve the beneficial effects derived from the direct activation of CBRs but limiting potential side effects mostly associated with direct CB1R agonists. Moreover, in MS patients, there is a significant alteration of the metabolic enzymes, mainly of FAAH and of MAGL (Benito et al. 2007; Chiurchiù et al. 2013). In particular, different studies using TMEV-infected mice showed that the inhibition of FAAH determines an improvement of the motor symptoms, with a reduction of inflammatory response and the downregulation of macrophage and of microglial function (Mestre et al. 2005; Ortega-Gutiérrez et al. 2005). Furthermore, it was demonstrated that chronic and long-term inhibition of FAAH, via genetic ablation, produces clinical remission and ameliorates long-term results in EAE mouse model (Webb et al. 2008).
Other studies showed that 2AG-treatment ameliorated the acute phase of disease with delay of disease onset and reduced disease mortality and long-term clinical disability in EAE models (Lourbopoulos et al. 2011). Moreover, the expression of cannabinoid receptors was increased and it was accompanied by an increase of the M2-macrophages in the perivascular infiltrations. These results indicated that 2-AG treatment may provide direct (via cannabinoid receptors) and immune (via M2 macrophages)-mediated neuroprotection in the EAE model (Lourbopoulos et al. 2011).
As 2-AG is the main endocannabinoid present in the brain, and it is a full agonist of both cannabinoid receptors, many studies were performed on the MAGL inhibitors. However, it was demonstrated that chronic MAGL inhibition and subsequently increase of 2-AG in the brain provoke a functional antagonism of the cerebral ECS, with tolerance to the analgesic effects of acute enzymatic inhibition, cross-tolerance to CB1R agonists, reduction of expression and function of the CB1Rs, and interruptions in endocannabinoid-dependent synaptic plasticity (Schlosburg et al. 2010).
In contrast, a recent work reported that the chronic administration of JZL184 reduced the neurological consequences of disease progression in EAE mice, reducing the myelin loss and inflammation of spinal cord white matter (Bernal-Chico et al. 2015). Furthermore, it was demonstrated that the repeated administration of JZL184 at a dose of 8 mg kg−1 did not provoke changes in CB1R expression in the hippocampus, and there was not tolerance to the anxiolytic and analgesic effects of the MAGL inhibitor (Bernal-Chico et al. 2015).
Recently, Brindisi et al. reported the β-lactam-based compound 4a (Table 8.3) as a very potent hMAGL inhibitor, with high selectivity toward FAAH, other serine hydrolases and cannabinoid receptors (Brindisi et al. 2016). This compound exerted a surprising beneficial effect on the progression of the MS disease in EAE model, due to the CB1R- and CB2R-mediated action. Histological evaluation of myelin demonstrated a significant reduction of the demyelinated area in the EAE mice treated with compound 4a (Brindisi et al. 2016). Finally, oral administration of 4a at 1 mg kg−1 dose dependently reversed the lowering of the threshold to cold stimuli (cold plate test) induced by oxaliplatin (OXP), indicating its efficacy in the treatment of neuropathic pain, which clearly depends on the increased levels of 2-AG and the subsequent indirect modulation of cannabinoid receptors (Brindisi et al. 2016).
As above reported, the irreversible MAGL inhibition causes pharmacological tolerance and receptor desensitization. For these reasons, some studies on reversible inhibitors were developed. An interesting example is given by the compound 21 (Table 8.3) synthesized by Hernández-Torres et al. (Hernández-Torres et al. 2014). This compound showed a sub-micromolar IC50 value for MAGL inhibition and very good selectivity against FAAH, ABDH6, ABHD12, and both CBRs (Hernández-Torres et al. 2014). In EAE mouse, it demonstrated to significantly increase the levels of 2-AG in spinal cord, improving clinical symptoms and decreasing tissue damage in the spinal cords. Importantly, catalepsy or other motor impairments that are observed after the administration of irreversible MAGL inhibitors, did not occurred.
The negative effects due to the prolonged inhibition of MAGL enzymes do not occur by FAAH inhibition. Indeed, it was demonstrated that the prolonged inhibition of FAAH produced no tolerance or no changes in the expression or function of the CB1R (Pryce et al. 2013). Pryce et al. demonstrated that potent FAAH inhibitors such as CAY10402 (Table 8.3) and CAY10400 (Table 8.3) inhibited spasticity but did not induce any hypothermia, typical of cannabimimetic effects. However, CAY10400 and CAY10402 have poor pharmacokinetics, and therefore, their development is unlikely as therapeutic drugs (Pryce et al. 2013).
A valid alternative is represented by compound URB597 (Table 8.3), which is a potent irreversible FAAH inhibitor with an improved pharmacokinetic profile (Pryce et al. 2013). The administration of URB597 induced spasticity alleviation immediately without an increased effect after four daily doses. However, the use of this inhibitor was not associated with CB1R tolerance. Actually, the study emphasized the benefit because the level of spasticity at the baseline after four administrations was lower than the baseline before treatment.
Nevertheless, the inhibition of the above-reported enzymes, MAGL and FAAH, can also drive to enhanced neurotoxicity due to an increase in the availability of endocannabinoids, which, together with elevated COX-2 activity, may convert endocannabinoids in new oxygenated derivatives, so-called prostamides (derived from AEA) or prostaglandin-glycerylesters (derived from 2-AG), which may be highly toxic for neurons (Alhouayek and Muccioli 2014).
Another effective approach to modulate the ECS is to act on AEA reuptake, and on this basis, many selective inhibitors of cellular reuptake of AEA have been developed. In particular, compounds O-3246 and O-3262 (Table 8.3) were reported to have very high potency as inhibitors of AEA cellular uptake and a negligible activity as FAAH inhibitors, CB1R and CB2R ligands, and TRPV1 agonists. These compounds have been shown to inhibit spasticity in CREAE mice, confirming the potential utility of selective AEA uptake inhibitors as antispasticity drugs in MS (Ligresti et al. 2006).
Furthermore, it has been found that UCM707 (Table 8.3), a potent and selective inhibitor of the AEA reuptake (Ortega-Gutiérrez et al. 2005), was able to improve the motor function in a TMEV-IDD mouse model, and at the histological level, it reduced microglial activation, diminished major histocompatibility complex class II antigen expression and decreased cellular infiltrates in the spinal cord (Ortega-Gutiérrez et al. 2005). Additionally, in microglial cells, UCM707 decreases the production of the proinflammatory cytokines tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, and IL-6; reduces nitric oxide levels and inducible nitric oxide synthase expression; and is able to potentiate the action of a subeffective dose of the endocannabinoid anandamide. These results confirm the role played by the ECS at the level of immunomodulation, and they are in agreement with experiments that describe how the blockade of microglial activation represses the development of the EAE model of MS (Ortega-Gutiérrez et al. 2005).
8.4 Conclusions
Millions of people worldwide are affected by MS, a progressive neurodegenerative disease without any effective cure so far and whose symptoms are still difficult to manage. The modulation of distinct components of ECS (CBRs, degrading enzymes, and AEA transporters) may represent a new and promising therapeutic strategy to control symptoms and disease progression of MS, as demonstrated by recent studies performed in animal models of MS. It has been reported that cannabinoids can relieve symptoms of MS by essentially activating the CB1R. The increase of endocannabinoid levels through the inhibition of the degrading enzymes of AEA and/or 2-AG (FAAH and MAGL, respectively) and of the AEA transporter can lead to the amelioration of spasticity. Moreover, the changes reported for the ECS in different MS models have been associated with adaptive responses for limiting neuronal damage. Specifically, the activation of CB1R regulates glutamate homeostasis and excitotoxic damage, providing neuroprotection. Furthermore, it has been has been shown in preclinical models of MS that CB2R activation has a protective effect in microglial cells upon inflammatory-induced CNS damage. Finally, an enhancing trophic and metabolic support to neurons is mediated by astroglial CB1R and/or CB2R, thus reducing excitotoxicity and leading neuroprotection. On the basis of the above results, it is reasonable to conceive a synergistic action between the simultaneous modulation of more targets of ECS and conventional therapies, producing more beneficial effects. Therefore, the study of multitarget modulators of ECS has been emerging in the last few years, to achieve this goal. These agents offer the possibility of modulating the ECS in a safer and more effective way with respect to a single target modulation, directly and indirectly modulating cannabinoid receptor activity through different mechanisms of action (Chicca et al. 2018). Although there is still a need for extensive preclinical studies, we can hypothesize that multitarget modulators of ECS could be able to control disease progression and symptoms MS, possibly having a great translational potential and representing promising candidates for clinical development.
References
Alberti TB, Barbosa WL, Vieira JL et al (2017) (−)-β-Caryophyllene, a CB2 receptor-selective Phytocannabinoid, suppresses motor paralysis and Neuroinflammation in a murine model of multiple sclerosis. Int J Mol Sci 18:691
Alhouayek M, Muccioli GG (2014) COX-2-derived endocannabinoid metabolites as novel inflammatory mediators trends. Pharmacol Sci 35:284–292
Andrzejewski K, Barbano R, Mink J (2016) Cannabinoids in the treatment of movement disorders: a systematic review of case series and clinical trials. Basal Ganglia 6:73–181
Arevalo-Martin A, Vela JM, Molina-Holgado E et al (2003) Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci 23:2511–2516
Atwood BK, Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160:467–479
Baker D, Pryce G, Croxford JL et al (2000) Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404:84–87
Ben Amar M (2006) Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol 105:1–25
Benito C, Romero JP, Tolón RM et al (2007) Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci 27:2396–2402
Bernal-Chico A, Canedo M, Manterola A et al (2015) Blockade of monoacylglycerol lipase inhibits oligodendrocyte excitotoxicity and prevents demyelination in vivo. Glia 63:163–176
Brindisi M, Maramai S, Gemma S et al (2016) Development and pharmacological characterization of selective blockers of 2-arachidonoyl glycerol degradation with efficacy in rodent models of multiple sclerosis and pain. J Med Chem 59:612–2632
Browne P, Chandraratna D, Angood C et al (2014) Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 83:1022–1024
Calabrese M, Magliozzi R, Ciccarelli O et al (2015) Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci 16:147–158
Cambron M, D’Haeseleer M, Laureys G et al (2012) White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J Cereb Blood Flow Metab 323:413–424
Centonze D, Mori F, Kock G et al (2009) Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci 30:531–534
Chicca A, Arena C, Bertini S et al (2018) Polypharmacological profile of 1,2-dihydro-2-oxo-pyridine-3-carboxamides in the endocannabinoid system. Eur J Med Chem 154:155–171
Chiurchiù V, Battistini L, Maccarrone M (2015a) Endocannabinoid signaling in innate and adaptive immunity. Immunology 144:352–364
Chiurchiù V, Cencioni MT, Bisicchia E et al (2013) Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol 73:626–636
Chiurchiù V, Leuti A, Maccarrone M (2015b) Cannabinoid signaling and neuroinflammatory diseases: a melting pot for the regulation of brain immune responses. J Neuroimmune Pharmacol 10:268–280
Chiurchiù V, van der Stelt M, Centonze D et al (2018) The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: clues for other neuroinflammatory diseases. Prog Neurobiol 160:82–100
Clark AJ, Ware MA, Yazer E et al (2004) Patterns of cannabis use among patients with multiple sclerosis. Neurology 62:2098–2100
Clifford DB (1983) Tetrahydrocannabinol for tremor in multiple sclerosis. Ann Neurol 13:669–671
Collin C, Davies P, Mutiboko IK et al (2007) Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 14:290–296
Collin C, Ehler E, Waberzinek G et al (2010) A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res 32:451–459
Compston A, Coles A (2008) A Multiple sclerosis. Lancet 372:1502–1517
Conte A, Bettolo CM, Onesti E et al (2009) Cannabinoid-induced effects on the nociceptive system: a neurophysiological study in patients with secondary progressive multiple sclerosis. Eur J Pain 13:472–477
Docagne F, Muñetón V, Clemente D et al (2007) Excitotoxicity in a chronic model of multiple sclerosis: neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci 34:551–561
Dunn SE, Gunde E, Lee H (2015a) Sex-based differences in multiple sclerosis (MS): part II: rising incidence of multiple sclerosis in women and the vulnerability of men to progression of this disease. Curr Top Behav Neurosci 26:57–86
Dunn SE, Lee H, Pavri FR et al (2015b) Sex-based differences in multiple sclerosis (part I): biology of disease incidence. Curr Top Behav Neurosci 26:29–56
Dutta R, Trapp BD (2011) Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 93:1–12
Fernández-Ruiz J, García C, Sagredo O, Gómez-Ruiz M et al (2010) The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets 14:387–404
Fernández-Ruiz J, Romero J, Ramos JA (2015) Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease and others. Handb Exp Pharmacol 231:233–259
Fife TD, Moawad H, Moschonas C et al (2015) Clinical perspectives on medical marijuana (cannabis) for neurologic disorders. Neurol Clin Pract 5:44–351
Freeman RM, Adekanmi O, Waterfield MR et al (2006) The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicenter, randomized placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct 17:636–641
Fu W, Taylor BK (2015) Activation of cannabinoid CB2 receptors reduces hyperalgesia in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Neurosci Lett 595:1–6
Galve-Roperh I, Chiurchiù V, Díaz-Alonso J et al (2013) Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res 52:633–650
Giacoppo S, Bramanti P, Mazzon E (2017) Sativex in the management of multiple sclerosis-related spasticity: an overview of the last decade of clinical evaluation. Mult Scler Relat Disord 17:22–31
Gloss DS, Maa EH (2015) Medical marijuana. Between a plant and a hard place. Neurol Clin Pract 5:281–284
Hafler DA, Compston A, Sawcer S et al (2007) Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357:851–862
Han S, Zhang FF, Qian HY et al (2015) Development of Quinoline-2,4(1H,3H)-diones as potent and selective ligands of the cannabinoid type 2 receptor. J Med Chem 58:5751–5769
Heremans H, Dillen C, Groenen M et al (1996) Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in mice: enhancement by monoclonal antibodies against interferon-gamma. Eur J Immunol 26:2393–2398
Hernández-Torres G, Cipriano M, Hedén E et al (2014) A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew Chem Int Ed Engl 53:13765–13770
Howlett AC, Barth F, Bonner TI et al (2002) International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202
Huynh JL, Casaccia P (2013) Epigenetic mechanisms in multiple sclerosis: implications for pathogenesis and treatment. Lancet Neurol 12:95–206
Jawahar R, Oh U, Yang S et al (2013) A systematic review of pharmacological pain management in multiple sclerosis. Drugs 73:1711–1722
Johnson C (2013) Shared care guideline: Nabilone in the Management of Chronic Neuropathic Pain that has failed to respond to other first and second line treatments. Lincolnshire in Association with United Lincolnshire Hospitals Trust, NHS
Karst M, Wippermann S, Ahrens J (2010) Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 70:2409–2438
Kavia RB, De Ridder D, Constantinescu CS et al (2010) Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler 16:1349–1359
Keating GM (2017) Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal spray (Sativex®): a review in multiple sclerosis-related spasticity. Drugs 77:563–574
Killestein J, Hoogervorst EL, Reif M et al (2002) Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 58:1404–1407
Killestein J, Hoogervorst EL, Reif M et al (2003) Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J Neuroimmunol 137:140–143
Klegeris A, Bissonnette CJ, McGeer PL (2003) Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol 139:775–786
Koch-Henriksen N, Sørensen PS (2010) The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 9:520–532
Koch-Henriksen N, Thygesen LC, Stenager E et al (2018) Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 90:e1954–e1963
Kong W, Li H, Tuma RF et al (2014) Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol 287:1–17
Koppel BS, Brust JCM, Fife T et al (2014) Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the guideline development Subcommittee of the American Academy of neurology. Neurology 82:1556–1563
Lakhan SE, Rowland M (2009) Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol 9:59
Langford RM, Mares J, Novotna A et al (2013) A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol 260:984–997
Lassmann H, Bradl M (2017) Multiple sclerosis: experimental models and reality. Acta Neuropathol 133:223–244
Lassmann H, van Horssen J, Mahad D (2012) Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 8:647–656
Leocani L, Nuara A, Houdayer E et al (2014) Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: a double-blind, placebo-controlled, crossover study. Mult Scler 20:498
Ligresti A, Cascio MG, Pryce G et al (2006) New potent and selective inhibitors of anandamide reuptake with antispastic activity in a mouse model of multiple sclerosis. Br J Pharmacol 147:83–91
Lourbopoulos A, Grigoriadis N, Lagoudaki R et al (2011) Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res 1390:126–141
Lyman WD, Sonett JR, Brosnan CF et al (1989) D9-Tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol 23:73–81
Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14:183–193
Martyn CN, Illis LS, Thom J (1995) Nabilone in the treatment of multiple sclerosis. Lancet 345:579
McCarthy DP, Richards MH, Miller SD (2012) Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler’s virus-induced demyelinating disease. Methods Mol Biol 900:381–401
Mecha M, Carrillo-Salinas FJ, Feliú A et al (2016) Microglia activation states and cannabinoid system: therapeutic implications. Pharmacol Ther 166:40–55
Mecha M, Carrillo-Salinas FJ, Mestre L et al (2013) Viral models of multiple sclerosis: Neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog Neurobiol 101–102:46–64
Mestre L, Correa F, Arévalo-Martín A et al (2005) Pharmacological modulation of the endocannabinoid system in a viral model of multiple sclerosis. J Neurochem 92:1327–1339
Mills RJ, Yap L, Young CA (2007) Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev 1:CD005029
Morales P, Gomez-Canas M, Navarro G et al (2016) Chromenopyrazole, a versatile cannabinoid scaffold with in vivo activity in a model of multiple sclerosis. J Med Chem 59:6753–6771
Musella A, Sepman H, Mandolesi G et al (2014) Pre- and postsynaptic type-1 cannabinoid receptors control the alterations of glutamate transmission in experimental autoimmune encephalomyelitis. Neuropharmacology 79:567–572
Navarrete C, Carrillo-Salinas F, Palomares B et al (2018) Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: implications for multiple sclerosis therapy. J Neuroinflammation 15:64–83
Nielsen S, Germanos R, Weier M et al (2018) The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Curr Neurol Neurosci Rep 18:8
Novotna A, Mares J, Ratcliffe S et al (2011) A randomized, doubleblind, placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 18:1122–1131
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665
Ortega-Gutiérrez S, Molina-Holgado E, Arévalo-Martín A et al (2005) Activation of the endocannabinoid system as therapeutic approach in a murine model of multiple sclerosis. FASEB J 19:1338–1340
Peferoen L, Kipp M, van der Valk P et al (2014) Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141:302–313
Petro DJ, Ellenberger C (1981) Treatment of human spasticity with delta-9-tetrahydrocannabinol. J Clin Pharmacol 21:413S–416S
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 692:292–302
Pryce G, Cabranes A, Fernández-Ruiz J et al (2013) Control of experimental spasticity by targeting the degradation of endocannabinoids using selective- fatty acid amide hydrolase inhibitors. Mult Scler 19:896–1904
Rahimi A, Faizi M, Talebi F et al (2015) Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience 290:79–287
Rog DJ, Nurmikko TJ, Friede T et al (2005) Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 65:812–819
Rog DJ, Nurmikko TJ, Young CA (2007) Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open label, 2-year extension trial. Clin Ther 29:2068–2079
Russo E, Guy GW (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66:34–246
Schlosburg JE, Blankman JL, Long JZ et al (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119
Shakespeare DT, Boggild M, Young C (2003) Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev 4:CD001332
Sharma C, Al Kaabi JM, Nurulain SM et al (2016) Polypharmacological properties and therapeutic potential of -caryophyllene: a dietary phytocannabinoid of pharmaceutical promise. Curr Pharm Des 22:3237–3264
Shi Y, Duan YH, Ji YY et al (2017) Amidoalkylindoles as potent and selective cannabinoid type 2 receptor agonists with in vivo efficacy in a mouse model of multiple sclerosis. J Med Chem 60:7067–7083
Stys PK, Zamponi GW, van Minnen J (2012) Will the real multiple sclerosis please stand up? Nat Rev Neurosci 137:507–514
Svendsen KB, Jensen TS, Bach FW (2004) Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomized double blind placebo controlled crossover trial. BMJ 329:253
Turcotte D, Doupe M, Torabi M et al (2015) Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Med 16:149–159
Ungerleider JT, Andrysiak T, Fairbanks L et al (1987) Δ-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse 7:39–50
Wade DT, Makela P, House H et al (2006) Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 12:639–645
Wade DT, Makela P, Robson P et al (2004) Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 10:434–441
Wang T, Collet JP, Shapiro S et al (2008) Adverse effects of medical cannabinoids: a systematic review. Can Med Assoc J 178:1669–1678
Webb M, Luo L, Ma JY et al (2008) Genetic deletion of fatty acid amide hydrolase results in improved long-term outcome in chronic autoimmune encephalitis. Neurosci Lett 439:106–110
Whiting PF, Wolff RF, Deshpande S et al (2015) Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313:2456–2473
Wirguin I, Mechoulam R, Breuer A et al (1994) Suppression of experimental autoimmune encephalomyelitis by cannabinoids. Immunopharmacology 28:209–214
Wissel J, Haydn T, Muller J et al (2006) Low dose treatment with the synthetic cannabinoid nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. J Neurol 253:1337–1341
Zajicek J, Ball S, Wright D et al (2013) Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomized, placebo-controlled trial. CUPID investigator group. Lancet Neurol 12:857–865
Zajicek J, Fox P, Sanders H et al (2003) Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo-controlled trial. Lancet 362:1517–1526
Zajicek JP, Sanders HP, Wright DE et al (2005) Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 76:1664–1669
Zhang M, Martin BR, Adler MW et al (2009) Modulation of cannabinoid receptor activation as a Neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol 4:249–259
Zhornitsky S, Potvin S (2012) Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals 5:529–552
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Manera, C., Bertini, S. (2021). Cannabinoid-Based Medicines and Multiple Sclerosis. In: Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M. (eds) Cannabinoids and Neuropsychiatric Disorders. Advances in Experimental Medicine and Biology, vol 1264. Springer, Cham. https://doi.org/10.1007/978-3-030-57369-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-57369-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57368-3
Online ISBN: 978-3-030-57369-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)