Abstract
The concept of the central nervous system (CNS) as an immune-privileged site, essentially due to the presence of the blood brain barrier, appears to be overly simplistic. Indeed, within healthy CNS immune activities are permitted and are required for neuronal function and host defense, not only due to the presence of the resident innate immune cells of the brain, but also by virtue of a complex cross-talk of the CNS with peripheral immune cells. Nonetheless, long-standing and persisting neuroinflammatory responses are most often detrimental and characterize several neuroinflammatory diseases, including multiple sclerosis, Alzheimer’s disease and amyotrophic lateral sclerosis. A growing body of evidence suggests that Cannabis sativa-derived phytocannabinoids, as well as synthetic cannabinoids, are endowed with significant immunoregulatory and anti-inflammatory properties, both in peripheral tissues and in the CNS, through the activation of cannabinoid receptors. In this review, the immunomodulatory effects of cannabinoid signaling on the most relevant brain immune cells will be discussed. In addition, the impact of cannabinoid regulation on the overall integration of the manifold brain immune responses will also be highlighted, along with the implication of these compounds as potential agents for the management of neuroinflammatory disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis (Cannabis sativa) has been known since ancient times and has been used for recreational and medicinal purposes for more than 5000 years (Russo 2001; Mechoulam et al. 2014). Although the first plant-derived cannabinoids or phytocannabinoids (phyCBs), like cannabinol (CBN) and cannabidiol (CBD), were isolated during the first half of the 20th century, it was only in 1964 that Mechoulam’s group isolated and characterized for the first time the main psychotropic principle of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC). Mechoulam’s elegant work formed indeed the basis of cannabinoid research as we know it today (Gaoni and Mechoulam 1964). Later on, the advent of synthetic cannabinoids or syntho-cannabinoids (syCBs) led to the discovery and cloning of type-1 and type-2 cannabinoid receptors (CB1 and CB2) (Pertwee et al. 2010), and showed that cannabis and cannabimimetic compounds clearly act on animal systems via physiological frameworks that normally regulate natural processes. The isolation of the endogenous ligands of these receptors in the first half of 1990s, termed “endocannabinoids”, led to the discovery of the two main substances: arachidonoylethanolamide (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) (Devane et al. 1992; Mechoulam et al. 1995), along with the understanding of their molecular mechanisms at the dawn of 21st century. This knowledge closed a circle but opened a new avenue of research based on the development of an ever-growing number of syCBs possessing better specificity towards cannabinoid receptors, and aimed at better understanding the role of cannabinoids in the control of several pathophysiological processes.
Plant-Derived Cannabinoids and Synthetic Cannabinoids
PhyCBs belong to a class of compounds, mostly found in Cannabis sativa and Cannabis indica, that are produced by decarboxylation of their acid precursor. They include a wide array of terpenophenolic compounds (at least 85 to date) with strong structure-activity related properties and grouped into 11 different classes that depend on carbon skeleton configuration (ElSohly 2002): cannabigerol (CBG)-type, cannabichromene (CBC)-type, cannabidiol (CBD)-type, Δ9-tetrahydrocannabinol (Δ9-THC)-type, Δ8-tetrahydrocannabinol (Δ8-THC)-type, cannabicyclol (CBL)-type, cannabielsoin (CBE)-type, cannabitriol (CBT)-type, cannabinol (CBN)-type, cannabinodiol (CBND)-type and a last class that includes compounds with miscellaneous structure (e.g., cannabicitran and cannabifuran). Indeed, CBN- and CBND-type cannabinoids are thought to be oxidation artifacts of THC (ElSohly 2002; Elsohly and Slade 2005; Pertwee 2006; El-Alfy et al. 2010). Although a great number of phyCBs has been reported to have different biological properties, THC, CBN and CBD have received particular attention due to their broad spectrum of actions, which include analgesic and anti-inflammatory effects (Pertwee 2006; Howlett et al. 2002; Mechoulam et al. 2007). Nonetheless, THC is the main psychoactive cannabinoid due to its well-known actions in the CNS, and its use as a recreational drug (El-Alfy et al. 2010). Despite CBD is also able to activate serotonine receptors (Russo et al. 2005), its congeners mostly bind to CB1 and CB2, yet with different affinities. Deeper understanding of the chemical and structural features of phyCBs, as well as of their receptor affinity, led to the synthesis of new compounds, the syCBs, whose development was boosted in search of higher selectivity, needed to discriminate the specific role of each receptor in the different pathophysiological processes (and to ultimately exploit them as better therapeutics with a few or none at all side effects). SyCBs are an extremely heterogeneous class of artificial compounds, that fall into 3 main chemical groups: (i) classical syCBs, which include dibenzopyran derivatives that are structurally similar to THC, the most notable example of which is (−)-11-hydroxy-Δ8-THC-dimethylheptyl (HU210); (ii) non classical syCBs, which include byciclic or tricyclic analogues of Δ9-THC lacking a pyran ring, CP55,940 being the most prominent member; and (iii) pyrrol-, indene- and indole-derivates, which consist of various compounds without any structural resemblance with either phyCBs or syCBs. The last class can be further divided into several subtypes, depending on the molecular structure and including aminoalkylindoles (e.g., WIN55,212-2), benzoylindoles (e.g., AM694), naphtoylindoles (e.g., JWH-015), naphtylmethylindoles, naphtylmethylindenes, naphthoylpyrroles and phenylacetyl-indoles. Since HU210 and CP55,940 bind both receptors with almost the same affinity, research and drug-design have invested a great deal of attention towards the development of gradually more selective compounds, namely JWH- and AM-series (respectively developed by John W. Huffman and Alexandros Makrijannis, hence their name) (Huffman and Dai 1994; Makriyannis and Deng 2000). Of note, JWH- or AM- suffixes do not necessarily reflect their belonging to a particular class of syCBs (for instance JWH-133, one of the most potent CB2 selective agonist, does not belong to the third class because it is chemically a dibenzopyran). Not surprisingly, development of highly selective cannabinomimetic compounds has led in recent years to their use as “legal highs” under brand names such as Spice or K2. As a consequence, many of these compounds have been forbidden in several countries due to their psychoactive effects similar to THC (yet with greater intensity), which led to medical and psychiatric emergencies (ElSohly et al. 2014; Castaneto et al. 2014).
Target Receptors and Signaling Pathways
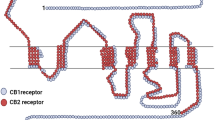
PhyCBs and syCBs bind to and functionally activate their target receptors, initiating various signaling pathways and leading to several biological effects on different tissues. The main receptor targets for eCBs are type-1 (CB1) and type-2 (CB2) G protein-coupled cannabinoid receptors (Pertwee et al. 2010; Maccarrone et al. 2014) (Fig. 1). CB1 is widely expressed in the nervous system and in many different extra-neural sites, where it is involved in the regulation of cognitive, memory and motor functions, as well as analgesia. Instead, CB2 is mainly expressed by the cells of the immune system where it is commonly associated with the regulation of different immune functions (Basu and Dittel 2011). However, CB2 has also been identified in brainstem neurons, microglial cells and astrocytes, where its presence was mainly correlated with cell activation or insult (Atwood and Mackie 2010). Indeed, upregulation of CB2 is associated with chronic inflammation of the nervous system, as well as with several cardiovascular and bone disorders (Patel et al. 2010; Galve-Roperh et al. 2013). CB1 and CB2 are metabotropic receptors that usually couple to heterotrimeric Gi/o proteins, thus leading to reduced cAMP levels through adenylyl cyclase inhibition and subsequent inactivation of protein kinase A (PKA). CB1 and CB2 also activate various effector protein kinase cascades involved in cell proliferation and survival. Among these, the most relevant are phosphatidylinositol 3-kinase/protein kinase B (PI3K/PKB), mitogen-activated protein kinase (MAPK) p38, extracellular-signaling regulated protein kinase (ERK)-MAPK, and focal adhesion kinase (FAK) (Galve-Roperh et al. 2013; Maccarrone et al. 2014). Other signaling pathways include coupling to ion channels (N- and P/Q-type Ca2+ channels and voltage-gated K+ channels), activation of phospholipase-C beta (PLCβ) and ceramide biosynthesis (Galve-Roperh et al. 2013; Maccarrone et al. 2014). THC and CBN bind to both CB1 and CB2 with high affinity, with the latter being more avid for CB2 (Huffman 2000; Mahadevan et al. 2000). Instead CBD, a non-psychoactive component, shows little affinity for both receptors (Mechoulam et al. 2007). Additionally to CB1 and CB2, phyCBs and syCBs can engage other non-CB targets and these include the transient receptor potential vanilloid 1 (TRPV1) channel, expressed in sensory neurons and in epithelial, endothelial and immune cells (Xia et al. 2011); peroxisome proliferator-activated receptor (PPAR) α and γ (Pistis and Melis 2010), which belong to a family of nuclear receptors capable of regulating lipid turnover and metabolism, as well as the orphan G protein-coupled receptor GPR55 (Moriconi et al. 2010). The presence of these additional targets suggests that the term “cannabinoid receptor” might be reconsidered in the near future, in order to embrace the heterogeneity of the different molecular targets identified so far for both phyCBs and syCBs.
Signaling networks of cannabinoid receptors by phyCBs and syCBs. PhyCBs and syCBs elicit their biological effects by engaging both membrane and intracellular receptors. Upon activation of CB1 and CB2 G protein-coupled receptors, several downstream signaling pathways are initiated, including: inhibition of adenylyl cyclase, thus lowering cAMP intracellular levels; phosphorylation of p38 and ERK1/2 MAP-kinases in a PLC- and PI3K/AKT-dependent manner, or of FAK; promotion of ceramide biosynthesis. Moreover, they CB1 and CB2 modulate Ca2+ and K+ channels. Upon activation of GPR55, phyCBs and syCBs induce activation of Rho signaling and phosphorylation of p38 MAP-kinase; instead, activation of TRPV1 and PPARs leads to Ca2+-dependent signaling and regulation of gene expression, respectively. Abbreviations: phyCBs phytocannabinoids, syCBs synthocannabinoids, cAMP cyclic adenosylmonophosphate, GTP guanosyltriphosphate, MAPK mitogen-activated protein kinase, ERK extracellular signal-regulated kinase; PLC phospholipase C, PI3K phosphatidylinositol 3-kinase, PKA protein kinase A, PKB protein kinase B, FAK focal adhesion kinase, VDCC voltage-dependent calcium channels, GIRK G protein-coupled inwardly-rectifying potassium channels, GPR55, G protein-coupled receptor 55, TRPV1 transient receptor potential cation channel subfamily V member 1; PPAR peroxisome proliferator-activated receptor
Cannabinoid Regulation of Brain Immune Responses
Typical immune cells, such as lymphocytes, eosinophils, basophils, and plasma cells, are not normally found in the CNS parenchyma, mostly because of the tight endothelial junctions of the blood–brain barrier (BBB), which strictly limits their entry. Only during intense immune activation or chronic systemic inflammation (such as infections), entry of leukocytes from the periphery occurs quite rapidly through specific areas with an open BBB, termed circumventricular organs (i.e., choroid plexus, subfornical organ, organum vasculosum of the laminia terminalis, and median eminence) (Xanthos and Sandkuhler 2014). Compelling evidence now suggests that the brain is endowed with its own innate immune cells, primarily microglia but also astrocytes and endothelial cells. Indeed, microglia, astrocytes, endothelial cells, and even neurons can release cytokines during disturbances of CNS homeostasis (Rivest 2009). In this scenario, the role of cannabinoid signaling in the regulation of microglia and astrocytes immune responses has been the most investigated and best understood so far, and will be the main focus of this review. This was possible thanks to deeper and recent knowledge on the differential expression of cannabinoid receptors in the various resident cells of the CNS (Table 1). Also its role on the integration of brain immune responses and in major neuroinflammatory diseases will be discussed herein.
Cannabinoid Signaling in the Innate Cellular Soldiers of the CNS
Microglia
Microglial cells are a type of glial cells that are the resident macrophages of the CNS, thus acting as the primary immune sentinels of the brain and spinal cord. They share several features with peripheral macrophages, including their immunophenotype and functional traits. The ontogeny of microglial cells is a controversial issue and to date two hypotheses have been formulated to explain it (Prinz and Priller 2014). The commonly accepted idea is that resident microglia differentiate from mesodermal/mesenchymal monocyte precursors that enter the brain during embryonic, fetal and perinatal stages (Nayak et al. 2014). However, in recent years another interesting hypothesis challenged the classical one, depicting microglial cells as descending from amoeboid non-hematopoietic microglial precursors in the yolk sac that express typical monocyte/macrophage-associated markers; the latter enter the brain in early stages of embryonal development (Monier et al. 2007; Ginhoux et al. 2013). Furthermore, experiments conducted on mice demonstrated that most of resident macrophages of different systems (including brain microglia) can differentiate through non-hematopoiesis-associated pathways (Perdiguero et al. 2014). It is possible that these two mechanisms work together during development, to ensure in different tissues a resident population of myeloid cells that act as innate guardians of the pathophysiology of the organ. Microglial cells act in the CNS as phagocytes/antigen-presenting cells (APCs) and scavengers, carrying out crucial tasks such as defense of the neural parenchyma against infections, tumors, ischemia, trauma and neurodegeneration (Hanisch and Kettenmann 2007). They are believed to remain in a dormant or surveying state in the healthy brain (resting microglia). However, these cells can switch from a resting to a primed state by an initial immune stimulus, even not excessively intense, and subsequently can be rapidly activated upon disturbance of brain homeostasis (Nayak et al. 2014). Activated microglial cells undergo a plethora of dramatic structural and functional changes, such as loss of ramified morphology in favor of amoeboid, phagocytic phenotype, release of radical species and of several inflammatory mediators. On the one hand, such a process is meant to promptly contrast invading microorganisms, and eliminate noxious cellular debris; on the other hand, it resolves inflammation and promote tissue repair (Hu et al. 2014). Indeed, much alike macrophages, microglia are extremely heterogeneous and there is evidence that several types of microglia exist, from neuroprotective to neurodestructive. However, persistency of such an activated stage can be deleterious in that it can fiercely expand tissue damage, conversely hindering full recovery of tissue functions. Although for a long time studies on tissue and cell distribution of both CB1 and CB2 indicated that the former was mainly expressed in the CNS while the latter was exclusively present in tissues and cells of the immune system, now one can expect to find appreciable levels of CB1 in microglia and no traces of CB2. Surprisingly, resting microglia of healthy brain do not particularly express neither CB1 nor CB2 (Stella 2010). Further studies suggested that, although absent from the CNS under normal conditions, CB2 is induced in glial cells, particularly in reactive microglia, in response to different damaging conditions associated with local inflammatory events, and its amount varies depending on the type of neuropathology (Fernandez-Ruiz et al. 2007; Viscomi et al. 2009). For instance, CB2 is remarkably upregulated in spinal cord injury (Zhang et al. 2003) as well as in response to inflammatory challenges (Maresz et al. 2005). High levels of CB2 are also found in plaques-associated activated microglia of brain tissue from Alzheimer’s and multiple sclerosis (MS) patients (Benito et al. 2003; Yiangou et al. 2006). Moreover, recent studies have proposed that CB2 receptors may be present in the brain even in healthy conditions (Onaivi et al. 2006), despite this issue has remained controversial due to uncertainty of experimental approaches used or of some methodological tools available. The unexpected presence of CB2 rather than CB1 in microglia is actually of no surprise, considering that these cells are part of the immune system. Similarly to CB2, also GPR55 is differentially expressed in microglia, as the expression of this receptor is induced in both primary mouse microglia and the BV-2 mouse microglial cell line upon cell activation with LPS and IFN-γ (Pietr et al. 2009). As yet, only two reports investigated the role of GPR55 on the modulation of microglia inflammatory responses, and suggest that its activation by its selective agonists such as abnormal-CBD and the synthetic compound O-1602 protected neurons by dampening microglia activation (Janefjord et al. 2014). While the activation of microglial CB1 receptors has only been investigated on mollusk and rat microglia, where CP55,940 exerts opposite effects on NO production (Stefano et al. 1996; Waksman et al. 1999), the stimulation of CB2 receptors by cannabinoids significantly affects the immune responses of activated microglia. Indeed, activation of CB2 with selective compounds like JWH133, AM1241 or SR144528 has been reported to potentiate microglial cell proliferation and migration, while reducing the release of proinflammatory mediators like TNFα, IL-1 and IL-6 and reactive species (Walter et al. 2003; Carrier et al. 2004; Kim et al. 2006; Dirikoc et al. 2007; Eljaschewitsch et al. 2006; Ramirez et al. 2005). Moreover, in a viral model of MS, the nonselective cannabinoid agonist WIN55,212-2 reduced microglial activation (Mestre et al. 2009). More recently, the use of the most potent and selective CB2 agonist GP1a revealed that receptor activation leads to reduced infiltration of microglial cells in spinal cord, an effect that was paralleled by a decreased expression of several proinflammatory cytokines and chemokines from T cells (Kong et al. 2014). The potential mechanism underlying some of these anti-inflammatory effects is supposed to be mediated by enhancing release of anti-inflammatory molecules, such as IL-1ra (Molina-Holgado et al. 2003; Fernandez-Ruiz et al. 2005, 2007). Another study reported that, although both psychoactive THC and non-psychoactive CBD exert inhibitory effects on the production of inflammatory cytokines in activated microglial cells in culture, their activities involve both different and overlapping intracellular pathways (Kozela et al. 2010). These effects are not mediated via CB1, CB2, nor via abnormal-CBD-binding receptors. For instance, CBD was recently reported to enhance microglial phagocytosis via transient receptor potential vanilloid 1 and 2 (Hassan et al. 2014). In addition, CBD was found to inhibit microglial activation in a mouse model of MS (Kozela et al. 2011). Additionally, CBD and WIN55,212-2 inhibit microglial activation and migration, both in vitro and in vivo (Martin-Moreno et al. 2011). The same effect on microglial cell infiltration is induced in vivo by chronic treatment with JWH133 (Martín-Moreno et al. 2012), supporting a CB2-mediated neuroprotective role.
Astrocytes
These cells are the most abundant in the whole CNS, and like neurons and oligodendrocytes they derive from the neuroectoderm (Chan et al. 2007). Astrocytes regulate almost every physiological aspect of the CNS, inasmuch as they are responsible for a plethora of functions, including nutritional and neuro-signaling support, neurotransmitters turnover, synaptic plasticity, control and constitution of the BBB, regulation of cerebral blood flow and energy metabolism (Jensen et al. 2013). Astrocytes are usually classified into three main cellular subtypes, depending on their shape, role and distribution within the CNS: (i) protoplasmic astrocytes are major regulators of the synaptic function and reside in the grey matter; (ii) fibrous astrocytes in the white matter are in physical contact with oligodendrocytes, and play a crucial role in myelination; (iii) radial astrocytes reside in the periventricular space and regulate neuronal migration during embryogenesis (Sofroniew and Vinters 2010; Pekny and Pekna 2014). There is also growing evidence that astrocytes play a pivotal role in central immunity. Indeed, upon insults they react to pathogens or damages (such as strokes, trauma, infections, neurodegeneration) behaving like an immune cell in a morpho-functional process that is usually referred to as reactive gliosis or astrogliosis. The latter is characterized by a marked hypertrophy of cellular processes, as well as by secretion of many cytokines and chemokines to influence effector cells, thus modulating the BBB and forming glial scars (Pekny and Pekna 2014). This process, though intended to restore tissue homeostasis, can degenerate and limit neuronal functional recovery, rather than promoting it. Indeed, astrogliosis is a hallmark of many neurodegenerative diseases (Sofroniew and Vinters 2010). Furthermore, astrocytes can sense the inflammatory environment by responding to pro- and anti-inflammatory cytokines, danger-associated epitopes via pattern-recognition receptors, and can respond to both by changing their cell phenotype to perform immune-related tasks as well as by directing the appropriate adaptive immune response, in concert with microglia. Although they do not serve as professional APCs, it seems likely that astrocytes are particularly active in the detection of, and defense against, CNS viral infection (Jensen et al. 2013). Few studies have addressed the expression profile and functional significance of cannabinoid receptors in astrocytes. It is noteworthy that cultured astrocytes from different species or brain regions show great variation in cannabinoid receptors expression; for instance, rat astrocytes express CB1, whereas mouse astrocytes do not, and the activation of this receptor by THC on rat astrocytes increases the rate of glucose oxidation and ketogenesis, both crucial for the energy supply of the brain (Blazquez et al. 1999; Sanchez et al. 1998). As a matter of fact, most studies have been centered on CB1, proving that it holds a physiological importance in communication between astrocytes and neurons, and in modulation of synaptic plasticity, by acting on release of gliotransmitters, energy supply and neuroprotection (Magistretti 2009; Chen and Swanson 2003). More strictly related to the immunomodulatory role of astrocytes, activation of CB1 on these cells restricts the production of inflammatory mediators, such as NO induced by LPS and IL-1β (Molina-Holgado et al. 2002; Sheng et al. 2005). Indeed, WIN55,212-2 inhibited the expression of iNOS and corresponding NO production by IL-1β-stimulated astrocytes, and the release of TNF-α and CXCL10, CCL2 and CCL5 chemokines. Many of these effects were partially antagonized by both CB1- and CB2-specific antagonists SR141716A and SR144528, respectively (Sheng et al. 2005). Similarly, WIN55,212-2 and CBN dose-dependently inhibited NO production and iNOS expression in C6 rat glioma cells, but only in a CB1-dependent manner (Esposito et al. 2001). However, at the same time another study reported that WIN55,212-2 strongly inhibited IL-1β-induced production of ICAM-1 and VCAM-1 adhesion molecules, as well as of IL-8 chemokine from astrocytoma cells. These effects were independent of CB receptors, and rather engaged inhibition of NF-kB activity (Curran et al. 2005). Interestingly, THC was found to regulate a group of biologically relevant genes and proteins in human astrocytes, associated with inflammation and the immune response (Bindukumar et al. 2008). To date, the presence of CB2 in astrocytes remains quite controversial, and its expression appears to be higher at lesioned sites or in astrocytomas (Fernandez-Ruiz et al. 2005; Stella 2010). One of the few reports showing a direct role of CB2 in the regulation of astrocytic immune responses documented that WIN55,212-2 suppresses IL-1β-triggered production of the neuroprotective CX3CL1 in a CB2-dependent manner, triggering p38 MAPK phosphorylation (Sheng et al. 2009). Altogether these findings, summarized in Fig. 2, support the concept that phyCBs and syCBs bear relevant anti-inflammatory and neuroprotective properties on both glial cells, and that these compounds may have true therapeutic potential for the treatment or management of neuroinflammatory disorders.
Beneficial effects of phyCBs and syCBs on brain immune cells. Both phyCBs and syCBs inhibit microglial activation and migration, as well as release of proinflammatory cytokines or reactive species, thus preventing neuronal death and axonal loss. Moreover, both groups of substances inhibit release of proinflammatory mediators from astrocytes, either preventing neuronal death or limiting further microglial activation. Most of these effects are mediated by mixed or selective CB1/CB2 agonists (see text for details). Abbreviations: ROS reactive oxygen species, NO nitric oxide
Cannabinoid-Mediated Integration of Brain Immunity
After the CNS, the immune system is considered the most complex in the body, providing a dynamic, highly versatile and, in many instances, a very specific defense. It is now clear that both systems do not function independently of each other, but are rather intimately connected, showing manifold interactions that drive the overall body health. Indeed, a bidirectional relationship links the immune system with the CNS, and such a communication pathway serves as the foundation for the multidisciplinary field of neuroimmunology (Wrona 2006). Immune cells and neuroimmune molecules such as cytokines, chemokines, and growth factors modulate brain functions through multiple signaling pathways throughout the lifespan. The CNS is under constant monitoring from both the adaptive and innate immune system. Throughout development and adult life, the immune system detects, and responds to, changes in cell identity and neural connectivity. Deregulation of both adaptive and acquired immune responses, impairment of crosstalks between these two systems, and alterations in the deployment of innate immune mechanisms can predispose the CNS to autoimmunity and neurodegeneration (Schwartz and Baruch 2014; Banks 2014). Among the main check points through which the immunosurveillance and inflammatory responses of the brain are regulated, the role of Toll-like receptors (TLRs), and of the recruitment of leukocytes from the periphery through the BBB, seem to be crucial and it is well-known that they are modulated by phyCBs and syCBs (Downer 2011; Klein 2005). Incidentally, in both microglia and astrocytes THC, CBD, WIN55,212-2 and CP55,940 ablate proinflammatory mediators production and neuroinflammatory changes mediated by TLR4 (Facchinetti et al. 2003; Waksman et al. 1999; Froger et al. 2009; Cabral et al. 2001; Molina-Holgado et al. 2002). WIN55,212-2 acts also as a novel regulator of TLR3, by selectively enhancing TLR3-induced expression of the antiviral IFN-β (Downer et al. 2011). TLRs are key players in infectious and non-infectious diseases of CNS, and their responses can be beneficial or detrimental, depending on the strength and timing of the activating signal (Kawasaki and Kawai 2014). Although additional data are required to further elucidate the regulatory role of phyCBs and syCBs on other TLR-dependent cascades, evidence for a cannabinoid-based modulation of these receptors suggests that these compounds are also crucial in achieving coordinated responses that are appropriate for maintaining brain homeostasis. Another checkpoint is represented by the expression of major histocompatibility complex (MHC) molecules, mainly MHC class I and class II, which play a key role in the induction and regulation of immune responses. The former class presents intracellular antigens and is expressed in most nucleated cells, whereas the latter class presents extracellular antigens is expressed only on APC, mature B cells and activated T cells. MHC molecules are particularly induced upon cell maturation and activation by specific transactivators, the most important of which is MHC class II transactivator CIITA (Reith et al. 2005). Very few reports have investigated the role of phyCBs and syCBs on the regulation of MHC expression, yet there is a general consensus that these compounds may reduce MHC molecules either directly (Wacnik et al. 2008) or through downregulation of CIITA (Gongora et al. 2004).
Concerning the infiltration of blood leukocytes within the CNS, which is a classical paradigm associated with neuroinflammation that leads to detrimental effects on neuronal functioning and glial responses (Wrona 2006), both phyCBs and syCBs have been shown to impact on such a process. The first evidence came from Guaza’s group, who reported that WIN55,212-2 is able to reduce ICAM-1- and VCAM-1-mediated CD4+ T lymphocytes infiltration in brain endothelium (Mestre et al. 2009). Subsequently, this finding was confirmed by the same group using the non-psychotropic CBD, which also involved down-regulation of chemokines and of IL-1β (Mecha et al. 2013). In addition, further studies demonstrated that selective activation of either CB1 or CB2 by syCBs inhibit leukocyte entry into the CNS in models of brain ischemia (Murikinati et al. 2010), MS (Mestre et al. 2011; Rossi et al. 2011; Kong et al. 2014), encephalitis (Ramirez et al. 2012), and uveitis (Toguri et al. 2014).
Interestingly, further evidence has recently shown that the regulation of the overall integration of immune responses and the evolution of several chronic inflammatory diseases are mediated by epigenetic mechanisms (Huang and Wells 2014). Of note, cannabinoids have been recently reported to regulate epigenetic modifications in both health and disease via chemical interactions with epigenetic enzymes, and through interactions with DNA repair mechanisms (D’Addario et al. 2013; Pucci et al. 2013; Lotsch et al. 2013; Yang et al. 2014). This appears particularly important, because targeting cannabinoid signaling might serve as a potentially innovative strategy to suppress the expression of proinflammatory genes, while activating that of anti-inflammatory genes, overall regulating the intricate immunologic responses within the brain.
Cannabinoid-Based Modulation of Neuroinflammatory Diseases
In the light of their anti-inflammatory and neuro-protective properties, cannabinoids are currently under investigation for the treatment or management of several neuroinflammatory diseases, including MS, Alzheimer’s disease (AD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD) (Ashton 2007; Koppel et al. 2014). Indeed, neuroinflammation and neurodegeneration, both characterized by hyperactive glial cells accompanied by infiltration of blood leukocytes (which both release a myriad of proinflammatory mediators), are a hallmark of these neurodegenerative diseases. Most of these findings have stemmed from animal models, which have proven to be valuable settings for the study of pharmacological modulation of CB receptors. However, there is still controversy on the origin of the different cells that populate the CNS in rodents and humans; therefore, a distinction between mouse models or patients affected by the disorders of interest has been made in this section.
AD is characterized by a progressive decline in cognitive function and extensive neuronal loss due to numerous amyloid plaques and neurofibrillary tangles that cause neuronal death. Amyloid plaques are primarily composed of aggregates of β-amyloid (Aβ), as well as other protein aggregates (e.g., hyperphosphorylated Tau, ubiquitin, and presenilins 1 and 2), whereas neurofibrillary tangles are aggregates of hyperphosphorylated Tau protein (Gosselet et al. 2013; Chiurchiù and Maccarrone 2011). Activated microglia are, indeed, found within Aβ plaques and both CB1 and CB2 are increased, suggesting a role for cannabinoids in the modulation of inflammatory processes during AD. Indeed, THC has been found to inhibit acetylcoline esterase-induced aggregation of Aβ (Eubanks et al. 2006), while CBD reduced the transcription and expression of glial proinflammatory molecules in the hippocampus of an in vivo mouse model of Aβ-induced neuroinflammation (Esposito et al. 2007). In independent studies, WIN55,212-2 and SR141716A have been found to prevent Aβ-induced microglial activation in AD patients (Ramirez et al. 2005), and amnesia in the AD mouse model (Mazzola et al. 2003).
HD is characterized by loss of muscle coordination, cognitive decline and behavioral symptoms caused by a genetic defect (in the gene encoding for the huntingtin protein), that causes abnormal protein processing and aggregation, ultimately leading to cytotoxic effects (Ross and Tabrizi 2011). In HD, there is a reduction of CB1 in the basal ganglia of either rat and mouse models of HD or post-mortem brains of HD patients, where the most prominent cell loss occurs (Blazquez et al. 2011; Lastres-Becker et al. 2002). Furthermore, THC has been found to attenuate motor coordination deficits and protein aggregation in a mouse model of HD (Blazquez et al. 2011), whereas oral doses of CBD have been used for a clinical trial in patients with HD, but its efficacy was almost the same as that of the placebo (Consroe et al. 1991). SyCBs like WIN55,212-2 and HU210 have also been found to exert partial neuroprotection in animal models of HD (Sagredo et al. 2012), yet further studies are needed to understand how and if cannabinoids can be used in clinical practice for the treatment of this disorder.
PD is characterized by muscular rigidity, bradykinesia, tremor of resting limbs, and loss of postural balance. The basic neuropathology of PD involves degeneration of pigmented neurons in substantia nigra, resulting in depletion of striatal dopamine and its metabolites and subsequent impairment of dopaminergic neurotransmission in the basal ganglia (Schapira and Tolosa 2010; Chiurchiù and Maccarrone 2011). Due to the increased activity of CB1 in the basal ganglia and its role in regulating neurotransmitter release and motor activity, agonists for this receptor have proven to be useful therapeutics against PD. Indeed, in mouse models of PD WIN55,212-2 has been shown to protect nigrostriatal dopamine neurons and microglial activation (Price et al. 2009), while SR141716A attenuated the hypokinesia induced by 6-hydroxydopamine injection (Gonzalez et al. 2006). A clinical trial is currently investigating the effect of phyCBs on tremors associated to PD, and results from this study are expected by the end of 2015 (ClinicalTrials.gov Identifier: NCT02028858). ALS is a neurodegenerative disease that affects primarily motor neurons in the spinal cord and brain stem, ultimately leading to progressive weakness and atrophy of skeletal muscles, weakness of chest muscles and diaphragm, and dysfunction of the larynx and pharynx, thus leading to respiratory problems, and ultimately to death. Currently the only licensed therapy available for the treatment of ALS is the anti-glutamatergic agent Riluzole, which has limited therapeutic effects. However, there is increasing evidence that cannabinoids and manipulation of the cannabinoid system may have therapeutic value in ALS. Although evidence on cannabinoids in ALS is scarce, the ability of these compounds to target multiple neurotoxic pathways and to exert neuroprotective and symptomatic effects in this disorder in both animal models of ALS and in true patients (Raman et al. 2004; Kim et al. 2006; Shoemaker et al. 2007; Rossi et al. 2010) boosted several clinical trials with phyCBs (Carter et al. 2010; Weber et al. 2010; Joerger et al. 2012), also using a Sativex®-like combination of THC and CBD (Moreno-Martet et al. 2014). Yet, the use of cannabinoids to treat ASL needs to be further investigated, and should focus on strategies that selectively activate CB2 receptors.
Undoubtedly, the most promising clinical use of cannabinoids concerns MS. This is a demyelinating, chronic inflammatory immune-mediated disease of the CNS, and is characterized by either episodic acute periods of exacerbations (relapses or attacks), gradual progressive deterioration of neurologic function, or combinations of both (Compston and Coles 2008; Chiurchiù and Maccarrone 2011). As a matter of fact, the hallmarks of MS are inflammation and neurodegeneration where, upon BBB damage, a massive infiltration of highly proinflammatory and autoreactive leukocytes occurs, thus causing demyelination as well as oligodendrocyte death, axon damage, and even neuronal loss (Weissert 2013). These autoimmune processes are paralleled by a continuous activation of resident macrophages/microglia, which potentiate the inflammatory response by producing proinflammatory cytokines and chemokines, along with reactive oxidants (Gandhi et al. 2010). Especially thanks to animal models of MS, i.e., experimental autoimmune encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD), a great deal of evidence has been accumulated for a role of cannabinoids in the immunopathogenesis of MS. To date, it is clear that not only the cannabinoid system is profoundly altered in MS patients (Centonze et al. 2007; Maccarrone et al. 2011; Chiurchiu et al. 2013), but also that phyCBs and syCBs have the potential to exert a myriad of immunomodulatory and neuroprotective effects (Pryce and Baker 2012; Granja et al. 2012; Jawahar et al. 2013). These findings paved the way to multiple clinical trials with cannabinoids and, at present, the cannabinoid oral spray Nabiximol®, which is a 1:10 mixture of the two cannabinoids THC and CBD, is available in the UK, in some European and Asian countries, but not yet in the U.S.A. (Sanchez and Garcia-Merino 2012). Nabiximol® was developed by GW Pharmaceuticals, and is currently prescribed for the neuropathic pain and spasticity associated with MS.
Nonetheless, despite the wealth of data describing cannabinoid-based and CB1/CB2 –targeting drugs as promising approaches in the treatment of neurodegenerative diseases and neuroinflammatory conditions, interesting new findings unveiled a possible unexpected dark side of cannabinoids and cannabimimetic compounds. Indeed, recently chronic exposure to cannabis components has been shown to cause microglial activation and subsequent cerebellar dysfunction through deregulated release of glutamate by cerebellar neurons (Cutando et al. 2013). Such an effect opens the possibility that, at least under particular circumstances, phyCBs and syCBs could lead to neuroinflammation instead of neuroprotection. Hence, the effects of these compounds are considerably complex, and the outcome of their action on the immunopathological features of neurodegeneration and neuroinflammation could either depend on their direct action on the cellular components of inflammation or on secondary events, in a rather intricate manner. Future therapies will have to consider both sides of the coin, as well as the possibility of additional side effects of cannabinoid-related drugs, including volume reduction of pivotal memory-associated brain areas (Lorenzetti et al. 2014), and onset of psychopathological issues (Radhakrishnan et al. 2014; Sanchez-Blazquez et al. 2014).
References
Ashton JC (2007) Cannabinoids for the treatment of inflammation. Curr Opin Investig Drugs 8:373–384
Atwood BK, Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160:467–479
Banks WA (2014) The blood–brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav Immun
Basu S, Dittel BN (2011) Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res 51:26–38
Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J (2003) Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci: Off J Soc Neurosci 23:11136–11141
Bindukumar B, Mahajan SD, Reynolds JL, Hu Z, Sykes DE, Aalinkeel R, Schwartz SA (2008) Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Res 1191:1–11
Blazquez C, Sanchez C, Daza A, Galve-Roperh I, Guzman M (1999) The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. J Neurochem 72:1759–1768
Blazquez C et al (2011) Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain: a J Neurol 134:119–136
Cabral GA, Harmon KN, Carlisle SJ (2001) Cannabinoid-mediated inhibition of inducible nitric oxide production by rat microglial cells: evidence for CB1 receptor participation. Adv Exp Med Biol 493:207–214
Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ (2004) Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol 65:999–1007
Carter GT, Abood ME, Aggarwal SK, Weiss MD (2010) Cannabis and amyotrophic lateral sclerosis: hypothetical and practical applications, and a call for clinical trials. Am J Hosp Palliat Care 27:347–356
Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA (2014) Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend
Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, De Chiara V, Battistini L, Bernardi G, Bernardini S, Martino G, Maccarrone M (2007) The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain: J Neurol 130:2543–2553
Chan WY, Kohsaka S, Rezaie P (2007) The origin and cell lineage of microglia: new concepts. Brain Res Rev 53:344–354
Chen Y, Swanson RA (2003) Astrocytes and brain injury. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 23:137–149
Chiurchiù V, Maccarrone M (2011) Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15:2605–2641
Chiurchiu V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, Centonze D, Battistini L, Maccarrone M (2013) Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol 73:626–636
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517
Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K (1991) Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav 40:701–708
Curran NM, Griffin BD, O’Toole D, Brady KJ, Fitzgerald SN, Moynagh PN (2005) The synthetic cannabinoid R(+)WIN 55,212-2 inhibits the interleukin-1 signaling pathway in human astrocytes in a cannabinoid receptor-independent manner. J Biol Chem 280:35797–35806
Cutando L, Busquets-Garcia A, Puighermanal E, Gomis-Gonzalez M, Delgado-Garcia JM, Gruart A, Maldonado R, Ozaita A (2013) Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. J Clin Investig 123:2816–2831
D’Addario C, Di Francesco A, Pucci M, Finazzi Agro A, Maccarrone M (2013) Epigenetic mechanisms and endocannabinoid signalling. FEBS J 280:1905–1917
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Dirikoc S, Priola SA, Marella M, Zsurger N, Chabry J (2007) Nonpsychoactive cannabidiol prevents prion accumulation and protects neurons against prion toxicity. J Neurosci: Off J Soc Neurosci 27:9537–9544
Downer EJ (2011) Cannabinoids and innate immunity: taking a toll on neuroinflammation. Sci World J 11:855–865
Downer EJ, Clifford E, Gran B, Nel HJ, Fallon PG, Moynagh PN (2011) Identification of the synthetic cannabinoid R(+)WIN55,212-2 as a novel regulator of IFN regulatory factor 3 activation and IFN-beta expression: relevance to therapeutic effects in models of multiple sclerosis. J Biol Chem 286:10316–10328
El-Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D, Khan I, ElSohly M, Ross S (2010) Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav 95:434–442
Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O (2006) The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 49:67–79
ElSohly M (2002) Chemical constituents of Cannabis. In: Grotenhermen F, Russo E (eds) Cannabis and cannabinoids—pharmacology, toxicology and therapeutic potential. Haworth Press, New York, pp 27–36
Elsohly MA, Slade D (2005) Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci 78:539–548
Elsohly MA, Gul W, Wanas AS, Radwan MM (2014) Synthetic cannabinoids: analysis and metabolites. Life Sci 97:78–90
Esposito G, Izzo AA, Di Rosa M, Iuvone T (2001) Selective cannabinoid CB1 receptor-mediated inhibition of inducible nitric oxide synthase protein expression in C6 rat glioma cells. J Neurochem 78:835–841
Esposito G, Scuderi C, Savani C, Steardo L Jr, De Filippis D, Cottone P, Iuvone T, Cuomo V, Steardo L (2007) Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol 151:1272–1279
Eubanks LM, Rogers CJ, Beuscher AE, Koob GF, Olson AJ, Dickerson TJ, Janda KD (2006) A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol Pharm 3:773–777
Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A (2003) Cannabinoids ablate release of TNFalpha in rat microglial cells stimulated with lypopolysaccharide. Glia 41:161–168
Fernandez-Ruiz J, Gonźalez S, Romero J, Ramos JA (2005) Cannabinoids in neurodegeneration and neuroprotection. In: Mechoulam R (ed) Cannabinoids as Therapeutics (MDT). Birkhäuser Verlag, Switzerland, pp 79–109
Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M (2007) Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci 28:39–45
Froger N, Orellana JA, Cohen-Salmon M, Ezan P, Amigou E, Saez JC, Giaume C (2009) Cannabinoids prevent the opposite regulation of astroglial connexin43 hemichannels and gap junction channels induced by pro-inflammatory treatments. J Neurochem 111:1383–1397
Galve-Roperh I, Chiurchiu V, Diaz-Alonso J, Bari M, Guzman M, Maccarrone M (2013) Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res 52:633–650
Gandhi R, Laroni A, Weiner HL (2010) Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol 221:7–14
Gaoni Y, Mechoulam R (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647
Ginhoux F, Lim S, Hoeffel G, Low D, Huber T (2013) Origin and differentiation of microglia. Front Cell Neurosci 7:45
Gongora C, Hose S, O’Brien TP, Sinha D (2004) Downregulation of class II transactivator (CIITA) expression by synthetic cannabinoid CP55,940. Immunol Lett 91:11–16
Gonzalez S, Scorticati C, Garcia-Arencibia M, de Miguel R, Ramos JA, Fernandez-Ruiz J (2006) Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson’s disease. Brain Res 1073–1074:209–219
Gosselet F, Saint-Pol J, Candela P, Fenart L (2013) Amyloid-beta peptides, Alzheimer’s disease and the blood–brain barrier. Curr Alzheimer Res 10:1015–1033
Granja AG, Carrillo-Salinas F, Pagani A, Gómez-Cañas M, Negri R, Navarrete C, Mecha M, Mestre L, Fiebich BL, Cantarero I, Calzado MA, Bellido ML, Fernandez-Ruiz J, Appendino G, Guaza C, Muñoz E (2012) A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J Neuroimmune Pharm 7:1002–1016
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Hassan S, Eldeeb K, Millns PJ, Bennett AJ, Alexander SP, Kendall DA (2014) Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br J Pharmacol 171:2426–2439
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (2002) International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2014) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol
Huang E, Wells CA (2014) The ground state of innate immune responsiveness is determined at the interface of genetic, epigenetic, and environmental influences. J Immunol 193:13–19
Huffman JW (2000) The search for selective ligands for the CB2 receptor. Curr Pharm Des 6:1323–1337
Huffman JW, Dai D (1994) Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett 4:563–566
Janefjord E, Maag JL, Harvey BS, Smid SD (2014) Cannabinoid effects on beta amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell Mol Neurobiol 34:31–42
Jawahar R, Oh U, Yang S, Lapane KL (2013) A systematic review of pharmacological pain management in multiple sclerosis. Drugs 73:1711–1722
Jensen CJ, Massie A, De Keyser J (2013) Immune players in the CNS: the astrocyte. J Neuroimmune Pharm: Off J Soc NeuroImmune Pharm 8:824–839
Joerger M, Wilkins J, Fagagnini S, Baldinger R, Brenneisen R, Schneider U, Goldman B, Weber M (2012) Single-dose pharmacokinetics and tolerability of oral delta-9- tetrahydrocannabinol in patients with amyotrophic lateral sclerosis. Drug Metab Lett 6:102–108
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Front Immunol 5:461
Kim K, Moore DH, Makriyannis A, Abood ME (2006) AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. Eur J Pharmacol 542:100–105
Klein TW (2005) Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 5:400–411
Kong W, Li H, Tuma RF, Ganea D (2014) Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol 287:1–17
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D (2014) Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 82:1556–1563
Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z (2010) Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem 285:1616–1626
Kozela E, Lev N, Kaushansky N, Eilam R, Rimmerman N, Levy R, Ben-Nun A, Juknat A, Vogel Z (2011) Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol 163:1507–1519
Lastres-Becker I, Berrendero F, Lucas JJ, Martin-Aparicio E, Yamamoto A, Ramos JA, Fernandez-Ruiz JJ (2002) Loss of mRNA levels, binding and activation of GTP-binding proteins for cannabinoid CB1 receptors in the basal ganglia of a transgenic model of Huntington’s disease. Brain Res 929:236–242
Lorenzetti V, Solowij N, Whittle S, Fornito A, Lubman DI, Pantelis C, Yücel M (2014) Gross morphological brain changes with chronic, heavy cannabis use. Brit J Psychiatr
Lotsch J, Schneider G, Reker D, Parnham MJ, Schneider P, Geisslinger G, Doehring A (2013) Common non-epigenetic drugs as epigenetic modulators. Trends Mol Med 19:742–753
Maccarrone M, Bernardi G, Finazzi Agro A, Centonze D (2011) Cannabinoid receptor signalling in neurodegenerative diseases: a potential role for membrane fluidity disturbance. Br J Pharmacol 163:1379–1390
Maccarrone M, Guzman M, Mackie K, Doherty P, Harkany T (2014) Programming and reprogramming neural cells by (endo-)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci 15:786–801
Magistretti PJ (2009) Neuroscience. Low-cost travel in neurons. Science 325:1349–1351
Mahadevan A, Siegel C, Martin BR, Abood ME, Beletskaya I, Razdan RK (2000) Novel cannabinol probes for CB1 and CB2 cannabinoid receptors. J Med Chem 43:3778–3785
Makriyannis A, Deng H (2000) Cannabimimetic indole derivatives. US Patent Office, Washington
Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN (2005) Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95:437–445
Martin-Moreno AM, Reigada D, Ramirez BG, Mechoulam R, Innamorato N, Cuadrado A, de Ceballos ML (2011) Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol 79:964–973
Mazzola C, Micale V, Drago F (2003) Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur J Pharmacol 477:219–225
Mecha M, Feliu A, Inigo PM, Mestre L, Carrillo-Salinas FJ, Guaza C (2013) Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis 59:141–150
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO (2007) Cannabidiol–recent advances. Chem Biodivers 4:1678–1692
Mechoulam R, Hanus LO, Pertwee R, Howlett AC (2014) Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci 15:757–764
Mestre L, Docagne F, Correa F, Loria F, Hernangomez M, Borrell J, Guaza C (2009) A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol Cell Neurosci 40:258–266
Mestre L, Inigo PM, Mecha M, Correa FG, Hernangomez-Herrero M, Loria F, Docagne F, Borrell J, Guaza C (2011) Anandamide inhibits Theiler’s virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB(1) receptors. J Neuroinflammation 8:102
Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ (2002) Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J Neurosci Res 67:829–836
Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, Rothwell NJ (2003) Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci: Off J Soc Neurosci 23:6470–6474
Monier A, Adle-Biassette H, Delezoide AL, Evrard P, Gressens P, Verney C (2007) Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol 66:372–382
Moreno-Martet M, Espejo-Porras F, Fernandez-Ruiz J, de Lago E (2014) Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex((R)) -like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther 20:809–815
Moriconi A, Cerbara I, Maccarrone M, Topai A (2010) GPR55: Current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr Med Chem 17:1411–1429
Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M (2010) Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J: Off Publ Fed Am Soc Exp Biol 24:788–798
Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402
Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR (2006) Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci 1074:514–536
Patel KD, Davison JS, Pittman QJ, Sharkey KA (2010) Cannabinoid CB(2) receptors in health and disease. Curr Med Chem 17:1393–1410
Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94:1077–1098
Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald H (2014) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. doi:10.1038/nature13989
Pertwee RG (2006) Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 147(Suppl 1):S163–S171
Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA (2010) International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62:588–631
Pietr M, Kozela E, Levy R, Rimmerman N, Lin YH, Stella N, Vogel Z, Juknat A (2009) Differential changes in GPR55 during microglial cell activation. FEBS Lett 583:2071–2076
Pistis M, Melis M (2010) From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr Med Chem 17:1450–1467
Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, Strong R, Lutz B, Marsicano G, Roberts JL, Giuffrida A (2009) WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Euro J Neurosci 29:2177–2186
Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312
Pryce G, Baker D (2012) Potential control of multiple sclerosis by cannabis and the endocannabinoid system. CNS Neurol Disord Drug Targets 11:624–641
Pucci M, Rapino C, Di Francesco A, Dainese E, D’Addario C, Maccarrone M (2013) Epigenetic control of skin differentiation genes by phytocannabinoids. Br J Pharmacol 170:581–591
Radhakrishnan R, Wilkinson ST, D’Souza DC (2014) Gone to Pot - a review of the association between cannabis and psychosis. Front Psychiatr 5:54
Raman C, McAllister SD, Rizvi G, Patel SG, Moore DH, Abood ME (2004) Amyotrophic lateral sclerosis: delayed disease progression in mice by treatment with a cannabinoid. Amyotroph Lateral Scler Motor Neuron Disord: Off Publ World Fed Neurol Res Group Motor Neuron Dis 5:33–39
Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML (2005) Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci: Off J Soc Neurosci 25:1904–1913
Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, Persidsky Y (2012) Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood–brain barrier dysfunction under inflammatory conditions. J Neurosci: Off J Soc Neurosci 32:4004–4016
Reith W, LeibundGut-Landmann S, Waldburger JM (2005) Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol 5:793–806
Rivest S (2009) Regulation of innate immune responses in the brain. Nat Rev Immunol 9:429–439
Ross CA, Tabrizi SJ (2011) Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 10:83–98
Rossi S, De Chiara V, Musella A, Cozzolino M, Bernardi G, Maccarrone M, Mercuri NB, Carri MT, Centonze D (2010) Abnormal sensitivity of cannabinoid CB1 receptors in the striatum of mice with experimental amyotrophic lateral sclerosis. Amyotroph Later Scler: Off Publ World Fed Neurol Res Group Motor Neuron Dis 11:83–90
Rossi B, Zenaro E, Angiari S, Ottoboni L, Bach S, Piccio L, Pietronigro EC, Scarpini E, Fusco M, Leon A, Constantin G (2011) Inverse agonism of cannabinoid CB1 receptor blocks the adhesion of encephalitogenic T cells in inflamed brain venules by a protein kinase A-dependent mechanism. J Neuroimmunol 233:97–105
Russo EB (2001) Hemp for headache: an in-depth historical and scientific review of cannabis in migraine treatment. J Cannabis Ther 2:21–92
Russo EB, Burnett A, Hall B, Parker KK (2005) Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 30:1037–1043
Sagredo O, Pazos MR, Valdeolivas S, Fernandez-Ruiz J (2012) Cannabinoids: novel medicines for the treatment of Huntington’s disease. Recent Patents CNS Drug Discov 7:41–48
Sanchez AJ, Garcia-Merino A (2012) Neuroprotective agents: cannabinoids. Clin Immunol 142:57–67
Sanchez C, Galve-Roperh I, Rueda D, Guzman M (1998) Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol Pharmacol 54:834–843
Sanchez-Blazquez P, Rodriguez-Munoz M, Garzon J (2014) The cannabinoid receptor 1 associates with NMDA receptors to produce glutamatergic hypofunction: implications in psychosis and schizophrenia. Frontiers Pèharmacology 4:169
Schapira AH, Tolosa E (2010) Molecular and clinical prodrome of Parkinson disease: implications for treatment. Nat Rev Neurol 6:309–317
Schwartz M, Baruch K (2014) Breaking peripheral immune tolerance to CNS antigens in neurodegenerative diseases: boosting autoimmunity to fight-off chronic neuroinflammation. J Autoimmun
Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK (2005) Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia 49:211–219
Sheng WS, Hu S, Ni HT, Rock RB, Peterson PK (2009) WIN55,212-2 inhibits production of CX3CL1 by human astrocytes: involvement of p38 MAP kinase. J Neuroimmune Pharm: Off J Soc NeuroImmune Pharmacol 4:244–248
Shoemaker JL, Seely KA, Reed RL, Crow JP, Prather PL (2007) The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem 101:87–98
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Stefano GB, Liu Y, Goligorsky MS (1996) Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J Biol Chem 271:19238–19242
Stella N (2010) Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58:1017–1030
Toguri JT, Lehmann C, Laprairie RB, Szczesniak AM, Zhou J, Denovan-Wright EM, Kelly ME (2014) Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis. Br J Pharmacol 171:1448–1461
Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M (2009) Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci: Off J Soc Neurosci 29:4564–4570
Wacnik PW, Luhr KM, Hill RH, Ljunggren HG, Kristensson K, Svensson M (2008) Cannabinoids affect dendritic cell (DC) potassium channel function and modulate DC T cell stimulatory capacity. J Immunol 181:3057–3066
Waksman Y, Olson JM, Carlisle SJ, Cabral GA (1999) The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther 288:1357–1366
Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N (2003) Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci: Off J Soc Neurosci 23:1398–1405
Weber M, Goldman B, Truniger S (2010) Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatr 81:1135–1140
Weissert R (2013) The immune pathogenesis of multiple sclerosis. J Neuroimmune Pharmacol: Off J Soc NeuroImmune Pharmacol 8:857–866
Wrona D (2006) Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol 172:38–58
Xanthos DN, Sandkuhler J (2014) Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15:43–53
Xia R, Samad TA, Btesh J, Jiang LH, Kays I, Stjernborg L, Dekker N (2011) TRPV1 signaling: mechanistic understanding and therapeutic potential. Curr Top Med Chem 11:2180–2191
Yang X, Hegde VL, Rao R, Zhang J, Nagarkatti PS, Nagarkatti M (2014) Histone modifications are associated with Delta9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J Biol Chem 289:18707–18718
Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P (2006) COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol 6:12
Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D (2003) Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Euro J Neurosci 17:2750–2754
Acknowledgments
We apologize in advance to all investigators whose research could not be appropriately cited owing to space limitations. We wish to thank Professor Alessandro Finazzi Agrò and Professor Giorgio Bernardi for their continuing support to our studies on cannabinoid signaling. Financial support from Fondazione Italiana Sclerosi Multipla (FISM grant 2013/R/8) to V.C., and from Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN grant 2010–2011) to M.M. is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiurchiù, V., Leuti, A. & Maccarrone, M. Cannabinoid Signaling and Neuroinflammatory Diseases: A Melting pot for the Regulation of Brain Immune Responses. J Neuroimmune Pharmacol 10, 268–280 (2015). https://doi.org/10.1007/s11481-015-9584-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-015-9584-2