Abstract
Objective: To test whether cannabinoids reduce urge incontinence episodes without affecting voiding in patients with multiple sclerosis. This was part of the multicentre trial of the Cannabinoids in Multiple Sclerosis (CAMS) study. Subjects and methods: The CAMS study randomised 630 patients to receive oral administration of cannabis extract, Δ9-tetrahydrocannabinol (THC) or matched placebo. For this substudy subjects completed incontinence diaries. Results: All three groups showed a significant reduction, p<0.01, in adjusted episode rate (i.e. correcting for baseline imbalance) from baseline to the end of treatment: cannabis extract, 38%; THC, 33%; and placebo, 18%. Both active treatments showed significant effects over placebo (cannabis extract, p=0.005; THC, p=0.039). Conclusion: The findings are suggestive of a clinical effect of cannabis on incontinence episodes in patients with MS. This is in contrast to the negative finding of the CAMS study, where no difference was seen in the primary outcome of spasticity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a close association between clinical spinal cord pathology and lower urinary tract symptoms (LUTS) in people with multiple sclerosis (MS) [1]. Overall, the prevalence of LUTS in MS is approximately 80% [2]. Up to 96% of those with MS for more than 10 years may be affected [3]. The most common LUTS associated with MS are urinary frequency, urgency, urge and reflex incontinence [4]. In 60–80% of patients the cause is neurogenic detrusor overactivity (NDO) [2] due to demyelination in the posterior and lateral columns of the cervical spinal cord [5]. Approximately 50% of them have coexistent voiding difficulty due to detrusor-sphincter-dyssynergia (D-S-D) [3, 6], which can result in incomplete bladder emptying, high post-void residual volumes, infection and potential upper tract damage. In another 20–30% detrusor areflexia/acontractility might exist, probably due to pathology in the lower spinal cord [7]. In the majority of patients with NDO and D-S-D, the aim of therapy is to reduce detrusor overactivity, improve voiding function and incontinence whilst protecting the upper tracts from the effects of high intravesical pressure.
Current therapies include bladder-retraining regimens, oral and intravesical administration of anticholinergic drugs [8], intravesical administration of vanilloids such as capsaicin and resiniferatoxin [9, 10] and botulinum toxin [11]. Unfortunately, results of drug therapy are inconsistent, and all the currently available drugs have potentially bothersome side effects. As the neurological condition progresses, bladder dysfunction becomes more difficult to treat, and incontinence remains a significant feature, causing morbidity and patient frustration [12]. Ultimately, long-term catheterisation is often required [8].
Anecdotal reports from people with MS have suggested that cannabis might have a beneficial effect on LUTS, but it could also produce too much detrusor-relaxant effect, resulting in urinary retention [13]. A questionnaire study of MS patients regularly using cannabis for symptom relief [14] found that of those subjects with urinary problems, over half claimed improvement in urgency. A recent uncontrolled study of cannabinoids has demonstrated improvement in LUTS and urodynamic parameters in patients with severe MS [15].
This ‘substudy’ presents the results for urge incontinence from the Cannabinoids in Multiple Sclerosis (CAMS) study [16], which had failed to show a significant effect on the primary outcome of spasticity.
Methods
Subjects
Patients were recruited for the main CAMS study from 33 neurology and rehabilitation centres in the UK. Details of inclusion and exclusion criteria have already been reported [16].
All patients recruited to the CAMS study were assessed for urge incontinence episodes (UIEs), with the exception of those with a permanent catheter. As a result some patients who did not have incontinence were included, due to the relapsing and remitting nature of MS, at enrolment. However, they were not excluded because they were part of the intention-to-treat (ITT) group, and it was an opportunity to assess new-onset incontinence in these patients. Ethical approval was obtained from the South West Multicentre Research Ethics Committee (MREC) as part of the main CAMS study. Approval for the urodynamics was obtained from local ethics committees at four recruiting centres, where a subsample of patients underwent urodynamics and 24-h pad tests.
Assessment of incontinence
All patients were asked to complete a 3-day urinary diary (which has been shown to have a similar reliability as a 7-day diary [17]) recording the number of UIEs at baseline and at completion (week 13). At the same two time points, specific questions were asked about incontinence within the United Kingdom Neurological Disability Score (UKNDS) (used in the CAMS study) and the King’s Health Questionnaire [18] in keeping with the recommendations of the International Continence Society (ICS) for outcome measures of studies on incontinence [19]. In the CAMS study at week 15, patients were asked four specific questions about the overall effect of treatment on spasticity, tremor, pain and ‘bladder function’ by the treating physician [16].
Urodynamic studies
These were performed in the subsample (four centres) at baseline and at week 13. All methods, definitions and units for urodynamic investigations were in accordance with the recommendations of the ICS [20].
Prior to each urodynamic test, patients completed a 24-h ICS pad test [21].
Outcome measure
The main outcome of this substudy was a reduction in UIEs, as judged by the 3-day diary.
Procedure
Capsules were manufactured to contain 2.5 mg of Δ9-tetrahydrocannabinol (THC) equivalent, and in the case of cannabis extract, 1.25 mg of cannabidiol with less than 5% other cannabinoids per capsule. Placebo capsules contained the respective vegetable oil vehicle. Dose of study medication was based on bodyweight, with a maximum possible dose of 25 mg daily. Full details of allocation sequence, implementation, attendances, dose adjustment and adverse-event monitoring are presented elsewhere [16]. Specific adverse events relevant to LUTS, e.g. urinary tract infection (UTI), voiding difficulty, and urinary retention, were recorded.
Statistical methods
Sample size for the main CAMS study was determined on its primary outcome, muscle spasticity. A power calculation was applied to ensure this sample gave reasonable power for this study of incontinence episodes. Because there were no published data available to indicate the distributions and differences to be expected for urge incontinence, data from the questionnaire survey of MS patients using illicit cannabis were used [14]. Assuming 67% of patients have urinary symptoms, and this reduces to 33% in both active groups, a sample size of 100 would have an 87% power to detect this difference. Therefore, the whole of the CAMS sample of 660 subjects was likely to provide sufficient power for any clinically important changes in UIEs.
Data entry and error checking were carried out by the CAMS investigators [16]. Comparison of treatment groups followed the approach specified for the CAMS study. SPSS for Windows release 9 and MLwiN version 1 were used for the analysis. All analyses were carried out according to an analysis plan drawn up before unblinding and was by ITT. A p value of 0.05 was taken to be significant.
Urge incontinence episodes were recorded at baseline and at the end of treatment. According to the protocol all counts were based on 3 days. The data were analysed using a multilevel (two-level) log-linear model with a Poisson response [22]. A Poisson response was modelled because the data were typically highly skewed, as would be expected where the responses were small counts. This mixed model is similar to the ordinary general linear model but allows for correlation between the observations [23] and incorporates random effects representing differences in rates of urge incontinence between patients. The latter allows estimates to be adjusted for baseline imbalance. No adjustments were made for multiple comparisons.
Results
Baseline data
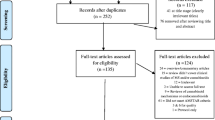
The dispositions by treatment group and flow of participants in this substudy are presented in Fig. 1. Likewise, baseline characteristics and demographics are presented in Table 1.
There was imbalance in the proportion of patients with urinary symptoms in each of the three main treatment groups, both in those patients who fully completed the urinary diaries (255) and in those who did not (267). More patients randomised to THC reported urge incontinence before treatment and had a higher rate of episodes. The imbalance between treatment groups is present both in the completed diary group and in the incomplete diary group. However, Ashworth scores (a validated measure of spasticity and the primary outcome for the CAMS trial) for these two groups were identical (mean scores 21.1 and 20.3, and SD 9.1 and 8.5, respectively), suggesting these variations were random. There were proportionately less patients from the incomplete data group who reported being incontinent, but this was evenly distributed in all three treatment arms, suggesting these data were missing at random, and the estimates are unlikely to be biased greatly by these missing data [23].
Urge incontinence episodes
Between 54 and 75% of patients had baseline episodes of fewer than two per day (cannabis extract, 72%; THC, 54%; and placebo, 75%). This included continent patients as all CAMS patients were included (657), and the intention was to see if any developed incontinence during the course of the study. Also, the analysis was based on ITT. Eighty-eight patients were continent at baseline, of whom 21 became incontinent and 67 remained continent.
Of the total 630 patients in the ITT analysis of CAMS, the questions on ‘bladder problems’ and ‘incontinence’ in the UKNDS at baseline were completed by 592 (94%) and 471 (75%) patients, respectively. The numbers reporting incontinence in the last month at baseline on UKNDS, excluding those with indwelling catheters were 26 (38.8%) for cannabis extract, 41 (60.3%) for THC and 26(40.6%) for placebo in the incomplete data group, and for the completed data group, 42(56.8%) for cannabis extract, 50 (70.4%) for THC and 39 (66.1%) for placebo.
As can be seen there was a baseline imbalance, which was controlled for using the statistical method described under statistical methods. All three groups showed a significant reduction, p<0.01, in adjusted episode rate (i.e. correcting for the baseline imbalance) from baseline to the end of treatment (Table 2), cannabis extract 38%; THC, 33%; and placebo, 18%. There was also a significant treatment effect for each of the cannabis groups over placebo; cannabis extract showed a 25% reduction (p=0.005), and THC showed a 19% reduction (p=0.039) relative to placebo.
In the main CAMS study patients were asked to assess the treatment benefit at week 15. Those reporting ‘improvement in bladder function’ (not defined) were 44% for cannabis extract, 40% for THC and 33% for placebo [16].
King’s Health Questionnaire
This was completed by subjects who reported a bladder problem on UKNDS. There were no significant differences in any of the domains.
Pad test
Reductions in pad test weight were greater in the active treatment groups than in placebo [mean reduction of 44.7 ml for cannabis extract (n=9), reduction of 43.2 for THC (n=10), and 43.9 ml for both cannabis treatments combined vs a mean increase of 8.3 ml for placebo (n=7)] (mean difference combined cannabis groups vs placebo=52.1 ml, 95% CI=13.4–90.9 ml, p=0·001).
Urodynamic measures
There were no significant differences in changes in any of the urodynamic parameters between the groups. At voiding cystometry there was a trend to increasing voided volume (mean increase of 40.7 ml for cannabis extract (n=15), 13.3 ml for THC (n=13), and a decrease of 73.7 ml for placebo (n=13)) and reduced post-void residual (mean reduction of 4.6 ml for cannabis extract, 39.6 ml for THC and 56.7 ml increase for placebo). However, the sample was small and variability was large, and the changes were not statistically significant.
Lower urinary tract adverse events
For each group adverse events involving the urinary tract were reported in 64 of the cannabis extract group, 62 THC and 73 placebo. Most were UTIs (33, 35 and 42, respectively). No episodes of urinary retention occurred in the cannabis extract group, but there were two in the THC group and three in the placebo group.
For those patients in the substudy who had urodynamics, there were four diagnoses of D-S-D before treatment (three in THC group and one in placebo) and two after treatment (one cannabis extract and one THC).
Discussion
This extra investigation to the main CAMS study set out to evaluate the usefulness of cannabis in treating MS-related urge incontinence. It was an “add-on” study to CAMS, which was assessing spasticity. As a result, patients were selected on this symptom rather than on incontinence. Due to the obvious difficulties in obtaining cannabis this was the only possible method of studying the effect on urge incontinence in these patients.
The priority for data collection by the CAMS investigators was the primary outcome (spasticity), and as a result, a lesser degree of supervision and encouragement for completing the urinary diaries might have occurred. This is a possible explanation for the large amount of missing data. Whilst this was disappointing, and could bias the results, the comparison of characteristics between those completing and not completing diaries suggests the data were missing at random, reducing the likelihood of bias. The group completing the diaries appeared to be representative of the whole population.
The results of this substudy have shown a significant reduction in UIEs from baseline in both cannabis groups of between 33 and 38% from baseline compared with an 18% reduction for placebo. It has already been shown that a 25% reduction in urge incontinence might be clinically significant [24]. Further support for a positive treatment effect comes from the pad test results that showed lower volume loss in the treatment groups, although the tests were performed on a small sample only.
This is the first attempted randomised controlled trial (RCT) of cannabis for urge incontinence in patients with MS. While the findings should be interpreted cautiously, because of missing data, nonetheless, over 250 patients were assessed, and the results suggest a possible treatment effect (the statistical model controlled for the baseline imbalance [23]).
Why there was an objective improvement in UIEs (in this study) and subjective change in other bladder symptoms (in CAMS), yet no improvement in spasticity, is unknown. It is possible that the effects on different symptoms might be dose-related.
Urodynamics
On urodynamics there were no statistically significant changes (although trends exist), possibly because of the high dropout rate. This was an extra investigation to those of the main CAMS study, and many patients refused to undergo urodynamics.
A larger urodynamic study alone is therefore required. However, some data from an uncontrolled trial have shown improvement in urodynamic measures following cannabinoids [15].
Quality of life
The lack of improvement in quality of life (QoL) as judged by the King’s Health Questionnaire despite a clinically significant reduction in UIEs [24] is unclear. Patients had more severe MS, so spasticity and immobility were possibly more bothersome than incontinence. However, it is also possible that cannabis did not improve incontinence-related QoL for these patients.
Adverse events
There were few adverse events related to the urinary tract other than UTI. The main concern of worsening of D-S-D and urinary retention occurred in only two patients with Δ9-THC compared with three taking placebo. Other side-effects have already been reported in the main study [16], and the drug appeared to be well tolerated.
Mechanism of action
It is possible that cannabinoids relax the detrusor smooth muscle during filling and so improve NDO, while not affecting the striated muscle of the rhabdosphincter (thus not worsening D-S-D). Support for this comes from animal studies [25] and the demonstration of cannabinoid receptor (CB1) immunoreactivity in the lower urinary tract [26, 27]. It is possible that cannabinoids act both centrally and peripherally for LUTS [28].
Overall evidence
Most MS patients are treated with anticholinergic drugs with or without self-catheterisation [8]. Anticholinergics often produce inconsistent results with a high incidence of side effects. Intravesical administration of vanilloids such as capsaicin [10] have shown encouraging results but can cause pain due to the initial stimulation of C-fibres. Resinoferatoxin [9] produces less pain, but few RCTs have been performed as of yet. The long-term effects of intravesical administration of vanilloids on the bladder mucosa are unknown.
Botulinum toxin inhibits acetylcholine release at the pre-synaptic cholinergic junction, and when injected into the detrusor, it has improved incontinence in spinal cord injury patients and in those with refractory overactive bladder [11, 29]. Non-invasive electrical stimulation or implanted sacral nerve stimulation might be considered, but evidence of their efficacy in MS is lacking. Often, catheterisation or surgery is required for many patients.
The findings from this and other studies [15] suggest that cannabis might be a treatment option before permanent indwelling catheterisation or invasive treatments such as surgery, but further large RCTs randomising on the basis of incontinence are required.
References
Betts CD, Dmellow MT, Fowler CJ (1993) Urinary symptoms and the neurological features of bladder dysfunction in multiple-sclerosis. J Neurol Neurosurg Psychiatry 56(3):245–250
Goldstein I, Siroky MB, Sax DS, Krane RJ (1982) Neurourologic abnormalities in multiple-sclerosis. J Urol 128(3):541–545
Hinson JL, Boone TB (1996) Urodynamics and multiple sclerosis. Urol Clin North Am 23(3):475
Sliwa JA, Bell HK, Mason KD, Gore RM, Nanninga J, Cohen B (1996) Upper urinary tract abnormalities in multiple sclerosis patients with urinary symptoms. Arch Phys Med Rehabil 77(3):247–251
Nathan PW, Smith NC (1982) The centrifugal pathway for micturition with the spinal cord. J Neurol Neurosurg Psychiatry 21:177
Chancellor MB, Blaivas JG (1994) Urological and sexual problems in multiple-sclerosis. Clin Neurosci 2(3–4):189–195
Gonor SE, Carroll DJ, Metcalfe JB (1985) Vesical dysfunction in multiple-sclerosis. Urology 25(4):429–431
Madersbacher H, Wyndaele JJ, Igawa Y, Chancellor M, Chartier-Kastler E, Kovindha A (2002) Conservative management in neuropathic urinary incontinence. In: Abrams P et al (eds) Incontinence: 2nd international consultation on incontinence, Health Publication Ltd., Plymouth, pp 697–754
De Ridder D, Baert L (2000) Vanilloids and the overactive bladder. BJU Int 86(2):172–180
de Seze M, Wiart L, Ferriere JM, de Seze MP, Joseph PA, Barat M (1999) Intravesical instillation of capsaicin in urology: a review of the literature. Eur Urol 36(4):267–277
Rapp DE, Lucioni A, Katz EE, O’Connor RC, Gerber GS, Bales GT (2004) Use of botulinum-a toxin for the treatment of refractory overactive bladder symptoms: an initial experience. Urology 63:1071–1075
Litwiller SE, Frohman EM, Zimmern PE (1999) Multiple sclerosis and the urologist. J Urol 161(3):743–757
Burton TA (1979) Urinary retention following cannabis ingestion. JAMA 242(4):351
Consroe P, Musty R, Rein J, Tillery W, Pertwee R (1997) The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol 38(1):44–48
Brady CM, DasGupta R, Wiseman OJ, Dalton CM, Berkley KJ, Fowler CJ (2002) The effect of cannabis based medicinal extract on lower urinary tract dysfunction in advanced multiple sclerosis: preliminary results. J Neurol Neurosurg Psychiatry 72(1):139
Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, Thompson A (2003) Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet 362(9395):1517–1526
Groutz A, Blaivas JG, Chaikin DC, Resnick NM, Engleman K, Anzalone D, Bryzinski B, Wein AJ (2000) Noninvasive outcome measures of urinary incontinence and lower urinary tract symptoms: a multicenter study of micturition diary and pad tests. J Urol 164(3):698–701
Kelleher CJ, Cardozo LD, Khullar V, Salvatore S (1997) A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 104(12):1374–1379
Lose G, Fantl JA, Victor A, Walter S, Wells TL, Wyman J, Mattiasson A (1998) Outcome measures for research in adult women with symptoms of lower urinary tract dysfunction. Neurourol Urodyn 17(3):255–262
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A (2002) The standardisation of terminology of lower urinary tract function: report from the of the International Standardisation Sub-Committee Continence Society. Neurourol Urodyn 21(2):167–178
Artibani W, Andersen JT, Gajewski JB, Ostergard DR, Raz S, Tubaro A (2002) Imaging and other investigations. in: Abrams P et al (eds) Incontinence: 2nd international consultation on incontinence, Health Publication Ltd., Plymouth, pp 697–754
Goldstein H (1995) Multilevel statistical models, 2nd edn. Arnold, London
Cnaan A, Laird NM, Slasor P (1997) Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 16(20):2349–2380
Coyne KS, Matza LS, Thompson CL (2005) The responsiveness of the Overactive Bladder Questionnaire (OAB-q). Qual Life Res 14:849–855
Greenland JE, Brading A (1995) The in-vitro effect of delta-9 tetrahydrocannabinol on the detrusor smooth muscle of the pig. Proceedings of the International Continence Society, Sydney, pp 87–88
Ost D, Van der Aa F, De Ridder D (2001) Immunohistochemical study of the cannabinoid receptors CB1 and CB2 in the human bladder. Mult Scler 7(1):S24
Pertwee RG, Fernando SR (1996) Evidence for the presence of cannabinoid CB1 receptors in mouse urinary bladder. Br J Pharmacol 118(8):2053–2058
Blyweert W, Van der Aa F, De Ridder D (2003) Cannabinoid therapy in detrusor overactivity: local versus systemic effect in a spinalised rat model. Neurourol Urodyn 22(5):379–380
Schurch B, Hauri D, Rodic B, Curt A, Meyer M, Rossier AB (1996) Botulinum-A toxin as a treatment of detrusor-sphincter dyssynergia: a prospective study in 24 spinal cord injury patients. J Urol 155(3):1023–1029
Acknowledgements
This study was funded by a grant from the Multiple Sclerosis Society. The Medical Research Council sponsored the main CAMS study. Solvay Healthcare Ltd. and the Institute for Clinical Research, IKF, Berlin, provided medication. We thank all patients who took part in the study. We acknowledge the assistance of the CAMS study team, particularly Jane Vickery for trial coordination and help with drafting the paper, Wendy Ingram for assistance with figure construction, Hilary Sanders for further statistical support as well as Suzi Reilly, Judy Willcocks, Sue Varley, Mark Warner, Lara Teare, Felicity Coates and Rosemary Bishop (urodynamic nurse).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freeman, R.M., Adekanmi, O., Waterfield, M.R. et al. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J 17, 636–641 (2006). https://doi.org/10.1007/s00192-006-0086-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-006-0086-x