Abstract
Anesthesia and surgery have a significant effect on hematological parameters, including hemoglobin, hematocrit, coagulation factors, natural anticoagulants, fibrinolytic system etc. that may vary from person to person, mostly due to their unique genetic profile. Anesthesia drugs differ in their metabolism and efficacy, based on the person’s profile, which indicates the importance of pharmacogenetic/pharmacogenomic. Pharmacogenomic, the cornerstone of personalized medicine, can make clearer the physician’s comprehension of the individual’s response to treatment based on his or her genome profile. With detailed hematological parameters and new genome sequencing technologies such as next-generation sequencing (NGS) or high-throughput sequencing, appropriate anesthetic technique and anesthetic agents for the patient can be chosen for safe, less eventful anesthesia and surgery. Since thrombotic and hemorrhagic disorders are potentially serious perioperative complications, with the development of diagnostic tools and our knowledge, preoperative genetic analysis can be useful in predicting the risks, and optimizing the patient’s management. Genetic research and cutting-edge technologies are progressing at a rapid pace, leading to safer, and more particular, personalized anesthesia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
In spite of 99.5% within the species-homology in the human genome, humans have their unique response to diseases and stimulators such as drugs. Hematological disorders are not excluded from responses to stimulators (Pati and Sharma 2017). Emergence of new genome sequencing technologies, such as next-generation sequencing (NGS) or high-throughput sequencing, is enabling us to tailor a person’s treatment to his or her unique gene profile (Gil et al. 2018). Anesthesia is a highly sensitive procedure which closely correlates with hematological conditions that emphasize the need for close and timely collaboration between hematologist and anesthetist/anesthesiologist. Both general and regional anesthesia can affect a person’s respiratory, hemostatic, hemodynamic and endocrine systems (Brueckner et al. 2003; Torpy et al. 2011; Sano et al. 2016; Smith and Goldman 2018). Anesthesia methods have varied effects on a patient’s physiological systems. By combining conventional and precision medicine, cutting-edge technologies can provide better patient management (Badalian-Very 2014; Collins and Varmus 2015). Anesthesia has potential effects on hematological parameters, including a patient’s blood profile and coagulation indices. Hence, clinical and familial history, medication and patient characteristics, along with preoperative blood tests such as complete blood count (CBC), prothrombin time (PT)/international normalized ratio (INR) and activated partial prothrombin time (APTT), should be considered (Akhunzada et al. 2018; Alzahrani et al. 2019). Anesthesia drugs differ in their metabolism and efficacy, based on the genetic profile of each person, which indicates the importance of pharmacogenetic/pharmacogenomic. Pharmacogenomic, the cornerstone of personalized medicine, can make clearer the physician’s comprehension of individual response to treatment based on one’s genome profile (Behrooz 2015; Gabriel et al. 2017).
Hematological Considerations for Anesthesia
CBC, blood film evaluation and coagulation tests are common preoperative laboratory assays. These tests can help in the diagnosis of immunodeficiencies, infection, anemia, polycythemia, some malignancies, and hemorrhage. Along with clinical features, they can facilitate choosing the appropriate type of anesthesia and surgery (George-Gay and Parker 2003; Kumar and Srivastava 2011). As each patient has a unique gene profile, one might exhibit a significant feature such as a congenital or acquired abnormality. Therefore, each patient should be prepared and treated according to their genetic profile, along with the results of basic and specific tests (Vogenberg et al. 2010).
Anesthetic Considerations in Erythroid Disorders
Anemia
From the point of view of anemia, genome sequencing can help us determine which medical care would be most useful for patients, because some gene variations determine how a patient responds to a drug or procedure. These data can be provided by micro-array and whole-genome sequencing technologies such as NGS and Sanger sequencing. The patient’s lifestyle, environment, socio-economic status, diet, age, and sex also should be considered for better treatment (Iolascon et al. 2015). For example, treatment of chronic hepatitis virus (HCV) infection with ribavirin (RBV) has the potential to induce acute hemolytic anemia (AHA). It has been demonstrated that inosine triphosphatase (ITP) deficiency is an overriding protective factor against RBV-induced AHA. Knowledge of patients’ genetic variations can improve their medical care or enable us to anticipate some complications (Fellay et al. 2010; Clark et al. 2013; Iolascon et al. 2015). In personalized medicine, the detection of anemia can be started by an individual by him/herself anytime, anywhere, instantaneously and remotely, with the help of new smartphones and specific applications (Mannino et al. 2018). Preoperative anemia is a great threat to a patient. Published protocols have recommended that red blood cell (RBC) indices such as hemoglobin and hematocrit should be checked at least 1 month before surgery to provide proper intervention (Burton et al. 2018). Erythrocyte sedimentation rate (ESR) usually is performed before, and occasionally after, surgery. However, several studies have shown that anesthesia has no bearing on an increase in ESR (Caglayan et al. 2006; Mirzayan et al. 2009; Bilehjani et al. 2017). Furthermore, not only is anemia a challenge to anesthesia, sometimes anesthesia can cause anemia. In both situations, considering patient status, specific measures should be taken to avoid further complications. In the matter of erythrocyte recovery after surgery, a study that compared the effects of three anesthetic techniques—epidural anesthesia, general anesthesia, and the combination of both techniques—on RBC endogenous recovery showed that epidural anesthesia administration had a notably higher recovery rate of the circulating erythrocyte mass than those who received general anesthesia or the combination.
Non-immune Hemolytic Anemia
Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency
The most prevalent enzyme deficiency in the world is glucose-6-phosphate dehydrogenase (G6PD) deficiency, an X-linked non-immune hemolytic anemia that can become an impediment to anesthesia and surgery. It affects more than 400 million people worldwide, most commonly of African, Mediterranean, and Asian descent (Dorgalaleh et al. 2013b; Richardson and O’Malley 2018). G6PD protects cells, especially RBCs, against oxidative damage from the ingestion of fava beans, infections, metabolic conditions like diabetic ketoacidosis, and certain anesthetic drugs such as benzocaine, lidocaine, prilocaine, and articaine. Importantly, in the anesthetic management of G6PD patients to avoid hemolysis, any oxidative agent, such as 8-aminoquinolines for treating malaria, sulphur-containing antibiotics, rasburicase, high doses of aspirin, and paracetamol should be limited as all can cause hemolysis (Elyassi and Rowshan 2009; Valiaveedan et al. 2011; Luzzatto and Seneca 2014; Hwang et al. 2018). Nitrate is part of anesthetist’s armamentarium; though hemolysis by nitrate can be induced without G6PD deficiency, G6PD deficient patients are more vulnerable to it (Wong et al. 2013). Additionally, a very recent study found that administration of sevoflurane, a common anesthesia drug, can lead to a temporary increase in the antioxidant defense system of cells (Zhou et al. 2018). To date, more than 190 genetic variations of G6PD have been found that can change the course of a patient’s treatment due to vulnerability to assorted drugs and agents. It has been suggested that G6PD be classified as a candidate in the matter of pharmacogenomic in its hotspot zones, which might lead to specific treatment (Fig. 11.1) (Manjurano et al. 2015; Goh 2018).
Furthermore, a new quantitative point-of-care test, called The SD Biosensor STANDARD G6PD test, provides sensitivity and specificity of 100% and 97%, respectively. Potentially, this test can improve health outcomes (Pal et al. 2019).
Hereditary Spherocytosis
In cases of non-immune hemolytic anemia, especially in cases with liver dysfunction, jaundice, and splenomegaly, anesthetic agents and surgery can be very challenging because most anesthetic drugs are metabolized by the liver. Some hereditary spherocytosis (HS) case studies have reported that epidural and general anesthesia have the potential to decrease the liver’s blood flow, which may lead to anesthesia-induced hepatotoxicity due to oxygen deprivation. It is highly recommended that preoperative anemia be rectified before any critical surgery (Gelman 1987). In cases of immediately necessary surgery, packed red cell transfusion can overcome the complication (Khatavkar et al. 2016).
Hemoglobinopathies
Sickle Cell Disease (SCD)
Sickle cell disease (SCD) is caused by a specific point mutation in the human β-globin (HBB) gene that results in the production of insoluble hemoglobin S (HbS). The severe form of SCD is called sickle cell anemia (Kato et al. 2018). More than 30 million people world-wide have SCD, with hotspots in Africa, the Middle East and central India (Tsaras et al. 2009). Hypoxia, alcohol, stress, cold weather, acidosis, and dehydration can trigger vaso-occlusive crises (VOC) in SCD patients. Preoperative assessment should review the hematological profile to avoid crises. Preoperative care for the SCD patient should combine the efforts of medical professionals, including surgeon, hematologist, anesthetist/anesthesiologist, and recovery room staff. Additionally, some major complications of SCD must be managed before surgery, including VOCs, chronic anemia, chronic pain, fever, infections, stroke, and pulmonary hypertension (Adjepong et al. 2018; Mangla et al. 2019). Some of the prophylactic measures for SCD include antibiotic prophylaxis, pneumococcal vaccination, thermoregulation, oxygenation and intravenous fluid administration. Also, there is a need for preoperative blood transfusion to reduce HbS to less than 30%. All of these considerations may help to manage complications (Hirst and Williamson 2001; Stanley and Christian 2013). Hydroxyurea primarily increases fetal hemoglobin (HbF) in SCD patients. This results in improvement of their signs and symptoms. But pharmacogenomic studies have shown that even the maximum tolerated dose (MTD) of hydroxyurea results in variable levels of HbF. Further investigations have shown that some single nucleotide polymorphisms (SNPs), in HBG2, BCL11A, and HMIP genes, can influence HbF levels following the consumption of hydroxyurea (Kolliopoulou et al. 2017; Yahouédéhou et al. 2018). Not only hydroxyurea effects are variable in these patients. Opioid medications such as codeine and hydrocodone, used in the management of VOC, elicit different responses because these drugs are converted to morphine and hydromorphone by cytochrome P450 2D6 (CYP2D6 gene polymorphism). All in, pharmacogenetic and genome sequencing can play a significant role in the management of SCD (Iolascon et al. 2015; Kolliopoulou et al. 2017; Rampersaud et al. 2018). Choosing the correct anesthesia for SCD patients is complex and controversial because of differing studies. The determination of regional or general anesthesia should be based on the patient’s underlying medical condition. It is crucial to select an anesthesia approach that prevents and attenuates the vaso-occlusive episodes. Essentially, pain due to VOC should be distinguished from chronic sickle cell pain to ensure proper treatment (Bakri et al. 2015; Ghaffari et al. 2018). Neuraxial anesthesia has been advised over general anesthesia, due to its association with sympathectomy and vasodilation. It has, additionally, the potential to decrease vaso-occlusive episodes, but its drawbacks include headaches, hypotension, bradycardia (Khurmi et al. 2017).
Thalassemia
Thalassemia is a common microcytic hypochromic hemolytic anemia caused by mutations in the HBB or human α-globin (HBA) genes, resulting in decreased synthesis of affected globin chains. These mutations can partially or completely decrease the production of the affected globin. Thalassemia, mostly thalassemia major, is a challenge due to its severity and life-long requirement for constant care and blood transfusion (Naderi et al. 2013; Jahantigh et al. 2014; Needs and Lynch 2018). Regular blood transfusion leads to iron overload, which requires iron-chelating agents like deferoxamine, deferiprone, and deferasirox. Pharmacogenomic studies have shown that some patients have adverse drug reactions while some have none due to the UTG1A6 SNPs (Alizadeh et al. 2014; Iolascon et al. 2015). Further pharmacogenetic studies are being conducted on human pluripotent stem cells to find better treatment options for these patients (Suh 2017). Cutting-edge genome editing techniques—such as clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) and transcription activator-like effector nucleases (TALEN)—show promise in curing thalassemia (Finotti et al. 2015). Thalassemia patients are in a hypercoagulable state with increased platelet activation and aggregation, which makes them prone to thromboembolic events such as pulmonary embolism (PE), deep venous thrombosis (DVT) and portal vein thrombosis, or bleeding due to thrombocytopenia. In the case of surgery, in preoperative preparations, the use of procoagulants such as antifibrinolytics and factor concentrates should be limited unless a particular situation demands their use (Taher et al. 2008; Avery IV and Klick 2018). The anesthetist/anesthesiologist confronts some challenges in these cases: insufficient oxygen-carrying capacity due to severe anemia, difficulty in the airway due to extramedullary hematopoiesis, pulmonary hypertension, and diseases related to blood transfusion like hepatitis (Jyothi et al. 2015). Epidural anesthesia has been recommended to overcome some conditions, such as the need for postoperative mechanical ventilation due to the low capacity of RBCs to carry oxygen, and postoperative nausea and vomiting. In terms of decreasing oxygen need, epidural anesthesia is more satisfactory in enhancing the patient’s recovery. In lowering the demand for neuromuscular blocking agents and intravenous analgesia, epidural anesthesia results in quicker awakening despite anemia and acidosis due to the anaerobic metabolism. In patients with a platelet count less than 100,000 × 109/L, the chance of bleeding is low. In such cases, regional anesthesia should be applied after evaluating risks and advantages (Huang et al. 2014; Govil et al. 2019).
Immune Hemolytic Anemia
Autoimmune hemolytic anemia (AHA) and Evans syndrome also can be obstacles to surgery. For the anesthetic management of these patients, hemodilutional autologous transfusion has been recommended as a useful measure to minimize complications related to bleeding and immune hemolytic reactions (Nagano et al. 2000; Igarashi et al. 2002; Soudabeh et al. 2014).
Aplastic Anemia
Aplastic anemia, associated with immunosuppression and pancytopenia, makes anesthetic management a challenge, particularly in pregnancy. Thrombocytopenia is a common complication in patients with aplastic anemia, which contraindicates regional anesthesia. General anesthesia seems to be the only option here (Table 11.1) (Marsh et al. 2009; Kaur et al. 2012a; Riveros-Perez et al. 2018). Propofol is a general anesthetic drug used for aplastic anemia patients (Ahmed and Monem 2005; Kaur et al. 2012b). Metabolism of propofol in the liver depends on several genes, including CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1 and NQO1, Disruption of these genes has the potential to alter the effect of propofol. Besides, the underlying mechanism for propofol’s action somehow depends on the GAPAG2 receptor gene, and polymorphism of this particular gene may affect propofol anesthetic effects (Mikstacki et al. 2013, 2017).
Anemia Caused by Anesthetic Drugs
Anesthetic agents are one of several factors that can affect the RBC and its indices. For example, nitrous oxide (N2O) , known as laughing gas, has the potential to cause acquired megaloblastic anemia by irreversible inactivation of vitamin B12. Usually, prolonged exposure to N2O can lead to the depletion of vitamin B12 and elevation of plasma homocysteine that are characteristics of B12 deficiency (Emmanouil and Quock 2007). This may make the patient vulnerable to cardiovascular disease (Duma et al. 2015; Ganguly and Alam 2015; Chi 2018). Interestingly, a comprehensive study on the medical staff who have been exposed to N2O in for at least 5 years has demonstrated that N2O gas can cause DNA damage. This study also compared the genotoxicity effects of sevoflurane and isoflurane with N2O, which showed there is no genotoxicity related to sevoflurane and isoflurane (Wronska-Nofer et al. 2009).
Erythrocyte Recovery
In the matter of erythrocyte recovery, a study that compared the effects of epidural anesthesia, general anesthesia, and combination of both on red blood cell endogenous recovery showed that epidural anesthesia resulted in a higher rate of the circulating erythrocyte mass than general anesthesia or a combination (Borghi et al. 2005).
Polycythemia
Polycythemia or erythrocytosis is a condition related to an absolute increase in RBC mass, in which hemoglobin and hematocrit levels are above the normal range based on gender and age. This can be primary, where a genetic factor plays a role, or secondary, where the cause is non-genetic (McMullin 2008). Generally, secondary erythrocytosis follows hypoxia. Hypoxia during general anesthesia in surgery is a major clinical occurrence in which the response can range from mild hypertension and elevation in EPO to bradycardia.
Patients with pre-existing hypoxia should be identified and managed before surgery; conditions include respiratory disorders: chronic obstructive pulmonary disease (COPD); Pickwickian syndrome, cyanotic heart disease with right-to-left shunts, renal disorders and heavy smoking. Hemoglobinopathies with hypoxia include high-affinity hemoglobins like Hb Yakima and methemoglobinemia. Hypoxia also can be a consequence of EPO administration, anabolic steroid use, and testosterone replacement therapy (Bendixen and Laver 1965; Tojo et al. 2015; Pillai and Babiker 2018). Often primary polycythemias such as polycythemia vera (PV) and Chuvash polycythemia have an underlying mutation in the erythropoiesis signaling pathway. Next, we should consider that the EPO level usually is normal in the primary type (McMullin 2008; Aljabry 2018). Mutation in the von Hippel-Lindau (VHL) gene leads to VHL syndrome and Chuvash polycythemia (Richard et al. 2002; Pastore et al. 2003; Perrotta et al. 2006). VHL syndrome is associated with spinal or retinal hemangioblastoma, renal cell carcinoma, or pheochromocytoma, a usually noncancerous tumor frequently occurring in the adrenal gland. For anesthetic management of the patient with VHL syndrome, the presence of pheochromocytoma must be considered. In such cases, epidural and general anesthesia can be used, based on patient condition, but in cases of hemangioblastoma, epidural blockades are limited (Murthy et al. 2006; Jalbani et al. 2015). Also, N2O cannot always be used for maintenance of anesthesia during surgery. For example, in a VHL case with cerebellar hemangioblastoma and renal cell carcinoma, N2O was avoided due to the possibility of carbon dioxide pneumoperitoneum; desflurane in a mixture of oxygen and air made that surgery uneventful (Sahni et al. 2019).
Anesthetic Considerations in Hematological Malignancies
Hematological malignancies such as leukemias, lymphomas, myeloproliferative neoplasms (MPN) and myelodysplastic syndromes (MDS) do not require surgery as such, but some situations require surgery and anesthetic management. Among them are infection, bone marrow (BM) biopsy or aspiration, chemotherapy and radiotherapy, orthopedic surgery, splenectomy, appendectomy, port implantation, and open-heart surgery. Such patients, especially children, are prone to infection due to immunosuppression, and in most cases require careful medical consideration. The collaboration of a skillful team of surgeons and medical oncologists is warranted. Since most hematological malignancies come with vast genetic variation, patients must be precisely evaluated and carefully managed before undergoing surgery (Spiers 1973; Hohenberger and Buchheidt 2005).
Leukemia
Anesthetic management and related therapy of leukemias should proceed carefully. Numerous genetic abnormalities can cause the different types of leukemia, making management of these patients more complex than most. Some leukemia patients develop anemia, coagulation disorders, immunosuppression or combinations thereof. Moreover, the infiltration of blast cells into oropharyngeal tissue may lead to difficult intubation and/or pharyngeal hemorrhage. At times hyperleukocytosis (white blood cell (WBC) > 100,000 × 109/L) may trigger leukostasis syndrome, with acute respiratory failure and increased blood viscosity, which may cause bleeding and thrombosis (Naderi et al. 2014). Considering that surgery may induce this syndrome, the reduction of WBC before general anesthesia seems necessary. Importantly, packed red cells should be transfused sparingly, to avoid the development of the syndrome (Groeben et al. 1992; Giammarco et al. 2017).
Bleeding and thrombosis, especially in the acute leukemias, are considered major risk factors for early death. Acute promyelocytic leukemia (APL) induces a hypercoagulable state which should be carefully managed to avoid further complication. Anticoagulant therapy may put these patients at high hemorrhagic risk, which makes this therapy a challenge for managing APL. While, with the rise of all-trans retinoic acid (ATRA), APL has been largely managed, the rate of early hemorrhagic death has not significantly decreased; ATRA may play a role in promoting thrombosis (Rickles et al. 2007). However, treating patients with ATRA and arsenic trioxide (ATO) has a lower risk of thrombosis. In these patients, bleeding associated with disseminated intravascular coagulation (DIC) may occur. Cytoreductive therapy and supportive care such as platelets, fresh frozen plasma, cryoprecipitate, or fibrinogen concentrates are recommended to prevent bleeding (Thachil et al. 2015; Lad et al. 2017).
Chronic myeloid leukemia (CML) is an MPN that can occasionally proceed to blast crisis, with elevation of blast cells above 20%. Some studies suggest that spinal or epidural anesthesia be evaluated prior to use as it increases the risk of central nervous system (CNS) contamination by circulating blast cells (Owsiak and Bullough 2016; Rebahi et al. 2018).
Susceptibility to infection due to immunosuppression is a major obstacle among the many that challenge surgery in a leukemic patient, especially a child. This immunosuppression can be caused by BM dysfunction and/or treatment with steroids and radio- or chemo-therapy. While most infections are caused by gram-negative bacteria, patients are also prone to fungi like Aspergillus which affect patients undergoing chemotherapy, viruses such as cytomegalovirus (CMV) and Epstein–Barr (EBV) and parasites (Oduro-Dominah and Brennan 2013; Dong et al. 2019). Management consists of supportive care and treatment of the underlying cause. Patients, especially children, frequently have an indwelling central venous catheter (CVC). As a CVC can be a source of contamination it should be placed by a professional under aseptic conditions. One should ensure that these patients’ surgeries are scheduled before surgery of any infective cases. The risk/benefit ratio of using regional anesthesia should be evaluated, since this kind of anesthesia, especially the neuraxial block, may also increase the risk of infection. Intramuscular injection might lead to formation of an abscess and should be avoided. Further, it is strongly recommended that patients be vaccinated against S. pneumoniae, N. meningitidis, and H. influenzae type b prior to procedures like splenectomy (Bonanni et al. 2017).
Polycythemia Vera and Essential Thrombocythemia
PV is an MPN, characterized by erythrocytosis, hyperviscosity and thrombocytosis and/or leukocytosis. Patients with PV are at increased risk of hemorrhagic events and/or thromboembolism (Gerds and Dao 2017). Although there is evidence of abnormal hemostasis in these patients, preoperative coagulation tests may be normal (Rigby and Leavell 1960). Keeping hematocrit below 45% by consumption of aspirin once or twice daily, with phlebotomy 2–4 months before surgery, is required to reduce thrombohemorrhagic complications. Cytoreductive measures for high-risk patients have been recommended, the main choice being hydroxyurea. Interferon-α, pipobroman, P-32 and busulfan are alternative options, because 20 and 25% of patients develop resistance to hydroxyurea (Sosis 1990; Sever et al. 2014; Tefferi and Barbui 2019). Adequate preoperative management of these patients, as enumerated above, minimizes complications, according to a comprehensive study which also noted that preoperatively uncontrolled patients and controlled patients had 36% and 5% mortality rates, respectively (Wasserman and Gilbert 1964). The bleeding diathesis in these patients mandates careful use of regional anesthesia (Sosis 1990; Saini et al. 2019). Another study found general anesthesia to be associated with more postoperative DVT than regional anesthesia in patients with PV (McKenzie et al. 1985).
Another MPN is essential thrombocythemia (ET), characterized by constant thrombocytosis with an inclination to thrombosis and hemorrhage (Brière 2007). Preoperative management of ET follows that of PV. In ET, reduction of the platelet count by cytoreductive therapy should be considered. Moreover, regional anesthesia, especially spinal anesthesia, should be managed carefully because it may cause a hematoma, which could lead to spinal cord compression and paraplegia (García-Ferreira et al. 2005; Sever et al. 2014; Tefferi and Barbui 2019).
Anesthesia and Hemostasis
One of the most common complications of surgical procedures is postoperative hypercoagulability. Major surgeries are associated with hypercoagulable and proinflammatory states which can continue into the postoperative phase. DVT and PE are among the most common coagulopathies of major surgical procedures, particularly lower-extremity vascular and orthopedic surgeries (Tuman et al. 1991; Christopherson et al. 1993; Rosenfeld et al. 1993). The exact underlying mechanism of the hypercoagulability remains unclear. Anesthesia and surgery, being stressful conditions, have significant effects on the hemostasis system. Hemostatic changes are due to several factors, including “stress response”, consumption of coagulation proteins at the site of injury, impaired synthesis, hemodilution and the effects of anesthesia on these proteins. Although surgery can increase acute phase reactants such as fibrinogen, FVIII, VWF and α-antiplasmin, many changes in the hemostasis system are triggered when the patient is anesthetized, even before surgery begins. This shows that both anesthetic and surgical stresses induce the changes (Table 11.2) (Collins Jr et al. 1977; Breslow et al. 1993; Rosenfeld et al. 1994).
Anesthesia and Thrombophilia
Perioperative thrombophilia is one of the main causes of morbidity and mortality in patients undergoing major surgery. Both acquired and inherited thrombophilia factors can increase the risk. Major surgery is associated with proinflammatory states that provoke hypercoagulability and, consequently, vaso-occlusive and thrombotic complications, as a major cause of surgery-related deaths.
Some perioperative changes such as elevated factor (F) VIII and von Willebrand factor (VWF) levels, decreased level of the natural anticoagulants such as heparin cofactor II, protein C and antithrombin, increased platelet function, and decreased fibrinolytic activities, can increase the risk of thrombosis (Ygge 1970; O’Brien et al. 1974; Collins Jr et al. 1977; Kluft et al. 1985; Andersson et al. 1987).
In a study on coronary artery disease (CAD), it is reported that in peoples who have A1A1 genotype the plasma FVIIa levels is epigenetically downregulated via methylation of F7 promoter. Hypo-methylation of F7 promoter in A1A1 genotypes increases the risk of CAD (Friso et al. 2012).
About the role of miR-223 in the thrombosis and atherosclerosis, it is suggested that hypo-methylation of miR-223 promoter increases the risk of atherosclerotic cerebral infarction (Rangrez et al. 2013; Li et al. 2017). Furthermore, hyper-methylation of Thrombomodulin (TM) promoter correlates with the lower TM mRNA levels and higher plasma level of homocysteine and could be associated with increased risk of cerebral infarction (Yang et al. 2016).
The preoperative level of plasminogen activator inhibitor-1 (PAI-1) is a predictor for risk of postoperative thrombosis, while this is not true for fibrinogen. The plasma level of these proteins may differ between individuals, due to their unique genetic profiles. Due to normal gene variations, some patients may have higher levels, and be more prone to postoperative thrombotic events. The PAI-1 plasma level partly is regulated by the promoter guanidine insertion/deletion (4G/5G) polymorphism in the PAI-1 gene. Patients with PAI-1 4G/4G polymorphism are at higher risk of perioperative thrombosis (Dorgalaleh et al. 2013a). The FV Leiden and prothrombin G20210A mutations are among the most common gene variations and are associated with thromboembolic events (Table 11.3).
In fact, individuals with inherited thrombophilia are at lifelong risk of venous and arterial thrombosis, particularly in response to challenging situations such as pregnancy or surgery (Ogasawara et al. 2003; Hazirolan et al. 2004; Hosseini et al. 2015). Preoperative thrombophilia testing is not routine in patients without a history of thrombosis. The growing role of perioperative coagulation genomic in determining the risk of bleeding and thrombosis remains to be established (Morozowich et al. 2006). Cutting-edge technologies like NGS can help in developing the coagulation genomic, and improving personal anesthesia management of patients requiring surgery. The anesthetic considerations of thrombophilia range from perioperative use of prophylactic and/or therapeutic anticoagulation to major resuscitation following a PE (Kalantari et al. 2013; Gonzalez-Fiol and Eisenberger 2014). Regional anesthesia has a lower risk of thrombosis than general anesthesia (Hollmann et al. 2001). However, in patients receiving therapeutic anticoagulation, most regional anesthetics, particularly spinal and epidural anesthesia, are contraindicated (Gonzalez-Fiol and Eisenberger 2014).
FV Leiden, the most common inherited risk factor for venous thromboembolism (VTE), is more common in Scandinavian and northern European ethnicities, while rare in Asian and African populations (Dahlbäck 1995; Ornstein and Cushman 2003). Heterozygous carriers and homozygotes have a 4 in 10, and 100-fold increased risk of DVT, respectively (Cooley et al. 2007). For parturients with FV Leiden and spontaneous, induced or elective cesarean delivery under spinal or epidural anesthesia, it has been suggested that the underlying disease process, anticoagulant dosing alterations, and anesthetic options, be discussed early in the third trimester. Transition from low molecular weight heparin (LMWH) to unfractionated heparin (UH) must be done prior to the 38th gestational week (Harnett et al. 2005). However, the majority of data do not support the alteration of anesthesia technique in patients with the known FV Leiden genotype (Donahue 2004).
Protein C level is also decreased postoperatively. In addition to surgery-related protein C deficiency, its decreased level is observed as an inherited deficiency. Anesthetic management of patients with protein C deficiency include preoperative protein C replacement therapy with fresh frozen plasma (FFP), atraumatic procedure and provision of enough air leak around the endotracheal tube (Wetzel et al. 1986). Perioperative management of protein C deficiency has included the use of tailored atraumatic anesthetics, avoidance of tissue compression and dehydration, protein C replacement and anticoagulation (Kumagai et al. 2001; Batool et al. 2011; Tiwari et al. 2012). In protein S deficiency, any anesthetic technique can be used after protein S replacement therapy (Abramovitz and Beilin 1999; Sugimoto et al. 2018).
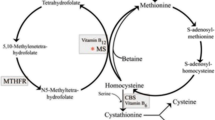
Several mutations are associated with methylenetetrahydrofolate reductase (MTHFR) deficiency, among which the C677T polymorphism is the most detrimental. Clinical manifestations of patients with MTHFR deficiency include neurological symptoms, cardiovascular and thromboembolic disorders (Gales et al. 2018). Anesthesiologist should avoid the use of N2O in patients with MTHFR deficiency (Shay et al. 2007). N2O irreversibly inhibits methionine synthesis and leads to decreased homocysteine re-methylation, thereby increasing systemic homocysteine, which increases the risk of thrombosis (Mayer et al. 1996; Badner et al. 2001). Such exposure also is associated with severe neurological and hematological manifestations. Hence, patients with MTHFR deficiency, especially homozygous patients, should not be anesthetized with N2O-containing general anesthesia and should be anticoagulated sufficiently whenever possible during their hospitalization. Spinal or epidural anesthesia can be considered except for patients receiving therapeutic anticoagulation. Thrombotic events in patients with MTHFR deficiency can cause kidney damage. Thus, one should consider altered drug clearance in patients receiving anticoagulants (Shay et al. 2007).
Antithrombin-III, the main physiological inhibitor of thrombin, while decreasing progressively during the early postoperative phase, more rapidly returns to the preoperative level following epidural rather than general anesthesia (Mayer et al. 1996; Corral et al. 2018). Both increased FVIII level and decreased antithrombin level can increase the risk of thrombosis. The risk is higher in patients with severe trauma. The antithrombin level was observed to fall up to 20% with both types of anesthesia. This degree of decrease can increase thrombosis risk four-fold (Konkle et al. 2003; Martinelli et al. 2014; Jooste et al. 2019).
Inherited antithrombin deficiency is the strongest risk factor for thrombosis, which presents in up to 1% of patients with venous thrombosis. Up to 80% of patients with antithrombin deficiency have SERPINC1 gene defects (Martinelli et al. 2014; Corral et al. 2018). There are no randomized clinical trials, guidelines or consensus statements about the use of antithrombin concentrate. However, a randomized, double-blind, placebo-controlled trial demonstrated that supplementation of antithrombin improves heparin sensitivity during cardiopulmonary bypass in infants (Jooste et al. 2019). According to the case reports and series, general or regional anesthesia management of anesthesia in patients with antithrombin deficiency includes perioperative use of recombinant or hemo-derivative antithrombin, placement of a filter in the inferior vena cava to prevent PE, active mobilization of lower extremities and perioperative anticoagulation (Konkle et al. 2003; Okamoto and Minami 2003; Pamnani et al. 2010; Piper and Farrell 2015; Yu et al. 2018). In some patients, decreased levels of antithrombin-III and protein C were sufficient for spontaneous thrombosis, as can be observed in patients with inherited deficiencies of these factors (Martinelli et al. 2014; Corral et al. 2018). Antithrombin-III and α-antiplasmin are sensitive indices of coagulation and activation of fibrinolysis, respectively (Konkle et al. 2003; Pamnani et al. 2010; Jooste et al. 2019). Multiple studies to date have unequivocally demonstrated that regional anesthesia, particularly epidural anesthesia, can ameliorate postoperative hypercoagulability. In a study on 20 premenopausal women undergoing abdominal hysterectomy, FVIII and VWF levels were observed to be lower in epidural than general anesthesia (Bredbacka et al. 1986). Several randomized clinical or controlled trials on patients with lower-extremity vascular reconstruction, total hip replacement, and abdominal hysterectomy, demonstrate that epidural anesthesia can improve fibrinolytic activity by hindering the postoperative release of PAI-1 protein (Simpson et al. 1982; Modig et al. 1983; Donadoni et al. 1989; Rosenfeld et al. 1993). Fibrinolytic activity is increased, and surgery related “stress response” is reduced, by epidural anesthesia. Surgical stress, with significant adrenocortical response, can increase fibrinolytic activity, while epidural anesthesia can decrease fibrinolysis by decreasing adrenocortical response (Rosenfeld et al. 1993). When a long-acting local anesthetic such as bupivacaine is used, in combination with adrenaline and ephedrine, it can increase fibrinolytic activity. Even a small amount of adrenaline can increase fibrinolytic activity (Rosenfeld et al. 1993). A study on the flow of blood in the calf during total hip replacement, using venous occlusion plethysmography (VOP) on the non-operated leg, demonstrated that generally anesthetized patients had significantly lower blood flow and venous capacity in the lower limb at the end of surgery than another group with epidural blockade. It suggested that a constant decrease of the blood flow in the deep veins of the lower limbs may increase the risk of DVT (Modig et al. 1980). Absorption of local anesthetic agents from the epidural space has systemic effects such as direct impairment of platelet aggregation (Feinstein et al. 1976; Borg and Modig 1985; Henny et al. 1986). Patients who were given general anesthesia and a postoperative epidural infusion of bupivacaine-fentanyl showed a nine-fold decreased incidence of vascular graft occlusion than patients with general anesthesia and patient-controlled opioid analgesia (PCA) (Tuman et al. 1991). Patients undergoing peripheral vascular surgery under epidural anesthesia had five-fold lower need for reoperation due to graft failure than patients who were given general anesthesia (Christopherson et al. 1993). An equivalent decrease in thromboembolic events has been observed in patients who had knee arthroplasty, or open prostatectomy, under epidural anesthesia (Hendolin et al. 1981; Jørgensen et al. 1991).
Furthermore, the use of anticoagulants, antiplatelets, and fibrinolytic drugs has a great effect on the perioperative VTE. The simultaneous use of regional anesthesia and antithrombotic agents may increase the risk of bleeding. In a patient with a known coagulopathy, anesthesiologists encounter challenging situations that require risk/benefit decision-making. Any coagulopathy is a challenge for regional anesthesia as it may cause serious complications.
Fibrinogen levels remain increased for a relatively long time (7 days) after major surgeries. Fibrinogen, FVIII and VWF levels are increased postoperatively. Although this increase can be observed in general and regional anesthesia, the FVIII and VWF level increase is significantly higher in general versus regional anesthesia. This increase is attributed to beta-adrenergic receptors; epidural anesthesia can attenuate this increase (Rosenfeld et al. 1993).
Epidural anesthesia can improve the blood flow in the lower extremities, while general anesthesia decreases this flow in the deep veins and can increase the risk of graft occlusion. Postoperative arterial thrombosis is higher in patients with a higher preoperative level of PAI-1 under general anesthesia. The postoperative PAI-1 level is higher in regional than general anesthesia. The rate of arterial thrombosis is higher in patients under general than regional anesthesia. Impaired fibrinolysis may relate to postoperative arterial thrombosis. It seems that regional anesthesia combined with epidural fentanyl analgesia may decrease the risk of arterial thrombosis due to lower PAI-1 levels in patients with lower extremity revascularization (Rosenfeld et al. 1993). PAI-1, a specific, rapidly acting inhibitor of tissue- (tPA) and urokinase-plasminogen activators (uPA), is primary regulator of plasminogen. PAI-1 deficiency and increased levels are associated with bleeding and thrombosis, respectively (Rosenfeld et al. 1993). Regional anesthesia attenuates surgery-related cortisol and catecholamine response, which change is suggested as main cause of a number of hemostatic changes under general and regional anesthesia. Dexamethasone administration can increase the PAI-1 level and impair fibrinolysis function (Rosenfeld et al. 1993; Corral et al. 2018). Increased PAI-1 level is also associated with the risk of myocardial infarction (MI).
Perioperative morbidity and mortality is higher in patients receiving general rather than regional anesthesia, while incidence of postoperative DVT is lower in patients with regional rather than general anesthesia. Increased levels of coagulation factors are smaller in patients receiving general rather than regional anesthetic.
A meta-analysis of randomized control trials demonstrated that neuraxial blocks decrease death by 30%, DVT by 44%, and PE by 55% (Modig et al. 1983; Bredbacka et al. 1986).
Anticoagulation Therapy and Anesthesia
Anesthetic Considerations for Patients Receiving Fibrinolytic Therapy
Fibrinolytic drugs can cause severe bleeding, especially in invasive procedures. Neuraxial hemorrhage can be observed in patients undergoing heparin and/or antiplatelet therapy. Patients with a history of lumbar puncture, epidural or spinal anesthesia and concomitant steroid injection into the epidural space should be closely monitored. Patients receiving thrombolytic drugs should not undergo puncture of a non-compressible vessel within 10 days after surgery. Patients undergoing fibrinolytic therapy should not receive epidural or spinal anesthesia, except under specific circumstances. Should patients receive concurrent neuraxial blocks and thrombolytic agents, evaluation of their neurologic status every two hours during surgery is recommended. Epidural catheter infusions must be restricted to medications inhibiting the risk of sensory and motor block to ensure better monitoring of neurological status. Unforeseen requirements for thrombolytic drugs may arise in patients with a neuraxial catheter. There is no clear-cut recommendation for catheter removal in such situations. However, fibrinogen assay can determine remaining fibrinolytic activity and the appropriate time for catheter removal (Horlocker et al. 2019).
Anesthetic Considerations for Patients on Unfractionated Heparin (UFH)
The medical history of patients should be reviewed to determine the possible use of coagulation modifiers. Two- or three-time daily thromboprophylaxis administration of 5000 U (low-dose) UFH is not contraindicated with the neuraxial techniques, if anesthesia is administered 4–6 h after heparin administration. Patients with a UFH dose greater than 10,000 U have an increased risk of surgical bleeding. However, the safety of neuraxial blockade for patients with daily or twice-daily administration of UHF has not been determined. Hence, risk-benefit assessment of use of UHF three times per day should be evaluated individually. However, in pregnant patients receiving preoperative thromboprophylaxis of high-dose (7500–10,000 U) UFH, daily or twice-daily dose no more than 20,000 U, neuraxial blockade is recommended to be administered 12 h after subcutaneous heparin administration, after evaluation of the patient’s hemostatic state. In preoperative administration of high-dose (more than 10,000 U per dose or total >20,000 U daily) UFH in pregnant patients, it is recommended that neuraxial blockade be administered 24 h after the subcutaneous heparin administration. Due to the higher risk of heparin-induced thrombocytopenia (HIT) in patients with more than 4 days of heparin therapy, a platelet count is recommended prior to catheter removal and the neuraxial blockade.
Some specific issues are recommended for patients anesthetized with neuraxial techniques and concomitant administration of intravenous heparin, including:
-
1.
Patients with other coagulopathies should not be anesthetized with neuraxial techniques.
-
2.
There must be a one-hour delay in heparin administration, after needle positioning.
-
3.
Neuraxial catheters should be removed 4–6 h after administration of the last heparin dose, with resumption of heparin administration one hour after catheter removal.
-
4.
For rapid recognition of a motor blockade, the continuous post-operative state of patient stability should be recorded. The lowest concentration of regional anesthetics should be administered to promote immediate diagnosis of a spinal hematoma.
-
5.
When needle positioning is difficult and/or accompanied by hemorrhage, discontinuing neuraxial anesthesia is recommended. In this situation, a suitable risk-benefit decision should be made by the surgeon and anesthesiologist. It is unclear whether the risk of neuraxial hematoma is increased while the anesthetic is accompanied by full anticoagulation. Postoperative monitoring of neurological function is recommended and decision-making about the type of analgesia should minimize the degree of sensory and motor blockade, yielding a subsequently easy recognition of unexpected neuro-difficulties (Table 11.4) (Horlocker et al. 2019).
Anesthetic Considerations for Patients on Low Molecular Weight Heparin (LMWH)
The current recommendations are not capable of entirely eradicating the possibility of spinal hematoma. Patients receiving chronic anticoagulation therapy via high-dose LMVH are at high risk of destabilization of their coagulation state. Despite the advantages of monitored high-dose LMWH therapy, measurement of plasma levels of anti-FXa is not a good predictor of bleeding risk. No residual anti-FXa level ensures a safe neuraxial blockade. Concomitant administration of antiplatelet agents, dextran, or other oral anticoagulants to a patient with LMWH may increase the risk of spinal hematoma. Due to the risk of HIT in patients with more than 4 days of LMWH therapy, a platelet count is recommended prior to catheter removal.
In a patient receiving preoperative prophylactic doses of LMWH, needle positioning should be done no earlier than 12 h after the LMWH administration. Delaying surgery is not necessarily recommended in bloody needle and catheter positioning. In this situation, the surgeon should be consulted and postoperative re-administration of LMWH postponed for 24 h. In patients receiving a high dose of LMWH, it is recommended that needle insertion be postponed at least 24 h after administration of LMWH to ensure the optimal hemostasis status. The monitoring of remaining anti-FXa activity should be considered, especially in senile patients and those with renal dysfunction For general surgery, the recommendation is against the use of neuraxial techniques if patients have received LMWH 2 h preoperatively. Due to maximum antithrombotic activity in this interval, needle positioning might be accompanied with a high bleeding tendency.
Enoxaparin is used for the management of patients with DVT or PE. For enoxaparin, positioning or removal of a neuraxial catheter must be postponed for no less than 12 h following administration of a prophylactic dose. For patients receiving therapeutic doses, the time interval should be 24 h. A postoperative dose of enoxaparin should be administered 4 h following catheter removal (Horlocker et al. 2019).
Anesthetic Considerations for Patients on Fondaparinux
Fondaparinux is a synthetic pentasaccharide that can selectively inhibit FXa. This anticoagulant can be used after extensive orthopedic surgery, or the management of PE (Turpie et al. 2002; Matisse Investigators 2003). Available data about the risk of spinal hematoma related to the administration of fondaparinux and its postoperative initial dosing are insufficient. The recommendations are against the administration of fondaparinux in patients with a residing epidural catheter. The recommended time-interval between interruption of fondaparinux and the pain blockade process is 3–4 days. The recommended time-interval between the pain blockade process and re-administration of fondaparinux is 24 h (Narouze et al. 2015).
Anesthetic Considerations for Patients on Oral Anticoagulants
Warfarin
Contrary to the achieved adequate FVII activity observed by decreasing INR, hemostatic conditions (FII and X activity levels) may not be enough to prevent hemorrhage during the first 24–72 h after interrupting a durable warfarin therapy (Xi et al. 1989; Ansell et al. 2008). Thus, care must be taken when a patient with interrupted durable warfarin therapy has to undergo a neuraxial technique. It is recommended that the surgical procedure be performed preferably 5 days after interruption of the durable warfarin therapy; initiation of the neuraxial blockade depends on the normalized INR. Recommendations are against the simultaneous administration of other oral anticoagulants with warfarin, the effects of which may not be reflected in INR (such as non-steroidal anti-inflammatory drugs (NSAIDs), UHF, LMWH and thienopyridine derivatives) but that affect the other coagulation mechanisms. At the beginning of warfarin therapy, less than 24 h prior to surgery, the neuraxial blockade should be accompanied by regular monitoring that INR is at the normal level. Daily INR checking is also suggested during the epidural analgesia for patients receiving low doses of warfarin. The neurological functions of patients receiving warfarin therapy should be monitored regularly during the epidural analgesia. Decision on the type of analgesia should strive to minimize the degree of sensory and motor blockade. In patients receiving prophylactic doses of warfarin, removal of neuraxial catheters should be limited to those with an INR of 1.5 and lower (activity of coagulation factors is considered to be more than 40%). Epidural catheter removal the first 12–24 h after administration of warfarin is not associated with increased risk of bleeding. However, epidural catheter removal at 48 h is not safe enough. It seems helpful to patients with an INR between 1.5 and < 3 to surgically remove a residing catheter. The administration history of other modulators of hemostasis that may have no effect on the INR (clopidogrel bisulfate, LMWH, UHF, aspirin and other NSAIDs) also should be checked. It is suggested that the neurological function form be monitored until the desired INR is achieved, before catheter removal. In patients undergoing neuraxial catheter infusion with an INR 3 or higher, it is strongly recommended that warfarin administration be reduced or suspended, because there is no reliable advice for simplifying catheter removal in these patients (Horlocker et al. 2019).
Genetic-Guided Dosing of Warfarin
Some evidence indicates that genetic-based differences in the warfarin metabolism could affect the functionality of the drug. For instance, CYP2C9 (cytochrome P450 family 2 subfamily C member 9) is a polymorphic gene which encodes a monooxygenase involved in the metabolization of more potent enantiomer of warfarin (S-warfarin) (Ansell et al. 2008). The more prevalent and well-investigated alleles of this enzyme are 2C9*2 (rs1799853: C430T) and 2C9*3 (rs1057910: A1075C) which have impact on pharmacokinetics of warfarin. Carriers of such alleles have reduced capacity to metabolize S-warfarin, resulting in reduced warfarin requirement compared to people with wild-type enzyme (2C9*1) (Ansell et al. 2008). Warfarin plays its anticoagulant role by inhibiting the vitamin K epoxide reductase (VKOR) enzyme in the vitamin K cycle. There are several variants of VKORC1 gene producing enzymes with different impacts on the pharmacodynamics of warfarin. For example, the H1 and H2 haplotypes are warfarin-sensitive and the H7, H8, and H9 represent warfarin-resistant haplotype (Ansell et al. 2008). The polymorphism of G3673A (rs9923231) is in the promoter region of VKORC1 gene affecting the pharmacokinetics of warfarin. The carriers of A allele have lower expression and activity of the VKOR enzyme. The A allele is more common in Asian populations. This issue provides an explanation for lower doses of warfarin in individuals of Asian descent. G9041A (rs7294) is a SNP in the 3′UTR of VKORC1 gene and it might be correlated with higher doses of warfarin (Owen et al. 2010).
Direct Oral Anticoagulants (DOACs)
Direct Oral FXa Inhibitors
Direct FX inhibitors, including rivaroxaban, apixaban, edoxaban, and betrixaban, have a growing role in the management of patients with thromboembolic disorders. Neuraxial blockade is recommended to be done 3 days after interruption of direct anti-FXa agents. Earlier than 3 days, the blockade should be done after considering anti-FXa activity level. However, an allowable level of remaining anti-FXa activity to be reached before administering neuraxial blockade remains unspecified. The catheter must be removed 6 h before the first postoperative dose of direct anti-FXa agent. This time interval is 5 h for betrixaban. Recommendations are against implementation of neuraxial blockade in patients with betrixaban therapy and a creatinine clearance (CrCl) rate of less than 30 mL/min. In patients with a residing catheter and an unexpected requirement for anti-FXa agents, it is recommended to that the agents be administered in a specific time interval (rivaroxaban 22–26 h, apixaban 26–30 h, edoxaban 20–28 h, and betrixaban 72 h) or after a calibrated anti-FXa assay prior to the catheter removal has been performed (Horlocker et al. 2019).
Direct Oral Thrombin Inhibitors
The renal clearance of dabigatran may be affected by surgery. It is recommended that the neuraxial blockade be done 5 days after discontinuation of dabigatran. For shorter intervals, patients should be checked by dilute thrombin time (dTT) and ecarin clotting times (ECT). An allowable level of remaining dabigatran activity, before receiving neuraxial blockade, remains unspecified. In patients with a residing catheter and an unexpected need for dabigatran, it is recommended to dabigatran dosing be held for 34–36 h, or that dTT and ECT be evaluated, prior to the catheter removal. Patients receiving parenteral thrombin inhibitors, including desirudin, bivalirudin, and argatroban, should not use neuraxial anesthetics (Horlocker et al. 2019).
Anesthetic Considerations for Patients on Antiplatelet Drugs
The antiplatelet drugs such as aspirin, platelet adenosine diphosphate receptor (P2Y12 subtype) antagonists (thienopyridine derivatives): clopidogrel, ticlopidine and prasugrel; GPIIb/IIIa antagonists: tirofiban, eptifibatide and abciximab; have different effects on platelet function. Bleeding time as a monitoring test of platelet function is not reliable. Thus, preoperative monitoring of patients receiving antiplatelet therapy is mandatory to minimize the risk of bleeding. Clinical manifestations, including petechiae, purpura, mucosal bleeding and easy bruising, also should be considered. The NSAIDs like aspirin appear to be associated with no additional risk of spinal hematoma in patients undergoing spinal or epidural anesthesia. There is no interference with neuraxial blockade in patients receiving NSAIDs. Also, there is no particular worry about the scheduling of catheter technique, monitoring of neurological functionality or the scheduling of catheter removal. The recommendations are against the implementation of neuraxial techniques in patients receiving NSAIDs with possible concomitant administration of antithrombotic drugs such as LMWH, UFH and oral anticoagulants at the initial time after surgery. Cyclooxygenase-2 (COX2) inhibitors such as celecoxib, etoricoxib and rofecoxib have a slight impact on platelet function; only concomitant administration with antithrombotic drugs might considerably increase the effect.
It is recommended that neuraxial blockade be performed 10 days after stopping ticlopidine, 5–7 days after clopidogrel, and 7–10 days after prasugrel. Twenty-four hours post-operative re-administration of thienopyridine derivatives is suggested. When neuraxial blockade is required 5–7 days after discontinuation of clopidogrel, platelet function should be reassessed to ensure elimination of drug effects on the platelets. The recommendations are against a sustained neuraxial catheter in patients receiving prasugrel or ticagrelor, because the drugs are quick-onset and do not require long-term catheterization. Due to the notion that both ticlopidine and clopidogrel are slow-onsets, a sustained neuraxial catheter might be kept for 24–48 h if an initial higher dose of antiplatelet medication is not given at the beginning of treatment. The re-administration of thienopyridine derivatives and ticagrelor following needle positioning or catheter removal is limited to the lack of an initial higher dose of thienopyridine derivatives and ticagrelor given at the beginning of a course of treatment. If not, it is recommended that the catheter be removed 6 h after re-administration of thienopyridine derivatives and ticagrelor. It is recommended that neuraxial blockade be performed 5–7 days after interruption of ticagrelor therapy.
The neuraxial blockade in patients receiving platelet GPIIb/IIIa inhibitors should be limited to a time in which the drug effects have been eliminated and platelet aggregation has recovered. Time duration for abciximab is 1–2 days, and for eptifibatide and tirofiban 4–8 h. Although the administration of GPIIb/IIIa antagonists is contraindicated until 4 weeks after surgery, it is recommended that neurological functionality be monitored, and the GP IIb/IIIa antagonist be administrated simultaneously during the postoperative phase.
Cilostazol is a platelet aggregation inhibitor with vasodilating properties that selectively inhibits the phosphodiesterase type 3. Due to the lack of a known risk of bleeding related to neuraxial blockade in a patient receiving cilostazol, it is recommended to apply a 2-day interval between the neuraxial blockade and cilostazol interruption. Re-administration of the first postoperative cilostazol dose should be done 6 h after catheter removal.
Dipyridamole and cangrelor are platelet aggregation inhibitors that inhibit the P2Y12 receptor. The risk of bleeding related to neuraxial blockade in patients receiving cangrelor is unclear. Concomitant administration of dipyridamole with aspirin might increase the risk of hemorrhage. According to the drug’s half-life, it is recommended to wait 24 and 3 h between neuraxial blockade and interruption of the dipyridamole and cangrelor, respectively. Re-administration of the first postoperative dipyridamole and cangrelor dose should be done 6 and 8 h after catheter removal, respectively (Horlocker et al. 2019).
Anesthetic Considerations for Patients on Herbal Medications
Plants with medicinal properties such as garlic—reduction of blood pressure and inhibition of platelet aggregation; ginkgo—inhibition of platelet-activating factor; and ginseng—prolongation of thrombin time and partial thromboplastin time in animals (Horlocker et al. 2003) seem not to be associated with additional risk of spinal hematoma in patients undergoing epidural or spinal anesthesia. Nor will they interfere with neuraxial blockade (Horlocker et al. 2019).
Anesthetic Considerations for Patients with Underlying Bleeding Disorders
Patients with congenital bleeding disorders require special precautions to reduce the risk of perioperative bleeding (Tabibian et al. 2016; Naderi et al. 2018). VWD is the most common congenital bleeding disorder with estimated incidence of 1% in the general population. The disorder is more common in some populations such as the Swedes. The disorder can be considered a potential risk factor for anesthesia. Most patients with VWD are affected by VWD type 1, diagnosis of which is challenging. Although molecular analysis of the VWF gene could be used for precise diagnosis, it is time-consuming and laborious. Most anesthetic recommendations are for patients with hemophilia, not for other rare or common congenital bleeding disorders (Dorgalaleh et al. 2018a, b). In fact, perioperative bleeding is a significant risk factor in all patients with congenital bleeding disorders, even in patients with mild disorders such as FXI deficiency and combined FV and FVIII deficiency (Dorgalaleh et al. 2016, 2017; Naderi et al. 2016; Tabibian et al. 2016; Bamedi et al. 2017) (Tables 11.5 and 11.6).
For anesthetic management of patients with bleeding disorders, pre-operative administration of deficient coagulation factor should be considered. For instance, one anesthetic consideration for patients with hemophilia A is preoperative administration of hemoderivative or recombinant FVIII to increase the plasma FVIII level. The recommendations are against the administration of NSAIDs in hemophilia patients due to the increased risk of hemorrhage. Hemophilia patients who are supposed to undergo a lumbar puncture should first be managed with a hemoderivative or recombinant coagulation factor to restore coagulation factor activity. Hemophilia patients with active pharyngeal bleeding are exposed to risk of airway obstruction resulting from unsafe airway protection, even with administration of FVIII concentrate. Hence, elective mechanical ventilation provided by insertion of an endotracheal tube into the airway is helpful to avoid airway obstruction. Recommendations for post-operative pain management of patients with hemophilia are against the intramuscular injection of analgesia. Pre-operative evaluation of hemophilia patients must involve inhibitor screening and assay, if resumed plasma factor activity level is less than anticipated. About the timing of surgery it is better to be scheduled at the start of the week, and first of the day, for the best laboratory and blood bank support, if needed. The dosing regimen and duration of coagulation factor concentrate coverage, depending on the type of surgery, should be enough to maintain levels until the postoperative phase (Membership of the Working Party et al. 2013). In patients with hemophilia who generate antibody against FVIII in response to given hemoderivatives, administration of recombinant products is indicated (Membership of the Working Party et al. 2013). Generally, it is suggested that if a patient with an investigated coagulation malfunction is supposed to be anesthetized via a regional technique, an anesthesiologist expert in this field should conduct the process. This is probably because an expert regional anesthesiologist will require fewer tries to reach the successful blockade (Membership of the Working Party et al. 2013).
A meta-analysis of randomized control trials revealed that neuraxial blockade reduces postoperative bleeding requiring transfusion and preoperative blood transfusion > 2 blood units.
FXII deficiency, or Hageman deficiency, is a relatively common autosomal recessive coagulopathy. The estimated incidence of the disorder is 1.5–3% in the general population. Patients with FXII deficiency do not have a bleeding tendency, while they do have a high rate (8–10%) of thromboembolic events. Patients with the disorder are in a hypercoagulable state due to impaired fibrinolysis. Perioperative thromboembolic risk is relatively high in patients with FXII deficiency.
References
Abramovitz SE, Beilin Y. Anesthetic management of the parturient with protein S deficiency and ischemic heart disease. Anesth Analg. 1999;89:709.
Adjepong KO, Otegbeye F, Adjepong YA. Perioperative management of sickle cell disease. Mediterr J Hematol Infect Dis. 2018;10:e2018032.
Ahmed A, Monem A. Perioperative anaesthetic management of a patient with relapsed aplastic Anaemia. J Pak Med Assoc. 2005;55:257–9.
Akhunzada NZ, Tariq MB, Khan SA, Sattar S, Tariq W, Shamim MS, Dogar SA. Value of routine preoperative tests for coagulation before elective cranial surgery. Results of an Institutional Audit and a Nationwide Survey of Neurosurgical Centers in Pakistan. World Neurosurg. 2018;116:e252–7.
Alizadeh S, Bavarsad MS, Dorgalaleh A, Khatib ZK, Dargahi H, Nassiri N, Hamid F, Rahim F, Jaseb K, Saki N. Frequency of beta-thalassemia or beta-hemoglobinopathy carriers simultaneously affected with alpha-thalassemia in Iran. Clin Lab. 2014;60:941–9.
Aljabry MS. Primary familial and congenital polycythemia; The forgotten entity. J Appl Hematol. 2018;9:39.
Alzahrani A, Othman N, Bin-Ali T, Elfaraidi H, Al Mussaed E, Alabbas F, Sedick Q, Albatniji F, Alshahrani Z, Asiri M. Routine preoperative coagulation tests in children undergoing elective surgery or invasive procedures: are they still necessary? Clin Med Insights Blood Disord. 2019;12:1179545X18821158.
Andersson T, Berner N, Larsen M, Odegaard O, Abildgaard U. Plasma heparin cofactor II, protein C and antithrombin in elective surgery. Acta Chir Scand. 1987;153:291–6.
Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:160S–98S.
Avery EG IV, Klick JC. The patient with anemia and coagulation disorders. In: Longnecker DE, editor. Anesthesiology. 3rd edn. New York: McGraw Hill; 2018.
Badalian-Very G. Personalized medicine in hematology—A landmark from bench to bed. Comput Struct Biotechnol J. 2014;10:70–7.
Badner NH, Freeman D, Spence JD. Preoperative oral B vitamins prevent nitrous oxide-induced postoperative plasma homocysteine increases. Anesth Analg. 2001;93:1507–10.
Bakri MH, Ismail EA, Ghanem G, Shokry M. Spinal versus general anesthesia for Cesarean section in patients with sickle cell anemia. Korean J Anesthesiol. 2015;68:469.
Bamedi T, Dadashizadeh G, Sarabandi A, Tabibian S, Shams M, Dorgalaleh A. Genetic risk factors for inhibitor development in patients with hemophilia and rare bleeding disorders. J Cell Mol Anesth. 2017;2:19–23.
Batool T, Babur B, Tasneem T. Anesthetic management of the parturient with combined protein C and S deficiency. Anaesth Pain Intensive Care. 2011;15:54–6.
Behrooz A. Pharmacogenetics and anaesthetic drugs: implications for perioperative practice. Ann Med Surg. 2015;4:470–4.
Bendixen H, Laver M. Hypoxia in anesthesia: a review. Clin Pharmacol Therap. 1965;6:510–39.
Bilehjani E, Fakhari S, Farzin H, Yaghoubi A, Mirinazhad M, Shadvar K, Dehghani A, Aboalaiy P. The correlation between preoperative erythrocyte sedimentation rate and postoperative outcome in adult cardiac surgery. Int J Gen Med. 2017;10:15.
Bonanni P, Grazzini M, Niccolai G, Paolini D, Varone O, Bartoloni A, Bartalesi F, Santini MG, Baretti S, Bonito C. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum Vaccin Immunother. 2017;13:359–68.
Borg T, Modig J. Potential anti-thrombotic effects of local anaesthetics due to their inhibition of platelet aggregation. Acta Anaesthesiol Scand. 1985;29:739–42.
Borghi B, Casati A, Iuorio S, Celleno D, Michael M, Serafini PL, Alleva R. Effect of different anesthesia techniques on red blood cell endogenous recovery in hip arthroplasty. J Clin Anesth. 2005;17:96–101.
Bredbacka S, Blombäck M, Hägnevik K, Irestedt L, Raabe N. Per-and postoperative changes in coagulation and fibrinolytic variables during abdominal hysterectomy under epidural or general anaesthesia. Acta Anaesthesiol Scand. 1986;30:204–10.
Breslow MJ, Parker SD, Frank SM, Norris EJ, Yates H, Raff H, Rock P, Christopherson R, Rosenfeld BA, Beattie C. Determinants of catecholamine and cortisol responses to lower extremity revascularization. The PIRAT Study Group. Anesthesiology. 1993;79:1202–9.
Brière JB. Essential thrombocythemia. Orphanet J Rare Dis. 2007;2:3.
Brueckner S, Reinke U, Roth-Isigkeit A, Eleftheriadis S, Schmucker P, Siemens H-JG. Comparison of general and spinal anesthesia and their influence on hemostatic markers in patients undergoing total hip arthroplasty. J Clin Anesth. 2003;15:433–40.
Burton BN, Alison M, Brovman EY, Scott MJ, Urman RD, Gabriel RA. Optimizing preoperative anemia to improve patient outcomes. Anesthesiol Clin. 2018;36:701–13.
Caglayan O, Buyukkocak U, Kara FK, Sert O. The decrease in erythrocyte sedimentation rate related to general anesthesia. Clin Hemorheol Microcirc. 2006;35:459–62.
Chi SI. Complications caused by nitrous oxide in dental sedation. J Dent Anesth Pain Med. 2018;18:71–8.
Christopherson R, Beattie C, Frank SM, Norris EJ, Meinert CL, Gottlieb SO, Yates H, Rock P, Parker SD, Perler BA. Perioperative morbidity in patients randomized to epidural or general anesthesia for lower extremity vascular surgery. Anesthesiology. 1993;79:422–34.
Clark P, Aghemo A, Degasperi E, Galmozzi E, Urban T, Vock D, Patel K, Thompson A, Rumi M, D’Ambrosio R. Inosine triphosphatase deficiency helps predict anaemia, anaemia management and response in chronic hepatitis C therapy. J Viral Hepat. 2013;20:858–66.
Collins GJ Jr, Barber JA, Zajtchuk R, Vahek D, Malogne LA. The effects of operative stress on the coagulation profile. Am J Surg. 1977;133:612–6.
Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–5.
Cooley BC, Chen C-Y, Schmeling G. Increased venous versus arterial thrombosis in the factor V Leiden mouse. Thromb Res. 2007;119:747–51.
Corral J, de la Morena-Barrio ME, Vicente V. The genetics of antithrombin. Thromb Res. 2018;169:23–9.
Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gin mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost. 1995;73:139–48.
Donadoni R, Baele G, Devulder J, Rolly G. Coagulation and fibrinolytic parameters in patients undergoing total hip replacement: influence of the anaesthesia technique. Acta Anaesthesiol Scand. 1989;33:588–92.
Donahue BS. Factor V Leiden and perioperative risk. Anesth Analg. 2004;98:1623–34.
Dong M, Li X, Liu J, Song Z, Zhao H, Wei S, Chen G, Chen J. Successful surgical management of invasive pulmonary fungal infection in patients with leukemia. Infect Drug Resist. 2019;12:1675.
Dorgalaleh A, Alizadeh S, Tabibian S, Taregh B, Karimi M. The PAI-I Gene 4G/5G polymorphism and central nervous system bleeding in factor XIII deficiency. Blood. 2013a;122(21):4782.
Dorgalaleh A, Shahzad MS, Younesi MR, Moghaddam ES, Mahmoodi M, Varmaghani B, Khatib ZK, Alizadeh S. Evaluation of liver and kidney function in favism patients. Med J Islam Repub Iran. 2013b;27:17.
Dorgalaleh A, Dadashizadeh G, Bamedi T. Hemophilia in Iran. Hematology. 2016;21:300–10.
Dorgalaleh A, Motlagh H, Tabibian S. Burden of congenital factor XIII deficiency in Iran. J Cell Mol Anesth. 2017;2:142–5.
Dorgalaleh A, Tabibian S, Shams M, Ala F, Bahoush G, Jazebi M, Manafi R, Tavasoli B, Baghaipour MR. Von Willebrand disease in Iran: diagnosis and management. Ann Blood. 2018a;3.
Dorgalaleh A, Tabibian S, Shiravand Y, Favaloro EJ. von Willebrand disease. In: Congenital bleeding disorders. Springer; 2018b. p. 57–102.
Duma A, Cartmill C, Blood J, Sharma A, Kharasch E, Nagele P. The hematological effects of nitrous oxide anesthesia in pediatric patients. Anesth Analg. 2015;120:1325.
Elyassi CAR, Rowshan MHH. Perioperative management of the glucose-6-phosphate dehydrogenase deficient patient: a review of literature. Anesth Prog. 2009;56:86–91.
Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54:9–18.
Feinstein M, Fiekers J, Fraser C. An analysis of the mechanism of local anesthetic inhibition of platelet aggregation and secretion. J Pharmacol Exp Therap. 1976;197:215–28.
Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, Little LD, Qiu P, Bertelsen AH, Watson M. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405.
Finotti A, Breda L, Lederer CW, Bianchi N, Zuccato C, Kleanthous M, Rivella S, Gambari R. Recent trends in the gene therapy of β-thalassemia. J Blood Med. 2015;6:69.
Friso S, Lotto V, Choi S-W, Girelli D, Pinotti M, Guarini P, Udali S, Pattini P, Pizzolo F, Martinelli N. Promoter methylation in coagulation F7 gene influences plasma FVII concentrations and relates to coronary artery disease. J Med Genet. 2012;49:192–9.
Gabriel RA, Ehrenfeld JM, Urman RD. Preoperative genetic testing and personalized medicine: changing the care paradigm. J Med Syst. 2017;41:185.
Gales A, Masingue M, Millecamps S, Giraudier S, Grosliere L, Adam C, Salim C, Navarro V, Nadjar Y. Adolescence/adult onset MTHFR deficiency may manifest as isolated and treatable distinct neuro-psychiatric syndromes. Orphanet J Rare Dis. 2018;13:29.
Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6.
García-Ferreira J, Hernández-Palazón J, García-Candel A, Verdú-Martínez T. Subarachnoid block in a patient with essential thrombocytemia. Anesth Analg. 2005;101:300.
Gelman S. General anesthesia and hepatic circulation. Can J Physiol Pharmacol. 1987;65:1762–79.
George-Gay B, Parker K. Understanding the complete blood count with differential. J Perianesth Nurs. 2003;18:96–117.
Gerds AT, Dao K-H. Polycythemia vera management and challenges in the community health setting. Oncology. 2017;92:179–89.
Ghaffari S, Dehghanpisheh L, Tavakkoli F, Mahmoudi H. The effect of spinal versus general anesthesia on quality of life in women undergoing cesarean delivery on maternal request. Cureus. 2018;10(12):e3715.
Giammarco S, Chiusolo P, Piccirillo N, Di Giovanni A, Metafuni E, Laurenti L, Sica S, Pagano L. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol. 2017;10:147–54.
Gil J, Laczmanska I, Pesz KA, Sasiadek MM. Personalized medicine in oncology. New perspectives in management of gliomas. Contemp Oncol. 2018;22:1.
Goh S. Pharmacogenomics and drug therapy. Austr Prescriber. 2018;41:6.
Gonzalez-Fiol A, Eisenberger A. Anesthesia implications of coagulation and anticoagulation during pregnancy. In: Seminars in perinatology. Elsevier; 2014. p. 370–7.
Govil N, Adabala V, Challa RB, Lohani S, Ahmed I. Anesthetic management with epidural analgesia in a case of thalassemia intermedia. Arch Anesthesiol Crit Care. 2019;5:69–70.
Groeben H, Heyll A, Peters J. Pathophysiologic and anesthesiologic characteristics of patients with leukemia. Anaesthesist. 1992;41:438–47.
Harnett MJ, Walsh ME, McElrath TF, Tsen LC. The use of central neuraxial techniques in parturients with Factor V Leiden mutation. Anesth Analg. 2005;101:1821–3.
Hazirolan T, Perler BA, Bluemke DA. Floating thoracic aortic thrombus in “protein S” deficient patient. J Vasc Surg. 2004;40:381.
Hendolin H, Mattila M, Poikolainen E. The effect of lumbar epidural analgesia on the development of deep vein thrombosis of the legs after open prostatectomy. Acta Chir Scand. 1981;147:425–9.
Henny C, Odoom J, CATE HT, CATE JT, Oosterhoff R, Dabhoiwala N, Sih I. Effects of extradural bupivacaine on the haemostatic system. BJA Br J Anaesth. 1986;58:301–5.
Hirst C, Williamson L. Preoperative blood transfusions for sickle cell disease. Cochrane Database Syst Rev. 2001.
Hohenberger P, Buchheidt D. Surgical interventions in patients with hematologic malignancies. Crit Rev Oncol Hematol. 2005;55:83–91.
Hollmann MW, Wieczorek KS, Smart M, Durieux ME. Epidural anesthesia prevents hypercoagulation in patients undergoing major orthopedic surgery. Reg Anesth Pain Med. 2001;26:215–22.
Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking KF, Heit JA, Mulroy MF, Rosenquist RW, Rowlingson J, Tryba M. Regional anesthesia in the anticoagulated patient: defining the risks. In: The second ASRA consensus conference on neuraxial anesthesia and anticoagulation; 2003.
Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines. Obstet Anesth Digest. 2019;39:28–9.
Hosseini S, Kalantar E, Hosseini MS, Tabibian S, Shamsizadeh M, Dorgalaleh A. Genetic risk factors in patients with deep venous thrombosis, a retrospective case control study on Iranian population. Thromb J. 2015;13:35.
Huang J, McKenna N, Babins N. Utility of thromboelastography during neuraxial blockade in the parturient with thrombocytopenia. AANA J. 2014;82:127–30.
Hwang S, Mruk K, Rahighi S, Raub AG, Chen C-H, Dorn LE, Horikoshi N, Wakatsuki S, Chen JK, Mochly-Rosen D. Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat Commun. 2018;9:4045.
Igarashi K, Sakurai K, Takahata O, Saito T, Sakamaki Y, Sengoku K, Iwasaki H. Anesthetic management of a patient with Evans syndrome—benefit of perioperative hemodilutional autologous blood transfusion. Masui Jpn J Anesthesiol. 2002;51:1260–2.
Iolascon A, Andolfo I, Russo R. Red cells in post-genomic era: impact of personalized medicine in the treatment of anemias. Haematologica. 2015;100:3.
Jahantigh M, Naderi M, Dorgalaleh A, Tabibian S. Prevalence of diabetes and impaired glucose tolerance test in patients with thalassemia major. Zahedan J Res Med Sci. 2014;16:86–8.
Jalbani IK, Nazim SM, Abbas F. Pheochromocytoma associated with von Hippel-lindau disease in a Pakistani family. Urol Ann. 2015;7:120.
Jooste EH, Scholl R, Wu Y-H, Jaquiss RD, Lodge AJ, Ames WA, Homi HM, Machovec KA, Greene NH, Donahue BS. Double-blind, randomized, placebo-controlled trial comparing the effects of antithrombin versus placebo on the coagulation system in infants with low antithrombin undergoing congenital cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33:396–402.
Jørgensen L, Rasmussen L, Nielsen P, Leffers A, Albrecht-Beste E. Antithrombotic efficacy of continuous extradural analgesia after knee replacement. Br J Anaesth. 1991;66:8–12.
Jyothi B, Sushma K, Syeda S, Raza SO. Anaesthetic management of beta thalassemia major with hypersplenism for splenectomy in pediatric age group: report of four cases. Anesth Essays Res. 2015;9:266.
Kalantari E, Dorgalaleh A, Rozei A, Jafari M, Naderi M, Tabibian S, Bamedi T. Molecular analysis of patients with deep venous thrombosis. In: American Society of Hematology; 2013.
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA, Weatherall DJ, Costa FF. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010.
Kaur M, Gupta B, Sharma A, Sharma S. Child with aplastic anemia: anesthetic management. Saudi J Anaesth. 2012a;6:298.
Kaur M, Gupta B, Sharma A, Sharma S. Child with aplastic anemia: anesthetic management. Saudi J Anaesth. 2012b;6:298–300.
Khatavkar SS, Thatte WS, Kazi SM, Paul A. Anesthetic management of a case with hereditary spherocytosis for splenectomy and open cholecystectomy. Med J Dr DY Patil Univ. 2016;9:267.
Khurmi N, Gorlin A, Misra L. Perioperative considerations for patients with sickle cell disease: a narrative review. Can J Anesth. 2017;64:860–9.
Kluft C, Verheijen J, Jie A, Rijken D, Preston F, Sue-Ling H, Jespersen J, Aasen A. The postoperative fibrinolytic shutdown: a rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest. 1985;45:605–10.
Kolliopoulou A, Stratopoulos A, Siamoglou S, Sgourou A, Ali BR, Papachatzopoulou A, Katsila T, Patrinos GP. Key pharmacogenomic considerations for sickle cell disease patients. Omics J Integr Biol. 2017;21:314–22.
Konkle BA, Bauer KA, Weinstein R, Greist A, Holmes HE, Bonfiglio J. Use of recombinant human antithrombin in patients with congenital antithrombin deficiency undergoing surgical procedures. Transfusion. 2003;43:390–4.
Kumagai K, Nishiwaki K, Sato K, Kitamura H, Yano K, Komatsu T, Shimada Y. Perioperative management of a patient with purpura fulminans syndrome due to protein C deficiency. Can J Anesth. 2001;48:1070–4.
Kumar A, Srivastava U. Role of routine laboratory investigations in preoperative evaluation. J Anaesthesiol Clin Pharmacol. 2011;27:174.
Lad D, Jain A, Varma S. Complications and management of coagulation disorders in leukemia patients. Blood Lymphat Cancer. 2017;7:61.
Li Z, Yu F, Zhou X, Zeng S, Zhan Q, Yuan M, Yang Q, Liu Y, Xia J. Promoter hypomethylation of microRNA223 gene is associated with atherosclerotic cerebral infarction. Atherosclerosis. 2017;263:237–43.
Luzzatto L, Seneca E. G6 PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol. 2014;164:469–80.
Mangla A, Ehsan M, Maruvada S. Sickle cell anemia. In: StatPearls. Treasure Island, FL: StatPearls; 2019.
Manjurano A, Sepulveda N, Nadjm B, Mtove G, Wangai H, Maxwell C, Olomi R, Reyburn H, Riley EM, Drakeley CJ. African glucose-6-phosphate dehydrogenase alleles associated with protection from severe malaria in heterozygous females in Tanzania. PLoS Genet. 2015;11:e1004960.
Mannino RG, Myers DR, Tyburski EA, Caruso C, Boudreaux J, Leong T, Clifford G, Lam WA. Smartphone app for non-invasive detection of anemia using only patient-sourced photos. Nat Commun. 2018:9.
Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70.
Martinelli I, De Stefano V, Mannucci PM. Inherited risk factors for venous thromboembolism. Nat Rev Cardiol. 2014;11:140.
Matisse Investigators. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695–702.
Mayer EL, Jacobsen DW, Robinson K. Homocysteine and coronary atherosclerosis. J Am Coll Cardiol. 1996;27:517–27.
McKenzie P, Wishart H, Gray I, Smith G. Effects of anaesthetic technique on deep vein thrombosis: a comparison of subarachnoid and general anaesthesia. BJA Br J Anaesth. 1985;57:853–7.
McMullin M. The classification and diagnosis of erythrocytosis. Int J Lab Hematol. 2008;30:447–59.
Membership of the Working Party, Harrop-Griffiths W, Cook T, Gill H, Hill D, Ingram M, Makris M, Malhotra S, Nicholls B, Popat M. Regional anaesthesia and patients with abnormalities of coagulation: the association of anaesthetists of Great Britain & Ireland the obstetric anaesthetists’ association regional anaesthesia UK. Anaesthesia. 2013;68:966–72.
Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, Zakerska-Banaszak O, Szalata M, Slomski R. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58:9–14.
Mikstacki A, Zakerska-Banaszak O, Skrzypczak-Zielinska M, Tamowicz B, Prendecki M, Dorszewska J, Molinska-Glura M, Waszak M, Slomski R. The effect of UGT1A9, CYP2B6 and CYP2C9 genes polymorphism on individual differences in propofol pharmacokinetics among Polish patients undergoing general anaesthesia. J Appl Genet. 2017;58:213–20.
Mirzayan MJ, Gharabaghi A, Samii M, Frömke C, Tatagiba M, Krauss JK, Rosahl SK. The diagnostic value of erythrocyte sedimentation rate in management of brain tumors. Neurol Res. 2009;31:514–7.
Modig J, Malmberg P, Karlström G. Effect of epidural versus general anaesthesia on calf blood flow. Acta Anaesthesiol Scand. 1980;24:305–9.
Modig J, Borg T, Bagge L, Saldeen T. Role of extradural and of general anaesthesia in fibrinolysis and coagulation after total hip replacement. Br J Anaesth. 1983;55:625–9.
Morozowich ST, Donahue BS, Welsby IJ. Genetics of coagulation: considerations for cardiac surgery. In: Seminars in cardiothoracic and vascular anesthesia. Thousand Oaks, CA: Sage; 2006. p 297–313.
Murthy T, Pratyush G, Prabhakar T, Singh P, Mohan C. Anaesthetic implication of von Hippel Lindau disease. Med J Armed Forces India. 2006;62:181.
Naderi M, Sadeghi-Bojd S, Valeshabad AK, Jahantigh A, Alizadeh S, Dorgalaleh A, Tabibian S, Bamedi T. A prospective study of tubular dysfunction in pediatric patients with Beta thalassemia major receiving deferasirox. Pediatr Hematol Oncol. 2013;30:748–54.
Naderi M, Miri-Moghaddam E, Alizadeh S, Dorgalaleh A, Tabibian S. Acute lymphoblastic leukemia in two patients with β-thalassemia major. Zahedan J Res Med Sci. 2014;16:50–1.
Naderi M, Tabibian S, Shamsizadeh M, Dorgalaleh A. Miscarriage and recurrent miscarriage in patients with congenital factor V deficiency: a report of six cases in Iran. Int J Hematol. 2016;103:673–5.
Naderi M, Rahamani P, Alizadeh S, Razavi H, Dorgalaleh A. Bleeding episodes among patients with congenital fibrinogen disorders, a study on 12 new Iranian patients. J Cell Mol Anesth. 2018;3:14–7.
Nagano K, Yamanaka I, Tajiri O, Sakamoto M, Okada Y, Endo M, Saito S. Anesthetic management for a patient with autoimmune hemolytic anemia. Masui Jpn J Anesthesiol. 2000;49:417–9.
Narouze S, Benzon HT, Provenzano DA, Buvanendran A, De Andres J, Deer TR, Rauck R, Huntoon MA. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of pain Medicine, the international Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2015;40:182–212.
Needs T, Lynch DT. Beta thalassemia. In: StatPearls [Internet]. StatPearls; 2018.
O’Brien J, Tulevski V, Etherington M, Madgwick T, Alkjaersig N, Fletchee A. Platelet function studies before and after operation and the effect of postoperative thrombosis. J Lab Clin Med. 1974;83:342–54.
Oduro-Dominah L, Brennan LJ. Anaesthetic management of the child with haematological malignancy. BJA Educ. 2013;13:158–64.
Ogasawara N, Kijima Y, Ike S, Nakagawa Y, Takagi T, Hata T, Suehisa E, Kawasaki T, Miyata T. Hereditary protein s deficiency with a history of recurrent myocardial infarction. Circ J. 2003;67:166–8.
Okamoto T, Minami K. Anesthesia for a child with a congenital antithrombin deficiency. Can J Anesth. 2003;50:311.
Ornstein DL, Cushman M. Factor V Leiden. Circulation. 2003;107:e94–7.
Owen RP, Gong L, Sagreiya H, Klein TE, Altman RB. VKORC1 pharmacogenomics summary. Pharmacogenet Genomics. 2010;20:642.
Owsiak J, Bullough A. Chronic myeloid leukemia in pregnancy: an absolute contraindication to neuraxial anesthesia? Int J Obstet Anesth. 2016;25:85–8.
Pal S, Bansil P, Bancone G, Hrutkay S, Kahn M, Gornsawun G, Penpitchaporn P, Chu CS, Nosten F, Domingo GJ. Evaluation of a novel quantitative test for glucose-6-phosphate dehydrogenase deficiency: bringing quantitative testing for glucose-6-phosphate dehydrogenase deficiency closer to the patient. Am J Trop Med Hyg. 2019;100:213–21.
Pamnani A, Rosenstein M, Darwich A, Wolfson A. Neuraxial anesthesia for labor and cesarean delivery in a parturient with hereditary antithrombin deficiency on recombinant human antithrombin infusion therapy. J Clin Anesth. 2010;22:450–3.
Pastore Y, Jedlickova K, Guan Y, Liu E, Fahner J, Hasle H, Prchal JF, Prchal JT. Mutations of von Hippel-Lindau tumor-suppressor gene and congenital polycythemia. Am J Hum Genet. 2003;73:412–9.
Pati HP, Sharma P. Pharmacogenetics and personalized medicine in hematology. Indian J Hematol Blood Transfus. 2017;33:301–2.
Perrotta S, Nobili B, Ferraro M, Migliaccio C, Borriello A, Cucciolla V, Martinelli V, Rossi F, Punzo F, Cirillo P. Von Hippel-Lindau-dependent polycythemia is endemic on the island of Ischia: identification of a novel cluster. Blood. 2006;107:514–9.
Pillai AA, Babiker HM. Polycythemia. In: StatPearls [Internet]. StatPearls; 2018.
Piper B, Farrell P. Hereditary antithrombin III deficiency and neuraxial anaesthesia. Anaesth Intensive Care. 2015;43:782–5.
Rampersaud E, Palmer LE, Hankins JS, Sheehan VA, Bi W, Mulder H, Kang G, Estepp JH, Wang S, Thrasher A. Precision medicine for sickle cell disease through whole genome sequencing. Blood. 2018;132(Suppl_1):3641.
Rangrez AY, Kumari M, Frey N. An emerging role of microRNA miR-223 in cardiovascular pathophysiology. microRNAs in. Cardiovasc Res. 2013;1:23–33.
Rebahi H, Ait Sliman M, El Adib A-R. Chronic myeloid leukemia and cesarean section: the anesthesiologist’s point of view. Case Rep Obstet Gynecol. 2018.
Richard S, Croisille L, Yvart J, Casadeval N, Eschwège P, Aghakhani N, David P, Gaudric A, Scigalla P, Hermine O. Paradoxical secondary polycythemia in von Hippel-Lindau patients treated with anti-vascular endothelial growth factor receptor therapy. Blood. 2002;99:3851–3.
Richardson SR, O’Malley GF. Glucose 6 phosphate dehydrogenase (G6PD) deficiency. In: StatPearls [Internet]. StatPearls; 2018.
Rickles FR, Falanga A, Montesinos P, Sanz MA, Brenner B, Barbui T. Bleeding and thrombosis in acute leukemia: what does the future of therapy look like? Thromb Res. 2007;120:S99–S106.
Rigby PG, Leavell BS. Polycythemia vera: a review of fifty cases with emphasis on the risk of surgery. Arch Intern Med. 1960;106:622–7.
Riveros-Perez E, Hermesch AC, Barbour LA, Hawkins JL. Aplastic anemia during pregnancy: a review of obstetric and anesthetic considerations. Int J Womens Health. 2018;10:117.
Rosenfeld BA, Beattie C, Christopherson R, Norris EJ, Frank SM, Breslow MJ, Rock P, Parker SD, Gottlieb SO, Perler BA. The effects of different anesthetic regimens on fibrinolysis and the development of postoperative arterial thrombosis. Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology. 1993;79:435–43.
Rosenfeld BA, Faraday N, Campbell D, Dise K, Bell W, Goldschmidt P. Hemostatic effects of stress hormone infusion. Anesthesiology. 1994;81:1116–26.
Sahni N, Masatkar S, Bora GS, Mavuduru RS. Anesthetic management of patient with Von Hippel-Lindau disease undergoing Robot-assisted nephron sparing surgery. Anaesth Pain Intensive Care. 2019; 68–70.
Saini S, Singhal S, Wason R. Polycythemia vera: perioperative anesthetic challenges and review of literature. In: The Indian Anaesthetists Forum. Medknow; 2019. p 32.
Sano Y, Ito S, Yoneda M, Nagasawa K, Matsuura N, Yamada Y, Uchinaka A, Bando YK, Murohara T, Nagata K. Effects of various types of anesthesia on hemodynamics, cardiac function, and glucose and lipid metabolism in rats. Am J Physiol Heart Circ Physiol. 2016;311:H1360–6.
Sever M, Newberry KJ, Verstovsek S. Therapeutic options for patients with polycythemia vera and essential thrombocythemia refractory/resistant to hydroxyurea. Leuk Lymphoma. 2014;55:2685–90.
Shay H, Frumento RJ, Bastien A. General anesthesia and methylenetetrahydrofolate reductase deficiency. J Anesth. 2007;21:493–6.
Simpson P, Radford SG, Forster S, Cooper GM, Hughes A. The fibrinolytic effects of anaesthesia. Anaesthesia. 1982;37:3–8.
Smith G, Goldman J. General anesthesia for surgeons. In: StatPearls [Internet]. StatPearls; 2018.
Sosis MB. Anesthesia for polycythemia vera. J Clin Anesth. 1990;2:31–4.
Soudabeh H, Ebrahim K, Nakisa H, Akbar D, Rozita HS, Bamedi T. Evaluation of complement regulatory components in patients with atypical hemolytic uremic syndrome. Cent Eur J Immunol. 2014;39:67.
Spiers A. Surgery in management of patients with leukaemia. Br Med J. 1973;3:528–32.
Stanley AC, Christian JM. Sickle cell disease and perioperative considerations: review and retrospective report. J Oral Maxillofac Surg. 2013;71:1027–33.
Sugimoto K, Kadosaki M, Egawa A, Tokitou R, Urayama M, Takeuchi M. Preventative management against thromboembolism using fresh frozen plasma in a coronary artery bypass graft patient with protein S deficiency: a case report. JA Clin Rep. 2018;4:17.
Suh W. A new era of disease modeling and drug discovery using induced pluripotent stem cells. Arch Pharm Res. 2017;40:1–12.
Tabibian S, Kazemi A, Dorgalaleh A. Laboratory diagnosis of congenital factor V deficiency, routine, specific coagulation tests with molecular methods. J Cell Mol Anesth. 2016;1:87–90.
Taher AT, Otrock ZK, Uthman I, Cappellini MD. Thalassemia and hypercoagulability. Blood Rev. 2008;22:283–92.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am J Hematol. 2019;94:133–43.
Thachil J, Falanga A, Levi M, Liebman H, Di Nisio M. Management of cancer-associated disseminated intravascular coagulation: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13:671–5.
Tiwari AK, Tomar GS, Tayal S, Chadha M, Kapoor CM. Anaesthetic significance and management of a child with neonatal purpura fulminans. Indian J Anaesth. 2012;56:283.
Tojo Y, Sekine H, Hirano I, Pan X, Souma T, Tsujita T, S-i K, Takeda N, Takeda K, Fong G-H. Hypoxia signaling cascade for erythropoietin production in hepatocytes. Mol Cell Biol. 2015;35:2658–72.
Torpy JM, Lynm C, Golub RM. Regional anesthesia. JAMA. 2011;306:781.
Tsaras G, Owusu-Ansah A, Boateng FO, Amoateng-Adjepong Y. Complications associated with sickle cell trait: a brief narrative review. Am J Med. 2009;122:507–12.
Tuman KJ, McCarthy RJ, March RJ, DeLaria GA, Patel RV, Ivankovich AD. Effects of epidural anesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesth Analg. 1991;73:696–704.
Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002;162:1833–40.
Valiaveedan S, Mahajan C, Rath GP, Bindra A, Marda MK. Anaesthetic management in patients with glucose-6-phosphate dehydrogenase deficiency undergoing neurosurgical procedures. Indian J Anaesth. 2011;55:68.
Vogenberg FR, Barash CI, Pursel M. Personalized medicine: part 1: evolution and development into theranostics. Pharm Therap. 2010;35:560.
Wasserman LR, Gilbert HS. Surgical bleeding in polycythemia vera. Ann N Y Acad Sci. 1964;115:122–38.
Wetzel RC, Marsh BR, Vaster M, Casella JF. Anesthetic implications of protein C deficiency. Anesth Analg. 1986;65:982–4.
Wong A, Koutsogiannis Z, Greene S, McIntyre S. A case of hemolysis and methemoglobinemia following amyl nitrite use in an individual with G6PD deficiency. JACME. 2013;3:23–5.
Wronska-Nofer T, Palus J, Krajewski W, Jajte J, Kucharska M, Stetkiewicz J, Wasowicz W, Rydzynski K. DNA damage induced by nitrous oxide: study in medical personnel of operating rooms. Mutat Res. 2009;666:39–43.
Xi M, Beguin S, Hemker H. The relative importance of the factors II, VII, IX and X for the prothrombinase activity in plasma of orally anticoagulated patients. Thromb Haemost. 1989;61:788–91.
Yahouédéhou SCMA, Adorno EV, da Guarda CC, Ndidi US, Carvalho SP, Santiago RP, Aleluia MM, de Oliveira RM, de Souza Gonçalves M. Hydroxyurea in the management of sickle cell disease: pharmacogenomics and enzymatic metabolism. Pharmacogenomics J. 2018;18:730–9.
Yang Z, Wang L, Zhang W, Wang X, Zhou S. Plasma homocysteine involved in methylation and expression of thrombomodulin in cerebral infarction. Biochem Biophys Res Commun. 2016;473:1218–22.
Ygge J. Changes in blood coagulation and fibrinolysis during the postoperative period. Am J Surg. 1970;119:225–32.
Yu S, Khalpey ZI, Wong RK, Huynh T, Nielsen VG. Complete antithrombin replacement for anticoagulation for cardiopulmonary bypass to repair severe infective mitral valve endocarditis. Blood Coagul Fibrinolysis. 2018;29:123–5.
Zhou X, Lu D, Chen X-h, Yang X-y, Chen X, Zhou Z-b, Ye J-H, Feng X. Sevoflurane affects oxidative stress and alters apoptosis status in children and cultured neural stem cells. Neurotox Res. 2018;33:790–800.
Acknowledgment
We highly appreciate Daisy Morant’s valuable work in improving English language of the chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dorgalaleh, A., Bahraini, M., Ahmadi, S.E. (2021). Personalized Anesthesia in Hematology. In: Dabbagh, A. (eds) Personalized Medicine in Anesthesia, Pain and Perioperative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-53525-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-53525-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53524-7
Online ISBN: 978-3-030-53525-4
eBook Packages: MedicineMedicine (R0)