Abstract
Anesthesia-induced neurotoxicity in immature animals has raised concerns about similar effects occurring in young children. Our study investigated two commonly used anesthetics—sevoflurane and propofol—for neurotoxicity in young children. Forty-seven children (aged 12–36 months) undergoing hypospadias repair surgery were randomized to receive sevoflurane (SG, n = 24) or propofol (PG, n = 23) general anesthesia. Venous blood was collected at three different times—immediately after induction, 2 h, and 3 days after surgery. The cellular portion was assessed for antioxidant defense and DNA damage, using enzyme assay kits and qRT-PCR, respectively, while serum was used to treat cultured neural stem cells (NSCs). MTT assay and TUNEL staining were performed, and the mRNA levels of antioxidant enzymes and apoptosis indicators were evaluated by qRT-PCR. Antioxidant defense and apoptosis status in the SG group were significantly higher than in the PG group at 2 h after surgery. Additionally, exposure of NSCs to postoperative serum of the SG group resulted in decreased cell density and viability, increased TUNEL-positive cells, elevated mRNA levels of antioxidant enzymes, and cleaved caspase-3 expression. Our data shows for the first time that in young children, administration of sevoflurane, but not propofol, leads to temporally increased antioxidant defense and apoptosis status as well as damage of NSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preclinical studies have demonstrated that exposure to various anesthetics during crucial brain development stages causes neurodegeneration and permanent neurocognitive defects later in life (Fredriksson et al. 2007; Sun 2010; Takaenoki et al. 2014; Xiong et al. 2014). However, epidemiological studies analyzing long-term neurodevelopmental effects of anesthesia in children have been equivocal, with some showing a likely association between anesthetic exposure and cognitive impairments (DiMaggio et al. 2011; Flick et al. 2011; Ing et al. 2012; Wilder et al. 2009), and others concluding that alternative reasons for this observation need to be ruled out (Davidson et al. 2016; Sprung et al. 2009; Sun et al. 2016).
Sevoflurane and propofol are the most common agents used in clinical anesthesia. Preclinical evidence shows that while sevoflurane has neurotoxic effects on the developing brain (Feng et al. 2012; Makaryus et al. 2015), propofol (a short-acting intravenous anesthetic agent widely used in surgery), despite reports of neurotoxicity in neonatal rats (Creeley et al. 2013), has demonstrated neuroprotective effects in various neuronal injury models (Fan et al. 2015; Xiong et al. 2014). The aim of this study was to understand the effects of sevoflurane and propofol on the developing human brain and their potential mechanisms. The immature brain is vulnerable to oxidative stress (Tataranno et al. 2015), which has been linked to cell death and neurodegenerative conditions (Radi et al. 2014). So we used antioxidant enzyme assays for glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) to evaluate for oxidative stress, and we measured caspase-3 mRNA levels to show apoptosis status. We further evaluated whether biochemical changes in the serum of children after anesthetic exposure induce neurotoxicity in primary cultured neural stem cells (NSCs).
Methods
Patient Selection
This prospective, single-center trial (NCT02711280) was conducted after approval from the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University and obtaining written informed consent from the guardians of the children enrolled. In total, 47 patients with the American Society of Anesthesiology (ASA) I-II, aged 12–36 months, undergoing selective surgery for hypospadias repair were included in this trial. Exclusion criteria included a history of developmental delay or mental retardation, a history of central nervous system diseases, a known or suspected coagulopathy, a known allergy to any of the study drugs, abnormalities of the sacrum, and any signs of infection at the site of the proposed caudal block. The subjects enrolled in this randomized, single-blind controlled clinical trial, received either sevoflurane general anesthesia (SG, n = 24 boys) or propofol general anesthesia (PG, n = 23 boys).
Anesthetic Technique
The subjects were premedicated with scopolamine (0.01 mg kg−1, maximum 0.3 mg) and lumina (2–4 mg kg−1, maximum 0.1 g) by intramuscular injection 30 min prior to surgery. On arrival to the operation room, peripheral venous access was established; standard monitors (Detex-Ohmeda, Louisville, USA) were attached, and baseline parameters were recorded. For patients in the SG group, anesthesia was induced with 8% sevoflurane with 8 L min−1 oxygen. For patients in the PG group, anesthesia was induced with propofol 4 mg kg−1. After induction, maintenance of the airway was through a laryngeal mask airway (LMA, LMAUnique, Singapore) with patients breathing spontaneously. In the SG group, anesthesia was maintained with 1–3% sevoflurane in oxygen/air mixture. In the PG group, anesthesia was maintained with propofol 7–12 mg kg−1 h−1. The concentration of anesthetics was adjusted based on intraoperative hemodynamics. After LMA insertion, caudal block was performed by a consultant anesthetist to provide intraoperative analgesia with 2 mg kg−1 of 0.2% ropicaine. At the end of the surgery, all patients received rectal paracetamol. The children were weaned from ventilatory support as soon as they were normothermic and had achieved adequate level of consciousness, after which they were sent to the postanesthesia care unit (PACU). In PACU, postoperative pain, which is an important indicator for determining the effect of caudal block, was assessed by an independent investigator using FLACC shown in Table 1. FLACC includes the facial expression, limb movement, activity, cry, and consolability. Each of the domains receives a score between 0 and 2 for a total score of between 0 and 10. When leaving the PACU, scores less than 3 were considered as successful caudal block and pain management. Noninvasive monitoring of systolic blood pressure (SBP), heart rate (HR), and breath rate (BR) were collected at the beginning of operation (t1), 30 min (t2), 60 min (t3), the end of operation (t4), and 90 min (t5).
Blood Samples
A total of 10 ml venous blood was collected from all the subjects immediately after induction (T1), at 2 h after the surgery (T2), and at 3 days (T3) after the surgery. After separation of the whole blood, erythrocytes were used for the assessment of GPx, SOD, and CAT activities, lymphocytes were used for apoptosis assay, and serum was used for cultured NSCs. All samples were coded.
Enzymatic Activities
The blood sample for analyzing SOD and CAT was drawn into vacuum tubes (Becton Dickinson) with the addition of ethylenediamine tetraacetic acid (K3-EDTA) as an anticoagulant. For analyzing GPx, vacuum tubes (Becton Dickinson) were used with the addition of lithium heparin. The enzyme activity was measured at a temperature of 37 °C within 1 h of collection, in adherence to the manufacturer’s protocol. GPx activity was determined using the Glutathione Peroxidase Assay Kit which utilizes its catalysis of the oxidation of reduced glutathione (GSH) by H2O2, then quantified using spectrophotometry, at a wavelength of 340 nm. The activity of the SOD was measured using the SOD Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and quantified by spectrophotometry at a wavelength of 550 nm (BeckMan Coulter DU730). The activity of CAT was assessed using the Catalase Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), which measures the degradation rate of H2O2 to water and molecular oxygen, and was quantified using spectrophotometry at a wavelength of 240 nm. All the results were expressed as U.g−1 hemoglobin (Hb).
Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)
Lymphocytes and cells after being treated were used for RT-PCR. RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). For cDNA synthesis, 1 μg of total RNA was reverse transcribed using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). For RT-PCR, KAPA SYBR FAST Universal 2X qPCR Master Mix (Kapa Biosystems) was added to appropriate cDNA samples and primers. Quantitative analysis of gene expression was performed for expression of ACTIN RNA (Human) or GAPDH (Mouse) in corresponding samples. The experiments for each group were conducted separately, in triplicate wells. Primers used are listed in Table 2.
Isolation and In Vitro Culture of the Primary Neural Stem Cell
All animal experiments were performed in compliance with the procedures approved by the Animal Center of Sun Yat-sen University (Guangzhou, China) and according to the guidelines of the Sun Yat-sen University Institutional Animal Care and Use Committee.
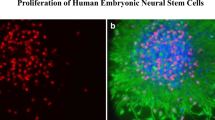
NSCs were obtained as described previously (Tropepe et al. 1999). Briefly, C57BL/6 mouse fetuses on embryonic day 14.5 were isolated, and cortical tissues were collected to obtain a single cell suspension. The dissociated cells were seeded in NSC medium that was DMEM/F12 (Hyclone) supplemented with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) (both at 20 ng ml−1, Pceprotech, Rocky Hill, USA), B27 (2%, Gibco), N2 (1%, Gibco), and 1% penicillin and streptomycin to form primary neurospheres. NSCs were grown in 5% CO2 in air, at 37 °C. To expand, the primary neurospheres were passaged every 3–4 days. NSCs at passage 3 and passage 4–6 were used in following experiments. The treated NSCs have been cultured for approximately 10–18 days since primary seeded, concerning the different passages of NSCs. The purity of NSCs was identified at passage 3 using immunofluorescence staining of NSC marker, Nestin.
Immunofluorescence Staining
For immunofluorescence, cells were fixed in 4% PFA. The cells were blocked with normal goat serum for 40 min and then incubated with primary antibodies overnight at 4 °C, followed by incubation with secondary antibodies (1:500 dilution) protected from light at room temperature for 1 h. The following primary antibodies were used: anti-Nestin (1:500, Millipore). The following secondary antibodies were used: Alexa Flour 488 donkey anti-mouse (Invitrogen). All images were obtained using a fluorescent microscope (Leica DMi8) and were analyzed using the Image J software.
Cell Treatment Protocols
Venous blood samples from the subjects for cell treatment were drawn into coagulation-promoting tubes (Becton Dickinson) and centrifuged at 3000r m−1 for 4 min to separate serum. To evaluate the effect of serum on NSCs, we had NSCs conditioned with differentiation medium (NSC medium with 2% serum, without EGF or bFGF). Cells were assigned into three groups: control (Con), cultured in conditioned medium with 2% FBS (Gibco); PG, cultured in conditioned medium with 2% serum of patients in the above discussed group PG; SG, cultured in conditioned medium with 2% serum of patients in the above discussed group SG. Cells were treated for 24 h before the following experiments.
Cell Viability Assay
NSCs were plated at a density of 1 × 104 cells/well in poly-L-Lysine-coated 96-well plates, and the cell viability was determined using the MTT assay (Sigma-Aldrich, St. Louis, MO). Briefly, at the end of the experiments, the culture medium was replaced, and the cells were treated with 5 mg ml−1 MTT and cultured for 4 h at 37 °C. The reaction product formazan was dissolved in DMSO (200 ul/well), and the absorbance at 490 nm was measured using Biohit BP800 (Multiskan MK3, Thermo Scientific) plate reader. The experiments were performed separately in triplicate wells, for each group. DMSO was only used at the end of the experiments in all groups to resolve the reaction product formazan, when the MTT reaction has been completed. The results were presented as a percentage of the control.
TUNEL Assay
TUNEL staining was performed using an In Situ Cell Death Detection kit, TMR red (Roche Diagnostics GmbH, Germany), as per the manufacturer’s instruction. Briefly, NSCs were cultured on poly-L-Lysine pre-coated glass coverslips in 24-well plates. Cells were then rinsed and fixed with 4% paraformaldehyde, and DNA fragmentation was assessed. DAPI was used to stain the nuclei, and the cells were imaged using the Zeiss LSM-780 Confocal Microscope (Carl Zeiss, Germany). Apoptosis/necrosis was quantified by the ratio of TUNEL-positive nuclei to total cell nuclei. Each experiment was performed in triplicate wells for each group.
Statistical Analysis
Values were presented as mean ± SEM. The SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Comparisons of means between two groups were performed using Student’s t test or the Wilcoxon W test. p < 0.05 was considered to be statistically significant.
Results
Sevoflurane Increases Oxidative Stress and Apoptosis Level in Postoperative Blood Samples
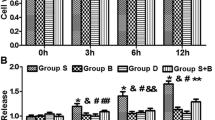
As indicated in Table 3, there was no significant difference between the sevoflurane-exposed subjects (SG) and propofol-exposed subjects (PG) with respect to age, weight, duration of surgery and anesthesia, and physical condition (ASA classification). For FLACC assessment, all the subjects were less than 3 points which indicated successful caudal block and pain management, and there was no significant difference between two groups (p > 0.05) shown in Table 3. Moreover, there was no significant difference in the vital signs between two groups (p > 0.05) shown in Table 4. Remarkably, at 2 h after surgery (T2), venous blood samples showed significant differences in antioxidant enzyme activity—particularly a higher SOD activity in the SG group compared to the PG group (p < 0.0001). Similarly, the activity of GPx from the SG group was significantly higher than that from the PG group (p = 0.0133) (Fig. 1a, b). These changes returned to normal level several days after surgery. No difference was found in CAT activity between the two groups at all time points tested (Fig. 1c).
Oxidative stress and apoptosis parameters in plasma of PG and SG group patients at different time points. Increased activity of SOD, GPx, and Caspase-3 in SG group patients postoperatively (T2) compared to PG group. No significant difference was found preoperatively (T1) or 3 days after surgery (T3). Data are presented as the mean ± SEM. ◆p < 0.05, ◆◆◆◆p < 0.0001. CAT catalase, GPx glutathione peroxidase, SOD superoxide dismutase
There was also a significant difference in apoptosis level in lymphocytes between the two groups. Specifically, caspase-3 mRNA level was significantly higher in the SG group at 2 h after surgery in the SG group than that in the PG group (p < 0.0001) (Fig. 1d).
Reduction in Cell Density and Cell Viability of NSCs in Postoperative Serum of SG Group
We next evaluated whether biochemical changes in the serum of children after sevoflurane or propofol exposure induces neurotoxicity in primary cultured NSCs. The NSCs grew as suspended stem cell colonies. Characterization of NSCs was assessed by immunofluorescence staining of NSC marker. More than 95% of the DAPI+ cells expressed the NSC marker, Nestin (Fig. 2). The in vivo evaluation showed no difference of oxidative stress and apoptosis level 3 days after surgery. Therefore, we only investigated the preoperative and postoperative serum in the following in vitro study.
Characterization of isolated murine neural stem cells (NSCs). Cells at the third passage were stained with anti-Nestin antibody. Nestin positive (green). Nuclear staining by DAPI (blue). The percentage of Nestin-positive cells over total cells in five different fields was calculated, and > 95% of cells were detected to express Nestin. Scale bar, 100 μm
Photographs of adherent NSCs after serum treatment showed a significant decrease in cell density of NSCs treated with the postoperative serum of the SG group, but not in those treated with the preoperative serum (Fig. 3a). The MTT assay showed a significant reduction in MTT uptake in NSCs treated with the postoperative serum of the SG group. This data suggests that sevoflurane exposure might have an adverse outcome to the viability of NSCs (Fig. 3b).
Neural stem cells (NSCs) exposed to postoperative serum of SG group shows a decreased cell density and cell viability compared to PG group. a Photographs of cultured NSCs after treated with serum in PG and SG group. Decreased cell density was observed in postoperative serum of SG group. Scale bar, 200 μm. b Effects of serum on cell viability of cultured NSCs determined by MTT assay. Cell viability in postoperative serum in SG group was significantly lower than that in PG group. No significant difference was found in preoperative serum. Data are presented as mean (SEM). ◆◆◆p < 0.001
Antioxidant Enzyme Levels Are Higher in NSCs Treated with Postoperative Serum of SG Group than Those of PG Group
To further investigate the effects of postoperative serum on NSCs oxidative stress status, we compared the mRNA levels of antioxidant enzymes of NSCs using qRT-PCR analysis. Consistent with the above results except the CAT activity (Fig. 1a–c), the mRNA expression levels of SOD1, GPx1, and CAT of NSCs treated with postoperative serum of the SG group were significantly higher than those treated with postoperative serum of the PG group (Fig. 4a–c).
Antioxidant enzyme (SOD1, GPx1, CAT) and caspase-3 mRNA expression level in murine NSCs exposed to serum of both groups evaluated by qRT-PCR. NSCs exposed to postoperative serum in SG group shown significantly higher mRNA expression level of the four indicators. No significant difference was found in preoperative serum. Data are presented as the mean ± SEM. ◆◆p < 0.01, ◆◆◆◆p < 0.0001
Apoptosis of NSCs Induced by Postoperative Serum of SG Group
As our results indicated a higher oxidative stress in the SG group (Fig. 4), knowing its vital role in apoptosis (Kannan and Jain 2000), we sought to determine whether the apoptosis level in the SG group would also be higher. Indeed, Caspase-3 mRNA level, measured by qRT-PCR, was higher in NSCs exposed to postoperative serum of the SG group compared to that of the PG group (Fig. 4d). Further confirming this difference, the TUNEL assay revealed a significantly higher percentage of TUNEL-positive cells with typical nuclear fragmentation and condensation in NSCs exposed to the SG group postoperative serum when compared to the PG group (Fig. 5).
Changes in apoptosis in NSCs exposed to serum determined by TUNEL assay. a Increased TUNEL-positive cell in postoperative serum from SG group compared to PG group. Detected by Confocal microscope LSM-780. TUNEL positive (red). Nuclear staining by DAPI (blue). Scale bar, 100 μm. b TUNEL-positive cell percentage of TUNEL assay. Cells were counted under a high magnification (400) for at least five fields (n = 3). TUNEL-positive cell percentage was increased in NSCs treated with postoperative serum of SG group. No significant difference was found in preoperative serum. Data was presented as the mean ± SEM. ◆◆p < 0.01
Discussion
The major findings of this study include postoperative elevated antioxidant defense and apoptosis status seen in blood samples of sevoflurane-administered children. However, these changes disappeared in 3 days’ postoperative blood. Additionally, NSCs exposed to the serum of sevoflurane-exposed subjects demonstrate decreased cell viability and increased TUNEL-positive cells, along with elevated antioxidant enzymes and caspase-3 mRNA expression levels. From this, it can be concluded that in young children, sevoflurane, but not propofol, leads to temporally increased antioxidant defense and apoptosis status and might further affect the NSCs.
A retrospective study indicated that children undergoing surgery at an age younger than 3 years had a 60% greater risk of being diagnosed with developmental and behavioral disorders later in life (DiMaggio et al. 2011). However, these can occur as a result of anesthesia, surgery, or even the illness itself. To rule out interference factors like surgery, illness, gender, or other intravenous analgesia drugs, we enrolled children with the same illness, used LMA for airway control, and caudal block to provide intraoperative analgesia during the hypospadias repair surgery. To represent the time of growth spurt in humans, which occurs from the time of birth to age 3, we included only children aged 12–36 months in the study (Huttenlocher and Dabholkar 1997). Here, we compared two commonly used anesthetics, sevoflurane and propofol. In this study, the doses of those two drugs provided to children are based on clinical practice in order to imitate the real situation. And the concentration of anesthetics was adjusted according to intraoperative hemodynamics. Bispectral index (BIS) was not used here. Because current researches have validated monitoring tools, such as BIS, for the use in older children, but does not provide strong support for use in younger children (Davidson 2006). Children enrolled in our study were aged 12–36 months including toddlers and young children. It is assumed that the monitor’s calibration for adult EEG brain activity patterns during general anesthesia cannot be reliably transferred to developing brains, potentially due to differences in brain-state dynamics under general anesthesia (Sciusco et al. 2017).
Animal studies have shown that general anesthetics used during a vulnerable period of brain development cause neurotoxicity with functional deficits (Hansen 2015). Whether sevoflurane or propofol would cause neurotoxicity in developing brains is still under debate. Some preclinical studies have demonstrated that sevoflurane has neurotoxic effects on the immature brain (Feng et al. 2012; Makaryus et al. 2015), while others indicate no deteriorative effect (Haseneder et al. 2013; Wei et al. 2005). Similarly, experimental studies demonstrating effects of propofol on the developing nervous system also yields diverse conclusions (Creeley et al. 2013; Fan et al. 2015; Xiong et al. 2014). Recently, clinical trials concerning this controversial topic showed no long-term neurotoxicity effect of single exposure to sevoflurane in young children (Davidson et al. 2016; Sun et al. 2016). However, evidences of the molecular changes during this process in human are still limited. In our study, we aimed to determine the effects of sevoflurane and propofol on antioxidant and apoptosis status in children.
We assessed the apoptosis of lymphocyte to show the genotoxic effect of anesthetics in young children. The increased apoptosis of lymphocyte indicates higher DNA damage level and clastogenic effect, which might be harmful for the developing brain. Also, elevated levels of oxidative stress aggravate cell death and facilitate the progress of neurodegenerative disease (Radi et al. 2014). Our results show a higher oxidative stress status along with an increased apoptosis index of blood cells in children under sevoflurane anesthesia postoperatively, and we assume these changes might participate in the process of anesthetics’ effects on the developing brain. In the current study, we used systemic antioxidant biomarkers (SOD, GPx, and CAT) to investigate the general oxidative stress status of children. H2O2, produced partly SOD, plays a key role in the process of oxidative stress. It can be converted into more damaging substances, which are involved in neurotoxicity in the developing brain (Lovell et al. 1995). Luckily, it can be decomposed by GPx and CAT. Our results showed that sevoflurane, not propofol, increased the SOD and GPx activity after surgery, while no significant change was found in CAT activity. We assume that SOD and GPx level were increased as a compensatory defense mechanism to deal with the excessive formation of intracellular ROS formation. The elevated GPx level in the sevoflurane group also protects the cell from oxidative damage induced by H2O2 accumulation resulting from increased SOD level (Cohen et al. 1994). However, these changes only sustained temporally, and no change was found 3 days after surgery. It is possible that single usage of sevoflurane had no long-term effect on oxidative stress or apoptosis. But it did not mean that no deteriorate effects happened during that process and have further harmful effects which are unknown and warranted further study.
Previous human and animal studies with different models draw a similar conclusion to ours that sevoflurane can induce local or systemic oxidative stress (Abou-Elenain 2010; Yue et al. 2015). A recent study revealed that sevoflurane might affect the intracerebral oxidative stress response and aggravate the Abeta-induced cognitive deficit (Abou-Elenain 2010). On the contrary, propofol has been reported to have antioxidant properties (Yue et al. 2015). Another clinical trial compared sevoflurane with propofol in oxidative stress, and found no difference between them during surgical correction of congenital heart defects in non-cyanotic children (Dumaresq et al. 2011). The difference in outcome from that of our study can be possibly attributed to the age range of children—from 1 day to 14 years—and the type of the surgery which was quite different from ours.
Neurogenesis inhibition contributes to the neurotoxicity caused by anesthetics found in animal models (Lin et al. 2014). To further investigate whether the changes in oxidative stress and apoptosis status would result in neurotoxic effects, cultured NSCs were treated with serum of the patients in both groups. Here, we chose NSCs from C57BL/6 instead of SD or Wistar rats. Because 99% of mouse genes have human counterparts, which means that the results come out from mice may be more convincible for clinical circumstances. Meanwhile, their physiology and genetics have been studied extensively, and can be compared to human easily. Furthermore, C57BL/6 are often applied to build transgenic mice models and are used as a background strain for the generation of congenics with both spontaneous and induced mutations, which might make our follow-up studies easier (Johnson 2016). Concerning our previous culturing experience and experimental demand of this study, we chose C57BL/6 for NSCs isolation to provide better reference to clinical circumstances.
NSCs are a subtype of progenitor cells in the nervous system which are generally located in the subventricular zone and hippocampus (Temple 2001). They continuously generate new-born neurons and play an important role in cognitive functions (Akers et al. 2014). Recently, strategies promoting neurogenesis—including NSCs transplantation—have been proved to be effective in neurodegenerative disease (Miltiadous et al. 2013; Moghadam et al. 2009; Zhang et al. 2014), which reveals that the state of NSCs is closely related to neurogenesis disorders. In this study, we found that NSCs treated with the postoperative serum of sevoflurane-exposed patients showed a significant decrease in cell viability as well as elevated antioxidant levels and apoptosis status, thus revealing temporally potential neurotoxicity after sevoflurane exposure. In accordance with our findings, a newly published research reported that embryonic stem cell self-renewal and neurogenesis is inhibited by sevoflurane (Yi et al. 2016). Additionally, a previous report showed that the increased oxidative stress level of NSCs was involved in tissue injury after ischemic stroke (Sakata et al. 2012).
In summary, the current study indicates that administration of sevoflurane in children, instead of propofol, leads to temporally increasing antioxidant defense and apoptosis status. Potentially cytotoxic effect shortly after single sevoflurane exposure found in our study may cause adverse outcomes. However, reverted changes 3 days after surgery indicated the safe single usage both in sevoflurane and propofol. To our knowledge, this is the first study to evaluate the effect of the serum of sevoflurane- and propofol-exposed subjects in cellular parameters using cultured NSCs. Given the implications for public health, further investigations are needed to determine whether multiple exposures would aggravate the changes shown in our study, along with the cellular and molecular mechanisms by which sevoflurane affects neurogenesis in developing brains.
References
Abou-Elenain K (2010) Study of the systemic and pulmonary oxidative stress status during exposure to propofol and sevoflurane anaesthesia during thoracic surgery. Eur J Anaesthesiol 27:566–571. https://doi.org/10.1097/EJA.0b013e3283392c1d
Akers KG et al (2014) Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344:598–602. https://doi.org/10.1126/science.1248903
Cohen SM, Olin KL, Feuer WJ, Hjelmeland L, Keen CL, Morse LS (1994) Low glutathione reductase and peroxidase activity in age-related macular degeneration. Br J Ophthalmol 78:791–794
Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A (2013) Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 110(Suppl 1):i29–i38. https://doi.org/10.1093/bja/aet173
Davidson AJ (2006) Measuring anesthesia in children using the EEG. Paediatr Anaesth 16:374–387. https://doi.org/10.1111/j.1460-9592.2006.01877.x
Davidson AJ et al (2016) Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387:239–250. https://doi.org/10.1016/S0140-6736(15)00608-X
DiMaggio C, Sun LS, Li G (2011) Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg 113:1143–1151. https://doi.org/10.1213/ANE.0b013e3182147f42
Dumaresq DM, Vasconcelos RC, Guimaraes SB, Cavalcante SL, Garcia JH, Vasconcelos AR (2011) Metabolic and oxidative effects of sevoflurane and propofol in children undergoing surgery for congenital heart disease. Acta Cir Bras / Soc Bras Desenvolvimento Pesqui Cir 26(Suppl 1):66–71
Fan W, Zhu X, Wu L, Wu Z, Li D, Huang F, He H (2015) Propofol: an anesthetic possessing neuroprotective effects. Eur Rev Med Pharmacol Sci 19:1520–1529
Feng X et al (2012) Single sevoflurane exposure decreases neuronal nitric oxide synthase levels in the hippocampus of developing rats. Br J Anaesth 109:225–233. https://doi.org/10.1093/bja/aes121
Flick RP et al (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128:e1053–e1061. https://doi.org/10.1542/peds.2011-0351
Fredriksson A, Ponten E, Gordh T, Eriksson P (2007) Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology 107:427–436. https://doi.org/10.1097/01.anes.0000278892.62305.9c
Hansen TG (2015) Anesthesia-related neurotoxicity and the developing animal brain is not a significant problem in children. Paediatr Anaesth 25:65–72. https://doi.org/10.1111/pan.12548
Haseneder R et al (2013) Sevoflurane anesthesia improves cognitive performance in mice, but does not influence in vitro long-term potentation in hippocampus CA1 stratum radiatum. PLoS One 8:e64732. https://doi.org/10.1371/journal.pone.0064732
Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387:167–178
Ing C et al (2012) Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 130:e476–e485. https://doi.org/10.1542/peds.2011-3822
Johnson M (2016) Laboratory mice and rats Mater Meth 2 https://doi.org/10.13070/mm.en.2.113
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiol: Off J Int Soc Pathophysiol / ISP 7:153–163
Lin EP, Soriano SG, Loepke AW (2014) Anesthetic neurotoxicity. Anesthesiol Clin 32:133–155. https://doi.org/10.1016/j.anclin.2013.10.003
Lovell MA, Ehmann WD, Butler SM, Markesbery WR (1995) Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 45:1594–1601
Makaryus R et al (2015) Brain maturation in neonatal rodents is impeded by sevoflurane anesthesia. Anesthesiology 123:557–568. https://doi.org/10.1097/ALN.0000000000000762
Miltiadous P, Kouroupi G, Stamatakis A, Koutsoudaki PN, Matsas R, Stylianopoulou F (2013) Subventricular zone-derived neural stem cell grafts protect against hippocampal degeneration and restore cognitive function in the mouse following intrahippocampal kainic acid administration. Stem Cells Transl Med 2:185–198. https://doi.org/10.5966/sctm.2012-0074
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H (2009) Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differ Res Biol Diversity 78:59–68. https://doi.org/10.1016/j.diff.2009.06.005
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. Journal of Alzheimer’s disease : JAD 42(Suppl 3):S125–S152. https://doi.org/10.3233/JAD-132738
Sakata H, Niizuma K, Wakai T, Narasimhan P, Maier CM, Chan PH (2012) Neural stem cells genetically modified to overexpress cu/zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke 43:2423–2429. https://doi.org/10.1161/STROKEAHA.112.656900
Sciusco A, Standing JF, Sheng Y, Raimondo P, Cinnella G, Dambrosio M (2017) Effect of age on the performance of bispectral and entropy indices during sevoflurane pediatric anesthesia: a pharmacometric study. Paediatr Anaesth 27:399–408. https://doi.org/10.1111/pan.13086
Sprung J et al (2009) Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology 111:302–310. https://doi.org/10.1097/ALN.0b013e3181adf481
Sun L (2010) Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 105(Suppl 1):i61–i68. https://doi.org/10.1093/bja/aeq302
Sun LS et al (2016) Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 315:2312–2320. https://doi.org/10.1001/jama.2016.6967
Takaenoki Y, Satoh Y, Araki Y, Kodama M, Yonamine R, Yufune S, Kazama T (2014) Neonatal exposure to sevoflurane in mice causes deficits in maternal behavior later in adulthood. Anesthesiology 120:403–415. https://doi.org/10.1097/ALN.0000435846.28299.e7
Tataranno ML, Perrone S, Longini M, Buonocore G (2015) New antioxidant drugs for neonatal brain injury. Oxidative Med Cell Longev 2015:108251. https://doi.org/10.1155/2015/108251
Temple S (2001) The development of neural stem cells. Nature 414:112–117. https://doi.org/10.1038/35102174
Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D (1999) Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 208:166–188. https://doi.org/10.1006/dbio.1998.9192
Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG (2005) Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res 1037:139–147. https://doi.org/10.1016/j.brainres.2005.01.009
Wilder RT et al (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804. https://doi.org/10.1097/01.anes.0000344728.34332.5d
Xiong M et al (2014) Propofol exposure in pregnant rats induces neurotoxicity and persistent learning deficit in the offspring. Brain Sci 4:356–375. https://doi.org/10.3390/brainsci4020356
Yi X, Cai Y, Zhang N, Wang Q, Li W (2016) Sevoflurane inhibits embryonic stem cell self-renewal and subsequent neural differentiation by modulating the let-7a-Lin28 signaling pathway. Cell Tissue Res. https://doi.org/10.1007/s00441-016-2394-x
Yue T, Shanbin G, Ling M, Yuan W, Ying X, Ping Z (2015) Sevoflurane aggregates cognitive dysfunction and hippocampal oxidative stress induced by beta-amyloid in rats. Life Sci 143:194–201. https://doi.org/10.1016/j.lfs.2015.11.002
Zhang W, Wang PJ, Sha HY, Ni J, Li MH, GJ G (2014) Neural stem cell transplants improve cognitive function without altering amyloid pathology in an APP/PS1 double transgenic model of Alzheimer’s disease. Mol Neurobiol 50:423–437. https://doi.org/10.1007/s12035-014-8640-x
Acknowledgements
The authors appreciate the editing of Dr. Rose Paulose
Funding
This work was funded by the National Natural Science Foundations of China (grant number 81571032, X.F; 81701047 X.Z) and the Guangdong Medical Scientific Technology Foundation (grant number 2016114123748455).
Author information
Authors and Affiliations
Contributions
Xue Zhou
Role: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Dihan Lu
Role: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Wen-da Li
Role: This author helped design the study, conduct the study, and analyze the data.
Xiao-hui Chen
Role: This author helped design the study and conduct the study.
Xiao-yu Yang
Role: This author helped conduct the study.
Xi Chen
Role: This author helped conduct the study.
Zhi-bin Zhou
Role: This author helped conduct the study.
Jiang-Hong Ye
Role: This author helped design the study, analyze the data, and write the manuscript.
Xia Feng
Role: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Corresponding authors
Ethics declarations
This prospective, single-center trial (NCT02711280) was conducted after approval from the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University and obtaining written informed consent from the guardians of the children enrolled. All animal experiments were performed in compliance with the procedures approved by the Animal Center of Sun Yat-sen University (Guangzhou, China) and according to the guidelines of the Sun Yat-sen University Institutional Animal Care and Use Committee.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhou, X., Lu, D., Li, Wd. et al. Sevoflurane Affects Oxidative Stress and Alters Apoptosis Status in Children and Cultured Neural Stem Cells. Neurotox Res 33, 790–800 (2018). https://doi.org/10.1007/s12640-017-9827-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9827-5