Abstract
The article focuses on the results of the surface morphology and dielectric properties research of the plasma electrolytic oxidation (PEO) coatings produced in the alkaline-silicate electrolytes in galvanostatic (GS) mode using rectified anode current. PEO coatings were formed on the samples of wrought aluminum alloy D16Tused normally for the manufacturing of the diamond grinding wheels bodies. The influence of PEO factors on the porosity (quantity, shape, size, structure and its distribution on the surface) and dielectric properties (volume resistivity, electrolytic strength) was studied. It was established that through porosity increases in the series of solutions: 12 g/L LG (“liquid glass” (LG), a technical-grade sodium silicate solution) < 1 g/L КOH + 6 g/L LG (alkaline-silicate solution) < 2 g/L КOH + 12 g/L LG. The accordingly sizes of pores are 10…15 μm, up to 1 μm and 2…10 μm. The through porosity increases in each electrolyte with increasing of anode current density from 5 to 15 A/dm2. The smallest relative increase in porosity is observed in the samples oxidated in sodium liquid glass solution 12 g/L LG. It was demonstrated that the dielectric properties research results qualitatively correlate with the micro geometric and morphology characteristics. The electrolyte composition is a major factor affecting the volume resistivity and electrolytic strength. The coatings produced in 12 g/l LG technical-grade sodium solution have the best dielectric properties, corresponding to the smallest through the porosity of these samples.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Morphology
- Through porosity
- Surface
- Galvanostatic mode
- Alkaline-silicate electrolyte
- Liquid glass
- Electricity mode
- Current density
- Volume resistivity
- Electrolytic strength
1 Introduction
Plasma electrolytic oxidation coatings (PEO coatings) on light alloys, including aluminum, have a wide range of functional properties and are used in various industries, in particular, mechanical engineering, for restoring worn surfaces of parts, providing wear resistance or resistance to aggressive environments. PEO coatings are commonly applied to friction pairs, plain bearings, gears, pistons, cylinders, face seals for internal combustion engines and machine tools.
A rather new and promising trend in this field is increasing the efficiency of using a diamond-abrasive tool in a combined diamond-spark grinding (DSG) by creating the selective dielectric protection on mounting surfaces. The most important requirements for the coatings of this application are high dielectric properties – electric strength, electrical resistance, etc.
These properties largely depend on the morphological characteristics of PEO coatings.
2 Literature Review
Morphological surface features of PEO coatings on light alloys (Al, Ti, Mg) are characterized by extremely developed relief, high roughness, and porosity indices. In general, these features can both improve and degrade the characteristics of products, depending on the exploitation conditions of their applications.
Sufficient attention is devoted to the study of surface morphology of PEO coatings on aluminum alloys, due to their unique properties, such as high specific strength, corrosion resistance, weldability, and deformability. In addition to the effect of increasing the corrosion resistance, the application of PEO coatings also increases the hardness of the surface layer, its wear resistance, and dielectric properties. It allows extending the application areas of the appropriate products significantly and makes them multifunctional [1,2,3,4,5,6].
Among the characteristics of surface morphology of PEO coatings, the most attention is devoted to the study of porosity, primarily through, caused by the nature of the oxidation process. Porosity is an integral structural component of PEO coatings and undoubtedly affects the efficiency of their use.
Most of the works deal with the effect of porosity on corrosion and wear resistance, which determines the most common application areas of coatings [1, 7,8,9,10,11]. The studies indicate a negative effect of through porosity on the corrosion properties of coatings. Since porosity formation cannot be prevented when the PEO process, it is important to search the ways of reducing the size and relative amount of pores to obtain the quality layers uniform in a structure. It is determined that nature, qualitative and quantitative indicators of through porosity are affected by many parameters – the electrolyte composition and concentration, the base material structure, as well as current density, voltage, temperature and oxidation time. The results of studies on through porosity reduction by controlling the oxidation parameters are presented in [4,5,6, 12,13,14,15].
In particular, according to the statement about the key role of the electrolyte composition in the formation of the coatings morphological features, PEO processes in electrolyte suspensions containing powders of varying dispersion degrees (from nanometers to tens of micrometers) and nature (carbides, nitrides, diamond, graphite, etc.) are quite promising [13, 14, 16,17,18,19]. The decrease in porosity is explained by the incorporation of the filler solid particles into the structure of the coatings. For example, the addition of AL2O3 sol in the electrolyte solution reduces through the porosity of the coatings almost 90 times [19].
The problem of porosity can also be solved by the use of a two-stage process, which involves the formation of a pre-porous layer and its further filling with sol-gel oxide by chemical technology [20].
The study of coatings operation in the wear mode has shown that porosity is an ambiguous factor in this case. In general, the porosity reduces the wear resistance of coatings [1, 8, 9, 21]. But in some applications, for example in friction pairs, medical products (bioactive coatings), a porous structure may be useful. In particular, during friction in the lubricating medium, the oil used enters the pores of coatings, which are a kind of reservoir for its accumulation. Further, the lubricant sequentially enters the friction zone, which significantly reduces the wear process [1, 12]. Thus, porosity plays a positive role in improving process efficiency. The paper [12] also notes that a similar pore designation can be used to incorporate the corrosion inhibitors.
The relationship between porosity as a structural component of the surface of the coating and their dielectric properties is much less. These properties are decisive for PEO coatings in diamond-abrasive tools in the DSG process [22,23,24].
When using PEO coatings for electrical insulation, it is to be expected that porosity, particularly through, can impair dielectric properties. First of all, this applies to electric strength, since it is much lower for air than for the main substance.
The purpose of this work is a qualitative assessment of through porosity effect on the dielectric characteristics of PEO coatings on D16T alloy, depending on the formation mode (the electrolyte composition, coatings thickness, current density).
3 Research Methodology
D16T wrought aluminum alloy was the experimental sample material due to its usual use for the manufacture of diamond grinding wheels in the DSG process.
To obtain insulating coatings, the samples were oxidated at a laboratory installation in the galvanostatic (GS) mode using rectified anode current. The main advantage of the GS mode is a high efficiency, i.e. high build-up rate of functional protective coatings controlled by current density.
Plasma electrolytic oxidation was carried out in different solutions of alkaline-silicate electrolytes – technical-grade sodium liquid glass (silicate) – LG solution, technical-grade sodium liquid glass and alkali solution (KOH + LG) with the corresponding concentrations: 12 g/l LG; 2 g/l KOH + 12 g/l LG; 1 g/l KOH + 6 g/l LG.
The selection of electric modes for the study was carried out based on the literature review and taking into account the results of previous studies [9, 10].
The thickness of the coatings on D16T alloy was measured by a non-destructive method using the NOVOTEST TP-1 eddy current thickness gauge. The measurement error was ±1.5 μm. The GS mode in all the tested electrolytes provided the formation of coatings with a thickness of 30…45 μm meeting the requirements for insulating properties.
To study the through porosity, after oxidation, the samples were washed with running water, degreased (τ~1 min) in soda ash solution (40 g/l) heated to 45…50 °C. After washing, the samples were immersed (τ~15 s) in the pickling solution – a mixture of nitric and hydrofluoric acids (1:1) and then in the contact copper isolation solution (τ~5 min) containing 20 g/l copper sulfate and 10 g/l sulfate and hydrochloric acids.
The washed and dried samples were examined using the USB Supereyes B008 microscope to obtain general information on the relative number and distribution of pores over the surface; magnification – 30…80. Additionally, to identify the shape, structure, and size of pores, the studies were performed using the JEOLJSM-840 electron scanning microscope at a magnification of 200…2000.
Volume resistivity and electric strength were used as indicators of the dielectric properties of PEO coatings.
Electrical resistance ρ was measured according to the standard procedure (Fig. 1). The method involves determining the volume leakage current, depending on the applied voltage. The E6-13 teraohmmeter was used for measurements, the operating voltage was 100 V.
Volume resistivity ρv, Ω·m, was calculated by the results of the electrical resistance ρ measurement:
where Rv is the total electrical resistance of the insulating coating limited by two metal electrodes, Ω; S is the cross-sectional area of the contact electrode, m2; h is the coating thickness, m.
The anode current (50 Hz) measurement of the electric strength of the coatings (breakdown voltage) was performed. The equipment included a high-voltage transformer, protective resistor of 5 kΩ and device for smooth voltage control. The measurements were carried out at the high-voltage side. The number of breakdowns was at least 5. The circuit of needle electrodes when measuring the breakdown voltage was similar to the electrical resistance circuit (Fig. 1).
The electric strength of the coatings E, V/μm, was calculated by the formula:
where n is the number of measurements; Ui is the breakdown voltage, V; h is the average coating thickness, μm.
4 Results
The electrolyte composition and current density were selected of many oxidation parameters as the most influential in the formation of the surface structure of PEO coatings, as shown by previous studies [10].
Analysis of the results of microscopic examination of the surface of insulating coatings on D16T alloy produced in the GS mode in different electrolytes at the same density (Fig. 2) allows making appropriate generalizations.
In particular, the lowest regular through porosity is characteristic for PEO coatings produced in 12 g/l LG (0:12) technical-grade sodium liquid glass solution (Fig. 2a). The deposits of contact copper, the egress of which is a direct sign of the through character of pores, are observed only in separate crater structural elements of rather large size of 10…15 μm (Fig. 2a), which are probably produced during the microarc-to-arc transition.
On the coatings produced in sodium liquid glass and alkali solution with the concentration of 2 g/l KOH + 12 g/l LG (2:12), a large number of systemic open pores of size 2…10 μm and copper inclusions of 3…6 μm are recorded over the entire surface, indicating the presence of through porosity (Fig. 2b). Along with porosity, open porosity is also a possible negative factor affecting the dielectric properties. Different shapes of open pores (round, oval, irregular) (Fig. 2b) may indicate the nonlinear pore propagation in the coatings, as well as the different degrees of their closing. Comparing to the surface of 0:12 solution coatings, the copper deposits on the samples oxidated in 2:12 solutions are more evenly distributed.
Relatively lower (by number and linear size) open porosity is characteristic for the oxide layers produced in 1 g/l KOH + 6 g/l LG (1:6) solution (Fig. 2c). Copper inclusions are also smaller in size, up to 1 μm.
It should be noted that the thickness of the coating in this electrolyte is the smallest – 37 μm (43 and 44 μm in 0:12 and 2:12 solutions, respectively). This factor also affects the through porosity index, in particular, impairs it.
In general, if we evaluate the surface quality of the coatings by the relative number and size of pores, we can conclude that through porosity increases in the series of solutions: 0:12 < 1:6 < 2:12.
Analysis of micrographs in Fig. 3, reflecting the surface of the coatings produced in the GS mode at different current densities j, shows that through porosity increases in each electrolyte with increasing j from 5 to 15 A/dm2.
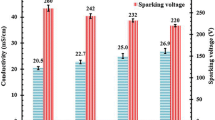
According to the obtained results, there is a significant dependence of volume resistivity on the electrolyte composition: the highest ρv values are characteristic for the coatings produced in 0:12 solution, the smallest – for the electrolyte oxidation coatings produced in 2:12 solution.
These data correlate qualitatively with the results of microscopic studies of PEO coatings, Figs. 2 and 3.
An analysis of ρv values of the coatings on D16T alloy produced in the GS mode at different current densities j shows that volume resistivity is almost independent of this indicator. Reduction of ρv for the coatings obtained in 0:12 solution is within the margin of error. A slight trend of ρv decrease with increasing j for the samples oxidated in 1:6 and 2:12 solutions is most likely due to the increase in the number of pores.
The nature of changes in electric strength E, depending on the studied factors (electrolyte composition, current density) has a certain analogy with the corresponding dependencies of volume resistivity (Fig. 4a, b) and also correlates qualitatively with the results of microscopic analysis.
In particular, the E indicator is more sensitive to the qualitative and quantitative electrolyte composition. The lowest values of the maximum breakdown voltage and, accordingly, electric strength is determined on the PEO coatings obtained in a more concentrated 2:12 electrolyte (Fig. 4b), where the largest number of through pores is recorded. Rather higher E and ρv values are characteristic for the coatings produced in silicate (0:12) and dilute alkaline silicate (1:6) solutions, where the number of through pores is smaller compared to the samples oxidated in 2:12 solution.
5 Conclusions
Based on the research performed, it was established that there is a qualitative correlation between the nature of through porosity in the plasma electrolytic oxidation coatings on D16T alloy and their dielectric properties.
The through porosity increases in the series of solutions: 12 g/L LG < 1 g/L КOH + 6 g/L LG < 2 g/L КOH + 12 g/L LG with increasing of anode current density from 5 to 15 A/dm2. The accordingly sizes of pores are 10…15 μm, up to 1 μm and 2…10 μm.
Increased through porosity of the coatings obtained in the galvanostatic mode in the 2 g/l KOH + 12 g/l LG electrolyte is one of the major reasons for the deterioration of their dielectric properties – the lowest values of resistivity and electric strength.
The coatings produced in 12 g/l LG technical-grade sodium liquid glass solution have the best dielectric properties, corresponding to the smallest through the porosity of these samples.
Further studies are related to the realization of the PEO process in suspension electrolytes to reduce the porosity and, accordingly, to improve the dielectric characteristics of the coatings on aluminum alloys.
It is also planned to continue research on the surface morphology and dielectric properties of PEO coatings on cast aluminum alloys to expand the range of structural materials for the manufacture of grinding wheels used in combined diamond-spark grinding.
References
Hussein, R.O., Northwood, D.O.: Production of anti-corrosion coatings on light alloys (Al, Mg, Ti) by plasma-electrolytic oxidation (PEO). In: Developments in Corrosion Protection, 201–239. InTech, Rijeka (2014)
Suminov, I.V., Belkin, P.N., Apelfeld, A.V., Ludin, V.B., Krit, B.L., Borisov, A.M.: Plasma-Electrolytic Modification of the Surface of Metals and Alloys: Book 2, vol. 2. Technosphera, Moscow (2011). (in Russian)
Curran, J.A., Clyne, T.W.: Porosity in plasma elecrtrolytic oxide coatings. Acta Mater. 54(7), 1985–1993 (2006)
Orlova, D.V., Trushkina, T.V., Vakhteev, E.V., Alyakretsky, R.V.: The study of porosity of oxide coatings on aluminum alloys. In: MAI Proceedings: Network Scientific Magazine vol. 68, p. 14327084 (2013). (in Russian)
Famiyeh, L., Xiaohu, H.: Plasma electrolytic oxidation coatings on aluminum alloys: microstructures, properties, and applications. Mod. Concepts Mater. Sci. 2(1), 000526 (2019)
Sotomonte, S.F., Pinzon, C.B., Vegrara S.G.: Growth of PEO ceramic coatings on AA 2024-T3 aluminium alloy. J. Phys.: Conf. Ser. 687, 012037 (2016)
Zhang, P., Nie, X., Hu, H.: Wear and galvanic corrosion protection of Mg alloy via plasma. SAE Technical Paper 2009-01-0790 (2009)
Montoya, R.D.Z., Vera, E.L., Pineda, Y.T., Cedeno, M.L.: Effect of the layer of anodized 7050-T6 aluminum corrosion properties. J. Phys.: Conf. Ser. 786, 012032 (2017)
Xie, H., Cheng, Y., Li, S., Cao, J., Cao, L.: Wear and corrosion resistant coatings on surface of cast A356 aluminum alloy by plasma electrolytic oxidation in moderately concentrated aluminate electrolytes. Trans. Nonferrous Met. Soc. China 27(2), 336–351 (2017)
Ji, S., Weng, Y., Wu, Z., Ma, Z., Tian, X., et al.: Excellent corrosion resistance of P and Fe modified micro-arc oxidation coating on Al alloy. J. Alloys Compd. 710, 452–459 (2017)
Shao, L., Li, H., Jiang, B., Liu, C., Gu, X., Chen, D.: A comparative study of corrosion behavior of hard anodized and micro-arc oxidation coatings on 7050 aluminum alloy. Metals 8(3), 165 (2018)
Walsh, F.C., Low, C.T.J., Wood, R.J.K., Stevens, K.T., Archer, J., Poeton, A.R., Ryder, A.: Plasmaelectrolytic oxidation (PEO) for production of anodized coatings on light weight metal (Al, Mg, Ti) alloy. Trans. Inst. Metal Finish. 87(3), 122–135 (2009)
Wang, X., Wu, X., Wang, R., Qiu, Z.: Effect of Na3AlF6 on the Structure and Mechanical Properties of Plasma Electrolytic Oxidation Coatings on 6061 Al alloy. Int. J. Electrochem. Sci. 8, 4986–4995 (2013)
Aliramezani, R., Raeissi, K., Santamaria, M., Hakimizad, A.: Characterization and properties of PEO coatings on 7075 Al alloy grown in alkaline silicate electrolyte containing KMnO4 additive. Surf. Coat. Technol. 329, 250–261 (2017)
Hussein, R.O., Zhang, P., Xia, Y., Nie, X., Northwood, D.O.: The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 206(7), 1990–1997 (2011)
Wang, P., Pu, J., Xiao, Y.-T., Hu, W.-J., Wu, T., Cao, W.-J., Gong, Z.-Y., Huang, M.: Effect of graphite additives in electrolytes on characteristics of micro-arc oxidation coatings on 7E04 aluminum alloy. Surf. Rev. Lett. 26(4), 18501780 (2019)
Wu, X., Qin, W., Guo, Y., Xie, Z.: Self-fabricative coating grown by micro-plasma oxidation on aluminum alloys in the solution of aluminate-graphite. Appl. Surf. Sci. 254(20), 6395–6599 (2008)
Liu, Y., Xu, J., Gao, J., Yuan, Y., Gao, C.: Influences of additive on the formation and corrosion resistance of micro-arc oxidation ceramic coatings on aluminum alloy. Phys. Procedia 32, 107–112 (2012)
Laleh, M., Sabour Rouhaghdam, A., Shahrabi, T., Shanghi, A.: Effect of alumina sol addition to micro-arc oxidation electrolyte on the properties of MAO coatings formed on magnesium alloy AZ91D. J. Alloys Compd. 496(1–2), 548–552 (2010)
Xu, X., Lu, P., Guo, M., Fang, M.: Cross-linked gelatin/nanoparticles composite coating on microarc oxidation film for corrosion and drug release. Appl. Surf. Sci. 256(8), 2367–2371 (2010)
Arrabal, R., Mohedano, M., Matykina, E., Pardo, A.: Characterization and wear behaviour of PEO coatings on 6082-T6 aluminium alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 269, 64–73 (2015)
Gutsalenko, Yu.G., Sevidova, E.K., Stepanova, I.I., Strel’nitskij, V.E.: Evaluation of dielectric properties of micro-arc coatings on deformable aluminum alloys. Probl. At. Sci. Technol. 114, 125–127 (2018)
Gutsalenko, Yu.G., Sevidova, E.K., Stepanova, I.I.: Evaluation of technological capability to from dielectric coatings on AK6 alloy, using a method of microarc oxidation. Surf. Eng. Appl. Electrochem. 55(5), 602–606 (2019)
Sevidova, E., Gutsalenko, Yu., Rudnev, A., Pupan, L., Titarenko, O.: The study of surface microgeometry and morphology of plasma electrolytic oxidation dielectric coatings on aluminum alloys. In: Ivanov, V., et al. (eds.) Advances in Design, Simulation and Manufacturing II. DSMIE-2019, LNME, pp. 302–310. Springer, Cham (2020)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Sevidova, E., Pupan, L., Gutsalenko, Y., Rudnev, A., Titarenko, O. (2020). Effect of Morphological Features on Dielectric Properties of Plasma Electrolytic Oxidation Coatings on D16T Aluminum Alloy. In: Ivanov, V., Trojanowska, J., Pavlenko, I., Zajac, J., Peraković, D. (eds) Advances in Design, Simulation and Manufacturing III. DSMIE 2020. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-50794-7_53
Download citation
DOI: https://doi.org/10.1007/978-3-030-50794-7_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50793-0
Online ISBN: 978-3-030-50794-7
eBook Packages: EngineeringEngineering (R0)