Abstract
The importance of the microenvironment in tumor development and their resistance to drugs is increasingly well known. This microenvironment is composed of different cell types, among which cells with stemness properties such as cancer stem cells (CSCs) and mesenchymal stem cells (MSCs) are distinguished for their relevant role in tumor proliferation, angiogenesis, metastasis, and drug resistance. The relationship between these stem cells (SCs) and tumor microenvironment is conducted by the secretome, consisting of several factors, cytokines, chemokines, and hormones released to the surrounding stroma, which plays a deterministic role in tumor hallmarks. Knowing the intrinsic and complex communication network that SCs establish with the microenvironment will allow to address the tumor processes responsible for cancer progression and the generation of new targeted therapeutic approaches useful in the clinic arena.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cancer stem cells
- Mesenchymal stem cells
- Cancer-associated fibroblasts
- Tumor microenvironment
- Secretome
- Growth factors
- Cytokines
- Extracellular matrix
- Niche
- Angiogenesis
- Hypoxia
- Metastasis
- Epithelial-to-mesenchymal transition
- Homing
- Inflammation
8.1 Introduction
Over the decades, tumor origin and development were attributed only to cancer cells; however, tumor cells do not act alone since they are immersed in a tumor microenvironment (TME) that comprises several cell types, a characteristic extracellular matrix (ECM) and a complex cytokines and growth factors network. The TME consists of the niche where the tumor develops, and it is unique for each tumor and patient, and also highly dynamic over time [1, 2]. The role played by TME in tumor hallmarks [3] such as high proliferation, invasion, angiogenesis, metastasis, and resistance to drugs [4,5,6,7] is increasingly well known. The TME contains several cell types including the tumor cells, cells from the immune system, CSCs, MSCs, fibroblasts, endothelial precursors, and a series of chemical components and biophysical signals [2]. This niche participates in the carcinogenesis by a complex network of cytokines, growth factors, and inflammatory and matrix-remodeling enzymes [8].
An essential process that must be given in the TME is the new vessel formation. Tumor neovascularization allows tumor growth and involves both tube-forming endothelial cells and their supporting pericytes, as well as tumor and stromal cells [9]. The new branched vessels of the existing vasculature and the development of neovascularization from endothelial cells and their associated pericytes or from cancer stem cells (CSCs) (in a process called vascular mimicry) depend on angiogenic signals from hypoxia regions or soluble factors from the TME [10, 11]. The resulting vasculature is chaotic and abnormally fulfills its functions, which facilitates the metastatic spread of cancer cells, increases hypoxia in the tumor [2, 8], and prevents the correct extravasation of immune cells and diffusion of drugs, helping tumor survival [12].
In TME development, cancer-associated fibroblasts (CAFs) are essential cells that secrete growth factors and cytokines, which stimulate the growth and survival of malignant cells [13,14,15] and contribute to drug resistance [16,17,18]. CAFs secrete also factors with chemoattractant properties, which stimulate the migration of other types of stromal cells and their progenitors to the TME, and promote angiogenesis by attracting pro-angiogenic myeloid cells and stimulating endothelial recruitment [19, 20].
Furthermore, the TME presents a wide diversity of infiltrating immune cells (IICs) , among which are tumor-associated macrophages (TAMs), dendritic cells, lymphocytes, natural-killers, and neutrophils, which as a whole can perform both protumor and antitumor functions depending on a large extent on the signals from the TME [21]. IICs deliver to the TME growth mediators that stimulate the proliferation of both tumor and stromal cells and activate angiogenic processes [22]. Also, IICs promote invasive cellular phenotypes, contribute to therapeutic resistance, and improve protumor inflammation [5, 8, 23].
Beyond the contributions of different cell types to the TME, the ECM is another key component, and involves not only the physical scaffolding of the cells in the niche, but also a source of different factors and cytokines that model tumor behavior. CAFs, TAMs, and tumor cells secrete heparanases and matrix metalloproteinases (MMPs) that degrade the ECM, releasing these factors to the TME [14, 19, 24, 25]. Through them, ECM mediates in angiogenesis, inflammatory processes, dysregulation of stromal cells, and tumor proliferation [26].
In addition to the cell types described above in the TME, main role is played by characteristics SCs such as MSCs and CSCs. Both kinds of SCs have several common features and participate actively in the TME, being essential for tumor growth. In this chapter, we first present the similarities and specific characteristics of both SCs. Second, we describe the specific particularities of the secretome released by these cells and how it participates and regulates the TME and the pathogenic processes associated with tumor development.
8.2 Stem Cells in the Tumor Microenvironment
Stem cells are rare cells defined by the capacity to self-renew themselves and being able to differentiate into mature cells from a tissue [27]. In the TME, these cells will acquire special importance as responsible to manage its origin and particular characteristics (Fig. 8.1).
In the first place, although there are different proposals about how a tumor is generated, there is evidence of the existence of a minority subpopulation in the TME called CSCs, responsible for tumor growth, metastasis, and cancer recurrence [28]. CSCs present similar characteristics to MSCs in terms of their capacity for self-renewal, the expression of embryonic SCs transcription factors, similar regulation of several signaling pathways, and gene expression modulation by short noncoding miRNAs [29]. CSCs characterization is a complex challenge due to surface markers not being universal for any cancer type, the existence of heterogeneous CSC pools in the same tumor, and the instability of the phenotype [30]. However, several markers have been useful to identify CSCs like CD133 and CD44 [31], aldehyde dehydrogenase 1 (ALDH1) activity [32], and its ability to exclude Hoechst 33342 (side population) [33].
The importance of CSCs in the TME also lies in tumor recurrence and metastasis [34, 35]. Moreover, CSCs provide tumor resistance to radio- and chemotherapy due to the overexpression of membrane proteins of multidrug resistance (MDR) and their ability to detoxify or mediate the outflow of cytotoxic agents [33, 36], high ALDH1 activity [36, 37], rapid reparative response to DNA damage [38], and their ability to maintain a quiescent state [39]. However, CSCs require the TME to regulate their proliferation and self-maintenance, interacting closely with the cells that comprise it [40, 41]. It is known that CSCs not only get adapted to TME, but also contribute aggressively to its generation and cell composition; thanks to the development of a powerful interactive network composed of cytokines, growth factors, chemokines, hormones, miRNA, microvesicles, and exosomes through which CSCs can recruit and activate different cells types like MSCs or vascular endothelial cells [41]. As well, the ECM is remodeled by the CSCs to maintain stem cell properties through anchorage, cell–cell and cell–ECM contact signals, and biomechanical properties [25].
On the other hand, one of the cell types recruited by the TME includes the MSCs, multipotent SCs that reside in many human organs and comprise a heterogeneous population with self-renewal ability [42]. Although their morphology, immunophenotype, and differentiation potentials are dependent on their tissue of origin [42], three criteria have been defined for their identification: (i) must be plastic-adherent when maintained in standard culture conditions, (ii) must express certain membrane markers, and (iii) must differentiate in vitro to osteoblasts, adipocytes, and chondroblasts [43, 44].
MSCs could be also found in the circulatory system and can arrive to inflammatory sites, where they seem to perform a restorative function, not only by structural repair of tissue, but also modulating the local environment due to its immunomodulatory and anti-inflammatory properties [42]. The role played by MSCs in the TME is not exempt from controversy [45]; however, in a relevant way, it has been shown that these cells are recruited by the TME [46,47,48]. It has been amply demonstrated that MSCs contribute to tumor growth and proliferation [48,49,50,51], increase the metastatic potential of tumor cells by promoting their motility, invasiveness [52, 53], the epithelial-to-mesenchymal transition (EMT) [54], and angiogenesis [55, 56], and participate in the appear of CAFs in the TME [51, 57]. Moreover, they play a key role in the tumor niche formation and support CSCs maintenance [58, 59]. Recently, our research group has shown that the MSCs secretomes, among which are interleukine-6 (IL-6) and hepatocellular growth factor (HGF) stand out, support the selection of CMCs with specific chromosomal alterations characterized by a translocation in the long arm of chromosome number 17 (17q25), that makes them more aggressive [58].
8.3 Stem Cell-Secreted Factors
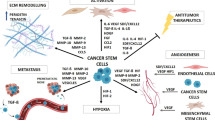
In normal adult tissues, the presence of MSCs generates an environment termed as “stem cell niche,” and the communication between the MSCs and their microenvironment is fundamental for normal tissue homeostasis, SCs maintenance, differentiation, and immunomodulation [60]. In cancer, this SC niche is modified with altered intercellular communication, be transformed in a TME that allows tumor growth changes over tumor progression and re-adapting [60,61,62]. All cells that constitute the TME display altered or modified secretomes compared to normal tissues, with simultaneous up- and downregulation of several factors [63]. SCs communicate with their microenvironment through the release of microvesicles and exosomes, as well as a wide range of soluble factors that include chemokines, cytokines, growth factors, hormones, and metabolites [64]. Specifically, factors released by tumor SCs promote several associated tumor processes, including tumor growth, invasion, metastasis, and promotion of angiogenesis, in addition to other processes such as influencing in cell phenotype, homing, differentiation, inflammation and immunodulation processes, and drug resistance mechanisms [63] (Fig. 8.2).
8.3.1 Angiogenesis
A decisive factor in tumor development is the presence of blood vessels, which provide both the nutrients and oxygen needed, and offer support for the metastasis. Several studies show that tumor SCs secrete vascular endothelial growth factor (VEGF) , which is the principal growth factor promoting vascularization [65, 66]. Furthermore, it has been observed that the secreted VEGF itself has the potential to induce differentiation of MSCs into endothelial cells (ECs) [66, 67]. However, this factor not only has this fundamental role in the TME, but it also stimulates CSCs proliferation and maintenance through the stimulation of neuropilin-1, a coreceptor of VEGF receptor 2. In addition, VEGF overexpression accelerates tumor growth, promoting CSCs division [65, 68, 69] (Fig. 8.1).
Nevertheless, VEGF is not the only factor related with angiogenesis, IL-6 secreted by MSCs increases the secretion of endothelin-1 (ET-1) in cancer cells, which induces the activation of Akt and ERK pathways in ECs, leading to the development of mature vessels [70]. Also, a recent study situates another cytokine secreted by MSCs, the interleukin-8 (IL-8), as responsible for endothelial proliferation induction and tube formation, demonstrating the paracrine pro-angiogenic effect of IL-8 [71] (Fig. 8.1).
8.3.2 Hypoxia
A hallmark in solid TME is hypoxia, which is directly related with tumor progression and therapeutic response failure. Within the tumor, the oxygen concentration is variable, appearing in distinct areas with different oxygen contents. The responsible for the adaptation to hypoxic microenvironment is the hypoxia-inducible factor (HIF) family of transcription factors, and plays crucial roles in diverse tumor processes such as angiogenesis, treatment and immune system resistance, proliferation, tumor cell plasticity, metastasis, and maintenance of CSCs [72]. It was observed that under hypoxic conditions, MSCs increase HIF-1α secretion and their proliferative capacity. In addition, elevated release of energy metabolism-associated genes such as lactate dehydrogenase, GLUT-1, and PDK1 was observed, thereby leading to acidosis in the tumor microenvironment, and all this results in a feedback of the hypoxia environment [73]. On the other hand, the expression of HIF-1α and HIF-2α is different between non-SCs and CSCs. HIF-1α is produced by stem and nonstem tumor cells, and is only stabilized under acute hypoxic conditions, but HIF-2α is significantly secreted by CSCs and is accumulated under low levels of hypoxia or even normal physiological oxygen levels [74]; so, the role of the two HIF isoforms depends on the timely characteristics of the TME. In addition, HIF-1α produced by SCs stimulates tumor angiogenesis through the enhanced expression of angiogenic proteins like VEGF [75]. Also, several studies evidence that hypoxia plays a determinant role in CSCs maintenance, enhancing the self-renewal capacity, and retaining the undifferentiated state of CSCs, state that is reversible when normoxic conditions are resettled [76,77,78].
8.3.3 Metastasis
Cells from the primary tumor present intravasation capacity, which allows them to enter into the surrounding blood and lymphatic vessels, and around 0.2% of these cells survive in circulation and have extravasation ability; finally, they colonize distant organs producing metastasis [79]. As can be seen, metastasis is a very complex process that requires a set of factors that support it to achieve success. MSCs present different roles in the metastatic process, on the one hand, they increase the metastatic potential of tumor cells, and on the other hand, they present the ability to prepare the metastatic niche in the distant tissue [48]. Related to the increment of metastatic potential, the release of chemokine CCL5 by MSCs activates its receptor CCR5 on breast cancer cells thereby promoting altered breast cancer development and metastasis [52]. In addition, ovarian CSCs present CCR1, CCR3, and CCR5 upregulated, being more sensitive to CCL5 induction, enhancing invasiveness through nuclear factor κB (NF-κB) activation and the consequently elevated MMP9 secretion [80]. Others MMPs are highly secreted in the TME, such as MMP10 and MMP13 that are released by CSCs, and this fact promotes ECM degradation and remodeling, which enhances metastatic behavior [81, 82]. As other factors described, MMPs also perform different functions, such as MMP10 that has an essential role in CSCs maintenance and treatment resistance through the activation of Wnt signaling [83]. Main factors of other tumor processes also participate in metastasis, for example, hypoxia promotes metastasis through the activation and enhancement expression of HIF, which mediates paracrine signaling between cancer cells and MSCs mediated by CXCL10 and CCL5 and its respective receptors CXCR3 and CCR5 in cancer cells [84].
EMT phenomenon and the intravasation are essential processes in metastasis, and are processes driven by a complex network of cytokines and factors. For example, MSCs secretome in general, and IL-6, IL-8, and TGFβ in particular, have the capacity to upregulate EMT specific markers (N-cadherin, Vimentin, Twist, and Snail) via activation of PI3K/AKT pathway [85,86,87]. Once the cells are in the blood vessel, they have to perform the extravasation to be able to colonize the new tissue, and TGFβ displays an indispensable role in this process [88]. TGFβ induces angiopoietin-like 4 via the Smad signaling pathways in cancer cells, and these cells enter the circulation to metastasize to the lungs. After that, circulating cells that retain angiopoietin-like 4 release this cytokine and disrupt endothelial cell–cell adhesions in lung capillaries, facilitating the target organ invasion [89].
The TME also includes the metastatic niche, a niche in which there are also SCs and the factors secreted by them, making metastasis a successful process. Kaplan et al. first described the formation of a premetastatic niche where MSCs that express VEGFR1 present the capacity to migrate and form premetastatic niches through the production of MMP9, preparing it before the arrival and establishment of tumor cells [90]. Also, periostin (an ECM molecule) is highly expressed in CSCs [91] and when it binds to Wnt ligands, promotes stemness [92] so that the first CSCs that reach the premetastatic niche could favor the stemness of the new cells through this molecule. All these data together show that metastasis is a process induced by original TME secretome, where SCs are a principal player that can handle such complex processes as traveling through blood and lymphatic vessels and establishing a new tumor in a different organ.
8.3.4 Inflammation and Immunomodulation Processes
Inflammation and immunomodulation play a critical role in tumor development through the production of several molecules that participate in diverse tumor processes [93, 94]. In the TME there are several immune system cellular types including macrophages, neutrophils, mast cells, eosinophils, and myeloid-derived suppressor cell, which are attracted by TME through the tumor cell secretome, as well as ECM-degrading enzymes that allow invasion [75, 95]. The transcription factors NF-κB and Stat3 regulate multiple aspects and serve as a central inflammatory mediator that responds to a large variety of immune stimulus [93, 94, 96], and as described in previous sections, these factors are very active in tumors. MSCs constitutively secrete several factors implicated in the immune suppressive role of these cells which include IL-1β, IL-6, IL-8, IL-10, HGF, TNFα, GM-CSF, TGFβ-1, prostaglandin E2, human leukocyte antigen-G5, and tryptophan-degrading enzyme indoleamine 2,3- dioxygenase (IDO) [97, 98]. In addition, CSCs have been demonstrated to have immunomodulatory properties through the release of inflammatory factors like IL-6, IL-17, and TGFβ, inducing Foxp3-positive regulatory T cells and pathogenic Th17 cells that can make the TME unresponsive to the recognizance of immune cells [99].
8.3.5 Homing
As described in the introduction section, the TME is composed of different cell types that interact to create the most optimal TME, as well as the different processes associated with tumor evolution; but to achieve this, it is necessary that the tumor “recruits” these cells. MSCs are attracted and activated by IL-6 released by different cell types, among them the CSCs, and in turn, the MSCs recruited produce CXCL7 that favors the maintenance of CSCs, generating a positive feedback loop [100]. Also, IL-1β, that shows higher expression in CSCs compared to their more differentiated counterparts [101], promotes MSCs migration through the expression of MMP1, which then activates the PAR1 and G-protein-coupled signal pathways [102].
Definitely, MSCs homing to tumor requires the participation of a complex molecule network that includes several cytokines and factors released by CSCs such as TGFβ1, VEGF, FGF, CCL2, CXCL8, and TNFα [103]. But MSCs do not respond only to signals from other cell types; for example, autocrine signaling of SDF-1 leads to the activation of Jak2/STAT3 and ERK1/2 signaling, thereby promoting FAK activation that finally promotes MSCs’ migration to the TME [104]. TAMs also are critical modulators of the TME and support tumor progression; their recruitment is done through the chemokine CCL2 and its receptor CCR2, secreted by MSCs, as well as VEGF in a HIF-1α-dependent manner released by both SCs [105, 106].
8.3.6 Cell Phenotype Maintenance or Differentiation Induction
The maintenance or alteration of the cell phenotype or the stemness state is highly influenced by the TME. CSCs phenotype, proliferation, and invasiveness are regulated by MSCs and the CSCs themselves that activate NF-κB pathway through the release of several growth factor and cytokines, such as CXCL12, CXCL7, IL-6, IL-8, HGF, VEGF, HIF, and Gremlin 1 (see previous sections) [59, 100, 107, 108]. TGFβ is one of the key factors produced by the CSCs, and helps to transform fibroblasts and MSCs to cancer-associated fibroblasts (CAFs); thanks to the activation of TGFBR1/Smad pathways, and these CAFs participate in several TME process through its secretome network, like angiogenesis, EMT, and metastasis [62, 109, 110]. Moreover, MSCs also present the capacity to differentiate into pericytes and ECs under the effect of VEGF produced by both SCs [111]. The balance between the differentiated-dedifferentiated state of the CSCs is essential for tumor evolution and treatment resistance, and the balance between both states depends of NF-κB signaling (and related molecules describes above), enhancing Wnt activation that drives tumor cells dedifferentiation [112].
8.4 Future Trends
CSCs are responsible for tumor development, metastasis, and relapses, but the entire responsibility of a tumor process should not be associated only with a single cell type, since the TME is composed of different cell types that are interconnected by a complex network of chemokines, cytokines, growth factors, hormones, and metabolites. MSCs and CSCs create the stem niche and participate in several indispensable tumor processes such as angiogenesis, hypoxia, cell recruitment, inflammation, undifferentiated phenotype maintenance or cell differentiation, and metastasis. The potential of future therapeutic approaches is based on the knowledge of the TME, and especially of both types of SCs, as well as the complex communication network between them and with the rest of tumor subpopulations. For example, both SCs release VEGF to induce angiogenesis that supports CSCs maintenance and metastasis, and many novel approach drugs are focused on disrupting this growth factor pathway, including tyrosine kinase inhibitors [113, 114]. In the same way, high HIF expression correlates with poor glioma patient survival [115], so new therapies against this factor and its signaling pathway will allow the disruption of the hypoxic environment that affects several tumor processes and characteristics, including angiogenesis. A key factor in future therapeutic approaches is to avoid CSCs maintenance/protection. As described in this chapter, the entire TME in general, and the SCs in particular, has developed a complex cellular communication directed to CSCs preservation; therefore, new therapies should focus on this connection, and, in fact, there are already several studies and clinical trials aimed at these cytokines and specific factors, such as IL-6 [116, 117], IL-8 [118], HGF [119], and TGFβ [120]. In conclusion, the molecules released in the TME form a complex network that determines the success of the hallmarks of cancer [3], and may constitute a powerful tool in the therapeutic targeting of precision and personalized oncology.
References
Caiado F, Silva-Santos B, Norell H (2016) Intra-tumour heterogeneity – going beyond genetics. FEBS J 283:2245–2258
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Joyce J, Quail D (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437
Junttila MR, De Sauvage FJ (2013) Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501(7467):346–354
Hui L, Chen Y (2015) Tumor microenvironment: sanctuary of the devil. Cancer Lett 368(1):7–13
Yeldag G, Rice A, del Rio Hernández A (2018) Chemoresistance and the self-maintaining tumor microenvironment. Cancers (Basel) 10(12):pii: E471
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125:5591–5596
Qian C-N, Tan M-H, Yang J-P, Cao Y (2016) Revisiting tumor angiogenesis: vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation. Chin J Cancer 35:10
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370
Turley SJ, Cremasco V, Astarita JL (2015) Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol 15:669–682
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59(19):5002–5011
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R et al (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348
Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A et al (2008) Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 68(3):918–926
Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z et al (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15:21–34
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J et al (2012) Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487:500–504
Paraiso KHT, Smalley KSM (2013) Fibroblast-mediated drug resistance in cancer. Biochem Pharmacol 85:1033–1041
Räsänen K, Vaheri A (2010) Activation of fibroblasts in cancer stroma. Exp Cell Res 316:2713–2722
Vong S, Kalluri R (2011) The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer 2:1139–1145
Zamarron BF, Chen W (2011) Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 7:651–658
Eyileten C, Majchrzak K, Pilch Z, Tonecka K, Mucha J, Taciak B et al (2016) Immune cells in cancer therapy and drug delivery. Mediat Inflamm 2016:1–13
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124:263–266
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S et al (2004) TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303:848–851
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406
Pickup MW, Mouw JK, Weaver VM (2014) The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15:1243–1253
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Hernández-Camarero P, Jiménez G, López-Ruiz E, Barungi S, Marchal JA, Perán M (2018) Revisiting the dynamic cancer stem cell model: importance of tumour edges. Crit Rev Oncol Hematol 131:35–45
Ni C, Huang J (2013) Dynamic regulation of cancer stem cells and clinical challenges. Clin Transl Oncol 15:253–258
Visvader JE, Lindeman GJ (2012) Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10:717–728
Jang J-W, Song Y, Kim S-H, Kim J, Seo HR (2017) Potential mechanisms of CD133 in cancer stem cells. Life Sci 184:25–29
Charafe-Jauffret E, Ginestier C, Birnbaum D (2009) Breast cancer stem cells: tools and models to rely on. BMC Cancer 9:202
Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U et al (2004) A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A 101:14228–14233
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M et al (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1:313–323
Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr H-A, Delaloye J-F et al (2011) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481:85–89
Dean M (2009) ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia 14:3–9
Eyler CE, Rich JN (2008) Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol 26:2839–2845
Maugeri-Saccà M, Bartucci M, De Maria R (2012) DNA damage repair pathways in cancer stem cells. Mol Cancer Ther 11:1627–1636
Moore N, Lyle S (2011) Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol 2011:pii: 396076
Wels J, Kaplan RN, Rafii S, Lyden D (2008) Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev 22:559–574
Ye J, Wu D, Wu P, Chen Z, Huang J (2014) The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumor Biol 35:3945–3951
Klimczak A, Kozlowska U (2016) Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int 2016:4285215
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Bergfeld SA, DeClerck YA (2010) Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 29:249–261
Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM et al (2007) Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res 13:5020–5027
Senst C, Nazari-Shafti T, Kruger S, Höner Zu Bentrup K, Dupin CL, Chaffin AE et al (2013) Prospective dual role of mesenchymal stem cells in breast tumor microenvironment. Breast Cancer Res Treat 137:69–79
Ridge SM, Sullivan FJ, Glynn SA (2017) Mesenchymal stem cells: key players in cancer progression. Mol Cancer 16:31
Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J et al (2003) Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102:3837–3844
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y et al (2012) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315:28–37
Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B et al (2009) Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 4(4):e4992
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563
Nabha SM, dos Santos EB, Yamamoto HA, Belizi A, Dong Z, Meng H et al (2008) Bone marrow stromal cells enhance prostate cancer cell invasion through type I collagen in an MMP-12 dependent manner. Int J Cancer 122:2482–2490
Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C et al (2010) Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 124:317–326
Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J et al (2011) Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med 17:579–587
Zhang T, Lee Y, Rui Y, Cheng T, Jiang X, Li G (2013) Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther 4:70
Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP et al (2008) Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68:4331–4339
Hossain A, Gumin J, Gao F, Figueroa J, Shinojima N, Takezaki T et al (2015) Mesenchymal stem cells isolated from human gliomas increase proliferation and maintain stemness of glioma stem cells through the IL-6/gp130/STAT3 pathway. Stem Cells 33:2400–2415
Jiménez G, Hackenberg M, Catalina P, Boulaiz H, Griñán-Lisón C, García MÁ et al (2018) Mesenchymal stem cell’s secretome promotes selective enrichment of cancer stem-like cells with specific cytogenetic profile. Cancer Lett 429:78–88
Kuhn NZ, Tuan RS (2010) Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol 222:268–277
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17:320–329
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437
Paltridge JL, Belle L, Khew-Goodall Y (1834) The secretome in cancer progression. Biochim Biophys Acta 2013:2233–2241
Melzer C, von der Ohe J, Lehnert H, Ungefroren H, Hass R (2017) Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Mol Cancer 16:28
Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A et al (2011) A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478:399–403
Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J et al (2008) VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 99:622–631
Zhang K, Shi B, Chen J, Zhang D, Zhu Y, Zhou C et al (2010) Bone marrow mesenchymal stem cells induce angiogenesis and promote bladder cancer growth in a rabbit model. Urol Int 84:94–99
Xu C, Wu X, Zhu J (2013) VEGF promotes proliferation of human glioblastoma multiforme stem-like cells through VEGF receptor 2. Sci World J 2013:1–8
Mercurio AM (2019) VEGF/neuropilin signaling in cancer stem cells. Int J Mol Sci 20(3):pii: E490
Huang W-H, Chang M-C, Tsai K-S, Hung M-C, Chen H-L, Hung S-C (2013) Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 32:4343–4354
Conroy S, Kruyt FAE, Wagemakers M, Bhat KPL, den Dunnen WFA (2018) IL-8 associates with a pro-angiogenic and mesenchymal subtype in glioblastoma. Oncotarget 9:15721–15731
Lequeux A, Noman MZ, Xiao M, Sauvage D, Van Moer K, Viry E et al (2019) Impact of hypoxic tumor microenvironment and tumor cell plasticity on the expression of immune checkpoints. Cancer Lett 458:13–20
Lavrentieva A, Majore I, Kasper C, Hass R (2010) Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun Signal 8:18
Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S et al (2009) Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15:501–513
Kitamura T, Qian B-Z, Pollard JW (2015) Immune cell promotion of metastasis. Nat Rev Immunol 15:73–86
Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD et al (2009) Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene 28:3949–3959
Lee D-H, Oh SC, Giles AJ, Jung J, Gilbert MR, Park DM (2017) Cardiac glycosides suppress the maintenance of stemness and malignancy via inhibiting HIF-1α in human glioma stem cells. Oncotarget 8(25):40233–40245
Jacobsson H, Harrison H, Hughes É, Persson E, Rhost S, Fitzpatrick P et al (2019) Hypoxia-induced secretion stimulates breast cancer stem cell regulatory signalling pathways. Mol Oncol 13(8):1693–1705
Zeeshan R, Mutahir Z (2017) Cancer metastasis – tricks of the trade. Bosn J Basic Med Sci 17:172–182
Long H, Xie R, Xiang T, Zhao Z, Lin S, Liang Z et al (2012) Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells 30:2309–2319
Justilien V, Regala RP, Tseng I-C, Walsh MP, Batra J, Radisky ES et al (2012) Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS One 7:e35040
Inoue A, Takahashi H, Harada H, Kohno S, Ohue S, Kobayashi K et al (2010) Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int J Oncol 37:1121–1131
Mariya T, Hirohashi Y, Torigoe T, Tabuchi Y, Asano T, Saijo H et al (2016) Matrix metalloproteinase-10 regulates stemness of ovarian cancer stem-like cells by activation of canonical Wnt signaling and can be a target of chemotherapy-resistant ovarian cancer. Oncotarget 7:26806–26822
Chaturvedi P, Gilkes DM, Wong CC, Kshitiz, Luo W, Zhang H et al (2013) Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest 123:189–205
So KA, Min KJ, Hong JH, Lee J-K (2015) Interleukin-6 expression by interactions between gynecologic cancer cells and human mesenchymal stem cells promotes epithelial-mesenchymal transition. Int J Oncol 47:1451–1459
Ritter A, Friemel A, Fornoff F, Adjan M, Solbach C, Yuan J et al (2015) Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget 6(33):34475–34493
Wu S, Wang Y, Yuan Z, Wang S, Du H, Liu X et al (2018) Human adipose-derived mesenchymal stem cells promote breast cancer MCF7 cell epithelial-mesenchymal transition by cross interacting with the TGF-β/Smad and PI3K/AKT signaling pathways. Mol Med Rep 19:177–186
McAndrews KM, McGrail DJ, Ravikumar N, Dawson MR (2015) Mesenchymal stem cells induce directional migration of invasive breast cancer cells through TGF-β. Sci Rep 5:16941
Padua D, Zhang XH-F, Wang Q, Nadal C, Gerald WL, Gomis RR et al (2008) TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133:66–77
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H et al (2012) Cancer stem cell-related gene periostin: a novel prognostic marker for breast cancer. PLoS One 7:e46670
Wang X, Liu J, Wang Z, Huang Y, Liu W, Zhu X et al (2013) Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLoS One 8:e72962
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67
Mantovani A (2010) Molecular pathways linking inflammation and cancer. Curr Mol Med 10:369–373
De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J et al (2012) Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 12:574–591
Liubomirski Y, Lerrer S, Meshel T, Morein D, Rubinstein-Achiasaf L, Sprinzak D et al (2019) Notch-mediated tumor-stroma-inflammation networks promote invasive properties and CXCL8 expression in triple-negative breast cancer. Front Immunol 10:804
Yoshimura A, Muto G (2011) TGF-β function in immune suppression. Curr Top Microbiol Immunol 350:127–147
Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F et al (2011) Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 71:614–624
Nomura A, Gupta VK, Dauer P, Sharma NS, Dudeja V, Merchant N et al (2018) NFκB-mediated invasiveness in CD133 + pancreatic TICs is regulated by autocrine and paracrine activation of IL1 signaling. Mol Cancer Res 16:162–172
Chen M-S, Lin C-Y, Chiu Y-H, Chen C-P, Tsai P-J, Wang H-S (2018) IL-1β-induced matrix metalloprotease-1 promotes mesenchymal stem cell migration via PAR1 and G-protein-coupled signaling pathway. Stem Cells Int 2018:3524759
Wang S, Miao Z, Yang Q, Wang Y, Zhang J (2018) The dynamic roles of mesenchymal stem cells in colon cancer. Can J Gastroenterol Hepatol 2018:1–8
Gao H, Priebe W, Glod J, Banerjee D (2009) Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27:857–865
Zhang J, Lu Y, Pienta KJ (2010) Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst 102:522–528
Chanmee T, Ontong P, Konno K, Itano N (2014) Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 6:1670–1690
Cabarcas SM, Mathews LA, Farrar WL (2011) The cancer stem cell niche – there goes the neighborhood? Int J Cancer 129:2315–2327
Davis H, Irshad S, Bansal M, Rafferty H, Boitsova T, Bardella C et al (2015) Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat Med 21:62–70
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401
Nwabo KAH, Kamga PT, Simo RT, Vecchio L, Seke EPF, Muller JM et al (2017) Mesenchymal stromal cells’ role in tumor microenvironment: involvement of signaling pathways. Cancer Biol Med 14:129
Orecchioni S, Gregato G, Martin-Padura I, Reggiani F, Braidotti P, Mancuso P et al (2013) Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res 73:5880–5891
Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK et al (2013) Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152:25–38
Ebos JML, Kerbel RS (2011) Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8:210–221
Ding L, Ni J, Yang F, Huang L, Deng H, Wu Y et al (2017) Promising therapeutic role of miR-27b in tumor. Tumor Biol 39:101042831769165
Liu Q, Cao P (2015) Clinical and prognostic significance of HIF-1α in glioma patients: a meta-analysis. Int J Clin Exp Med 8:22073–22083
Li J, Xu J, Yan X, Jin K, Li W, Zhang R (2018) Targeting interleukin-6 (IL-6) sensitizes anti-PD-L1 treatment in a colorectal cancer preclinical model. Med Sci Monit 24:5501–5508
Kampan NC, Xiang SD, McNally OM, Stephens AN, Quinn MA, Plebanski M (2018) Immunotherapeutic interleukin-6 or interleukin-6 receptor blockade in cancer: challenges and opportunities. Curr Med Chem 25:4785–4806
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á et al (2017) Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev 60:24–31
Papaccio F, Della Corte C, Viscardi G, Di Liello R, Esposito G, Sparano F et al (2018) HGF/MET and the immune system: relevance for cancer immunotherapy. Int J Mol Sci 19:3595
Colak S, ten Dijke P (2017) Targeting TGF-β signaling in cancer. Trends Cancer 3:56–71
Acknowledgments
This work has been partially funded by the Ministerio de Economía y Competitividad (MINECO, FEDER funds, grant numbers MAT2015-62644.C2.2.R and RTI2018-101309-B-C22), the Consejería de Economía, Conocimiento, Empresas y Universidad de la Junta de Andalucía (European Regional Development Fund (ERDF), ref. SOMM17/6109/UGR), grants from the Ministry of Economy and Competitiveness, Instituto de Salud Carlos III (FEDER funds, projects no. PIE16/00045 and DTS17/00087), and from the Chair “Doctors Galera-Requena in cancer stem cell research” (CMC-CTS963).
Conflicts of Interest
None of the authors have a conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jiménez, G., López de Andrés, J., Marchal, J.A. (2020). Stem Cell-Secreted Factors in the Tumor Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironment . Advances in Experimental Medicine and Biology, vol 1277. Springer, Cham. https://doi.org/10.1007/978-3-030-50224-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-50224-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50223-2
Online ISBN: 978-3-030-50224-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)