Abstract
Despite recent progresses in tumor therapy and increased knowledge in tumor biology, tumor remains a common and lethal disease worldwide. Cancer stem cells (CSCs) are a subset of cancer cells with a stem cell-like ability, which may drive tumor growth and recurrence and are resistant to many current anticancer treatments. Solid tumors are regarded as “organs” which are comprised of cancer cells and the tumor stroma. The tumor microenvironment makes up the stroma of the tumor, which occupies the majority of the tumor mass, including the extracellular matrix (ECM), mesenchymal stem cells (MSCs), endothelial cells, immune cells, and, what is more, networks of cytokines and growth factors. The microenvironment or niche surrounding CSCs largely governs their cellular fate. Recent work has revealed that the microenvironment supports CSC self-renewal and simultaneously serves as a physical barrier to drug delivery. The tumor microenvironment plays pivotal roles in each stage of tumor development. Knowledge about the interactions of CSCs with their microenvironment would seem to be of most importance for developing new treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer has been a frequent disease and the first or second most common cause of death worldwide. Growing evidence has implicated that cancer is a disease of stem cells. A novel paradigm in tumor biology suggests that tumors are composed of a mixture of cells with different characteristics that fulfill different roles in tumor growth and a small proportion of the cells within a tumor named cancer stem cells (CSCs) are predicted as a critical population of tumor progression. In recent years, the CSC theory has received much attention, and the CSC model can explain clinical events such as therapy resistance, metastasis, and tumor recurrence. The CSC model presents tumor as a hierarchically organized tissue with CSC population at the top that generates the more differentiated bulk of the tumor cells. The first identification of cancer stem-like cells in acute myeloid leukemia was soon followed by similar results in solid malignancies, including colorectal, breast, brain, prostate, liver, and pancreas [1–4]. CSCs share important properties with normal stem cells, including self-renewal and differentiation capacity. They can produce heterogeneous lineages of cancer cells and can cause expansion of fully differentiated tumor cells [5, 6]. CSCs are both structurally and functionally distinct from the other cells within a tumor mass and are regarded as playing a central role in tumor progression. They are characterized by their resistance to anticancer therapeutics and are the root of tumor metastasis and recurrence [7]. Current radio- and chemotherapies kill the bulk of cancer cells but often are not able to eliminate the critical CSCs, which are protected by specific resistance mechanisms.
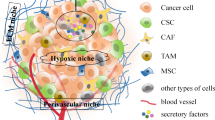
Solid tumors are regarded as “organs” which are comprised of cancer cells and the tumor microenvironment. The tumor microenvironment includes the extracellular matrix (ECM), mesenchymal stem cells (MSCs), endothelial cells, immune cells, and, what is more, networks of cytokines and growth factors [8]. CSCs also require a special microenvironment which is known as “CSC niche” to regulate their stemness and proliferation and save stem cells from depletion [9, 10]. There is now overwhelming evidence that CSCs interplay closely with the tumor microenvironment and disrupting this niche microenvironment impairs CSC self-renewal and thereby significantly inhibits the growth of tumors. Some cancer cells require coinjection with stromal cells to form tumors, indicating that CSCs are supported by their surrounding microenvironmental factors [11]. CSCs may promote tumor angiogenesis, and significant therapeutic advantage can be achieved by treatment with an angiogenesis inhibitor. It is known that CSCs not only merely adapt to the existing niche but more aggressively generate such environment. Understanding of the interaction between CSCs and their niche could be a paradigm shift in the treatment of cancer, thus improving therapeutic outcome (Fig. 1).
Schematic representation of interactions between cancer stem cells and their tumor microenvironment. Tumor progression requires a cooperative interplay between CSCs and their niche. The reciprocal feedback between CSCs and ECM molecules, stromal cells, and, what is more, networks of cytokines and exosomes enhances the proliferation and survival of CSCs and also has a direct effect on cancer cell malignancy. This figure highlights the cell types and molecules mentioned in this review
Properties of the ECM in the cancer stem cell niche
Analogous to the regulation of normal stem cells by their “niche,” CSCs may represent plasticity whose phenotype and function are continuously modulated by the tumor microenvironment. The niche must have both anatomic and functional dimensions, which specifically enable CSC reproduction or self-renewal [10].
ECM is secreted molecules, composed of biochemically distinct components including protein, glycoprotein, proteoglycan, and polysaccharide which have different physical and biochemical properties. ECM is versatile, not only maintains tissue structure but also performs a diverse set of regulatory functions. As a major component of the microenvironment, ECM takes part in most basic cell behaviors, from cell proliferation, migration, to cell differentiation. Also, ECM is likely to play important roles in tumor angiogenesis and lymphangiogenesis [12, 13].
Mounting evidences suggest that ECM is an essential noncellular component of the adult stem cell niche [12]. Contact with ECM is necessary for cells to acquire or maintain stem cell properties. In contrast, the loss of ECM contact reduces the number of stem cells in different vertebrate and invertebrate systems [12]. In solid tumors, ECM has been reported to be a physical barrier affecting the effectiveness of therapeutics through blocking of intratumoral diffusion, and this can protect the CSCs away from chemotherapeutic agents [14]. It is shown that both tumor cell differentiation and CSC characteristics are influenced by the mechanical properties of the niche. In hepatocellular cancer, increasing matrix stiffness promotes proliferation and chemotherapeutic resistance, whereas surviving cells from soft supports had significantly higher clonogenic capacity with enhanced expression of CSC-related markers, including CD44, CD133, c-kit, CXCR4, OCT4, and NANOG [15].
ECM is an obvious barrier to migrating cells, such as cancer cells, MSCs, and immune cells, while being a promoter of cellular movement after cleavage and remodeling [13]. Linearized cross-linked collagen bundles potentiate cell migration, whereas a dense network of stiff cross-linked matrix fibers impedes migration [12]. ECM dynamics may result from changes of the absolute amount or composition of ECM. Its anomalies also deregulate the behavior of tumor stromal cells, facilitate tumor-associated angiogenesis and inflammation, and thus lead to generation of a tumor microenvironment [12].
Key cellular players of the cancer stem cell niche
Plenty of efforts have been devoted to determining how cellular components of the tumor microenvironment initiate and promote cancer development. CSCs are considered to form their own microenvironment by recruiting and activating specific cell types. MSCs are one of the critical components which secrete a variety of cytokines that have both paracrine and autocrine functions in the tumor milieu. They are immunoprivileged, serve significant roles in cases of malignancy, and provide an advantageous tumor microenvironment for the restoration of CSCs. Breast CSCs can produce interleukin-6 which attracts and activates MSCs to produce the CSC supportive cytokine CXCL7 [16]. MSCs promote tumor growth and favor angiogenesis in the ovarian tumor niche [17]. Also, MSCs have been implicated in tumorigenesis through multiple mechanisms, including promoting cancer cell proliferation and metastasis and fostering angiogenesis, in addition to the generation of an immunosuppressive microenvironment [18, 19].
Angiogenesis is a key factor in the growth and dissemination of cancer, with significant implications for its clinical management [20]. The connection between CSCs and endothelial cells seems to be critical. Endothelial cells may impact biological behaviors of CSCs in the tumor microenvironment by direct interaction with tumor cells as well as producing factors [21]. Vascular endothelial cells of the niche can maintain the stem-like state of brain CSCs; in turn, CSCs promote angiogenesis through the release of vascular endothelial growth factor (VEGF) and stromal-derived factor 1 [22, 23]. In head and neck squamous cell carcinomas, endothelial cell-secreted factors promote self-renewal of CSCs, as demonstrated by the upregulation of Bmi-1 expression [24]. The soluble form of Jagged-1, which is produced by endothelial cells, leads to Notch activation and thus promotes the CSC phenotype in colorectal cancer [25]. Endothelial cells are potent inducers of the epithelial-mesenchymal transition in breast epithelial cells with stem cell properties [26]. The combination of chemotherapy and antiangiogenic therapy is able to reduce the number of CSCs significantly.
The immune system has important roles in limiting the spread of tumor. However, an immunosuppressive tumor microenvironment may eliminate the role of the immune system. Tumor-infiltrating immune cells can promote chemoresistance and metastatic spread in aggressive tumors. Tumor-associated macrophages (TAM), the distinct alternatively activated M2 polarized population, have been shown to promote tumor angiogenesis, invasion, and metastasis in addition to their immunosuppressive role [27, 28]. Reduction of the number of TAM can decrease the number of CSCs in pancreatic tumors [29]. In both human glioma samples and animal models, the distribution of TAM in the marginal area correlated with the location of CD133+ glioma cells, and TAM can significantly enhance the invasive capability of glioma stem cells through paracrine production of TGF-β1 [30]. Interestingly, CSCs are the major subset which can render macrophages with the ability to facilitate the production of tumorigenic factors such as MFGE8 and IL-6 [21]. Regulatory T cell (Treg) recruitment plays a pivotal role in forming a VEGF-A-rich microenvironment and facilitating angiogenesis. On the contrary, CSCs also impact on Treg mainly through their recruitment and function and also regulate Tregs through a cell-to-cell contact mechanism induced by B7-H1, a group of costimulatory inhibitory molecules [31].
Cytokine networks are important regulators
The CSCs are regulated by complex interactions with the components of the tumor microenvironment. Cytokines play pivotal roles in intercellular communication, and many of them regulate the stem cell phenotype in a variety of contexts including tumor. Cells in the tumor microenvironment can produce factors that include VEGF, TGF-β, matrix metalloproteinases (MMPs), FGF, HGF, EGF, SDF-1, IGF, PDGF, Wnt, Notch ligand, and Hedgehog ligands, which stimulate CSC self-renewal, induce angiogenesis, and recruit TAM, neutrophils, and mast cells, which secrete additional growth factors that promote tumor cell invasion and metastasis [32–34].
Brain CSCs are able to promote recruitment and formation of blood vessels by secreting high levels of VEGF which is the primary mediator in proangiogenic factors in this region of the brain [35, 36]. In turn, exogenous VEGF stimulates CSC proliferation via VEGFR2, while VEGFR1 has a negative feedback effect on VEGFR2 when cells are exposed to a higher concentration of VEGF [37]. VEGF promotes tumor progression in different tumor models in an autocrine and paracrine manner. Recent evidence indicates that tumor angiogenesis may be induced by CSCs due to angiogenic factor expression in the tumor microenvironment. Antiangiogenesis therapy targeting VEGF can deplete the tumor vasculature and ablate self-renewing CSCs [38].
Recent evidence shows that cells which undergo epithelial-mesenchymal transition (EMT) acquire stem cell-like properties [39]. TGF-β elicits tumor-promoting effects through its ability to induce EMT; in addition, it exerts suppression of immune surveillance and stimulation of angiogenesis via effecting on nonmalignant cells of the tumor [40]. TGF-β is a ubiquitous cytokine that plays an active role in many cellular processes and it has opposing roles. Normal cell types have the ability to secrete TGF-β which functions as a tumor suppressor, and conversely, in tumor contexts, TGF-β promotes tumor cell invasion and metastasis [41]. In glioma, CSCs can become more invasive upon treatment with TGF-β [30]. TGF-β can increase the number of CSCs through inducing EMT. Accumulating evidence has demonstrated that TGF-β is often elevated in the tumor microenvironment and associated with advanced tumor staging and in the regulation of CSC function, and CSC phenotypes can be abrogated by the novel TGF-β-targeting peptides. Furthermore, TGF-β can play as a chemoattractant for infiltrating macrophage into the tumor microenvironment. Macrophage in the niche is known to promote tumor invasion and metastasis through facilitating angiogenesis and extracellular matrix breakdown, as well as reinforcing the immunosuppressive environment through further release of TGF-β [41].
Remodeling of the ECM is important in both physiological and pathological processes, including angiogenesis, inflammation, and tumor growth. MMPs are a family of zinc-dependent endopeptidases that primarily degrade components of ECM and further facilitate angiogenesis, tumor cell invasion, and metastasis [42]. Also, MMPs constitute key players in releasing cytokines, growth factors, and other cell surface molecules [43]. MMP10 is highly expressed in CSCs, and repression of MMP10 leads to a loss of stem cell-related gene expression. A strong positive correlation is between tumor MMP10 expression and metastatic behavior in many human tumor types [44].
The cancer stem cell niche, hypoxia, and HIF
Hypoxia is one of the fundamental biological phenomena that are intricately associated with a variety of solid tumors and can enhance the phenotypes of CSCs. It is an essential feature of the tumor microenvironment because of the chaotic vasculature and poor oxygen diffusion in solid cancers. Increasing evidence propose that CSCs exist in hypoxic microenvironment, which may be beneficial for the maintenance of these cells in virtually all tissues of the body. Hypoxia is a good indicator of disease outcome because of selecting the most invasive cancer cells and promotes resistance to therapies [45].
Hypoxia is a predominant feature of glioblastomas which can promote the self-renewal capability of glioblastoma cells with upregulation of important stem cell factors, such as OCT4, NANOG, and c-Myc, and slow down the growth of glioblastoma cells which are in a relatively quiescent stage, increasing the colony-forming efficiency and migration of glioblastoma cells [46]. Hypoxia also enhances immunosuppression through potentiating the CSC-mediated inhibition of T cell proliferation and activation in glioma and further inhibiting macrophage phagocytosis compared to a normoxia condition [47]. Hypoxia affects not only tumor cell autonomous functions, such as invasion and resistance to therapy, but also nonautonomous processes, such as angiogenesis. The reason why hypoxia poses a challenge to the field of anticancer therapeutics is that it provides a niche for quiescent, drug-resistant cells, which may be identical to CSCs [48].
Hypoxia-inducible factor (HIF) orchestrates the expression of a wide variety of genes thought to be critical for adaptation to low oxygen [49]. Among the several angiogenic growth factors, the HIF gene family is underscored as a prime driving factor for the angiogenic switch. It becomes clear that tumors harbor a myriad of ways to build their own vascular network. HIF1α is required for migration and invasion and is able to induce VEGF and VEGFR2. HIF1α expression is correlated significantly with microvessel density, which suggests that HIF1α may play a role in angiogenesis and tumor progression via regulation of VEGF in colorectal cancer [50]. Several studies have verified that hypoxia and high HIF1α can promote the EMT phenotype [51]. Moreover, as a potential target for tumor therapy, HIF1α can eliminate toxic metabolic waste products generated which occur during hypoxia [52]. HIF2α, which is essential only in CSCs, and multiple HIF-regulated genes are preferentially expressed in CSCs in comparison to differentiated tumor cells [53]. Analysis of a molecular database reveals that HIF2α expression correlates with poor glioma patient survival and may represent a promising target for therapy [54]. Moreover, in breast cancer, hypoxia can promote metastasis through activation of HIF which mediates paracrine signaling between cancer cells and MSCs [55].
Exosomes: biogenesis and functions
Most studies focus on the cytokines or enzymes, through which the intercellular communication is mediated. Intercellular exchange of bioinformation-containing exosomes/microparticles is an increasingly important mode of cell-cell communication [56]. Exosomes are lipid vesicles that are <1 μm in diameter and are secreted by many cells, including MSCs, macrophages, some cancer cells, dendritic cells, B cells, T cells, mast cells, and endothelial cells. They are membrane vesicles equipped to mediate intercellular communication via the transfer of information, which are released following a process of budding and detachment from donor cells [57]. Bidirectional exchange of genetic information between cancer cell horizontal and the niche plays an important role to maintain the tumor. By facilitating the transfer of bioactive molecules such as mRNAs and microRNAs (miRNAs), they are now thought to have vital roles in tumor invasion and metastasis, inflammation, and stem cell renewal [58]. Multidrug resistance (MDR) is caused by the overexpression of the efflux transporters P-glycoprotein (P-gp) and multidrug resistance-associated protein 1 (MRP1). Intercellular transfer of functional P-gp via exosomes is a novel mechanism for the acquisition of MDR in cancer cells [59].
Nowadays, greater attentions come into miRNAs due to their regulatory effects on cancer cell progression. miRNAs are small, evolutionarily conserved, noncoding RNAs of 18–25 nucleotides in length that have an important function in gene regulation and can be deregulated in cancer [60]. miRNAs have been shown to act as tumor suppressors or oncogenes and also been found to play important roles in normal and CSC function. miR-328 inhibits the number of SP cells and reverses drug resistance in colorectal cancer [61]. miR-130b exhibits an increased ability of proliferation, self-renewal, and resisting standard chemotherapy in hepatocellular CSCs [62]. miR-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian CSCs [63]. Increased miR-200c level can significantly inhibit the malignant CSC-like properties of ALDH (+)/CD44 (+) cells in head and neck cancer [64]. miRNAs exert a broad regulatory role on tumor development; therefore, miRNA-based therapeutics that specifically target CSCs establish a strong rationale for effective cancer therapies.
More recent data have demonstrated that macrophages are able to produce exosomes which shuttle miRNAs into adjacent tumor cells within the microenvironment and miR-223 is found increasing in breast cancer cells following coculture with TAM [65, 66]. MSCs also can facilitate miRNA-mediated intercellular communication by secreting pre-miRNA-enriched exosomes [67]. In the tumor microenvironment, miRNAs can be engulfed by immune cells surrounding cancer cells and bind to toll-like receptors (TLR8). As a result, the binding miRNAs lead to NF-κB signaling activation and promote cancer cell growth and metastasis [68]. There is an intriguing possibility that stem cells can alter the expression of genes in neighboring cells by transferring miRNAs contained in exosomes [69].
There are two main hypotheses about the cellular uptake of miRNAs. For exosomes containing miRNAs, it has been proposed that they are delivered to cells either by a process involving endocytosis or by membrane fusion [70, 71]. Exosomes may be useful therapeutic tools for transferring mRNA, miRNAs, and protein to cells and may be important mediators of signaling within the CSC niche. In addition, the niche microenvironment seems to be of crucial importance, and the interaction between CSCs and their niche microenvironments creates the dynamic system necessary for the ultimate design of cancer therapeutics [35, 72].
Summary and clinical implications
The CSC theory is tremendously attractive to both researchers and physicians, because the CSCs are central to cancer cell biology and cancer therapy. The proposed plasticity of CSCs suggests that not only CSCs produce more differentiated descendants but also non-CSCs can be converted to CSCs through EMT mediated by microenvironmental signals [73]. Cancer development is not solely a cell-intrinsic process driven by the collection of epigenetic or genetic mistakes in transformed cells, but one that also depends on their microenvironments. The microenvironment surrounding CSCs plays multiple roles including as a mechanical anchorage for the stem cells and in cross-talk communication mediated by direct contact and/or indirect extracellular factors. CSCs protect their niche and, vice versa, the niche contributes to enhance therapy resistance of CSCs [74, 75].
We anticipate that a therapeutic benefit may be gained by targeting several of the described components of the microenvironment including depleting the ECM, antiangiogenesis, and elimination or reprogramming of the immunosuppressive tumor microenvironment. Antiangiogenesis agents are opening new avenues for targeted cancer therapy. Interfering with tumor endothelial cell growth and survival could inhibit not only angiogenesis but also the self-replication of CSCs [76]. Thus, therapeutic targeting of the vasculature could disrupt the CSC niche microenvironment as well as simply achieving tumor eradication. A rapidly developing field in cancer therapeutics is targeting miRNAs, which exhibit aberrant levels in cancer cells. Functional studies of specific miRNA regulation involving CSCs and their microenvironment are necessary to find a therapeutic target. Moreover, as we mentioned above, pro-dedifferentiation and CSC maintenance cytokine blockade in the tumor microenvironment could attenuate tumor formation. Our research found that IFN-γ showed synergistic effects with the conventional anticancer drug oxaliplatin to eliminate both CSCs and differentiated cancer cells in colorectal cancer [75]. Therefore, understanding the cross talk between CSCs and the niche may help us design new therapeutic strategies and pave the way for the development of newer strategies for treating cancer [8].
References
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8.
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7.
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8.
Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annual Rev Med. 2007;58:267–84.
Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–5.
Hatfield S, Ruohola-Baker H. microRNA and stem cell function. Cell Tissue Res. 2008;331:57–66.
Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–9.
Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78.
Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development. 2007;134:2001–6.
Li L, Cole J, Margolin DA. Cancer stem cell and stromal microenvironment. Ochsner J. 2013;13:109–18.
Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406.
Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspectives in Biology 2011;3. doi:10.1101/cshperspect.a005058.
Wong GS, Rustgi AK. Matricellular proteins: priming the tumour microenvironment for cancer development and metastasis. Br J Cancer. 2013;108:755–61.
Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205.
Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–24.
Waterman RS, Henkle SL, Betancourt AM. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PloS One. 2012;7:e45590.
Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adhes Migr. 2012;6:220–30.
Nishimura K, Semba S, Aoyagi K, Sasaki H, Yokozaki H. Mesenchymal stem cells provide an advantageous tumor microenvironment for the restoration of cancer stem cells. Pathobiol : J Immunopathol, Mol Cell Biol. 2012;79:290–306.
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6.
Jinushi M, Baghdadi M, Chiba S, Yoshiyama H. Regulation of cancer stem cell activities by tumor-associated macrophages. Am J Cancer Res. 2012;2:529–39.
Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–4.
Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8.
Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70:9969–78.
Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–85.
Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, Fridriksdottir AJ, Ringner M, Villadsen R, et al. Endothelial induced EMT in breast epithelial cells with stem cell properties. PloS One. 2011;6:e23833.
Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7.
Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–52.
Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–41.
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189:444–53.
Yu X, Li H, Ren X. Interaction between regulatory T cells and cancer stem cells. Int J Cancer J Int du Cancer. 2012;131:1491–8.
Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Investig. 2010;120:485–97.
Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Investig. 2007;117:3988–4002.
Ma Y, Liang D, Liu J, Axcrona K, Kvalheim G, Giercksky KE, et al. Synergistic effect of SCF and G-CSF on stem-like properties in prostate cancer cell lines. Tumour Biol : J Int Soc Oncodevelopmental Biol Med. 2012;33:967–78.
Yi SY, Hao YB, Nan KJ, Fan TL. Cancer stem cells niche: a target for novel cancer therapeutics. Cancer Treat Rev. 2012;39:290–6.
Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–62.
Xu C, Wu X, Zhu J. VEGF promotes proliferation of human glioblastoma multiforme stem-like cells through VEGF receptor 2. Sci World J. 2013;2013:417413.
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z, et al. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PloS One. 2013;8:e60687.
Ye J, Wu D, Shen J, Wu P, Ni C, Chen J, et al. Enrichment of colorectal cancer stem cells through epithelial–mesenchymal transition via CDH1 knockdown. Mol Med Rep. 2012;6:507–12.
Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–70.
Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-β pathway for cancer therapy. Clin Cancer Res : Off J Am Assoc Cancer Res. 2012;18:4514–21.
Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012;20:210–6.
Noel A, Gutierrez-Fernandez A, Sounni NE, Behrendt N, Maquoi E, Lund IK, et al. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front Pharmacol. 2012;3:140.
Justilien V, Regala RP, Tseng IC, Walsh MP, Batra J, Radisky ES, et al. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PloS One. 2012;7:e35040.
Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2013. doi:10.1038/onc.2013.121
Li P, Zhou C, Xu L, Xiao H. Hypoxia enhances stemness of cancer stem cells in glioblastoma: an in vitro study. Int J Med Sci. 2013;10:399–407.
Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, et al. Hypoxia potentiates glioma-mediated immunosuppression. PloS One. 2011;6:e16195.
Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res : Off J Am Assoc Cancer Res. 2012;18:4266–76.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85.
Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer J Int du Cancer. 2003;105:176–81.
Gammon L, Biddle A, Heywood HK, Johannessen AC, Mackenzie IC. Sub-sets of cancer stem cells differ intrinsically in their patterns of oxygen metabolism. PloS One. 2013;8:e62493.
Kappler M, Taubert H, Schubert J, Vordermark D, Eckert AW. The real face of HIF1α in the tumor process. Cell Cycle. 2012;11:3932–6.
Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–84.
Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer cell. 2009;15:501–13.
Chaturvedi P, Gilkes DM, Wong CC, Luo W, Zhang H, Wei H, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Investig. 2013;123:189–205.
Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–60.
Jaiswal R, Luk F, Gong J, Mathys JM, Grau GE, Bebawy M. Microparticle conferred microRNA profiles—implications in the transfer and dominance of cancer traits. Mol Cancer. 2012;11:37.
Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Science. 2010;123:1603–11.
Jaiswal R, Luk F, Dalla PV, Grau GE, Bebawy M. Breast cancer-derived microparticles display tissue selectivity in the transfer of resistance proteins to cells. PloS One. 2013;8:e61515.
Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–55.
Xu XT, Xu Q, Tong JL, Zhu MM, Nie F, Chen X, et al. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like SP cells in colorectal cancer. British J Cancer. 2012;106:1320–30.
Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, et al. miR-130b promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7:694–707.
Cheng W, Liu T, Wan X, Gao Y, Wang H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012;279:2047–59.
Lo WL, Yu CC, Chiou GY, Chen YW, Huang PI, Chien CS, et al. MicroRNA-200c attenuates tumour growth and metastasis of presumptive head and neck squamous cell carcinoma stem cells. J Pathol. 2011;223:482–95.
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leuk : Off J Leukemia Soc Am, Leuk Res Fund, UK. 2006;20:1487–95.
Yang M, Chen J, Su F, Yu B, Lin L, Liu Y, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117.
Jing Y, Han Z, Liu Y, Sun K, Zhang S, Jiang G, et al. Mesenchymal stem cells in inflammation microenvironment accelerates hepatocellular carcinoma metastasis by inducing epithelial–mesenchymal transition. PloS One. 2012;7:e43272.
Fabbri M. TLRs as miRNA receptors. Cancer Res. 2012;72:6333–7.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PloS One. 2009;4:e4722.
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66.
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66.
Chumsri S, Phatak P, Edelman MJ, Khakpour N, Hamburger AW, Burger AM. Cancer stem cells and individualized therapy. Cancer Genomics Proteomics. 2007;4:165–74.
Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012;30:363–71.
Sottoriva A, Sloot PM, Medema JP, Vermeulen L. Exploring cancer stem cell niche directed tumor growth. Cell Cycle. 2010;9:1472–9.
Ni C, Huang J. Dynamic regulation of cancer stem cells and clinical challenges. Clin Transl Oncol : Off Publ Fed Span Oncol Soc Nat Cancer Inst Mex. 2013;15:253–8.
Gu JW, Rizzo P, Pannuti A, Golde T, Osborne B, Miele L. Notch signals in the endothelium and cancer “stem-like” cells: opportunities for cancer therapy. Vasc Cell. 2012;4:7.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no. 91019005, no. 81272672) and Zhejiang Provincial Natural Science Foundation of China (no. Y2110034).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jun Ye, Dang Wu, and Pin Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ye, J., Wu, D., Wu, P. et al. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumor Biol. 35, 3945–3951 (2014). https://doi.org/10.1007/s13277-013-1561-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1561-x