Abstract
The development of efficient catalysts in terms of activity and selectivity has always been a major topic for researchers since several decades. The use of colloidal metallic nanoparticles received an increasing interest due to their large surface area leading to high catalytic activities. The main problems for these nanoparticles arise from the control of their size and also their aggregation before and during the catalytic process. In this context, the use of cyclodextrins as protective agents proved to be effective and allowed the rise of a large variety of catalytic systems at the nanoscale.
In this chapter, we reviewed all the articles related to the metallic nanoparticles synthesized in the presence of cyclodextrin for catalytic applications since the 1980s to early 2020. The major points are (1) the possibility of development of metal nanoparticles in solution or immobilized onto a support in the presence of cyclodextrin; (2) the multiple roles of cyclodextrin such as reducing agent, mass transfer agent, and stabilizing/dispersing agent leading to the increase of the stability of metal nanoparticles and better catalytic activities or specific selectivities; and (3) the control and the use of more complex catalytic systems where cyclodextrin is playing the main role as a supramolecular host.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- History
- Catalysis
- Metal nanoparticles
- Cyclodextrin-assisted synthesis
- Cyclodextrin derivatives
- Structures
- Inclusion complexes
- Supramolecular chemistry
5.1 Introduction
Metal nanocatalysts are an interesting research field for the scientific community due to the high control of both the size and the shape of the metal nanoparticles in order to find a good compromise between stability and reactivity of the catalytic system. Metal nanoparticles can be synthesized through two approaches: the fragmentation of a bulk metal, called the top-down approach, or the chemical transformation of a metal precursor, called the bottom-up approach. The key parameter of a metal nanoparticle-based catalyst, in solvent-dispersed form or immobilized on a support, is the choice of the stabilizing agent which ensures the good dispersion of the active phase with the desired particle size. Taking into account this, the need to develop more eco-friendly metal nanoparticle synthesis without harmful solvents, the use of water-soluble capping agents has grown since the beginning of the twenty-first century. Ammonium salts, phosphanes, dendrimers, polymers, or oligosaccharides are generally found in the literature and show interesting results in terms of catalytic activity and stability. Among these capping agents, cyclodextrins are interesting candidates in the field of nanocatalysis due to their low cost, non-toxicity, high capacity to interact with metal ions, and the possibility to form inclusion complexes with reactants in order to bring them close to the active site.
According to a bibliographic survey up to 2020 involving cyclodextrin, catalysis, and metal nanoparticles as keywords, a huge number of publications have been found. Therefore, we decided to restrict our study to the cases where cyclodextrins are present during the catalytic process using metal nanoparticles, either in solvent-dispersed form or immobilized on a support, as active phase. This means that the works dealing with cyclodextrin-assisted syntheses of supported metal nanoparticles including a calcination/carbonization step or a washing step to remove the cyclodextrin have not been considered here.
This chapter is divided into two distinct parts. In the first part, we focus on the publications concerning the solvent-dispersed nanoparticles. Several parameters such as the size of the cavity, the functionalization of the cyclodextrin rims, and the presence of co-stabilizers (surfactants, phosphanes, or polymers) or cyclodextrin-based polymers have been studied to get stable, active, and recyclable nanoheterogeneous catalysts. Cyclodextrins can be either added during the nanoparticle synthesis or after the metal nanoparticle synthesis and can consequently play the role of mass transfer agent to bring the substrate in the vicinity of the active species and improve the activity and/or the selectivity. The second part is dedicated to catalysts with nanoparticles immobilized onto and/or into a support matrix in the presence of cyclodextrins. In this case, different strategies are discussed for the synthesis of heterogeneous catalysts consisting of nanoparticles immobilized on a support, such as inorganic supports, carbonaceous materials, or polymers, in the presence of cyclodextrins either non-covalently grafted or covalently grafted on the supports. Nanoparticles can be synthesized (i) before their adsorption/incorporation onto or into the support or (ii) in the presence of the support and also of the cyclodextrins. It should be noticed that the structure of the cyclodextrin remains intact whatever the strategy. Consequently, in line with the first part, the relationships between the supramolecular structures and their activities are discussed, and the different roles of the cyclodextrin are further underlined.
5.2 Nanoparticles Stabilized by Cyclodextrins in Solution
5.2.1 Nanoparticles Stabilized by Native Cyclodextrins
The first example of the synthesis of metal nanoparticles stabilized by native cyclodextrins (α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin) was reported by Komiyama and Hirai (1983). An aqueous Rh(III) salt solution in the presence of native cyclodextrin and ethanol was refluxed in order to give Rh nanoparticles. The stability of the resulting particles was associated to the ability of the cyclodextrin to prevent aggregation via strong hydrophobic interactions between the cyclodextrin cavity and the metal surface. β-cyclodextrin gave the best colloidal dispersion with a Rh average diameter of 2.8 nm. When γ-cyclodextrin was used, no colloidal suspension was observed, and sedimentation took place. The resulting Rh particles stabilized by β-cyclodextrin were then evaluated in the catalytic hydrogenation of water-soluble α,β-conjugated carbonyls under mild experimental conditions (30 °C, 1 bar of hydrogen) with the catalytic activities ranging from 0.06 to 0.24 molH2 g(Rh)−1 s−1. The role of β-cyclodextrin was highlighted by observing no activity for the hydrogenation of 3-buten-2-one in the presence of cyclohexanol that was a competing guest forming an inclusion complex with β-cyclodextrin.
The ability of β-cyclodextrin to stabilize solvent-dispersed metal colloids was also observed by Willner in 1987 by synthesizing TiO2 and CdS nanoparticles with, respectively, an average diameter of 8 nm and 10 nm (Mandler and Willner 1987). The catalytic activity of the abovementioned semiconductor particles was evaluated in the photoreduction of a relay molecule (N, N′-dioctyl-4, 4′-bipyridinium) with semiconductor particles (Fig. 5.1). An increase of the local concentration of the relay in the vicinity of the semiconductor interface was explained by the association of this relay with the cavity of the β-cyclodextrin (Kass = 5.6 103 M−1). Moreover, inhibition experiments using phenol as substrate were performed where phenol was associated to β-cyclodextrin, leading to a bad electron transfer.
Supposed operation of the receptor-semiconductor colloid. The interfacial electron transfer has been improved from the excited semiconductor to the relay substrate because of the increase of the local concentration of the relay substrates at the semiconductor interface due to the association of the relay with the β-cyclodextrin. (Adapted from Willner and Mandler 1987)
The same authors studied the synthesis of palladium nanoparticles stabilized by β-cyclodextrin by warming an aqueous solution of Na2PdCl4 containing β-cyclodextrin (1% in weight) at 60 °C via the classical polyol process (Willner and Mandler 1989). The non-reduced palladium was removed by addition of an Amberlyst® resin, and the resulting colloids were centrifuged to separate the precipitated palladium colloids. This preparation resulted in an active catalyst for the photosensitized reduction of sodium bicarbonate to sodium formate by visible light in the presence of deazariboflavin as photosensitizer, N, N′-dimethyl-4,4′-bipyridinium as the first electron donor, and sodium oxalate as the sacrificial electron donor. After kinetic studies, the authors emphasized the biomimetic character of these colloids acting as artificial enzymes. By comparing their Pd nanoparticles to other Pd nanoparticles stabilized by classical agents such as glucose or poly(N-vinyl-2-pyrrolidone), the authors clearly showed that the best catalytic activity was obtained with their colloids.
Despite numerous studies concerning the synthesis of gold nanoparticles in the presence of cyclodextrins, only a few of them was dedicated to catalytic applications. The first catalytic application was reported in 2009, when the group of Qi synthesized α-cyclodextrin-capped gold nanoparticles by reduction of HAuCl4 in alkaline solution at 60 °C where α-cyclodextrin was playing the dual role of reducing agent of the metal precursor and stabilizing agent of Au nanoparticles (Huang et al. 2009). As in the case of palladium, Au(III) can be reduced via the polyol process in alkaline medium using the cyclodextrin hydroxyls as reductant. The concentration of α-cyclodextrin was the key parameter to get the smallest particles with a narrow size distribution; the higher the α-cyclodextrin concentration, the smaller the nanoparticles. The sodium hydroxide concentration played also an important role. For pH values lower than 10.5, α-cyclodextrin failed to reduce the gold precursor, but too high pH (pH = 12) led to the irreversible agglomeration of Au nanoparticles. These colloidal suspensions were effective hydrogen activators for the reduction of 4-nitrophenol to 4-aminophenol with a large excess of sodium borohydride. The conversion was determined by the time-dependent decay of the 4-nitrophenol absorbance at 400 nm. The kinetic reaction rate constants were inversely proportional to the particle size. These results pointed out that these α-cyclodextrin-capped Au nanoparticles showed catalytic activity and that cyclodextrin did not disturb the metal surface.
More recently, monodisperse Au nanoparticles with diameter of 15–20 nm were synthesized by the polyol process (100 °C in a phosphate buffer solution) using native cyclodextrins (α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin) as both reducing agents of metal precursor and protective agents of Au colloidal suspensions. These Au nanoparticles were used for applications in fluorescent sensing, self-assembly, and cascade catalysis (Zhao et al. 2016). The FTIR analysis clearly showed the decrease of the intensity of the hydroxyl group absorbance band. In the same time, the appearance of carboxyl groups was observed by X-ray photoelectron spectroscopy due to the oxidation of the cyclodextrins. All of these analyses respectively justified the reduction of the gold precursor in the presence of cyclodextrins and the stabilization of the resulting Au nanoparticles through the carboxylate functions. In addition to the conventional host-guest interaction-based properties, the gold nanoparticles stabilized by these cyclodextrins showed interesting catalytic activities and exhibited mimicking properties of both glucose oxidase and horseradish peroxidase. Especially, the cascade reaction (oxidation of glucose with generation of gluconic acid and H2O2 followed by the oxidation of TMB (3,3′,5,5′-tetramethylbenzidine)) was well-achieved using the Au nanoparticles as the sole catalyst.
Several cucurbit[n]urils and native cyclodextrins were used as stabilizers of metastable gold nanoparticles, i.e., gold nanoparticles synthesized by chemical reduction of Au(III) with NaBH4 and then the addition of the desired macromolecules (del Pozo et al. 2018). Whatever the receptor, transmission electron microscopy images showed spherical gold nanoparticles which, on the one hand, were organized into non-ordered structures with cyclodextrins and, on the other hand, were homogeneously dispersed in the case of cucurbit[n]urils with mean diameters ranging from 5.2 nm to 10.7 nm. The catalytic activity of these colloidal suspensions was evaluated in the reduction of 4-nitrophenol using NaBH4 as reducing agent in the presence of these gold nanoparticles. The different stabilizers were compared by evaluating the catalytic activities with normalized rate constants depending on the gold amount (named kc) or depending on the total gold surface (named ks) or by determining a loss of efficiency after 2 months (Table 5.1).
The metastable gold nanoparticles, i.e., the particles with no added stabilizer, showed the highest catalytic activities which can be explained by weak interactions between the boron species and the gold nanoparticles. Nevertheless, a significant loss of performance was observed after keeping this colloidal suspension during 1 month under stirring. It clearly showed that the addition of a stabilizing agent was necessary to keep a long-term stability and also a good catalytic activity. Finally, the efficiency of the catalysts was explained by the surface coverage of nanoparticles obtaining high reaction rates with low surface coverage.
Other metal nanocatalysts were prepared using the polyol process strategy with native cyclodextrins. For example, Li et al. (2017) studied the synthesis of silver nanoparticles in alkaline medium in the presence of β-cyclodextrin at room temperature. FTIR analysis of the resulting Ag nanoparticles showed a characteristic peak at 1647 cm−1, which corresponds to the oxidation of hydroxyl groups during the polyol process. In addition, slight red shift compared with normal vibration wavelength indicated the coordination of β-cyclodextrin onto the surface of the Ag nanoparticles. Interestingly, the key parameters to get small particles with a narrow distribution (pH value, temperature, and β-cyclodextrin/Ag molar ratio) are the same as in the study of Qi concerning the gold nanoparticles. According to the authors, not only the cyclodextrin deprotonation led to the formation and the stabilization of the silver nanoparticles but also the insoluble silver oxide formation in alkaline medium which allowed a better control of the growth of the silver nanoparticles. The authors also found that the optimal synthesis temperature was 35 °C. The intensity of absorption peak increased with the temperature, but very high temperature led to Ag2O decomposition and the loss of the control of the particle growth. For the catalytic results, the authors suggested that H bonding interactions between the cyclodextrin and the substrate are the key factor. These Ag nanoparticles that were used in the catalytic reduction of 4-nitrophenol have proven to be more active than traditional Ag nanoparticles stabilized by sodium citrate. This better catalytic activity was explained by hydrogen bonds between 4-nitrophenol and the hydroxyl groups of β-cyclodextrin which allowed a better diffusion of the substrate onto the surface of Ag nanoparticles.
Another study developed γ-cyclodextrin-capped Ag nanoparticles for the enhancement of the antibacterial efficiency of chloramphenicol (Gannimani et al. 2016). The formation of nanoparticles was confirmed by UV-Vis spectroscopy and the appearance of the surface plasmon resonance band at 412 nm. The formation of inclusion complexes was studied by 1H NMR. The observed changes in the shifts could be considered as direct evidence of the existence of host-guest non-covalent interactions between γ-cyclodextrin and chloramphenicol. Moreover, an increase of the antibacterial activity of chloramphenicol was observed when it was used in combination with γ-cyclodextrin-capped Ag nanoparticles because of supramolecular interactions leading to the immobilization of chloramphenicol onto the Ag nanoparticle surface.
Using simple, economical thermal pyrolysis approach and tin(II) stearate as eco-friendly organometallic precursor, SnO2 quantum dots with mean diameters less than 10 nm were synthesized (Haw et al. 2016) and used in both aqueous and non-aqueous media thanks to β-cyclodextrin employed for surface-ligand exchange. It was suggested that the formation of inclusion complexes between stearate-stabilized quantum dots and β-cyclodextrin could allow the phase transfer from the non-aqueous phase to the aqueous phase. Moreover, the use of the SnO2 quantum dots for the photocatalytic hydrogen gas evolution was studied and compared with commercial SnO2 nanoparticles. The results demonstrated higher photocatalytic activity of the former compared to the latter (~31.3% higher yield of hydrogen). The higher photocatalytic activity of SnO2 quantum dots was attributed to their smaller size, higher surface area, and lower rates of photogenerated e−/h+ recombination. Noteworthy, β-cyclodextrin could enhance the surface moiety and the hydrophilicity of SnO2 particles and consequently improve their dispersion in the aqueous solution.
An efficient combination between nanoparticles of zinc oxide and β-cyclodextrin in water for a three-component reaction has been highlighted by Sagir et al. (2016). More precisely, an ortho-aminothiophenol, with aromatic aldehydes and isocyanides, can give the corresponding 3-aryl-4H-benzo[1.4]thiazin-2-amine in the presence of a Lewis acid catalyst in aqueous medium. The authors prepared ZnO nanoparticles with a spherical shape with a diameter between 15 and 25 nm and compared their activity to classical Lewis acids. The best catalytic result was obtained using 5 mol% of ZnO nanoparticles. In order to enhance the catalytic activity, phase-transfer agents were applied (10 mol% of β-cyclodextrin, cetyltrimethylammonium bromide, or tetradecyltrimethylammonium bromide), and the best activity was obtained in the presence of β-cyclodextrin (respectively, 83% of yield after 30 min, 86% after 40 min, and 84% after 50 min). Recycling experiments showed that the catalyst could be reused during five consecutive runs without any loss of its activity. The authors compared their results to other homogeneous or nanoheterogeneous systems, and the chosen reaction conditions (water, 60 °C, 40 min of reaction) furnished a yield of 92%.
ZrO2-β-cyclodextrin composite was synthesized by co-precipitation using ZrOCl2 and β-cyclodextrin in an ammonium hydroxide solution (Girish et al. 2015). These composite nanoparticles were prepared for the solvent-free synthesis of 2, 4, 5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles. The catalyst could be recycled for three runs without any appreciable loss of activity and selectivity.
The synthesis of iron-platinum core-shell nanoparticles (Fe@Pt) for aqueous hydrogenation reactions was investigated in order to limit the use of Pt monometallic-based catalyst (Mori et al. 2009). Fe@Pt nanoparticles were synthesized by thermal decomposition of Fe(CO)5 followed by chemical reduction of Pt(acac)2 in the presence of oleic acid and oleylamine. After precipitation and dispersion in hexane, the particle organic-water transfer occurred after the addition of a γ-cyclodextrin aqueous solution. Transmission electron microscopy images showed a homogeneous dispersion with an average diameter of 2.5 nm. The core shell structure (iron as the core, platinum as the shell) was determined by X-ray absorption measurements. The catalytic properties of the Fe@Pt nanoparticles were evaluated in the aqueous hydrogenation of allylic alcohol under 1 atm of hydrogen. γ-cyclodextrin-capped nanoparticles were more efficient in water than those in organic solvent without cyclodextrin. This difference of activity could be explained by host-guest complexation between the substrate and the γ-cyclodextrin bringing the substrate close to the active phase. The catalyst could be recycled at least three times.
Even if water appeared as the ideal solvent (non-toxic, cheap, and readily available), its use is limited because a wide range of organic compounds are not water soluble or are unstable in this solvent. Ionic liquids had promising results, but their environmental safety is still discussed (Kunz and Häckl 2016). Recently, low melting mixtures (solvents prepared by mixing high melting point starting materials, which form a liquid by hydrogen bond interactions) (Francisco et al. 2013) were developed for the catalytic applications with homogeneous metal catalyst (Ferreira et al. 2015). These low melting mixtures are generally cheap and easy to prepare from readily available materials. Zhao et al. (2014) investigated the palladium-catalyzed Suzuki coupling of phenyl boronic acid with aryl bromides in different carbohydrate-urea-inorganic salt mixtures. A β-cyclodextrin/N-methylurea mixture was used as solvent for the stabilization of Pd nanoparticles. 80 °C is generally required to get active Pd nanoparticles for C-C coupling reactions, and N-methylurea was necessary to achieve this temperature. Cyclodextrin reduced the palladium ions to get palladium nanoparticles. The model reaction was the coupling between bromobenzene and phenylboronic acid with 0.05 mol% Pd, using K2CO3 as base giving 90% of biphenyl after 2 h. A catalytic amount of water improved the yield up to 95%. Other aryl halides were tested, and the catalytic system showed a great tolerance toward a broad range of functional groups such as –NO2, –NH2, and –CN functionalities. The recyclability was studied, and the catalytic system preserved its activity and stability after four runs.
A second type of non-conventional media has been developed and consisted into performing the reaction without any solvent. In this case, where the catalyst and the substrate are in the solid state, mechanochemistry appeared as an interesting alternative strategy. Gold nanoparticles were mechano-synthesized and were used as nanocatalysts in the reduction of substituted nitrobenzene derivatives by ball milling (Menuel et al. 2016). Several cyclodextrins and saccharide additives were tested to afford well-dispersed Au nanoparticles. The smallest Au average particle size was obtained with β-cyclodextrin. X-ray photoelectron spectroscopy data confirmed the presence of zerovalent Au nanoparticles. Several parameters were studied to see their influence on the activity of the gold nanoparticles during the nitroarene reduction. First, the nature of the saccharide was evaluated. Except in the case of methylated saccharides, all additives improved the catalytic activity of Au nanoparticles, especially in the case of cyclodextrins. The best enhancement was obtained with β-cyclodextrin. Water played a crucial role during the catalytic process. Recycling experiments were performed, and the best results were obtained for β-cyclodextrin where no loss of the activity was noticed after three successive runs. Several nitrobenzene compounds were tested, and, whatever the substrate, the para-substituted derivatives showed lower activities than the ortho- or meta-ones. The authors explained these differences by favored/unfavored routes due to, respectively, unstable/stable complexes between the cyclodextrin and the substrate (Fig. 5.2). The para-substituted compounds can form stable complexes, which limit the approach of the substrate close to Au nanoparticle surface.
Schematic representation of dynamics of exchange between cyclodextrins and halogenonitrobenzene derivatives. The catalytic activity depends upon the strength of the inclusion complex between the substrate and the cyclodextrin or between the product and the cyclodextrin. The conversion is decreasing when the inclusion complex between the substrate (or the product) and cyclodextrin is stronger. (Adapted from Menuel et al. 2016)
5.2.2 Nanoparticles Stabilized by Functionalized Cyclodextrins
In order to improve the stability of the solvent-dispersed metal nanoparticles, the use of molecular functionalized cyclodextrins was investigated. According to our literature survey, these functionalized cyclodextrins could be divided in two families: (1) the thiolated cyclodextrins and (2) the alkylated cyclodextrins.
5.2.2.1 Nanoparticles Stabilized by Thiolated Cyclodextrins
Alvarez et al. (2000) reported the use of thiolated cyclodextrins for the stabilization of platinum and palladium nanoparticles in water for the catalytic hydrogenation of allylamine. More precisely, solutions of MCl42− (M = Pt or Pd) sodium salts in a DMSO:H2O mixture were reduced with sodium borohydride in the presence of per-6-thio-β-cyclodextrin (Fig. 5.3) leading to a dark precipitate.
Synthesis of per-6-thio-β-cyclodextrin. per-6-thio-β-cyclodextrin has been synthesized into two steps with a final yield of 85%. (Adapted from Alvarez et al. 2000)
The interaction of the thiolated cyclodextrin with the metal nanoparticles was justified by the disappearance of the S–H stretching peak at 2560 cm−1 in the FTIR spectra of the resulting materials. Transmission electron microscopy measurements showed spherical metal nanoparticles with an average diameter of 14.1 ± 2.2 and 15.6 ± 1.3 nm, respectively, for Pt and Pd particles. The catalytic activity of these nanoparticles was evaluated in the hydrogenation of allylamine under 1 atmosphere of hydrogen, at room temperature in D2O solution in order to follow the reaction by 1H NMR. Full conversions were obtained for both catalytic systems after 6 h of reaction with a selectivity of 100% toward propylamine. The same authors also optimized the synthesis of the palladium nanoparticles with an average particle size decrease (from 15.6 nm to 3.5 nm) by increasing the thiolated cyclodextrin amount, which is generally observed in the synthesis of solvent-dispersed nanoparticles. These new aqueous dispersed nanoparticles were tested in hydrogenation (Liu et al. 2001) and Suzuki coupling reactions (Strimbu et al. 2003) (Fig. 5.5). For the hydrogenation study, the authors tried to tune the catalytic activity of per-6-thio-β-cyclodextrin-stabilized Pd nanoparticles by ordering host-guest interactions between the receptors and chosen guests in the solution (Table 5.2).
The addition of molecules such as adamantanol or ferrocenyl ammonium derivatives, which strongly interact with cyclodextrins via host-guest inclusion complexes, led to a decrease of the catalytic activity of the palladium nanoparticles. The highest inhibitive effect was observed with a ferrocenyl ammonium derivative (Fig. 5.4).
Deactivation of active catalytic sites by binding ferrocenyl-based molecules to the cyclodextrin hosts. The addition of a cationic ferrocene derivative is leading to a decrease of the catalytic activity due to the creation of a Coulomb barrier when the positively charged substrate is approaching the surface of the Pd nanoparticle. (Adapted from Liu et al. 2001)
For the Suzuki reaction (Fig. 5.5), the catalytic activities are similar whatever the functional group on the aromatic group. Higher activities were obtained in the case of iodo derivatives in comparison to bromo derivatives (respectively, turnover frequency from 41 to 48 h−1 and from 7.8 to 13 h−1) which is generally observed in C-C coupling reactions. Interestingly, the authors compared their values to poly(N-vinyl-2-pyrrolidone)-stabilized Pd nanoparticles (Li et al. 2002). These lower values can be explained by too strong interactions between the thiolated cyclodextrin with the palladium nanoparticles. In the case of iodoferrocene, substrate which can form an inclusion complex with the thiolated cyclodextrin, the catalytic activity of the corresponding palladium nanoparticles was improved in the presence of the cyclodextrin. It is important to note that the authors awarded the opposing solubility requirements which constitute the use of cyclodextrin-capped metal nanoparticles.
Suzuki cross-coupling reaction using per-6-thio-β-cyclodextrin-capped Pd nanoparticles. Isolated yields ranging from 77% to 98% were obtained depending on the nature of the substituents R and X. (Adapted from Strimbu et al. 2003)
Mhadgut et al. (2005) studied the hydrogenation of isophorone in the presence of per-thiolated-β-cyclodextrin (PSH-β-cyclodextrin)-stabilized palladium nanoparticles dispersed in water (Table 5.3).
A synergistic effect between per-thiolated-β-cyclodextrin (phase-transfer catalyst) and Pd nanoparticles on the overall catalytic process was observed using adamantane as a competitive substrate in the hydrogenation of isophorone (entry 3 vs. entry 5). In 2007, they extended the use of these nanocatalysts in the Sonogashira reaction without phosphine and copper (Xue et al. 2007). The synthesized nanoparticles showed high catalytic activity in aqueous medium with isolated yields ranging from 52% to 93% depending of the nature of the substrates. These good activities were explained by the good dispersion of Pd nanoparticles as well as the mass transfer ability of the cyclodextrins confirmed by the catalytic test realized in the presence of adamantane.
The development of combined catalysts based on thiolated cyclodextrin-modified gold nanoparticles with homogeneous complexes is rare. Li et al. (2008) synthesized gold nanoparticles stabilized by per-6-thiol-β-cyclodextrin and used them as a support for triethylenetetramine-adamantane-based copper complexes via supramolecular assembly (Fig. 5.6). These dual catalysts showed a typical Michaelis-Menten kinetics for the cleavage of 4, 4′-dinitrophenylcarbonate. The kinetic analyses indicated the synergistic action of bimetallic catalytic centers and three-dimensional structure of gold nanoparticles for the rate improvement of carbonate hydrolysis.
Copper complexes adsorbed onto gold nanoparticle through adamantyl inclusion into cyclodextrin cavity. This cyclodextrin-modified gold nanozyme proved to be catalytically active in the carbonate hydrolysis. The kinetic analyses clearly showed that a synergistic effect was observed between the multi-metal catalytic centers and the Au nanoparticles leading to an increase of the activity. (Adapted from Li et al. 2008)
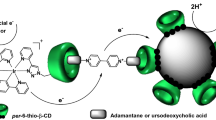
Contreras Carballada et al. (2012) developed a similar approach with platinum nanoparticles stabilized by per-thiolated cyclodextrin combined with a ruthenium or iridium complexes (Fig. 5.7). These catalytic systems were used for the reduction of proton for the production of hydrogen, and the authors have clearly shown that the combination of homogeneous complex with Pt nanoparticles had a beneficial effect on the catalytic activity. This high hydrogen production is also due to the good stabilization of the platinum colloids by the per-thiolated cyclodextrin.
Self-assembled three-component catalytic system for the photo-induced electron transfer. This catalytic system is including an iridium complex as the photosensitizer, methyl viologen as electron relay, and thiolated-β-cyclodextrin-coated platinum nanoparticles as the catalyst. During the production of hydrogen, the photosensitizer is consumed and has to be regenerated using EDTA as sacrificial donor. (Adapted from Contreras Carballada et al. 2012)
More recently, aqueous Cu nanoparticles with an average size of 2 nm were synthesized using mono-6-thio-β-cyclodextrin as stabilizer and hydrazine as reducing agent (Zhong et al. 2016) (Fig. 5.8). The FTIR spectrum of the synthesized nanoparticles compared with that of mono-6-thio-β-cyclodextrin showed several shifts, indicating the interactions between the thiolated cyclodextrin and the surface of the nanoparticles (3356 and 1415 cm−1 vs. 3375 and 1371 cm−1 for the hydroxyl bands). These copper nanoparticles were tested as enzyme mimic, and the cyclodextrin had a strong effect on the reaction rate. The peroxidase-like catalysis of Cu nanoclusters showed the Michaelis-Menten kinetics, which was similar to that of horseradish peroxidase. On the basis of its unique and attractive catalytic activity, a simple and selective colorimetric assay for H2O2 and glucose has been developed. Compared with the natural enzymes, Cu nanoclusters as a mimic peroxidase showed several advantages such as the ease of preparation, the low cost, as well as the high stability and activity under harsh conditions, which made it a promising candidate as enzyme mimics in biotechnology and clinical diagnosis applications.
Schematic illustration of β-cyclodextrin-protected Cu nanoclusters as peroxidase mimics for colorimetric detection of H2O2 and glucose. In this catalytic system, mono-6-thio-β-cyclodextrin is used as stabilizing agent of Cu nanoparticles but also modulator in order to increase the peroxidase-like catalytic rate. (Adapted from Zhong et al. 2016)
5.2.2.2 Nanoparticles Stabilized by Alkylated Cyclodextrins
Alkylated cyclodextrins such as randomly methylated β-cyclodextrin (RaMe-β-cyclodextrin) and hydroxypropylated β-cyclodextrin (HP-β-cyclodextrin) (Fig. 5.9) have also been used for the stabilization of metal nanoparticles.
The synthesis of ruthenium nanoparticles in water using randomly methylated cyclodextrins as protective agents was reported for the first time by Monflier and Roucoux (Nowicki et al. 2006; Denicourt-Nowicki et al. 2007). These cyclodextrins had some advantages such as high solubility in water, low cost, non-toxicity, and availability and had already proven to be efficient phase-transfer catalysts (Leclercq et al. 2007) (Table 5.4).
Ru nanoparticles were synthesized by chemical reduction of ruthenium trichloride with an excess of sodium borohydride in aqueous solution of randomly methylated cyclodextrins with different sizes (α, β, γ) and substitution degrees. The best compromise between the stability and activity was obtained with a cyclodextrin/Ru molar ratio of 10, which had been considered as the standard ratio.
According to the transmission electron microscopy, ruthenium nanoparticles were dispersed into non-ordered superstructures with an average particle size of 2.5 nm. The catalytic activity of these ruthenium nanoparticles was evaluated in the hydrogenation of various unsaturated substrates such as long-chain alkenes under 1 bar of hydrogen at room temperature (Table 5.5).
These alkenes were totally hydrogenated to their saturated analogues with turnover frequencies (TOFs) ranging from 12 to 22 h−1. These TOFs are modest in comparison to other aqueous nanocatalytic systems, but the experimental conditions are less harsh. It is worth noting that the catalytic activity decreased with increasing the chain length (C10 > C12 > C14). This phenomenon could be attributed to a lower solubility of the resulting inclusion complex in water. These colloids were also evaluated in the hydrogenation of various arene derivatives (Table 5.6).
Very interestingly, the hydrogenation of aromatic rings depended both on the type of methylated cyclodextrin (α, β, γ) and on the substitution degree. Indeed, when RaMe-α-cyclodextrin was used as the stabilizer, the aromatic rings were not hydrogenated. In contrast, their total hydrogenation was observed with RaMe-γ-cyclodextrin-stabilized Ru nanoparticles. These results can be explained by the cavity size of the different cyclodextrins which leads to more or less important interactions with the substrates. In the case of the RaMe-β-cyclodextrin, the selectivities were related to the substitution degree. These catalytic results can be correlated to the deeper hydrophobic host cavity of the RaMe-β-cyclodextrin with the highest degree of substitution which can wrap more efficiently the aromatic rings of the substrate avoiding their hydrogenation. The turnover frequency values are modest compared to other catalytic systems, but the reactions were carried out at room temperature under atmospheric hydrogen pressure.
More recently, the synthesis of Ru nanoparticles stabilized in the aqueous phase by RaMe-β-cyclodextrin was performed following two new optimized strategies (Guerrero et al. 2013) (Fig. 5.10).
Two methodologies for Ru nanoparticles stabilized by RaMe-β-cyclodextrin in water. The one-pot approach consists into the reduction of Ru metal precursor reduced under atmospheric hydrogen pressure in the presence of RaMe-β-cyclodextrin. The cascade approach consists into the chemical reduction of the Ru metal precursor by sodium borohydride in a first step followed by the addition of an aqueous solution of RaMe-β-cyclodextrin in a second step. (Adapted from Guerrero et al. 2013)
The comparison had been made on the size and the dispersion of the resulting particles on the one hand and also on the stability, the catalytic activity, and the selectivity in the hydrogenation of various hydrophobic substrates on the other hand. The one-pot approach consisted in the reduction of ruthenium trichloride salt by hydrogen in the presence of RaMe-β-cyclodextrin in water. In contrast, the cascade method was carried out in two successive steps. A Ru hydrosol was obtained by controlled NaBH4 reduction with dropwise addition, to avoid particle agglomeration. Then, RaMe-β-cyclodextrin was added in the abovementioned hydrosol. Whatever the strategy, stable colloidal suspensions were obtained, confirming that RaMe-β-cyclodextrin was an efficient stabilizer for Ru nanoparticles. As evidenced by transmission electron microscopy measurements, both approaches led to well-dispersed nanoparticles. The one-pot strategy allowed stabilizing particles with an average diameter of 1.0 ± 0.2 nm, while the cascade method led to a mean particle size of 1.4 ± 0.2 nm. To have a deeper insight into the interactions between the randomly methylated cyclodextrins and metal surface in both methods, diffusion ordered spectroscopy experiments had been carried out in D2O solution. Whatever the synthesis strategy, similar values of diffusion coefficients and hydrodynamic radius were obtained. Contrary to what is usually observed with strongly interacting ligands, such as phosphines, the 1H NMR experiments exhibited no significant difference in the chemical shifts of the cyclodextrins, indicating weak interactions between RaMe-β-cyclodextrin and the surface of the metal nanoparticles. Consequently, the authors gave a dispersive agent behavior to the cyclodextrin instead of a stabilizing effect of a classical ligand. The catalytic activity of the Ru nanoparticles prepared by each strategy was evaluated in the hydrogenation of several model substrates, including 3-methylanisole, methyl-2-acetamidoacrylate, and ethyl pyruvate, under 20 bar of hydrogen. For ethyl pyruvate and methyl-2-acetamidoacrylate, the catalytic activities related to the nanoparticles prepared by the cascade method were slightly higher. The possibility of recycling these catalytic systems was investigated on the hydrogenation of ethyl pyruvate, and whatever the strategy, it showed that four successive runs were achieved without any significant loss of stability and activity.
More recently, these methodologies (one-pot and cascade approaches) were extended to prepare ruthenium nanoparticles stabilized by randomly methylated β-cyclodextrins grafted with chiral amino acid moieties, such as l-leucine and l-alanine (Chau et al. 2013) (Fig. 5.11).
Structure of grafted RaMe-β-cyclodextrin with chiral amino acid moieties as stabilizers and mass transfer agents for catalytically active ruthenium nanoparticles dispersed in water for asymmetric hydrogenation of acetophenone, ethyl pyruvate, methyl-2-acetamidoacrylate, and 3-methylanisole under 20 bar of H2 at room temperature in aqueous phase. (Adapted from Chau et al. 2013)
The influence of the ligand and synthesis methodology on the size, dispersion, and surface properties was studied. These Ru nanoparticles stabilized by amino acid-grafted RaMe-β-cyclodextrin were evaluated in the hydrogenation of prochiral model substrates such as acetophenone, ethyl pyruvate, methyl-2-acetamidoacrylate, and 3-methylanisole under 20 bar of H2 at room temperature. The stability of the aqueous colloidal suspensions under reaction conditions was established, indicating that RaMe-β-cyclodextrin bearing optically active moieties acted as an efficient protective agent around the nanoparticle surface. In addition, the catalytic data showed that, whatever the strategy, Ru nanoparticles stabilized by RaMe-β-cyclodextrin-trz-Leu were more active than those capped by RaMe-β-cyclodextrin-trz-Ala (respectively, 100% vs. 54% of ethyl-2-hydroxypropanoate). However, no significant enantiomeric excess was measured probably due to the weak or deficient interaction between the chirally modified cyclodextrin and the nanoparticle surface.
The first work of Malta on the synthesis of palladium nanoparticles stabilized by hydroxypropyl-α-cyclodextrin for catalytic applications was reported in 2009 (Senra et al. 2009). Interestingly, the authors reported that hydroxypropyl-α-cyclodextrin could play several roles such as capping agent like the randomly methylated cyclodextrins or thiolated cyclodextrins but also as reducing agent. A black precipitate was obtained after the addition of hydroxypropyl-α-cyclodextrin in an aqueous PdCl2 solution. The nanoparticles were characterized by several physicochemical techniques that revealed the formation of spherical particles in the size range of 2–7 nm. Further analyses by FTIR spectroscopy and 1H NMR did not show covalent bonds between cyclodextrins and palladium nanoparticles, suggesting that hydroxypropyl-α-cyclodextrin was only physically adsorbed on the metal surface. These observations were presumably due to hydrophobic interactions enabling the limitation of the mutual coalescence of nanoclusters. The catalytic activity of these colloids was evaluated in several Pd-catalyzed C-C coupling reactions, such as Suzuki, Heck, and Sonogashira reactions, in water with good yields, and Pd nanoparticles stabilized by hydroxypropyl-α-cyclodextrin were reused during four consecutive runs without any significant loss of activity. More recently, the influence of the size of the hydroxypropyl cyclodextrin (α, β, γ) and the Pd-to-cyclodextrin ratio was studied to observe eventual changes in the particle size of the resulting particles and to exploit the surface/cavity effects in the Suzuki-Miyaura reaction (Senra et al. 2016). The differences in catalytic activities between the Pd nanoparticles stabilized by different cyclodextrins were explained by the formation of inclusion complex, and the best activities were obtained with hydroxypropyl-β-cyclodextrin which had the strongest complexation capacity.
The use of hydroxypropyl cyclodextrin as capping agent for the synthesis of silver nanoparticles was reported for the first time in 2013 (Devi and Mandal 2013). A series of hydroxypropyl-cyclodextrin-capped Ag nanoparticles was synthesized by the reduction of silver nitrate in alkaline aqueous medium at 60 °C. The resulting nanoparticles were tested in the reduction of p-nitrophenol using sodium borohydride. The catalytic activity of hydroxypropyl-cyclodextrin-stabilized Ag nanoparticles was much higher than other cyclodextrin-capped nanoparticles. These catalytic results were correlated with the size and morphology of the particles. Whereas native α-cyclodextrin and β-cyclodextrin seemed to form aggregated nanostructures, hydroxypropyl cyclodextrin (α and β) formed a chain-like assembly.
A novel hybrid nanocomposite prepared from hydroxypropyl-β-cyclodextrin and alginate was used as stabilizer to synthesize Ag nanoparticles (Nguyen et al. 2018). Aqueous extract of J. subtriplinerve leaves was used as reducing agent in order to avoid too toxic compounds such as NaBH4 or hydrazine. The silver nanoparticles were tested in the catalytic degradation of methyl orange, rhodamine, and 4-nitrophenol. 4-nitrophenol was completely converted into 4-aminophenol within 25 min when rhodamine and methyl orange were completely converted within 22 min.
Bhoi et al. (2016) prepared spherical monodispersed silver and gold nanoparticles dispersed in alkaline medium by chemical reduction of the corresponding metallic precursor using cyclodextrins such as α-cyclodextrin, β-cyclodextrin, γ-cyclodextrin, and hydroxypropyl-β-cyclodextrin as both stabilizing and reducing agent (Fig. 5.12).
Schematic representation for the formation of mono- and bimetallic nanoparticles by chemical reduction in alkaline medium of the corresponding metal precursors (AgNO3 and HAuCl4) by cyclodextrins. The chosen cyclodextrins (α, β, γ, and hydroxypropyl-β) played a dual role of stabilizer and reducing agent, and the resulting metal nanoparticles showed high catalytic activity for the radical scavenging reaction of the stable free radical 2, 2′-diphenyl-1-picrylhydrazyl. (Adapted from Bhoi et al. 2016)
The corresponding core-shell (Ag@Au and Au@Ag) were also synthesized via the polyol process, and the particle size of the corresponding core-shell nanoparticles was determined by static light scattering measurements (Table 5.7). All these colloidal suspensions showed radical scavenging behavior from the quenching of 2, 2′-diphenyl-1-picrylhydrazyl light adsorption. A kinetic study had been particularly done in the case of Ag nanoparticles, and in this case, a pseudo first-order rate constant of 1.79 × 10−2 min−1 was obtained, but a deviation of the linearity was observed after 80 min of reaction time which could be explained by the decrease of Ag nanoparticle concentration.
5.2.3 Nanoparticles Stabilized by Polymers in the Presence of Cyclodextrin
5.2.3.1 Nanoparticles Stabilized by a Physical Mixture of Polymer and Cyclodextrin
Another way to stabilize aqueous dispersed metal nanoparticles is the possibility of using water-soluble polymer in the presence of cyclodextrins. The use of cyclodextrins as additives in the synthesis of poly(N-vinyl-2-pyrrolidone)-stabilized metal nanoparticles was studied (Herbois et al. 2012). For this purpose, Ru nanoparticles were synthesized in the presence of poly(N-vinyl-2-pyrrolidone)/cyclodextrin mixture with a controlled ratio, and the transmission electron microscopy images of the corresponding particles were carefully compared. For the standard Ru nanoparticles, i.e., Ru nanoparticles stabilized by poly(N-vinyl-2-pyrrolidone) alone, the particles were entrapped in string-like assemblies by the effect of poly(N-vinyl-2-pyrrolidone) chains. The morphology did not seem to be altered by the presence of cyclodextrin. However, a slight decrease in the mean particle size was noticed when cyclodextrins were added to the poly(N-vinyl-2-pyrrolidone) (2.5 and 2.3 nm, respectively, for β-cyclodextrin and RaMe-β-cyclodextrin against 3.0 nm without cyclodextrin). The well-known aggregation of cyclodextrins in aqueous solutions, which can give rise to large agglomerates in the 200–300 nm range, was deeply disturbed by the presence of poly(N-vinyl-2-pyrrolidone). According to the dynamic light scattering experiments, it was observed that these cyclodextrin aggregates had the tendency to significantly disappear in the presence of poly(N-vinyl-2-pyrrolidone) in favor of small-sized assemblies of only two or three cyclodextrin units as evidenced by the presence of a population centered on a mean diameter about 2–3 nm. The disaggregated cyclodextrins were assumed to interact easier with the soluble Ru(III) precursor than poly(N-vinyl-2-pyrrolidone), thus increasing the efficiency in controlling the growth of the Ru nanoparticles after the reduction step (Fig. 5.13).
Schematic representation of a proposal for the Ru nanoparticle synthesis in the presence of a mixture of poly(N-vinyl-2-pyrrolidone) and cyclodextrin. Each part of the mixture has a specific role: the cyclodextrin can be seen as a metal particle size controller, whereas poly(N-vinyl-2-pyrrolidone) ensures the long-term stability of the Ru particles but also allows breaking cyclodextrin aggregates to get monomeric species to improve ruthenium ion and cyclodextrin interactions before the reduction step. The size of the synthesized metal nanoparticles depends of the nature of the cyclodextrins (2.3 nm for randomly methylated cyclodextrins against 2.5 nm for the native ones). (Adapted from Herbois et al. 2012)
To confirm this hypothesis, an additional experiment was realized, in which cyclodextrins were added to a preformed poly(N-vinyl-2-pyrrolidone)-stabilized Ru colloidal suspension and kept under stirring during 24 supplementary hours. Notably, the size range of the particles was the same to that of the control preparation without cyclodextrins. It is important to mention that the presence of poly(N-vinyl-2-pyrrolidone) was crucial in order to ensure long-term stability of the Ru nanoparticles, knowing that a ratio of poly(N-vinyl-2-pyrrolidone) to Ru higher than 8 was absolutely required.
The influence of the quantity of cyclodextrin on the catalytic activity of the resulting Ru nanoparticles was studied, performing for the hydrogenation of furfural, a biosourced substrate, under mild experimental conditions (30 °C, 10 bar of H2) (Table 5.8). Whatever the poly(N-vinyl-2-pyrrolidone)/cyclodextrin ratio, the Ru nanoparticles were visually stable, and no sedimentation was observed at the end of the catalytic test. According to the results gathered in Table 5.8, only a small amount of cyclodextrin was required to get an activity improvement. The obtained results were rationalized in terms of size and morphology control of the Ru nanoparticles. In line with what is generally observed in nanocatalysis, the decrease in the particle size resulted in the increase of the number of available surface active sites and, consequently, increased the catalytic efficiency.
In another study, Kuklin et al. (2016) investigated the role of free and supported cyclodextrin on the selective hydrogenation of phenol to cyclohexanone in aqueous media and a n-hexyltriethylammonium bromide ionic liquid (N6222Br) using Rh nanoparticles stabilized by polyacrylic acid. Noteworthy, the catalytic activity and selectivity of the Rh nanoparticles depended on the presence of cyclodextrin. It was attributed to the fact that both substrate and the cyclohexenol, which was formed as an intermediate, could form inclusion complexes with cyclodextrin (Fig. 5.14).
Proposed mechanism for the phenol hydrogenation without cyclodextrin (a) and with the assistance of cyclodextrin (b). Phenol was firstly reduced to cyclohexenol and then to cyclohexanol. Just a small amount of cyclohexenol could desorb from the catalyst and could equilibrate itself to cyclohexanone which did not adsorb on catalyst and could not be reduced to cyclohexanol (path a). In the presence of cyclodextrin (path b), all the cyclohexenol amount was desorbed from the catalyst by making an inclusion complex and avoided the hydrogenation of this molecule toward cyclohexanol. (Adapted from Kuklin et al. 2016)
Upon formation of inclusion complex with the intermediate, it would be desorbed from Rh nanoparticles. High stability of this complex made the repeated adsorption of cyclohexenol unlikely (Fig. 5.14). The presence of ionic liquid was also crucial as it could form inclusion complexes with cyclodextrin and fix it in the surface layer and facilitate the desorption of cyclohexenol from the surface of Rh nanoparticles. Other factors which affect the reaction were the nature of the cyclodextrin and the reaction media. Using optimum reaction condition, 80 °C, P(H2) = 10–40 bars, the product was obtained with the yield of 100% in 1 h.
The influence of cyclodextrin derivatives X-cyclodextrins (X = OH, NH2 or SH) on the aerobic oxidative kinetic resolution of racemic secondary alcohols using poly(N-vinyl-2-pyrrolidone)-stabilized Au nanoparticles was studied (Hirano et al. 2019). 1.8 ± 0.6 nm gold nanoparticles stabilized by poly(N-vinyl-2-pyrrolidone) were obtained after the chemical reduction of HAuCl4 by NaBH4. The effect of the coordinating ability of different cyclodextrins on the catalytic performance of the Au nanoparticles was determined by the comparison of the s factor in the case of the oxidation of a racemic mixture of 1-(2-naphtyl)ethanol. Native β-cyclodextrin (X = OH) brought no modification on the selectivity of the reaction. The thiolated-β-cyclodextrin improved the selectivity but with a dramatic activity decrease. Amino-β-cyclodextrin (X = NH2) gave the best results both in activity and selectivity. The authors suggested, by comparing the size of the NH2-cyclodextrin and by using a competitive guest, that the enantioselective oxidation could be explained by an eventual inclusion of the alcohol into the chiral cavity of the cyclodextrin.
5.2.3.2 Nanoparticles Stabilized by Cyclodextrin-Grafted Polymer
The first example of metal nanoparticles stabilized by a cyclodextrin-based polymer was referenced by Shiraishi’s group (Shiraishi et al. 2007; Taylor et al. 2010). Palladium nanoparticles were prepared by refluxing an alcohol/water solution of palladium acetate in the presence of the cross-linked cyclodextrin-based polymer. The catalytic activity was evaluated in the hydrogenation of unsaturated carboxylic acids. The cavity of the cyclodextrin played a crucial role by forming inclusion complex with the substrate. Pt nanoparticles were synthesized by the photoreduction of hexachloroplatinic acid by a UV irradiation in the presence of a cyclodextrin-based polymer. According to transmission electron micrographs, Pt colloids with average particle size of 1–6 nm range were observed with an increase of the average particle size with the cyclodextrin size. The Pt colloids were tested in the superoxide anion quenching reaction and showed higher activity than polyacrylic acid-stabilized Pt nanoparticles. These results could be explained by too strong interactions between the metal nanoparticles and the polyacrylic acid.
Meo et al. (2012) developed a novel heterogeneous catalysts based on stabilization of Ag nanoparticles as a core with poly-(6-N,N-dimethyl-propylenediamino)-(6-deoxy)-β-cyclodextrin (AmCD) as a capping agent which surrounded the nanoparticles like a shell. The synthetic procedure involved reaction of [Ag(NH3)2]+ complex in the presence of a proper amount of AmCD with an excess of formaldehyde at 40 °C for 90 min. It was believed that the shell could act as a steric and electrostatic barrier to avoid the aggregation. The obtained nanoparticles were then used as catalysts for the reduction of nitroarenes in the presence of NaBH4. The authors studied the kinetics of the reaction and the effect of the para-substituent on the substrate and the trends of the induction period observed at the beginning of the reaction. It was established that the presence of electron-donating groups on the nitroarene could influence the reaction course, implying that the nanoparticle surface acted as an electrophile toward the nitro group. Nevertheless, the presence of negatively charged or bulky groups on the nitroarene had a slightly detrimental effect, which could emerge from the difficulty for the substrate to approach the nanoparticle surface. The AmCD-covered catalyst surface seemed less active, because of the intrinsic stability of the substrate-AmCD inclusion complex.
In line with the stabilization of Ru nanoparticles by functionalized cyclodextrins, Noël et al. (2012) studied the synthesis of metal nanoparticles in the presence of cyclodextrin-based polymer leading to greater complexity of the protective agent structure. Rhodium trichloride was reduced by sodium borohydride in the presence of poly(mono(β-cyclodextrin-2-yl)-maleate-alt-maleate-alt-methylvinylether) (Fig. 5.15). It could be noticed that a control experiment was performed using the same polymer backbone but without any presence of grafted cyclodextrin. The two colloidal suspensions were characterized by transmission electron microscopy, and the corresponding images clearly showed two different types of nanoparticle organization.
Structure of the two polymers used: poly(mono-(β-cyclodextrin- 2-yl)-maleate-alt-maleate-alt-methylvinylether) (x = 0.04) and poly(maleate-alt-methylvinylether) (x = 0) for the synthesis of aqueous rhodium nanoparticles. The cyclodextrin-functionalized polymer afforded to get well-dispersed metal nanoparticles and better catalytic activities due to this good dispersion but also to the intrinsic mass transfer property of the cyclodextrin. (Adapted from Noël et al. 2012)
Thus, for the Rh nanoparticles stabilized by the polymer containing cyclodextrins, a mono and well-dispersed size distribution was clearly obtained, whereas those prepared from the cyclodextrin-free polycarboxylate (control polymer) was organized into non-ordered superstructures, in which the nanoparticles were entrapped in string-like assemblies with a bigger average particle size. The catalytic properties were evaluated in the hydrogenation of various alkene and arene derivatives. For instance, in the case of 1-tetradecene, the turnover frequency was equal to 2000 h−1 and 420 h−1 for the hydrogenation reaction catalyzed by Rh nanoparticles stabilized by polyCOONa-β-cyclodextrin and polyCOONa, respectively. These results were correlated with both the average particle size and the dispersion of the rhodium nanoparticles stabilized by the above stabilizers. In order to have a better insight into the beneficial effect of the structure of the polymer, an additional experiment has been performed using a physical mixture, i.e., adding the same amount of β-cyclodextrin in the polycarboxylate solution. The turnover frequency was very close to the value of the polycarboxylate alone, but the colloidal suspension after the catalytic test was unstable. All these experiments undoubtedly supported the view that the β-cyclodextrin covalently linked to the polymer chain induced a significant effect in terms of activity and reusability. The recyclability was studied by reusing the aqueous catalytic layer during five successive hydrogenation runs of 1-tetradecene with no loss of stability and activity. The rhodium leaching in the organic phase of each catalytic test was very low (<0.2 ppm), while the transmission electron microscopy experiments confirmed the robustness of the colloidal suspensions, with no change in terms of particle size and morphology.
A deeper study on the stability and the catalytic activity of the rhodium nanoparticles was performed by modifying the pH value of the solution before the reduction step and by using different ratios of grafted cyclodextrin (Noël et al. 2014). According to transmission electron microscopy experiments, homogeneous dispersions of the metal nanoparticles were observed for the lowest initial pH value (5.1), and, on the contrary, unstable colloids were observed for highest pH value (7.7). It seemed that there was no link between the mean particle size and the amount of grafted cyclodextrin onto the polymer backbone. The catalytic activities were evaluated in the hydrogenation of methyl linoleate under 10 bar of hydrogen at 30 °C. Several Rh nanoparticles called controls were also synthesized (Rh nanoparticles stabilized by a β-cyclodextrin and polycarboxylate mixture with a ratio corresponding to the grafted polycarboxylates). According to the catalytic results, the grafted polycarboxylates gave higher activities, and, in terms of recovery, the colloids with the physical mixture showed stable emulsions which led to non-recoverable catalytic systems.
Herbois et al. (2015) reported another work which consisted into the encapsulation of fine water-dispersible Ru nanoparticles (with size of ~1.8 nm) into 3D β-cyclodextrin-based polymer (poly(CTR-β-cyclodextrin) derived from controlled polycondensation of β-cyclodextrin with citric acid). The synthetic procedure included reduction of Ru precursor, ruthenium nitrosyl nitrate, by NaBH4 in the presence of as-prepared poly(CTR-β-cyclodextrin). It was confirmed that carboxylate moieties in the backbone of the polymer could render the surface of the globules negatively charged and consequently stabilize them due to electrostatic repulsion. Moreover, these functionalities played an important role in the interaction with Ru3+, affecting the nucleation of metal clusters in the initial stages of reduction. Finally, the catalytic utility of the hybrid system as an efficient catalyst for aqueous phase hydrogenation of biomass-derived 2-furaldehyde and 3-(2-furyl)acrolein at 1 MPa pressure and 303 K was confirmed. Notably, the catalyst was reusable for five reaction runs, and the transmission electron microscopy analyses demonstrated that the reuse of the catalyst did not induce any significant change in the morphology of the catalyst. It was suggested that the high catalytic activity and stability came from the ability of poly(CTR-β-cyclodextrin) to provide a good balance of stabilizing properties with the metal surface, both in terms of steric and electrostatic interactions without hindering the catalytic activity. Additionally, each globule could be considered as a confined space, microreactor, for promoting the hydrogenation process.
Another cyclodextrin-based polymer was used to stabilize ruthenium nanoparticles in aqueous medium. This polymer was synthesized by reacting β-cyclodextrin with epichlorohydrin and glycidyltrimethylammonium chloride in alkaline medium (Noël et al. 2017). The resulting Ru nanoparticles were tested in the hydrogenation of different petro and biosourced substrates and showed a catalytic activity close to the Ru/poly(CTR-β-cyclodextrin) nanoparticles. Interestingly, these nanoparticles could be used in acidic medium (pH 3) for the hydrogenation of unsaturated carboxylic acids, especially linoleic and oleic acid. The catalytic system showed a strong robustness because no loss of activity and stability had been observed by transmission electron microscopy experiments after ten consecutive runs in the tetradecene hydrogenation.
In an interesting study, Au/Ag bimetallic core-shell nanoparticles with different core diameters were synthesized by Haldar et al. (2014) through a β-cyclodextrin-assisted synthetic procedure. The authors investigated the effect of core size (10–100 nm) on the catalytic properties of the nanoparticles for the reduction of 4-nitrophenol in the presence of sodium borohydride. It was found that the catalytic activity was influenced by the size of the core and differed from 41.8% to 96.5%. Noteworthy, the core-shell system with core size of 100 nm was 12 times more efficient than Au nanoparticles of the same size, indicating the effect of the core-shell structure on the catalytic activity.
Vasconcelos et al. (2016) reported the synthesis of polyurethane nanosponges through reaction of hexamethylenediisocyanate and β-cyclodextrin and used the prepared nanosponges as a template for the synthesis of Aun quantum clusters, by the core etching of glutathione-capped Au nanoparticles. The authors investigated the effect of the Au: nanosponge ratio on the nanocluster formation step. It was established that the longer reaction time, the smaller clusters were formed. Initially, the clusters formed in the cavities of cyclodextrin (Au7 and Au15), while for longer reaction time, the cluster concentration in cavities increased. Further increase in the reaction time led to the formation of larger clusters that could not be hosted in the cavities of cyclodextrin but may interact with other functional groups such as carbonyls and nitrogenated groups. The catalytic activity of Aun clusters for reduction of 4-nitrophenol in the presence of NaBH4 was also studied. It was found that Langmuir-Hinshelwood kinetic model fitted the reaction and the reaction proceeded with no induction time.
Subnanometer size noble metal colloids were synthesized using a cyclodextrin polymer-based network (from azido-β-cyclodextrin and diethynylbenzene using click chemistry) as stabilizing agent. Transmission electron microscopy images showed ultra-small Pd nanoparticles which were well-dispersed in the polymeric matrix. The catalytic activity of these colloids was evaluated in the reduction of 4-nitrophenol and in a C–C coupling reaction. In the case of the 4-nitrophenol reduction, the authors emphasize the role of the cavity of the cyclodextrin. The cyclodextrin, whose secondary face is free, formed inclusion complex and allowed the guest molecule to be close to the metal surface.
5.2.4 Nanoparticles Stabilized by Cyclodextrin-Based Inclusion Complex
The combination of cyclodextrins with other stabilizing agents, i.e., alkyl ammonium salts, phosphanes, and dendrimers, was also reported for the synthesis of metallic nanoparticles dispersed into the aqueous phase. Another strategy consisted into the combination of cyclodextrins with polymers likely to form supramolecular complexes leading to the development of a new reaction medium named hydrogel. Whatever the strategy, the catalytic activity of these colloidal suspensions was also evaluated for different kinds of reactions.
5.2.4.1 Nanoparticles Stabilized by Quaternary Ammonium-Based Salts/Cyclodextrin Inclusion Complexes
Ionic surfactants such as alkyl ammonium salts are known to be good candidates to stabilize active metallic nanoparticles. Moreover, this family of surfactants is well known to strongly interact with β-cyclodextrin derivatives. Randomly methylated cyclodextrins combined with ammonium salts bearing a lipophilic chain was used for the synthesis of aqueous Ru nanoparticles (Hubert et al. 2009) (Fig. 5.16).
Aqueous ruthenium nanoparticle synthesis in the presence of RaMe-β-cyclodextrin/HEA16Cl. The ruthenium nanoparticles were stabilized by a mixture of free cyclodextrins, alkyl ammoniums, and inclusion complexes. It was suggested that RaMe-β-cyclodextrin affected the adsorption of the surfactant on the metal surface and prevented the double layer formation which is observed for Ru nanoparticles stabilized by HEA16Cl alone. The cyclodextrin could act as a spacer between the alkyl chains and reduced the intermolecular interactions, thus allowing a better mobility and diffusion of the substrate at the vicinity of the metal surface. (Adapted from Hubert et al. 2009)
NMR spectroscopic studies were performed to prove the formation of an inclusion complex between RaMe-β-cyclodextrin and the chloride salt of N, N-dimethyl, N-hexadecyl, N-(2-hydroxyethyl)ammonium (HEA16Cl) in water. The continuous variation technique, named Job’s method, and transverse rotating-frame Overhauser effect spectroscopy experiments emphasized the existence of such a complex. The resulting nanoparticles were characterized by transmission electron microscopy and showed a homogeneous distribution with an average size of about 4 nm. The particles were organized into superstructures similarly to those previously observed with the Ru nanoparticles stabilized by free RaMe-β-cyclodextrin. The catalytic activity of the Ru colloidal suspension stabilized by RaMe-β-cyclodextrin:HEA16Cl mixture with a ratio of 1:1 was evaluated in the hydrogenation of several functionalized aromatic compounds (i.e., anisole, toluene, and styrene), and their performances were compared to the Ru nanoparticles prepared using only the ammonium salt surfactant (Table 5.9).
Whatever the substrate, the RaMe-β-cyclodextrin/HEA16Cl-stabilized Ru nanoparticle was the most active catalytic system, indicating a beneficial effect of the inclusion complex as stabilizer. In the case of anisole, the catalytic activity of the above nanoparticles was three times higher than the nanoparticles stabilized by the ammonium surfactant. The same tendency was observed for toluene and styrene hydrogenation. The difference of the activity was related to a different organization of the stabilizer around the metal surface. Indeed, whereas the Ru nanoparticles stabilized by HEA16Cl were protected by a surfactant double layer, as already described by other groups, it was suggested that RaMe-β-cyclodextrin affected the adsorption of the surfactant on the metal surface and prevented the double layer formation. The cyclodextrin could act as a spacer between the alkyl chains and reduced the intermolecular interactions, thus allowing a better mobility and diffusion of the substrate at the vicinity of the metal surface.
More recently, Thanh Chau et al. (2016) developed a novel protective agent for the formation of very fine Rh nanoparticles. To prepare the inclusion complexes, the authors used randomly methylated β-cyclodextrin or its leucine-grafted analogue (RaMeCDLeu) and an optically active ammonium salt (QCD16Br = (1S,2R,4S,5R)-(+)-N-hexadecyl-5-vinyl-2-quinuclidinium-methanol bromide) or N, N-dimethyl-N-hexadecyl-N-(2-hydroxyethyl)-ammonium chloride (HEA16Cl) as surfactants. Typically, catalysts were synthesized by reduction of Rh salts by NaBH4 in the presence of cyclodextrin and surfactant. Notably, to reach stable nanoparticles, the cyclodextrin/surfactant ratio must be optimized. By transmission electron microscopy analyses, it was found that the dispersion of the spherical Rh nanoparticles was very good and their size varied from 1.2 nm to 1.5 nm depending on the type of cyclodextrin and surfactant. It was also proven that these systems could act as efficient and stable catalysts for promoting biphasic hydrogenation of various substrates including ketones and olefins such as methyl-2-acetamidoacrylate, ethyl pyruvate, or acetophenone with high catalytic activities and interesting specific activity under mild experimental conditions.
In another study, gold nanoparticles were synthesized using α-cyclodextrin as stabilizing agent in aqueous medium (Peng et al. 2014). The addition of a trans-azo-benzene-based surfactant led to the phase transfer of the gold nanoparticles. By UV irradiation, the azo-benzene compound isomerized itself giving the cis-azo-benzene and consequently leading to the inverse phase transfer in water (Fig. 5.17). These gold nanoparticles were tested in the 4-nitrophenol reduction using NaBH4. The recovery and recycling can be achieved by a visible irradiation which gave again trans form which bring the nanoparticles in organic phase.
Photoreversible inclusion of azo-ligand onto α-cyclodextrin-coated Au nanoparticles. The gold nanoparticles are dispersed in organic phase. The photo isomerization of trans-azo-benzene to cis-azo-benzene by UV light irradiation prevents the inclusion of this latter into the cyclodextrin cavity, and a phase transfer of the gold nanoparticles is observed from organic to aqueous phase. The catalytic nitrophenol reduction can also be performed in aqueous phase. The catalyst recovery can be effective by the reverse isomerization reaction. (Adapted from Peng et al. 2014)
5.2.4.2 Nanoparticles Stabilized by Phosphanes/Cyclodextrin Inclusion Complexes
Cyclodextrins have been used as mass transfer promoters in biphasic aqueous phase catalysis processes using water-soluble organometallic complexes with sulfonated phosphines as ligands since a long time. Spectroscopic studies demonstrated that, depending on the nature and the position of the substituents on the aromatic ring, sulfonated phosphines could interact with RaMe-β-cyclodextrin by forming inclusion complexes and tune the catalytic performances of the metal centers. The idea was to combine the advantages of a sulfonated diphosphine as water-soluble stabilizer of nanoparticles with a cyclodextrin for its shuttle and supramolecular control effects in biphasic aqueous phase catalysis (Monflier et al. 1999). Thus, ruthenium nanoparticles were synthesized by hydrogen reduction of the organometallic ruthenium complex [Ru(cod)(cot)] and stabilized either with 1,4-bis[(di-m-sulfonatophenyl)phosphine]butane (dppbs) or its combination with RaMe-β-cyclodextrin in tetrahydrofuran (Guerrero et al. 2013, 2014) (Fig. 5.18). The so-obtained Ru nanoparticles were isolated by precipitation and finally easily re-dispersed in water.
Synthesis of dppbs: RaMe-β-cyclodextrin Ru nanoparticle system. (Adapted from Guerrero et al. 2013)
The synthesized nanoparticles (diphosphine alone and phosphine-cyclodextrin mixture) were fully characterized by several techniques (transmission electron microscopy, high-resolution transmission electron microscopy, wide-angle X-ray scattering, dynamic light scattering, liquid and solid NMR spectroscopy). Whatever the stabilizer (without or with cyclodextrin as co-additive), transmission electron microscopy images showed small and well-dispersed particles with an average diameter between 1.2 nm and 1.5 nm. The nanoparticle environment in solution was studied by dynamic light scattering. The results showed that the hydrodynamic radius depended on the amount of cyclodextrin present during the nanoparticle synthesis which was a strong indication that the cyclodextrins surround the metal nanoparticle surface. Resonance shifts in 1H and 31P NMR in D2O confirmed the presence of a weak interaction between the dppbs and RaMe-β-cyclodextrin (aromatic protons and protons which are in the cyclodextrin cavity). However, the addition of RaMe-β-cyclodextrin on a preformed dppbs-stabilized Ru nanoparticle solution did not change the NMR spectra, thus evidencing that the dppbs/cyclodextrin complex was formed only if the diphosphine and cyclodextrin were both present at the beginning of the synthesis.
In order to investigate the influence of the cyclodextrin on the catalytic performances of the diphosphine-stabilized nanoparticles, the hydrogenation of model compounds such as styrene, acetophenone, and m-methylanisole was carried out. Based on turnover frequency values, whatever the substrate, an activity improvement was observed with increasing the initial amount of cyclodextrin. More interestingly, in the case of m-methylanisole, it was observed that the quantity of cyclodextrin dramatically influenced the stereoselectivity toward the preferential formation of the cis isomer (51% vs. 100% for dppbs and dppbs/5.0 cyclodextrin, respectively).
5.2.4.3 Nanoparticles Stabilized by Dendrimers
Astruc et al. (2012) exploited mono-, bis-, and tris(4-ferrocenyl-1,2,3-triazolylmethyl)arene-β-cyclodextrin adducts, i.e., 4-methoxy-1-(4-ferrocenyl-1,2,3-triazolylmethyl) benzene-β-cyclodextrin, p-bis(4-ferrocenyl-1,2,3-triazolylmethyl)-benzene-β-cyclodextrin, and 1,3,5-tris(4-ferrocenyl-1,2,3-triazolylmethyl) benzene-β-cyclodextrin, for stabilizing Pd nanoparticles (Fig. 5.19). The reasons behind this design were the facts that ferrocene could form an inclusion complex with cyclodextrin and led to water solubilization of the catalytic system and the role of triazolyl group as ligand for stabilization of the Pd nanoparticles. The transmission electron microscopy analyses showed that fine Pd nanoparticles (5–6 nm) as well as large aggregates could be formed. The authors studied the catalytic activities of the catalysts for C–C coupling reactions including Miyaura-Suzuki and Heck reactions that were, respectively, performed at ambient temperature and 80 °C. The comparison of the catalytic performances of three catalysts established that the bolaamphiphile-like bis- and tris-cyclodextrin systems led to much better pre-catalysts, indicating the efficiency of encapsulation of a hydrophobic catalytic system with two or three peripheral water-solubilizing cyclodextrin caps. The authors studied the effect of mol ratio of Pd/cyclodextrin. Interestingly, in the case of Miyaura-Suzuki reaction, the yield improved by increasing this value from 1 to 2. In the Heck reaction, however, the effect of this value was more pronounced. The authors attributed the different effect of Pd/cyclodextrin ratio for Heck and Miyaura-Suzuki reaction to the different reaction temperatures (higher temperature could render the ferrocenyl encapsulation reversible and allowed free cyclodextrins to inhibit the reaction through hosting Pd nanoparticles) and mechanism.
Preparation of Pd nanoparticle materials. Here, the tris(4-ferrocenyl-1,2,3-triazolylmethyl) benzene in combination with β-cyclodextrin was used as stabilizer of Pd nanoparticles in aqueous medium. The Pd(II) reduction using NaBH4 gave Pd nanoparticles but also mono Pd(0) atoms which are stabilized by the triazol groups. (Adapted from Liang et al. 2012)
Taking advantage of CuI-catalyzed azide-alkyne cycloaddition, also known as click reaction, Wang et al. (2016a) developed a series of ligands containing bifunctional 1,2,3-triazole for stabilization of water-solubilized gold nanoparticles of 3.0–11.2 nm. In one designed ligand, β-cyclodextrin and biocompatible polyethylene glycol were substituted on the triazole ring. The authors believed that the synthesized gold nanoparticles could have potential applications for encapsulation, catalysis, and sensing and confirmed the catalytic activity of the gold nanoparticles for reduction of 4-nitrophenol in the presence of NaBH4. Noteworthy the catalytic activity of the Au nanoparticles stabilized by triazoles was superior (k = 7.0 × 10−3 s−1, when 0.5% Au nanoparticles is used) to that of the thiolate Au nanoparticles. This observation was attributed to the easier removal of the triazole ligands by the substrate from the fine gold nanoparticles.
5.2.4.4 Nanoparticles Stabilized by Cyclodextrin-Based Supramolecular Hydrogels
Moreover, more and more sophisticated catalytic systems were developed in order to furnish active and selective catalysts playing on the confinement of the metal nanoparticles with the substrate. Among the reported systems, hydrogel applications have become popular in a wide range of applications such as medicine, materials, and catalysis. The embedment of metal nanoparticles into the supramolecular-structured hydrogels based on host-guest interactions in the presence of cyclodextrins was investigated.
The first example had been developed by the group of Zhang in 2009. Silver nanoparticles were embedded in a supramolecular hydrogel made from Pluronic® F-68, an amphiphilic block copolymer of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) and α-cyclodextrin (Ma et al. 2009). These colloidal suspensions were finally used for the catalytic reduction of methylene blue in the presence of sodium borohydride. The colloidal suspension was synthesized within two successive steps. The first step consisted into the reduction of a silver nitrate in an aqueous solution of Pluronic® F-68 which played the role of reducing agent leading to the formation of Ag nanoparticles. The second step evolved the addition of an α-cyclodextrin solution to the pre-synthesized colloidal suspension conducting to the formation of a gel due to the supramolecular self-assembly between α-cyclodextrin and the block copolymer. These colloidal suspensions in the gel state were fully characterized by viscosimetry measurements, wide-angle X-ray diffraction, and scanning electron microscopy. The authors clearly showed that gelation time decreased with the increase of block copolymer concentration and that the supramolecular hybrid hydrogels demonstrated to have predominantly a solid-like behavior. The beneficial effect of α-cyclodextrin was evidenced in the catalytic reduction of methylene blue. Indeed, when Ag nanoparticles were used in an aqueous solution of Pluronic® F-68, the relative absorbance of methylene blue decreased very slowly, and when Ag nanoparticles were used in a hybrid hydrogel, the catalytic activity widely increased demonstrated by a sharp decrease of the relative absorbance of methylene blue.
A similar strategy had been reported by Wang et al. (2010) where colloidal silver hydrosols were synthesized using Pluronic® F-68 and then incorporated in situ into an α-cyclodextrin-assisted supramolecular hydrogel. An absorption peak around 430 nm was observed for the synthesized silver nanoparticles that were attributed to the characteristic surface plasmon resonance effect, showing the reduction and stabilization performances of the Pluronic®F-68. According to transmission electron microscopy images, the silver nanoparticles were deposited on the surface of Pluronic® aggregates. Pluronic® F-68 can also interact with α-cyclodextrin, and it was shown that the higher the Pluronic® concentration, the better the gelation process. The effects of the silver nanoparticles on the gelation process and the hydrogel strength were investigated through several physicochemical analyses. The catalytic activity of the resulting hydrogel-embedded silver nanoparticles was evaluated for the reduction of methylene blue using sodium borohydride. In the presence of the hybrid hydrogel, methylene blue was consumed (80% converted in 10 min) which was not the case in the absence of it. Léger et al. (2012) developed a thermoresponsive hydrogel for the synthesis of Ru nanoparticles and their activation for hydrogenation reactions. The hydrogel template properties have been taken to access size-controlled Ru nanoparticles. More precisely, once metal nanoparticles have been embedded into the supramolecular matrix, the system was heated above the sol-gel transition temperature to give a sol phase where the catalytic reaction took place. First, the polypseudorotaxane template was prepared from the mixture of the N-alkylpyridinium amphiphilic [py-N-(CH2)12OC6H3-3, 5-(OMe) 2]+ (Br−) and α-cyclodextrin. The self-assembly of these molecules yielded a thermoresponsive hydrogel with a sol-gel transition temperature of 42 °C. The synthesis of Ru nanoparticles was realized by classical chemical reduction of ruthenium metallic salt solubilized in hydrogel at the sol state (50 °C) by an excess of sodium borohydride. Transmission electron microscopy analysis clearly showed homogeneous dispersion of spherical Ru nanoparticles with an average diameter of 1.6 nm within the hydrogel network, which is smaller than that observed using surfactants or ionic liquids as Ru nanoparticle stabilizers. This result emphasized the effective control exerted by the hydrogel internal network structure over the Ru nanoparticle growth. The catalytic hydrogenation of various substrates, ranging from hydrophobic long-chain to hydrophilic olefins such as 2-methyl-3-buten-2-ol, was evaluated. Under H2 pressure ranging from 10 to 40 bar at 50 °C, turnover frequency comprised between 4 and 350 h−1 was obtained. At the end of the catalytic test, after cooling to ambient temperature, the hydrogel spontaneously returned to the gel state, and consequently the products could be easily recovered (Fig. 5.20). The recycling of the nanoparticles entrapped in the hydrogel had been successfully performed using 1-decene.
Thermoregulated Ru nanoparticle-catalyzed hydrogenation of alkenes. The ruthenium nanoparticles were embedded in the rotaxane-based gel phase. When temperature has increased, the gel-sol transition occurred, and cyclodextrins could play the role of mass transfer and “bring” alkenes into the Ru nanoparticle aqueous phase for their conversion into the corresponding alkanes. The catalytic system was recovered by the return of the temperature beyond the transition phase temperature. (Adapted from Léger et al. 2012)
The same group had also reported a similar strategy involving the synthesis of gold nanoparticles embedded into a thermoresponsive hydrogel made from the combination of Tetronic® 90R4 and α-cyclodextrin and their use in the catalytic hydrogenation of alkenes, alkynes, and aldehydes (Chevry et al. 2019). In this case, the first step consisted into the reduction of AuCl3 metal precursor in a Tetronic® 90R4 solution at 40 °C leading to the formation of Au nanoparticles where poloxamine played the role of the reducing agent. After heating of this solution, α-cyclodextrin was added to the colloidal suspension, and after cooling down to room temperature, Au nanoparticles were incorporated into α-cyclodextrin/Tetronic® 90R4 hydrogel. The addition of α-cyclodextrin was very important because it allowed the formation of the supramolecular hydrogel coming from the sliding of α-cyclodextrin along the copolymer chain. Moreover, by repeating heating/cooling cycles, the authors clearly showed that the presence of α-cyclodextrin improved the long-term stability of the Au nanoparticles by additional steric stabilization effects. Colloidal suspensions were synthesized considering Au concentration ranging from 0.1 mM to 2 mM. No significant influence of the Au concentration was noticed on the final mean diameters of Au nanoparticles always centered around 7 nm. The catalytic activity of the colloidal suspension prepared with Au concentration of 0.5 mM was particularly studied in the hydrogenation of terminal and internal alkenes and also alkynes under mild experimental conditions. Conversions were ranging from 5% to 54% depending on the nature of the substrate. The recyclability study was also realized in the styrene hydrogenation, and Au nanoparticles could be reused during five successive runs without any loss of activity, showing as well the robustness of this kind of catalytic system.
Ag nanoparticles were synthesized into thermosensitive poly(NIPAAm-co-AMPS) hydrogels by chemical reduction of silver nitrate with sodium borohydride (Wang et al. 2016c). Small-sized Ag nanoparticles were homogeneously dispersed into this hydrogel with a mean diameter centered on 3.5 nm. In this study, the silver colloidal suspensions were used in the catalytic reduction of 4-nitrophenol, and α-cyclodextrin was added before the catalytic test. The catalytic activity of Ag nanoparticles was better in the presence of α-cyclodextrin, and it was explained by the formation of an inclusion complex between the cyclodextrin and the substrate and a decrease of the activation energy.
Jia et al. (2015) reported a simple method for the immobilization of catalytically active gold nanoparticles in acrylate α-cyclodextrin-modified poly(N-vinylcaprolactam) microgels. According to transmission electron microscopy images, monodispersed gold nanoparticles with an average diameter of 5–6 nm were obtained without addition of any reducing agent. The reduction of the gold ions can be explained by the presence of the hydroxyl groups of poly(N-vinylcaprolactam) microgels. The homogeneous distribution of the particles could be attributed to the acrylate α-cyclodextrin through the capping efficiency on the surface of the gold particles due to the carboxylate-gold interactions. The immobilization of the gold particles did not influence the swelling-deswelling properties of the microgels. The poly(N-vinylcaprolactam)-α-cyclodextrin gold composite particles showed efficient catalytic activity for the reduction of aromatic nitro compounds. The host-guest capacity of cyclodextrin was proven through the reduction of p-nitrophenol, and the catalytic activity was much higher than in the case of dimethyl-p-nitrophenol.
In the same way, a thermosensitive catalytic system based on silver nanoparticles immobilized into a network of poly(NIPAAm-co-AMPS) hydrogels was studied (Wang et al. 2016c). The catalytic performances could be adjusted by choosing the adequate reaction temperature. The post-addition of α-cyclodextrin improved the catalytic activity by forming a complex with the 4-nitrophenol.
The complexation of guest molecules in the cyclodextrin cavity had also been studied with polymers. For example, Li et al. (1994) had put forward the formation of inclusion complexes called polypseudorotaxanes between poly(ethyleneglycol) and α-cyclodextrin in the 1990s. In order to immobilize cyclodextrin along the polymer chain, a large number of polymers with stoppers, called polyrotaxanes, had been reported. Thus, Wu et al. (2010) succeeded in stabilizing platinum nanoparticles in a water/ethylene glycol mixture, in the presence of a polyrotaxane in which α-cyclodextrin was threated onto poly(ethyleneglycol) chains capped with triazole-β-cyclodextrins (Fig. 5.21). The Pt nanoparticles were tested in the reduction of 4-nitrophenol by sodium borohydride. The authors assume that the high catalytic activity came from the high density and high surface area of the nanoparticles that had been adsorbed on the surface of the template.
Polyrotaxane synthesized by Gao and co-workers for the deposition of platinum nanoparticles in order to get nanowires. The catalytic activity is evaluated in the 4-nitrophenol reduction using sodium borohydride. (Adapted from Wu et al. 2010)
5.3 Nanoparticles Immobilized on a Support in the Presence of Cyclodextrin
Another strategy consists into the immobilization of metal nanoparticles on a support in order to improve the stability of these nanoheterogeneous catalysts. Moreover, the interaction between the support and nanoparticles could also influence the catalytic activity and the selectivity of the reaction. Considering the state of the art for nanoparticles immobilized on a support in the presence of cyclodextrin, two main strategies have been reported; whatever the nature of the support, it means inorganic supports or organic supports. It could be done regarding two different ways of preparation: (1) the one-pot method where the synthesis of nanoparticles is realized in the presence of the support and (2) the two-step method where the synthesis of nanoparticles is realized before the addition of the support. Whatever the strategy which will be considered, the multiple roles of the cyclodextrin described in the first part of this chapter related to the synthesis of the nanocatalysts and also to the catalytic processes will be further discussed in this part. All of the strategies mentioned before will be detailed above in this second part (Fig. 5.22).
General scheme involving the different ways of synthesis of nanoparticles immobilized on a support in the presence of cyclodextrin. The one-step method involves multi-task properties of the cyclodextrin: stabilizing agent, active phase dispersing agent, mass transfer agent, reducing agent in some cases. The two-step method involves the immobilization of preformed metal particles where cyclodextrins have different roles: stabilizing agent for the synthesis of the metal particles or preformed metal nanoparticle dispersing agent immobilized onto/into the support
5.3.1 Nanoparticles Immobilized on a Support Considering One-Step Method
5.3.1.1 Nanoparticles Immobilized on a Support in the Presence of Non-grafted Cyclodextrin
A cooperation of cyclodextrin with heterogeneous metal nanocatalyst was reported by Tang et al. (2015). They synthesized silver nanoparticles supported on cellulose nanocrystals and studied the reduction of 4-nitrophenol using NaBH4 as reducing agent. The initial rate was improved in the presence of native β-cyclodextrin, which can be explained by host-guest interactions between 4-nitrophenol and β-cyclodextrin.
In an attractive research, Li et al. (2014) benefited from the hybrid of β-cyclodextrin and graphene nanosheets as a support for the synthesis of Pt nanoparticles. This hybrid material was prepared through a simple wet chemical method which included the formation of graphene nanosheets-cyclodextrin material via reduction of graphene in the presence of cyclodextrin and hydrazine followed by the addition of PtCl62- and its subsequent reduction by sodium borohydride or formic acid leading to a heterogeneous catalyst with a hydrangea-like morphology. The authors believed that combination of cyclodextrin and graphene nanosheets could furnish a support with high surface area, conductivity, and recognition property. Noteworthy, the content of cyclodextrin was estimated to be 32 wt% which made the hybrid system water soluble. The hybrid system was successfully used as an electrocatalyst for the oxidation of MeOH with the catalytic activity and CO tolerance superior to those of conventional catalysts such as Pt/graphene nanosheets and Pt/Vulcan X72R. The higher catalytic activity, which made this electrocatalyst very promising for the use in direct methanol fuel cell, was attributed to the well dispersion of Pt nanoparticles and their specific morphologies.
Patil et al. (2018) prepared ruthenium nanoparticles supported on cyclodextrin-modified graphene oxide for the selective aerobic oxidation of alcohols in aqueous medium but also in stilbene derivatives’ hydrogenation. NaBH4 reduced both ruthenium precursor and graphene oxide in the presence of RaMe-β-cyclodextrin in a one-pot strategy. RaMe-β-cyclodextrin not only acted as a capping agent for Ru nanoparticles but also intercalated between the layers of graphene oxide and functionalized the surface of reduced graphene oxide by H bonding. The amount of RaMe-β-cyclodextrin in rGO@Ru-RaMe-β-cyclodextrin was found to be 42.33 wt% by thermogravimetric analysis when the amount of Ru, determined by ICP-AES analysis, was about 2.5%. The best catalytic results related to the piperonyl alcohol oxidation were obtained with rGO@Ru-RaMe-β-cyclodextrin in the presence of K2CO3. After this optimization step, the catalyst was tested for a wide range of benzylic alcohols with various substituents such as Cl, NO2, NH2, or OMe. Yields ranging from 88% to 94% were obtained with selectivities higher than 99%. This heterogeneous catalyst was also active in the reduction of several alkenes such as stilbene and its derivatives. The recyclability of rGO@Ru-RaMe-β-cyclodextrin was studied, and a slight decrease of the catalytic activity was observed after five runs which could came from the leaching of Ru, confirmed by ICP-AES, and also the leaching of RaMe-β-cyclodextrin, confirmed by thermogravimetric analysis.
The group of Montazer studied the influence of the amount of native β-cyclodextrin in the synthesis of a nanocomposite. Ag/TiO2 was prepared by addition of a solution containing cyclodextrin and silver to a TiO2 dispersion under irradiation (Attarchi et al. 2013). They studied the influence of the amount of β-cyclodextrin, and according to dynamic light scattering experiments, the average hydrodynamic diameter in water increased with the amount of cyclodextrin which was still present in the composite. The methylene blue photodegradation was studied with these composite nanomaterials, and the reactivity was faster with Ag/TiO2/β-cyclodextrin (65% of degraded methylene) than pure TiO2 (39%) or Ag/TiO2 (52%). These results can be explained by the strong affinity of cyclodextrin toward TiO2 surface via the outer hydroxyl groups of the cyclodextrin making a bridge between the titanium oxide and methylene blue. Moreover, cyclodextrin could act as a host molecule. However, the use of excess cyclodextrin had a detrimental role in the degradation rate.
Another study reported the synthesis of Ag-β-cyclodextrin/TiO2 loaded onto activated carbon by using microwave-assisted procedure and its use for the photocatalytic degradation of naphthalene (Chen et al. 2018). In comparison to some control catalysts, the best catalytic activities were obtained with Ag-β-cyclodextrin/TiO2/AC catalyst due to host-guest interactions between β-cyclodextrin and naphthalene leading to an increase of the interactions between the substrate and TiO2 surface.
A nanocomposite based on iron oxide nanoparticles and β-cyclodextrin (Fe3O4@β-cyclodextrin) was prepared via a one-pot strategy by mixing iron salts and β-cyclodextrin in sulfuric acid aqueous solution (Wang et al. 2016b). According to transmission electron microscopy images, Fe3O4 and Fe3O4@β-cyclodextrin particles were spherical with an average diameter between 10 nm and 20 nm. X-ray diffraction patterns indicated a spinel structure with no crystal structure modification in the presence of β-cyclodextrin, but the intensity of the peaks decreased. This composite was tested in the 4-chorophenol degradation using hydrogen peroxide. According to the kinetic following, the composite was 2.3 times more active than the iron oxide alone. This catalytic activity enhancement can be explained by host-guest interactions between β-cyclodextrin and the 4-chorophenol.
Hydroxypropyl-β-cyclodextrin was used both as fullerene [60] dispersing agent in water and stabilizing agent for the synthesis of supported Pd nanoparticles for electrocatalytic applications (Zhang et al. 2015). Two controls, i.e., Pd nanoparticles synthesized without hydroxypropyl-β-cyclodextrin and one without fullerene, were considered to show the beneficial effect of the C60-hydroxypropyl-β-cyclodextrin combination. According to transmission electron microscopy experiments, a uniform size distribution and a good adsorption of the Pd nanoparticles on fullerene were observed on the composite material. Aggregates were obtained without cyclodextrin, and a less uniform distribution was obtained without fullerene. According to X-ray diffraction patterns, neither hydroxypropyl-β-cyclodextrin nor C60 had influence on the nanoparticle crystallinity, but the adsorption of hydroxypropyl-β-cyclodextrin on the fullerene surface was observed by elemental mapping measurement. According to thermogravimetric analysis, the cyclodextrin content was around 5%. The catalytic activity of Pd nanoparticles supported on the composite material was evaluated in the ethanol electrooxidation. Pd supported on hydroxypropyl-β-cyclodextrin/C60 showed better electrocatalytic activity which was explained by a smaller size and a better distribution of the nanoparticles onto the support.
The synthesis of carbon-based cobalt oxide (Co3O4/C) and gold nanoparticles immobilized on Co3O4/C using several ways (impregnation, microwave irradiation, impregnation, and microwave irradiation) in aqueous medium in the presence of β-cyclodextrin was recently reported (Kepenienė et al. 2020). These materials were evaluated in the catalytic oxygen reduction reaction, and the Au supported on Co3O4/C gave the highest activities.
5.3.1.2 Nanoparticles Immobilized on a Cyclodextrin-Grafted Support
Another strategy consisted in the use of cyclodextrin-decorated magnetic nanoparticles. In this context, γ-Fe2O3@SiO2-cyclodextrin core-shell hollow sphere can be mentioned (Sadjadi et al. 2017). The hybrid system was developed by initial synthesis of γ-Fe2O3@SiO2 core-shell system followed by amine functionalization by using 3-N-(2-(trimethoxysilyl)ethyl)methanediamine and subsequent reaction with tosylated cyclodextrin. The system was then applied for the immobilization of silver nanoparticles which were reduced and capped by Hollyhock flower extract. Notably, upon introduction of non-magnetic component, the maximum saturation magnetization (Ms) value of the hybrid system did not decreased remarkably compared to bare Fe2O3. Finally, the catalytic activity of the ternary hybrid was studied for ultrasonic-assisted A3 and KA2 coupling reactions of phenyl acetylene, amines, aldehydes, or ketones. The results established the excellent performance of the magnetic hybrid system, which was superior to some of previous reports. Hot filtration test confirmed that the silver leaching was considerably controlled and the catalyst could be reused up to four reaction runs.
In another example, Azaroon and Kiasat (2018) developed an organometallic magnetic catalyst for selective reduction of nitro functionality to corresponding amine compounds. To prepare the catalyst, magnetic hydroxyapatite (HAp) γ-Fe2O3@HAp was first synthesized, and its surface was amine functionalized. The final catalyst was obtained through reaction with 1,1-carbonyldiimidazole-β-cyclodextrin followed by incorporation of silver nanoparticles. The authors believed that the cavity of cyclodextrin could stabilize silver nanoparticles and avoid them from aggregation. Notably, the catalyst, γ-Fe2O3@HAp-cyclodextrin.Ag, could be recovered magnetically and recycled for five reaction runs with negligible loss of the catalytic activity.
Chalasani and Vasudevan (2013) developed cyclodextrin-functionalized Fe3O4@TiO2 core-shell nanoparticle via a two-step process. Initially, spherical Fe3O4 nanocrystals were prepared via thermal decomposition of FeOOH in a high boiling octadecene solution and covered by a titania shell through the hydrolysis of tetrabutyl titanate. The resultant compound was then capped with carboxymethyl-β-cyclodextrin in the presence of carbodiimide (Fig. 5.23). The transmission electron microscopy analysis revealed that the nanocrystals had the mean diameter of 12 nm and core of about 9 nm. The hybrid system was used as a photocatalyst for the degradation of bisphenol A and dibutyl phthalate, in aqueous media. Notably, the catalytic performance of cyclodextrin-functionalized Fe3O4@TiO2 core-shell nanoparticle was higher than that of Fe3O4@TiO2 core-shell nanoparticles. This could be attributed to the role of carboxymethyl-β-cyclodextrin coming from its ability to disperse the nanoparticles in the aqueous phase and to form an inclusion complex with pollutants. The authors believed that this cost-effective catalyst could be simply recovered and reused.
Preparation of carboxymethyl-β-cyclodextrin-Fe3O4@TiO2. Firstly, magnetic nanocrystals as cores were prepared through thermal decomposition of FeOOH and then coated with titania shell. Then, the nanoparticles were capped with carboxymethyl-β-cyclodextrin. (Adapted from Chalasani and Vasudevan 2013)
Sadjadi (2018) reported β-cyclodextrin-decorated halloysite nanoclay (Hal) as a potential support for the immobilization of Pd nanoparticles. The hybrid catalyst, Pd@Hal-T-cyclodextrin, was simply prepared through Cl-functionalization of halloysite nanoclay and its subsequent reaction with thiourea and tosylated cyclodextrin (Fig. 5.24). Pd@Hal-T-cyclodextrin was successfully used for catalyzing copper and ligand-free Sonogashira coupling reaction under mild reaction conditions. Noteworthy, the catalyst was recyclable and could be successfully recovered and recycled up to five reaction runs.
The schematic process for the fabrication of Pd@Hal-T-cyclodextrin. Halloysite nanoclay was first Cl-functionalized and then reacted with thiourea and tosylated cyclodextrin. The prepared support was applied for Pd stabilization. It is presumed that the presence of cyclodextrin in the structure of the catalyst could act both as a capping agent for Pd nanoparticles and a phase-transfer agent for the hydrophobic substrate in coupling reaction. (Adapted from Sadjadi 2018)
Khalafi-Nezhad and Panahi (2014) introduced an organic-inorganic hybrid catalyst, PdNP-silica cyclodextrin, by the synthesis of 1–10 nm Pd nanoparticles in the presence of cyclodextrin grafted onto silica. The catalytic activity of the hybrid catalyst was studied for Heck coupling reaction. Notably, the catalyst exhibited high reusability and could be reused up to six reaction runs with only negligible loss of the catalytic activity.
In another attempt, Martina et al. (2016) developed an efficient, green, and rapid microwave-assisted protocol for ligand-free Suzuki and Heck C–C coupling reaction as well as semi-hydrogenation of phenyl acetylene by using a novel catalyst, Pd/Si-cyclodextrin. The catalyst was prepared though reaction of (3-glycidoxypropyl)methyltriethoxysilane and silica SIPERNAT. The modified silica, Si-GPMS, reacted with 10-undecynil-1-amine under microwave irradiation to afford Si-G-Und. The latter reacted with 6-monoazido-β-cyclodextrin in the presence of ascorbic acid and CuSO4·4H2O to furnish Si-cyclodextrin which subsequently tolerated reaction with Pd(OAc)2. Pd nanoparticle immobilization on Si-cyclodextrin was achieved through reduction of Pd salt in EtOH or H2O. This step could be carried out under ultrasonic irradiation. It was found that the presence of cyclodextrin could increase the Pd content in the final catalyst. Moreover, this value was affected by the solvent and the reaction condition used in the final step. The highest loading, 6 wt%, was observed upon using EtOH under reflux conditions. Noteworthy, cyclodextrin also affected the size distribution of Pd nanoparticles. Studying the reusability of the catalyst for four reuses established only slight loss of the catalytic activity. High yields, low reaction times, and low amount of the catalyst were other benefits of this protocol.
Considering the potential of cyclodextrin as both reducing agent and dispersant, Li et al. (2015) designed and synthesized a β-cyclodextrin and multiwalled carbon nanotube hybrid material for deposition of PtRh nanoparticles through a one-pot hydrothermal approach (Fig. 5.25). Briefly, a solution of β-CD in water was added to the suspension of multiwalled carbon nanotubes and then sonicated. The solutions of metal precursors were introduced, and the obtained mixture was subjected to the hydrothermal treatment. FTIR analysis of this composite material was done, and it clearly showed that β-cyclodextrin was attached to the surface of the pristine carbon nanotubes. Moreover, the amount of β-cyclodextrin attached to the multiwalled carbon nanotubes was determined by thermogravimetric analysis experiment and was about 9.3% in the PtRh/β-cyclodextrin-CNT material. The obtained hybrid system, PtRh/β-cyclodextrin-CNTs, was used as an electrocatalyst for promoting methanol oxidation. The authors also compared the efficiency of the cyclodextrin-containing hybrid with the PtRh nanoparticles supported on multiwalled acid-treated carbon nanotubes (AO-CNTs) which was obtained through reduction by NaBH4. The results established the smallest size and highest dispersion of the metallic components in PtRh/β-cyclodextrin-CNTs and consequently excellent electrocatalytic activity compared to PtRh/AO-CNTs. Notably, the authors studied the effect of Pt/Rh atomic ratio and found the atomic ratio of 1:1 as the best choice.
Schematic illustration of the fabrication of PtRh/acid-treated carbon nanotubes (upper) and PtRh/β-cyclodextrin-carbon nanotubes (below). As depicted, for the preparation of PtRh/β-cyclodextrin-carbon nanotubes, an aqueous solution of cyclodextrin and multiwalled carbon nanotube suspension was sonicated, and then the metal precursors were added, and the mixture was hydrothermally treated. In the case of PtRh/AO-carbon nanotubes, however, multiwalled carbon nanotube was acid treated, and the metallic particles were obtained via reduction by NaBH4. Notably, in the case of PtRh/β-cyclodextrin-carbon nanotubes, the nanoparticles with smaller sizes and better dispersion were obtained. (Adapted from Li et al. 2015)
In another report, Sadjadi et al. (2018b) covalently conjugated carbon nanotubes (CNT) with cyclodextrin nanosponges (CDNS) and applied the hybrid system as a heterogeneous support for the immobilization of Pd nanoparticles. The hybrid system Pd@CDNS-CNT that benefited from the chemistry of both carbon nanotubes and cyclodextrin nanosponges chemistry was then used as an efficient catalyst for catalyzing the ligand and copper-free Sonogashira and Heck coupling reactions in aqueous media. The comparison of the catalytic activity of Pd@CDNS-CNT with that of control catalysts, Pd@CNT, Pd@CDNS, and Pd@CNT + CDNS, established that the hybrid catalyst exhibited superior catalytic activity, indicating that hybridization of carbon nanotubes and cyclodextrin nanosponges was more effective than the use of each one separately or as individual. The recyclability of the catalyst up to six reaction runs was also confirmed.
Taking advantage of β-cyclodextrin-decorated reduced graphene oxide (rGO) as support, β-cyclodextrin-rGO, Ran et al. (2017) developed a mild and efficient procedure for the synthesis of very small (size of 2 nm) and well-dispersed Pd-Pt bimetallic nanoclusters, Pd-Pt@β-cyclodextrin-rGO (Fig. 5.26). The synthetic process took place in an aqueous solution at ambient temperature and considering a very short reaction time of 30 min in the absence of organic solvents. FTIR experiment was done, and it clearly showed that β-cyclodextrin was attached to reduced graphene oxide. By thermogravimetric analysis, the amount of β-cyclodextrin was determined and was about 28 wt%. This grafting of β-cyclodextrin could prevent the aggregation of the bimetallic clusters. The hybrid system was used as an electrocatalyst for promoting methanol and ethanol oxidation. The comparison of the catalytic activity of this hybrid system with conventional Pd/C and Pd@β-CD-rGO, Pt@β-CDrGO, and Pd-Pt@rGO confirmed its superior catalytic activity, poison tolerance, and durability which could emerge from very tiny size, high monodispersity, and uniformity of bimetallic catalyst, using a hybrid support with high surface area and synergistic effects due to the coexistence of Pd and Pt atoms on the surface.
Schematic presentation for the preparation of the Pd-Pt@β-cyclodextrin-reduced graphene oxide nanohybrid using the in situ reduction method. The presence of cyclodextrin in the structure of the support prevented the bimetallic clusters from aggregation and led to the formation of small particles with high dispersion. (Adapted from Ran et al. 2017)
Putta et al. (2015) disclosed a novel hybrid system, Pd@cyclodextrin-graphene nanosheets, containing Pd nanoparticles, β-cyclodextrin, and graphene nanosheets with the utility as a catalyst for promoting phosphine-free Suzuki-Miyaura, Heck-Mizoroki, and C-C coupling reactions in aqueous media. To prepare cyclodextrin-graphene nanosheets, the suspension of graphene oxide in deionized water was well dispersed by using ultrasonic irradiation and mixed with the solution of β-cyclodextrin and ammonia. Then, hydrazine solution was added, and the resulting mixture reacted under stirring to afford cyclodextrin-graphene nanosheets. To incorporate Pd nanoparticles, cyclodextrin-graphene nanosheets in ethanol were dispersed and reacted with PdCl2. Ethanol played the role of green solvent and in situ reducing agent. The Pd loading in the final catalyst was calculated to be 6.2 wt%. The authors believed that the presence of cyclodextrin on graphene nanosheets could stabilize the Pd nanoparticles, improve their dispersion, and avoid agglomeration. Moreover, the cyclodextrin cavity could serve as an inclusion site for the reagents. These factors as well as “Breslow effect” and formation of ternary cyclodextrin/substrate/additive complexes on the Pd-graphene nanosheet surface led to high catalytic activity of the hybrid system. Notably, the catalyst was reusable and could promote four successive reaction runs with loss of the catalytic activity to some extent (about 20% for the fourth reaction run compared to the first run). The hot filtration test as well as ICP-AES analysis proved 0.31 wt% Pd leaching in the reused catalyst after three reaction runs compared to fresh catalyst. Low amount of the catalyst, high yields, green and simple procedure, and wide substrate scope were the merits of this strategy.
To improve the water disposability of fullerene, C60, it was modified with hydroxypropyl-β-cyclodextrin. The hybrid system was then applied for supporting Pd nanoclusters with mean particle size of 2.5 nm (Zhang et al. 2015). Hydroxypropyl-β-cyclodextrin acted as a coordination agent and surfactant and improved the disparity and compositional uniformity of nanoparticles. The hybrid system with an electrochemical surface area of 41.6 m2 g−1 exhibited outstanding electrocatalytic activity for the oxidation of formic acid. Moreover, the much more negative onset potentials and better stability compared to electrodes modified by other electrocatalysts were observed, implying the promising utility of this system for use in a direct formic acid fuel cell.
Considering the high electrocatalytic activity of Au nanoparticles, the recognition ability and hydrophilicity of cyclodextrin, and high surface area and electrochemical features of hollow carbon nanospheres (HCNS), Yi et al. (2015) developed an Au-based hybrid system, β-cyclodextrin-AuNPs/HCNS, based on the initial synthesis of silica@polydopamine followed by carbonization to afford hollow carbon nanospheres and subsequent introduction of gold nanoparticles and β-cyclodextrin (Fig. 5.27). The resulting nanocomposite was used as an electrode material to detect RhB by electrochemical method. The comparison of the performance of β-cyclodextrin-Au nanoparticles/HCNS/GCE (GCE stands for glassy carbon electrode) with HCNS/GCE and Au nanoparticles/HCNS/GCE established the superior performance of the former, the detection limit of 0.96 μg L−1, indicating the synergistic effects between the three components, cyclodextrin, hollow carbon nanospheres, and gold nanoparticles.
Representation of electrochemically sensing of RhB (the blue star) based on β-cyclodextrin-Au-nanoparticles/hollow carbon nanosphere nanohybrids. As shown, for the synthesis of the catalyst, SiO2 particles were reacted with dopamine (DA) to form silica@polydopamine. The latter was then carbonized and silica template was removed by treating with HF. Subsequently, the resulting hollow carbon nanospheres were used for Au immobilization. Finally, Au-nanoparticles/hollow carbon nanospheres were mixed with cyclodextrin and sonicated. (Adapted from Yi et al. 2015)
Among the different organic supports which could be considered in order to support metal nanoparticles, the use of polymer network has also been reported. Huang et al. (2019) had reported in 2019 the use of cyclodextrin polymer networks decorated by different metal nanoparticles. In this case, ultra-small noble metal nanoparticles based on Pd, Ag, Pt, Au, and Rh were prepared by a simple chemical reduction of the metal precursor by sodium borohydride. Ultra-small nanoparticles were obtained with mean diameters ranging from 0.5 nm to 0.9 nm, respectively, for Ag nanoparticles and Pd nanoparticles. The Pd nanoparticles deposited onto cyclodextrin polymer networks were fully characterized by powder X-ray diffraction, HAADF-STEM, X-ray photoelectron spectroscopy, and ICP. The authors clearly showed the importance of 1,2,3-triazolyl groups in order to obtain very small nanoparticles dispersed homogeneously onto the cyclodextrin polymer network. Finally, the catalytic activity of these Pd nanoparticles was evaluated in the reduction of 4-nitrophenol. The beneficial effect of the cyclodextrin was evidenced by a reaction conducted in the presence of 1-adamantane carboxylate sodium salt (ACNa) which is known to strongly interact with β-cyclodextrin. A decrease of the conversion was observed in the presence of ACNa in comparison with the catalytic test made without ACNa. Indeed, the adsorption of 4-nitrophenol onto Pd nanoparticle surface could be enhanced by the complexation of 4-nitrophenol with β-cyclodextrin. The recyclability of this catalytic system was also studied, and it was reused during seven successive catalytic runs without any loss of activity and increase of the mean diameter of the Pd nanoparticles. Moreover, these Pd nanoparticles revealed to be active in the hydrogenation of a wide variety of nitroarenes and also in the Suzuki-Miyaura coupling reaction.
Palladium nanoparticles were immobilized on cyclodextrin-modified poly(amidoamine)s (PAAs) by a chemical reduction of Pd(OAc)2 in dimethylsulfoxide (Zhang et al. 2019). FTIR data confirmed the presence of the polymer and the cyclodextrin structures in the material. Scanning electron microscopy image showed that the Pd nanoparticles were uniformly dispersed onto the PAAs-cyclodextrin with a globular radius of 10 nm. The catalytic activity of these supported Pd nanoparticles was evaluated in the Suzuki reaction in aqueous medium. The temperature and the nature of the base were studied for the optimization of the reaction between the phenylboronic acid and 4-bromoethoxybenzene. The catalyst showed good activities which is probably due to the cyclodextrin moiety which ensured a good dispersion of the heterogeneous catalyst in water. The beneficial effect of the cyclodextrin through inhibitive experiments using adamantane was confirmed by an activity decrease. The study was then extended to a variety of aryl halides, and the cross-coupling products were obtained from moderate to excellent yields. The catalytic system could finally be recycled by maintaining activity after six successive runs.
The development of nanofibers has received an increasing interest since several years. This kind of material could be considered for the development of heterogeneous catalysts by the deposition or the incorporation of metal nanoparticles onto and into the nanofibers. The group of Uyar is a precursor in this field. Indeed, in 2019, they reported the incorporation of Ag nanoparticles into nanofibers made from the electrospinning of hydroxypropyl-β-cyclodextrin solution containing Ag nanoparticles (Celebioglu et al. 2019b). In this case, Ag nanoparticles were synthesized before the electrospinning step using hydroxypropyl-β-cyclodextrin as reducing agent of silver metal precursor in an alkaline solution. Hydroxypropyl-β-cyclodextrin was also used for the production of the nanofiber mats. The catalytic nanofibers were prepared starting from a dimethylformamide solution or an aqueous solution. Ag nanoparticles were homogeneously dispersed onto the nanofiber mats, and the mean diameter of Ag nanoparticles increased when the nanofibers were prepared in dimethylformamide instead of aqueous solutions and when the amount of silver increased in the nanofiber mats from 1 wt. % to 2 wt. %. Indeed, the mean size of Ag nanoparticles in the fiber matrix produced from dimethylformamide increased from 3.5 nm to 4.8 nm for, respectively, 1 wt. % and 2 wt. % of Ag loading. And the mean size of Ag nanoparticles in the fiber matrix produced from aqueous solutions increased from 1.9 nm to 2.3 nm for, respectively, 1 wt. % and 2 wt. %. The same tendency was observed for the mean diameter of the electrospun nanofibers. The antibacterial properties of the nanofiber mats against gram-negative (E. coli) and gram-positive (S. aureus) were finally evaluated. These hydroxypropyl-β-cyclodextrin/Ag nanoparticles composite nanofibers showed antibacterial activities, and it was directly due to the presence of Ag nanoparticles in the nanofiber mats.
The same strategy was considered by the same team for the incorporation of Pd nanoparticles into cyclodextrin nanofibers which were used for the catalytic reduction of nitroarene (Celebioglu et al. 2019a). These nanocomposite nanofibers were prepared by electrospinning a hydroxypropyl-β-cyclodextrin solution containing Pd(OAc)2 as palladium precursor. Hydroxypropyl-β-cyclodextrin played the role of reducing agent of the palladium source and was very important in order to manufacture the electrospun nanofibers. Different parameters such as the Pd loading and the nature of the solvent were studied. These composite nanofibers were fully characterized by FTIR, transmission electron microscopy, scanning electron microscopy, and X-ray photoelectron spectroscopy. Pd nanoparticles were homogeneously dispersed into and onto the nanofibers with mean diameter ranging from 3.7 and 4.9 nm depending on the nature of the solvent or the Pd loading. These nanofibers containing Pd nanoparticles were catalytically active in the reduction of 4-nitrophenol into 4-aminophenol with turnover frequency ranging from 10.17 h−1 to 12.25 h−1. The authors clearly showed the multiple role of hydroxypropyl-β-cyclodextrin for the preparation of these catalytic nanofibers; it means the role of reducing agent of palladium metal precursor, the role of stabilizing agent of Pd nanoparticles, and also the role of handy nanofibrous carrier matrix.
Another strategy has been developed by the same team and consisted into the production of cyclodextrin nanofibers by the electrospinning of an aqueous solution of hydroxypropyl-β-cyclodextrin containing a multifunctional cross-linker (1,2,3,4-butanetetracarboxylic acid, BTCA) in order to improve the robustness of the nanofibers. After manufacturing of these poly-cyclodextrin nanowebs, Pd nanoparticles were supported onto this material by atomic layer deposition (Celebioglu et al. 2017). Transmission electron microscopy measurements clearly showed that Pd nanoparticles were homogeneously dispersed onto the nanofiber mats with a mean diameter centered on 4.34 nm. These nanofibers were used for the catalytic reduction of 4-nitrophenol which was completely converted into 4-aminophenol within 35 min. The reusability of these catalytic nanofibers was also studied, and it was possible to reuse the Pd nanoparticles deposited onto electrospun nanofibers during five successive runs without any loss of activity which clearly showed that the Pd nanoparticles were strongly anchored onto the poly-cyclodextrin nanowebs.
Yi et al. (2013) employed β-cyclodextrin for modification of multiwalled carbon nanotubes (MWCNT) immobilized on Ti plates to prepare a novel electrode on which binary Pd-Ni nanoparticles were electrodeposited. It was confirmed that incorporation of β-cyclodextrin could improve the dispersion of nanoparticles and led to nanoparticles with smaller sizes compared with the material without β-cyclodextrin. The authors studied the electrocatalytic performance of the electrode for alcohol electrooxidation and compared it with those of PdNi/MWCNT/Ti and PdNi/Ti. The results established the superior electrocatalytic activity of the former.
Shen et al. (2013) synthesized a composite material based on reduced graphene oxide rGO/β-cyclodextrin/TiO2 in a one-pot hydrothermal strategy. FTIR analysis clearly showed that β-cyclodextrin molecules were still attached to the surface of rGO. Moreover, by transmission electron microscopy, the author explained that the sheets tend to form small aggregates due to the cross-linked rGO sheets with β-cyclodextrin molecules. In this composite material, β-cyclodextrin acted as a linker between rGO and TiO2 nanoparticles. The photocatalytic and adsorption efficiencies are higher than rGO/TiO2 or rGO/β-cyclodextrin materials which can be explained by the interactions between the three components.
Sadjadi et al. (2018a) disclosed the utility of a hybrid system composed of halloysite nanotube and cyclodextrin nanosponges (CDNS), prepared from reaction of cyclodextrins monomers and diphenyl carbonate, for the immobilization of Pd nanoparticles. To suppress the leaching of Pd nanoparticles, graphitic carbon nitride, g-C3N4, was also introduced to the hybrid system via hydrothermal treatment (Fig. 5.28). The final catalytic system, Pd@Hal-CDNS-g-C3N4, was successfully used for promoting ligand and copper-free Sonogashira and Heck coupling reactions under mild and environmentally benign conditions. The authors believed that cyclodextrin nanosponges could contribute to the catalysis through formation of inclusion complex with the substrates and closing them to the catalytic sites. Notably, the catalyst showed high recyclability, up to ten consecutive reaction runs with slight loss of the catalytic activity and Pd leaching. Using control catalysts, the authors also confirmed the contribution of each hybrid components to the catalysis as well as the synergism between them.
The procedure for the preparation of Pd@Hal-CDNS-g-C3N4. As shown, cyclodextrin nanosponges (CDNS) was first prepared and amine functionalized and then reacted with Cl-functionalized halloysite nanoclay. In the next step, the as-prepared hybrid was palladated and hydrothermally reacted with g-C3N4. It is believed that cyclodextrin nanosponges served as a phase-transfer agent, while g-C3N4 could suppress Pd leaching. (Adapted from Sadjadi et al. 2018b)
5.3.2 Nanoparticles Immobilized on a Support Considering Two-Step Method
Among the different methods reported for obtaining well-dispersed supported metallic nanoparticles, the deposition of metallic nanoparticles onto a porous support from stabilized colloidal suspensions has received attention since 2005. For example, Ponchel and co-workers have studied the preparation of carbon-supported ruthenium catalysts for gas-phase hydrogenation reactions (Denicourt-Nowicki et al. 2008; Wyrwalski et al. 2011). The idea was to take advantage of an efficient anchoring of the metallic nanoparticles onto the carbon support via hydrophobic interactions coming from the presence of the cyclodextrin. To validate the strategy, a series of carbon-supported ruthenium nanocatalysts were prepared by the adsorption on a porous activated carbon of Ru nanoparticles preformed in aqueous solution by chemical reduction of RuCl3 in the presence of RaMe-cyclodextrin (α, β, and γ). Nitrogen adsorption measurements showed that the immobilization of the RaMe-cyclodextrin-stabilized Ru nanoparticles deeply affected the textural properties of the porous carbon material. Moreover, thermogravimetric measurements proved that the prepared catalysts were thermally stable up to 235 °C under both inert and reducing atmospheres. Finally, the study of the dispersion and morphology of the supported particles was carried out by transmission electron microscopy analysis. For instance, the transmission electron microscopy characterization of the Ru-3-β-cyclodextrin/C sample showed that nanoparticles had a spherical shape with an average diameter of 2.4 nm. The catalytic activity of the Ru nanoparticles was evaluated in the hydrogenation of xylene isomers in gas phase at 85 °C. The catalytic results had clearly shown that the cyclodextrin-based Ru catalysts were more efficient than the control Ru/C. Moreover, the catalytic activity depended on the cyclodextrin size and initial cyclodextrin/Ru ratio. In terms of stereoselectivity, the trans to cis ratio was improved in the presence of cyclodextrin-based catalysts and thus whatever the substrate. These results can be explained by several factors such as the dispersion of the active species through a promoting effect of the cyclodextrin and the host-guest interactions occurring between the substrate and cyclodextrin, which is adsorbed onto the nanoparticles.
The generation of materials from the incorporation of metal particles into polymer matrix received a growing interest due to applications in electrocatalysis such as methanol oxidation (Chen et al. 2014) or dioxygen electroreduction (Gopalan et al. 2006; Chen et al. 2015). For example, gold nanoparticles were stabilized by an inclusion complex of cyclodextrin with 4-aminothiophenol. These nanoparticles were then electrochemically deposited on glass electrode forming Au(0) nanoparticles and poly(aminothiophenol). A repairable catalytic system was considered on the basis of pre-synthesized gold nanoparticles stabilized by thiolated-β-cyclodextrin and porous nickel (PNi) containing azobenzene compounds to adsorb these metal nanoparticles by the formation of an inclusion complex (Zhou et al. 2017). The high specific surface area and connected porous structure of porous nickel provided a good opportunity to achieve the multivalent interactions between β-cyclodextrin-Au nanoparticles and PNi@IPTS-Azo. Additionally, the reactant solution could be catalyzed by flowing through the pores of the PNi@IPTS-Azo@β-CD-AuNPs. This catalytic model showed a high efficiency close to 95%. Because of the reversible multivalent host-guest interactions between thiolated β-cyclodextrin and azobenzene, the catalytic system could be regenerated by removing the deactivated Au nanoparticles with UV light irradiation and recombining new ones through in situ multivalent interactions. Because of the large specific surface area and connected porous structure of nickel, a large number of Au nanoparticles could be anchored on the surface through multivalent host-guest interactions, and the reactant solution could be catalyzed by flowing through the pores. Because of the photoisomerization property of azobenzene, the multivalent host-guest interactions between β-cyclodextrin and azobenzene could be removed by UV irradiation. Thus, the gold nanoparticles could be anchored on or removed from the surface of the porous nickel. Different electrocatalysts were prepared by increasing the gold amount. These electrocatalysts had been evaluated in the oxygen electroreduction and compared to the unmodified electrode. The authors observed an activity enhancement for the poly(aminothiophenol)-Aunano which can be explained by small crystallite sizes and good distribution of gold nanoparticles on the surface of the polymer. Platinum nanoworms self-assemble with a β-cyclodextrin polymer/reduced graphene oxide via the use of 4-aminothiophenol as nanoparticle capping agent incorporated in the cyclodextrin cavities (Gopalan et al. 2006). The cyclodextrin-based polymer was synthesized by cross-linking β-cyclodextrin with epichlorohydrin and the graphene oxide from natural graphite. The polymer of cyclodextrin was modified on graphene oxide, and the resulting composite was dispersed in ethanol and mixed with polyaminothiophenol. The modified graphene oxide was reduced by hydrazine and filtered before the addition of Pt nanoworms. The interactions of the 4-aminothiophenol and the polymer of cyclodextrin were evaluated by UV-Vis spectroscopy and by FTIR spectroscopy. One interaction gave a red shift corresponding to the thiol/amino group from 251 nm to 256 nm. Moreover, all the characteristic peaks of 4-aminothiophenol were not observed because 4-aminothiophenol molecules were embedded inside the cyclodextrin cavity, lowering the vibrations of absorption bands. Energy-dispersive X-ray spectroscopy experiments were performed to determine the content of the platinum nanoworms anchoring on the composite. The main elements which were found were Pt, C, N, O, and S, confirming that both inclusion complex-based polymer and platinum were anchored on reduced graphene oxide. Thermogravimetric analysis confirmed the above results. This heterogeneous catalyst had proved to be electrocatalytic active for oxygen reduction reaction and was stable because no significant decrease of the current response was observed after 1 week.
In 2015, pre-synthesized gold nanoparticles were adsorbed on the same composite (cyclodextrin-based polymer with reduced graphene oxide) (Chen et al. 2015). Moreover, the surface properties were characterized by static contact angles where the more polymer is adsorbed on reduced graphene oxide, the stronger the hydrophilicity of the composite is. The interactions of the 4-aminothiophenol and the polymer of cyclodextrin was evaluated by UV-Vis and 1H NMR spectroscopy. The presence of the gold nanoparticles on the composite material was confirmed by UV-Vis spectroscopy by the observation of one red shift of the surface plasmon resonance of gold nanoparticles, from 522 nm to 568 nm, due to the interaction of the 4-aminothiophenol with the particles. This interaction gave another red shift corresponding to the thiol/amino group from 251 nm to 253 nm. Structural analyses were also performed (X-ray diffraction and energy-dispersive X-ray spectroscopy). The catalytic activity of these materials was evaluated in the oxygen electroreduction in a 0.1 M H2SO4 solution under several atmospheres. According to the voltammetric data, the Au nanoparticles supported on 4-ATP_β-CDP/rGO showed a good activity toward oxygen reduction. The catalytic activity increased with the Au nanoparticle loading.
Immobilized gold nanoparticles (Fe3O4@Au) were synthesized through a self-assembly route between thiolated-β-cyclodextrin gold nanoparticles and ferrocenyl-functionalized iron oxide Fe3O4 nanoparticles (Qu et al. 2018). This catalyst showed a high catalytic activity in the reduction of 4-nitrophenol in comparison of control experiments. The Fe3O4@Au ensured good recyclability by an easy recovery due to the magnetic properties of the support and by keeping the catalytic activity even after ten runs. The catalyst could be disassembled via redox properties by using hydrogen peroxide. This latter oxidizes the ferrocenyl moiety, and the corresponding oxidized product could not interact with the cyclodextrin cavity, leading to the disassembly process.
Combining the advantages of click chemistry and supramolecular assembly, Li et al. (2016) developed a novel hybrid nanocatalyst containing a movable platinum nanocluster encapsulated in temperature- and pH-responsive polymer brushes decorated through a template-assisted protocol. The authors studied the catalytic activity of the inorganic-polymer nanocomposite for the reduction of 4-nitrophenol in the presence of NaBH4. The results established the high catalytic activity and reusability of the catalyst. It is suggested that the hairy hybrid nanorattles which contained the hydrophilic poly(N-vinylcaprolactam) brushes on their surface could improve the dispersion in the aqueous media. Notably, diverse catalysts of this type could be prepared by altering the thickness of P[MAA-co-(PMA-click-β-cyclodextrin)] shell and the length of poly(N-vinylcaprolactam) brushes and changing the size of SiO2 intertemplate layer during the sol-gel process into a cross-linked β-cyclodextrin polymer network.
5.4 Conclusion
This chapter has highlighted several historical roles of the cyclodextrin in catalysis using metal nanoparticles as active phase; whatever the nature of the catalyst, it means solvent-dispersed nanoparticles or nanoparticles immobilized on a support. Indeed, cyclodextrin as stabilizing agent of metal nanocatalyst has been widely studied since the first study of Komiyama and Hirai in 1983. The stability, the catalytic activity, and the recyclability of the resulting nanoparticles have been improved by using more complex cyclodextrin-based protective agents. From the first studies using native cyclodextrins to cyclodextrin-based polymers or rotaxanes, native and functionalized cyclodextrins have proven their ability to protect metal nanoparticles against agglomeration via different stabilizing properties (electrostatic, steric, and electrosteric). Most of the examples reported in this chapter clearly showed that cyclodextrin-based systems have improved both the average size decrease and the dispersion of the metal nanoparticles in comparison to their free cyclodextrin controls leading to the enhancement of their catalytic activity. According to several studies, cyclodextrins could be also used as reducing agents of metal precursor by their sugar-like reducing properties.
Due to a dynamic organization at the surface of metal nanoparticles, the classical mass transfer property of cyclodextrins has also been exploited with solvent-dispersed nanoparticles or nanoparticles immobilized on a support. The catalytic activity is generally improved thanks to the inclusion complex formed between the substrate and the cyclodextrin via hydrophobic interactions. This inclusion complex could also, in some cases, improve the selectivity of the reaction by inhibiting some side reactions. Cyclodextrins could also serve as agent to get confining catalytic systems by forming supramolecular hydrogels where the metal nanoparticles can be embedded. This confinement could improve the selectivity of the reaction, the catalytic activity by enhancing the proximity between the substrate and the metal particles, and also the stability leading to longer lifetime which is very important from an economical point of view.
The combination of cyclodextrin and polymer in a physical mixture and as cyclodextrin-based polymer where the cyclodextrin is directly incorporated into the polymer structure has recently proven to be very promising both in the use of nanoparticles dispersed into a solvent or immobilized on cyclodextrin polymer where it could play the role of stabilizing agent and also the role of support.
In the near future, the perspectives and the challenges are numerous concerning the development of nanoheterogeneous catalysis in the presence of cyclodextrin. Indeed, in terms of nanocatalyst design, in order to draw a parallel with the supramolecular chemistry defined by Jean-Marie Lehn, it would be very interesting, in the case of cyclodextrin-based nanoheterogeneous catalytic systems, to develop more complex and more controlled structures in order to work at a higher-dimensional scale. The preparation of supramolecular materials could lead to some synergistic effects with the aims to improve the catalytic activity or selectivity, for example. The design of cyclodextrin-based metal-organic frameworks containing metal nanoparticles could answer to some of these issues. From a catalytic point of view, always keeping in mind the multi-task agent properties of cyclodextrin for the development of nanoheterogeneous catalysis, it would be innovative to consider multimetallic nanocatalysts applied for cascade reactions for the valorization of biosourced compounds.
Abbreviations
- ACNa:
-
1-Adamantane carboxylate sodium salt
- AmCD:
-
Poly-(6-N, N-dimethyl-propylenediamino)-(6-deoxy)-β-cyclodextrin
- AO-CNTs:
-
Acid-treated carbon nanotubes
- C60:
-
Fullerene[60]
- CD:
-
Cyclodextrin
- CDNS:
-
Cyclodextrin nanosponges
- CNTs:
-
Carbon nanotubes
- FTIR:
-
Fourier transform infrared spectroscopy
- GCE:
-
Glassy carbon electrode
- Hal:
-
Halloysite nanoclay
- HEA16Cl:
-
N, N-dimethyl, N-hexadecyl, N-(2-hydroxyethyl)ammonium chloride
- ICP-AES:
-
Inductively coupled plasma atomic emission spectroscopy
- IPTS:
-
(3-isocyanatopropyl) triethoxysilane
- RaMe-β-CD:
-
Randomly methylated-β-cyclodextrin
- rGO:
-
Reduced graphene oxide
References
Alvarez J, Liu J, Román E, Kaifer AE (2000) Water-soluble platinum and palladium nanoparticles modified with thiolated β-cyclodextrin. Chem Commun:1151–1152. https://doi.org/10.1039/b002423f
Astruc D, Liang LY, Rapakousiou A, Ruiz J (2012) Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials. Acc Chem Res 45:630–640. https://doi.org/10.1021/ar200235m
Attarchi N, Montazer M, Toliyat T (2013) Ag/TiO2/β-CD nano composite: preparation and photo catalytic properties for methylene blue degradation. Appl Catal A Gen 467:107–116. https://doi.org/10.1016/j.apcata.2013.07.018
Azaroon M, Kiasat AR (2018) Silver nanoparticles engineered β-cyclodextrin/γ-Fe2O3@ hydroxyapatite composite: efficient, green and magnetically retrievable nanocatalyst for the aqueous reduction of nitroarenes. Catal Lett 148:745–756. https://doi.org/10.1007/s10562-017-2272-5
Bhoi VI, Kumar S, Murthy CN (2016) Cyclodextrin encapsulated monometallic and inverted core–shell bimetallic nanoparticles as efficient free radical scavengers. New J Chem 40:1396–1402. https://doi.org/10.1039/C5NJ02511G
Celebioglu A, Ranjith KS, Eren H, Biyikli N, Uyar T (2017) Surface decoration of pt nanoparticles via ALD with TiO2 protective layer on polymeric nanofibers as flexible and reusable heterogeneous nanocatalysts. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-13805-2
Celebioglu A, Topuz F, Uyar T (2019a) Facile and green synthesis of palladium nanoparticles loaded into cyclodextrin nanofibers and their catalytic application in nitroarene hydrogenation. New J Chem 43:3146–3152. https://doi.org/10.1039/c8nj05133j
Celebioglu A, Topuz F, Yildiz ZI, Uyar T (2019b) One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr Polym 207:471–479. https://doi.org/10.1016/j.carbpol.2018.12.008
Chalasani R, Vasudevan S (2013) Cyclodextrin-functionalized Fe3O4@TiO2: reusable, magnetic nanoparticles for photocatalytic degradation of endocrine-disrupting chemicals in water supplies. ACS Nano 7:4093–4104. https://doi.org/10.1021/nn400287k
Chau NTT, Guégan JP, Menuel S, Guerrero M, Hapiot F, Monflier E, Philippot K, Denicourt-Nowicki A, Roucoux A (2013) β-Cyclodextrins grafted with chiral amino acids: a promising supramolecular stabilizer of nanoparticles for asymmetric hydrogenation? Appl Catal A Gen 467:497–503. https://doi.org/10.1016/j.apcata.2013.08.011
Chen M, Meng Y, Zhou J, Diao G (2014) Platinum nanoworms self-assemble on β-cyclodextrin polymer inclusion complexes functionalized reduced graphene oxide as enhanced catalyst for direct methanol fuel cells. J Power Sources 265:110–117. https://doi.org/10.1016/j.jpowsour.2014.04.031
Chen M, Shen X, Liu P, Wei Y, Meng Y, Zheng G, Diao G (2015) β-Cyclodextrin polymer as a linker to fabricate ternary nanocomposites AuNPs/pATP-β-CDP/rGO and their electrochemical application. Carbohydr Polym 119:26–34. https://doi.org/10.1016/j.carbpol.2014.11.022
Chen X, Liu D, Wu Z, Cravotto G, Wu Z, Ye BC (2018) Microwave-assisted rapid synthesis of Ag-β-cyclodextrin/TiO2/AC with exposed {001} facets for highly efficient naphthalene degradation under visible light. Catal Commun 104:96–100. https://doi.org/10.1016/j.catcom.2017.10.026
Chevry M, Menuel S, Léger B, Noël S, Monflier E, Hapiot F (2019) Hydrogenation of hydrophobic substrates catalyzed by gold nanoparticles embedded in tetronic/cyclodextrin-based hydrogels. New J Chem 43:9865–9872. https://doi.org/10.1039/c8nj06081a
Contreras Carballada P, Mourtzis N, Felici M, Bonnet S, Nolte RJM, Williams RM, De COla L, Feiters MC (2012) Variation of the viologen electron relay in cyclodextrin-based self-assembled systems for photoinduced hydrogen evolution from water. Eur J Org Chem:6729–6736. https://doi.org/10.1002/ejoc.201200886
del Pozo M, Blanco E, Hernández P, Casas JA, Quintana C (2018) Catalytic efficiency of macrocyclic-capped gold nanoparticles: cucurbit[n]urils versus cyclodextrins. J Nanopart Res 20:121–130. https://doi.org/10.1007/s11051-018-4230-6
Denicourt-Nowicki A, Ponchel A, Monflier E, Roucoux A (2007) Methylated cyclodextrins: an efficient protective agent in water for zerovalent ruthenium nanoparticles and a supramolecular shuttle in alkene and arene hydrogenation reactions. Dalton Trans 43:5714–5719. https://doi.org/10.1039/B713989F
Denicourt-Nowicki A, Roucoux A, Wyrwalski F, Kania N, Monflier E, Ponchel A (2008) Carbon-supported ruthenium nanoparticles stabilized by methylated cyclodextrins: a new family of heterogeneous catalysts for the gas-phase hydrogenation of arenes. Chem Eur J 14:8090–8093. https://doi.org/10.1002/chem.200801323
Devi LB, Mandal AB (2013) Self-assembly of Ag nanoparticles using hydroxypropyl cyclodextrin: synthesis, characterisation and application for the catalytic reduction of p-nitrophenol. RSC Adv 3:5238–5253. https://doi.org/10.1039/C3RA23014G
Ferreira M, Jérôme F, Bricout H, Menuel S, Landy D, Fourmentin S, Tilloy S, Monflier E (2015) Rhodium catalyzed hydroformylation of 1-decene in low melting mixtures based on various cyclodextrins and N,N′-dimethylurea. Catal Commun 63:62–65. https://doi.org/10.1016/j.catcom.2014.11.001
Francisco M, van den Bruinhorst A, Kroon MC (2013) Low-transition-temperature mixtures (LTTMs): a new generation of designer solvents. Angew Chem Int Ed Engl 52:3074–3085. https://doi.org/10.1002/anie.201207548
Gannimani R, Ramesh M, Mtambo S, Pillay K, Soliman ME, Govender P (2016) γ-Cyclodextrin capped silver nanoparticles for molecular recognition and enhancement of antibacterial activity of chloramphenicol. J Inorg Biochem 157:15–24. https://doi.org/10.1016/j.jinorgbio.2016.01.008
Girish YR, Sharath Kumar KS, Thimmaiah KN, Rangappa KS, Shashikanth S (2015) ZrO2-β-cyclodextrin catalyzed synthesis of 2,4,5-trisubstituted imidazoles and 1,2-disubstituted benzimidazoles under solvent free conditions and evaluation of their antibacterial study. RSC Adv 5:75533–75546. https://doi.org/10.1039/C5RA13891D
Gopalan AI, Lee K-P, Manesh KM, Santhosh P, Kim JH (2006) Gold nanoparticles dispersed into poly(aminothiophenol) as a novel electrocatalyst – fabrication of modified electrode and evaluation of electrocatalytic activities for dioxygen reduction. J Mol Catal A Chem 256:335–345. https://doi.org/10.1016/j.molcata.2006.05.027
Guerrero M, Coppel Y, Chau NTT, Rucoux A, Denicourt-Nowicki A, Monflier E, Bricout H, Lecante P, Philippot K (2013) Efficient ruthenium nanocatalysts in liquid-liquid biphasic hydrogenation catalysis: towards a supramolecular control through a sulfonated diphosphine-cyclodextrin smart combination. ChemCatChem 5:3802–3811. https://doi.org/10.1002/cctc.201300467
Guerrero M, Chau NTT, Roucoux A, Nowicki-Denicourt A, Monflier E, Bricout H, Philippot K (2014) Organometallic synthesis of water-soluble ruthenium nanoparticles in the presence of sulfonated diphosphines and cyclodextrins. Mater Res Soc Symp Proc 1675:219–225. https://doi.org/10.1557/opl.2014.888
Haldar KK, Kundu S, Patra A (2014) Core-size-dependent catalytic properties of bimetallic Au/Ag core-shell nanoparticles. ACS Appl Mater Interfaces 6:21946–21953. https://doi.org/10.1021/am507391d
Haw C, Chiu W, Khanis NH, Rahman SA, Khiew P, Radiman S, Abd-Shukor R, Abdul Hamid MA (2016) Tin stearate organometallic precursor prepared SnO2 quantum dots nanopowder for aqueous- and non-aqueous medium photocatalytic hydrogen gas evolution. J Energy Chem 25:691–701. https://doi.org/10.1016/j.jechem.2016.04.006
Herbois R, Noël S, Léger B, Bai L, Roucoux A, Monflier E, Ponchel A (2012) Cyclodextrins as growth controlling agents for enhancing the catalytic activity of PVP-stabilized Ru(0) nanoparticles. Chem Commun 48:3451–3453. https://doi.org/10.1039/c2cc17355g
Herbois R, Noël S, Léger B, Tilloy S, Menuel S, Addad A, Martel B, Ponchel A, Monflier E (2015) Ruthenium-containing β-cyclodextrin polymer globules for the catalytic hydrogenation of biomass-derived furanic compounds. Green Chem 17:2444–2454. https://doi.org/10.1039/c5gc00005j
Hirano K, Takano S, Tsukuda T (2019) Asymmetric aerobic oxidation of secondary alcohols catalyzed by poly(N-vinyl-2-pyrrolidone)-stabilized gold clusters modified with cyclodextrin derivatives. Chem Commun 55:15033–15036. https://doi.org/10.1039/C9CC06770A
Huang T, Meng F, Qi L (2009) Facile synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced Raman scattering. J Phys Chem C 113:13636–13642. https://doi.org/10.1021/jp903405y
Huang T, Sheng G, Manchanda P, Emwas AH, Lai Z, Nunes SP, Peinemann KV (2019) Cyclodextrin polymer networks decorated with subnanometer metal nanoparticles for high-performance low-temperature catalysis. Sci Adv 5:1–11. https://doi.org/10.1126/sciadv.aax6976
Hubert C, Denicourt-Nowicki A, Roucoux A, Landy D, Léger B, Crowyn G, Monflier E (2009) Catalytically active nanoparticles stabilized by host-guest inclusion complexes in water. Chem Commun:1228–1230. https://doi.org/10.1039/b818786j
Jia H, Schmitz D, Ott A, Pich A, Lu Y (2015) Cyclodextrin modified microgels as “nanoreactor” for the generation of Au nanoparticles with enhanced catalytic activity. J Mater Chem A 3:6187–6195. https://doi.org/10.1039/C5TA00197H
Kepenienė V, Stagniūnaitė R, Tamašauskaitė-Tamašiūnaitė L, Pakstas V, Jasulaitiene V, Léger B, Rousseau J, Ponchel A, Monflier E, Norkus E (2020) Co3O4/C and Au supported Co3O4/C nanocomposites – peculiarities of fabrication and application towards oxygen reduction reaction. Mater Chem Phys 241:122332. https://doi.org/10.1016/j.matchemphys.2019.122332
Khalafi-Nezhad A, Panahi F (2014) Size-controlled synthesis of palladium nanoparticles on a silica-cyclodextrin substrate: a novel palladium catalyst system for the heck reaction in water. ACS Sustain Chem Eng 2:1177–1186. https://doi.org/10.1021/sc5000122
Komiyama M, Hirai H (1983) Colloidal rhodium dispersions protected by cyclodextrins. Bull Chem Soc Jpn 56:2833–2834. https://doi.org/10.1246/bcsj.56.2833
Kuklin S, Maximov A, Zolotukhina A, Karakhanov E (2016) New approach for highly selective hydrogenation of phenol to cyclohexanone: combination of rhodium nanoparticles and cyclodextrins. Catal Commun 73:63–68. https://doi.org/10.1016/j.catcom.2015.10.005
Kunz W, Häckl K (2016) The hype with ionic liquids as solvents. Chem Phys Lett 661:6–12. https://doi.org/10.1016/j.cplett.2016.07.044
Leclercq L, Bricout H, Tilloy S, Monflier E (2007) Biphasic aqueous organometallic catalysis promoted by cyclodextrins: can surface tension measurements explain the efficiency of chemically modified cyclodextrins? J Colloid Interface Sci 307:481–487. https://doi.org/10.1016/j.jcis.2006.12.001
Léger B, Menuel S, Ponchel A, Hapiot F, Monflier E (2012) Nanoparticle-based catalysis using supramolecular hydrogels. Adv Synth Catal 354:1269–1272. https://doi.org/10.1002/adsc.201100888
Li J, Harada A, Kamachi M (1994) Sol-gel transition during inclusion complex formation between α-cyclodextrin and high molecular weight poly(ethylene glycol)s in aqueous solution. Polym J 26:1019–1026. https://doi.org/10.1295/polymj.26.1019
Li Y, Boone E, El-Sayed MA (2002) Size effects of PVP-Pd nanoparticles on the catalytic Suzuki reactions in aqueous solution. Langmuir 18:4921–4925. https://doi.org/10.1021/la011469q
Li X, Qi Z, Liang K, Bai X, Xu J, Liu J, Shen J (2008) An artificial supramolecular nanozyme based on β-cyclodextrin-modified gold nanoparticles. Catal Lett 124:413–417. https://doi.org/10.1007/s10562-008-9494-5
Li Z, Zhang L, Huang XM, Ye LT, Lin S (2014) Shape-controlled synthesis of Pt nanoparticles via integration of graphene and β-cyclodextrin and using as a noval electrocatalyst for methanol oxidation. Electrochim Acta 121:215–222. https://doi.org/10.1016/j.electacta.2013.12.174
Li L, Tian C, Yang J, Zhang X, Chen J (2015) One-pot synthesis of PtRh/β-CD-CNTs for methanol oxidation. Int J Hydrog Energ 40:14866–14874. https://doi.org/10.1016/j.ijhydene.2015.09.021
Li X, Cai T, Kang ET (2016) Hairy hybrid nanorattles of platinum nanoclusters with dual-responsive polymer shells for confined nanocatalysis. Macromolecules 49:5649–5659. https://doi.org/10.1021/acs.macromol.6b00945
Li P, Li S, Wang Y, Zhang Y, Han GZ (2017) Green synthesis of β-CD-functionalized monodispersed silver nanoparticles with enhanced catalytic activity. Colloids Surf A Physicochem Eng Asp 520:26–33. https://doi.org/10.1016/j.colsurfa.2017.01.034
Liang L, Diallo AK, Salmon L, Ruiz J, Astruc D (2012) Catalysis of C-C cross-coupling reactions in aqueous solvent by bis- and tris(ferrocenyltriazolylmethyl)arene-β-cyclodextrin-stabilized Pd nanoparticles. Eur J Inorg Chem 17:2950–2958. https://doi.org/10.1002/ejic.201200098
Liu J, Alvarez J, Ong W, Roman E, Kaifer AE (2001) Tuning the catalytic activity of cyclodextrin-modified palladium nanoparticles through host−guest binding interactions. Langmuir 17:6762–6764. https://doi.org/10.1021/la015563i
Ma D, Xie X, Zhang LM (2009) A novel route to in-situ incorporation of silver nanoparticles into supramolecular hydrogel networks. J Polym Sci Part B Polym Phys 47:740–749. https://doi.org/10.1002/polb.21677
Mandler D, Willner I (1987) Effective photoreduction of carbon dioxide/bicarbonate to formate using visible light. J Am Chem Soc 109:7884–7885. https://doi.org/10.1021/ja00259a048
Martina K, Baricco F, Caporaso M, Berlier G, Cravotto G (2016) Cyclodextrin-grafted silica-supported Pd nanoparticles: an efficient and versatile catalyst for ligand-free C-C coupling and hydrogenation. ChemCatChem 8:1176–1184. https://doi.org/10.1002/cctc.201501225
Menuel S, Léger B, Addad A, Monflier E, Hapiot F (2016) Cyclodextrins as effective additives in AuNP-catalyzed reduction of nitrobenzene derivatives. Green Chem 18:5500–5509. https://doi.org/10.1039/c6gc00770h
Meo PL, Anna FD, Gruttadauria M, Riela S, Noto R (2012) Synthesis and characterization of new polyamino-cyclodextrin materials. Carbohydr Res 347:32–39. https://doi.org/10.1016/j.carres.2011.10.029
Mhadgut SC, Palaniappan K, Thimmaiah M, Hackney SA, Torok B, Liu J (2005) A metal nanoparticle-based supramolecular approach for aqueous biphasic reactions. Chem Commun:3207–3209. https://doi.org/10.1039/b502181b
Monflier E, Tilloy S, Meliet C, Mortreux A, Fourmentin S, Landy D, Surpateanu G (1999) First evidence of molecular recognition between cyclodextrins and a water-soluble ligand used in aqueous phase organometallic catalysis. New J Chem 23:469–472. https://doi.org/10.1039/a900898e
Mori K, Yoshioka N, Kondo Y, Takeuchi T, Yamashita H (2009) Catalytically active, magnetically separable, and water-soluble FePt nanoparticles modified with cyclodextrin for aqueous hydrogenation reactions. Green Chem:1337–1342. https://doi.org/10.1039/b905331j
Nguyen TD, Dang CH, Mai D-T (2018) Biosynthesized AgNP capped on novel nanocomposite 2-hydroxypropyl-β-cyclodextrin/alginate as a catalyst for degradation of pollutants. Carbohydr Polym 197:29–37. https://doi.org/10.1016/j.carbpol.2018.05.077
Noël S, Léger B, Herbois R, Ponchel A, Tilloy S, Wenz G, Monflier E (2012) Carboxylated polymers functionalized by cyclodextrins for the stabilization of highly efficient rhodium(0) nanoparticles in aqueous phase catalytic hydrogenation. Dalton Trans 41:13359–13363. https://doi.org/10.1039/c2dt31596c
Noël S, Léger B, Ponchel A, Hapiot F, Monflier E (2014) Effective catalytic hydrogenation of fatty acids methyl esters by aqueous rhodium(0) nanoparticles stabilized by cyclodextrin-based polymers. Chem Eng Trans 37:337–342. https://doi.org/10.3303/CET1437057
Noël S, Bourbiaux D, Tabary N, Ponchel A, Martel B, Monflier E, Léger B (2017) Acid-tolerant cyclodextrin-based ruthenium nanoparticles for the hydrogenation of unsaturated compounds in water. Cat Sci Technol 7:5982–5992. https://doi.org/10.1039/c7cy01687e
Nowicki A, Zhang Y, Léger B, Rolland JP, Bricout H, Monflier E, Roucoux A (2006) Supramolecular shuttle and protective agent: a multiple role of methylated cyclodextrins in the chemoselective hydrogenation of benzene derivatives with ruthenium nanoparticles. Chem Commun 42:296–298. https://doi.org/10.1039/B512838B
Patil MR, Kapdi AR, Vijay Kumar A (2018) A recyclable supramolecular – ruthenium catalyst for the selective aerobic oxidation of alcohols on water: application to total synthesis of brittonin A. ACS Sustain Chem Eng 6:3264–3278. https://doi.org/10.1021/acssuschemeng.7b03448
Peng L, You M, Wu C, Han D, Ocsoy I, Chen T, Chen Z, Tan W (2014) Reversible phase transfer of nanoparticles based on photoswitchable host-guest chemistry. ACS Nano 8:2555–2561. https://doi.org/10.1021/nn4061385
Putta C, Sharavath V, Sarkar S, Ghosh S (2015) Palladium nanoparticles on β-cyclodextrin functionalised graphene nanosheets: a supramolecular based heterogeneous catalyst for C–C coupling reactions under green reaction conditions. RSC Adv 5:6652–6660. https://doi.org/10.1039/C4RA14323J
Qu H, Yang L, Yu J, Wang L, Liu H (2018) Host-guest interaction induced rapid self-assembled Fe3O4@Au nanoparticles with high catalytic activity. Ind Eng Chem Res 57:9448–9456. https://doi.org/10.1021/acs.iecr.8b00894
Ran X, Yang L, Qu Q, Li S, Chen Y, Zuo L, Li L (2017) Synthesis of well-dispersive 2.0 nm Pd-Pt bimetallic nanoclusters supported on β-cyclodextrin functionalized graphene with excellent electrocatalytic activity. RSC Adv 7:1947–1955. https://doi.org/10.1039/c6ra24893d
Sadjadi S (2018) Palladium nanoparticles immobilized on cyclodextrin-decorated halloysite nanotubes: efficient heterogeneous catalyst for promoting copper- and ligand-free Sonogashira reaction in water-ethanol mixture. Appl Organomet Chem 32:4211. https://doi.org/10.1002/aoc.4211
Sadjadi S, Malmir M, Heravi MM (2017) A green approach to the synthesis of Ag doped nano magnetic γ-Fe2O3@SiO2-CD core-shell hollow spheres as an efficient and heterogeneous catalyst for ultrasonic-assisted A3 and KA2 coupling reactions. RSC Adv 7:36807–36818. https://doi.org/10.1039/C7RA04635A
Sadjadi S, Heravi MM, Malmir M (2018a) Pd@HNTs-CDNS-g-C3N4: a novel heterogeneous catalyst for promoting ligand and copper-free Sonogashira and Heck coupling reactions, benefits from halloysite and cyclodextrin chemistry and g-C3N4 contribution to sup. Carbohydr Polym 186:25–34. https://doi.org/10.1016/j.carbpol.2018.01.023
Sadjadi S, Heravi MM, Raja M (2018b) Combination of carbon nanotube and cyclodextrin nanosponge chemistry to develop a heterogeneous Pd-based catalyst for ligand and copper free C-C coupling reactions. Carbohydr Polym 185:48–55. https://doi.org/10.1016/j.carbpol.2018.01.020
Sagir H, Rahila RP, Singh PK, Siddiqui LR (2016) ZnO nanoparticle–β-cyclodextrin: a recyclable heterogeneous catalyst for the synthesis of 3-aryl-4H-benzo[1,4]thiazin-2-amine in water. New J Chem 40:6819–6824. https://doi.org/10.1039/C5NJ03273C
Senra JD, Malta LFB, da Costa MEHM, MIchel RC, Aguiar LCS, Simas ABC, Antunes OAC (2009) Hydroxypropyl-α-cyclodextrin-capped palladium nanoparticles: active scaffolds for efficient carbon-carbon bond forming cross-couplings in water. Adv Synth Catal 351:2411–2422. https://doi.org/10.1002/adsc.200900348
Senra JD, Viana GM, Malta LFB, Simas ABC, Aguiar LCS (2016) Selectivity studies towards the synthesis of novel biaryl ureas by (hetero)nanocatalysis: size control and support effects. ChemCatChem 8:192–199. https://doi.org/10.1002/cctc.201500889
Shen J, Li N, Ye M (2013) Supramolecular photocatalyst of RGO-cyclodextrin-TiO2. J Alloys Compd 580:239–244. https://doi.org/10.1016/j.jallcom.2013.05.090
Shiraishi Y, Hayashi M, Toshima N (2007) Preparation and catalysis of poly(β-cyclodextrin)-stabilized palladium nanoparticles. Kobunshi Ronbunshu 64:74–76
Strimbu L, Liu J, Kaifer AE (2003) Cyclodextrin-capped palladium nanoparticles as catalysts for the Suzuki reaction. Langmuir 19:483–485. https://doi.org/10.1021/la026550n
Tang J, Shi Z, Berry RM, Tam KC (2015) Mussel-inspired green metallization of silver nanoparticles on cellulose nanocrystals and their enhanced catalytic reduction of 4-nitrophenol in the presence of β-cyclodextrin. Ind Eng Chem Res 54:3299–3308. https://doi.org/10.1021/acs.iecr.5b00177
Taylor P, Shiraishi Y, Hashimura M, Nakao M, Ishizu T, Kazita M, Miyamoto Y, Toshima N (2010) Syntheses of poly (cyclodextrin)-stabilised metal nanoparticles and their quenching abilities of active oxygen species. Supramol Chem 23:37–41. https://doi.org/10.1080/10610278.2010.521831
Thanh Chau NT, Menuel S, Colombel-Rouen S, Guerrero M, Monflier E, Philippot K, Denicourt-Nowicki A, Roucoux A (2016) Active hydrogenation Rh nanocatalysts protected by new self-assembled supramolecular complexes of cyclodextrins and surfactants in water. RSC Adv 6:108125–108131. https://doi.org/10.1039/C6RA21851B
Vasconcelos DA, Kubota T, Santos DC, Araujo MV, Teixeira Z, Gimenez IF (2016) Preparation of Aun quantum clusters with catalytic activity in β-cyclodextrin polyurethane nanosponges. Carbohydr Polym 136:54–62. https://doi.org/10.1016/j.carbpol.2015.09.010
Wang J, Gao P, Ye L, Feng Z (2010) Solvent and thermoresponsive polyrotaxanes with cyclodextrin dispersed/aggregated structures on a pluronic F127 backbone. J Phys Chem B 114:5342–5349. https://doi.org/10.1021/jp101068b
Wang C, Salmon L, Li Q, Igartua ME, Moya S, Ciganda R, Ruiz J, Astruc D (2016a) From mono to tris-1,2,3-triazole-stabilized gold nanoparticles and their compared catalytic efficiency in 4-nitrophenol reduction. Inorg Chem 55:6776–6780. https://doi.org/10.1021/acs.inorgchem.6b01092
Wang M, Fang G, Liu P, Zhou D, Ma C, Zhang D, Zhan J (2016b) Fe3O4@β-CD nanocomposite as heterogeneous Fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP). Appl Catal B Environ 188:113–122. https://doi.org/10.1016/j.apcatb.2016.01.071
Wang M, Wang J, Wang Y, Liu C, Liu J, Qiu Z, Xu Y, Lincoln SF, Guo X (2016c) Synergetic catalytic effect of α-cyclodextrin on silver nanoparticles loaded in thermosensitive hydrogel. Colloid Polym Sci 294:1087–1095. https://doi.org/10.1007/s00396-016-3867-x
Willner I, Mandler D (1987) The oxidation states of the elements and their potentials in aqueous solutions. J Chem Soc Faraday Trans 109:6834
Willner I, Mandler D (1989) Characterization of palladium-β-cyclodextrin colloids as catalysts in the photosensitized reduction of bicarbonate to formate. J Am Chem Soc 111:1330–1336. https://doi.org/10.1021/ja00186a028
Wu J, He H, Gao C (2010) β-Cyclodextrin-capped polyrotaxanes: one-pot facile synthesis via click chemistry and use as templates for platinum nanowires. Macromolecules 43:2252–2260. https://doi.org/10.1021/ma902255v
Wyrwalski F, Léger B, Lancelot C, Roucoux A, Monflier E, Ponchel A (2011) Chemically modified cyclodextrins as supramolecular tools to generate carbon-supported ruthenium nanoparticles: an application towards gas phase hydrogenation. Appl Catal A Gen 391:334–341. https://doi.org/10.1016/J.apcata.2010.07.006
Xue C, Palaniappan K, Arumugam G, Hackney SA, Liu J, Liu H (2007) Sonogashira reactions catalyzed by water-soluble, β-cyclodextrin-capped palladium nanoparticles. Catal Lett 116:94–100. https://doi.org/10.1007/s10562-007-9108-7
Yi Q, Sun L, Liu X, Nie H (2013) Palladium-nickel nanoparticles loaded on multi-walled carbon nanotubes modified with β-cyclodextrin for electrooxidation of alcohols. Fuel 111:88–95. https://doi.org/10.1016/j.fuel.2013.04.051
Yi Y, Sun H, Zhu G, Zhang Z, Wu X (2015) Sensitive electrochemical determination of rhodamine B based on cyclodextrin-functionalized nanogold/hollow carbon nanospheres. Anal Methods 7:4965–4970. https://doi.org/10.1039/C5AY00654F
Zhang Q, Bai Z, Shi M, Yang L, Qiao J, Jiang K (2015) High-efficiency palladium nanoparticles supported on hydroxypropyl-β-cyclodextrin modified fullerene [60] for ethanol oxidation. Electrochim Acta 177:113–117. https://doi.org/10.1016/j.electacta.2015.01.207
Zhang W, Yaob ZJ, Deng W (2019) Palladium nanoparticles supported on β-cyclodextrin functionalised poly(amido amine)s and their application in Suzuki-Miyaura. J Braz Chem Soc 30:1667–1677. https://doi.org/10.21577/0103-5053.20190067
Zhao X, Liu X, Lu M (2014) β-cyclodextrin-capped palladium nanoparticle-catalyzed ligand-free Suzuki and Heck couplings in low-melting β-cyclodextrin/NMU mixtures. Appl Organomet Chem 28:635–640. https://doi.org/10.1002/aoc.3173
Zhao Y, Huang Y, Zhu H, Zhu Q, Xia Y (2016) Three-in-one: sensing, self-assembly, and cascade catalysis of cyclodextrin modified gold nanoparticles. J Am Chem Soc 138:16645–16654. https://doi.org/10.1021/jacs.6b07590
Zhong Y, Deng C, He Y, Ge Y, Song G (2016) Exploring a monothiolated β-cyclodextrin as the template to synthesize copper nanoclusters with exceptionally increased peroxidase-like activity. Microchim Acta 183:2823–2830. https://doi.org/10.1007/s00604-016-1915-3
Zhou DH, Liang CC, Nie J, Zhu XQ (2017) Construction of a repairable fixed porous catalytic bed loaded with gold nanoparticles via multivalent host-guest interactions. ACS Sustain Chem Eng 5:7587–7593. https://doi.org/10.1021/acssuschemeng.7b00879
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Noël, S., Ponchel, A., Sadjadi, S., Monflier, E., Léger, B. (2020). Metal Nanoparticles and Cyclodextrins for Catalytic Applications. In: Crini, G., Fourmentin, S., Lichtfouse, E. (eds) The History of Cyclodextrins. Environmental Chemistry for a Sustainable World, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-030-49308-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-49308-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49307-3
Online ISBN: 978-3-030-49308-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)