Abstract
An artificial nanozyme model was developed by the supramolecular complexation of a β-cyclodextrin-modified gold nanoparticle and metal catalytic centers. The cyclodextrin-based monolayer was first constructed on the surface of gold nanoparticle by using the thiol modified cyclodextrin, subsequently the cyclodextrin-modified gold nanoparticle was utilized as a backbone to install metal catalytic centers by supramolecular assembly of the copper complex of triethylnetetramine-adamantane and β-cyclodextrin receptors immobilized on the surface of gold nanospheres via hydrophobic interaction. The catalytic behaviors of β-cyclodextrin-modified gold nanoparticles with adjacent multi-metal catalytic centers were investigated as an esterase mimic. Strong hydrolase activities for catalyzing the cleavage of an active ester 4,4′-dinitrodiphenyl carbonate (DNDPC) were observed. A detailed kinetic study on nanozyme-catalyzed hydrolysis of ester DNDPC has been described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development of new functionalized nanomaterials constitutes an area of current interests in the fields of nanotechnology, drug delivery, electronic applications and catalysis [1–3]. Recently, a special interest focuses on the construction of colloid nanoparticles for a variety of applications, for example, various organic and biological molecules such as sulfur-based compounds, proteins, DNA, enzymes [4–6] and cyclodextrins [7, 8], have been used to modify nanoparticles for new functions. Among these nanomaterials, the construction of novel water-soluble nanocatalysts based on colloidal particles has received special interests for chemists and biologists [9–13].
Cyclodextrins are a series of cyclic saccharides possessing a hydrophobic inner cavity which enables the complexation of various organic, inorganic and biological molecules to form stable host-guest inclusion complexes [14, 15] and supramolecular aggregates [16, 17] in aqueous solution. Recently, the complexation of cyclodextrins and gold nanoparticles received special attention. A number of gold nanoparticles capped with thiolated cyclodextrins have been reported by Kaifer [18], Matsui [8], and Reinhoudt [19]. However, most of these works focus on the preparation, characterization and the assembly of these CD-modified gold nanoparticles. The development of functional nanomaterials based on these CD-modified gold nanoparticles is still rare. Herein, we constructed a nanostructure hydrolase model which employed supramolecular complex of preformed catalytic sites on CD-modified gold nanoparticles (AuNPs) [20] as three-dimensional scaffolds for immobilizing the catalytic metal centers. And the copper complex of the tetramine-modified adamantane is associated through the inclusion of adamantal group within the CD cavities (Scheme 1) [21]. The gold nanoparticles supporting multi-metal catalytic centers exhibited strong rate enhancement for the catalysis of the hydrolysis of DNDPC. A detailed kinetic study of catalyzed hydrolysis of ester DNDPC by these complexes has been described.
2 Materials and Methods
2.1 General Materials and Procedure
All reagents and solvents unless otherwise were of analytical reagent grade commercially available and all the solvents were used dried by normal procedure. A Milli-Q grade water was used for preparation of sample solutions. β-Cyclodextrin (β-CD) of reagent grade was recrystallized twice from water and dried under vacuum at 120 °C for 24 h prior to use. 1H-NMR was measured on a Bruker AM-500 spectrometer. Molecular weight was obtained from the LDI-1700 MALDI-TOF-MS. The kinetic study was carried out using a Shimadzu 2450 UV–vis recording spectrophotometer, and data were calculated and analyzed by using Origin 7.5 software.
2.2 Synthesis of the Triethylnetetramine-Adamantane
To a solution of triethylnetetramine and triethylamine (molar ratio of 1:1) in dry DMF, 1-bromoadamantane in dry DMF (20 mL) was added dropwise during 2 h under stirring and was heated at 70 °C for 4 h. The solvent removed and crude residue was purified by column chromatography. 1HNMR (500 MHz, D2O): δ = 2.6–2.8 (12H), 2.2 (3H), 2.0 (5H) 1.8–1.84 (6H), 1.5 (5H), 1.1 (1H).
2.3 Synthesis of Per-6-Deoxy-(6-Thio)-β-Cyclodextrin (CD-SH)
This compound was prepared in two steps from β-CD according to previous method reported by Kaifer [18]. Per-6-iodo-β-cyclodextrin (1.5 g) and thiourea (0.468 g) were dissolved in DMF, the reaction mixture was heated at 70 °C for 19 h under a nitrogen atmosphere. The DMF was concentrated under reduced pressure and the residue was dissolved in water. Sodium hydroxide (0.41 g) was added and the reaction mixture refluxes for 1 h under a nitrogen atmosphere, and pH was adjusted with KHSO4. The precipitate was filtered off, and washed thoroughly with water, and then dried. The product was suspended in water (10 mL) and the minimum amount of potassium hydroxide was added to give a clear solution, the product was then reprecipitated by acidifying with aqueous KHSO4. 1HNMR (500 MHz, DMSO): δ = 4.93 (m, 7H), 3.62–3.68 (m, 7H), 3.59–3.60 (m, 7H), 3.19–3.30 (m, 14H), 2.72–2.78 (m, 7H) 2.08–2.15 (m, SH); MALDI-TOF: calcd. 1247, found [M + Na+] 1270.
2.4 Prepare of CD-Modified Gold Nanospheres (1)
The β-cyclodextrin-capped gold nanoparticles were prepared as previously reported [17] using perthiolated β-cyclodextrin as capping molecules. To prepare the gold modified gold nanospheres, a 50 mg of HAuCl4 was dissolved in 20 mL DMSO. This solution was quickly mixed with second 20 mL of DMSO containing 75.5 mg NaBH4 and a 16.8 mg of the perthiolated CD. The reaction mixture was stirred for 24 h at room temperature. The precipitate formed by 40 mL CH3CN was added, and was collected by centrifugation. The obtained solid product was further washed with 60 mL of CH3CN: DMSO (1:1 v/v) and 60 mL of ethanol, isolated by centrifugation, and dried under vacuum for 24 h. 1HNMR (500 MHz, D2O): δ = 4.9–5.1 (7H), δ = 3.3–4.16 (41H).
2.5 Copper Complex of CD-Modified Gold Nanoparticles (2)
Nanoparticle 1 containing perthiolated β-cyclodextrin (16.8 mg) and copper complex of triethylnetetramine-adamantane (4.6 mg) in molar ratio of 1:1 was dissolved in water (15 mL) then stirred for 2 days, and finally the complex was obtained. 1HNMR (500 MHz, D2O) δ = 5.0–5.2 (7H), δ = 3.7–4.3 (41H), δ = 2.17 (3H), δ = 1.85 (6H), δ = 1.28–1.35 (5H), δ = 1.11 (1H).
2.6 Hydrolysis of 4, 4′-Dinitrodiphenyl Carbonate (DNDPC)
The hydrolysis rate of 4, 4′-dinitrodiphenyl carbonate (DNDPC) was measured by following the increase in the 400 nm (ε = 8,700 M−1 cm−1, pH 7.0) [22] absorption of the released 4-nitrophenolate (NP) using a computer-linked UV-2450 spectrophotometer. The reaction solution was maintained at 25 °C and pH = 7.0 with 20 mM HEPES buffer and 50% (v/v) acetonitrile (CH3CN) aqueous solution.
3 Results and Discussion
3.1 Synthesis of the Catalysts
Gold nanoparticles protected by a monolayer of perthiolated β-cyclodextrin were prepared by the reported procedure [18], and the theoretical molar ratio of CD to AuCl4− is calculated to be 0.14:1. The resulting water-soluble gold nanoparticles (1) were characterized by 1H NMR and UV–vis spectroscopic data. From the UV–vis image described in Fig. 1 (λmax = 506 nm), their average diameter of CD-capped gold nanoparticles was measured to be 4.8 ± 0.5 nm using Malven ZETAS12-ERNANOSERIES (see Fig. 2). Since β-CD is capable of forming highly stable inclusion complexes with adamantane derivatives in aqueous solution, the enzyme model 2 was expediently prepared by the supramolecular assembly of the copper complex of triethylnetetramine-adamantane and β-CD receptors immobilized on the surface of gold nanospheres via hydrophobic interaction. The synthesis route of enzyme model 2 has been shown in Scheme 2.
3.2 Catalytic Active of Cu–Ad–CD Catalysts
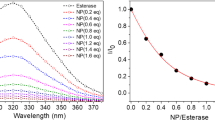
The catalytic properties of the gold nanoparticle-based nanozyme 2 were investigated through the hydrolysis of the activated ester DNDPC. The reaction rates of the ester bond cleavage of DNDPC were measured by following the increase of 400 nm absorption of 4-nitrophenolate in 20 mM HEPES containing 50% (v/v) CH3CN aqueous solution at 25 °C. By varying the initial substrate concentration a kinetic profile of the reaction towards saturation was obtained, which suggests that the cleavage reaction proceeds through a pre-equilibrium step between the complex and the substrate and the saturation kinetics was observed in high substrate concentrations as shown in Fig. 3. The copper complex of CD-modified gold nanoparticles 2 showed a typical Michaelis–Menten kinetics for the cleavage of carbonates.
To further assess the hydrolysis ability of this supermolecular nanozyme, a detailed kinetic study was undertaken. The kinetic parameters for the hydrolysis of DNDPC have been shown in Table 1. These values were deduced from the double-reciprocal plots. From these date a remarkable turnover number of k cat = 1.38 min−1 and a Michaelis constant K m = 3.06 mM were calculated for DNDPC hydrolysis catalyzed by Au nanoparticle-based nanozyme 2, the rate acceleration (k cat/k uncat) is approximately 2,654-fold compared to the reaction rate for the uncatalyzed hydrolysis of DNDPC (k uncat = 5.20 × 10−4 min−1) in the same buffer solution. However, for the inclusion complex CD–Ad–Cu(II) lacking Au nanoparticle backbone, it showed a low k cat = 0.014 min−1, and the value of k cat/k uncat was only found to be 27.5-fold under the same conditions, and it is at least 2 orders of magnitude lower than that of 2. Comparison of Michaelis constant K m with nanoenzyme 2 and CD–Ad–Cu(II) indicates that the rate enhancement of the hydrolysis was induced by the CD-modified gold nanoparticle backbone of nanoenzyme 2, but not by the change in substrate affinity. Furthermore, in the case of compound Ad–Cu (II), only a 1.15-fold rate enhancement over the uncatalyzed reaction were observed, and weakly binding ability of substrate to Ad–Cu (II) was obtained (K m = 8.7 mM). The catalytic efficiency k cat/K m of the investigated complexes follows the order nanoenzyme 2> CD–Ad–Cu(II)> Ad–Cu (II), and suggests that the high rate acceleration of 2 may be attributed to a contribution of the three-dimensional backbone of Au nanoparticle and catalytic synergic action of the two Cu(II) centers in process of hydrolysis reaction.
The pH-rate profile for DNDPC cleavage catalyzed by 2 are depicted in Fig. 4. Nanoenzyme 2 displayed rate enhancement with the increase of pH, the pH-dependent profile against value v0 show that it is acid–base mechanism for catalytic hydrolysis of DNDPC by nanoenzyme 2 [23]. The possible mechanism for the catalysis by nanoenzyme was conjectured as shown in Scheme 3. In the catalytic process both Cu(II) centers bind the oxyanion and where a Cu(II) bound hydroxide ion as a general base attacked the substrate. For to further prove this possible mechanism, a detailed kinetics at pH = 9.0 was studied (see Table 1). The kinetic parameters showed that a catalytic efficiency of nanoenzymes 2 is stronger at higher pH. The results suggest that the Cu–OH is the kinetically active species. The K m in pH 9.0 is somewhat higher than that of pH 7.0, the metal bound hydroxide ions at high pH may lead to the high K m and reduced efficient binding for the substrate.
4 Conclusion
In this paper, we have successfully prepared cyclodextrin-modified gold nanozyme and demonstrated that the nanoenzyme exhibited excellent hydrolysis ability. The study show that cyclodextrin modified gold nanoparticles are suitable building blocks for the design of effective enzyme models. The kinetic analyses indicated that the synergic action of multi-metal catalytic centers and three-dimensional structure of Au nanoparticle may be the possibility for rate enhancement of carbonate hydrolysis. Furthermore, the exploration of the biochemical ability of functional gold nanoparticles will expand the application of nanoparticles in biological approach.
References
Dujardin E, Hsin LB, Wang CRC, Mann S (2001) Chem Commun 14:1264
Zanchet D, Micheel CM, Parak WJ, Gerion D, Alivisatos AP (2001) Nano Lett 1:32
Zhou X, Zhang X, Yu X (2008) Biomaterials 29:111
Maxwell DJ, Taylor JR, Nie S (2002) J Am Chem Soc 124:9606
Sato K, Hosokawa K, Maeda M (2003) J Am Chem Soc 125:8102
Kanaras AG, Wang Z, Bates AD, Cosstick R, Brust M (2003) Angew Chem Int Ed 42:191
Liu J, Alvarez J, Ong W, Kaifer AE (2001) Nano Lett 1:57
Banerjee IA, Yu L, Matsui H (2003) J Am Chem Soc 125:9542
Villalonga R, Cao R, Fragoso A, Damiao AE, Ortiz PD, Caballero J (2005) J Mol Catal B Enzym 35:79
Kinsella JM, Shalaev MV, Ivanisevic A (2007) Chem Mater 19:3586
Pasquato L, Pengo P, Scrimin P (2004) J Mater Chem 14:3481
Pengo P, Baltzer L, Pasquato L, Scrimin P (2006) Angew Chem Int Ed 45:1
Manea F, Houillon FB, Pasquato L, Scrimin P (2004) Angew Chem Int Ed 43:6165
Venema F, Nelissen HFM, Berthault P, Birlirakis N, Rowan AE, Feiters MC, Nolte RJM (1998) Chem Eur J 4:2237
Connors KA (1997) Chem Rev 97:1325
Dvornikovs V, House BE, Kaetzel M, Dedman JR, Smithrud DB (2003) J Am Chem Soc 125:8290
Liu Y, Wang H, Chen Y, Ke CF, Liu M (2005) J Am Chem Soc 127:657
Liu J, Mendoza S, Román E, Lynn MJ, Xu R, Kaifer AE (1999) J Am Chem Soc 121:4304
Huskens J, Deij MA, Reinhoudt DN (2002) Angew Chem Int Ed 41:4467
Rojas MT, Koeniger R, Stoddart JF, Kaifer AE (1995) J Am Chem Soc 117:336
Rekharsky MV, Inoue Y (1998) Chem Rev 98:1875
Nomura A, Sugiura Y (2004) Inorg Chem 43:1708
Liu J, Ong W, Román E, Lynn MJ, Kaifer AE (2000) Langmuir 16:3000
Dong SD, Breslow R (1998) Tetrahedron Lett 39:9343
Acknowledgments
This work was supported by the Natural Science Foundation of China (No: 20534030, 20471023), the National Basic Research Program (2007CB808006), and the Innovative Research Team in University (IRT0422).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Qi, Z., Liang, K. et al. An Artificial Supramolecular Nanozyme Based on β-Cyclodextrin-Modified Gold Nanoparticles. Catal Lett 124, 413–417 (2008). https://doi.org/10.1007/s10562-008-9494-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9494-5