Abstract
Bone is a complex and dynamic tissue that, in addition to its biomechanical properties, harbors stem cells and precursor cells of the hematopoietic and immune system. The skeleton undergoes continuous remodeling processes to maintain proper bone homeostasis. The equilibrium and coupling of bone resorption with bone formation is fine-tuned by the controlled actions of distinct cell types: bone-forming osteoblasts, bone-resorbing osteoclasts, and osteocytes as key communicators. This ensures proper bone mass and quality. Under pathophysiological conditions, the impairment of these mechanisms triggers bone remodeling dysregulation, causing loss in bone mass and/or alteration in bone microarchitecture.
This chapter deals with the function and regulation of cellular contributors of bone homeostasis – osteoblasts, osteoclasts, osteocytes, and immune cells. Further, we discuss current knowledge on the coupling of bone resorption and bone formation. Finally, we give an overview about the role of inter-organ crosstalk through bone-secreted factors that act as communicators to peripheral organs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Bone is a multi-functional organ. The skeletal system serves as connective tissue but also exhibits mechanical, metabolic, and endocrine functions [1]. It provides mechanical support to protect the brain, as well as other soft organs within the rib cage from damage. Bone is a storage site for calcium and phosphate and serves as a target organ for paracrine and endocrine factors that maintain mineral homeostasis and regulate energy outlay. Moreover, as an endocrine organ, the skeletal system contributes to body homeostasis through secreted molecules that exhibit effects on peripheral organs [2,3,4]. The skeleton is a complex and dynamic tissue that, in addition to its biomechanical properties, harbors stem cells and specific precursor cells of the hematopoietic and immune system [5, 6].
To maintain physiological bone mass, the skeleton undergoes constant remodeling processes. Bone remodeling is a lifelong dynamic process that guarantees efficient degradation of old bone in balance with replacement by new mineral. Bone mass is determined by the coordinated actions of four distinct bone cell types: bone-forming osteoblasts, bone-resorbing osteoclasts, bone-lining cells, and osteocytes. Bone-lining cells build a monolayer that covers the bone surface in the quiescent state. Osteocytes, embedded within the bone matrix during skeletal maturation, are important mechanosensing cells and therefore key skeletal communicators [7]. Under physiological conditions, the equilibrium and coupling of bone resorption with bone formation is tightly fine-tuned to ensure proper bone mass and quality. Bone remodeling occurs in four steps in a highly vascularized special space called “bone remodeling compartment” (BMC, Fig. 16.1) [8]. Remodeling initiates by recruitment of osteoclasts to BMC by osteocytes. Differentiated and functional active osteoclasts cause bone resorption and concurrent recruitment of mesenchymal stem cells. Osteoblasts differentiate and form new bone. In the final step, the osteoid mineralizes [7].
Bone remodeling occurs in the bone remodeling compartment through the coordinated action of osteocytes, osteoblast, and osteoclasts. The molecular machinery in each cell contributes to proper cellular function. Images below: Left – osteocytes (red arrow) in murine cortical bone, silver precipitation technique: Nuclei were visualized using thionin counter stain. Middle – primary mouse osteoclast differentiated in vitro from bone marrow progenitors using M-CFS and RANKL. Immunofluorescence staining of actin rings (green), trap (red). Nuclei were visualized using DAPI (blue). Right – osteoblasts (yellow arrow) in human trabecular bone specimen embedded undecalcified in poly(methyl methacrylate), toluidine blue staining. The figure was partially created using Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License
In many pathophysiological conditions, the impairment of these mechanisms triggers a dysregulation of bone remodeling, causing unbalanced between bone resorption and formation. A consequential loss in bone mass and/or alteration in bone microarchitecture can lead to skeletal destruction and increased fracture risk, in conditions like osteoporosis, rheumatoid arthritis, and skeletal metastases [9].
Osteoblasts
Osteoblasts stem from mesenchymal stem cells (MSC), similar to chondrocytes, adipocytes, myocytes, and other stromal cells. More recently, studies characterized the skeletal stem cell more precisely, showing that osteoblasts, chondrocytes, and stromal cells share a progenitor cell that does not give rise to adipocytes or myocytes, indicating that these cells are more closely related in the hierarchical tree [10]. Importantly, these observations also hold true for human skeletal stem cells [11]. Osteoblasts are specialized cells that produce the bone matrix by first laying down the organic matrix (i.e., osteoid), which mostly consists of type I collagen and other non-collagenous proteins such as osteocalcin or osteopontin, and then contributing to the mineralization of the matrix. During matrix production, osteoblasts have a cuboidal shape and are packed with endoplasmic reticulum and mitochondria. Hydroxyapatite-based microcrystals are deposited into the collagen matrix, which requires two phases: (I) the removal of acid evolved during hydroxyapatite nucleation by the osteoblast to allow for matrix deposition, and (II) mineral maturation from amorphous calcium phosphate to hydroxyapatite, which is associated with important changes in mineral orientation and organization, leading to the dense lamellar structure of mature bone. This process is critically dependent on pH and the function of tissue non-specific alkaline phosphatase (TNAP) as a supplier of phosphate , which works best at an alkaline pH [12,13,14].
Besides producing the bone matrix, osteoblasts have additional functions including providing a niche for hematopoietic stem cells [5, 15], as well as regulation of energy production [16, 17], male fertility [18, 19], and brain function by producing osteocalcin [20], which has been identified as a factor with hormonal actions on distant organ sites. Most importantly, however, osteoblasts are key regulators of bone resorption by osteoclasts, as they produce receptor activator of NF-κB ligand (RANKL) and its endogenous inhibitor osteoprotegerin (OPG), to determine the rate of osteoclastogenesis and bone resorption [21,22,23,24]. Thus, bone formation and bone resorption are tightly coupled processes that underlie a strict regulation within not only the communication between osteoblasts and osteoclasts but also other cells that influence their communication, including osteocytes and immune cells.
Osteoblast Differentiation
Osteoblast differentiation is a multi-step process, beginning with maturation from stem cell into an osteoblast progenitor, then to a pre-osteoblast, and finally to a mature osteoblast. These stages are paralleled by different functional steps, including cell proliferation, matrix production, and mineralization. Once fully matured, osteoblasts either undergo apoptosis, become quiescent bone-lining cells that can be reactivated quickly after proper stimuli (e.g., parathyroid hormone (PTH) or inhibition of sclerostin), or terminally differentiate into osteocytes. How the osteoblast fate is chosen remains largely unknown.
To differentiate, two transcription factors are absolutely necessary: Runt-related transcription factor 2 (Runx2) and osterix (Osx). Deficiency of either of these transcription factors in mice results in a non-mineralized skeleton made of only cartilage [25,26,27]. Both intramembranous and endochondral ossification are completely lacking in Runx2−/− and Osx−/− mice. These transcription factors are important not only in mice but also in humans. Mutations in the gene encoding for Runx2 are associated with cleidocranial dysplasia [28]. Several growth factor signals that are crucial for osteoblast differentiation, such as bone morphogenetic protein (BMP) signaling, fibroblast-like growth factor, or insulin-like growth factor signaling, use either Runx2 or Osx as their downstream transcriptional mediators. Besides Runx2 and Osx, Msx1 and Msx2 are critical for osteoblast differentiation in cranial bone [29]. In addition, several other transcription factors are important for osteoblastic differentiation at various sites including Dlx5, Dlx6, AP1, ATF4 , NFATs, and Twist proteins [30].
Regulatory Pathways
Osteoblast differentiation is regulated by several growth factor pathways and systemic hormones (e.g., vitamin D or glucocorticoids). However, studies in mice and humans have shown that two pathways are central regulators of ossification and osteoblast differentiation. These are the BMP and Wnt signaling pathways.
BMPs belong to the transforming growth factor beta (TGFβ) superfamily and have essential functions during embryogenesis, organogenesis, as well as cell proliferation and stem cell differentiation. Of the 15 identified BMPs, BMP2, 4, 6, 7, and 9 exhibit osteoinductive capabilities. They promote the commitment of mesenchymal stem cells to osteoprogenitors and further stimulate osteoblastogenesis and bone mineralization [31,32,33]. BMP2 and BMP4 deficiencies are embryonically lethal. Conditional knockout of BMP2 and BMP4 alone or in combination in osteoblasts results in impaired bone formation caused by dysfunctional chondrocyte and osteoblast differentiation as well as function [33, 34]. Besides the ligands, BMP receptors (e.g., ALK2, ALK3, ACVR1) in osteoblasts were also shown to play a role in ossification [35,36,37,38]. Constitutive activation and/or different ligand susceptibilities of mutated ALK2 lead to a rare genetic disease called fibrodysplasia ossificans progressiva, which is characterized by excessive heterotopic ossification [39, 40].
The BMP signal transduction is initiated upon BMP ligand binding to a receptor heterodimer complex, which consists of type I and type II BMP transmembrane serine/threonine kinase receptors (BMPR) . The constitutively active BMPR type II activates BMPR type I through phosphorylation upon ligand binding. The SMAD-dependent pathway is the classical, canonical BMP pathway and is based on the activation of R-Smads (Smad 1, 5, 8) and co-Smads (Smad 4), which, when translocated into the nucleus, can bind to BMP responsive elements and initiate the transcription of osteoblastic genes. Furthermore, BMP signaling can induce non-canonical signaling pathways, including mitogen-activated protein kinases such as extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and P38 mitogen-activated protein kinases (P38) to induce osteopromoting outcomes [33]. Also the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway has been implicated to play a role in BMP-mediated signaling in osteoblasts [41, 42].
The BMP signaling pathway is regulated via numerous inhibitors including inhibitory SMADs (Smad 6, Smad 7) as well as extracellular antagonists such as gremlin, chordin, and noggin [43]. Noggin has been investigated in great detail in the context of bone. Either overexpression or conditional knockout of noggin leads to low bone mineral density and reduced bone formation [44, 45], suggesting that optimal levels of BMP signaling are required for proper bone formation.
The Wnt signaling pathway is among the most critical pathways to regulate osteoblast differentiation. Mutations in components of the Wnt signaling pathway result in drastic skeletal alterations in both mice and humans. In brief, activation of Wnt signaling leads to enhanced osteoblast differentiation and bone formation, while inhibition results in decreased osteoblastogenesis. Thus, Wnt signaling has also been a major target to develop new treatments for osteoporosis [46].
Wnt signaling exhibits a similarly complex pathway as BMP signaling, containing 19 Wnt ligands, 10 Frizzled-receptor (FZD) variants , and multiple intracellular and extracellular inhibitors [46]. Moreover, Wnt signaling is divided between canonical Wnt signaling, which uses β-catenin as transcriptional mediator, and non-canonical β-catenin-independent pathways [47]. Canonical Wnt signaling is activated when canonical Wnt ligands (e.g., Wnt1, Wnt3a, Wnt10b) bind to the FZD receptors and LDL receptor-related protein (LRP)5/6 co-receptors. This leads to the resolution of the β-catenin destruction complex, which consists of scaffold protein axin, adenomatous polyposis coli, casein kinase 1, and constitutively active glycogen synthase kinase 3. Destruction of this complex leads to phosphorylation of cytosolic β-catenin, resulting in its ubiquitination and degradation. The non-degraded β-catenin accumulates in the cytosol and eventually translocates into the nucleus where it activates the transcription of target genes by recruiting transcriptional activators to the T cell factor/lymphoid enhancer factor (TCF/LEF) transcription complex [46,47,48]. In contrast to the β-catenin-dependent activation of transcription factors in canonical Wnt signaling, non-canonical Wnt signaling is independent of LRPs and activates other pathways including small Rho GTPases/JNK signaling, calcium-dependent NFAT signaling, and PI3K/Akt signaling that are known to control planar cell polarity, cell differentiation, and tissue patterning. Both canonical and non-canonical signals are important regulators of trabecular and cortical bone homeostasis. Mutations in the genes encoding Wnt signaling molecules (e.g., Wnt1, Wnt5a, Wnt16) lead to altered bone phenotypes, affecting either osteoblast differentiation or osteoblast-mediated communication [49,50,51]. As such, human mutations in WNT1 are associated with early-onset osteoporosis, and mutations in the WNT5A gene lead to autosomal dominant Robinow syndrome-1, which is characterized by short stature [52, 53]. In the case of LRP5, gain-of-function mutations lead to high bone mass in mice and humans, while loss-of-function mutations lead to osteopenia [54, 55].
As with all developmental pathways, Wnt signaling is tightly regulated. It can be blocked at various levels including extracellular and intracellular inhibitors. At the ligand level, secreted frizzled-related proteins and Wnt inhibitory factor 1 associate with the Wnt ligands themselves, sequestering them and thus inhibiting their binding to FZD [46, 47]. The family of Dickkopf (Dkk) proteins and sclerostin, on the other hand, bind to LRP5/6, thereby preventing their interaction with FZD. In the bone context, Dkk1 and sclerostin have been shown to be main regulators of bone mass. Deficiency of either protein leads to a high bone mass, whereas overexpression of Dkk1 or sclerostin leads to low bone mass [56,57,58,59]. Sclerostin is mainly produced by osteocytes, whereas Dkk1 is expressed in different organs including bone, skin, placenta, and the prostate. Due to the site-specific expression of sclerostin, neutralizing antibodies were developed that are now available as one of the few bone-anabolic options to treat postmenopausal osteoporosis [60]. Interestingly, however, deficiency or suppression of Dkk1 or sclerostin leads to a compensatory increase in the other Wnt inhibitor, thereby limiting the long-term efficacy of blocking sclerostin or Dkk1 [56, 61, 62]. Thus, at least experimentally, bi-specific antibodies targeting Dkk1 and sclerostin show the greatest increase in bone mass and, therefore, may be the most potent option to induce bone anabolism in the future [61].
Osteoclasts
The physiological removal of old bone by osteoclasts is necessary for growth, development, and bone remodeling to adapt the adult skeleton to function and external conditions. Loss of bone mass is the consequence of increased skeleton degradation rate relative to its formation. Pathophysiological osteoclast differentiation and activation plays a key role in osteolysis (e.g., osteoporosis). Thus, understanding the complex biology and regulatory mechanisms of osteoclasts is necessary to develop strategies to prevent pathologic bone resorption.
Osteoclasts exclusively resorb bone. They are terminally differentiated cells derived from the hematopoietic linage and therefore phenotypically related to macrophage and dendritic cells [7]. Osteoclasts are giant multinucleated cells (Fig. 16.1) that form from maturation and fusion of mononuclear precursor cells, which differentiate from bone marrow monocytes/macrophages [7]. The origin of osteoclasts was demonstrated using a parabiosis model more than 40 years ago [63]. Recent evidence shows that osteoclasts derive from embryonic erythro-myeloid progenitors and are in fact long-lived cells that use iterative fusion of monocytic precursor cells for their maintenance throughout life [64]. Osteoclast precursor cells express cell surface receptors, like macrophage-1 (mac-1), macrophage colony-stimulating factor (M-CFS) receptor c-fms, receptor activator of NF-κB (RANK), and receptors for co-stimulatory molecules such as osteoclast-associated receptor (OSCAR) [65, 66]. It is suggested that the recruitment of osteoclast precursors from the bone marrow occurs by either crossing the bone-lining cells or from capillaries that penetrate into BMC [67, 68].
The generation of mature osteoclasts capable of resorbing bone is a multi-step process. It includes the presence and priming of osteoclastic precursor cells, multinucleation and maturation, and finally activation that triggers specific cytoskeletal rearrangements, cell motility, attachment to bone matrix, and resorption [7].
Osteoclast Differentiation
There are only two essential and sufficient cytokines for osteoclastogenesis in vitro: (I) macrophage colony-stimulating factor (M-CFS) that participates in promoting cell proliferation and survival of osteoclast precursors and (II) RANKL that drives osteoclast differentiation, fusion, activation, activity, and survival [24, 69, 70].
M-CFS stimulates the expression of RANK – the signaling receptor for RANKL – in osteoclast precursor cell [7]. The central role of RANKL in osteoclast differentiation led to the successful development a neutralizing antibody, denosumab, which inhibits bone resorption and reduces fracture risk [71]. Mature osteoclasts tightly adhere to bone and release hydrogen ions that acidify the interface between bone and osteoclast, leading to bone resorption.
Physiological osteoclast differentiation requires the presence of RANKL-secreting bone-resident cells, like osteoblasts, marrow stromal cells, and osteocytes [72]. Mice lacking RANK or RANKL develop osteopetrosis due to lack of osteoclasts, demonstrating the importance of the RANK/RANKL system [73, 74].
When RANKL binds to RANK expressed on osteoclast precursors, a set of transcription factors (e.g., NF-κB, activator protein-1 (AP-1), and nuclear factor of activated T cells (NFAT)) are activated that modulate the expression of osteoclast-specific effector proteins [75].
Binding of M-CFS to its receptor c-fms activates the receptor by dimerization and promotes alteration in the cytoskeleton and expression of RANK on osteoclast precursors , increasing susceptibility to RANKL binding [7]. The transcription factor PU.1 promotes the expression c-fms. In fact, mice lacking PU.1 show a failure in macrophage differentiation [76] and inhibition of osteoclastogenesis [77].
RANK lacks intrinsic kinase activity. Therefore, the activation of RANK by RANKL causes the recruitment of intracellular adaptor protein tumor necrosis factor receptor-associated factors (TRAFs) to three different motifs of the intracellular domain of RANK [78, 79]. As a consequence, a cascade-like intracellular signaling transduction initiates osteoclast formation, function, and survival and inhibits osteoclast apoptosis via several signaling pathways. The TRAF-6 recruiting motif activates NFκB, JNK, ERK, p38, and Akt. NFκB signaling pathway then activates NFATc1 [80]. TRAF-6-deficient mice exhibit an osteopetrosis bone phenotype [81]. The role and function of the two other TRAF motifs are not clear yet [82].
Concurrent activation of co-stimulatory molecules/immunoreceptor tyrosine-based activation motif (ITAM) signaling (like osteoclast-associated receptor (OSCAR), DNAX-activating protein of 12 kDa (DAP12), or FC receptor common gamma subunit (FcRγ)) modulates RANK-mediated processes [83]. This causes Ca-dependent activation of calcineurin that directly activates NFATc1. Deficiency of DAP12 or FcRγ results in subtle osteoclastic defects, while deletion of both molecules causes severe osteopetrosis [84]. NFATc1 is an established master transcription factor of osteoclastogenesis. As part of a transcription factor complex with MIFT, PU1, CREB, and AP1, NFATc1 participates in differentiation and fusion of osteoclasts. NFATc1-regulated genes are cathespinK, MMP9, H+ ATPase, and CIC7 [85].
Osteoclast Resorptive Capacity
The bone matrix consists of inorganic (mainly crystalline hydroxyapatite) and organic components (e.g., collagen type I) [86]. Therefore, the resorption has to involve the dissolution of hydroxyapatite followed by the proteolytic cleavage of organic components.
The osteoclast activation is primarily characterized by the establishment of two essential structural and functional features for proper resorption: the ruffled border and the isolated resorption compartment [7]. The resorption compartment is isolated from the general extracellular space. It is formed by the attachment of osteoclasts to the bone matrix though a structural feature called the sealing zone. This forms a closed microenvironment between the underlying matrix and the osteoclast also called “clear zone” that is key for resorptive events. This physical interaction of bone matrix and osteoclast is mediated by integrin αvβ3 and corresponding proteins in the extracellular matrix (e.g., osteopontin, vitronectin, or bone sialoprotein) [87]. The interaction induces cytoskeleton re-organization cooperatively with M-CFS via the c-Src-signaling complex. Osteoclasts polarize fibrillary actin to circular structures to create the typical actin ring surrounding of the ruffled border that generates the isolated resorptive compartment [88]. In vitro and in vivo studies demonstrated that integrin αvβ3 is the main integrin mediating bone resorption [89]. Integrin αvβ3-deficient mice develop osteopetrosis [90].
The ruffled border is the resorptive organelle of osteoclasts and a morphological characteristic to increase the surface area [7]. The transport of protons and proteolytic enzymes into the resorption compartment is required to dissolve mineral and degrade bone matrix proteins [91]. The ruffled border is formed by migration and insertion of acidified vesicles loaded with proton pumps and cathepsin K through microtubules and actin to the plasma membrane facing the bone leading to the expression of vacuolar H + -adenosine triphosphate (H + -ATPase) and chloride channels, and release of proteolytic enzymes into the clear zone by endocytosis [92]. Diminished function of each of the three proteins results in human disease with excess bone mass demonstrating their critical role in bone resorption [92]. H + -ATPase then transports protons to decrease the pH (to about 4) of the resorption compartment, while chloride ions are transported to maintain electro-neutrality. Cytoplasmic carbonic acid is the main source of protons [93]. Passive chloride-bicarbonate exchanger supplies chloride ions. Acidification mobilizes hydroxyapatite crystals from the bone and provides an optimal activity environment for proteolytic enzymes like cathepsin K [92]. Collagen type I degradation products are removed by transcytosis. Products are endocytosed and transported in vesicles to basolateral surface of the cell and discharged into surrounding intracellular fluid [94].
Fine-Tuning of Osteoclast Formation and Function
The RANKL signaling cascade is regulated at multiple levels to ensure proper controlled osteoclastogenesis. OPG is a neutral antagonist that controls the interaction between RANK and RANKL [23]. OPG has a high affinity to RANKL and acts as soluble decoy receptor. Cells of the mesenchymal origin secrete OPG basally and in response to regulatory signals like cytokines or bone-targeting steroids [95]. The OPG/RANKL system ensures physiological balance of bone resorption and formation. Genetic deletion of OPG in humans and mice causes osteoporosis, while OPG overexpression results in osteopetrosis [23, 96]. Under pro-inflammatory conditions, cytokines suppress OPG and increase RANKL, causing increased osteoclast formation and activity [95].
RANKL-independent osteoclastogenesis remains controversial. The combination of TNFα with TGFβ or IL6 can induce osteoclastogenesis dependent on NFATc1 and DAP12 under pathophysiological conditions [97,98,99]. Other studies showed that IL-1 and TNFα stimulate osteoclastogenesis via TRAF-6 and TRAF-2 in postmenopausal osteoporosis and rheumatoid arthritis but cannot induce it independent of RANKL [100]. IL-1 and TNFα seem to increase the expression of M-CFS and RANKL [101], concluding that pro-inflammatory cytokines synergize RANKL-induced osteoclastogenesis.
Interferon (IFN)γ may serve as an anti-osteoclastogenic cytokine [102]. It has been shown to be a suppressor of osteoclast formation and function via a negative feedback pathway in vitro [102], but existing data are conflicting [103]. RANKL induces IFNγ, which simultaneously suppresses RANKL and its downstream genes.
Other cytokines have also been suggested, but the data remain unclear. The lingering confusion may be related to a lack of translation of mouse observations in human physiology. For example, another c-fms ligand interleukin (IL)-34 can support osteoclastogenesis together with RANKL [104]. However, since mouse models that lack functional M-CFS reveal reduced osteoclast, IL-34 may only play a role in pathophysiological conditions like rheumatoid arthritis [105].
Osteocytes
Within the mineralized bone matrix, a cellular network of specialized bone cells directs bone homeostasis. Osteocytes are star-shaped cells that possess several long dendritic processes connecting osteocytes to one another and cells on the bone matrix surface to form a global communicative cell network in bone. Nutrient supply and waste product removal are managed by the surrounding fluid-filled interstitium. The osteocyte cell body lies within a lacuna and each process in a fluid-filled canaliculus. Through exercise and body movements, the bone matrix and thereby the fluid inside the lacuno-canalicular system are stretched and compressed and put a strain upon the osteocytes. The resultant mechanotransduction that dictates bone remodeling resulted in osteocytes being termed the “mechanosensor of bone”. Osteocytes can direct the function of both bone-producing osteoblasts and bone-resorbing osteoclasts, adapting the local bone matrix to withstand loading.
Regulatory Pathways Involved in Osteocytogenesis
Osteocytes are considered terminally differentiated osteoblasts belonging to the mesenchymal cell lineage. Once a seam of active osteoblasts “finish” their work of producing the collagen type I-rich osteoid, three potential cell fates have been discovered: I) Osteoblasts can undergo apoptosis as a final endpoint [106]. II) Some osteoblasts lose their cuboidal, active phenotype and transform into flat bone-lining cells covering inactive bone surfaces, waiting to be reactivated by signals (e.g., parathyroid hormone (PTH)) [107]. III) A selective group of osteoblasts will be embedded into the newly formed matrix. The latter process of embedding requires the osteoblast to lose most of its cytoplasm, and cell membrane extends to form dendritic processes [108]. This morphological transformation occurs stepwise to allow for an initial dendrite budding into the osteoid laid out below the cell body. During embedding the entire osteocyte forms dendrites in all directions [109]. Several proteins are sequentially expressed during osteocytogenesis and characterize the transition from an early, matrix-embedding osteocyte to a late, deeply embedded osteocyte (Table 16.1).
The molecular drivers of osteocyte differentiation are of current scientific interest, and only a few puzzle pieces have been identified so far. Factors that induce bone formation by osteoblasts (reviewed in [110]) as well as the external matrix quality can drive osteocytogenesis [111]. Some specific molecules that promote the transition of osteoblasts to osteocytes have been identified. E11/podoplanin is one driver of osteocyte transition by promoting dendrite formation [112]. Fibroblast growth factor 2 (FGF2) was identified as regulator of E11 in osteocytes. FGF2 promotes osteocytogenesis by E11 translocation to the cell membrane and increases expression of dentin matrix protein 1 (Dmp1) and phosphate-regulating gene with homologies to endopeptidases on the X chromosome (Phex) [113].
Further, miRNAs are involved in the osteocyte differentiation. The potential contribution of miRNAs within bone became apparent through observations that the osteoblast progenitor-specific deletion of Dicer, a miRNA processing RNase III endonuclease, resulted in early mortality and mineralization defects in mice [114]. The miR23a cluster has been shown to regulate TGFβ signaling through the repression of PR-Domain Zinc Finger Protein 16 (Prdm16) and thereby stimulates osteocytogenesis in mice [115].
Regulatory Pathways Involved in Mineralization
During matrix embedding and osteocyte differentiation, osteocytes are also involved in the mineralization of the newly produced matrix. Several proteins that contribute to matrix mineralization are sequentially expressed during osteocyte differentiation and can be considered differentiation markers as mentioned above.
Dmp1 is an acidic phosphoprotein and a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family that is expressed in tooth and bone. Osteocytes produce large amounts of Dmp1 during the mid-stage of osteocytogenesis [116]. Dmp1 aids mineralization of the bone matrix by binding to initial calcium phosphate nanoparticles. This oligomerization stabilizes the calcium phosphate nanoparticles to direct their binding to the collagenous osteoid [117]. Initial studies also determined the mechanoresponsiveness of Dmp1 expression in osteocytes [118]. The role of Dmp1 in bone matrix mineralization was demonstrated by both Dmp1-deficient mice and the genetic analysis of patients with autosomal recessive hypophosphatemic rickets [119, 120]. Dmp1-deficient mice showed defective mineralization of bone that coincided with impaired osteocyte differentiation. Dmp1 regulation of mineralization is based on the full-length form of the protein, which is not found in bone. Three peptide forms of Dmp1 (37kD N-terminal, 57kD C-terminal, and a chondroitin-sulfate-linked N-terminal fragment) have been identified within the mineralized matrix that all bind to hydroxyapatite and could affect mineralization [121]. Subsequently, Wnt signaling induced by Wnt3a ligand was shown to inhibit mineralization and Dmp1 expression in osteocyte cultures, resulting in altered mineral properties with larger and poorly arranged crystals negatively affecting the mechanical properties of the mineral [122]. Also, Notch signaling appears to disturb mineralization as well as osteocytogenesis in an autocrine manner, causing a disassembled deposition of hydroxyapatite and by downregulation of Wnt signaling and impaired osteocytogenesis [123, 124].
Regulatory Pathways Involved in Phosphate Homeostasis
A process inevitably linked to mineralization is the regulation of the phosphate homeostasis, and several proteins expressed by osteocytes are found to be involved in this regulation. The Dmp1 null mice exhibited decreased serum phosphate levels and elevated serum fibroblast growth factor 23 (FGF23) levels caused by excessive expression of FGF23 by osteocytes [125]. This pathological situation resembles the autosomal recessive hypophosphatemic rickets [126] characterized by inactivating mutations in Dmp1, but also the dominant form of heritable hypophosphatemic rickets due to cleavage-resistant mutations in FGF23 [127] and X-linked hypophosphatemia with mutations in the Phex protein [128]. These diseases result in high FGF23 expression by osteocytes, elevated FGF23 serum levels, and renal phosphate wasting by FGF23-induced suppression of the abundance of phosphate-transporting molecules in the proximal renal tubule leading to reduced reabsorption of phosphate from the urine. The pathologies cause similar skeletal abnormalities, including defective mineralization of the bone matrix and dysplasia pronounced as rickets during childhood and osteomalacia during adulthood. The pathological role of FGF23 is also associated with chronic kidney disease, where osteocyte expression of FGF23 serves as an endocrine regulator of the phosphaturia in the kidneys and contributes to the increased cardiovascular mortality [129].
FGF23 can be induced by several humoral factors, e.g., PTH or vitamin D (reviewed in [130]). The ratio of the mineralization-substrate phosphate to the mineralization-inhibitor pyrophosphate appears particularly important in the regulation of FGF23 secretion [131]. Mice lacking the pyrophosphate generating enzyme ectonucleotide pyrophosphatase (ENPP1) present a rickets phenotype and elevated FGF23 levels [132]. FGF23 seems to direct bone mineralization by the transcriptional suppression of TNAP. This effect was independent of the FGF23 receptor α-Klotho that is expressed at a relevant level in bone tissue [133]. While the molecular pathways regulating FGF23 production are not entirely elucidated to date, FGF receptor 1 (FGFR1) signaling may be involved as the osteocyte-specific ablation of FGFR1 partially rescues FGF23 secretion in Hyp mice [134]. Gain-of-function mutations in FGFR1 can promote FGF23 secretion in patients [135]. The regulatory pathways to control FGF23 expression in osteocytes are tightly linked to Dmp1 and Phex expression. Double mutant Phex and Dmp1 mice (Hyp/Dmp1−/−) showed non-additive elevations of serum FGF23, resulting a similar osteomalacia and rickets phenotype as seen in the single mutants [136]. Dmp1 contains the so-called ASARM motif (small protease-resistant phosphorylated acidic serine aspartate-rich MEPE-associated motif) that can be bound by Phex and prevent FGF23 transcription [137].
The intricacies of FGF23 regulation appear endless considering the revealed complexity of the posttranslational modifications of FGF23, and the protein may be protected from cleavage by O-glycosylation via polypeptide N-acetylgalactosaminyltransferase 3 (GalNT3), or phosphorylated by family with sequence similarity 20, member C (FAM20C), to favor cleavage of FGF23 [138].
Regulatory Pathways Involved in Osteocytic Osteolysis
Osteocytes control their surrounding bone matrix – the perilacunar and pericanalicular matrix – by means of osteocytic osteolysis [139]. With lactation, a calcium demanding condition due to milk production, osteocytes contribute to osteoclast-driven bone resorption and generation of free calcium from their surrounding bone matrix [140]. During this process, osteocytes demineralize their perilacunar matrix by acidifying the microenvironment involving the vacuolar ATPase as a proton pump [141]. By mechanisms unclear to date, osteocytes can resist the acidic environment produced and sustain a significantly higher viability compared to other cell types in similar conditions [141]. The process is reversible and osteocytes likely contribute to bone re-formation post-lactation [142]. During the lytic process, osteocytes not only demineralize the matrix but also utilize proteases (e.g., matrix metalloproteinase 13 (MMP13), tartrate-resistant acid phosphatase (TRAP), and cathepsin K) to digest the organic part of the perilacunar matrix [140, 143]. TGFβ signaling has been shown to be involved in the process of osteocytic osteolysis involving MMP13 [144]. The osteocytic osteolysis with lactation involves the PTH related peptide (PTHrP)-induced PTHR1 signaling, which if blocked in Dmp1-expressing osteocytes prevents the enlargement of osteocyte lacunae with lactation [140].
The calcium hormone calcitonin is also implicated in osteocytic osteolysis. Lactating calcitonin receptor-deficient mice reveal larger osteocyte lacunae than littermate controls [145]. The protective role of calcitonin was indicated by calcitonin inhibition and periosteocytic demineralization in disuse osteoporosis [146].
The presence of osteocytic osteolysis remains seems a matter of controversy with immobilization or disuse (reviewed elsewhere [142]). The Wnt inhibitor sclerostin is involved in both unloading and osteocytic osteolysis. Sclerostin act in an autocrine/paracrine fashion to induce osteocyte expression of resorptive genes and lower the pH of culture medium in vitro [147].
Regulatory Pathways Involved in Mechanosensation and Mechanotransduction
The location within a fluid-filled lacuno-canalicular system in the mineralized bone matrix makes osteocytes well-suited to sense , transduce, and translate the mechanical load that is placed upon the skeleton. Early work by Frost et al. predicted an adaptive process of bone turnover that depends on the activity level of the individual [148]. In response to mechanical loading, osteocytes release secondary messengers including prostaglandin E2 (PEG2) and nitric oxide [149, 150], thereby regulating bone turnover. Osteocytes were shown to be highly sensitive to fluid flow in vitro that simulate forces that osteocytes could experience in vivo [151, 152]. Osteocytes also align to the load axis and possess different cytoskeletons if harboring in a non-load-bearing calvaria or a load-bearing fibula [153, 154], implying direct sensation of bone matrix deformation by osteocytes. These mechanosensation mechanisms are influenced by the morphological features of the osteocyte lacuno-canalicular network, since shape affects the applied shear stresses to osteocytes and the transmission of strain to the immediate osteocyte microenvironment [155, 156].
The mechanical forces transmitted by the fluid and matrix deformation to the osteocyte are most likely sensed by either tethering elements, connecting the dendrite to the pericanalicular matrix [157], the primary cilia of the osteocytes [158], or potentially mechanosensitive ion channels. While high-resolution imaging and experimental models support the theory that tethering elements along with strain amplification are the main osteocyte mechanosensor [159], the intracellular transmission is less clear. The primary cilium is a microtubule-based structure that extends outward from the cell. A lack of response to anabolic loading was found in in osteoblast-specific kinesin-like protein 3a (Kif3a)-deficient mice [160] and osteocyte-specific polycystic kidney disease 1 (PKD1)-deficient mice [161]. Both genes regulate the function and structure of the cilium. Recently, the mechanosensitive ion channel Piezo1 was shown to play an important role in mechanosensation of osteocytes [162].
Several key molecules have been identified that are important for mechanotransduction. One of the most prominent is connexin 43 (Cx43), which functions as part of the gap junctions connecting osteocyte dendrites to one another and as part of hemichannels mediating the release of the secondary messenger PGE2 into the fluid-filled lacuno-canalicular system [163]. The expression of Cx43 is induced in osteocytes of the alveolar bone in an in vivo model of tooth movement and in vitro induced by fluid shear stress, demonstrating the potential role of Cx43 in the regulation of bone mechanotransduction [164, 165]. Mice lacking Cx43 in osteocytes exhibit increased bone-anabolic mechanoresponsiveness in vivo and are protected against bone loss induced by unloading [166, 167], suggesting that osteocytes restrain bone responsiveness to the mechanical stimuli through the expression of Cx43.
Immune Cells That Participate in Skeletal Homeostasis
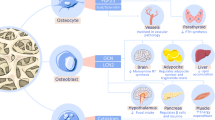
Over the past years, the reciprocal interaction between the immune and the skeletal system gained recognition in bone research, resulting in a new interdisciplinary research field called “osteoimmunology” [168,169,170]. The most important concepts in osteoimmunology arose from the observation of increased bone loss and fracture risk in various inflammatory and autoimmune diseases such as rheumatoid arthritis and chronic viral infections [171,172,173,174,175,176,177]. The discovery of RANK and RANKL, two molecules that were first identified as important factors expressed on T cells and dendritic cells, and their later identification as key osteoclastogenic molecules have clearly established the link between the immune and the skeletal system. The importance of RANKL as a key regulator of osteoclastogenesis especially during inflammation was further underlined by the discovery of Kong and colleagues, who identified that activated T cells in adjuvant arthritis are capable of inducing osteoclastogenesis through the RANKL/RANK/OPG axis [172]. Since then, a number of regulatory molecules including cytokines, receptors, signaling molecules, and transcription factors have been identified in both the skeletal and the immune system [178]. Therefore, changes in one system under physiological or pathological conditions likely affect the other. Thus, understanding the mechanisms of action of osteoimmunology will help in the development of new therapeutic drugs to treat bone loss in inflammatory diseases. In the following, we will discuss the impact of different immune cell populations on bone turnover (Fig. 16.2).
Interaction of bone and immune cells. RANKL secreted by activated T and B cells initiates osteoclastogenesis by binding to its receptor, RANK, which is expressed on preosteoclasts. OPG secreted by osteoblasts and Treg cells acts as a RANKL decoy receptor and subsequently prevents RANKL-RANK binding. Under physiologic conditions, OPG/RANKL is in equilibrium and preserves bone homeostasis. Under inflammatory conditions, RANKL is upregulated, while OPG is downregulated. T helper cells (Th1/Th2/Th17) secrete numerous pro-inflammatory cytokines, stimulating RANKL expression and thereby osteoclast formation and activity. Also pro-inflammatory M1 macrophages and mast cells are known to produce cytokines that promote bone resorption. Dendritic cells on the other hand are indirect players in inflammation-induced bone loss as they regulate T cell activity through a direct RANK-RANKL interaction
For instance, macrophages promote osteoblastogenesis by the secretion of interleukin-18 [179], whereas T cells are known to influence osteoclastogenesis mainly by secretion of various cytokines such as IL-1, IL-6, IFNγ, or IL-4 [180, 181].
T Lymphocytes
Among the immune cells, T lymphocytes play a predominant role in the regulation of bone turnover. According to the subunits that form the T cell receptor (TCR), these lymphocytes can be divided into two classes expressing either αβ or γδ TCR on their surface [182]. The majority of T lymphocytes are αβ T cells, which express either CD4 or CD8. Most of γδ T cells lack CD4 and CD8 expression, and their functions remain largely unknown [183]. How T cells affect bone remodeling depends on their activation state. Under pathological conditions such as estrogen deficiency [184, 185] and inflammatory diseases such as rheumatoid arthritis and periodontitis [172, 186], activated T cells (CD4+, CD8+, and TH17) modify bone homeostasis. One of the most important findings in this context is that activated T cells express high levels of RANKL, thereby promoting osteoclastogenesis and bone loss [181]. However, little is known about the role of T cells in physiological bone homeostasis and about their crosstalk with osteoblasts. Under physiological conditions, T cells are not considered a significant source of RANKL as T cell-deficient mice do not show diminished Rankl mRNA expression in their bone marrow [187]. Previous studies indicate that resting T cells blunt osteoclast formation in vitro [188] and attenuate bone resorption in vivo [187]. In line with this, T cell-deficient mice exhibit increased bone resorption, resulting in reduced bone density when compared to controls [189]. Indeed, depletion of CD4+ and CD8+ T lymphocytes in mice showed that T cells can also mediate anti-osteoclastogenic signals in vivo. The authors suggested that T cells enhance vitamin-D3-stimulated osteoclast formation in vitro by yet unknown mechanisms involving decreased OPG production [190]. CD4+ T cells can be subdivided into different subsets based on the produced cytokines (Th1 (IFNγ), Th2 (IL-4), and Th17 (IL-17)). Even though Th1 and Th2 produce cytokines that mediate osteoclast formation and function (e.g., TNF, IL-6), they might also be capable of inhibiting osteoclast differentiation by releasing IFNγ and IL-4, respectively [191]. The role of IL-17 in bone homeostasis was first demonstrated by Kotake et al. who identified IL-17 is a potent stimulator of osteoclastogenesis in the synovial fluid of rheumatoid arthritis patients [192]. Indeed, it is now validated that Th17 cells are increased in various bone diseases and that it significantly contributes to increased bone resorption [191, 193]. While the previously mentioned CD4+ T cell subsets have been shown to mainly promote osteoclastogenesis, anti-inflammatory CD4+ Tregs are known to inhibit osteoclast differentiation by secreting OPG [194,195,196]. Similar to Tregs, CD8+ T cells have a bone protective role, as they suppress osteoclastogenesis mainly due to soluble molecules [197].
Beside their function as regulators for osteoclastogenesis, an increasing body of evidence points toward an important role of T cells as activators of bone formation, as they are able to produce and secrete Wnt ligands (e.g., Wnt10b) that can activate Wnt signaling in osteoblastic cells [198,199,200]. A role of the canonical Wnt pathway in T cell biology (T cell development, CD8+ memory T cell formation, and regulatory T cell function) has been suggested in multiple experimental systems with varying results [201,202,203,204]. Chae et al. showed that Dkk1 is uniquely expressed at the cell membrane of Foxp3+ Treg cells to inhibit T cell-mediated autoimmune colitis [205]. Furthermore, we previously demonstrated that T cell-specific Dkk1 deletion resulted in a high bone mass primarily due to an increased bone formation (Colditz and Rauner, unpublished data). In line with this, co-culture experiments revealed that inactive T cells promoted osteoblast differentiation. It should be noted that bone cells spontaneously or in response to specific stimuli secrete cytokines, thereby inducing the differentiation of naïve helper T cells into mature T cell populations that in turn regulate bone homeostasis [206]. Interestingly, activated T cells suppressed osteogenic differentiation and thus had opposing effects on osteoblasts than resting T cells. It was already shown that the activation of T cells increases their cytokine production, which may exert negative effects on osteoblasts [207]. Taken together, T cells can have multiple effects on bone, depending mainly on their activation status and phenotype.
B Lymphocytes
B cells not only represent an important cell population of our immune system by producing antibodies and working as antigen-presenting cells, but they are also important regulators of bone resorption as they are considered the dominant producers of bone marrow OPG [187]. B cell-deficient mice exhibit a reduced bone mass, indicating that these immune cells play an important role in the maintenance of bone homeostasis [187]. Indeed, the osteopenic phenotype of B cell knockout mice was associated with a loss of OPG mRNA and protein expression [187]. While resting B cells have not been shown to produce high amount of RANKL, they are considered an important RANKL source under inflammatory conditions [208]. B cells in multiple myeloma have been shown to increase osteoclastogenesis directly by producing RANKL [209] or indirectly by IL-7 secretion [210, 211]. Malignant B cell-derived plasma cells in multiple myeloma patients also produce an increased amount of Dkk1 and sclerostin, two important inhibitors of osteoblast differentiation [212,213,214]. Additionally, B lymphopoiesis is upregulated during estrogen deficiency [215], while it is downregulated by estrogen treatment [216], suggesting a role of B cells in estrogen deficiency-induced bone loss [217]. Indeed B cells that express the B220 marker have not only been shown to be able to transdifferentiate into osteoclasts in vitro [218], but they are also more abundant in ovariectomized mice [219] and have been demonstrated to secrete RANKL in estrogen-deficient postmenopausal women [220]. In line with this, mice lacking RANKL in B cells are protected from ovariectomy-induced bone loss [221]. However, peripheral blood B cells have also been shown to inhibit osteoclast formation in vitro, which is partially mediated by TGFβ secretion [222], a cytokine that stimulates the apoptosis of osteoclasts [222,223,224]. Depletion of B cells in vivo aggravated periodontitis-induced bone loss, indicating that B cells do limit bone resorption under certain conditions [225]. Thus, the role of resting B cells in bone remodeling seems to be minimal, while activated B cells seem to play a more prominent role in many inflammatory or malignant diseases which are associated with bone changes.
Previous studies showed that T cell-deficient CD40 knockout mice are osteoporotic, suggesting that T and B cells cooperate with each other by enhancing OPG production by a CD40/CD40L mediated co-stimulation [187]. Accordingly, low bone density has been found in children with X-linked hyper-Ig syndrome, where CD40L production is impaired due to a mutation of the CD40L gene [226]. However , as T cells need to be activated to express CD40L and only a few activated T cells are found under basal conditions, the relevance of this mechanism for basal bone homeostasis remains unclear.
Mast Cells
Mast cells are found in most tissues including bone and are known to exert critical immunoregulatory roles in various immune disorders through the release of mediators such as histamine, leukotrienes, cytokines, and chemokines [227,228,229]. However, up until now, the exact molecular mechanisms by which mast cells contribute to the pathogenesis of arthritis, atherosclerosis, cancer, and obesity are unclear. Mast cells are known to not only produce TGF-β, TNF, IL-1, IL-3, and IL-6, cytokines known to stimulate bone resorption, but also RANKL [230, 231]. In line with this, a number of studies found an association between mastocytosis and reduced bone quality [146, 232,233,234,235]. Under physiological conditions, mast cells modulate bone homeostasis as mast cell-deficient mice exhibit enhanced osteoclastogenesis with concomitant slow formation of newly synthesized bone matrix, while mineralization rates remain normal [231]. Lack of chymase, a mast cell restricted protease, however, led to expansion of diaphyseal bone, which was associated with increased levels of a bone-anabolic serum marker and a higher periosteal bone formation rate [236]. Furthermore, mice that lack histamine decarboxylase, an enzyme required for the production of histamine, exhibited increased bone resorption [237]. An increased number of mast cells have been found in ovariectomized rat [238] as well as postmenopausal women suffering from osteoporosis [239], indicating an important role of mast cells in estrogen deficiency-induced bone loss. Overall, there is still a lack of knowledge on the role of mast cells on bone turnover under physiological and pathological conditions.
Monocytes/Macrophages
Monocytes and macrophages have crucial and distinct roles in tissue homeostasis and immunity. Macrophages are heterogeneous immune cells and are polarized into pro-inflammatory M1 and anti-inflammatory M2 macrophages depending on the environment [240]. Macrophages as well as macrophage-derived factors play an important role in the pathogenesis of inflammatory diseases. Macrophages have been shown to directly interact with bone cells and play a critical role in bone formation [241]. Under physiological conditions the majority of macrophages display an M2 phenotype, which maintain tissue homeostasis as they secrete IL-10 and TGF-β, thereby inhibiting bone resorption [242, 243]. However, under inflammatory conditions macrophages are activated and polarized to the M1 phenotype that contribute to tissue damage by the production of nitric oxide and pro-inflammatory cytokines (TNF-α, IL-1β, and IL-12), resulting in an induction of bone resorption [244]. Bone-resident macrophages, also called “osteomacs,” have been shown to promote bone formation, as removal of osteomacs from primary calvarial osteoblast culture resulted in a significant decrease in mineralization of osteoblasts [245]. Similar to resident macrophages, inflammatory macrophages can influence bone formation. While classically M1 macrophages have been shown to produce oncostatin M [246], which promotes osteogenesis, alternatively activated M2 macrophages have been shown to induce osteoblast formation in a oncostatin M-independent manner [247, 248]. It is well established that macrophages play a crucial role in the pathogenesis of rheumatoid arthritis, as they are considered the main source of the pro-inflammatory cytokines, which in turn activate endothelial cells, induce synovial inflammation, and increase osteoclastogenesis, thus finally leading to joint damage [249, 250]. Indeed, macrophages are able to promote T cell migration and polarization, as they secrete cytokines known to induce Th1 and Th17 polarization [251]. Furthermore the differentiation of macrophages into osteoclasts contributes to bone erosion due to induced expression of RANKL in synovial fibroblasts by pro-inflammatory cytokines [240]. Taken together, these results suggest that macrophages have crucial effects on bone remodeling during bone inflammation.
Apart from macrophages , monocytes can differentiate into dendritic cells (DC), which are the most efficient antigen-presenting cells and key players in the regulation of T cell immunity against pathogens and tumors [252]. DCs seem to not contribute to normal bone homeostasis, as DC-deficient mice have no altered bone phenotype [253]. While DCs are rarely localized in the bone under physiological conditions, active lesions in rheumatoid arthritis and periodontitis contain both mature and immature DC in different compartments of affected tissue surrounded by bone [254,255,256,257,258,259]. DCs have been described to interact through RANK-RANKL with T cells and are thereby considered active indirect players in inflammation-induced bone loss through regulation of T cell activity [254,255,256,257,258,259,260]. Apart from influencing T cell biology, DCs have been shown to transdifferentiate into osteoclasts in the presence M-CSF and RANKL and thereby might directly contribute to osteoclastogenesis [261]. Hence, DCs are of great importance in inflammatory conditions, while they do not seem to contribute to normal bone homeostasis .
Coupling of Bone Resorption and Formation
Bone tissue functions to withstand and enable our daily locomotion, while ensuring mineral homeostasis of the body, two intricate functions that rely on the timely and locally coupled activity of bone formation and bone resorption [262]. Osteocytes contribute to bone remodeling via osteocytic osteolysis and the regulation of mineralization; those functions will not be further elucidated here; rather the orchestration of osteoclast and osteoblast activities by osteocytes will be discussed.
The coordinated activity of bone remodeling relies on two major activities: (i) the demineralization and proteolytic degradation of the bone matrix by osteoclasts and (ii) the activity of osteoblasts to form the unmineralized type I collagen-rich osteoid. Bone remodeling may start with the formation of microcracks caused by an inappropriately high loading for the existing bone matrix quality. Thereby, RANKL is released as part of apoptotic bodies of microcrack-damaged osteocytes and is actively produced by neighboring osteocytes [263, 264]. RANKL produced by cells of the osteoblast lineage (osteoblasts and osteocytes) contributes in the activation of signaling pathways involved in osteoclast differentiation, osteoclast activity, and survival through the interaction with the surface receptor RANK to promote bone resorption [265]. With unloading, the role of osteocyte-released RANKL becomes apparent [266]. RANKL conditional knockout mice are protected from hind-limb unloading-induced bone resorption [72, 267]. RANKL is initially produced as a membrane-bound protein that once cleaved by proteases exists also in a soluble form (sRANKL). Both forms seem to have different roles in resorption activation, with sRANKL being indispensable for estrogen deficiency-induced bone loss [268]. Further, osteocytes produce the signaling molecules M-CFS and OPG to directly drive osteoclast differentiation or oppose osteoclast formation, respectively [269, 270].
Once the resorptive activity of osteoclasts ceases and the reversal phase is initiated, bone formation proceeds. Bone formation can be both induced and inhibited by osteocyte signals depending on the loading situation. Nitric oxide, a signaling product of mechanically loaded osteocytes [149], has emerged as a stimulator for osteogenic response. Inhibition of nitric oxide in rats has been shown to inhibit bone formation [271, 272]. Accumulating evidence exists that PGE2 secreted by mechanically loaded osteocytes through Cx43 hemichannels contribute to the bone’s adaptive response to mechanical stimuli [163, 273]. Mechanical loading results in increased production of cyclooxygenase-2 (COX2), the key enzyme required for PGE2 synthesis, as shown in bone cells of rat in vivo as well as in human and chicken osteocytes in vitro [150, 274]. On the other hand, in the absence of mechanical stimuli or with postmenopausal estrogen deficiency, the bone-anabolic Wnt signaling is inhibited in osteoblasts through the release of the Wnt inhibitors sclerostin and Dkk1 from osteocytes [56, 59]. Sclerostin inhibits the activation of Wnt/β-catenin signaling in osteoblasts, resulting in reducing bone formation activity, but also resulting in enhanced osteoclast recruitment via increased RANKL and reduced OPG expression [275, 276]. Osteocytes respond to different stimuli (e.g., exercise, PTH, and estrogen) by secreting less sclerostin, resulting in increased activity of the Wnt signaling pathway and enhanced osteoblast-mediated bone formation [277, 278].
In addition to the regulation by osteocytes, osteoclasts couple to osteoblasts and vice versa by utilizing several factors, some of which (e.g., RANKL and OPG) have already been mentioned. However, it may not always appear straightforward to imagine that this cellular crosstalk of osteoclasts and osteoblasts exists on the same bone surface at the same time [279]. There is a true multitude of factors known to signal in between the actors of bone remodeling, and intensive reviews can be found elsewhere [280, 281]. During bone resorption some coupling factors are released from the bone matrix and could potentially act upon osteoblast precursors, signaling their replication, recruitment, and differentiation. Here, platelet-derived growth factor (PDGF), BMP2 and 4, IGF1, and TGFβ1 play a role [282,283,284,285]. In addition, osteoclasts secrete coupling factors (e.g., sphingosine-1-phosphate, cardiotrophin-1, complement factor 3a, and many more) [286,287,288]. Though not widely accepted, a potential cell-to-cell contact might also direct interactions between osteoclasts and osteoblasts or their progenitors. Coupling factors that are membrane-bound have been defined, and EphrinB2 [39] and Semaphorin D were proposed to be of importance in bone homeostasis [289, 290]. Future research will demonstrate the biological relevance of the individual regulatory mechanisms of bone remodeling and most likely introduce further new players into the signaling machinery .
Bone Homeostasis Contributes to the Inter-organ Crosstalk Between Bone and Peripheral Organs
For many years, bone was only considered as an endocrine target organ that responds to hormones like PTH. However, several lines of evidence suggest that the skeletal system participates in inter-organ crosstalk through secreted factors called osteokines that have effects on peripheral organs such as the muscle, liver, kidney, pancreas, and brain and the cardiovascular system. Therefore bone actively contributes to whole body homeostasis (Fig. 16.3). Mainly osteoblasts and osteocytes produce and secret endocrine factors that are capable of altering distant tissue functions. In recent years, the function of bone-derived circulating FGF23 and osteocalcin was intensively studied.
The multifaceted inter-organ crosstalk between bone and peripheral organs through osteokines. Bone cells secrete osteokines like osteocalcin, fibroblast growth factor 23 (FGF23), dentin matrix protein-1 (DMP-1), matrix extracellular phosphoglycoprotein (MEPE), phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX), receptor activator of nuclear factor-kappa B ligand (RANKL), prostaglandin E2 (PEG2), and WNT-3A [291]. An exemplary list of peripheral organs is shown. The figure was created using Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License
FGF23
FGF23 is one of the most important osteocyte-secreted molecules [292]. The binding of FGF23 to its receptor FGFR is Klotho-dependent [293]. Targets organs of FGF23 are multifaceted (e.g., kidney, parathyroid gland, and the cardiovascular system).
The FGF23 bone-kidney axis plays an important role in phosphate homeostasis that is dependent on bone-secreted FGF23. FGF23 inhibits the expression of renal sodium/phosphate co-transporters as well as 1-α hydroxylase, which are essential for renal phosphate reabsorption and vitamin D metabolism [294, 295], causing increased urinary phosphate excretion and reduced phosphate absorption.
The signaling between bone and the parathyroid gland is also bidirectional. Parathyroid gland-secreted PTH increases FGF23 expression in osteocytes, which acts on the parathyroid gland to diminish PTH secretion [292].
A major milestone in the understanding of the bone-heart/vascular axis was the observation that circulating FGF23 levels associated with altered cardiac and vascular function [296]. High serum FGF23 levels were independently associated with elevated risk for cardiac hypertrophy and mortality in patients with and without chronic kidney disease [297,298,299,300]. There are numerous explanations for the prognostic finding, including FGF23-induced endothelial dysfunction [301], arterial stiffness [301, 302], vascular calcification [303, 304], inflammation [305], stimulation of the renin-angiotensin system [306], and left ventricular hypertrophy [300, 307].
Osteocalcin
Osteocalcin (OCN) acts as a marker for osteoblast activity and bone formation and regulator of osteoclast activity. OCN is a non-collagenous, vitamin K-dependent protein that acts on multiple peripheral organs, especially in its metabolic active uncarboxylated form [308]. OCN plays an important role in brain development and cognitive function [309] and in energy metabolism [16]. Evidence from animal studies showed that OCN can have an effect on pancreas, adipose tissue, male gonads, and muscle to regulate insulin secretion, insulin sensitivity, male fertility, and muscle power through the peripheral receptor G protein-coupled receptor 6a [17, 19, 310]. Interestingly, exogenous OCN treatment preceding exercise was sufficient to enhance exercise capacity in mice [17]. OCN also affects muscle tissue in human [311]. Recent data revealed that OCN acts as a mediator for vascular calcification in vivo that is mediated by the Wnt/β-catenin signaling pathway and mitochondrial dynamics [312].
Other Osteokines
Other osteocyte-derived osteokines (e.g., PHEX, DMP-1, sclerostin, RANKL, PGE2, and Wnt 3a) are known to have paracrine effects on peripheral tissue [292]. PGE2 and Wnt 3a, two molecules secreted in response to shear stress, support myogenesis and muscle function [313, 314]. Recently, it was shown that RANKL impairs muscles strength and insulin sensitivity in mouse and human [315].
Conclusion
The importance to maintain bone homeostasis is underlined by the tightly regulated process of bone cell communication via mechanical sensors and secreted factors. In this process each cell is highly specialized. A better understanding of bone homeostasis has led to the development of successful therapeutic strategies targeting distinct pathways (e.g., osteoclast differentiation by RANKL antibodies and osteocyte biology using sclerostin antibodies).
The knowledge that bone is not only an endocrine target organ but also signals via secreted factors to peripheral organs triggered the development of new research fields that study bidirectional inter-organ crosstalk (e.g., the bone-muscle axis (musculoskeletal research), bone-immune system axis (osteoimmunology), and bone-vascular axis). Clinical findings and animal models indicate an association between osteoporosis and cardiovascular calcification [316, 317]. Various in vitro and in vivo studies suggest that cellular and molecular processes in cardiovascular calcification are similar to those seen in pathological bone remodeling. However, whether the calcification paradox is a cause of shared risk factors and common underlying mechanisms or due to unknown bone-secreted factors is currently under debate.
Placing the bone as a central organ of our body, a deeper understanding of the role of physiological and pathophysiological bone homeostasis on the secretion of molecules that affect target organs will be helpful in the identification of new targets that prevent comorbidities.
References
Oldknow KJ, MacRae VE, Farquharson C. Endocrine role of bone: recent and emerging perspectives beyond osteocalcin. J Endocrinol. 2015;225(1):R1–19.
Guntur AR, Rosen CJ. Bone as an endocrine organ. Endocr Pract. 2012;18(5):758–62.
DiGirolamo DJ, Clemens TL, Kousteni S. The skeleton as an endocrine organ. Nat Rev Rheumatol. 2012;8(11):674–83.
Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304(8):E863–73.
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6.
Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53.
Feng X, Teitelbaum SL. Osteoclasts: new insights. Bone Res. 2013;1
Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16(9):1575–82.
Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9(9):522–36.
Chan CKF, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1-2):285–98.
Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, et al. Identification of the human skeletal stem cell. Cell. 2018;175(1):43–56.e21.
Gadaleta SJ, Paschalis EP, Betts F, Mendelsohn R, Boskey AL. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: new correlations between X-ray diffraction and infrared data. Calcif Tissue Int. 1996;58(1):9–16.
Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10(9):3815–26.
Yadav MC, Simao AMS, Narisawa S, Huesa C, McKee MD, Farquharson C, et al. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res. 2011;26(2):286–97.
Zhang J, Niu C, Ye L, Huang H, He X, Tong W-G, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69.
Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016;23(6):1078–92.
Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123(6):2421–33.
Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144(5):796–809.
Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155(1):228–41.
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–9.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76.
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–602.
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–54.
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29.
Sinha KM, Zhou X. Genetic and molecular control of osterix in skeletal formation. J Cell Biochem. 2013;114(5):975–84.
Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, et al. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat Genet. 1997;16(3):307–10.
Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24(4):391–5.
Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99(5):1233–9.
Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51.
Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005.
Wu M, Chen G, Li Y-P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009.
Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6(8A):32–52.
Kamiya N, Shuxian L, Yamaguchi R, Phipps M, Aruwajoye O, Adapala NS, et al. Targeted disruption of BMP signaling through type IA receptor (BMPR1A) in osteocyte suppresses SOST and RANKL, leading to dramatic increase in bone mass, bone mineral density and mechanical strength. Bone. 2016;91:53–63.
Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, et al. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res. 2008;23(12):2007–17.
Shi C, Mandair GS, Zhang H, Vanrenterghem GG, Ridella R, Takahashi A, et al. Bone morphogenetic protein signaling through ACVR1 and BMPR1A negatively regulates bone mass along with alterations in bone composition. J Struct Biol. 2018;201(3):237–46.
Wang Y, Sun J-C, Wang H-B, Xu X-M, Kong Q-J, Wang Y-J, et al. ACVR1-knockout promotes osteogenic differentiation by activating the Wnt signaling pathway in mice. J Cell Biochem. 2019;120:8185–94.
Hatsell SJ, Idone V, Wolken DMA, Huang L, Kim HJ, Wang L, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. 2015;7(303):303ra137.
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–7.
Lauzon M-A, Drevelle O, Daviau A, Faucheux N. Effects of BMP-9 and BMP-2 on the PI3K/Akt pathway in MC3T3-E1 preosteoblasts. Tissue Eng Part A. 2016;22(17-18):1075–85.
Valer JA, Sanchez-de-Diego C, Gamez B, Mishina Y, Rosa JL, Ventura F. Inhibition of phosphatidylinositol 3-kinase alpha (PI3Kalpha) prevents heterotopic ossification. EMBO Mol Med. 2019;11(9):e10567.
Lowery JW, Rosen V. The BMP pathway and its inhibitors in the skeleton. Physiol Rev. 2018;98(4):2431–52.
Canalis E, Brunet LJ, Parker K, Zanotti S. Conditional inactivation of noggin in the postnatal skeleton causes osteopenia. Endocrinology. 2012;153(4):1616–26.
Devlin RD, Du Z, Pereira RC, Kimble RB, Economides AN, Jorgetti V, et al. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144(5):1972–8.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92.
Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99.
Lerner UH, Ohlsson C. The WNT system: background and its role in bone. J Intern Med. 2015;277(6):630–49.
Luther J, Yorgan TA, Rolvien T, Ulsamer L, Koehne T, Liao N, et al. Wnt1 is an Lrp5-independent bone-anabolic Wnt ligand. Sci Transl Med. 2018;10(466):eaau7137.
Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18(3):405–12.
Moverare-Skrtic S, Henning P, Liu X, Nagano K, Saito H, Borjesson AE, et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20(11):1279–88.
Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013;50(5):345–8.
Robinow M, Silverman FN, Smith HD. A newly recognized dwarfing syndrome. Am J Dis Child. 1969;117(6):645–51.
Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–21.
Frontali M, Stomeo C, Dallapiccola B. Osteoporosis-pseudoglioma syndrome: report of three affected sibs and an overview. Am J Med Genet. 1985;22(1):35–47.
Colditz J, Thiele S, Baschant U, Niehrs C, Bonewald LF, Hofbauer LC, et al. Postnatal skeletal deletion of Dickkopf-1 increases bone formation and bone volume in male and female mice, despite increased sclerostin expression. J Bone Miner Res. 2018;33(9):1698–707.
Poole KES, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19(13):1842–4.
Rauner M, Baschant U, Roetto A, Pellegrino RM, Rother S, Salbach-Hirsch J, et al. Transferrin receptor 2 controls bone mass and pathological bone formation via BMP and Wnt signaling. Nat Metab. 2019;1(1):111–24.
van Bezooijen RL, Roelen BAJ, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–14.
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–43.
Florio M, Gunasekaran K, Stolina M, Li X, Liu L, Tipton B, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505.
Witcher PC, Miner SE, Horan DJ, Bullock WA, Lim K-E, Kang KS, et al. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight. 2018;3(11):98673.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–8.
Jacome-Galarza CE, Percin GI, Muller JT, Mass E, Lazarov T, Eitler J, et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature. 2019;568(7753):541.
Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190(12):1741–54.
Lorenzo J. Osteoclast precursor cells. Adv Exp Med Biol. 2007;602:77–82.
Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol. 2009;174(1):239–47.
Jensen PR, Andersen TL, Pennypacker BL, Duong LT, Engelholm LH, Delaisse JM. A supra-cellular model for coupling of bone resorption to formation during remodeling: lessons from two bone resorption inhibitors affecting bone formation differently. Biochem Biophys Res Commun. 2014;443(2):694–9.
Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N, et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998;246(1):199–204.
Itoh K, Udagawa N, Matsuzaki K, Takami M, Amano H, Shinki T, et al. Importance of membrane- or matrix-associated forms of M-CSF and RANKL/ODF in osteoclastogenesis supported by SaOS-4/3 cells expressing recombinant PTH/PTHrP receptors. J Bone Miner Res. 2000;15(9):1766–75.
McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res. 2012;27(7):1480–6.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4.
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–24.
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–23.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42.
Anderson KL, Smith KA, Conners K, McKercher SR, Maki RA, Torbett BE. Myeloid development is selectively disrupted in PU.1 null mice. Blood. 1998;91(10):3702–10.
Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, et al. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature. 1997;386(6620):81–4.
Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem. 1999;274(12):7724–31.
Jules J, Wang S, Shi Z, Liu J, Wei S, Feng X. The IVVY motif and tumor necrosis factor receptor-associated factor (TRAF) sites in the cytoplasmic domain of the receptor activator of nuclear factor kappaB (RANK) cooperate to induce osteoclastogenesis. J Biol Chem. 2015;290(39):23738–50.
Armstrong AP, Tometsko ME, Glaccum M, Sutherland CL, Cosman D, Dougall WC. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J Biol Chem. 2002;277(46):44347–56.
Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13(8):1015–24.
Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–64.
Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol Cell. 2008;31(3):422–31.
Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004;101(16):6158–63.
Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation. J Bone Metab. 2014;21(4):233–41.
Unal M, Creecy A, Nyman JS. The role of matrix composition in the mechanical behavior of bone. Curr Osteoporos Rep. 2018;16(3):205–15.
Nakamura I, Duong LT, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25(6):337–44.
Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421746.
Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest. 2003;111(5):749–58.
McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105(4):433–40.
Mulari MT, Zhao H, Lakkakorpi PT, Vaananen HK. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic. 2003;4(2):113–25.
Ng PY, Brigitte Patricia Ribet A, Pavlos NJ. Membrane trafficking in osteoclasts and implications for osteoporosis. Biochem Soc Trans. 2019;47(2):639–50.
Nordstrom T, Rotstein OD, Romanek R, Asotra S, Heersche JN, Manolson MF, et al. Regulation of cytoplasmic pH in osteoclasts. Contribution of proton pumps and a proton-selective conductance. J Biol Chem. 1995;270(5):2203–12.
Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276(5310):270–3.
Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–19.
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12(9):1260–8.
Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202(5):589–95.
Yokota K, Sato K, Miyazaki T, Kitaura H, Kayama H, Miyoshi F, et al. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014;66(1):121–9.
O'Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, et al. RANK-independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. 2016;68(12):2889–900.
Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49–60.
Amarasekara DS, Yun H, Kim S, Lee N, Kim H, Rho J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018;18(1):e8.
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–5.
Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117(1):122–32.
Bostrom EA, Lundberg P. The newly discovered cytokine IL-34 is expressed in gingival fibroblasts, shows enhanced expression by pro-inflammatory cytokines, and stimulates osteoclast differentiation. PLoS One. 2013;8(12):e81665.
Adamopoulos IE, Mellins ED. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11(3):189–94.
Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–37.
Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27(10):2075–84.
Holtrop ME. Light and electron microscopical structure of bone forming cells. In: Hall BK, editor. The osteoblast and osteocyte, vol. 1. Caldwell: The Telford Press; 1990.
Nijweide PJ, van der Plas A, Scherft JP. Biochemical and histological studies on various bone cell preparations. Calcif Tissue Int. 1981;33(5):529–40.
Chen X, Wang L, Zhao K, Wang H. Osteocytogenesis: roles of physicochemical factors, collagen cleavage, and exogenous molecules. Tissue Eng Part B Rev. 2018;24(3):215–25.
Mullen CA, Haugh MG, Schaffler MB, Majeska RJ, McNamara LM. Osteocyte differentiation is regulated by extracellular matrix stiffness and intercellular separation. J Mech Behav Biomed Mater. 2013;28:183–94.
Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26(12):4539–52.
Ikpegbu E, Basta L, Clements DN, Fleming R, Vincent TL, Buttle DJ, et al. FGF-2 promotes osteocyte differentiation through increased E11/podoplanin expression. J Cell Physiol. 2018;233(7):5334–47.
Gaur T, Hussain S, Mudhasani R, Parulkar I, Colby JL, Frederick D, et al. Dicer inactivation in osteoprogenitor cells compromises fetal survival and bone formation, while excision in differentiated osteoblasts increases bone mass in the adult mouse. Dev Biol. 2010;340(1):10–21.
Zeng HC, Bae Y, Dawson BC, Chen Y, Bertin T, Munivez E, et al. MicroRNA miR-23a cluster promotes osteocyte differentiation by regulating TGF-beta signalling in osteoblasts. Nat Commun. 2017;8:15000.
Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, et al. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res Off J Am Soc Bone Miner Res. 2001;16(11):2017–26.
He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, et al. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44(49):16140–8.
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, et al. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18(5):807–17.
Feng JQ, Ward LM, Liu S, Lu Y, Xie X, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–5.
Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res. 2005;20(12):2169–77.
Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, et al. Different forms of DMP1 play distinct roles in mineralization. J Dent Res. 2010;89(4):355–9.
Zhou Y, Lin J, Shao J, Zuo Q, Wang S, Wolff A, et al. Aberrant activation of Wnt signaling pathway altered osteocyte mineralization. Bone. 2019;127:324–33.
Shao J, Zhou Y, Lin J, Nguyen TD, Huang R, Gu Y, et al. Notch expressed by osteocytes plays a critical role in mineralisation. J Mol Med. 2018;96(3–4):333–47.
Shao J, Zhou Y, Xiao Y. The regulatory roles of Notch in osteocyte differentiation via the crosstalk with canonical Wnt pathways during the transition of osteoblasts to osteocytes. Bone. 2018;108:165–78.
Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295(2):E254–E61.
Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38(11):1248–50.
Kenneth E. White, Wayne E. Evans, Jeffery L.H. O’Riordan, Marcy C. Speer, Michael J. Econs, Bettina Lorenz-Depiereux, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8.
A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11(2):130–6.
Silver J, Naveh-Many T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat Rev Nephrol. 2013;9(11):641–9.
Erben RG. Pleiotropic actions of FGF23. Toxicol Pathol. 2017;45(7):904–10.
Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19(9):1093–104.
Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86(2):267–72.
Murali SK, Andrukhova O, Clinkenbeard EL, White KE, Erben RG. Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp mice. PLoS Biol. 2016;14(4):e1002427.
Xiao Z, Huang J, Cao L, Liang Y, Han X, Quarles LD. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS One. 2014;9(8):e104154.
White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76(2):361–7.
Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25(8):2551–62.
Rowe PS. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr. 2012;22(1):61–86.
Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A. 2014;111(15):5520–5.
Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44(1):11–6.
Qing H, Ardeshirpour L, Divieti Pajevic P, Dusevich V, Jähn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–29.
Jähn K, Kelkar S, Zhao H, Xie Y, Tiede-Lewis LM, Dusevich V, et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J Bone Miner Res. 2017;32(8):1761–72.
Tsourdi E, Jahn K, Rauner M, Busse B, Bonewald LF. Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact. 2018;18(3):292–303.
Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27(9):1936–50.
Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, et al. Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21(9):2585–96.
Clarke MV, Russell PK, Findlay DM, Sastra S, Anderson PH, Skinner JP, et al. A role for the calcitonin receptor to limit bone loss during lactation in female mice by inhibiting osteocytic osteolysis. Endocrinology. 2015;156(9):3203–14.
Duriez R, Duriez J. Periosteocyte demineralization in disuse osteoporosis. The effect of calcitonin. Int Orthop. 1981;5(4):299–304.
Kogawa M, Wijenayaka AR, Ormsby RT, Thomas GP, Anderson PH, Bonewald LF, et al. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J Bone Miner Res. 2013;28(12):2436–48.
Frost HM. Bone remodelling dynamics. Thomas Publishers. Arthritis ans Theumatology. 1964;7(5):545.
Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts–correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217(2):640–8.
Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes–a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225(1):62–8.
Burger EH, Klein-Nulend J, van der Plas A, Nijweide PJ. Function of osteocytes in bone – their role in mechanotransduction. J Nutr. 1995;125(7 Suppl):2020S–3S.
Klein-Nulend J, van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB. 1995;9(5):441–5.
Vatsa A, Breuls RG, Semeins CM, Salmon PL, Smit TH, Klein-Nulend J. Osteocyte morphology in fibula and calvaria – is there a role for mechanosensing? Bone. 2008;43(3):452–8.
Vatsa A, Semeins CM, Smit TH, Klein-Nulend J. Paxillin localisation in osteocytes–is it determined by the direction of loading? Biochem Biophys Res Commun. 2008;377(4):1019–24.
Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39(9):1735–43.
Hemmatian H, Jalali R, Semeins CM, Hogervorst JMA, van Lenthe GH, Klein-Nulend J, et al. Mechanical loading differentially affects osteocytes in fibulae from lactating mice compared to osteocytes in virgin mice: possible role for lacuna size. Calcif Tissue Int. 2018;103(6):675–85.
You L-D, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278(2):505–13.
Nguyen AM, Jacobs CR. Emerging role of primary cilia as mechanosensors in osteocytes. Bone. 2013;54(2):196–204.
Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004;101(47):16689–94.
Temiyasathit S, Tang WJ, Leucht P, Anderson CT, Monica SD, Castillo AB, et al. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One. 2012;7(3):e33368.
Xiao Z, Dallas M, Qiu N, Nicolella D, Cao L, Johnson M, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011;25(7):2418–32.
Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. elife. 2019;8:e49631.
Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16(7):3100–6.
Gluhak-Heinrich J, Gu S, Pavlin D, Jiang JX. Mechanical loading stimulates expression of connexin 43 in alveolar bone cells in the tooth movement model. Cell Commun Adhes. 2006;13(1–2):115–25.
Jiang JX, Cheng B. Mechanical stimulation of gap junctions in bone osteocytes is mediated by prostaglandin E2. Cell Commun Adhes. 2001;8(4-6):283–8.
Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthopaedic Res. 2013;31(7):1075–81.
Lloyd SA, Lewis GS, Zhang Y, Paul EM, Donahue HJ. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. J Bone Miner Res. 2012;27(11):2359–72.
Arron JR, Choi Y. Bone versus immune system. Nature 2000;408(6812):535–6.
Greenblatt MB, Shim J-H. Osteoimmunology: a brief introduction. Immune Network. 2013;13:111–5.
Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. 2007;7(4):292–304.
Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20(3):161–71.
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–9.
Konishi M, Takahashi K, Yoshimoto E, Uno K, Kasahara K, Mikasa K. Association between osteopenia/osteoporosis and the serum RANKL in HIV-infected patients. AIDS (London, England). 2005;19:1240–1.
Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44(5):1003–12.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93.
Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheumat. 2000;43(11):2523–30.
Stellon AJ, Davies A, Compston J, Williams R. Bone loss in autoimmune chronic active hepatitis on maintenance corticosteroid therapy. Gastroenterology. 1985;89(5):1078–83.
Mori, G., D’Amelio, P., Faccio, R. & Brunetti, G. The Interplay between the Bone and the Immune System. Clin Dev Immunol 2013;2013:720504.
Cornish J, Gillespie MT, Callon KE, Horwood NJ, Moseley JM, Reid IR. Interleukin-18 is a novel mitogen of osteogenic and chondrogenic cells. Endocrinology. 2003;144(4):1194–201.
Mirosavljevic D, Quinn JMW, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. T-cells mediate an inhibitory effect of Interleukin-4 on osteoclastogenesis. J Bone Miner Res. 2003;18(6):984–93.
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408(6812):600–5.
Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764–5.
Haas W, Pereira P, Tonegawa S. Gamma/Delta Cells. Annu Rev Immunol. 1993;11:637–85.
Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN–induced class II transactivator. Proc Natl Acad Sci. 2003;100(18):10405–10.
Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci. 2001;98:13960–5.
Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–98.
Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–48.
John V, Hock JM, Short LL, Glasebrook AL, Galvin RJ. A role for CD8+ T lymphocytes in osteoclast differentiation in vitro. Endocrinology. 1996;137(6):2457–63.
Toraldo G, Roggia C, Qian W-P, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci U S A. 2003;100(1):125–30.
Grcevic D, Lee S-K, Marusic A, Lorenzo JA. Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1,25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol. 2000;165:4231–8.
Yuan FL, Li X, Lu WG, Zhao YQ, Li CW, Li JP, et al. Type 17 T-helper cells might be a promising therapeutic target for osteoporosis. Mol Biol Rep. 2012;39(1):771–4.
Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Investig. 1999;103(9):1345–52.
Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–82.
Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68(5):744–50.
Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56(12):4104–12.
Zaiss MM, Frey B, Hess A, Zwerina J, Luther J, Nimmerjahn F, et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J Immunol. 2010;184:7238–46.
Choi Y, Woo KM, Ko SH, Lee YJ, Park SJ, Kim HM, et al. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8+ T cells. Eur J Immunol. 2001;31(7):2179–88.
Hardiman G, Albright S, Tsunoda JI, McClanahan T, Lee F. The mouse Wnt-10B gene isolated from helper T cells is widely expressed and a possible oncogene in BR6 mouse mammary tumorigenesis. Gene. 1996;172:199–205.
Ouji Y, Yoshikawa M, Shiroi A, Ishizaka S. Wnt-10b secreted from lymphocytes promotes differentiation of skin epithelial cells. Biochem Biophys Res Commun. 2006;342:1063–9.
Terauchi M, Li JY, Bedi B, Baek KH, Tawfeek H, Galley S, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10:229–40.
Ding Y, Shen S, Lino AC, Curotto De Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–9.
Guo Z, Dose M, Kovalovsky D, Chang R, O’Neil J, Look AT, et al. β-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007;109:5463–72.
van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, Van Boxtel R, Meerding J, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39:298–310.
Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175:7981–8.
Chae WJ, Park JH, Henegariu O, Yilmaz S, Hao L, Bothwell ALM. Membrane-bound Dickkopf-1 in Foxp3+regulatory T cells suppresses T-cell-mediated autoimmune colitis. Immunology. 2017;152(2):265–75.
Pacifici R. Osteoimmunology and Its Implications for Transplantation. Am J Transplant. 2013;13(9):2245–54.
Pietschmann P. Principles of osteoimmunology: molecular mechanisms and clinical applications. Springer Vienna. 2011. p. 1-280.
Horowitz MC, Fretz JA, Lorenzo JA. How B cells influence bone biology in health and disease. Bone 2010;47(3):472–9.
Heider U, Langelotz C, Jakob C, Zavrski I, Fleissner C, Eucker J, et al. Expression of receptor activator of nuclear factor κB ligand on bone marrow plasma cells correlates with osteolytic bone disease in patients with multiple myeloma. Clin Cancer Res. 2003;9(4):1436–40.
Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, et al. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106(7):2472–83.
Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, et al. Human myeloma cells stimulate the receptor activator of nuclear factor-κB ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100(13):4615–21.
Brunetti G, Oranger A, Mori G, Specchia G, Rinaldi E, Curci P, et al. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann N Y Acad Sci. 2011;1237:19–23.
Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Specchia G, et al. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011;1(6):e27.
Oranger A, Carbone C, Izzo M, Grano M. Cellular mechanisms of multiple myeloma bone disease. Clin Dev Immunol. 2013;2013:289458.
Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, et al. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090.
Erlandsson MC, Jonsson CA, Islander U, Ohlsson C, Carlsten H. Oestrogen receptor specificity in oestradiol-mediated effects on B lymphopoiesis and immunoglobulin production in male mice. Immunology. 2003;108:346.
Miyaura C, Onoe Y, Inada M, Maki K, Ikuta K, Ito M, et al. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc Natl Acad Sci. 2002;94:9360.
Sato T, Shibata T, Ikeda K, Watanabe K. Generation of bone-resorbing osteoclasts from B220+ cells: its role in accelerated osteoclastogenesis due to estrogen deficiency. J Bone Miner Res. 2001;16:2215.
Kanematsu M, Sato T, Takai H, Watanabe K, Ikeda K, Yamada Y. Prostaglandin E2 induces expression of receptor activator of nuclear factor-κB ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res. 2000;15:1321.
Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221.
Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC, et al. Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem. 2012;287:29851.
Weitzmann MN, Cenci S, Haug J, Brown C, DiPersio J, Pacifici R. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFβ. J Cell Biochem. 2000;78:318.
Chenu C, Pfeilschifter J, Mundy GR, Roodman GD. Transforming growth factor β inhibits formation of osteoclast-like cells in long-term human marrow cultures. Proc Natl Acad Sci USA. 1988;85:5683.
Hughes DE, Dai A, Tiffee JC, Li HH, Munoy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF−. Nat Med. 1996;2:1132.
Klausen B, Hougen HP, Fiehn NEE. Increased periodontal bone loss in temporarily B lymphocyte-deficient rats. J Periodontal Res. 1989;24:384.
Lopez-Granados E, Temmerman ST, Wu L, Reynolds JC, Follmann D, Liu S, et al. Osteopenia in X-linked hyper-IgM syndrome reveals a regulatory role for CD40 ligand in osteoclastogenesis. Proc Natl Acad Sci U S A. 2007;104:5056–61.
Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CMM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749.
Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45.
Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13(5):362–75.
Ali AS, Lax AS, Liljestrom M, Paakkari I, Ashammakhi N, Kovanen PT, et al. Mast cells in atherosclerosis as a source of the cytokine RANKL. Clin Chem Lab Med. 2006;44(5):672–4.
Silberstein R, Melnick M, Greenberg G, Minkin C. Bone remodeling in W/Wv mast cell deficient mice. Bone. 1991;12:227–36.
Rossini M, Zanotti R, Bonadonna P, Artuso A, Caruso B, Schena D, et al. Bone mineral density, bone turnover markers and fractures in patients with indolent systemic mastocytosis. Bone. 2011;49:880.
Seitz S, Barvencik F, Koehne T, Priemel M, Pogoda P, Semler J, et al. Increased osteoblast and osteoclast indices in individuals with systemic mastocytosis. Osteoporosis Int. 2013;24:2325.
Van Der Veer E, Van Der Goot W, De Monchy JGR, Kluin-Nelemans HC, Van Doormaal JJ. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy. 2012;67:431.
Guillaume N, Desoutter J, Chandesris O, Merlusca L, Henry I, Georgin-Lavialle S, et al. Bone complications of mastocytosis: a link between clinical and biological characteristics. Am J Med. 2013;126(1):75.e1.
Lind T, Gustafson AM, Calounova G, Hu L, Rasmusson A, Jonsson KB, et al. Increased bone mass in female mice lacking mast cell chymase. PLoS One. 2016;11:e0167964.
Fitzpatrick LA, Buzas E, Gagne TJ, Nagy A, Horvath C, Ferencz V, et al. Targeted deletion of histidine decarboxylase gene in mice increases bone formation and protects against ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2003;100:6027.
Lesclous P, Saffar JL. Mast cells accumulate in rat bone marrow after ovariectomy. Cells Tissues Organs. 1999;164:23–9.
Fallon MD, Whyte MP, Craig RB, Teitelbaum SL. Mast-cell proliferation in postmenopausal osteoporosis. Calcif Tissue Int. 1983;35:29–31.
Gu Q, Yang H, Shi Q. Macrophages and bone inflammation. J Orthop Translat. 2017;10:86–93.
Hume DA, Loutit JF, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984;66:189–94.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2015;5:683.
Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxidative Med Cell Longev. 2016;2016:2795090.
Chang MK, Raggatt L-J, Alexander KA, Kuliwabea JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1234.
Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762–72.
Fernandes TJ, Hodge JM, Singh PP, Eeles DG, Collier FM, Holten I, et al. Cord blood-derived macrophage-lineage cells rapidly stimulate osteoblastic maturation in mesenchymal stem cells in a glycoprotein-130 dependent manner. PLoS One. 2013;8:e73266.
Horwood NJ. Macrophage Polarization and Bone Formation: A review. Clin Rev Allergy Immunol. 2016;51(1):79–86.
Dimitroulas T, Nikas SN, Trontzas P, Kitas GD. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun Rev. 2013;12:958–66.
Yeo L, Adlard N, Biehl M, Juarez M, Smallie T, Snow M, et al. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann Rheum Dis. 2016;75:763.
Egan PJ, Van Nieuwenhuijze A, Campbell IK, Wicks IP. Promotion of the local differentiation of murine Th17 cells by synovial macrophages during acute inflammatory arthritis. Arthritis Rheum. 2008;58:3720.
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–26.
McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95(11):3489–97.
Cirrincione C, Pimpinelli N, Orlando L, Romagnoli P. Lamina propria dendritic cells express activation markers and contact lymphocytes in chronic periodontitis. J Periodontol. 2002;73:45.
Cutler CW, Jotwani R. Dendritic cells at the oral mucosal interface. Journal of dental research. 2006;85(8):678–89.
Highton J, Kean A, Hessian PA, Thomson J, Rietveld J, Hart DNJ. Cells expressing dendritic cell markers are present in the rheumatoid nodule. J Rheumatol. 2000;27:339.
Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168(10):5333–41.
Page G, Miossec P. RANK and RANKL expression as markers of dendritic cell-T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis Rheum. 2005;52:2307.
Thomas R, MacDonald KPA, Pettit AR, Cavanagh LL, Padmanabha J, Zehntner S. Dendritic cells and the pathogenesis of rheumatoid arthritis. J Leukoc Biol. 1999;66:286.
Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001;167:1758–68.
Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104(13):4029–37.
Seeman E. Bone modeling and remodeling. J Bone Miner Res. 2009;19:219–33.
Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50(5):1115–22.
Kennedy OD, Lendhey M, Mauer P, Philip A, Basta-Pljakic J, Schaffler MB. Microdamage induced by in vivo reference point indentation in mice is repaired by osteocyte-apoptosis mediated remodeling. Bone. 2017;95:192–8.
Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–7.
Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21(4):605–15.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41.
Xiong J, Cawley K, Piemontese M, Fujiwara Y, Zhao H, Goellner JJ, et al. Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat Commun. 2018;9(1):2909.
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30(12):3071–85.
Harris SE, MacDougall M, Horn D, Woodruff K, Zimmer SN, Rebel VI, et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50(1):42–53.
Fox SW, Chambers TJ, Chow JW. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Phys. 1996;270(6 Pt 1):E955–60.
Turner CH, Takano Y, Owan I, Murrell GA. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Phys. 1996;270(4 Pt 1):E634–9.
Lara-Castillo N, Kim-Weroha NA, Kamel MA, Javaheri B, Ellies DL, Krumlauf RE, et al. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone. 2015;76:58–66.
Westbroek I, Ajubi NE, Alblas MJ, Semeins CM, Klein-Nulend J, Burger EH, et al. Differential stimulation of prostaglandin G/H synthase-2 in osteocytes and other osteogenic cells by pulsating fluid flow. Biochem Biophys Res Commun. 2000;268(2):414–9.
van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16(3):319–27.
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900.
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res Off J Am Soc Bone Miner Res. 2009;24(10):1651–61.
Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006;6(4):354.
Sims NA, Martin TJ. Coupling signals between the osteoclast and osteoblast: how are messages transmitted between these temporary visitors to the bone surface? Front Endocrinol (Lausanne). 2015;6:41.
Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481.
Lerner UH, Kindstedt E, Lundberg P. The critical interplay between bone resorbing and bone forming cells. J Clin Periodontol. 2019;46(Suppl 21):33–51.
Hock JM, Canalis E. Platelet-derived growth factor enhances bone cell replication, but not differentiated function of osteoblasts. Endocrinology. 1994;134(3):1423–8.
Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87(3):305–12.
Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095–101.
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15(7):757–65.
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105(52):20764–9.
Matsuoka K, Park KA, Ito M, Ikeda K, Takeshita S. Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J Bone Miner Res. 2014;29(7):1522–30.
Walker EC, McGregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JM, et al. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res. 2008;23(12):2025–32.
Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4(2):111–21.
Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17(11):1473–80.
Maurel DB, Jahn K, Lara-Castillo N. Muscle-bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines. 2017;5(4):E62.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocr Rev. 2013;34(5):658–90.
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4.
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297(2):F282–91.
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8.
Stohr R, Schuh A, Heine GH, Brandenburg V. FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol. 2018;9:351.
Souma N, Isakova T, Lipiszko D, Sacco RL, Elkind MSV, DeRosa JT, et al. Fibroblast growth factor 23 and cause-specific mortality in the general population: the northern Manhattan study. J Clin Endocrinol Metab. 2016;101(10):3779–86.
Brandenburg VM, Kleber ME, Vervloet MG, Tomaschitz A, Pilz S, Stojakovic T, et al. Fibroblast growth factor 23 (FGF23) and mortality: the Ludwigshafen risk and cardiovascular health study. Atherosclerosis. 2014;237(1):53–9.
Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152(10):640.
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408.
Silswal N, Touchberry CD, Daniel DR, McCarthy DL, Zhang SQ, Andresen J, et al. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab. 2014;307(5):E426–E36.
Mirza MAI, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205(2):385–90.
Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporosis Int. 2012;23(7):2017–25.
Ozkok A, Kekik C, Karahan GE, Sakaci T, Ozel A, Unsal A, et al. FGF-23 associated with the progression of coronary artery calcification in hemodialysis patients. BMC Nephrol. 2013;14:241.
David V, Francis C, Babitt JL. Ironing out the cross talk between FGF23 and inflammation. Am J Physiol-Renal. 2017;312(1):F1–8.
Wohlfahrt P, Melenovsky V, Kotrc M, Benes J, Jabor A, Franekova J, et al. Association of fibroblast growth factor-23 levels and angiotensin-converting enzyme inhibition in chronic systolic heart failure. Jacc-Heart Fail. 2015;3(10):829–39.
Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep. 2017;7:1993.
Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047.
Shan C, Ghosh A, Guo XZ, Wang SM, Hou YF, Li ST, et al. Roles for osteocalcin in brain signalling: implications in cognition- and motor-related disorders. Mol Brain. 2019;12(1):23.
Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014;561:137–46.
Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, et al. Under carboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone. 2014;64:8–12.
Rashdan NA, Sim AM, Cui L, Phadwal K, Roberts FL, Carter R, et al. Osteocalcin regulates arterial calcification via altered Wnt signaling and glucose metabolism. J Bone Miner Res. 2019;35(2):357–67.
Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6(3):223–9.
Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway. JBMR Plus. 2017;1(2):86–100.
Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019;129(8):3214–23.
Hjortnaes J, Bouten CV, Van Herwerden LA, Grundeman PF, Kluin J. Translating autologous heart valve tissue engineering from bench to bed. Tissue Eng Part B Rev. 2009;15(3):307–17.
Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and lung and blood institute aortic stenosis working group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124(16):1783–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rauner, M., Jähn, K., Hemmatian, H., Colditz, J., Goettsch, C. (2020). Cellular Contributors to Bone Homeostasis. In: Aikawa, E., Hutcheson, J. (eds) Cardiovascular Calcification and Bone Mineralization. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-46725-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-46725-8_16
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-46724-1
Online ISBN: 978-3-030-46725-8
eBook Packages: MedicineMedicine (R0)