Abstract
Crops under both abiotic and biotic stress are the major constraints on productivity. A number of factors like physical disorders, disease susceptibility, toxicity, hormonal imbalance, and nutritional deficiency interfere with the growth and development of plant under stress condition. Under these circumstances, rhizoremediation with the help of the plant growth-promoting rhizobacteria can mitigate stress-induced adverse effects on crop productivity. Plant growth-promoting rhizobacteria and their associated molecules play dual role by affecting both nutrition and resistance concomitantly through overlapping mechanisms. These free-living plant growth-promoting rhizobacteria actively colonize plant roots, exerting beneficial effects using their own metabolism or by directly affecting the plant metabolism. Rhizobial symbiosis has great agricultural importance in terms of improving soil fertility and crop productivity due to their synergistic as well as antagonistic interactions with other microbes in the soil environment. Plant growth-promoting rhizobacteria trigger elicitors, produce siderophores which deprive iron nutrition, and also induce cell wall-degrading extracellular enzymes as defense responses against plant pathogens. PGPR have the ability to induce the secretion of phytohormones, volatile compounds, antibiotics, and toxins which play an important role in plant growth. Rhizobacteria trigger N-acyl homoserine lactones (AHLs) like autoinducer molecules to regulate the gene expression as a part of quorum sensing. Other than these, plant growth-promoting rhizobacteria stimulate endogenous hormones of hosts to enhance stress tolerance. The mutualistic symbiosis triggers NOD factors and NOP effectors, while nonsymbiotic bacterial molecules enhance plant nutrient acquisition and growth. Here in this chapter, we have discussed and reviewed comprehensively the effectivity and mechanisms of plant growth-promoting rhizobacteria for enhancing crop productivity under different stress conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Environmental change with rapidly increasing population throughout the world is becoming a big challenge to feed all the people. Within 2020, the population of the world will be about 8 billion (Glick 2012) and it would be 9 billion in 2050 (Vejan et al. 2016). About 50% of grain yield is required to be increased in most important crops like rice, wheat, and maize to feed all the people in 2050 (Shrivastava and Kumar 2015). Rapid changes to the environment mainly due to excessive use of chemical fertilizers and hazardous material in the field are decreasing crop productivity in one hand and increasing pollution on the other hand (Chakraborty et al. 2014; Roychowdhury 2014). Plant–microbes interactions draw a lot of attention to many scientists from time to time throughout the world. Deep relationship and interactions between plants, soil, soil microfauna, and microorganisms take place at rhizosphere (Antoun and Prevost 2005). Rhizospheric microorganisms that live in the rhizosphere of plants may or may not invade the plant root for shelter and make a symbiotic association with plants by providing some essential elements as well as protection. Rhizobacteria are involved in the promotion of plant growth and development, known as plant growth-promoting rhizobacteria, simply, PGPR. A bacterium can be called as PGPR when it shows three important characters or at least two characters as stimulation, fast colonization, and beneficial activity to plants on growth (Bhattacharyya and Jha 2012). Despite huge numbers of microbes present in the rhizosphere, only 7–15% microbial cells occupy the root surface (Gray and Smith 2005), and only 1–2% bacteria are responsible for the beneficial activity to the plants as PGPR (Beneduzi et al. 2012). There are two basic types of PGPR: intracellular PGPR (iPGPR), which makes root nodule and resides in it; another one is extracellular PGPR (ePGPR), which cannot make nodule and resides outside of the root. iPGPR can fix nitrogen symbiotically in the root nodule of the host; on the other hand, ePGPR helps by providing protection to plant, forming siderophores, increasing phytohormone production, enhancing the resistant potential to plants, etc. (Gray and Smith 2005). Basically, Rhizobia and Frankia are not called as PGPR (Antoun and Prevost 2005), but in this study, we will also focus on the Rhizobia as they have a direct effect on growth of the plants by nitrogen fixation.
The concept of nitrogen cycle was first inaugurated by Reyset in 1856 by describing the release of nitrogen from organic matter. Berthelot in 1885 was able to demonstrate chemical nitrogen fixing by lightning strike. Although biological nitrogen fixation by the microorganisms was first introduced by Jodinin in 1862. At the end of nineteenth to early twentieth century nitrogen-fixing microbes got much attention, and interest is increasing day to day (Elmerich 2007).
Being sessile, plant has to deal with different types of environmental hazards like biotic (pathogenic fungi, bacteria, virus, nematodes, etc.) as well as abiotic (extreme temperature, salt stress, flood, drought, high wind, etc.). Plants overcome these situations by modulating their mode of gene expression (Yang et al. 2009; Roychowdhury et al. 2014; Hasanuzzaman et al. 2015; Anumalla et al. 2016).
Acceleration of nutrient availability, assimilation of nutrients, suppression of disease-causing microorganisms, and enhancing growth and metabolisms are beneficial activities for plant which are commonly performed by PGPR (Perez-Montano et al. 2014). For example, PGPR and other plant beneficial microorganisms help plants to overcome the stress conditions by exhorting induced systemic tolerance (IST) (Yang et al. 2009).
In stress conditions, PGPR induces many stress-tolerating genes, proteins, enzymes, etc. In drought stress, transcription of ERD15 gene takes place in Arabidopsis thaliana by the activity of PGPR Paenibacillus polymyxa (Timmusk and Wagner 1999). Achromobacter piechaudii ARV8 is another example of PGPR, which produces 1-aminocyclopropane-1-carboxylate (ACC) deaminase in drought stress condition, inhibits the function of ethylene (responsible for the reduction of root and shoot length) in pepper (Capsicum annuum L.) and tomato (Solanum lycopersicum) plants (Mayak et al. 2004). HIGH-AFFINITY K+ TRANSPORTER 1 (HKT1) is a transporter protein expressed in the Arabidopsis responsible for the Na+ import to the root system; in presence of PGPR, its expression is decreased in salt stress condition (Yang et al. 2009).
According to the experiment of Guo and Chi 2014, presence of PGPR influenced Cd accumulation in root and it is balanced in rhizospheric region of Lolium multiflorum Lam. but in case of Glycine max L. Cd accumulation showed significantly decreases in both root as well as shoot.
However, in this review, we point out recent knowledge on PGPR, a very brief history, and its impact on the plant growth and development in stress conditions, like salt, drought, and heavy metal.
2 Impact of Environmental Stresses on Crop Productivity
2.1 Adaptation of Defense System Under Stress Conditions
Some plants are not able to take action against the pathogenic microorganisms. Usually, some physical and chemical materials are the basic weapons for the plant defense. Pathogenic microorganisms induce systemic acquired resistance (SAR) in plants. SAR is associated with the pathogen-related (PR) protein and salicylic acid. On the other hand, induced systemic resistance (ISR) is induced by PGPR. ISR is associated with ethylene and jasmonic acid production. PGPR induce the production of oxidative enzymes like peroxidase, superoxide dismutase, etc. which give protection to plant from different pathogens like bacteria, fungi, virus, etc. (Kumar and Verma 2019). Wheat plants treated with PGPR Dietzianatrono limnaea STR1, supplemented with 150 mM salt concentration, showed better dry weight and length against control condition due to the overexpression of ABA-responsive gene (ABARE) and TaOPR1 gene in root and shoot system (Bharti et al. 2016). Some Rhizobacteria produce exopolysaccharides that accumulate Na+ ions and relieve plants from salt stress (Arora et al. 2013). Physiological, biochemical, and morphological adaptations with different beneficial activities of PGPR help to induce the defense system in plants to overcome other abiotic stresses.
2.2 Nitrogen Fixation Under Stress Conditions
The eukaryotic organisms are not able to fix molecular nitrogen into their cells. Plants used to uptake nitrogenous compounds such as nitrate, ammonia, etc. from the environment through their root system. Here, the role of microorganisms is noticeable; many beneficial free-living and symbiotic microbes are well documented as nitrogen fixers in plants. But in stress condition, nitrogen fixation is also hampered dramatically. So, the basic metabolisms in plants get partially or fully arrested because of the low level of nitrogen-containing compounds resulting reduction of growth and development. Nitrogen fixation is a very energy-consuming procedure. PGPR is not too good as nitrogen fixers (Martínez-Viveros et al. 2010), but it helps plants directly or indirectly to overcome the stressed condition. Symbiotic association by Rhizobium sp. with nodule formation is restricted in the legume plants only, so nonleguminous plants are dependent on rhizospheric bacteria (Martínez-Viveros et al. 2010). The well-known nitrogen-fixing nonsymbiotic bacteria are as Azoarcus sp. (Reinhold-Hurek et al. 1993), Burkholderia sp. (Santos et al. 2001); Azospirillum sp. (Bashan and de-Bashan 2010), etc. nif gene is responsible for the biological nitrogen fixation , and this gene is present also in the PGPR (Gupta et al. 2015). Pseudomonas stutzeri A1501 is an ACC deaminase-producing strain containing acdS gene; besides ACC deaminase production in salt stress, it also regulates the function of nitrogenase, an important nitrogen-fixing enzyme, and increases crop yield in rice plant (Han et al. 2015).
3 Plant Growth-Promoting Rhizobacteria (PGPR)
3.1 History of PGPR
The term PGPR was first used by Joseph W. Kloepper in late 1970s (Vessey 2003) and defined by Kloepper and Schroth (Kloepper 1978). From the last decade of the nineteenth century, nitrogen-fixing bacteria act as a PGPR, and its molecular mechanism (Bhattacharyya and Jha 2012) has become an interesting topic to the scientists. At the very beginning, PGPR studies were restricted on beneficial activity regarding biological control of plant diseases only (Antoun and Prevost 2005).
The common plants which make symbiotic associations with the rhizobia are soybean (Glycine max), alfalfa (Medicago sativa), bean (Phaseolus vulgaris), pea (Pisum sativum), clover (Trifolium sp.), peanut (Arachis hypogaea), acacia (Acacia sp.), lentil (Lens culinaris), vetch (Coronilla sp.), birdsfoot trefoil (Lotus corniculatus), chickpea (Cicer arietinum), etc. (Gray and Smith 2005). Rhizobial species that can associate with these plants are as Bradyrhizobium japonicum E109 (Cassan et al. 2009), Bradyrhizobium japonicum 532C, etc. Many bacterial strains like Aeromonas hydrophila P73, Pseudomonas fluorescens 31-12, Serratia liquefaciens2-68, Pseudomonas putida G11-32, etc. (Zhang et al. 1996), Rhizobium sp. (Nyoki and Ndakidemi 2018), and Sphingomonas sp. LK11 (Bilal et al. 2018) make relationships with soybean (Glycine max). Ensifer meliloti (Cedeño-García et al. 2018; Kang et al. 2018), Rhizobium radiobacter, Rhizobium rosettiformans (Kang et al. 2018); Sinorhizobium meliloti, Achromobacter spanium, Serratia plymuthica (Aroua et al. 2018), Sinorhizobium meliloti with Paenibacillus mucilaginosus (Ju et al. 2019), etc. can make symbiotic relationship with alfalfa (Medicago sativa) plants. Bean (Phaseolus vulgaris) plants are associated with the common rhizobacteria as Azospirillum brasilense (Malinich and Bauer 2018) and Rhizobium tropici (Nogales et al. 2002) along with Rhizobium sp. (Ormeño-Orrillo et al. 2012), Rhizobium tropici, R. etli, R. gallicum, R. leguminosarum bv. phaseoli, R. giardinii (Fernandez-Aunión et al. 2010), etc. One of the common legume plant peas (Pisum sativum) is associated with rhizobacteria as Streptomyces lydicus WYEC108 (Tokala et al. 2002), Bacillus thuringeinsis-KR1, along with R. leguminosarum (Mishra et al. 2009), etc. Co-inoculation of PGPR with the Rhizobium is also extensively studied in case of the pea plant.
Many species of Bacillus and Pseudomonas belong to free-living PGPR, i.e., ePGPR (Beneduzi et al. 2012). Other bacterial species such as Enterobacter, Klebsiella, Azotobacter, Variovorax, Azospirillum, Serratia, Burkholderia (Nadeem et al. 2014; Vejan et al. 2016), etc. are also reported as ePGPR. These bacteria are involved directly or indirectly with the plant growth and development.
3.2 PGPR to Mitigating Stress
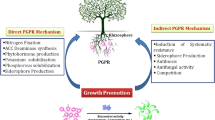
Different types of abiotic stresses like salinity, drought, heavy metal, water logging, temperature, water contamination, air pollutant, etc. and biotic stresses like pathogenicity, weeds, parasites, etc. are present in the environment (Saleem et al. 2007). By interactions with plants, PGPR help them to mitigate both abiotic and biotic stresses. Some examples of PGPR that mitigate stresses like salt by Achromobacter piechaudii, (Mayak et al. 1999) and Variovorax paradoxus5C-2 against drought on pea plants (Dodd et al. 2004); Pseudomonas putida UW4 to tomato plants (Grichko and Glick 2001); Burkholderia phytofirmans relieves potato plants in temperature (Bensalim et al. 1998); Pseudomonas fluorescens can reduce pathogenicity stress over Chamaecytisus proliferus plant (Donate Correa et al. 2005); Kluyvera ascorbata SUD165 has an effect on Brassica napus in heavy metal stress (Burd et al. 1998). ACC deaminase is the very common enzyme, produced by PGPR, which helps plant to mitigate all abovementioned stresses. Here, in this review, we just discuss the most important plant biotic and abiotic stresses (salt, drought, and heavy metal) and their mitigation strategies (Fig. 17.1).
3.3 Potential Synergistic As Well As Antagonistic Effects of PGPR
There are many biotic factors like bacteria, fungi, nematodes, parasites, etc. that directly and indirectly interact with plants. These organisms may involve in beneficial activities with plants in one hand, while in another, they can produce a detrimental effect on normal metabolism.
PGPR have the ability to interact with these factors and reduce their pathological activities by the production of antibiotic, siderophore, HCN, etc. (Gupta et al. 2015). For example, antibiotics are produced as amphisin, tropolone, oomycin A, phenazine, pyrrolnitrin, tensin, 2, 4-diacetylphloroglucinol (DAPG), pyoluteorin, and cyclic lipopeptides by different species of Pseudomonas (Loper and Gross 2007). From the study of Srivastava et al. 2010 it is revealed that Trichoderma, fluorescent Pseudomonas, and Glomus have a synergistic effect on the Fusarium wilting disease of potato. Alizadeh et al. in 2013 reported synergistic effects of Pseudomonas sp. Ps14 and fungus TrichodermaharzianumTr6 on Cucumis sativus to express some defense-related genes. In studies on cotton, Gossypium hirsutum, two different bacterial strains, Azospirillum sp. AZ204 and Pseudomonas fluorescens Pf1, showed growth promotion against normal conditions (Marimuthu et al. 2013). Also Pseudomonas aeruginosa PHU094, Trichoderma harzianum THU0816, and Mesorhizobium sp. RL091 have the capability to activate the phenylpropanoid pathway (Singh et al. 2014).
Some microorganisms have the ability to inhibit the growth of other microorganisms by secretion of some toxic chemicals like antibiotics. PGPR also produce some chemicals and destroy the growth of many pathogenic microbes (Siddiqui and Singh 2005) like synthesis of hydrolytic enzymes (protease, lipase, glucanase, etc.), competition for nutrient, regulation of ethylene production and siderophore and antibiotic production, etc. (Beneduzi et al. 2012). Siderophores or iron-chelating chemicals are a good weapon for the rhizospheric microbes. More than 100 types of siderophores produced by microbes are discovered until now. PGPR can produce siderophores, attract iron ions, and accumulate iron for their metabolic activity. So, pathogenic bacteria are deprived of iron and ultimately die. For example, siderophore pseudobactin is produced by Pseudomonas putida B10 that can inhibit the growth of Fusarium oxysporum (Kloepper et al. 1980). Bacteriocins are the chemicals produced by bacteria that are antagonistic to the same group of bacteria. E. coli, a gram-negative bacterium, produces bacteriocin and colicin, which is antagonistic to many gram-negative bacteria (Beneduzi et al. 2012). Chitinase and beta-glucanase are two important enzymes produced by PGPR that can inhibit the growth of fungi (Vejan et al. 2016). Induced systemic resistance (ISR) and systemic acquired resistance (SAR) synergistically affect against the biotic stress in presence of PGPR. Species of Pseudomonas are mainly responsible for the stimulation of these responses (Fig. 17.2).
4 Rhizoremediation to Mitigate Stress-Induced Adverse Effects on Crop Productivity
4.1 Effects of PGPR on Salty Crops
About 20% agricultural lands and 50% crop (about 5.2 billion hectares of fertile land, Numan et al. 2018) are under salt stress in the world (Paul and Lade 2014). When electrical conductivity of a saturated paste soil extract is ECe ≥ 4dS/m, it is known as saline soil (Forni et al. 2017). There are five different classes of soil salinity, such as nonsaline, slightly saline, moderately saline, strongly saline, and very strongly saline (Paul and Lade 2014). Among all, less number of salt-tolerable plants can grow in very strongly saline class. Soil salinity can inhibit many process in plant including protein synthesis, lipid metabolism, photosynthesis, etc.
Usually, salts induce ROS production, such as superoxide radicals (O2−), hydroxyl radicals (OH), and hydrogen peroxide (H2O2), are responsible for DNA damage, protein degradation, and lipid peroxidations of membranes. It also hampers seed set and crop yield and reduces flowering in different plants like wheat, barley, rice, cotton, etc. (Numan et al. 2018). Accumulation of sodium and chlorine ions in the soil can reduce the availability of other important essential elements; cause high osmotic potential; affect ion transport, DNA damage, cell viability (Jha and Subramanian 2014), plant morphology, and root and shoot growth; etc.
To overcome the salt stress, plants upregulate different enzyme production such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and proline catalase (CAT), essential to scavenge and detoxify the effects of ROS (Noreen et al. 2010). Na+/H+ anti-transporter plays a crucial role in sodium accumulation inside the vacuole of plant cell. Sometimes Na+ is transported and accumulated inside the older cells of plant, and ultimately these cells sacrifice themselves. SOS response genes (SOS1, SOS2, and SOS3) are expressed under tight regulations of salt stress in plants (Numan et al. 2018). In salinity stress, plants upregulate ABA production in root and shoot system (Cramer and Quarrie 2002; Kang et al. 2014a, b). Nitric oxide regulates the synthesis of H+ ATPase actively and forces to Na+/H+ exchange by H+ gradient formation which leads to the homeostasis of Na+ and K+ ultimately (Zhang et al. 2008a, b). Genes are expressed by plant to mitigate salt stress as P5CS mod in tobacco (Hong et al. 2000), BADH1 (betaine aldehyde dehydrogenase) in tomato (Zhang et al. 2001), DcHsp17.7 in carrot (Song and Ahn 2011), SOS1 in Brassica (Chakraborty et al. 2012), etc.
In the presence of PGPR, root length, shoot length, and dry weight of the rice are increased in both salty and normal conditions (Jha and Subramanian 2014). Studies have shown that different hormones, siderophores, HCN productions, phosphate solubilizations, etc. (Sarkar et al. 2018) have been accelerated in plants in the presence of PGPR. Auxins like indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), etc. and its precursor may be produced by the bacteria through its metabolism. IAA induces to produce a huge number of lateral roots, increase the length of the hypocotyl (Zhao et al. 2001) and shoot to root ratio, and reduce root elongation (Loper and Schroth 1986). Cytokinin and gibberellins, produced by the bacteria, are also directly involved to mitigate salt stress in plants by promoting its growth. GA1, GA19, GA20, and GA44 gibberellins are produced by different bacteria (Numan et al. 2018). Volatile organic compounds (VOCs) are produced by PGPR for plant stress management. Ryu et al. in 2004 reported that 2, 3-butanediol and acetoin are two VOCs produced by bacteria able to promote growth in Arabidopsis thaliana. Nonsymbiotic nitrogen fixation and organic and inorganic phosphate solubilizations are also done by these specific types of bacteria in salt stress. Downregulating the expression of K+ ion transporter (HKT1) and upregulating the same gene in shoot may provide protection to plants from salt stress causing less accumulation of Na+ (Zhang et al. 2008a, b). Activities of caspase-like protease, superoxide dismutase, lipid peroxidation, etc. are reduced in the presence of PGPR in rice plants (Jha and Subramanian 2014). Transcription factors such as TaMYB and TaWRKY are modulated by PGPR Dietzianatrono limnaea STR1 to activate the genes of wheat plants which are actively involved in salt elimination from the cytosol by proper expressions of transporter genes. TaST, a salt stress-induced gene; TaNHX1, TaHAK, and TaHKT1, ion transporter genes; and APX, MnSOD, CAT, POD, GPXb, and GR antioxidant proteins are also expressed on wheat plant in presence of PGPR D. natronolimnaea STR1 (Bharti et al. 2016) (Fig. 17.3).
Sulla carsona is a species of Leguminosae used as cattle food in salty regions of the world. Presence of PGPR-like Acinetobacter sp. Br3, Pseudomonas putida Br18, and Curtobacterium sp. Br20 along with Sulla carcona shows increases biomass, more chlorophyll content and antioxidant property. (Hmaeid et al. 2019). Other plant growth-regulating rhizobacteria and their impacts on plant against salt stress are discussed in Table 17.1.
4.2 Effects of PGPR on Thirsty Crops
In drought stress , plant faces very detrimental effect on the crop production (Vinocur and Altman 2005). Damage of photosynthetic apparatus and change of chlorophyll content in the plants (Ortiz et al. 2015) are major issues in this condition. Due to less amount of water, concentrations and viscosity of the cells increased dramatically; proteins or enzymes which are mixed in the cytoplasm may easily come near to each other and may deteriorate (Hoekstra et al. 2001).
According to Farooq et al. (2009), dry and fresh weight of the root and shoot is remarkably reduced and root length is increased in drought stress. Nutrition uptake and transpiration in plant are also hampered in water scarcity. Ions like SO24 −, NO3−, etc. are not assimilated due to the unavailability of energy (Grossman and Takahashi 2001) in drought condition which negatively affects plant growth and development.
PGPR produce phytohormone (abscisic acid, gibberellin, indole acetic acid, cytokinin); important enzymes like ACC deaminase, which is responsible for reduction of ethylene in root system; exopolysaccharide, which increases systemic tolerance in plant; etc. (Yang et al. 2009; Dimkpa et al. 2009; Timmusk and Nevo 2011; Kim et al. 2012; Timmusk et al. 2014). In 2007, Arkhipova et al. reported the effect of Bacillus sp. on lettuce plant and concluded the effect of bacterial cytokinin on plant growth in drying soil. ACC deaminase-producing bacteria Variovorax paradoxus showed better growth and development of pea plant in the drying soil by deactivating ethylene production (Belimov et al. 2009). ACC deaminase metabolizes ACC into ammonia and α-ketobutyrate (Saleem et al. 2007). In pepper plants, Bacillus licheniformis K11 can upregulate several genes like Cadhn, VA, sHSP, and CaPR-10 and their respective proteins such as dehydrin-like protein, vacuolar H+-ATPase, small heat shock protein, pathogenesis-related protein 10, etc. (Lim and Kim 2013). Pseudomonas putida MTCC5279 showed regulation of several important genes like DREB1A, NAC, LEA, DHN, etc. (Tiwari et al. 2016).
The activity of phosphatase and accumulation of the proline in roots and leaves, respectively, increased in lettuce plants due to the interaction of Pseudomonas mendocina; also activities of peroxidase and catalase are enhanced, and superoxidase dismutase is reduced (Kohler et al. 2008). Some examples of PGPR and their potentiality against drought stress are shown in Table 17.2.
4.3 Effects of PGPR on Crops Under Heavy Metal Stress
The environment is becoming polluted beyond our expectation because of progressive industrialization and urbanization. Besides other factors, excessive accumulation of heavy metals in soil, water, and air causes loss of soil fertility, which affects metabolic pathways of plants, ecosystem functioning, and health issues to humans and animals.
Heavy metals (>5 g/cm3) are classified into three types, namely, precious metals, radionuclides, and toxic metals. Precious metals [palladium (Pd), platinum (Pt), silver (Ag), gold (Au), ruthenium (Ru), etc.] are less reactive with high economic value; radionuclides [uranium (U), thorium (Th), radium (Ra), americium (Am), etc.] contain unstable nucleus and emit harmful rays like alpha, beta, and gamma; and toxic metals [mercury (Hg), chromium (Cr), lead (Pb), zinc (Zn), copper (Cu), nickel (Ni), cadmium (Cd), arsenic (As), cobalt (Co), tin (Sn), selenium (Se), etc.] are known for its potential toxicity mainly in environmental contexts. The nature and concentration of elements determine the toxicity of heavy metals. A little amount of heavy metals (copper, cobalt, molybdenum, etc.) are required for the metabolic pathways of organisms, but if the amount is high, then it could be harmful to the organisms (Roychowdhury and Tah 2011; Basu et al. 2012; Roychowdhury et al. 2018, 2019).
Heavy metals are nonbiodegradable in nature and they are hard to remove. There are many processes to mitigate heavy metals from the environment like ultrafiltration, immobilization, coagulation, electrodialysis, soil washing, chemical precipitation, stabilization, ion exchange, etc., but these processes are too expensive because it requires many chemical reagents and high energy sources (Gupta et al. 2016; Selatnia et al. 2004). The most efficient methods of removing heavy metals from environment are use of microbes, which have the ability to degrade heavy metals by means of its intrinsic properties or to convert it into toxic to nontoxic form (Gupta et al. 2016; Ledin 2000).
PGPR developed many mechanisms and play an important role in extraction process of heavy metal. These mechanisms include
-
(i)
Biotransformation or mineralization – alteration of highly toxic metals into low or nontoxic forms (Gupta and Diwan 2017).
-
(ii)
Metals bind with metal-binding proteins and peptides – metal-binding proteins like metallothioneins and phytochelatins (Mejare and Bulow 2001) and peptides composed of metal-binding amino acids (mainly cysteine and histidine residues).
-
(iii)
Methylation, volatilization, and demethylation processes mediated by microorganisms to remove toxic metals (Ullah et al. 2015).
-
(iv)
Extrusion metals that are extruded out from the bacterial cells through plasmid or chromosomal mediated methods (Tak et al. 2013).
-
(v)
Exclusion metal ions change in the position of target sites (Tak et al. 2013).
Overexpression of GSH synthetase in the cytosol of Indian mustard (Brassica juncea) by E. coli gshII gene enhanced accumulation and tolerance of Cd (Mosa et al. 2016). Mercuric ion reductase (encoded by merA gene) and organomercurial lyase (encoded by merB gene) present on bacterial cell help to convert the toxic form of mercury into less toxic forms (Meagher 2000; Dhankher et al. 2012) (Fig. 17.4).
Bacillus sp. SC2b isolated from Sedum plumbizincicola tolerate high concentration of Cd, Zn, and Pb (Ma et al. 2015). Microbacterium oxydans AY509223 isolated from Alyssum murale mobilized high concentration of Ni present in Ni-contaminated soil (Abou-Shanab et al. 2006). Some Cr-resistant PGPR play an important role to harbor the tolerance capacity of heavy metals like Cu, Pb, Zn, and Cd (Ma et al. 2015). A detailed account on heavy metal tolerance is given in Table 17.3.
5 Genetic Engineering Approaches of PGPR
As bacteria thrive in always changing environments, genetic material of microbes changes in many ways to overcome different types of stresses. Rhizospheric bacteria , Pseudomonas putida VTw33, shows ars operon with arsH gene (Chang et al. 2018); the same operon with ars R gene (Ramanathan et al. 1998) can bioremediate the arsenic metalloid stress. Mercury causes many severe diseases in human and is toxic to plants. Mercury-tolerating gene, mercury reductase (merA) , is present in some PGPR. This gene was incorporated in plants by genetic engineering and showed that transgenic plants were able to phytoremediate mercury (Karenlampi et al. 2000). The report says that many bacterial strains as Pseudomonas putida, Ralstonia eutropha, E. coli, Mycobacterium marianum, etc. are genetically modified and applied on the heavy metal-contaminated field (Sarma and Prasad 2019). Not only that, genetically modified plant species may be applied with the PGPR, and its synergistic activity is also very promising approach. Genetically engineered Arabidopsis thaliana can remove cadmium and lead when inoculated with Rhizobacteria (Bhattacharyya and Jha 2012).
6 Conclusion and Future Perspective
Food scarcity along with population burst throughout the world is becoming a very common problem, and we need to develop sustainable agriculture to feed all the people. Due to climate change and anthropogenic activities, plants are facing different biotic and abiotic stresses throughout their life. Besides this, we are using a tremendous amount of chemical fertilizers, chemical pesticides, and herbicides for increased crop production, leads to loss of agronomic fields and productivity from day to day. Stresses are different types; among them, drought stress, salt stress, and heavy metal stress are more detrimental and cause the main loss in agriculture. Plants’ own defense system is not enough to overcome these detrimental stressed conditions. Plant growth-promoting bacteria (PGPR) play a central role to mitigate stress by physiological, biochemical, and molecular modification of plant responses on stress. Synergistic effects of PGPR are also extensively studiedand it revealed that more than one microorganism gave the better result against the individual one. Bacteria with multiple functions against separate stresses may be a very useful tool for trace management and improvement of the crop. Among different bacterial strains, Pseudomonas sp. is much common in rhizosphere and potent bacteria for the member of synergistic activity. PGPR help in nitrogen fixation by their nif genes, which produce siderophore and antibiotics to inhibit the growth of other microorganisms, and through ACC deaminase activity ethylene content is reduced which leads to continuous growth and development of plants under stress conditions. Heavy metal-accumulating bacteria can accumulate different heavy metals like cadmium, lead, mercury, copper, arsenic, etc. Not only that, genetic engineering approaches help to insert desired microbial genes to the microorganisms and plants that can express microbial proteins which help to bioremediate heavy metal from the environment. The activity of eukaryotic organisms, i.e., fungi, in association with the prokaryotic bacteria to mitigate several stresses in plants is an interesting topic, and getting much more attention to fight against the common use of chemical fertilizers.
References
Abou-Shanab RA, Angle JS, Chaney RL (2006) Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol Biochem 38(9):2882–2889
Alizadeh H, Behboudi K, Ahmadzadeh M et al (2013) Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol Control 65(1):14–23
Antoun H, Prévost D (2005) Ecology of plant growth promoting rhizobacteria. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 1–38
Anumalla M, Roychowdhury R, Geda CK, Bharathkumar S, Goutam KD, TSS M (2016) Mechanism of stress signal transduction and involvement of stress inducible transcription factors and genes in response to abiotic stresses in plant. Int J Recent Sci Res 7(8):12754–12771
Arkhipova TN, Prinsen E, Veselov SU et al (2007) Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 292(1–2):305–315
Armada E, Roldán A, Azcon R (2014) Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb Ecol 67(2):410–420
Aroua I, Abid G, Souissi F et al (2018) Identification of two pesticide-tolerant bacteria isolated from Medicago sativa nodule useful for organic soil phytostabilization. Int Microbiol 22(1):111–120
Arora NK, Tewari S, Singh R (2013) Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs. In: Plant microbe symbiosis: Fundamentals and advances, Springer, New Delhi, p 411–449
Bal HB, Nayak L, Das S et al (2013) Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 366(1–2):93–105
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth-a critical assessment. Adv Agron 108:77–136
Basu A, Roychowdhury R, Bhattacharyya SS, Tah J (2012) Estimation of major heavy metals (Fe, Cu and Zn) in the fruit part of Cucumis sativus L. World J Sci Technol 2(7):01–03
Belimov AA, Dodd IC, Hontzeas N et al (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signaling. New Phytol 181(2):413–423
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): theirpotential as antagonists and biocontrol agents. Genet Mol Biol 35(4):1044–1051
Bensalim S, Nowak J, Asiedu SK (1998) A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am J Potato Res 75(3):145–152
Bharti N, Pandey SS, Barnawal D et al (2016) Plant growth promoting rhizobacteria Dietzianatronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28(4):1327–1350
Bidgoli RD, Azarnezhad N, Akhbari M et al (2019) Salinity stress and PGPR effects on essential oil changes in Rosmarinus officinalis L. Agric Food Secur 8(1):2
Bilal S, Khan AL, Shahzad R et al (2018) Mechanisms of Cr (VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr (VI) phytotoxic mitigating effects in soybean (Glycine max L.). Ecotoxicol Environ Saf 164:648–658
Bresson J, Varoquaux F, Bontpart T et al (2013) The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol 200(2):558–569
Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64(10):3663–3668
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46(3):237–245
Cassan F, Perrig D, Sgroy V et al (2009) Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol 45(1):28–35
Cedeño-García GA, Gerding M, Moraga G et al (2018) Plant growth promoting rhizobacteria with ACC deaminase activity isolated from Mediterranean dryland areas in Chile: effects on early nodulation in alfalfa. Chilean J Agric Res 78(3):360–369
Chakraborty K, Sairam RK, Bhattacharya RC (2012) Differential expression of salt overly sensitive pathway genes determines salinity stress tolerance in Brassica genotypes. Plant Physiol Biochem 51:90–101
Chakraborty S, Pattanayak A, Mandal S, Das M, Roychowdhury R (2014) An overview of climate change: causes, trends and implications. In: Roychowdhury R (ed) Crop improvement in the era of climate change. IK International Publishing House, New Delhi, pp 1–29
Chang JS, Yoon IH, Kim KW (2018) Arsenic biotransformation potential of microbial arsHresponses in the biogeochemical cycling of arsenic-contaminated groundwater. Chemosphere 191:729–737
Cramer GR, Quarrie SA (2002) Corrigendum to: abscisic acid is correlated with the leaf growth inhibition of four genotypes of maize differing in their response to salinity. Funct Plant Biol 29(1):111–115
Dary M, Chamber-Pérez MA, Palomares AJ et al (2010) “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater 177(1–3):323–330
Dell’Amico E, Cavalca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol Biochem 40(1):74–84
Dhankher OP, Pilon-Smits EA, Meagher RB et al (2012) Biotechnological approaches for phytoremediation. In: Plant biotechnology and agriculture. Academic, San Diego, pp 309–328
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32(12):1682–1694
Dodd IC, Belimov AA, Sobeih WY et al (2004) Will modifying plant ethylene status improve plant productivity in water-limited environments. In: Proceedings for the 4th international crop science congress, Brisbane, Australia 26
Donate-Correa J, León-Barrios M, Pérez-Galdona R (2005) Screening for plant growth-promoting rhizobacteria in Chamaecytisus proliferus (tagasaste), a forage tree-shrub legume endemic to the Canary Islands. Plant Soil 266(1–2):261–272
Elmerich C (2007) Historical perspective: from bacterization to endophytes. In: Associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Springer, Dordrecht, pp 1–20
Estrada-De Los Santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl Environ Microbiol 67(6):2790–2798
Farooq M, Wahid A, Kobayashi N et al (2009) Plant drought stress: effects, mechanisms and management. In: Sustainable agriculture. Springer, Dordrecht, pp 153–188
Fernandez-Aunión C, Hamouda TB, Iglesias-Guerra F et al (2010) Biosynthesis of compatible solutes in rhizobial strains isolated from Phaseolus vulgaris nodules in Tunisian fields. BMC Microbiol 10(1):192
Forni C, Duca D, Glick BR (2017) Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 410(1–2):335–356
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:1–15
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem 37(3):395–412
Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39(1):11–17
Grossman A, Takahashi H (2001) Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Annu Rev Plant Biol 52(1):163–210
Guo J, Chi J (2014) Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 375(1–2):205–214
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71
Gupta G, Parihar SS, Ahirwar NK et al (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 7(2):096–102
Gupta A, Joia J, Sood A et al (2016) Microbes as potential tool for remediation of heavy metals: a review. J Microb Biochem Technol 8(4):364–372
Han Y, Wang R, Yang Z et al (2015) 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas stutzeri A1501 facilitates the growth of rice in the presence of salt or heavy metals. J Microbiol Biotechnol 25(7):1119–1128
Hasanuzzaman M, Roychowdhury R, Karmakar J, Dey N, Nahar K, Fujita M (2015) Recent advances in biotechnology and genomic approaches for abiotic stress tolerance in crop plants. In: Devarajan T, Jeyabalan S (eds) Genomics and proteomics: concepts, technologies and applications. Apple Academic Press, Canada, pp 333–366
Hmaeid N, Wali M, Mahmoud OM et al (2019) Efficient rhizobacteria promote growth and alleviate NaCl-induced stress in the plant species Sulla carnosa. Appl Soil Ecol 133:104–113
Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6(9):431–438
Hong Z, Lakkineni K, Zhang Z et al (2000) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122(4):1129–1136
Jha Y, Subramanian RB (2014) PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol Mol Biol Plants 20(2):201–207
Ju W, Liu L, Fang L et al (2019) Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol Environ Saf 167:218–226
Kang SM, Khan AL, Waqas M et al (2014a) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9(1):673–682
Kang SM, Radhakrishnan R, Khan AL et al (2014b) Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol Biochem 84:115–124
Kang W, Shi S, Xu L (2018) Diversity and symbiotic divergence of endophytic and non-endophytic rhizobia of Medicago sativa. Ann Microbiol 68(5):247–260
Kärenlampi S, Schat H, Vangronsveld J et al (2000) Genetic engineering in the improvement of plants for phytoremediation of metal polluted soils. Environ Pollut 107(2):225–231
Karlidag H, Yildirim E, Turan M et al (2013) Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria× ananassa). HortScience 48(5):563–567
Kim YC, Glick BR, Bashan Y et al (2012) Enhancement of plant drought tolerance by microbes. In: Plant responses to drought stress. Springer, Berlin, pp 383–413
Kloepper JW (1978) Plant growth-promoting rhizobacteria on radishes. In Proceedings of of the 4th International conference on plant pathogenic bacteria, Station de Pathologie Vegetale et Phytobacteriologie, vol 2. INRA, Angers, France, pp 879–882
Kloepper JW, Leong J, Teintze M et al (1980) Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4(5):317–320
Kohler J, Hernández JA, Caravaca F et al (2008) Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol 35(2):141–151
Kohler J, Caravaca F, Roldán A (2010) An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol Biochem 42(3):429–434
Kumar A, Verma JP (2019) The role of microbes to improve crop productivity and soil health. In: Ecological wisdom inspired restoration engineering. Springer, Cham, pp 249–265
Ledin M (2000) Accumulation of metals by microorganisms—processes and importance for soil systems. Earth-Sci Rev 51(1–4):1–31
Lim JH, Kim SD (2013) Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11. Plant Pathol J 29(2):201–208
Liu ZF, Ge HG, Li C et al (2015) Enhanced phytoextraction of heavy metals from contaminated soil by plant co-cropping associated with PGPR. Water Air Soil Pollut 226(3):1–10
Loper JE, Gross H (2007) Genomic analysis of antifungal metabolite production by Pseudomonas fluorescens Pf-5. Eur J Plant Pathol 119:265–278
Loper JE, Schroth MN (1986) Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 76(4):386–389
Ma Y, Oliveira RS, Wu L et al (2015) Inoculation with metal-mobilizing plant-growth-promoting rhizobacterium Bacillus sp. SC2b and its role in rhizoremediation. J Toxicol Environ Health 78(13–14):931–944
Malinich EA, Bauer CE (2018) The plant growth promoting bacterium Azospirillum brasilense is vertically transmitted in Phaseolus vulgaris (common bean). Symbiosis 76(2):97–108
Marimuthu S, Ramamoorthy V, Samiyappan R et al (2013) Intercropping system with combined application of Azospirillum and Pseudomonas fluorescens reduces root rot incidence caused by Rhizoctonia bataticola and increases seed cotton yield. J Phytopathol 161(6):405–411
Martínez-Viveros O, Jorquera MA, Crowley DE et al (2010) Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant 10(3):293–319
Mayak S, Tirosh T, Glick BR (1999) Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul 18(2):49–53
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166(2):525–530
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3(2):153–162
Mejáre M, Bülow L (2001) Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol 19(2):67–73
Mishra PK, Mishra S, Selvakumar G et al (2009) Coinoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J Microb Biot 25(5):753–761
Molina-Favero C, Creus CM, Simontacchi M et al (2008) Aerobic nitric oxide production by Azospirillum brasilense Sp245 and its influence on root architecture in tomato. Mol Plant-Microbe Interact 21(7):1001–1009
Mondani F, Khani K, Honarmand SJ et al (2019) Evaluating effects of plant growth-promoting rhizobacteria on the radiation use efficiency and yield of soybean (Glycine max) under water deficit stress condition. Agric Water Manage 213:707–713
Mosa KA, Saadoun I, Kumar K et al (2016) Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci 7:303
Nadeem SM, Ahmad M, Zahir ZA et al (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32(2):429–448
Nogales J, Campos R, Ben Abdelkhalek H et al (2002) Rhizobium tropici genes involved in free-living salt tolerance are required for the establishment of efficient nitrogen-fixing symbiosis with Phaseolus vulgaris. Mol Plant-Microbe Interact 15(3):225–232
Noreen Z, Ashraf M, Akram NA (2010) Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J Agron Crop Sci 196(4):273–285
Numan M, Bashir S, Khan Y et al (2018) Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol Res 209:21–32
Nyoki D, Ndakidemi PA (2018) Root length, nodulation and biological nitrogen fixation of rhizobium inoculated soybean (Glycine max [L.] Merr.) grown under maize (Zea mays L.) intercropping systems and P and K fertilization. Adv Biores 9(1):173–180
Ormeño-Orrillo E, Menna P, Almeida LG et al (2012) Genomic basis of broad host range and environmental adaptability of Rhizobium tropici CIAT 899 and Rhizobium sp. PRF 81 which are used in inoculants for common bean (Phaseolus vulgaris L.). BMC Genomics 13(1):735
Ortiz N, Armada E, Duque E et al (2015) Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. J Plant Physiol 174:87–96
Paul D, Lade H (2014) Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron Sustain Dev 34(4):737–752
Pérez-Montaño F, Alías-Villegas C, Bellogín RA et al (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169(5–6):325–336
Prapagdee B, Chanprasert M, Mongkolsuk S (2013) Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere 92(6):659–666
Ramanathan S, Shi W, Rosen BP et al (1998) Bacteria-based chemiluminescence sensing system using β-galactosidase under the control of the ArsR regulatory protein of the ars operon. Anal Chim Acta 369(3):189–195
Reinhold-Hurek B, Hurek T, Gillis M et al (1993) Azoarcus gen. nov., nitrogen-fixing proteobacteria associated with roots of Kallar grass (Leptochloafusca (L.) Kunth), and description of two species, Azoarcusindigens sp. nov. and Azoarcuscommunis sp. nov. Int J Syst Evol Microbiol 43(3):574–584
Roychowdhury R (2014) Crop improvement in the era of climate change. IK International Publishing House, New Delhi, p 496
Roychowdhury R, Tah J (2011) Differential response by different parts of Solanum melongena L. for heavy metal accumulation. Plant Sci Feed 1(6):80–83
Roychowdhury R, Taoutaou A, Hakeem KR, Gawwad MR, Tah J (2014) Molecular marker-assisted technologies for crop improvement. In: Roychowdhury R (ed) Crop improvement in the era of climate change. IK International Publishing House, New Delhi, pp 241–258
Roychowdhury R, Khan MH, Choudhury S (2018) Arsenic in rice: an overview on stress implications, tolerance and mitigation strategies. In: Hasanuzzaman M, Nahar K, Fujita M (eds) Plants under metal and metalloid stress. Springer, Singapore, pp 401–415
Roychowdhury R, Khan MH, Choudhury S (2019) Physiological and molecular responses for metalloid stress in rice – a comprehensive overview. In: Hasanuzzaman M, Fujita M, Nahar K, Biswas J (eds) Advances in rice research for abiotic stress tolerance. Woodhead Publishing/Elsevier, USA, pp 341–369
Ryu CM, Farag MA, Hu CH et al (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134(3):1017–1026
Saleem M, Arshad M, Hussain S et al (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34(10):635–648
Sarkar A, Ghosh PK, Pramanik K et al (2018) A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Microbiol Res 169(1):20–32
Sarma H, Prasad MN (2019) Metabolic engineering of Rhizobacteria associated with plants for remediation of toxic metals and metalloids. In: Transgenic plant technology for remediation of toxic metals and metalloids. Academic, London, pp 299–318
Selatnia A, Boukazoula A, Kechid N et al (2004) Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochem Eng 19(2):127–135
Sharma P, Khanna V, Kumari P (2013) Efficacy of aminocyclopropane-1-carboxylic acid (ACC)-deaminase-producing rhizobacteria in ameliorating water stress in chickpea under axenic conditions. Afr J Microbiol Res 7(50):5749–5757
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131
Siddiqui ZA, Singh LP (2005) Effects of fly ash, Pseudomonas striata and Rhizobium on the reproduction of nematode Meloidogyne incognita and on the growth and transpiration of pea. J Environ Biol 26(1):117–122
Singh RP, Jha PN (2017) Analysis of fatty acid composition of PGPR Klebsiella sp. SBP-8 and its role in ameliorating salt stress in wheat. Symbiosis 73(3):213–222
Singh A, Jain A, Sarma BK et al (2014) Rhizosphere competent microbial consortium mediates rapid changes in phenolic profiles in chickpea during Sclerotium rolfsii infection. Microbiol Res 169(5–6):353–360
Sobariu DL, Fertu DI, Diaconu M et al (2017) Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. New Biotechnol 39:125–134
Song NH, Ahn YJ (2011) DcHsp17. 7, a small heat shock protein in carrot, is tissue-specifically expressed under salt stress and confers tolerance to salinity. New Biotechnol 28(6):698–704
Srivastava R, Khalid A, Singh US et al (2010) Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. Lycopersici for the management of tomato wilt. Biol Control 53(1):24–31
Tak HI, Ahmad F, Babalola OO (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev Environ Contam Toxicol 223(Springer):33–52
Timmusk S, Nevo E (2011) Plant root associated biofilms: perspectives for natural product mining. In: Bacteria in agrobiology: plant nutrient management. Springer, Heidelberg, pp 285–300
Timmusk S, Wagner EG (1999) The plant-growth-promoting rhizobacterium Paenibacilluspolymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant-Microbe Interact 12(11):951–959
Timmusk S, El-Daim IA, Copolovici L et al (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9(5):e96086
Tiwari S, Lata C, Chauhan PS et al (2016) Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99:108–117
Tokala RK, Strap JL, Jung CM et al (2002) Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol 68(5):2161–2171
Ullah A, Heng S, Munis MF et al (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Vaishnav A, Kumari S, Jain S et al (2015) Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J Appl Microbiol 119(2):539–551
Vejan P, Abdullah R, Khadiran T et al (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21(5):1–17
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255(2):571–586
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16(2):123–132
Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2018) Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep 37(11):1557–1569
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1):1–4
Zarea MJ, Hajinia S, Karimi N et al (2012) Effect of Piriformospora indica and Azospirillum strains from saline or non-saline soil on mitigation of the effects of NaCl. Soil Biol Biochem 45:139–146
Zhang F, Dashti N, Hynes RK et al (1996) Plant growth promoting rhizobacteria and soybean [Glycine max (L.) Merr.] nodulation and nitrogen fixation at suboptimal root zone temperatures. Ann Bot 77(5):453–460
Zhang HX, Hodson JN, Williams JP et al (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci 98(22):12832–12836
Zhang H, Kim MS, Sun Y et al (2008a) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant-Microbe Interact 21(6):737–744
Zhang H, Xie X, Kim MS et al (2008b) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56(2):264–273
Zhao Y, Christensen SK, Fankhauser C et al (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291(5502):306–309
Acknowledgments
Authors are thankful to UGC Centre for Advanced Studies, Department of Botany, The University of Burdwan, for pursuing the research work. AK is thankful to DHESTBT (WB-DBT) for providing the research fund [Memo no. 30 (Sanc.)-BT/ST/P/S&T/2G-48/2017]. KM and ML are grateful to UGC-JRF for supporting and proving fund to continue research work. U.H. is thankful to SRF (state-funded) for the finance assistance [Fc (Sc.)/RS/SF/BOT./2017-18/22].
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kabiraj, A., Majhi, K., Halder, U., Let, M., Bandopadhyay, R. (2020). Role of Plant Growth-Promoting Rhizobacteria (PGPR) for Crop Stress Management. In: Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S. (eds) Sustainable Agriculture in the Era of Climate Change. Springer, Cham. https://doi.org/10.1007/978-3-030-45669-6_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-45669-6_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45668-9
Online ISBN: 978-3-030-45669-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)