Abstract
Nanosized particles in the size range 1–100 nm are emerging as an alternative to conventional particles in technological applications. This is due to their small size, which presents them with a great surface area to volume ratio. This unique property of nanomaterials along with the capacity to tune important physicochemical characteristics, such as molecular detection capabilities, based on size and morphology has increased their utilization for novel and improved gas sensor development in various fields of application. Most gases, above their exposure threshold concentration level, can be toxic to humans and the environment.
The improved gas detection ability of nanomaterials along with the potential to synthesize them via green methods promotes their use in sustainable sensor development for noxious gas detection. The present chapter discusses examples of nanomaterials that are used to fabricate sensors with the ability to detect toxic gases and biological molecules. In addition, the drawbacks of nanomaterials as sensors, and the efficiency and limitations of green synthesized nanomaterials for sustainable toxic gas detection and biosensing applications are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanomaterials
- Biosensor
- Gas sensor
- Nanocomposites
- Biosynthesis
- Green chemistry
- Transducers
- Detectors
- Size reduction
- Smart devices
1 Introduction
Sensors are the general term that is used for the materials that are used to detect and sense a physical parameter and converts them to electrical current (Pallas-Areny and Webster 2012). Sensors consist of four main parts, namely analyte or an element to be detected, receptor, transducer, and signal processing unit (Töppel et al. 2018). The analyte or the molecule to be sensed is the key for the fabrication of sensors, based on which the type of sensors to be used for detection will be decided (Ajay Piriay et al. 2017). The transducer is an essential component of a sensor, which converts the physical quantities into electrical signals (Kocakulak and Butun 2017). These converted electrical signals are amplified and converted into readable signals via signal processors, which can be displayed in a digital device (Erden et al. 2016). There are numerous types of sensors that are under extensive research and have been used in several applications (Khaydukova et al. 2017). Among these sensors, gas sensors are the widely utilized sensor type in several industries as well as in commercial markets (Dey 2018). In 2016, the overall market of gas sensors throughout the world is about USD 812.3 million, which is expected to be about USD 1297.6 million in 2023 (Bogue 2014). The expected growth in the gas sensor market between 2017 and 2023 is about 6.83%, which is based on the gas type, technology, geography, and end-use application (Markets 2017). Companies such as Amphenol Corporation (United States) and Figaro Engineering Inc. (Japan) are the significant producers of gas sensors for end-use applications including aerospace, medical, transportation, and residential safety (Kato et al. 2000; Shan et al. 2018).
Generally, micro-sized particles are utilized for gas sensor fabrication to monitor distinct gas molecules that are toxic to humans above its threshold limit value (Lupan et al. 2016). These micro-sized particles exhibited enhanced potential in sensing various toxic gases and help to avoid their exposure toward humans (Acosta et al. 2009). However, the lack of precision in the detection, disability in detecting multiple gases, and formation of impurities during the detection of gases are the limitations of using micro-sized particles for detection of gases (Carregal-Romero et al. 2013; Kim and Kim 2014). Thus, nano-sized particles and materials are introduced as novel materials to fabricate gas sensors (Llobet 2013). These nanomaterials possess exclusive size-dependent entities with an elevated ratio of surface and volume as well as effects due to confinement in the quantum regime (Eranna 2016). Additionally, the size, morphology, and surface charge of the nanosized materials can be manipulated based on the desired sensor applications (Lyson-Sypien et al. 2015). These advantages of nanomaterials pave the way for a separate market for nanomaterial-based toxic gas sensors which are now gaining attention among industries and researchers (Šutka and Gross 2016).
The emergence of nanotechnology has led to the fabrication of biosensors, similar to toxic gas sensors (Pandey et al. 2008). These biosensors are the latest trend in biomedical sciences which is beneficial in detecting any form of biological analytes (Rai et al. 2012). The advantages of nanomaterials in toxic gas sensors have made the biomedical scientists to try out nano-sized particles for the fabrication of biosensors (Mishra and Rajakumari 2019). The incorporation of nanomaterials in biosensors has led to various wearable and in-situ biochip advancement that can help in the detection of biological analytes and in the diagnosis of diseases (Chandra and Segal 2016). Currently, the global market of biosensors is about 21.2 billion USD, which is expected to reach about 31.5 billion USD in 2024 (Markets 2019). Due to the high demand for novel toxic gas and biosensors, numerous nanomaterials are introduced to fabricate sensors with high detection efficiency (Mehrotra 2016). Metal, metal oxide, carbon and polymer nanoparticles, either in nanoformulated or nanocomposite forms are the nanomaterials, that are usually utilized for sensor fabrication in recent times. The diverse variety of nanomaterials used in the sensor fabrication has led to the development of semiconductor (Dey 2018), sensitive (Wang et al. 2016a), hybrid (Chatterjee et al. 2015), fluorescent (Zhou et al. 2016), room temperature (Shafiei et al. 2015), and impedimetric (Vignesh et al. 2015) sensors to detect toxic gases. Likewise, microbial (Ponamoreva et al. 2019), electrochemical (Rotariu et al. 2016), surface plasmon resonance (Olaru et al. 2015), whole-cell based (Saini et al. 2019), and lab-on-a-chip (Jamshaid et al. 2016) are the distinct biosensors that are fabricated using nanomaterials. Thus, the aim of the present chapter is to discuss about nanomaterials that are used to fabricate sensors with the ability to detect toxic gases and biological molecules. In addition, the drawbacks of nanomaterials as sensors, and the efficiency and limitations of green synthesized nanomaterials for sustainable, toxic gas detection, and biosensing applications are also discussed.
2 Nanomaterials as Toxic Gas Sensors
Nanomaterials are widely utilized to fabricate swift-responding nanosensors with electrochemical, optical, thermal, piezoelectric, and other properties for low concentration chemical compound detection (Tallury et al. 2010). Recently, nanomaterial-enhanced sensors such as carbon nanotubes, nanosized metal particles (particularly gold and silver), graphene, semiconductors, quantum dots, and silicon-based nanomaterials were tailored for detecting and measuring contaminants such as ions of heavy metals, hydrogen peroxide (H2O2), organophosphate pesticides, toxic gases, and industrial wastewaters (Su et al. 2012). Metals, oxides of metals, metal complexes, polymers, and carbon-based nanomaterials are extensively under research for the fabrication of toxic gas sensors.
2.1 Metal and Metal Oxide Nanomaterials
2.1.1 Metal Oxide Nanomaterials
Numerous metal oxide nanomaterials are reported to be appropriate for reducing, combustible, or oxidizing gas detection via measurements of modifications in conductivity. It has been proven that the oxide nanomaterials show a sensitive conductivity response upon detecting a gas molecule (Kanazawa et al. 2001). It is noteworthy that the choice of metal oxides for fabricating a gas sensor is based on their electronic structure. The oxides of metals possess an inclusive electronic structure range that is classified into oxides of transition metals and non-transition metals, which are further subclassified into oxides of pre-transition metals and post-transition metals. Oxides of pre-transition metals, such as MgO, are naturally inert, due to their hefty gaps in their electronic bands, which makes it tedious to form electrons or holes. These materials are infrequently designated to fabricate gas sensors as it is hard to measure their electrical conductivity (Kanazawa et al. 2001). The oxide materials of post-transition metals can alter their properties to perform as a better toxic gas sensors. Thus, these materials are reported to be highly sensitive than oxides of pre-transition metals toward the environment. Nevertheless, instability in structures and difficulties in optimizing parameters of these nanomaterials limits their usage in conductometric gas sensor applications. Only oxides of transition metals with electronic configurations of d0 and d10 are employed in real gas sensor application. The configuration of d0 is present in binary oxides of transition metals, whereas the configuration of d10 exists in the oxides of post-transition metals. Even though, several semiconductor oxides of metals that are delicate to noxious gas molecules are reported to be n-type semiconductors. Also, it was demonstrated that certain p-type semiconductors are also utilized as materials to fabricate gas sensors. It is reported that about 10% content of NiOx is required to alter n-type into p-type conductivity. When temperature elevates, an increase in the sensitivity of n-type film toward reducing gases can be noted, which is vice versa in p-type NiOx film (Wisitsoraat et al. 2009). Thus, semiconductors of p-type nature can operate in a comparatively lesser temperature than n-type, for toxic gas sensor applications.
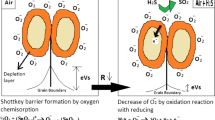
Sensors that are fabricated with semiconductors of metal oxides are primarily applied to sense-gas molecules via target gas and surface oxygen mediated redox reactions (Yamazoe and Shimanoe 2008). This process requires two initial steps, namely (1) redox reactions, where scattered oxygen ions over material surface will react with target gas molecules to create electronic variations in the surface of oxides; and later (2) the disparity is transferred via transduction into a noticeable change in the electrical resistance of sensors. The variations in resistance can be noticed by determining the capacitance alterations, function of work function, optical characteristics, mass, or reaction energy (Kanan et al. 2009).
Tin dioxide is a noteworthy metal oxide nanoparticles that are used for toxic gas detection. It is a granular n-type material with density-dependent electrical conductivity of surface pre-adsorbed oxygen ions. It is reported that the tin dioxide resistance alters, depending on the variations in the gas concentration (Batzill and Diebold 2005; Wang et al. 2010), with nonlinear association between concentration and resistance of target gas. Also, other semiconducting oxides of metals such as tungsten trioxide are generally utilized for noxious gas detecting applications. Oxides of tungsten nanomaterials as anode that are fabricated via electrochemical tungsten etching illustrate outstanding hydrogen and nitrogen oxide detection responses (Endres et al. 1996). However, the pure WO3 respond poorly toward NH3 with declined selectivity, due to the interference of NOx. Thus, vanadium and copper decorated WO3 are utilized as additives of catalysts to recover their response, eliminate anomalous sensor performance and to utilize WO3 in gas sensors (Hoefer et al. 1997). Other nanosized metal oxides (TiO2) are highly beneficial as layers of sensitivity to elevate dielectric permittivity of sensors to adsorb gas molecules (Fraiwan et al. 2011). Likewise, distinct ZnO nanostructures including nanowires, nanobelts, and tetrapods are fabricated as monitoring devices for ultrasensitive H2S and NO gas detection (Gupta et al. 2010).
Moreover, semiconducting sensors of metallic oxides that are fabricated using nanomaterials are gaining importance in large-scale applications. However, elevated operational temperature demand in certain sensor involves high budget and intricate arrangements than other room temperature sensors, which limits their large-scale application. Researchers have developed a unique method to overcome these limitations, which includes silicon-based integrated circuit fabricated microsensor mediated micro-heater technology (Lee et al. 1996). Additionally, pulsating operating temperature mode to heat at short intervals (Jaegle et al. 1999) was also introduced, which simplifies the sensor operation and reduces the consumption of power. Further, studies about nanosized oxides of metal structures have revealed that semiconductor nanowires can recover the response time and sensitivity of gas sensors (Comini 2006). Another challenge is the requirement of extended recovery period after exposure of each gas, which restricts their employment in certain e-nose like sensor devices, as the concentration of gas is rapidly and regularly under modifications. Moreover, the defects and instability in the structure of indicators also reduce the applications in the field of sensors. In recent times, there are numerous reports to prove the efficiency of metal oxide nanomaterials in detecting toxic gas molecules with high sensitivity (Dey 2018). However, novel solutions are required to overcome the limitations and defects of semiconducting oxides of metals, which is achievable via extensive sensor research in the future.
2.1.2 Metal Nanoparticles
Nanosized metal particles are deposited over substrate surface for elevating the ratio between area and volume and also to favor the adsorption of gas molecules. When these nanomaterials are bound with analyte, the molecules of analytes alter their substrate entities as gas molecules are adsorbed to the metal (Jimenez-Cadena et al. 2007). Mostly, the preparation for deposition is performed via metal precursor vaporization, to afford consequent annealing of the nanofilms or particles. For instance, an electromotive force (emf) measuring electrode in a cell with high concentration of oxygen was fabricated using a zirconium and yttrium metal component along with 5–10 nm-sized iridium nanoparticles. In the existence of oxygen at 650 °C, the sensor conductivity was altered within 10–20 s of response time. The sensor response was explained via oxidization reaction, where the nanosized iridium particles permit a better interface between the substrate and the molecules to provide an enhanced contact area for the analyte (Kimura and Goto 2005). Li et al. (2002) fabricated an electrode of platinum with surface embedded nanosized gold particles along with a silver–silver chloride composite as a reference electrode. The nanosized metal particles are reported to be beneficial as a layer of sensors to permit the detection of hydrogen and sulfate analytes with ultrahigh sensitivity and selectivity at low temperature (Li et al. 2002). Moreover, gold and platinum nanoclusters are widely accepted as a catalyst to upsurge sensor sensitivity. Generally, nanoclusters are integrated or fabricated into alloys with distinct nanomaterials to enhance their sensitivity, selectivity, and to increase their kinetic oxygen reduction limitation (Hallil et al. 2010). Further, Wang et al. (2010) prepared an extremely sensitive hydrogen gas nanosensor using platinum nanocluster-decorated graphene oxide (GO) between a prepatterned pair of titanium–gold electrodes with microgap (Xiang et al. 2010). Currently, there are several nanomaterials, especially biosynthesized nanoparticles, are employed in the fabrication of toxic gas nanosensors (Xu et al. 2018b). It is noteworthy that most of the studies reported the utilization of metal oxide nanomaterials, rather than a nano-metals, to fabricate substrates of nanosensor to detect toxic sensors. However, the utilization of nanosized metal particles on the substrate with inert molecules to fabricate noxious gas nanosensors should be studied extensively in the future.
2.1.3 Nanosensors with Metal Complexes
Transition metal nanomaterials are proficient in modifying interactions of atoms which makes them to be extensively beneficial as receptors to detect and analyze various gases. This behavior is subjugated to prepare an analyte with complex supra-molecules for selectivity enhancement of numerous toxic gas sensors. Elosua et al. (2006) stated that optical fiber sensor fabricated by coating gold and silver complex was used to detect volatile alcoholic compounds. Nanoscale Fizeau interferometer with complex alcoholic vapor dopants is proved to possess vapochromic property to act as recognition layer of the nanosensor. The solvents coordinate with centers of silver metal and break the silver and gold–silver bonds to provide initial orange to red color to the complex. The results revealed claim the nanosensor with metal nanoparticles can be used for at least 3 months without degradation for the effective detection of toxic gases (Elosúa et al. 2006).
Brousseau et al. (1997) revealed carbon dioxide detection using reactions of carbon dioxide with hydroxysilanes, alcohols, and amines under optimum pressure and temperature. The study utilized three bifunctional nano-receptors along with a group of amines that coordinates with ions of metal in the copper octane di-yl bis (phosphonate) thin film and are coated over a microbalance made up of quartz crystal. The sensor and the analyte reaction at optimum temperature were reversible which is highly dependent on the receptor and the concentration of carbon dioxide (Brousseau et al. 1997). The primary benefit of metallic sensing layer complexes as sensing layer is the interactions that are reversible between the devices and the analytes. The formation of coordination bonds in gas detection are fragmented by temperature increment or chemical alterations in the sensor. Besides, specific receptors are intended to cooperate with precise analytes to increase their selectivity. Thus, fabrication of sensors with these complexes is expensive and is used only to functionalize specific receptors that can be beneficial as nanostructured transducer materials (Jimenez-Cadena et al. 2007).
2.2 Polymer Nanomaterials
Polymers are used in gas sensor fabrication as they possess exceptional physicochemical properties and are classified into polymers with conducting and nonconducting properties.
2.2.1 Conducting Polymers
Polymers with electric conductive property are broadly considered as toxic gas sensors due to their sensitive electrical conductivity alterations via assorted inorganic and organic gas exposure. This property has led to critical material examination for fabricating layers that can detect gas molecules in sensors (McNaghten et al. 2009; Shrivas et al. 2007). Polymers with conducting property such as polyaniline (PAni), polypyrrole (PPy) , polythiophene (PTh), and their byproducts are widely employed as noxious gas determining nanosized materials. It can be noted that pure polymers with low conductivity to perform as an individual gas determining material. Hence, extended research to select specific polymers for nanocomposite fabrication with metallic nanoparticles is highly recommended in the future. Earlier reports demonstrated that polymer conductivity is upgraded via exclusive doping approach by reactions of protonation or redox. Later, polymers are converted into conductors or semiconductors after the reversible doping process. It is significant to note that the level of doping can be altered via chemical-based target gas and polymer reactions, making the analyte detection to be a more practical with conducting polymers. Several polymers are doped through redox reactions and specific polymers are utilized for gases, which are inactive at room temperature to redox reactions. For instance, redox reactions will not happen in CO at room temperature, however, PAni can lead to a change in their redox potential (Hatfield et al. 1994). Thus, conducting polymer-based nanomaterials are directly used as transducers in toxic gas detecting nanosensors to reflect electrical property modifications .
2.2.2 Nonconducting Polymers
Polymers without conducting property are broadly employed as absorptive coatings on diverse sensor devices, where general polymeric transducers with distinct physisorption are used as sensor fabricating material. For example, layers of polymer that lead to resonance frequency modifications, enthalpy, and dielectric constant upon analyte desorption or absorption are coated on the surface of surface transverse wave (STW), quartz crystal microbalance (QCM), calorimetric sensor devices, capacitive and surface acoustic wave (SAW) for sensitive toxic gas detection. Later, these sensor devices are transformed into an output electrical signal via monitored polymer properties (Bai and Shi 2007). Even though the basic polymer with nonconducting principles are logical in gas sensors, their recital is highly intricate, even after coating them on the sensor devices. For example, STW devices with resonance property are coated with sensitive thin nanosized layers of polymer to feature extensive rewards of relative sensitive gas probing, inclusive electrical property, and low sensor oscillator noise than bulk counterparts of SAW (Hagleitner et al. 2002). Furthermore, membrane of polymer with nonconducting properties was also proved to be utilized in semiconducting oxides of metal-based gas sensors as sieves of molecules, to augment their inclusive selectivity for sensitive layers of polymer introduction (Avramov et al. 2000).
Numerous approaches have been reviewed for polymer nanocomposite fabrication with exclusive properties for sensor applications (Kaushik et al. 2015). Among several approaches based on emulsion polymerization such as conventional emulsion, soapless emulsion, microemulsion, suspension, dispersion, and precipitation polymerization approaches (Wong et al. 1995), mini-emulsion polymerization is considered as a potential approach to fabricate functional and flexible nanomaterials (Anderson and Daniels 2003). Nohria et al. (2006) utilized a thin film of poly (anilinesulfonic acid) (SPANI) to construct a sensor to evaluate humidity. The 90 nm sized thin films were deposited via the spin coating method or by the layer-by-layer (LbL) nanoparticle assembly approach, which added negative charges on the coupled layer polycation substrate and a polyanion named poly (allylamine hydrochloride) (PAH), and poly (styrene sulfonate) (PSS), respectively. After the deposition of layers, the PSS is substituted by SPANI to yield a film with 26 nm of thickness. When these nanofilm sensors are under atmospheric exposure, its resistance declines with an increase in relative humidity along with 15–30 s of response time (Nohria et al. 2006).
Nanosized gas sensors with polymers have merits such as enhanced sensitivities at reduced response times, compared to bulk polymers. Furthermore, sensors with polymers function at room temperature, which make them superior than metal oxide nanoparticles as they detect toxic gases at elevated temperatures. Therefore, the lower consumption of energy by nano polymers permits their utilization in toxic gas detection units that are operated by batteries. Additionally, advantages including the low fabrication cost, simple structures, and portability, as well as reproducibility (Landfester 2006) make these nanostructures to gain focus of researchers to use them in nanosensor fabrication. Gas sensors with polymers also possess hindrances such as instability, poor selectivity, and irreversibility. Further, the performance can be altered due to the working environment. Besides, there are only few evidences to explain the actual working principle of toxic gas-sensing polymers. Despite these demerits, polymer-based nanosensors can be used as a lower power-consuming toxic gas sensors in the future.
2.3 Carbon-Based Nanomaterials
Carbon nanotubes (CNTs) and graphene show a high potential for miniaturized chemical sensor development , due to their excellent large surface-mediated adsorptive capacity, better electrical property modulation upon exposure to analytes via greater cross-sectional interaction zone, capability to fine-tune electrical nanostructure entities by tailoring their composition or size and the configuration ease into distinct geometries (Goustouridis et al. 2005; Munoz and Steinthal 1999). SWNTs are cylinders with individual diametric layers of 1–3 nm and micron length of rolled graphite. Similarly, multi-walled carbon nanotubes (MWNTs) are fabricated via concentric SWNTs with various nanosized diameters. Both SWNTs and MWNTs are employed to fabricate various sensors, due to their exclusive physico-electronic properties (Ellis and Star 2016). The adsorption processes are highly favored due to their unique atomic arrangement on SWNTs surface and their enhanced ratio between area and volume, which increases their sensitivity to various gas molecules in the atmosphere (Zaporotskova et al. 2016). Nanotubes can exhibit both metallic and semiconductor properties with their extraordinary electrical conductivity which depends on their structural chirality (Kavitha and Kalpana 2017). Semiconducting nanotubes are highly beneficial in device construction including field-effect transistors (FETs) . These nanotube devices are utilized for analyte determination via electrical surface conductivity modifications of CNTs (Tran and Mulchandani 2016). This can lead to consequences such as, transfer of charge from analyte to the nanotube, or may elevate the scattering potential of analyte (Park et al. 2017a). These exclusive effects of CNTs are utilized for electrode of third gate-mediated FET device modulation (Jang et al. 2016). Alternatively, the current will decline without altering the CNTs characteristics , if the center of scattering is an analyte. Even though CNTs possess sensitivity toward the surrounding chemical environment, they lack selectivity which is a major limitation to use them in large-scale sensor application. Thus, several functionalization processes have been implemented for sensitivity and selectivity enhancement of CNTs as gas sensors . Among functionalization processes, distinct polymeric material coating and doping or oxides and metal particle deposition are proven to be significant in improving the gas detection properties of CNTs at room temperature (Lee et al. 2018).
Gas sensors with graphene and pristine CNTs have firm restrictions, such as low analyte sensitivity, declined selectivity, irreversibility, and long recovery time (Bekyarova et al. 2004). Graphene and CNTs functionalized with diverse materials are essential to overcome the limitations of free CNTs via chemical property modifications and sensing performance enhancements. Functionalization is significant in altering the chemistry of carbon nanotubes and graphene and manipulating their chemical properties is highly essential for utilizing them in potential applications including sensor fabrication (Dong et al. 2004). There are numerous CNTs and graphene functionalization approaches that are reported in literatures (Wright 2017) such as defect method, covalent sidewall functionalization approach, noncovalent exohedral, and endohedral functionalization. CNTs functionalized with metal, metal oxides, and polymer nanoparticles can result in improved electronic properties, selectivity, and response to specific gases through target molecule interface with functional additives is distinct, compared to free and bulk carbon materials (Li et al. 2011).
2.3.1 Carbon Nanotubes Functionalized with Metal, Metal Oxide, and Polymer Nanomaterials
The conductivity of nanotubes can be altered significantly with sidewall functionalization of chemical reactants, which is essential to specific gas sensor application. Various approaches have been reported for covalent carbon nanotube functionalization such as creation of defect site and functionalization via defects, embedding acids of carboxyl group on caps of carbon nanotube end and following acid derivatization. Polymer surfactant nanotube wrapping for noncovalent functionalization is proven to reserve the physical entity and solubilization of nanotubes. Pure carbon nanotubes do not possess appreciable sensitivity toward certain gases, whereas CNTs decorated with nanoparticles comparatively improves gas-sensing performance. Nanosized metal particles including platinum, lead, aluminium, and tin are widely utilized to decorate CNTs, allowing determination of selective gases, such as H2, NH3, NO2, and CO (Fowler et al. 2009; Li et al. 2010; Stankovich et al. 2006; Yan et al. 2010). The oxide of metal NPs modified with CNT as hybrid was developed as sensing films that displayed advantages such as high catalytic activity, efficient charge transfer, and capacity to adsorb. Numerous studies have demonstrated the brilliant sensing ability of CNTs/SnO2 and CNTs/ZnO hybrid sensors for carbon monoxide, nitrogen dioxide, and ammonia gas detected at low temperatures (Ma et al. 2012). Polymer-functionalized CNTs also considered as an essential nanomaterial to recover the CNTs and polymers of organic nature’s compatibility in sensor fabrication and provide ultrahigh sensitivity and selectivity for the determination of gases. The unique characteristics of CNTs combined with polymer properties such as delocalized bonds, high permeability, and low density have established the possibility of detecting diverse gas molecules with swift response, excellent sensitivity, and great reproducibility (Alshammari et al. 2017). In addition, various other CNT modifications via doping or coating are recommended to further augment the CNT’s gas-detecting properties (Gardner and Bartlett 1993; Penza et al. 2008).
2.3.2 Graphene Functionalized with Metal, Metal Oxide, and Polymer Nanomaterials
In several gas-sensing applications, metal nanoparticles are embedded with graphene to upsurge the sensitivity, detection limit, selectivity, or a mixture of all these properties (Kim 2009). In several cases, graphene nanomaterials are modified via electrochemical metal salt reduction by using graphene flakes that are attained from oxide of graphene. In specific scenarios, the deposition of nanosized particles over graphene can be achieved via chemical metal salt reduction by adding reducing agent, followed by that are yielded after adsorption of nanosized particles in solution (Liu et al. 2011). Noble metals that are active as catalysts are extensively utilized to elevate graphene-based chemical sensor sensitivity toward a wide range of gases. Lately, the advancement of room temperature-operating sensors with oxides of metal along with enhanced sensitivity and truncated fabrication cost has fascinated much consideration. When molecules of gases are adsorbed on the surface of functionalized oxides of metal with graphene sensor film, nanosized oxide of metal particles act as sensing and element of transduction ability, whereas oxide of graphene act as a mesh with high conductivity. These nanomaterial-based sensor intensifies their transducing property resulting in larger alterations in conductance, compared to previous results demonstrated for chemical synthesized graphene-based sensors (Bekyarova et al. 2004). Numerous literatures also reported that the graphene is incorporated into matrices of polymer to yield novel nanosized composites. It was recommended that graphene nanosheets can provide highly active polyaniline nucleation sites and exclusive electron transfer pathways (Yuan and Shi 2013).
2.4 Nanocomposites
Generally, gas sensors are fabricated by two types of materials such as organic polymers with conductivity and inorganic oxides of metals. Gas sensors using coveted functional and conductive organic conducting polymers are proven to improve gas-detecting performance (Castro et al. 2011). Although these nanocomposites are unstable and exhibit comparatively meager sensitivity (Janata and Josowicz 2003) due to excellent polymers with conducting and affinity property toward environmental moisture and volatile organic compounds (VOCs) . Gas detectors fabricated via oxides of inorganic metals exhibited upsurge detecting qualities due to oxygen-mediated stoichiometric alterations and surface-active electrical charges (Capone et al. 2000). The function of these devices at higher temperatures led to gradual modifications in metal oxide nanostructure properties. The high-temperature function can lead to grain boundary fusion, which can alter nanostructure stability and shorten the sensor lifetime. In addition, high-temperature operating nanocomposite sensor needs a discrete assembly of temperature-controlled heating complex and consumes extra heating power. The shortcomings of organic materials such as low stability and conductivity, and inorganic materials such as functioning at high temperatures and sophisticated processability act as a hurdle to be employed in large-scale gas sensor fabrication. Thus, the nanocomposites are utilized to promote effective and peculiar gas-sensing ability and allow them to operate at low temperature. A promising approach for conductometric gas sensor improvement is to utilize composite nanomaterials that are fabricated via semiconducting metal oxide or metal or carbon nanoparticles along with organic polymer or inorganic matrix (Barsan et al. 2007).
Suri et al. (2002) described that nanosized magnetic composites possess a significant part in noxious gas sensor applications. Nanosized composites of iron oxide and poly pyrrole that are prepared via instantaneous polymerization and gelation process were utilized for the fabrication of sensors to determine humidity. The results revealed that these sensors are highly beneficial in toxic gas determination such as carbon dioxide, nitrogen, and methane. Further, the study also stated that the sensitivity of nanosensor was in the order of carbon dioxide > nitrogen > methane, due to their kinetic diameter variation of gas molecules (Suri et al. 2002). Moreover, various methods are utilized to combine CNMs with polymers to yield functional nanocomposites with specific extraordinary properties for technological purposes (Yu et al. 2017). Polyaniline (PANI), polypyrrole (PPy), polythiophene (PTh), and poly(3,4-ethylenedioxythiophene) (PEDOT) conducting polymers are exploited as matrices to integrate various CNMs (Xue et al. 2017). The polymer matrices embedded with carbon nanomaterials are an attractive approach for combining their electrical and mechanical entities (Liu and Kumar 2014). These novel nanosized composites created novel chances, not only in sensor applications, but also in electrochemical capacitor, solar cells, transistors, and molecular electronic devices (Christ et al. 2017). In recent times, nanosized composite with carbon polymers, and nanosized metal particles (MNPs) with unique alignments and proportions are broadly examined (Kondratiev et al. 2016; Monerris et al. 2016). Further, Trakhtenberg et al. (2012) reported numerous metal oxide nanocomposites that are utilized to fabricate efficient nanocomposites for the sensitive and selective determination of noxious gases in the environment at room temperature (Trakhtenberg et al. 2012). All these studies revealed that nanocomposites can be an excellent nanosensor fabrication material for gas sensor applications, compared to free nanoparticles .
3 Nanomaterials as Biosensors
Similar to toxic gas sensors, biosensors are also fabricated using metal, metal oxide, carbon, polymer, and composite nanomaterials.
3.1 Metal and Metal Oxide Nanomaterials
The noble metals including gold, silver, and platinum (Pt) are commonly utilized in the field of biosensor application. These metal nanoparticles (MNP) are significant in detecting microorganisms such as viruses, bacteria, and pathogens with enhanced sensitivity and specificity. These MNPs incorporated biosensors with biological recognition receptors namely antibody, enzyme, nucleic acid, and cells are also utilized for formidable disease monitoring applications such as cardiovascular and cancer diseases. The MNPs act as mediators to transfer the signals that are obtained via modification on their surface to transducers in the form of electrochemical, piezoelectric, and optical signals. The gold NPs are in conjugation with primary or secondary antibodies through gold–antibody ionic interaction, hydrophobic interaction, and dative binding phenomena. Conjugation of nanoparticles with antibodies is achieved via other chemical interactions such as thiol derivative chemisorption, bifunctional linkers, and via adapter molecules namely Streptavidin and biotin (Ijeh 2011; Ljungblad 2009).
3.1.1 Glucose Biosensors
The MNPs are conjugated with a glucose oxidase enzyme (GOx) and ferrocyanide for the glucose sensor signal enhancement. The most popular techniques for conjugation are amperometry, electrochemical impedance spectroscopy (EIS), and cyclic voltammetry (CV) . The nanosized gold particles are attached covalently to GOx and CV measurement is used to obtain the quantitative biological glucose level in complex samples. Similarly, the ferrocyanide acts as a mediator for transferring electrons during GOx reaction for glucose detecting nanosensors. A typical example of the fabrication of the glucose sensor with nanosized gold electrode particles was demonstrated by Zhang et al. (2005) to detect highly concentrated with enhanced sensitivity and 8.2 μM limit of detection (Zhang et al. 2005). Likewise, nanosized platinum particles are incorporated with GOx and Nafion on the highly oriented pyrolytic graphite (HOPG) surface to fabricate sensors with stability, controllability, and reproducibility. The results showed that these nanosensors exhibited a linear response (25 μM) with 15 mM limit for determining glucose (Liu and Huang 2012). Similarly, manganese dioxide NPs (MnO2 NPs) were also conjugated with Nafion and GOx on the graphene nanoribbon surface under the influence of 0.1 M phosphate buffer at pH 7.4. The amperometric glucose measurement for the nanosensor prepared with MnO2 NPs at an operating potential of +0.50 was reported to be as high as 56.32 μA/mmol cm2 (Vukojević et al. 2018). However, the detachment of GOx from the Nafion is common in the preparation, which is the major limitation for glucose biosensor fabrication. Thus, these challenges can be avoided by treating the initial electrode with positively charged polyethyleneimine (PEI) polymer, which was followed by adding 5% (w/v) of negatively charged Nafion on the PEI surface. Later, GOx has to be attached to the PEI/Nafion layer to initiate strong bond formation between them (Tsiafoulis et al. 2005). Furthermore, selenium nanoparticles are conjugated with mesoporous silica composite (MCM-41) matrix. In this study, the carbon paste electrode (CPE) was bound together with mesoporous silica composite material which was later attached to selenium nanoparticles to embed on the MCM-41 surface. Since GOx reaction liberates the electron, the amperometric measurement will yield the quantity of glucose present in the sample by slight modifications in the surface of MCM-41 that are initiated by selenium metal (Yusan et al. 2018).
3.1.2 DNA Biosensors
Nanosized gold and silver particles are widely utilized in DNA biosensor fabrication to detect the target DNA molecules. Metallic nanoparticles possess the ability to bind with target DNA molecule at 0.1 M of sodium chloride concentration, due to the salt aggregation effect. Further, an increment in the salt concentration to the 2 M of sodium chloride concentration will lead to aggregation of ssDNA molecules with NPs (Jamdagni et al. 2016). On the other hand, the peptide nucleic acid (PNA) and PNA–DNA bound with NPs are measured at 600 nm and 520 nm, respectively, in a UV–visible spectroscopy, which indicates the colorimetric DNA sensing ability of gold–silver nanoparticles (Kanjanawarut and Su 2009). The alterations in the DNA motif via gold NP are based on the pH variation in fluorescent quenching method (Liu et al. 2006). In this scenario, the DNA hybridization determination is possible via gold nanoparticles encapsulated by streptavidin and biotinylated oligomer target by stripping potentiometric method (Madhurantakam et al. 2018). Additionally, gold NPs linked with cysteamine-modified electrode that are encapsulated with oligonucleotide and a group of mercaptohexyl at the 5′-phosphate of DNA end and chitosan layer onto SAM-modified gold NPs are highly significant in electrochemical biosensor fabrication to detect target DNA (Mazloum-Ardakani et al. 2015).
3.1.3 Blood Pressure Sensors
A simple and common method for monitoring blood pressure is to use piezoelectric sensors that detect the pressure of blood flow in vessels, based on piezo resistivity, capacitance, and piezo electricity mechanism. Nanosized metal particles including gold, aluminium, silver, and copper are considered as the best materials for the fabrication of integrated circuits to be included in piezoelectric sensors (Xu et al. 2018a). Conversely, nanosized gold–copper alloy particles are used for uric acid determination, depending on an enzymatic reaction through electrochemical measurement (Wang et al. 2001). Moreover, the electro-catalytic effect of silver with platinum nanoparticles on reduced oxide of graphene is utilized for tumor necrosis factor-α (TNF-α) detection through electrochemical measurements (Pingarrón et al. 2008). It is noteworthy that TNF-α is an essential part of proinflammatory cytokines which is associated with salt-sensitive hypertension and is related to renal injury (Mehaffey and Majid 2017).
3.2 Carbon-Based Nanomaterials
The hollow cavity in carbon nanotubes (CNTs) attracts several researchers to use them in the fabrication of nanosized biosensors (Adhikari and Chowdhury 2010), as hollow cavities provide a chemically inert environment (Khlobystov et al. 2005; Manzetti 2013). Further, these hollow cavities also provide a potential electromagnetic or a magnetic response site for biosensor and nanoreactor development via electric or electromagnetic impulses (Khlobystov 2011). The CNT structures are classified according to the molecule of interest such as single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) via binding to their biosensor surfaces. However, SWCNTs with one carbon layer can easily transfer an electric signal after an analyte or mediator molecule attachment to their modified surfaces (Hirata et al. 2008).
CNTs are accepted as a potential material for electrochemical biosensor fabrication , owing to their enhanced electron transferability to the electrodes. Thus, CNTs are reported to be working on both second and third electrode systems using the electrochemical cell, which converts or transfers a biological element detection into electrochemical signal. However, the hydrophobic nature of CNTs and strong intermolecular p–p interactions are the major limitations, while developing CNT-based biosensors (Thirumalraj et al. 2017; Wang and Dai 2015). For instance, cationic surfactants effectively bind with negatively charged DNA for designing functionalized CNTs. In addition, cationic functionalities are introduced with amine groups to allow further attachment of other targeting molecules and fluorophore markers to track cells. Several studies demonstrated that RNAs, short double-stranded and single-stranded DNAs, possess the ability to dissolve into SWCNTs , whereas the macromolecules are attached to the open MWCNTs cavity in a nonspecific fashion (Sanz et al. 2011). Furthermore, water-soluble, PEGylated phospholipid functionalized SWCNTs were developed for cancer drug delivery and as a noninvasive diagnostic tool (Liu et al. 2008). In addition, MWCNTs conjugated with poly ethylenimine to enhance binding properties of DNA and these conjugated nanoparticles are proved to be highly sensitive and low toxicity for efficient detection and targeted delivery of genes (Liu et al. 2005).
Graphene sheets with high surface area are fabricated into semi-conductive films and conductive interfaces to be useful in biosensor development. Graphene-dependent biological sensors are broadly utilized in medical diagnostic applications. These biological sensors are utilized in the determination of significant biomolecules including cytochromes, reduced nicotinamide–adenine dinucleotide (NADH) form, hemoglobin (Hb), and individual amino acids (Kuila et al. 2011; Song et al. 2012). Further, these biosensors are involved in trace element detection that are present in urine samples (Tahernejad-Javazmi et al. 2018). Graphene-based biosensors also possess ability to detect heavy metals and larger biomolecules including DNA (Huang et al. 2015a; Mishra et al. 2017).
3.3 Polymer Nanomaterials
The polymeric nanoparticle-based biosensors are highly beneficial compared to metal and ceramic materials due to their mild synthetic conditions, scalable downstream processing, flexibility, low operating temperature, nontoxic, and biocompatibility. It can be noted that the conducting polymers are proven to possess high sensitivity, precise reproducibility, and eliminates nonspecific binding with the analytes in biosensors (Park et al. 2014). The macroscale hydrogel-based materials are durable, elastic, transparent, and biocompatible for the prosthetic device fabrication such as wearable or even implantable biosensor devices. Poly (3,4-ethylenedioxythiophene) (PEDOT), polyaniline (PANI), polypyrrole (PPy), and poly indole are the general conducting polymers that are employed in biosensor fabrication. These nanosized polymers are conjugated with CNTs, MNPs, metal oxides, and other nanomaterials to enhance their sensitivity and selectivity while detection biomolecules (Annabi et al. 2014; Oliva et al. 2017). The polylactic acid (PLA) and PLA–alginate nanoparticles are efficiently encapsulated platinum–porphyrin complex for oxygen detection based on fluorescent measurement. A linear fluorescence response of oxygen concentrations (0–6 mM) was recorded with sensitivity toward oxygen indirectly measures and represents the presence of 0–10 mM of glucose within 4 seconds via catalytic reaction. These biocompatible and implantable glucose biosensors are proposed to be highly beneficial as in-situ glucose sensors for diabetic patients (Pandey et al. 2019). Moreover, the glyphosate (Gly) herbicide was detected via p-aminothiophenol-functionalized AuNPs with Gly as a template molecule that is fabricated using the electro-polymerization technique. The study emphasized that a linear sweep voltammetry with [Fe (CN)6]4−and [Fe (CN)6]3− solution can be utilized for the quantitative detection of 1 pM and 1 μM concentration of Gly, respectively (Do et al. 2015).
The template-oriented nanosized polymer particles are utilized in innumerable forms including porous membranes, vesicles, micelles, and macromolecules for biosensor applications. The mesoporous conducting nanocomposite polymer of micro-sized flowers of graphene-nanosized polyaniline fibers (PANInf-GMF) was reported to be beneficial in cholesterol biosensor fabrication. This polymer material was placed over the glass substrate that is coated with Indium–tin oxide via drop-casting approach and this novel biosensor electrochemically sensed cholesterol with a 1.93 mg/dL as limit of detection (Lakshmi et al. 2016). Similarly, PPy was used for the electrochemical enhancement of skeletal muscle cell proliferation, whereas PEDOT and polystyrene sulfonate (PSS) were utilized for the detection of dextran sulfate. The conjugation of PPy, PEDOT and PSS as a nanomaterial was used in the fabrication of biosensors with enhanced conductivity that can increase skeletal cell differentiation and helps in monitoring in vitro dextran sulfate (Harman et al. 2015). Likewise, PANIs are formed via polymerization with a chiral monomer chondroitin sulfate (CHS) for chondroitin sulfate detection (Yuan and Kuramoto 2004). The incorporation of bio-dopants namely chondroitin sulfate, dextran sulfate, and alginate, into polymers of PEDOT are further used to detect biomolecules such as proteins, fibronectin, and collagen (Molino et al. 2014). Other studies with PPy and PANI showcased that the polymeric nanoparticles can be utilized to detect cell proliferation and dextran sulfate, respectively (Gilmore et al. 2009; Yuan and Kuramoto 2003). In addition, most biosensors fabricated via polymer are utilized for peptide and protein determination. For instance, a potentiometric biosensor for urea determination was developed via a copolymer conductive thiophene, and poly (3-hexylthiophene-co-3-thiopheneacetic acid). The urease-immobilized polymer film electrode was covalently surface linked with carboxyl group of polymer film to detect urea with a detection range of about 1–5 mM (Lai et al. 2017).
3.4 Nanocomposites
The construction of biosensors with nanocomposites provides additional reinforcement for the signal transaction and to use them in diverse applications. The nanocomposites are broadly classified into four types such as metal, ceramic, polymer, and magnetic composites. The metallic composites of rare earth elements are further divided into bimetallic colloids. Metallic oxides, including Al2O3 and MgO in combination with other metal oxides, CNT and platinum, iron, magnesium, and nickel bimetallic colloids in combination with other metals including ruthenium, copper, palladium, and silver (Janas and Liszka 2018; Khalil et al. 2018). The bimetallic nanocomposites are exclusively used in implantable biosensor preparation as they possess specific biological entities such as diffusion coefficient, biocompatibility, and biodegradation rate. It also helps in eliminating infected cells with the influence of external magnetic field (Pankhurst et al. 2016).
The graphene (GR)- and graphene oxide (GO)-based nanocomposites show high conductivity of electricity, enhanced area in the surface, defective site access, and better activity as electrocatalysts. These properties of GR and GO make them as a highly efficient material to fabricate nanocomposite-based biosensors. The functional groups of oxygen present in the GO are hydrophilic and hence, have high binding efficiency with MNPs, oxides of metals, nanosized semiconducting particles, polymers, and quantum-sized dots to recover their biosensing electrochemical ability. The nanocomposite-based biosensors are broadly classified into three types are enzymatic, nonenzymatic, and immunosensors, which is based on the type of biomarkers to be detected and the desired applications. The biomarkers such as hydrogen peroxides (H2O2) and DNA are detected using GO-based nanocomposite biosensors. The P-L-His–reduced GO and CeO2–reduced GO is used for H2O2 detection, whereas, chitosan–GO, GR–reduced GO and gold NPs–reduced GO are beneficial for DNA detection . It can be noted that GR and reduced GO-based nanocomposites are extensively utilized for glucose biosensor fabrication. Most of the enzymatic reactions in humans are demonstrated to be based on NADH-dependent reactions. These NADH molecules are detected through nanocomposites that contain gold and silver NPs with reduced GO such as gold NPs–reduce GO–PAH (poly (allylamine hydrochloride)), and gold–silver NPs–P(L-Cys)–ErGO. Further, the cholesterol molecules are detected with nanocomposites of GR and reduced GO, apart from gold, palladium, and platinum, the additional molecules in blood serum are detected by other nanocomposites that consist of chitosan, TiO2 nanowires, PPy (polypyrrole), and PVP (polyvinylpyrrolidone) (Istrate et al. 2016; Komathi et al. 2016; Pakapongpan and Poo-Arporn 2017; Pramanik et al. 2018; Radhakrishnan and Kim 2015; Tığ 2017; Vilian and Chen 2014; Wu et al. 2017; Zou et al. 2019).
Besides, graphene-based nanocomposite biosensors are employed for dopamine, bilirubin, ascorbic acid (AA), and uric acid (UA) determination via nonenzyme electrodes that are fabricated using multilayer graphene nanoflake films (MGNFs) (Shang et al. 2008; Thangamuthu et al. 2018). It is reported in various literatures that nanocomposites of GR and reduced GO with metal or metal oxides are highly beneficial in glucose, H2O2, and cholesterol sensors (Dhara et al. 2015; Gao et al. 2014; Lakshmi et al. 2016; Zor et al. 2014). Further, the graphene-based nanocomposites are used to develop immunosensors for biomarker determination such as human chorionic gonadotropin (hCG) , carcinoembryonic antigen (CEA) , interleukin-6 (IL-6) , and prostate-specific antigen (PSA) . The early detection of breast cancer markers such as ERBB2c and CD24c is quantified through gold–graphene oxide nanocomposites. Moreover, the tumor cells were identified by bio-probe that is fabricated via GO–polyaniline (PANI) nanocomposites along with CdSe quantum dot-functionalized polystyrene microspheres (Saeed et al. 2017; Wang et al. 2018). Similarly, graphene-coated gold and silver NPs as nanocomposites are used to determine CEA antigen (Huang et al. 2015b). Likewise, electrochemical label-free immunosensor was fabricated to sense PSA via nanosized chitosan–graphene–methylene blue composite (Yáñez-Sedeño et al. 2017). Also, circulating tumor cells (CTCs) were captured from the blood via graphene oxide conjugated with Neutravidin to restrain and detect the biotinylated epithelial cell that is adhesive to antibody (Yoon et al. 2013). Recently, Saeed et al. (2017) produced nanosized graphene oxide–gold composites modified with DNA aptamer (ERBB2c and CD24c) for early breast cancer detection. A sandwich-type strategy to build sensor was utilized for fabrication which has led to sensitive detection of ERBB2 and CD24 (Saeed et al. 2017). In addition, Wang et al. (2018) demonstrated that CdSe quantum dot-functionalized polystyrene microspheres can be used as a bio-probe along with GO–polyaniline (PANI) nanocomposites for ultrasensitive tumor cell detection. The results revealed that the detection limit was about 3 cells/mL, which was attributed to the high rate of electron transfer and enhanced loading of tumor cell on the surface of nanosized composites (Wang et al. 2018).
3.5 Other Novel Nanomaterials
The nanomaterial-based biosensors are showing promises to determine molecules and are in high demand in the current pharmaceutical market . The current scenario seems to be challenging to enhance the electrochemical sensors for real-time measurement, point-of-care (POC) analysis, portability, and direct analysis of multiple target analytes. Nanomaterials have been employed as immobilized bioreceptor, electrode modifiers, and electroactive labels, to increase the direct or indirect signals for the detection and quantify biomolecules (García-Mendiola et al. 2018). Since nanomaterials possess an enhanced surface area, the biomolecules are attached to the electrodes via physical adsorption through van der Waals forces (Yáñez-Sedeño et al. 2017). Thus, the biomolecules are immobilized to either supramolecular or coordinative binding biological species surfaces. For instance, SWCNT are coated with poly (adamantane–pyrrole), which is anchored to glucose oxidase (GOX) and gold nanoparticles are bound to β-cyclodextrin. The adamantane-tagged GOX was immobilized over the novel nanomaterials for the glucose biosensor fabrication and the glucose was measured by the potentiostatic method at 0.7 V (Holzinger et al. 2009).
The luminescent semiconducting nanocrystals are called as quantum dots (QDs) , which are predominant nanomaterial that are used for the biosensor fabrication. The in vitro immunoassays-based biosensors are fabricated via quantum dots for nucleic acid determination via fluorescence resonance energy transfer (FRET) method. The core-shell CdSe–ZnS QDs with size-tunable fluorescence entities are used for live tracking of cell, in vivo imaging, drug discovery, and other biological diagnostic purposes (Michalet et al. 2005). The bioluminescence resonance energy transfer (BRET) principle for QD655-Luc8 determination is reported to be useful for in vivo imaging of endogenous chromophores (So et al. 2006). The effective sensing of biomolecules using FRET and BRET is based on graphene oxide (GO) with quantum dots (QDs) quenching approach (Dong et al. 2010). The DNA and oligonucleotide are detected through FRET quenching, which reveals the optical transduction of QD (Freeman et al. 2013). The principles of FRET and BRET are used along with quenching approach to transfer charges and chemiluminescence resonance energy transfer (CRET) as light-mediated transducers for certain biosensing purposes with QDs (Algar et al. 2010). Further, streptavidin-conjugated QDs are utilized in the imaging of prostate-specific antigen (PSA) and ssDNA via near-infrared (NIR) light using surface plasmon resonance phenomena (Malic et al. 2011). Moreover, the ferromagnetic iron oxide nanomaterial is mostly used in the determination of numerous bio-analytes such as DNA, mRNA, proteins, enzymes, drugs, pathogens, and tumor cells (Haun et al. 2010).
4 Nanomaterials as Sustainable Gas and Biosensors
It is noteworthy from the previous sections that the nanomaterials are extensively utilized in the fabrication of enhanced toxic gas and biosensors. However, the toxicity and stability of nanomaterials toward humans and the environment are a major concern, while utilizing them in sensors (Jeevanandam et al. 2018). Several literatures reported that chemically synthesized nanomaterials are toxic to human cells and organs, irrespective of the type, concentration, or dose (Khan et al. 2017). Chemical-based nanomaterial synthesis approaches utilized toxic precursors, stabilizing and reducing agents, which encapsulate over nanomaterials to exhibit toxicity toward human cells (Andra et al. 2019). These toxic nanomaterials are impossible to be used as implantable biochips and other biomedical applications (Darwish et al. 2019). Even in toxic gas sensors, it is better to avoid toxic nanomaterials as they may lead to hazard effects toward the environment after its lifetime (Valsami-Jones and Lynch 2015). Thus, green or biosynthesis approaches are introduced for the fabrication of nanomaterials to be used as sensors (Mandal et al. 2018). These biosynthesized nanomaterials are formed by using biomolecules and hence they are less or nontoxic to human cells, compared to chemically synthesized nanomaterials (Yola et al. 2014). The recent trends in sensor applications are to utilize biosynthesized nontoxic nanomaterials for the fabrication of sustainable sensors that are not harmful to both humans and the environment (Omobayo Adio et al. 2016). Additionally, the energy used to fabricate sensors with green synthesized nanomaterial is much lesser compared to sensors derived from a chemical synthesis approach, which makes them as a significant method to develop sustainable sensors (Sharma et al. 2017).
4.1 Metal-Based Nanomaterials
Numerous metal, metal oxide nanoparticles , and nanocomposites were synthesized via green synthesis approach for the fabrication of efficient and sustainable toxic gas sensors. Pandey et al. (2013) fabricated an eco-friendly nanosized gold particle via reducing agent from the extracts of guar gum. These green-synthesized nanosized particles were employed as an optical sensor to detect aqueous ammonia via surface plasmon resonance. The obtained results emphasized that the green-synthesized gold nanoparticles possess properties of good reproducibility, excellent sensitivity with less than 10 s response time, and detection limit of 1 parts-per-billion (Pandey et al. 2013). Likewise, Zhao et al. (2015) synthesized spherical shaped, 6–10 nm-sized zinc oxide nanoparticles coated with silver using leaf extract from Tribulus terrestris to be used as a nontoxic and economic gas sensor at room temperature. The study revealed that the silver-coated zinc oxide nanoparticles possess enhanced and sustainable ethanol gas-sensing properties than pure zinc oxide nanoparticles (Zhao et al. 2015). Moreover, Li et al. (2008) fabricated porous tin dioxide nanoparticles by using an innovative ionic liquid template at room temperature named 1-hexadecyl-3-methylimidazolium bromide using a biogenic sol–gel approach. The obtained micro and mesopore nanoparticles were employed to detect gases such as carbon monoxide and hydrogen . The result revealed that the tin dioxide nanoparticles have the potential to be a sensitive and swift responding sustainable gas sensor (Li et al. 2008). Silver nanoparticles synthesized using ultraviolet radiation (Dubas and Pimpan 2008), Cyamopsis tetragonaloba (Pandey et al. 2012) and gold nanoparticles via locus bean gum (Tagad et al. 2014) are the other green synthesized nanomaterials that possess the ability to be fabricated as sustainable gas sensors.
Recently, Kalanur et al. (2015) fabricated palladium–tungsten oxide nanocomposites via polyvinyl pyrrolidone as a template using ultraviolet radiation-assisted photochemical method, without using any toxic chemicals. These green-synthesized metal nanocomposites exhibited excellent gas-sensing ability against hydrogen with excellent sensitivity (Kalanur et al. 2015). Likewise, Manoj et al. (2018) introduced novel green method to synthesized monodispersed copper nanoparticles using carboxymethyl cellulose. These green synthesized copper nanoparticles were combined with multi-walled carbon nanotubes and glassy carbon to detect nitrite oxidation. The result revealed that the novel nanocomposite possesses excellent nitrite oxidation detection property with great sensitivity (Manoj et al. 2018). Moreover, Tomer et al. (2019) reported that the silver nanoparticles fabricated via cyanobacterial extracts possess enhanced ammonia sensing ability (Tomer et al. 2019). Similarly, silver nanoparticles synthesized via Duranta erecta extract (Ismail et al. 2018), silver nanoparticle decorated carbon nanotube (Banihashemian et al. 2019), and palladium-deposited multi-walled nanotubes (Yoo et al. 2019) are the other significant green synthesized nanomaterials that are employed for sustainable toxic gas sensor fabrication.
Apart from toxic gas sensors, green-synthesized metal nanoparticles are also used to fabricate biosensors. Zhang et al. (2013) synthesized hybrid membrane structures of nanosized gold-reduced graphene oxide particles using uric acid, glucose, ascorbic acid, and dopamine as reducing agent to be beneficial as a hydrogen peroxide sensor. The result emphasized that the self-assembled hybrid membranes possess enhanced hydrogen peroxide detection potential with high selectivity, stability, and low detection limit (Zhang et al. 2013). Likewise, Liu et al. (2012a, b) reported a novel biogenic nanosized gold particles–graphene sheet nanohybrids using polyoxometalate as a reducing and encapsulating agent. The result demonstrated that the nanohybrids possess elevated catalytic activity with better sensitivity, stability, swift response, varied range of linearity, and low limit of detection (Liu et al. 2012a). Meanwhile, silver–graphene nanocomposites were synthesized via reducing tannic acid. The electrochemical and the Raman scattering results emphasized that the nanocomposites possess surface-enhanced Raman-scattering properties with enhanced hydrogen peroxide detection ability. The study confirmed that the nanocomposites can be used as an enzyme-less, amperometric hydrogen peroxide and glucose sensor with rapid response time of less than 2 s (Zhang et al. 2012b). Moreover, silver nanoparticles as optical fiber-based hydrogen peroxide sensors (Tagad et al. 2013), palladium-decorated poly(3,4-ethylenedioxythiophene) as a nonenzymatic biosensor to detect hydrogen peroxide (Jiang et al. 2013), bio-templated synthesized gold nanoparticles—bacteria cellulose nanofiber nanocomposites (Zhang et al. 2010), starch-stabilized silver nanoparticles (Vasileva et al. 2011), Sargassum alga synthesized palladium nanoparticles (Momeni and Nabipour 2015) are greener nanomaterials that are used in sustainable biosensor fabrication.
In recent times, Gayda et al. (2019) listed the metallic nanoparticles that are attained via biogenic method and used as a biosensor construction platform (Gayda et al. 2019). Bollella et al. (2017) fabricated nanosized gold and silver particles via reducing quercetin for biosensor applications. These spherical shaped, 5–8 nm sized, green-synthesized nanoparticles showed enhanced efficiencies as third-generation lactose biosensors (Bollella et al. 2017). Meanwhile, Su et al. (2016) fabricated nanostructure of cobalt oxide hydroxide using a photochemical approach without template, surfactant, or toxic solvents of organic nature at optimum temperature. The result showed that these nanostructures are highly beneficial in the sunlight mediated bifunctional detection of hydrogen peroxide and can be used to fabricate novel biosensors (Su et al. 2016). Silver-reduced graphene oxide–carbon nanotube nanocomposites were fabricated recently via a novel green synthesis approach for the biosensor applications . The result demonstrated that the nanocomposite with unique sensing properties exhibited excellent ability to detect dopamine with a response time of 12 s and 0.033 μM of detection limit (Khan et al. 2016). Similarly, green synthesized zinc oxide nanoparticles (Muthuchamy et al. 2018), graphene nanoribbon–silver nanoparticle–polyphenol oxidase composite (Sandeep et al. 2018), gold nanoparticles (Raj and Sudarsanakumar 2018), cyclodextrin-capped gold nanoparticles (Zhang et al. 2019), and molybdenum disulfide–gold nanoparticles (Wu et al. 2018) are the other recent nanomaterials that are used to develop sustainable biosensors .
4.2 Carbon-Based Nanomaterials
Similar to metals, carbon-based nanomaterials were also used to fabricate toxic gas and biosensors. It is noteworthy that most of the carbon-based nanomaterials are nanocomposites as carbon materials are highly reactive and cause toxic reactions toward humans and microbes. Nanosized composites of tin dioxide with reduced graphene oxide to detect nitrogen dioxide at low temperatures (Zhang et al. 2014), graphene–zinc oxide nanoparticle hybrid (Kavitha et al. 2012) and conjugated polymer–carbon nanotube (Dai et al. 2002) are widely used in the gas sensor applications. However, they are synthesized via chemical approaches that use toxic chemicals and are not suitable for biosensor applications. Nanosized sheets of graphene decorated with nanosized zirconia particle hybrid for the enzyme-less detection of methyl parathion (Gong et al. 2012), indium-doped tin dioxide nanoparticle-graphene nanohybrids as nitrogen dioxide sensors (Cui et al. 2013), tin dioxide nanoparticle-decorated graphene sheets as cataluminescence gas sensors (Song et al. 2011), and mesoporous spherical tin dioxide–graphene nanocomposites as highly sensitive formaldehyde sensor (Chen et al. 2016) are the carbon-based nanomaterials that are used as sustainable gas sensors. In recent times, zinc oxide loaded with nanosized silver particles along with reduced graphene oxide are designed as hybrid for low-temperature detection of acetylene gas (Iftekhar Uddin et al. 2015), Justicia glauca leaf extract synthesized graphene oxide reduced with nanosized silver particle decoration as nitrobenzene sensor (Karuppiah et al. 2015), graphene-based sensors (Wang et al. 2016c), and nanosized hybrid composite of iron oxide-reduced graphene oxide to detect nitrogen dioxide at room temperature (Zhang et al. 2017) are the latest graphene-based nanomaterials that are used in sustainable toxic gas sensor fabrication. Likewise, carbon nanotubes (Davis et al. 2003; Wanna et al. 2006) and nanodots (Sun and Lei 2017; Wang et al. 2016b) were also synthesized via green approaches and are used to fabricate sustainable gas sensors.
Several green synthesized carbon-based nanomaterials were also employed for biosensor fabrication. Nayak et al. (2013) demonstrated a novel green synthesis method for the fabrication of nanosized hybrid of graphene-carbon nanotube decorated with zinc oxide particles as composites using solar energy. These novel nanohybrids were subjected to perform as a transducer in an organophosphorus biosensor and the result revealed that the nanohybrids exhibited a linear Paraoxon detection response of 1–26 nM with a detection limit of 1 pM (Nayak et al. 2013). Likewise, a biogenic nanosized composite film that is fabricated with glucose oxidase, gold particles, polyvinyl alcohol, and carbon nanotubes by Zhang et al. (2011). The result from the study reported that the nanocomposite exhibited a linear amperometric response toward concentration of glucose (0.5–8 mM) with a sensitivity of 16.6 μA mM−1 cm−2 (Zhang et al. 2011). Similarly, nanosized composites of gold reduced graphene oxide were synthesized via aqueous extract of rose flower as reducing agent. These novel green synthesized nanocomposites exhibited enhanced efficiency to be an effective glucose sensor with a low (10 μM) limit of detection (Amouzadeh Tabrizi and Varkani 2014). Recently, carbon dots (Liu et al. 2015), nickel–cobalt oxide–graphene nanohybrids (Ko et al. 2017), photoluminescent self-doped carbon dots (Wang et al. 2017), carbon dots-manganese dioxide nanosheets (Qu et al. 2017), and composites made up of nanosized silver particle, reduced oxide of graphene and carbon nanotube (Lorestani et al. 2015) are synthesized using green methods and are used to fabricate sustainable biosensors .
4.3 Polymer-Based Nanomaterials
Polymer nanomaterials and individual nanomaterials functionalized with polymers were widely used to fabricate toxic gas and biosensors. Venditti et al. (2013) introduced an innovative osmosis-based method for polyaniline–gold nanoparticle fabrication. Further, these nanocomposites are doped with hydrogen sulfide to detect ammonia gas and the result demonstrated that these nanomaterials possess enhanced sensitivity against ammonia up to 10 parts-per-million, compared to other vapor organic compounds (Venditti et al. 2013). Moreover, Nia et al. (2015) reported that assembly of silver nanoparticle decorated graphene oxide over glassy carbon electrode via an amperometry method. Polypyrrole nanofibers were attached to the electrodes and are employed as nonenzymatic hydrogen peroxide sensor. The result showed that these nanocomposites possess enhanced ability to detect gases with a detection limit of 1.099 (Moozarm Nia et al. 2015). Likewise, Wang et al. (2008) produced antimony-doped tin dioxide nanoparticles via a new block copolymer with amphiphilic property named poly(ethylene-co-butylene)-block-poly (ethylene oxide). These doped nanoparticles were employed to detect formaldehyde gas in the atmosphere and the nanoparticles exhibited good and swift responses in detecting the gas with good recovery (Wang et al. 2008). In recent times, conducting polymer–nanoparticle composite based chemo-electrical gas sensors (Park et al. 2017b), zinc oxide thin film prepared with inorganic green sodium–carboxymethyl cellulose polymer as acetone sensors (Muthukrishnan et al. 2016), uniformly decorated silver nanoparticles on polypyrrole as hydrogen peroxide sensor (Nia et al. 2015) and polyaniline–samarium-doped titanium dioxide nanocomposites (Ramesan and Sampreeth 2018) are the green synthesized polymer nanomaterials that are used to fabricate sustainable toxic gas sensors.
Huang et al. (2011) fabricated a bio-electro-chemically active infinite coordination polymer nanoparticles and reported that they possess enhanced glucose detection property (Huang et al. 2011). Likewise, Zhang et al. (2010) synthesized nanocomposites via nanosized gold particles and bacterial cellulose nanofiber for hydrogen peroxide detection with 1 μM of detection limit (Zhang et al. 2010). Similarly, Asati et al. (2009) fabricated a polymer-coated nanosized cerium oxide particles for oxidation monitoring in cells and antioxidants (Asati et al. 2009). Moreover, Liu et al. (2012a, b) synthesized a nanodot polymer with doped nitrogen that is rich in carbon and with photoluminescent property for biosensor applications, especially for the label-free copper ion detection in body fluids (Liu et al. 2012b). Furthermore, Ruan et al. (2013) described a green synthesis approach for polydopamine–graphene composite film fabrication that is modified with enzyme electrode to be useful as a glucose sensor (Ruan et al. 2013). In recent times, novel carbon nanotube–poly (brilliant green), carbon nanotube–poly (3,4-ethylenedioxythiophene) as enzyme-based biosensors with electrochemical property (Barsan et al. 2016), gold nanoparticles stabilized by alcohol oxidase protein that is encapsulated with polyaniline as amperometric alcohol biosensor (Chinnadayyala et al. 2015) and silver nanoparticle embedded in bacterial cellulose nano-paper as plasmonic sensors (Pourreza et al. 2015) are the novel green synthesized polymeric nanomaterials that help in sustainable biosensor fabrication .
4.4 Novel Nanocomposites
Nanocomposites are the latest addition to the broad set of nanomaterials that are effective for sensor applications. Punicalagin green functionalized copper–copper oxide–zinc oxide nanocomposite as potential electrochemical transducer (Fuku et al. 2016), nanosized composites of graphene oxide reduced by zinc oxide for nitrogen dioxide gas-sensing application (Kumar et al. 2015a) and tin dioxide-based hierarchical nano-microstructures (Jiang et al. 2009) are the novel nanocomposites that are extensively under research as sustainable toxic gas sensors. Meanwhile, mesoporous spherical tin dioxide–graphene nanoparticles as formaldehyde gas sensors (Chen et al. 2016), copper oxide-y@ zinc oxide-α nanosized composites for improved room temperature nitrogen dioxide detection applications (Geng et al. 2017) and silver–iron oxide core–shell magnetic nanocomposite (Mirzaei et al. 2016) are the recent novel nano-sized composites that are used to fabricate sustainable toxic gas sensors. Similarly, photoluminescent carbon dots are synthesized with willow bark to fabricate gold nanoparticles-reduced graphene oxide nanocomposites , which is useful as a glucose sensor (Qin et al. 2013). Likewise, gold–chitosan nanocomposites as caffeic acid sensors (Di Carlo et al. 2012), silver nanoparticle-graphene oxide nanocomposite as tryptophan sensor (Li et al. 2013), palladium–gold–carbon dot nanocomposite to sense colitoxin DNA in human serum (Huang et al. 2017), graphene-nanosized gold particles hybrid as surface-enhanced Raman-scattering biosensor (Khalil et al. 2016) and nanosized flower-shaped reduced graphene oxide–iron oxide hybrid composites for sensing riboflavin (Madhuvilakku et al. 2017) are the novel nanocomposite-based sustainable biosensors that are under extensive research. Even though there are several nanomaterials that are synthesized using green and biosynthesis approaches, there exist certain limitations while using nanomaterials as sensors which have to be addressed before employing them for large-scale sensor applications .
5 Limitations and Future Perspective
The nanomaterial sensors are becoming an integral part of any sensor research due to their enormous applications (Luo et al. 2006). It is noteworthy that nanosensors are the hot topic in recent nanotechnology researches to develop enhanced sensors with rapid sensitivity and excellent detection limit (Ding et al. 2010). However, there exist several limitations to use nanomaterials in the fabrication of large-scale and commercial toxic gas and biosensors (Wang et al. 2013). Most of the commercial nanosized toxic gas and biosensors are fabricated via chemical approaches as shown in previous sections. These chemically synthesized nanomaterial-based sensors may lead to toxicity toward humans and other organisms (El-Safty et al. 2007). Moreover, it may accumulate as wastes over its lifetime and the toxic chemicals upon degradation may react with the environment (soil, air, or water) and lead to hazardous effects (Khin et al. 2012). This has led to the emergence of green and biosynthesis approaches to fabricate nanomaterials for sensor applications. These synthesis approaches have provided less or nontoxic nanomaterials for sensor fabrications (Zhang et al. 2012a). However, nanomaterials from green or biosynthesis are unstable most of the time and agglomerate to become micro-sized particles which alter their significant sensor properties (Wahab et al. 2018). Thus, it is highly essential to blend chemical, green, and biosynthesis approaches in the right proportion as a hybrid approach to fabricate sensors to avoid toxicity and improve stability with enhanced sensing properties. Moreover, the latest nanomaterials are focused on detecting a specific molecule, either gas or biomolecules, with enhanced detection limit and sensitivity (Yang et al. 2015). The future research of nanomaterial-based sensors must focus on multi-molecule detection , which can detect several toxic gases and biomolecules. This is highly possible by fabricating complex nanocomposites that can be tailored based on the requirement of sensors for desired applications.
The future of toxic gas and biosensors is based on their incorporation with wearable technologies and in biochips that can be implanted in the patients (Shafiee et al. 2018). Generally, nanomaterials are designed to either sense toxic gases or biomolecules (Smith et al. 2017). In future, multitasking nanomaterials will be designed to detect both toxic gas and biomolecules (Gu and Zhang 2018). These multitasking nanomaterial-based sensors will reduce the cost as well as the time and effort needed to produce such sensors (Casanova-Chafer and Llobet 2019). Moreover, these novel sensors will be incorporated into fabrics to sense toxic gases in the environment, inhalation or exposure level of toxic gas toward humans and detect biomolecules related to disease in patients (Hu et al. 2018; Jang et al. 2018). Recently, fabrics with sensors are available which possess sensors and can change color upon sensing toxic gas in the environment (He et al. 2019). Likewise, multitasking nanomaterial-based sensors can be incorporated in smartwatches and mobile phones to detect toxic gases as well as disease-related biomolecules (Tiwari et al. 2019). These smart technologies will serve as a display to know the level of detecting toxic gas or biomolecules and can be monitored by physicians (Tabatabaee et al. 2019). Such sensors will be the future of biomedical sciences which will reduce the burden of patients and physicians in continuous monitoring of analytes. It will also help in the fabrication of fabrics that can monitor both toxic gases and biomolecules, which can help firefighters, workers in mines, and the people who work in extreme conditions. It is noteworthy that nanomaterials can be used to fabricate sensor substrate (Kumar et al. 2015b), bind with the analyte for enhancing the detection (Cardinal et al. 2017), transducer (Zaretski et al. 2016), signal processors (Lu et al. 2011), and as an individual sensing material (Wang et al. 2003). Thus, nanomaterials will replace the current sensor market and enhance its ability to be present in any tools that are used by consumers for sensing toxic gases and will be used as implantable biochips in the future that can sense disease and deliver drugs in the target site. Figure 1 is the summary of futuristic nanomaterial-based sensors in different toxic gas and biosensor applications.
6 Conclusion
This chapter is a summary of distinct nanomaterials that are used for the fabrication of toxic gas and biosensors. Metal, carbon, polymer-based nanomaterials, and nanocomposites are widely used in sensor fabrication applications. However, it was noteworthy that chemically synthesized nanomaterials are proved to be toxic toward humans as well as environment and are not suitable for implantable biosensors. Thus, green and biosynthesis are introduced to fabricate sustainable, nontoxic, and stable sensors to detect toxic gases and biomolecules. Biosynthesis using extracts from plants and bacteria as well as green synthesis using sunlight, ultraviolet, visible, and infrared radiation are used as catalyst and reducing agent to fabricate nanomaterials for sensor applications. However, there are several limitations to utilize green and biosynthesized nanomaterials for large-scale and commercial sensor applications. In future, it is possible to overcome these limitations with the advantages and swift progresses in nanotechnology field for the emergence of futuristic sensors. These futuristic sensors can be incorporated into fabrics, smartwatch , phones, paints, and several other tools that a consumer will use in their day-to-day life for toxic gases and biomolecules detection.
Abbreviations
- Al2O3 :
-
Aluminium oxide
- AuNPs:
-
Gold nanoparticles
- BRET :
-
Bioluminescence resonance energy transfer
- CdSe:
-
Cadmium selenide
- CEA :
-
Carcinoembryonic antigen
- CeO2 :
-
Cerium dioxide
- CHS:
-
Chondroitin sulfate
- CNMs:
-
Carbon nanomaterials
- CNTs:
-
Carbon nanotubes
- CO:
-
Carbon monoxide
- CPE :
-
Carbon paste electrode
- CRET:
-
Chemiluminescence resonance energy transfer
- CTCs:
-
Circulating tumor cells
- CV :
-
Cyclic voltammetry
- DNA:
-
Deoxyribonucleic acid
- EIS :
-
Electrochemical impedance spectroscopy
- EMF:
-
Electromotive force
- FETs:
-
Field effect transistors
- Gly:
-
Glyphosate
- GO:
-
Graphene oxide
- GOx :
-
Glucose oxidase enzyme
- GR:
-
Graphene
- H2O2 :
-
Hydrogen peroxide
- H2S:
-
Hydrogen sulfide
- Hb :
-
Hemoglobin
- hCG :
-
Human chorionic gonadotropin
- HOPG :
-
Highly oriented pyrolytic graphite
- IL-6 :
-
Interleukin-6
- LbL:
-
Layer-by-layer
- MgO:
-
Magnesium oxide
- MnO2 NPs :
-
Manganese dioxide NPs
- MNPs :
-
Metal nanoparticles
- MWNTs:
-
Multi-walled carbon nanotubes
- NADH :
-
Nicotinamide–adenine dinucleotide
- NH3 :
-
Ammonia
- NiOx:
-
Nickel oxide
- NIR:
-
Near infrared
- NO:
-
Nitrogen oxide
- NPs:
-
Nanoparticles
- PAH :
-
Poly (allylamine hydrochloride)
- PAni:
-
Polyaniline
- PEDOT:
-
Poly (3,4-ethylenedioxythiophene)
- PEG:
-
Polyethylene glycol
- PEI:
-
Polyethyleneimine
- PNA :
-
Peptide nucleic acid
- POC :
-
Point-of-care
- PPy:
-
Polypyrrole
- PSA :
-
Prostate-specific antigen
- PSS:
-
Poly (styrene sulfonate)
- PSS:
-
Polystyrene sulfonate
- Pt :
-
Platinum
- PTh:
-
Polythiophene
- PVP :
-
Polyvinylpyrrolidone
- QCM:
-
Quartz crystal microbalance
- QDs :
-
Quantum dots
- RNAs:
-
Ribonucleic acid
- SAW:
-
Surface acoustic wave
- SnO2 :
-
Tin dioxide
- SPANI:
-
Poly (anilinesulfonic acid)
- ssDNA:
-
Single-stranded DNA
- STW:
-
Surface transverse wave
- SWNTs:
-
Single-walled carbon nanotubes
- TiO2 :
-
Titanium dioxide
- TMF-α:
-
Tumor necrosis factor-α
- USD:
-
United States Dollars
- UV:
-
Ultraviolet
- VOCs :
-
Volatile organic compounds
- WO3 :
-
Tungsten oxide
- ZnO:
-
Zinc oxide
- ZnS:
-
Zinc sulfide
References
Acosta MA, Ymele-Leki P, Kostov YV, Leach JB (2009) Fluorescent microparticles for sensing cell microenvironment oxygen levels within 3D scaffolds. Biomaterials 30:3068–3074
Adhikari S, Chowdhury R (2010) The calibration of carbon nanotube based bionanosensors. J Appl Phys 107:124322
Ajay Piriya VS, Joseph P, Daniel SCGK, Lakshmanan S, Kinoshita T, Muthusamy S (2017) Colorimetric sensors for rapid detection of various analytes. Mater Sci Eng C 78:1231–1245
Algar WR, Tavares AJ, Krull UJ (2010) Beyond labels: a review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal Chim Acta 673:1–25
Alshammari AS, Alenezi MR, Lai KT, SRP S (2017) Inkjet printing of polymer functionalized CNT gas sensor with enhanced sensing properties. Mater Lett 189:299–302
Amouzadeh Tabrizi M, Varkani JN (2014) Green synthesis of reduced graphene oxide decorated with gold nanoparticles and its glucose sensing application. Sensors Actuators B 202:475–482. https://doi.org/10.1016/j.snb.2014.05.099
Anderson CD, Daniels ES (2003) Emulsion polymerisation and latex applications, vol 14. iSmithers Rapra Publishing.
Andra S, Balu SK, Jeevanandham J, Muthalagu M, Vidyavathy M, San Chan Y, Danquah MK (2019) Phytosynthesized metal oxide nanoparticles for pharmaceutical applications. Naunyn Schmiedebergs Arch Pharmacol 392(7):755–771
Annabi N et al (2014) 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv Mater 26:85–124
Asati A, Santra S, Kaittanis C, Nath S, Perez JM (2009) Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Ed 48:2308–2312
Avramov ID, Rapp M, Voigt A, Stahl U, Dirschka M (2000) In: Comparative studies on polymer coated SAW and STW resonators for chemical gas sensor applications. IEEE, pp 58–65
Bai H, Shi G (2007) Gas sensors based on conducting polymers. Sensors 7:267–307
Banihashemian SM, Hajghassem H, Nikfarjam A, Azizi Jarmoshti J, Abdul Rahman S, Boon Tong G (2019) Room temperature ethanol sensing by green synthesized silver nanoparticle decorated SWCNT. Mater Res Express 6:065602. https://doi.org/10.1088/2053-1591/ab07de
Barsan N, Koziej D, Weimar U (2007) Metal oxide-based gas sensor research: how to? Sensors Actuators B 121:18–35
Barsan MM, Pifferi V, Falciola L, Brett CMA (2016) New CNT/poly (brilliant green) and CNT/poly (3,4-ethylenedioxythiophene) based electrochemical enzyme biosensors. Anal Chim Acta 927:35–45
Batzill M, Diebold U (2005) The surface and materials science of tin oxide. Prog Surf Sci 79:47–154
Bekyarova E, Davis M, Burch T, Itkis ME, Zhao B, Sunshine S, Haddon RC (2004) Chemically functionalized single-walled carbon nanotubes as ammonia sensors. J Phys Chem B 108:19717–19720
Bogue R (2014) Towards the trillion sensors market. Sens Rev 34:137–142
Bollella P, Schulz C, Favero G, Mazzei F, Ludwig R, Gorton L, Antiochia R (2017) Green synthesis and characterization of gold and silver nanoparticles and their application for development of a third generation lactose biosensor. Electroanalysis 29:77–86. https://doi.org/10.1002/elan.201600476
Brousseau LC, Aurentz DJ, Benesi AJ, Mallouk TE (1997) Molecular design of intercalation-based sensors. 2. Sensing of carbon dioxide in functionalized thin films of copper octanediylbis (phosphonate). Anal Chem 69:688–694
Capone S, Siciliano P, Quaranta F, Rella R, Epifani M, Vasanelli L (2000) Analysis of vapours and foods by means of an electronic nose based on a sol–gel metal oxide sensors array. Sens Actuators B 69:230–235
Cardinal MF, Vander Ende E, Hackler RA, McAnally MO, Stair PC, Schatz GC, Van Duyne RP (2017) Expanding applications of SERS through versatile nanomaterials engineering. Chem Soc Rev 46:3886–3903
Carregal-Romero S et al (2013) Multiplexed sensing and imaging with colloidal nano-and microparticles. Annu Rev Anal Chem 6:53–81
Casanova-Chafer J, Llobet E (2019) Carbon nanomaterials integrated in rugged and inexpensive sensing nanoscale materials for warfare agent detection. Nanoscience for Security 13
Castro M, Kumar B, Feller J-F, Haddi Z, Amari A, Bouchikhi B (2011) Novel e-nose for the discrimination of volatile organic biomarkers with an array of carbon nanotubes (CNT) conductive polymer nanocomposites (CPC) sensors. Sensors Actuators B 159:213–219
Chandra P, Segal E (2016) Nanobiosensors for personalized and onsite biomedical diagnosis. The Institution of Engineering and Technology
Chatterjee SG, Chatterjee S, Ray AK, Chakraborty AK (2015) Graphene–metal oxide nanohybrids for toxic gas sensor: a review. Sensors Actuators B 221:1170–1181
Chen S et al (2016) One-pot synthesis of mesoporous spherical SnO2@graphene for high-sensitivity formaldehyde gas sensors. RSC Adv 6:25198–25202
Chinnadayyala SR, Santhosh M, Singh NK, Goswami P (2015) Alcohol oxidase protein mediated in-situ synthesized and stabilized gold nanoparticles for developing amperometric alcohol biosensor. Biosens Bioelectron 69:155–161
Christ JF, Aliheidari N, Ameli A, Pötschke P (2017) 3D printed highly elastic strain sensors of multiwalled carbon nanotube/thermoplastic polyurethane nanocomposites. Mater Des 131:394–401
Comini E (2006) Metal oxide nano-crystals for gas sensing. Anal Chim Acta 568:28–40
Cui S et al (2013) Indium-doped SnO2 nanoparticle–graphene nanohybrids: simple one-pot synthesis and their selective detection of NO2. J Mater Chem A 1:4462–4467
Dai L, Soundarrajan P, Kim T (2002) Sensors and sensor arrays based on conjugated polymers and carbon nanotubes. Pure Appl Chem 74:1753–1772
Darwish A, Sayed GI, Hassanien AE (2019) The impact of implantable sensors in biomedical technology on the future of healthcare systems. In: Intelligent pervasive computing systems for smarter healthcare
Davis JJ, Coleman KS, Azamian BR, Bagshaw CB, MLH G (2003) Chemical and biochemical sensing with modified single walled carbon nanotubes. Chem A Eur J 9:3732–3739
Dey A (2018) Semiconductor metal oxide gas sensors: a review. Mater Sci Eng B 229:206–217
Dhara K, Ramachandran T, Nair BG, TGS B (2015) Single step synthesis of Au–CuO nanoparticles decorated reduced graphene oxide for high performance disposable nonenzymatic glucose sensor. J Electroanal Chem 743:1–9
Di Carlo G et al (2012) Green synthesis of gold–chitosan nanocomposites for caffeic acid sensing. Langmuir 28:5471–5479
Ding B, Wang M, Wang X, Yu J, Sun G (2010) Electrospun nanomaterials for ultrasensitive sensors. Mater Today 13:16–27
Do MH et al (2015) Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int J Environ Anal Chem 95:1489–1501
Dong XM, Fu RW, Zhang MQ, Zhang B, Rong MZ (2004) Electrical resistance response of carbon black filled amorphous polymer composite sensors to organic vapors at low vapor concentrations. Carbon 42:2551–2559
Dong H, Gao W, Yan F, Ji H, Ju H (2010) Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal Chem 82:5511–5517
Dubas ST, Pimpan V (2008) Green synthesis of silver nanoparticles for ammonia sensing. Talanta 76:29–33. https://doi.org/10.1016/j.talanta.2008.01.062
Ellis JE, Star A (2016) Carbon nanotube based gas sensors toward breath analysis. ChemPlusChem 81:1248–1265
Elosúa C, Bariáin C, Matías IR, Arregui FJ, Luquin A, Laguna M (2006) Volatile alcoholic compounds fibre optic nanosensor. Sensors Actuators B 115:444–449
El-Safty SA, Prabhakaran D, Ismail AA, Matsunaga H, Mizukami F (2007) Nanosensor design packages: a smart and compact development for metal ions sensing responses. Adv Funct Mater 17:3731–3745
Endres HE et al (1996) A thin-film SnO2 sensor system for simultaneous detection of CO and NO2 with neural signal evaluation. Sensors Actuators B 36:353–357
Eranna G (2016) Metal oxide nanostructures as gas sensing devices. CRC Press, Boca Raton
Erden F, Velipasalar S, Alkar AZ, Cetin AE (2016) Sensors in assisted living: a survey of signal and image processing methods. IEEE Signal Process Mag 33:36–44
Fowler JD, Allen MJ, Tung VC, Yang Y, Kaner RB, Weiller BH (2009) Practical chemical sensors from chemically derived graphene. ACS Nano 3:301–306
Fraiwan L, Lweesy K, Bani-Salma A, Mani N (2011) In: A wireless home safety gas leakage detection system. IEEE, pp 11–14
Freeman R, Girsh J, Willner I (2013) Nucleic acid/quantum dots (QDs) hybrid systems for optical and photoelectrochemical sensing. ACS Appl Mater Interfaces 5:2815–2834
Fuku X, Kaviyarasu K, Matinise N, Maaza M (2016) Punicalagin green functionalized Cu/Cu2O/ZnO/CuO nanocomposite for potential electrochemical transducer and catalyst. Nanoscale Res Lett 11:386
Gao W, Tjiu WW, Wei J, Liu T (2014) Highly sensitive nonenzymatic glucose and H2O2 sensor based on Ni(OH)2/electroreduced graphene oxide—multiwalled carbon nanotube film modified glass carbon electrode. Talanta 120:484–490
García-Mendiola T et al (2018) Carbon nanodots based biosensors for gene mutation detection. Sensors Actuators B 256:226–233
Gardner JW, Bartlett PN (1993) Design of conducting polymer gas sensors: modelling and experiment. Synth Met 57:3665–3670
Gayda ZG et al (2019) Metallic nanoparticles obtained via “green” synthesis as a platform for biosensor construction. Appl Sci 9. https://doi.org/10.3390/app9040720
Geng X, Zhang C, Luo Y, Debliquy M (2017) Preparation and characterization of CuxO1-y@ ZnO1-α nanocomposites for enhanced room-temperature NO2 sensing applications. Appl Surf Sci 401:248–255
Gilmore KJ et al (2009) Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials 30:5292–5304
Gong J, Miao X, Wan H, Song D (2012) Facile synthesis of zirconia nanoparticles-decorated graphene hybrid nanosheets for an enzymeless methyl parathion sensor. Sensors Actuators B 162:341–347. https://doi.org/10.1016/j.snb.2011.12.094
Goustouridis D, Manoli K, Chatzandroulis S, Sanopoulou M, Raptis I (2005) Characterization of polymer layers for silicon micromachined bilayer chemical sensors using white light interferometry. Sensors Actuators B 111:549–554
Gu B, Zhang Q (2018) Recent advances on functionalized upconversion nanoparticles for detection of small molecules and ions in biosystems. Adv Sci 5:1700609
Gupta SK, Joshi A, Kaur M (2010) Development of gas sensors using ZnO nanostructures. J Chem Sci 122:57–62
Hagleitner C, Lange D, Hierlemann A, Brand O, Baltes H (2002) CMOS single-chip gas detection system comprising capacitive, calorimetric and mass-sensitive microsensors. IEEE J Solid State Circuits 37:1867–1878
Hallil H, Chebila F, Menini P, Pons P, Aubert H (2010) In: Feasibility of wireless gas detection with an FMCW RADAR interrogation of passive RF gas sensor. IEEE, pp 759–762
Harman DG et al (2015) Poly (3,4-ethylenedioxythiophene): dextran sulfate (PEDOT: DS)—a highly processable conductive organic biopolymer. Acta Biomater 14:33–42
Hatfield JV, Neaves P, Hicks PJ, Persaud K, Travers P (1994) Towards an integrated electronic nose using conducting polymer sensors. Sensors Actuators B 18:221–228
Haun JB, Yoon TJ, Lee H, Weissleder R (2010) Magnetic nanoparticle biosensors. Wiley Interdisciplinary Rev Nanomed Nanobiotechnol 2:291–304
He T, Shi Q, Wang H, Wen F, Chen T, Ouyang J, Lee C (2019) Beyond energy harvesting-multi-functional triboelectric nanosensors on a textile. Nano Energy 57:338–352
Hirata T et al (2008) Chemical modification of carbon nanotube based bio-nanosensor by plasma activation. Japanese J Appl Phys 47:2068
Hoefer U, Böttner H, Felske A, Kühner G, Steiner K, Sulz G (1997) Thin-film SnO2 sensor arrays controlled by variation of contact potential—a suitable tool for chemometric gas mixture analysis in the TLV range. Sensors Actuators B 44:429–433
Holzinger M, Bouffier L, Villalonga R, Cosnier S (2009) Adamantane/β-cyclodextrin affinity biosensors based on single-walled carbon nanotubes Adamantane/β-cyclodextrin affinity biosensors based on single-walled carbon nanotubes. Biosens Bioelectron 24:1128–1134
Hu H, Sun Z, Jornet JM (2018) In: Through-the-body localization of implanted biochip in wearable nano-biosensing networks. IEEE, pp 278–283
Huang P, Mao J, Yang L, Yu P, Mao L (2011) Bioelectrochemically active infinite coordination polymer nanoparticles: one-pot synthesis and biosensing property. Chem A Eur J 17:11390–11393. https://doi.org/10.1002/chem.201101634
Huang H, Bai W, Dong C, Guo R, Liu Z (2015a) An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens Bioelectron 68:442–446
Huang J, Tian J, Zhao Y, Zhao S (2015b) Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sensors Actuators B 206:570–576
Huang Q, Lin X, Zhu J-J, Tong Q-X (2017) Pd-Au@ carbon dots nanocomposite: Facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum. Biosens Bioelectron 94:507–512
Iftekhar Uddin ASM, Phan D-T, Chung G-S (2015) Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sensors Actuators B 207:362–369. https://doi.org/10.1016/j.snb.2014.10.091
Ijeh MO (2011) Covalent gold nanoparticle-antibody conjugates for sensivity improvement in LFIA
Ismail M, Khan MI, Akhtar K, Khan MA, Asiri AM, Khan SB (2018) Biosynthesis of silver nanoparticles: a colorimetric optical sensor for detection of hexavalent chromium and ammonia in aqueous solution. Phys E Low-dimens Syst Nanostruct 103:367–376. https://doi.org/10.1016/j.physe.2018.06.015
Istrate O-M, Rotariu L, Marinescu VE, Bala C (2016) NADH sensing platform based on electrochemically generated reduced graphene oxide–gold nanoparticles composite stabilized with poly (allylamine hydrochloride). Sensors Actuators B 223:697–704
Jaegle M, Wöllenstein J, Meisinger T, Böttner H, Müller G, Becker T, Braunmühl CB-V (1999) Micromachined thin film SnO2 gas sensors in temperature-pulsed operation mode. Sensors Actuators B 57:130–134
Jamdagni P, Khatri P, Rana JS (2016) Nanoparticles based DNA conjugates for detection of pathogenic microorganisms. Int Nano Lett 6:139–146
Jamshaid T, Neto ETT, Eissa MM, Zine N, Kunita MH, El-Salhi AE, Elaissari A (2016) Magnetic particles: from preparation to lab-on-a-chip, biosensors, microsystems and microfluidics applications. TrAC Trends Anal Chem 79:344–362
Janas D, Liszka B (2018) Copper matrix nanocomposites based on carbon nanotubes or graphene. Mater Chem Front 2:22–35
Janata J, Josowicz M (2003) Conducting polymers in electronic chemical sensors. Nat Mater 2:19
Jang S, Kim S, Geier ML, Hersam MC, Dodabalapur A (2016) Inkjet printed carbon nanotubes in short channel field effect transistors: influence of nanotube distortion and gate insulator interface modification. Flexible Printed Electron 1:035001
Jang JS et al (2018) Flexible gas sensors: glass-fabric reinforced Ag nanowire/Siloxane composite heater substrate: sub-10 nm metal@ metal oxide nanosheet for sensitive flexible sensing platform (Small 44/2018). Small 14:1870201
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074
Jiang L-Y, Wu X-L, Guo Y-G, Wan L-J (2009) SnO2-based hierarchical nanomicrostructures: facile synthesis and their applications in gas sensors and lithium-ion batteries. J Phys Chem C 113:14213–14219
Jiang F, Yue R, Du Y, Xu J, Yang P (2013) A one-pot ‘green’synthesis of Pd-decorated PEDOT nanospheres for nonenzymatic hydrogen peroxide sensing. Biosens Bioelectron 44:127–131
Jimenez-Cadena G, Riu J, Rius FX (2007) Gas sensors based on nanostructured materials. Analyst 132:1083–1099
Kalanur SS, Yoo I-H, Lee Y-A, Seo H (2015) Green deposition of Pd nanoparticles on WO3 for optical, electronic and gasochromic hydrogen sensing applications. Sensors Actuators B 221:411–417. https://doi.org/10.1016/j.snb.2015.06.086
Kanan S, El-Kadri O, Abu-Yousef I, Kanan M (2009) Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 9:8158–8196
Kanazawa E, Sakai G, Shimanoe K, Kanmura Y, Teraoka Y, Miura N, Yamazoe N (2001) Metal oxide semiconductor N2O sensor for medical use. Sensors Actuators B 77:72–77
Kanjanawarut R, Su X (2009) Colorimetric detection of DNA using unmodified metallic nanoparticles and peptide nucleic acid probes. Anal Chem 81:6122–6129
Karuppiah C et al (2015) Green synthesized silver nanoparticles decorated on reduced graphene oxide for enhanced electrochemical sensing of nitrobenzene in waste water samples. RSC Adv 5:31139–31146. https://doi.org/10.1039/C5RA00992H
Kato K, Kato Y, Takamatsu K, Udaka T, Nakahara T, Matsuura Y, Yoshikawa K (2000) Toward the realization of an intelligent gas sensing system utilizing a non-linear dynamic response. Sensors Actuators B 71:192–196
Kaushik A, Kumar R, Arya SK, Nair M, Malhotra BD, Bhansali S (2015) Organic–inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem Rev 115:4571–4606
Kavitha M, Kalpana AM (2017) Carbon nanotubes: properties and applications—a brief review i-Manager’s. J Electron Eng 7:1
Kavitha T, Gopalan AI, Lee K-P, Park S-Y (2012) Glucose sensing, photocatalytic and antibacterial properties of graphene–ZnO nanoparticle hybrids. Carbon 50:2994–3000
Khalil I, Julkapli N, Yehye W, Basirun W, Bhargava S (2016) Graphene–gold nanoparticles hybrid—synthesis, functionalization, and application in a electrochemical and surface-enhanced Raman scattering biosensor. Materials (Basel) 9:406
Khalil I, Rahmati S, Julkapli NM, Yehye WA (2018) Graphene metal nanocomposites—recent progress in electrochemical biosensing applications. J Ind Eng Chem 59:425–439
Khan A, Khan AAP, Asiri AM, Abu-Zied BM (2016) Green synthesis of thermally stable Ag-rGO-CNT nano composite with high sensing activity. Compos Part B Eng 86:27–35. https://doi.org/10.1016/j.compositesb.2015.09.018
Khan I, Saeed K, Khan I (2017) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Khaydukova M, Medina-Plaza C, Rodriguez-Mendez ML, Panchuk V, Kirsanov D, Legin A (2017) Multivariate calibration transfer between two different types of multisensor systems. Sensors Actuators B 246:994–1000
Khin MM, Nair AS, Babu VJ, Murugan R, Ramakrishna S (2012) A review on nanomaterials for environmental remediation. Energ Environ Sci 5:8075–8109
Khlobystov AN (2011) Carbon nanotubes: from nano test tube to nano-reactor. ACS Nano 5:9306–9312
Khlobystov AN, Britz DA, GAD B (2005) Molecules in carbon nanotubes. Acc Chem Res 38:901–909
Kim SJ (2009) The effect on the gas selectivity of CNT-based gas sensors by binder in SWNT/Silane sol solution. IEEE Sens J 10:173–177
Kim JS, Kim G-W (2014) New non-contacting torque sensor based on the mechanoluminescence of ZnS: Cu microparticles. Sensors Actuators A Phys 218:125–131
Kimura T, Goto T (2005) Ir–YSZ nano-composite electrodes for oxygen sensors. Surf Coat Technol 198:36–39
Ko T-H, Radhakrishnan S, Seo M-K, Khil M-S, Kim H-Y, Kim B-S (2017) A green and scalable dry synthesis of NiCo2O4/graphene nanohybrids for high-performance supercapacitor and enzymeless glucose biosensor applications. J Alloys Compd 696:193–200
Kocakulak M, Butun I (2017) In: An overview of wireless sensor networks towards internet of things. IEEE, pp 1–6
Komathi S, Muthuchamy N, Lee KP, Gopalan AI (2016) Fabrication of a novel dual mode cholesterol biosensor using titanium dioxide nanowire bridged 3D graphene nanostacks. Biosens Bioelectron 84:64–71
Kondratiev VV, Malev VV, Eliseeva SN (2016) Composite electrode materials based on conducting polymers loaded with metal nanostructures. Russ Chem Rev 85:14
Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26:4637–4648
Kumar N, Srivastava AK, Patel HS, Gupta BK, Varma GD (2015a) Facile synthesis of ZnO-reduced graphene oxide nanocomposites for NO2 gas sensing applications. Eur J Inorg Chem 2015:1912–1923
Kumar R, Al-Dossary O, Kumar G, Umar A (2015b) Zinc oxide nanostructures for NO2 gas–sensor applications: a review. Nano-Micro Lett 7:97–120
Lai C-Y, Foot P, Brown J, Spearman P (2017) A urea potentiometric biosensor based on a thiophene copolymer. Biosensors 7:13
Lakshmi G, Sharma A, Solanki PR, Avasthi DK (2016) Mesoporous polyaniline nanofiber decorated graphene micro-flowers for enzyme-less cholesterol biosensors. Nanotechnology 27:345101
Landfester K (2006) Synthesis of colloidal particles in miniemulsions. Annu Rev Mat Res 36:231–279
Lee D-D, Chung W-Y, Choi M-S, Baek J-M (1996) Low-power micro gas sensor. Sensors Actuators B 33:147–150
Lee Y, Trocchia SM, Warren SB, Young EF, Vernick S, Shepard KL (2018) Electrically controllable single-point covalent functionalization of spin-cast carbon-nanotube field-effect transistor arrays. ACS Nano 12:9922–9930
Li H, Wang Q, Xu J, Zhang W, Jin L (2002) A novel nano-Au-assembled amperometric SO2 gas sensor: preparation, characterization and sensing behavior. Sens Actuators B 87:18–24
Li L-L et al (2008) Room temperature ionic liquids assisted green synthesis of nanocrystalline porous SnO2 and their gas sensor behaviors. Cryst Growth Des 8:4165–4172. https://doi.org/10.1021/cg800686w
Li Y, Fan X, Qi J, Ji J, Wang S, Zhang G, Zhang F (2010) Palladium nanoparticle-graphene hybrids as active catalysts for the Suzuki reaction. Nano Res 3:429–437
Li Z et al (2011) Graphene buffered galvanic synthesis of graphene–metal hybrids. J Mater Chem 21:13241–13246
Li J, Kuang D, Feng Y, Zhang F, Xu Z, Liu M, Wang D (2013) Green synthesis of silver nanoparticles–graphene oxide nanocomposite and its application in electrochemical sensing oftryptophan. Biosens Bioelectron 42:198–206
Liu A, Huang S (2012) A glucose biosensor based on direct electrochemistry of glucose oxidase immobilized onto platinum nanoparticles modified graphene electrode. Sci China Phys Mech Astron 55:1163–1167
Liu Y, Kumar S (2014) Polymer/carbon nanotube nano composite fibers—a review. ACS Appl Mater Interfaces 6:6069–6087
Liu Y et al (2005) Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew Chem Int Ed 44:4782–4785
Liu D, Bruckbauer A, Abell C, Balasubramanian S, Kang D-J, Klenerman D, Zhou D (2006) A reversible pH-driven DNA nanoswitch array. J Am Chem Soc 128:2067–2071
Liu Z, Robinson JT, Sun X, Dai H (2008) PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc 130:10876–10877
Liu Y, Chen M, Mohebbi M, Wang ML, Dokmeci MR (2011) In: The effect of sequence length on DNA decorated CNT gas sensors. IEEE, pp 2156–2159
Liu R et al (2012a) Facile synthesis of au-nanoparticle/polyoxometalate/graphene tricomponent nanohybrids: an enzyme-free electrochemical biosensor for hydrogen peroxide. Small 8:1398–1406. https://doi.org/10.1002/smll.201102298
Liu S et al (2012b) Hydrothermal treatment of grass: a low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer Nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv Mater 24:2037–2041. https://doi.org/10.1002/adma.201200164
Liu S, Zhao N, Cheng Z, Liu H (2015) Amino-functionalized green fluorescent carbon dots as surface energy transfer biosensors for hyaluronidase. Nanoscale 7:6836–6842
Ljungblad J (2009) Antibody-conjugated gold nanoparticles integrated in a fluorescence based biochip
Llobet E (2013) Gas sensors using carbon nanomaterials: a review. Sensors Actuators B 179:32–45
Lorestani F, Shahnavaz Z, Mn P, Alias Y, NSA M (2015) One-step hydrothermal green synthesis of silver nanoparticle-carbon nanotube reduced-graphene oxide composite and its application as hydrogen peroxide sensor. Sensors Actuators B 208:389–398
Lu G, Park S, Yu K, Ruoff RS, Ocola LE, Rosenmann D, Chen J (2011) Toward practical gas sensing with highly reduced graphene oxide: a new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 5:1154–1164
Luo X, Morrin A, Killard AJ, Smyth MR (2006) Application of nanoparticles in electrochemical sensors and biosensors. Electroanal Int J Devoted to Fundam Pract Asp Electroanal 18:319–326
Lupan O, Postica V, Cretu V, Wolff N, Duppel V, Kienle L, Adelung R (2016) Single and networked CuO nanowires for highly sensitive p-type semiconductor gas sensor applications. Phys Status Solidi Rapid Res Lett 10:260–266
Lyson-Sypien B, Radecka M, Rekas M, Swierczek K, Michalow-Mauke K, Graule T, Zakrzewska K (2015) Grain-size-dependent gas-sensing properties of TiO2 nanomaterials. Sensors Actuators B 211:67–76
Ma H, Zhou W, Yuan W, Jie Z, Liu H, Li X (2012) The gas sensing mechanism of the low-dimension carbon composites with metal oxide quantum dots. Phys Proc 32:31–38
Madhurantakam S, Babu KJ, Rayappan JBB, Krishnan UM (2018) Nanotechnology-based electrochemical detection strategies for hypertension markers. Biosens Bioelectron 116:67–80
Madhuvilakku R, Alagar S, Mariappan R, Piraman S (2017) Green one-pot synthesis of flowers-like Fe3O4/rGO hybrid nanocomposites for effective electrochemical detection of riboflavin and low-cost supercapacitor applications. Sens Actuators B 253:879–892
Malic L, Sandros MG, Tabrizian M (2011) Designed biointerface using near-infrared quantum dots for ultrasensitive surface plasmon resonance imaging biosensors. Anal Chem 83:5222–5229
Mandal D, Mishra S, Singh RK (2018) Green synthesized nanoparticles as potential nanosensors. In: Environmental, chemical and medical sensors. Springer, pp 137–164
Manoj D, Saravanan R, Santhanalakshmi J, Agarwal S, Gupta VK, Boukherroub R (2018) Towards green synthesis of monodisperse Cu nanoparticles: an efficient and high sensitive electrochemical nitrite sensor. Sensors Actuators B 266:873–882. https://doi.org/10.1016/j.snb.2018.03.141
Manzetti S (2013) Molecular and crystal assembly inside the carbon nanotube: encapsulation and manufacturing approaches. Adv Manuf 1:198–210
Markets Ma (2017) Gas sensors market by gas type (oxygen, carbon monoxide, carbon dioxide, ammonia, chlorine, hydrogen sulfide, nitrogen oxide, volatile organic compounds, hydrocarbons), technology, end-use application, geography—global forecast 2023. www.marketsandmarkets.com. https://www.marketsandmarkets.com/Market-Reports/gas-sensor-market-245141093.html. 2019
Markets Ma (2019) Biosensors market by type (sensor patch and embedded device), product (wearable and nonwearable), technology (electrochemical and optical), application (POC, home diagnostics, research lab, food & beverages), and geography—global forecast to 2024. www.marketsandmarkets.com. https://www.marketsandmarkets.com/PressReleases/biosensors.asp
Mazloum-Ardakani M, Hosseinzadeh L, Taleat Z (2015) Synthesis and electrocatalytic effect of Ag@ Pt core–shell nanoparticles supported on reduced graphene oxide for sensitive and simple label-free electrochemical aptasensor. Biosens Bioelectron 74:30–36
McNaghten ED, Parkes AM, Griffiths BC, Whitehouse AI, Palanco S (2009) Detection of trace concentrations of helium and argon in gas mixtures by laser-induced breakdown spectroscopy. Spectrochim Acta Part B Atom Spectrosc 64:1111–1118
Mehaffey E, Majid DSA (2017) Tumor necrosis factor-α, kidney function, and hypertension. Am J Physiol Renal Physiol 313:F1005–F1008
Mehrotra P (2016) Biosensors and their applications—a review. J Oral Biol Craniofac Res 6:153–159. https://doi.org/10.1016/j.jobcr.2015.12.002
Michalet X et al (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544
Mirzaei A, Janghorban K, Hashemi B, Bonyani M, Leonardi SG, Neri G (2016) A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram Int 42:18974–18982
Mishra RK, Rajakumari R (2019) Nanobiosensors for biomedical application: present and future prospects. In: Characterization and biology of nanomaterials for drug delivery. Elsevier, pp 1–23
Mishra RK, Nawaz MH, Hayat A, Nawaz MAH, Sharma V, Marty J-L (2017) Electrospinning of graphene-oxide onto screen printed electrodes for heavy metal biosensor. Sensors Actuators B 247:366–373
Molino PJ et al (2014) Influence of biodopants on PEDOT biomaterial polymers: using QCM-D to characterize polymer interactions with proteins and living cells. Adv Mater Interfaces 1:1300122
Momeni S, Nabipour I (2015) A simple green synthesis of palladium nanoparticles with Sargassum alga and their electrocatalytic activities towards hydrogen peroxide. Appl Biochem Biotechnol 176:1937–1949
Monerris MJ, Arévalo FJ, Fernández H, Zon MA, Molina PG (2016) Electrochemical immunosensor based on gold nanoparticles deposited on a conductive polymer to determine estrone in water samples. Microchem J 129:71–77
Moozarm Nia P, Lorestani F, Meng WP, Alias Y (2015) A novel non-enzymatic H2O2 sensor based on polypyrrole nanofibers–silver nanoparticles decorated reduced graphene oxide nano composites. Appl Surf Sci 332:648–656. https://doi.org/10.1016/j.apsusc.2015.01.189
Munoz BC, Steinthal G, Sunshine S (1999) Conductive polymer-carbon black composites-based sensor arrays for use in an electronic nose. Sensor Rev 19:300–305
Muthuchamy N, Atchudan R, Edison TNJI, Perumal S, Lee YR (2018) High-performance glucose biosensor based on green synthesized zinc oxide nanoparticle embedded nitrogen-doped carbon sheet. J Electroanal Chem 816:195–204. https://doi.org/10.1016/j.jelechem.2018.03.059
Muthukrishnan K, Vanaraja M, Boomadevi S, Karn RK, Singh V, Singh PK, Pandiyan K (2016) Studies on acetone sensing characteristics of ZnO thin film prepared by sol–gel dip coating. J Alloys Compounds 673:138–143
Nayak P, Anbarasan B, Ramaprabhu S (2013) Fabrication of organophosphorus biosensor using ZnO nanoparticle-decorated carbon nanotube–graphene hybrid composite prepared by a novel green technique. J Phys Chem C 117:13202–13209. https://doi.org/10.1021/jp312824b
Nia PM, Meng WP, Alias Y (2015) Hydrogen peroxide sensor: uniformly decorated silver nanoparticles on polypyrrole for wide detection range. Appl Surf Sci 357:1565–1572
Nohria R, Khillan RK, Su Y, Dikshit R, Lvov Y, Varahramyan K (2006) Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sensors Actuators B 114:218–222
Olaru A, Bala C, Jaffrezic-Renault N, Aboul-Enein HY (2015) Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit Rev Anal Chem 45:97–105
Oliva N, Jo C, Wang K, Artzi N (2017) Designing hydrogels for on-demand therapy. Acc Chem Res 50:669–679
Omobayo Adio S, Basheer C, Zafarullah K, Alsharaa A, Siddiqui Z (2016) Biogenic synthesis of silver nanoparticles; study of the effect of physicochemical parameters and application as nanosensor in the colorimetric detection of Hg2+ in water. Int J Environ Anal Chem 96:776–788
Pakapongpan S, Poo-Arporn RP (2017) Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater Sci Eng C 76:398–405
Pallas-Areny R, Webster JG (2012) Sensors and signal conditioning. Wiley, New York
Pandey P, Datta M, Malhotra BD (2008) Prospects of nanomaterials in biosensors. Anal Lett 41:159–209
Pandey S, Goswami GK, Nanda KK (2012) Green synthesis of biopolymer–silver nanoparticle nanocomposite: an optical sensor for ammonia detection. Int J Biol Macromol 51:583–589. https://doi.org/10.1016/j.ijbiomac.2012.06.033
Pandey S, Goswami GK, Nanda KK (2013) Green synthesis of polysaccharide/gold nanoparticle nanocomposite: an efficient ammonia sensor. Carbohydr Polym 94:229–234. https://doi.org/10.1016/j.carbpol.2013.01.009
Pandey G, Chaudhari R, Joshi B, Choudhary S, Kaur J, Joshi A (2019) Fluorescent Biocompatible platinum-porphyrin–doped polymeric hybrid particles for oxygen and glucose biosensing. Sci Rep 9:5029
Pankhurst Q, Jones S, Dobson J (2016) Applications of magnetic nanoparticles in biomedicine: the story so far. J Phys D Appl Phys 49:R167–R181
Park S, Kwon O, Lee J, Jang J, Yoon H (2014) Conducting polymer-based nanohybrid transducers: a potential route to high sensitivity and selectivity sensors. Sensors 14:3604–3630
Park RS, Hills G, Sohn J, Mitra S, Shulaker MM, HSP W (2017a) Hysteresis-free carbon nanotube field-effect transistors. ACS Nano 11:4785–4791
Park S, Park C, Yoon H (2017b) Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers 9:155
Penza M, Rossi R, Alvisi M, Cassano G, Signore MA, Serra E, Giorgi R (2008) Surface modification of carbon nanotube networked films with Au nanoclusters for enhanced NO2 gas sensing applications. J Sensors 2008:1–8
Pingarrón JM, Yañez-Sedeño P, González-Cortés A (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53:5848–5866
Ponamoreva ON, Rossinskaya IV, Alferov VA, Vlasova YA, Esikova TZ, Reshetilov AN (2019) Caprolactam detection using two types of microbial biosensors. Water Chem Ecol 118:30–35
Pourreza N, Golmohammadi H, Naghdi T, Yousefi H (2015) Green in-situ synthesized silver nanoparticles embedded in bacterial cellulose nanopaper as a bionanocomposite plasmonic sensor. Biosens Bioelectron 74:353–359
Pramanik K, Sarkar P, Bhattacharyay D, Majumdar P (2018) One step electrode fabrication for direct electron transfer cholesterol biosensor based on composite of polypyrrole, green reduced graphene oxide and cholesterol oxidase. Electroanalysis 30:2719–2730
Qin X, Lu W, Asiri AM, Al-Youbi AO, Sun X (2013) Green, low-cost synthesis of photoluminescent carbon dots by hydrothermal treatment of willow bark and their application as an effective photocatalyst for fabricating Au nanoparticles–reduced graphene oxide nanocomposites for glucose detection. Cat Sci Technol 3:1027–1035
Qu F, Pei H, Kong R, Zhu S, Xia L (2017) Novel turn-on fluorescent detection of alkaline phosphatase based on green synthesized carbon dots and MnO2 nanosheets. Talanta 165:136–142
Radhakrishnan S, Kim SJ (2015) An enzymatic biosensor for hydrogen peroxide based on one-pot preparation of CeO2-reduced graphene oxide nanocomposite. RSC Adv 5:12937–12943
Rai M, Gade A, Gaikwad S, Marcato PD, Durán N (2012) Biomedical applications of nanobiosensors: the state-of-the-art. J Braz Chem Soc 23:14–24
Raj DR, Sudarsanakumar C (2018) Colorimetric and fiber optic sensing of cysteine using green synthesized gold nanoparticles. Plasmonics 13:327–334
Ramesan MT, Sampreeth T (2018) In situ synthesis of polyaniline/Sm-doped TiO2 nanocomposites: evaluation of structural, morphological, conductivity studies and gas sensing applications. J Mater Sci Mater Electron 29:4301–4311
Rotariu L, Lagarde F, Jaffrezic-Renault N, Bala C (2016) Electrochemical biosensors for fast detection of food contaminants—trends and perspective. TrAC Trends Anal Chem 79:80–87
Ruan C et al (2013) One-pot preparation of glucose biosensor based on polydopamine–graphene composite film modified enzyme electrode. Senors Actuators B 177:826–832
Saeed AA, Sánchez JLA, O'Sullivan CK, Abbas MN (2017) DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 118:91–99
Saini R, Hegde K, Brar SK, Verma M (2019) Advances in whole cell-based biosensors in environmental monitoring. In: Tools, techniques and protocols for monitoring environmental contaminants. Elsevier, pp 263–284
Sandeep S, Santhosh AS, Swamy NK, Suresh GS, Melo JS, Chamaraja NA (2018) A biosensor based on a graphene nanoribbon/silver nanoparticle/polyphenol oxidase composite matrix on a graphite electrode: application in the analysis of catechol in green tea samples. New J Chem 42:16620–16629
Sanz V et al (2011) Optimising DNA binding to carbon nanotubes by non-covalent methods. Carbon 49:1775–1781
Shafiee A, Ghadiri E, Kassis J, Pourhabibi Zarandi N, Atala A (2018) Biosensing technologies for medical applications, manufacturing, and regenerative medicine. Curr Stem Cell Rep 4:105–115
Shafiei M, Hoshyargar F, Lipton-Duffin J, Piloto C, Motta N, O’Mullane AP (2015) Conversion of n-type CuTCNQ into p-type nitrogen-doped CuO and the implication for room-temperature gas sensing. J Phys Chem C 119:22208–22216. https://doi.org/10.1021/acs.jpcc.5b06894
Shan L, Freidman D, Kennedy C, Persak W, Lau K (2018) In: Backward compatible connectors for next generation PCIe electrical I/O. IEEE, pp 1798–1804
Shang NG et al (2008) Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv Funct Mater 18:3506–3514
Sharma V, Tiwari P, Mobin SM (2017) Sustainable carbon-dots: recent advances in green carbon dots for sensing and bioimaging. J Mater Chem B 5:8904–8924
Shrivas AG, Bavane RG, Mahajan AM (2007) In: Electronic nose: a toxic gas sensor by polyaniline thin film conducting polymer. IEEE, pp 621–623
Smith MK, Martin-Peralta DG, Pivak PA, Mirica KA (2017) Fabrication of solid-state gas sensors by drawing: an undergraduate and high school introduction to functional nanomaterials and chemical detection. J Chem Educ 94:1933–1938
So M-K, Xu C, Loening AM, Gambhir SS, Rao J (2006) Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol 24:339
Song H, Zhang L, He C, Qu Y, Tian Y, Lv Y (2011) Graphene sheets decorated with SnO2 nanoparticles: in situ synthesis and highly efficient materials for cataluminescence gas sensors. J Mater Chem 21:5972–5977
Song Y, He Z, Hou H, Wang X, Wang L (2012) Architecture of Fe3O4-graphene oxide nanocomposite and its application as a platform for amino acid biosensing. Electrochim Acta 71:58–65
Stankovich S et al (2006) Graphene-based composite materials. Nature 442:282
Su S, Wu W, Gao J, Lu J, Fan C (2012) Nanomaterials-based sensors for applications in environmental monitoring. J Mater Chem 22:18101–18110
Su C-Y, Lan W-J, Chu C-Y, Liu X-J, Kao W-Y, Chen C-H (2016) Photochemical green synthesis of nanostructured cobalt oxides as hydrogen peroxide redox for bifunctional sensing application. Electrochim Acta 190:588–595. https://doi.org/10.1016/j.electacta.2015.12.092
Sun X, Lei Y (2017) Fluorescent carbon dots and their sensing applications. TrAC Trends Anal Chem 89:163–180
Suri K, Annapoorni S, Sarkar AK, Tandon RP (2002) Gas and humidity sensors based on iron oxide–polypyrrole nanocomposites. Sensors Actuators B 81:277–282
Šutka A, Gross KA (2016) Spinel ferrite oxide semiconductor gas sensors. Sensors Actuators B 222:95–105
Tabatabaee RS, Golmohammadi H, Ahmadi SH (2019) Easy diagnosis of jaundice: a smartphone-based nanosensor bioplatform using photoluminescent bacterial nanopaper for point-of-care diagnosis of hyperbilirubinemia. ACS Sensors 4(4):1063–1071
Tagad CK, Dugasani SR, Aiyer R, Park S, Kulkarni A, Sabharwal S (2013) Green synthesis of silver nanoparticles and their application for the development of optical fiber based hydrogen peroxide sensor. Sens Actuators B 183:144–149. https://doi.org/10.1016/j.snb.2013.03.106
Tagad CK, Rajdeo KS, Kulkarni A, More P, Aiyer RC, Sabharwal S (2014) Green synthesis of polysaccharide stabilized gold nanoparticles: chemo catalytic and room temperature operable vapor sensing application. RSC Adv 4:24014–24019. https://doi.org/10.1039/C4RA02972K
Tahernejad-Javazmi F, Shabani-Nooshabadi M, Karimi-Maleh H (2018) Gold nanoparticles and reduced graphene oxide-amplified label-free DNA biosensor for dasatinib detection. New J Chem 42:16378–16383
Tallury P, Malhotra A, Byrne LM, Santra S (2010) Nanobioimaging and sensing of infectious diseases. Adv Drug Deliv Rev 62:424–437
Thangamuthu M, Gabriel W, Santschi C, Martin O (2018) Electrochemical sensor for bilirubin detection using screen printed electrodes functionalized with carbon nanotubes and graphene. Sensors 18:800
Thirumalraj B, Kubendhiran S, Chen S-M, Lin K-Y (2017) Highly sensitive electrochemical detection of palmatine using a biocompatible multiwalled carbon nanotube/poly-l-lysine composite. J Colloid Interface Sci 498:144–152
Tığ GA (2017) Highly sensitive amperometric biosensor for determination of NADH and ethanol based on Au-Ag nanoparticles/poly (l-cysteine)/reduced graphene oxide nanocomposite. Talanta 175:382–389
Tiwari S, Sharma V, Mujawar M, Mishra YK, Kaushik A, Ghosal A (2019) Biosensors for epilepsy management: state-of-art and future aspects. Sensors 19:1525
Tomer AK, Rahi T, Neelam DK, Dadheech PK (2019) Cyanobacterial extract-mediated synthesis of silver nanoparticles and their application in ammonia sensing. Int Microbiol 22:49–58. https://doi.org/10.1007/s10123-018-0026-x
Töppel T, Lausch H, Brand M, Hensel E, Arnold M, Rotsch C (2018) Structural integration of sensors/actuators by laser beam melting for tailored smart components. JOM 70:321–327
Trakhtenberg LI, Gerasimov GN, Gromov VF, Belysheva TV, Ilegbusi OJ (2012) Gas semiconducting sensors based on metal oxide nanocomposites. J Mater Sci Res 1:56
Tran T-T, Mulchandani A (2016) Carbon nanotubes and graphene nano field-effect transistor-based biosensors. TrAC Trends Anal Chem 79:222–232
Tsiafoulis CG, Trikalitis PN, Prodromidis MI (2005) Synthesis, characterization and performance of vanadium hexacyanoferrate as electrocatalyst of H2O2. Electrochem Commun 7:1398–1404
Valsami-Jones E, Lynch I (2015) How safe are nanomaterials? Science 350:388–389
Vasileva P, Donkova B, Karadjova I, Dushkin C (2011) Synthesis of starch-stabilized silver nanoparticles and their application as a surface plasmon resonance-based sensor of hydrogen peroxide. Colloids Surf A Physicochem Eng Asp 382:203–210
Venditti I, Fratoddi I, Russo MV, Bearzotti A (2013) A nanostructured composite based on polyaniline and gold nanoparticles: synthesis and gas sensing properties. Nanotechnology 24:155503. https://doi.org/10.1088/0957-4484/24/15/155503
Vignesh RH, Sankar KV, Amaresh S, Lee YS, Selvan RK (2015) Synthesis and characterization of MnFe2O4 nanoparticles for impedometric ammonia gas sensor. Sensors Actuators B 220:50–58
Vilian ATE, Chen S-M (2014) Simple approach for the immobilization of horseradish peroxidase on poly-l-histidine modified reduced graphene oxide for amperometric determination of dopamine and H2O2. RSC Adv 4:55867–55876
Vukojević V, Djurdjić S, Ognjanović M, Fabian M, Samphao A, Kalcher K, Stanković DM (2018) Enzymatic glucose biosensor based on manganese dioxide nanoparticles decorated on graphene nanoribbons. J Electroanal Chem 823:610–616
Wahab AW, Karim A, La Nafie N, Sutapa IW (2018) Synthesis of silver nanoparticles using Muntingia calabura L. leaf extract as bioreductor and applied as glucose nanosensor. Oriental J Chem 34:3088–3094
Wang Z, Dai Z (2015) Carbon nanomaterial-based electrochemical biosensors: an overview. Nanoscale 7:6420–6431
Wang J, Xu D, Kawde A-N, Polsky R (2001) Metal nanoparticle-based electrochemical stripping potentiometric detection of DNA hybridization. Anal Chem 73:5576–5581
Wang Y, Jiang X, Xia Y (2003) A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J Am Chem Soc 125:16176–16177
Wang YD, Djerdj I, Antonietti M, Smarsly B (2008) Polymer-assisted generation of antimony-doped SnO2 nanoparticles with high crystallinity for application in gas sensors. Small 4:1656–1660. https://doi.org/10.1002/smll.200800644
Wang C, Yin L, Zhang L, Xiang D, Gao R (2010) Metal oxide gas sensors: sensitivity and influencing factors. Sensors 10:2088–2106
Wang P, Jornet JM, Malik MGA, Akkari N, Akyildiz IF (2013) Energy and spectrum-aware MAC protocol for perpetual wireless nanosensor networks in the Terahertz Band. Ad Hoc Networks 11:2541–2555
Wang F et al (2016a) A highly sensitive gas sensor based on CuO nanoparticles synthesized via a sol–gel method. RSC Adv 6:79343–79349
Wang L et al (2016b) Facile, green and clean one-step synthesis of carbon dots from wool: application as a sensor for glyphosate detection based on the inner filter effect. Talanta 160:268–275
Wang T et al (2016c) A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett 8:95–119. https://doi.org/10.1007/s40820-015-0073-1
Wang R, Wang X, Sun Y (2017) One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sensors Actuators B 241:73–79
Wang J, Wang X, Tang H, Gao Z, He S, Li J, Han S (2018) Ultrasensitive electrochemical detection of tumor cells based on multiple layer CdS quantum dots-functionalized polystyrene microspheres and graphene oxide–polyaniline composite. Biosens Bioelectron 100:1–7
Wanna Y, Srisukhumbowornchai N, Tuantranont A, Wisitsoraat A, Thavarungkul N, Singjai P (2006) The effect of carbon nanotube dispersion on CO gas sensing characteristics of polyaniline gas sensor. J Nanosci Nanotechnol 6:3893–3896
Wisitsoraat A, Tuantranont A, Comini E, Sberveglieri G, Wlodarski W (2009) Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Films 517:2775–2780
Wong KKL, Tang Z, Sin JKO, Chan PCH, Cheung PW, Hiraoka H (1995) In: Study on selectivity enhancement of tin dioxide gas sensor using non-conducting polymer membrane. IEEE, pp 42–45
Wright KD (2017) Functionalization of carbon materials with metals
Wu S et al (2017) Development of glucose biosensors based on plasma polymerization-assisted nanocomposites of polyaniline, tin oxide, and three-dimensional reduced graphene oxide. Appl Surf Sci 401:262–270
Wu J et al (2018) Two-dimensional molybdenum disulfide (MoS2) with gold nanoparticles for biosensing of explosives by optical spectroscopy. Sens Actuators B 261:279–287
Xiang Q, Meng G, Zhang Y, Xu J, Xu P, Pan Q, Yu W (2010) Ag nanoparticle embedded-ZnO nanorods synthesized via a photochemical method and its gas-sensing properties. Sens Actuators B 143:635–640
Xu F, Li X, Shi Y, Li L, Wang W, He L, Liu R (2018a) Recent developments for flexible pressure sensors: a review. Micromachines 9:580
Xu K, Fu C, Gao Z, Wei F, Ying Y, Xu C, Fu G (2018b) Nanomaterial-based gas sensors: a review. Instrum Sci Technol 46:115–145
Xue L, Wang W, Guo Y, Liu G, Wan P (2017) Flexible polyaniline/carbon nanotube nanocomposite film-based electronic gas sensors. Sensors Actuators B 244:47–53
Yamazoe N, Shimanoe K (2008) Theory of power laws for semiconductor gas sensors. Sensors Actuators B 128:566–573
Yan J, Wei T, Shao B, Fan Z, Qian W, Zhang M, Wei F (2010) Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 48:487–493
Yáñez-Sedeño P, Campuzano S, Pingarrón J (2017) Carbon nanostructures for tagging in electrochemical biosensing: a review. J Carbon Res 3:3
Yang C, Denno ME, Pyakurel P, Venton BJ (2015) Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: a review. Anal Chim Acta 887:17–37
Yola ML, Gupta VK, Eren T, Şen AE, Atar N (2014) A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim Acta 120:204–211
Yoo I-H, Kalanur SS, Seo H (2019) Deposition of Pd nanoparticles on MWCNTs: Green approach and application to hydrogen sensing. J Alloys Compd 788:936–943. https://doi.org/10.1016/j.jallcom.2019.02.298
Yoon HJ et al (2013) Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat Nanotechnol 8:735
Yu X, Zhang W, Zhang P, Su Z (2017) Fabrication technologies and sensing applications of graphene-based composite films: advances and challenges. Biosens Bioelectron 89:72–84
Yuan G-L, Kuramoto N (2003) Synthesis and chiroptical properties of optically active poly (N-alkylanilines) doped and intertwined with dextran sulfate in aqueous solution. Macromolecules 36:7939–7945
Yuan GL, Kuramoto N (2004) Synthesis of helical polyanilines using chondroitin sulfate as a molecular template. Macromol Chem Phys 205:1744–1751
Yuan W, Shi G (2013) Graphene-based gas sensors. J Mater Chem A 1:10078–10091
Yusan S, Rahman MM, Mohamad N, Arrif TM, Latif AZA, Ma MA, Nik W (2018) Development of an amperometric glucose biosensor based on the immobilization of glucose oxidase on the Se-MCM-41 mesoporous composite. J Anal Methods Chem 2018:1–8
Zaporotskova IV, Boroznina NP, Parkhomenko YN, Kozhitov LV (2016) Carbon nanotubes: sensor properties. A review. Mod Electron Mater 2:95–105
Zaretski AV et al (2016) Metallic nanoislands on graphene as highly sensitive transducers of mechanical, biological, and optical signals. Nano Lett 16:1375–1380
Zhang S, Wang N, Yu H, Niu Y, Sun C (2005) Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 67:15–22
Zhang T, Wang W, Zhang D, Zhang X, Ma Y, Zhou Y, Qi L (2010) Biotemplated synthesis of gold nanoparticle–bacteria cellulose nanofiber nanocomposites and their application in biosensing. Adv Func Mater 20:1152–1160
Zhang H, Meng Z, Wang Q, Zheng J (2011) A novel glucose biosensor based on direct electrochemistry of glucose oxidase incorporated in biomediated gold nanoparticles–carbon nanotubes composite film. Sensors Actuators B Chem 158:23–27. https://doi.org/10.1016/j.snb.2011.04.057
Zhang Y, Gao G, Qian Q, Cui D (2012a) Chloroplasts-mediated biosynthesis of nanoscale Au-Ag alloy for 2-butanone assay based on electrochemical sensor. Nanoscale Res Lett 7:475
Zhang Y et al (2012b) One-pot green synthesis of Ag nanoparticles-graphene nanocomposites and their applications in SERS, H2O2, and glucose sensing. RSC Adv 2:538–545. https://doi.org/10.1039/C1RA00641J
Zhang P, Zhang X, Zhang S, Lu X, Li Q, Su Z, Wei G (2013) One-pot green synthesis, characterizations, and biosensor application of self-assembled reduced graphene oxide–gold nanoparticle hybrid membranes. J Mater Chem B 1:6525–6531. https://doi.org/10.1039/C3TB21270J
Zhang H, Feng J, Fei T, Liu S, Zhang T (2014) SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sensors Actuators B Chem 190:472–478
Zhang H, Yu L, Li Q, Du Y, Ruan S (2017) Reduced graphene oxide/α-Fe2O3 hybrid nanocomposites for room temperature NO2 sensing. Sensors Actuators B Chem 241:109–115. https://doi.org/10.1016/j.snb.2016.10.059
Zhang NMY et al (2019) One-step synthesis of cyclodextrin-capped gold nanoparticles for ultra-sensitive and highly-integrated plasmonic biosensors. Sensors Actuators B Chem 286:429–436
Zhao Z-Y, Wang M-H, Liu T-T (2015) Tribulus terrestris leaf extract assisted green synthesis and gas sensing properties of Ag-coated ZnO nanoparticles. Mater Lett 158:274–277. https://doi.org/10.1016/j.matlet.2015.05.155
Zhou X, Zeng Y, Liyan C, Wu X, Yoon J (2016) A fluorescent sensor for dual-channel discrimination between phosgene and a nerve-gas mimic. Angew Chem Int Ed 55:4729–4733
Zor E, Saglam ME, Akin I, Saf AO, Bingol H, Ersoz M (2014) Green synthesis of reduced graphene oxide/nanopolypyrrole composite: characterization and H2O2 determination in urine. RSC Adv 4:12457–12466
Zou N, Wei X, Zong Z, Li X, Wang Z, Wang X (2019) A novel enzymatic biosensor for detection of intracellular hydrogen peroxide based on 1-aminopyrene and reduced graphene oxides. J Chem Sci 131:28
Acknowledgments
The authors would like to acknowledge their respective departments for their support while drafting this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jeevanandam, J., Kaliyaperumal, A., Sundararam, M., Danquah, M.K. (2020). Nanomaterials as Toxic Gas Sensors and Biosensors. In: Inamuddin, Asiri, A. (eds) Nanosensor Technologies for Environmental Monitoring. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-45116-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-45116-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45115-8
Online ISBN: 978-3-030-45116-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)