Abstract
Green route for silver nanoparticle synthesis has gained increasing attention. Cyanobacteria are one of the promising organisms to produce a number of secondary metabolites that are capable of reducing silver ions to small-sized silver nanoparticles. In the present study, we employed an aqueous extract of the cyanobacterium Haloleptolyngbya alcalis KR2005/106 isolated from a soda lake for biosynthesis of silver nanoparticles (AgNPs). The extract acted as a reducing agent for AgNPs synthesis and resulted formation of nanoparticles < 50 nm in size. In this study, synthesis of AgNPs obtained only in the sample exposed to photosynthetically active radiation (PAR) while the synthesis of AgNPs was not observed in the samples kept in dark. The biogenic fabrication of AgNPs was carried out by optimizing several governing parameters such as concentration of the silver nitrate solution, pH, temperature, and amount of biomass. Results obtained through different analytical techniques revealed that cyanobacterial taxon H. alcalis isolated from saline-alkaline habitat is a potential candidate for biosynthesis of optimum-sized spherical AgNPs. Surface plasmon resonance (SPR) property of AgNPs was exploited for aqueous ammonia sensing and revealed that AgNPs synthesized using aqueous extract of cyanobacterium H. alcalis could be employed for colorimetric detection of dissolved ammonia for monitoring quality of water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology, a rapidly growing field has become an integral part of modern technology due to nanoparticles applications in pharmaceutical, industrial, and biotechnological purposes owing to their unique physical and chemical properties (Chernousova and Epple 2013; Schröfel et al. 2014). Generally, various physical, chemical, and biological methods are deployed for the synthesis of silver nanoparticles (Singh et al. 2015). In the recent years, researches have been focussing more on green synthesis of silver nanoparticles. Biosynthesis of nanoparticles using plants needs large area and relatively long growth periods, which ultimately limits the feasibility of the synthesis process (Rösken et al. 2014). Currently, amongst green chemistry approaches, cyanobacteria, one of the largest, diverse, and most important groups of photoautotrophic bacteria on earth have attracted special attention for synthesis of nanomaterials due to high growth rate and biomass productivity (Husain et al. 2015; Lengke et al. 2007). A number of cyanobacterial taxa belonging to order Nostocales (Morsy et al. 2014), Oscillatoriales, and Synchococcales (Lengke et al. 2007; Sudha et al. 2013; Roychoudhury et al. 2016) have been used for the synthesis of AgNPs. In case of Nostoc commune, water soluble extracellular polysaccharide was employed for the fabrication of AgNPs without using surfactant and capping agent (Morsy et al. 2014). Dry biomass of a marine cyanobacterium Phormidium fragile was used to synthesize silver nanoparticles. This approach is apparently cost effective due to reduction in the use of heavy energy and nutrient requirements for maintaining the algal cells in the bioreactors (Satapathy and Shukla 2017). In another study, the culture supernatant of eight cyanobacterial strains was screened for their ability to synthesize AgNPs in light and dark conditions (Patel et al. 2015). Previous study described the biological synthesis of AgNPs using a proteinaceous pigment phycocyanin extracted from Nostoc linckia as reducing agent (El-Naggar et al. 2017). Recently, an aqueous extract of the Nostoc linckia was used to synthesize AgNPs (Vanlalveni et al. 2018). The nanoparticles synthesized using whole cell is very complex process and cost-ineffective. Hence, use of cyanobacterial extract for the synthesis of nanoparticles is being widely practiced days (Sonker et al. 2017).
Ammonia, a colorless gas with a typical pungent smell, is widely used in various industries such as chemicals, textiles, fertilizers, cosmetics, automobiles, and paper product industries (Malins et al. 1999). It is considered as a major pollutant of the soil and water due to its harmful effects on aquatic animals and terrestrial plants (Srivastava and Jain 2015). This is also known to cause deleterious effects on eyes, nose, throat as well as skin, which can lead to vomiting, headache, pneumonia, and even death (Wetchakun et al. 2011). An exposure of ammonia above 25 ppm is known to be harmful leading to chronic illness (Besar et al. 2017; Sivalingam and Balasubramanian 2017). Thus, leakage of ammonia must be monitored for the sustenance of ambient environment. In this framework, various types of ammonia sensors, such as metal oxide semiconductor sensors, conductive plastic substrate sensors, electrochemical sensors, and surface acoustic wave (SAW) sensors have been developed (Besar et al. 2017; Li et al. 1995; Tang et al. 2014; Timmer et al. 2005). The major drawback of all techniques was their applicability only for ammonia gas and not for ammonia dissolved in water. However, there are a few spectrophotometric methods known to detect dissolved ammonia. The best known coloration methods are Nessler reaction and Berthelot reaction (Timmer et al. 2005). The Nessler reagent in the Nessler reaction is considered to be toxic that also aids in the formation of non-soluble reaction product, basic mercury(II) amido iodide, making them incapable of implementing as detection system (Ghauch et al. 1999; Timmer et al. 2005). On the other hand, Berthelot reaction makes use of less toxic chemicals and the reactant products produced are soluble in water. Thus, this strategy was suitable to be applied in miniaturized analysis systems. The major drawback that withheld this technique was its slower kinetics of reaction. Thus, this technique is still under development to detect ammonia dissolved in water (Timmer et al. 2005). Nowadays, amongst the mentioned techniques, optical sensors based on metallic nanoparticles have shown to be beneficial for analysis of liquid ammonia. Thus, metallic nanoparticles can be exploited due to its simplicity, high sensitivity, low cost, rapidity, and use of less complicated apparatus (Pandey et al. 2012). AgNPs synthesized by phycocyanin extract from a cyanobacterium (Nostoc linckia) have potential application in the detection of copper ions in diverse water environment (Wei et al. 2018). Similarly, AgNPs can be synthesized using phenolic compounds such as tannic acid in the presence of UV radiation at room temperature, which successfully sensed ammonia present in water (Ritthichai and Pimpan 2017). To the best of the authors’ knowledge, sensing of aqueous ammonia by biosynthesized AgNPs using extract of extreme tolerant cyanobacteria has not been carried out so far.
Extreme tolerant microorganisms are known for uniqueness in physiology, biochemistry, and can act as potential candidate for production of biological active components (Mesbah and Wiegel 2012). In general, there is a paucity of research on synthesis of AgNPs under photosynthetically active radiation (PAR) using cyanobacterial taxa inhabit extreme conditions (Singh et al. 2017). Thus, in the present study, green synthesis of AgNPs under PAR has been achieved using aqueous extract of the cyanobacterium Haloleptolyngbya alcalis KR2005/106 isolated from extreme environment and synthesized AgNPs characterized using different techniques and physicochemical parameters were optimized for their biosynthesis. Moreover, the sensing properties of the AgNPs solution were tested against increasing dissolved ammonia concentrations in water.
Material and methods
Material
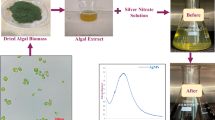
Haloleptolyngbya alcalis KR2005/106 (Fig. 1a), a non-heterocystous filamentous cyanobacterium belonging to order Oscillatoriales, has been isolated from soda lake Nakuru, Kenya (Dadheech et al. 2012), and clonal culture is being maintained in cyanobacterial culture collection at Central University of Rajasthan, Bandarsindri (Ajmer), Rajasthan, India, in BG-11 medium (Rippka et al. 1979) at 25 °C under 14 h:10 h light–dark regime.
a Microphotograph of cyanobacterial taxon Haloleptolyngbya alcalis used for biosynthesis of AgNPs, b visual observation of AgNPs synthesis, c UV-visible spectra of extract, reaction in dark and under exposure of PAR, d UV-visible spectra of reaction mixture at different temperature, e UV-visible spectra of synthesized AgNPs recorded at different time, f pH, g concentration of silver nitrate, and h biomass concentrations
Methods
Production of biomass and extract preparation
The cultures were inoculated in 400 ml of BG-11 media in 1 L Erlenmeyer flask for the biomass production with initial OD of 0.08. The inoculated cultures were incubated under white fluorescent light (6.1 W/m2) with 14 h:10 h light–dark regime at 25 °C. The cultures were hand shaked three times in a day to avoid shelf shading. The biomass of the organism was harvested from 15 days old culture and washed three times with sterile double distilled water by centrifugation at 5000 g for 10 min. The pellet was transferred to the sterile watch glass and kept under vacuum in a desiccator to obtain dried biomass (Patel et al. 2015). The dry cyanobacterial biomass of 48 mg was obtained after 15-day cultivation in 100 ml BG-11 medium. Twenty milligrams of dried biomass was dissolved in 20 ml of sterile double distilled water. The mixture was agitated at 40 °C at 1000 rpm under dark condition for 24 h and filtered through Whatman filter no. 1 and stored at 4 °C for further use (Husain et al. 2015).
Silver nanoparticles biosynthesis
The aqueous cell extract was distributed equally (5 ml each) into three round bottom flasks for biosynthesis of AgNPs. To one flask, silver nitrate (AgNO3) was added to attain a final concentration of 1 mM and a second flask contained only cell extract. The reaction mixture was stirred at 600 rpm under PAR (6.1 W/m2cool fluorescent light) at 30 °C. The third flask containing cyanobacterial extract and 1 mM AgNO3 was subjected to complete darkness by wrapping it with aluminium foil. One millimolar AgNO3 solution without cyanobacterial biomass extract was also kept in parallel under identical conditions as control. After 2 h, appropriate aliquots were withdrawn and the synthesis of AgNPs was confirmed by UV-Vis spectroscopy in the range of 300–700 nm using a HALO-30, UV-Visible double beam spectrophotometer, Dynamica (Husain et al. 2015).
Optimization of physicochemical parameters for AgNPs synthesis
Temperature
The effect of temperature was investigated on AgNPs biosynthesis reaction using different temperature ranges (30, 40, 50, 60, 70, and 80 °C). The reactions were conducted with a constant extract concentration of 1 mg/ml and 1 mM AgNO3. The reaction mixture was stirred at 600 rpm for 2 h under PAR at different temperature as aforementioned. The resulting AgNPs synthesis was analyzed using UV-Visible spectrophotometer.
Time
The impact of time on the AgNPs biosynthesis reaction was analyzed to find out initiation time (0, 30, 60, 90, and 120 min) and completion time (0, 12, 24, 36, and 48 h) of the reaction. Here, the temperature 30 °C was employed for the reaction with constant extract (1 mg/ml) and AgNO3 concentration (1 mM). The determination of AgNPs synthesis was done on UV-Visible spectrophotometer.
pH
The pH of the reaction was optimized by varying the pH (5, 6, 7, and 8) of the reaction mixture. Herein, the reaction was conducted for 2 h by keeping other parameters same as mentioned in the previous section. Thereafter, the resulting AgNPs production was analyzed using UV-Visible spectrophotometer.
Silver nitrate concentration
The reaction was performed with different concentrations of AgNO3 (0.5–2 mM) at optimum pH 7 with all other parameters as mentioned above. The synthesized AgNPs samples were scanned using UV-Visible spectrophotometer.
Biomass concentration
Different concentrations of dry biomass (0.5–2 mg/ml) were taken to prepare aqueous extract. Thereafter, the extract was used to synthesize AgNPs at optimum 1 mM AgNO3 concentration and all other parameters were same as employed in the aforementioned reactions. The resulting AgNPs were analyzed using UV-Visible spectrophotometer.
Characterization of biosynthesized AgNPs
In addition to UV-Vis spectrophotometry, the hydrodynamic size of the biosynthesized AgNPs and zeta potential were determined using Dynamic Light Scattering (DLS) particle size analyzer (Zetasizer Nano ZSP, Malvern, UK). The actual size and shape of AgNPs was determined by HRTEM (high-resolution transmission electron microscopy) equipped with energy dispersive spectroscopy (FEI Technai T20 G2, DTU, Denmark). The crude aqueous extract and biosynthesized AgNPs were subjected to Fourier transform infrared (FTIR) spectrometric analysis and spectra were recorded in the wave number frequency ranged from 4000 to 500 cm−1 using a Perkin Elmer FTIR instrument (Model No. L1860116). Crystalline nature of the AgNPs was analyzed by XRD (PANalytical-Empyrean).
Ammonia sensing by AgNPs
The biosynthesized AgNPs by aqueous extract of H. alcalis KR2005/106 were subjected to the sensing of water soluble ammonia. The different concentrations of ammonia solution (0, 10, 25, 50, 100, 150, 200, 300, 400, and 500 ppm) were prepared from 25% (250,000 ppm) of ammonia solution. In brief, 950 μl of silver nanoparticles (10 μg/ml) was mixed with 50 μl of ammonia. The sensing properties of the AgNPs were tested by monitoring the changes in localized surface plasmon resonance (LSPR) position and amplitude with a UV-Vis spectrophotometer (Pandey et al. 2012).
Results and discussion
Primary confirmation of AgNPs biosynthesis
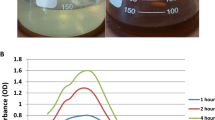
To evaluate the effect of PAR and darkness on biosynthesis of AgNPs using aqueous extract of cyanobacterium Haloleptolyngbya alcalis, samples were kept in white fluorescent light and dark by wrapping with aluminium foil to ensure complete darkness. The formation of nanoparticles was indicated by the change in color of the reaction from pale yellow to brown in 120 min (Fig. 1b). The synthesis of AgNPs was confirmed using UV-Vis spectrophotometry within the range of 700–300 nm. The characteristic absorbance peak between 450 and 400 nm denoted the specific surface plasmon resonance (SPR) of the AgNPs obtained only in the sample exposed to PAR while the synthesis of AgNPs was not observed in the samples kept in dark (Fig. 1c). This was found in accordance with previous study, where AgNPs synthesis assessed by employing cyanobacterial biomass and cell-free culture under light and dark conditions (Patel et al. 2015). It has been revealed that the requirement of light and dark conditions for AgNPs synthesis mainly depend on cyanobacterial strains by explaining that different strains produce diverse compounds capable of AgNPs synthesis, some of them require light activation while others do not (Patel et al. 2015). A single SPR band depicted the formation of spherical AgNPs, while increase in the number of SPR peaks may lead to decrease in the symmetry of nanoparticles (Mie 1908). Furthermore, SPR band depends on the size and molecules responsible for stabilization particles absorbed on the surface along with the dielectric constant of the reduction medium (El-Naggar et al. 2017; Mie 1908). The detection of single peak at 440 nm in the present study corresponded to spherical-shaped nanoparticles (Prathna et al. 2011). However, the SPR band can shift to longer wavelengths due to the formation of larger particles (El-Naggar and Abdelwahed 2014). It has been reported that only the extracellular compound produced by Chlorella sp. 142-5-2 was responsible for the synthesis of AgNPs under PAR. However, synthesis of AgNPs neither observed under light nor dark in conditions when live biomass was employed (Patel et al. 2015). Nevertheless, it is evident from our study that water soluble active compounds present in the dry biomass of H. alcalis are responsible for biosynthesis of AgNPs under exposure of PAR.
Optimization of AgNPs synthesis
In order to use bacteria for synthesis of nanoparticles in industrial scale, the yield and production rate are important issues to be considered (Singh et al. 2015). This could be achieved by optimizing several parameters influencing synthesis of AgNPs. Earlier study revealed that the governing parameters of the reaction such as concentration of the extract and silver nitrate, reaction time, temperature, and pH of the reaction played a vital role in synthesis of AgNPs (Iravani 2014). The optimum condition for synthesis of AgNPs by aqueous extract of H. alcalis was determined employing different physical conditions such as temperature, reaction time, amount of biomass, and pH.
The effect of temperature on biosynthesis of AgNPs was evaluated by performing reactions at different temperature conditions (30, 40, 50, 60, 70, and 80 °C). The reaction was conducted at silver nitrate concentration of 1 mM. It is evident that the AgNPs formation elevated with the increasing temperature and the maximum AgNPs synthesis was found at 80 °C temperature (Fig. 1d). This could be attributed to the fact that high temperature imparts increased kinetic energy that lead to faster synthesis rate (Birla et al. 2013). Notwithstanding, all experiments related to optimization of AgNPs conducted at 30 °C as it was the initiating temperature for biosynthesis of nanoparticles.
To optimize the reaction time, a time variation study was executed with 1 mM AgNO3 concentration at 30 °C. It is clearly depicted from the UV-Vis spectra of the nanoparticles that the AgNPs formation started after 90 min with continuous exposure under PAR. It was observed that the characteristic absorbance of AgNPs increased with an advancement in time of the reaction and reached to saturation point after 36 h and the synthesis did not show any further change in the absorbance peak after 36 h (Fig. 1e).
The optimum pH for biosynthesis of AgNPs varies with extract of a particular microalgae as optimum green synthesis of silver nanoparticles was observed at pH 7 (Singh et al. 2017), whereas in one of the study, the optimum pH 8 was recorded for the polysaccharide-mediated green synthesis of AgNPs (Vasquez et al. 2016). Furthermore, in some cases, maximum synthesis was observed at pH 10 with significant enhancement in the yield of silver nanoparticles within short time period (Gurunathan et al. 2009). During the present study, the maximum synthesis of AgNPs was attained at pH 7 when compared to other pH conditions (Fig. 1f).
Various living systems such as plant and microorganisms produce distinct composition of secondary metabolites and other components such as proteins in varying quantities that affect the nanoparticles synthesis (Patra and Baek 2014). The effect of component concentration available in aqueous extract was studied by taking 0.5, 1, 1.5, and 2 mg/ml of cyanobacterial biomass. It was found that the extract prepared with biomass of 1.5 mg/ml was optimum for reduction of 1 mM AgNO3 solution. However, no distinct peak indicating biosynthesis of AgNPs was displayed in the extract prepared using cyanobacterial biomass below (0.5 and 1.0 mg/ml) and above (2.0 mg/ml) to the optimum biomass. (Fig. 1h). Moreover, concentration of the substrate (AgNO3) was optimized to make the reaction more productive and economical. Thus, different concentrations of AgNO3 solution were investigated with a fixed extract concentration (1.5 mg biomass/ml). The silver nitrate solution at a concentration of 0.5 mM and 1 mM showed minimum and maximum AgNPs production, respectively. The AgNPs synthesis decreased upon increasing the AgNO3 concentration beyond 1 mM (Fig. 1g). However, photo-induced green synthesis of silver nanoparticles has been reported by increasing AgNO3 concentration up to 4 mM and increasing extract up to 5% (v/v) in the reaction mixture and synthesis was decreased beyond 5% (Singh et al. 2017).
Characterization of biosynthesized AgNPs
The size distribution and surface charge of the biosynthesized AgNPs were detected through DLS analysis. The surface charge of the nanoparticles also plays a vital role for interaction with the macromolecules and biochemical pathways in the cell and is known to play a critical role in biomedical sciences, drug delivery as well as biosensors (Mukherjee et al. 2014; Nayak et al. 2015). Particles with zeta potentials greater than + 30 mV and less than − 30 mV are considered stable for colloidal dispersion in the absence of stearic stabilization (Gurunathan 2015). In the present study, surface charge of the AgNPs was found to be − 41 ± 6 mV. Earlier, surface charge of green synthesized AgNPs using Sargassum siliquosum (a brown algae) was observed in the range of − 50.0 ± 3.49 mV (Vasquez et al. 2016).
The FTIR spectrum of the biosynthesized AgNPs was recorded to determine the components and functional groups present in aqueous extract of H. alcalis responsible for the reduction and capping of the AgNPs. The interaction of phytochemicals with the metallic nanoparticles showed characteristic peaks at 3066, 2330, 1550, 748, and 1730 cm−1 stretch (Fig. 2a). The peak at 3066 cm−1 depicted the presence of O–H bond indicating the involvement of H-bonded phenols and alcohols in AgNPs, while the peaks at 2330 cm−1 corresponded to the nitrile groups in AgNPs. Generally, phenolic compounds easily get converted to quinones after oxidation and the shift from 1580 to 1600 cm−1 may indicate the quinoid compound for the reduction of Ag+ ions to AgNPs (Naz et al. 2014). The FTIR spectrum displayed clear absorption bands at the range of 1730 cm−1, which showed the presence of amide group due to C=O stretch. Thus, the presence of amide group indicated the probable role of proteins in biosynthesis AgNPs with strong binding ability suggesting the formation of a layer around the particles and acting as a capping agent, thereby preventing agglomeration and providing stability to the medium (Baun et al. 2008; Garg 2012). The characteristic peaks at 748 cm−1 corresponded to phenyl group. Besides proteins, water soluble polysaccharides from cyanobacteria were found to be responsible for green synthesis of AgNPs (Morsy et al. 2014). Also, C=O stretching of quinine structure appeared at 1616 cm−1. Other vibrations at 1000 cm−1 and 2165 cm−1 attributes to the C–O groups stretching in ester and C=C stretching of aromatic ring respectively. Overall, it can be confirmed that phenolic compounds and proteins available in aqueous extract of H. alcalis helped in the reduction and capping of the AgNPs. It is well known that proteins are capable of binding to the AgNPs by free amines or cysteine groups present in the saponins, phenolic acids, or quinones support the stabilization of the formed particles (Pant et al. 2013; Saranyaadevi et al. 2014).
The X-ray diffraction pattern (XRD) of the synthesized AgNPs showed distinct diffraction peaks at 2θ values of 37.9°, 64.3°, and 76.5° (Fig. 2b) correlated to the Bragg’s reflections of metallic AgNPs crystallized in a face-centered cubic structure with basal (111), (220), and (311) lattice planes, respectively (Roy et al. 2015; Yu et al. 2012). The intense peaks further corroborated that the particles synthesized were crystalline in nature and also efficiently reduced by the extract. The role of extract in the reduction process can be discerned from other peaks such as 31.8° and 46.2° attributing to the bioorganic compounds on the surface of the AgNPs (Suvith and Philip 2014). Hence, it can be concluded that the biomolecules in the aqueous extract of investigated cyanobacterial taxon acted as a reducing agent for the biosynthesis of nanoparticles.
The AgNPs biosynthesized in the present study showed spherical shape in TEM examination (Fig. 3a, b) that are usually common to most of the microbial-mediated synthesis (Shivaji et al. 2011). In previous study, thirty cyanobacterial strains were screened for tailoring AgNPs, where most of the strains synthesized spherical AgNPs in the size ranging from 30 to 88 nm (Husain et al. 2015).
The actual size of the AgNPs synthesized by the extract of H. alcalis was found to be in the range 30–40 nm and showed invariable spherical shape. Thus, it could be a potential candidate for spherical and small-sized AgNPs production. The selected area electron diffraction (SAED) pattern with bright circular rings confirmed the crystalline nature of the AgNPs (Fig. 3c). The energy dispersive spectrum revealed the presence of elemental form of AgNO3 (Fig. 3d). The sharp peak at 3 Kev represents the presence of metallic silver due to surface plasmon resonance property (Das et al. 2013).
Ammonia sensing
High amount of ammonia dissolved in water produce deleterious effects to living organisms (Ostrensky and Wasielesky Jr 1995). Nowadays, it is desirable to develop a rapid and highly sensitive technique to detect ammonia dissolved in water bodies (Pandey et al. 2012). Recently, a technique was developed to detect ammonia in water by the selective formation of blue indophenol dye through modified Berthelot’s reaction on porous paper (Cho et al. 2018). In this context, UV-Vis spectroscopy was employed to analyze efficacy of synthesized AgNPs towards sensing of ammonia in aqueous solution. It is worth to mention that AgNPs biosynthesized by the extract of a cyanobacterium was used for the first time for sensing soluble ammonia in water. It was observed that there was a strong blue shift in the absorbance upon interaction of ammonia with the AgNPs in the solution (Fig. 4a). The color of the solution was also changed from brown to yellow within few seconds after adding ammonia. Earlier studies reported that the change in color and shift in SPR band of AgNPs mixed with ammonia occurred due to the formation of silver-ammonia complex (Ag(NH3)2+) (Amirjani and Fatmehsari 2018; Dubas and Pimpan 2008). The shift in SPR band can be attributed to the changes in inter-particle distance and dielectric constant of surrounding medium (Link and El-Sayed 2000). The absorbance peak of the AgNPs was found to be at 445 nm in the absence of ammonia, which eventually shifted to 426 nm and also decreased in the presence of ammonia illustrating the significant changes in the absorbance spectra. The absorbance peak intensity at 426 nm showed decreasing trend with increase in ammonia concentrations (0–500 ppm) (Fig. 4b). In one of the study, AgNPs synthesized using guar gum polysaccharides was investigated to sense aqueous ammonia. It was noted that SPR peak intensity at 440 nm shifted to 413 nm and also decreased as the ammonia content is increased (Pandey et al. 2012). Tannic acid was used as a reducing agent to synthesize AgNPs under UV exposure and showed two characteristic peak at around 370 nm and 452 nm. After adding ammonia, a new absorbance peak at 320 nm and a decrease in the intensities of the absorbance peaks at 370 and 425 nm were observed (Ritthichai and Pimpan 2017). Furthermore, the absorbance ratio (Abs426/Abs445) was plotted as a function of ammonia concentration and was found to be in a linear range with correlation factor R2 equal to 0.9764 for the concentrations 0–500 ppm (Fig. 4c, d) that has also been reported in earlier study (Pandey et al. 2012). It is presumed that AgNPs produced by the aqueous extract of H. alcalis have the properties to sense ammonia in liquid could be applied for rapid detection of ammonia dissolved in water bodies.
a Blue shift in UV-Visible spectra of AgNPs after adding ammonia solution (500 ppm), b changes in spectrum of synthesized AgNPs colloidal solution at various dissolved ammonia concentration, c variation of absorbance at 426 and 445 nm, and d ratio of the absorbance peak (Abs426/Abs445) which display a linear relationship at different ammonia concentrations (0–500 ppm) with correlation factor R2 equal to 0.9764
References
Amirjani A, Fatmehsari DH (2018) Colorimetric detection of ammonia using smartphones based on localized surface plasmon resonance of silver nanoparticles. Talanta 176:242–246
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17(5):387–395
Besar K, Dailey J, Zhao X, Katz HE (2017) A flexible organic inverter made from printable materials for synergistic ammonia sensing. J Mater Chem C 5(26):6506–6511
Birla SS, Gaikwad SC, Gade AK, Rai MK (2013) Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Sci World J 2013:1–12
Chernousova S, Epple M (2013) Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chemie Int Ed 52(6):1636–1653
Cho YB, Jeong SH, Chun H, Kim YS (2018) Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sensors Actuators B Chem 256:167–175
Dadheech PK, Mahmoud H, Kotut K, Krienitz L (2012) Haloleptolyngbya alcalis gen. et sp. nov., a new filamentous cyanobacterium from the soda lake Nakuru, Kenya. Hydrobiologia 691(1):269–283
Das J, Das MP, Velusamy P (2013) Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta Part A Mol Biomol Spectrosc 104:265–270
Dubas ST, Pimpan V (2008) Green synthesis of silver nanoparticles for ammonia sensing. Talanta 76(1):29–33
El-Naggar NE-A, Abdelwahed NAM (2014) Application of statistical experimental design for optimization of silver nanoparticles biosynthesis by a nanofactory Streptomyces viridochromogenes. J Microbiol 52(1):53–63
El-Naggar NE-A, Hussein MH, El-Sawah AA (2017) Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci Rep 7(1):10844
Garg S (2012) Rapid biogenic synthesis of silver nanoparticles using black pepper (Piper nigrum) corn extract. Int J Innov Biol Chem Sci 3:5–10
Ghauch A, Rima J, Charef A, Suptil J, Fachinger C, Martin-Bouyer M (1999) Quantitative measurements of ammonium, hydrogenophosphate and Cu (II) by diffuse reflectance spectrometry. Talanta 48:385–392
Gurunathan S (2015) Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J Ind Eng Chem 29:217–226
Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SRK, Muniyandi J, Hariharan N, Eom SH (2009) Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces 74(1):328–335
Husain S, Sardar M, Fatma T (2015) Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J Microbiol Biotechnol 31(8):1279–1283
Iravani S (2014) Bacteria in nanoparticle synthesis: current status and future prospects. Int Sch Res Notices 2014:359316
Lengke MF, Fleet ME, Southam G (2007) Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 23(5):2694–2699
Li RCC, Chan PCH, Cheung PW (1995) Analysis of a MOS integrated gas sensor using a surface chemistry based model. Sensors Actuators B Chem 28(3):233–242
Link S, El-Sayed MA (2000) Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int Rev Phys Chem 19(3):409–453
Malins C, Doyle A, MacCraith BD et al (1999) Personal ammonia sensor for industrial environments. J Environ Monit 1(5):417–422
Mesbah NM, Wiegel J (2012) Life under multiple extreme conditions: diversity and physiology of the halophilic alkalithermophiles. Appl Environ Microbiol 78(12):4074–4082
Mie G (1908) Contributions to the optics of cloudy media, especially colloidal metal solutions. Ann Phys 330(3):377–445
Morsy FM, Nafady NA, Abd-Alla MH, Elhady DA (2014) Green synthesis of silver nanoparticles by water soluble fraction of the extracellular polysaccharides/matrix of the cyanobacterium Nostoc commune and its application as a potent fungal surface sterilizing agent of seed crops. Univ J Microbiol Res 2(2):36–43
Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S (2014) Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 4(3):316–335
Nayak D, Pradhan S, Ashe S, Rauta PR, Nayak B (2015) Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci 457:329–338
Naz S, Khaskheli AR, Aljabour A, Kara H, Talpur FN, Sherazi STH, Khaskheli AA, Jawaid S (2014) Synthesis of highly stable cobalt nanomaterial using gallic acid and its application in catalysis. Adv Chem 2014:1–6
Ostrensky A, Wasielesky W Jr (1995) Acute toxicity of ammonia to various life stages of the São Paulo shrimp, Penaeus paulensis Pérez-Farfante, 1967. Aquaculture 132(3–4):339–347
Pandey S, Goswami GK, Nanda KK (2012) Green synthesis of biopolymer–silver nanoparticle nanocomposite: an optical sensor for ammonia detection. Int J Biol Macromol 51(4):583–589
Pant G, Nayak N, Prasuna RG (2013) Enhancement of antidandruff activity of shampoo by biosynthesized silver nanoparticles from Solanum trilobatum plant leaf. Appl Nanosci 3(5):431–439
Patel V, Berthold D, Puranik P, Gantar M (2015) Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep 5:112–119
Patra JK, Baek K-H (2014) Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater 2014:1–12
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A (2011) Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf B Biointerfaces 82(1):152–159
Rippka R, Deruelles J, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Ritthichai T, Pimpan V (2017) Ammonia sensing of silver nanoparticles synthesized using tannic acid combined with UV radiation: effect of UV exposure time. J King Saud Univ Sci 1:1–8
Rösken LM, Körsten S, Fischer CB et al (2014) Time-dependent growth of crystalline Au0-nanoparticles in cyanobacteria as self-reproducing bioreactors: 1. Anabaena sp. J Nanopart Res 16(4):1–14
Roy K, Sarkar CK, Ghosh CK (2015) Photocatalytic activity of biogenic silver nanoparticles synthesized using potato (Solanum tuberosum) infusion. Spectrochim Acta A Mol Biomol Spectrosc 146:286–291
Roychoudhury P, Ghosh S, Pal R (2016) Cyanobacteria mediated green synthesis of gold-silver nanoalloy. J Plant Biochem Biotechnol 25:73–78
Saranyaadevi K, Subha V, Ravindran RSE, Renganathan S (2014) Green synthesis and characterization of silver nanoparticle using leaf extract of Capparis zeylanica. Asian J Pharm Clin Res 7:44–48
Satapathy S, Shukla SP (2017) Application of a marine cyanobacterium Phormidium fragile for green synthesis of silver nanoparticles. Indian J Biotechnol 16:110–113
Schröfel A, Kratošová G, Šafařík I, Šafaříková M, Raška I, Shor LM (2014) Applications of biosynthesized metallic nanoparticles–a review. Acta Biomater 10(10):4023–4042
Shivaji S, Madhu S, Singh S (2011) Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem 46(9):1800–1807
Singh R, Shedbalkar UU, Wadhwani SA, Chopade BA (2015) Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl Microbiol Biotechnol 99(11):4579–4593
Singh AK, Tiwari R, Kumar V et al (2017) Photo-induced biosynthesis of silver nanoparticles from aqueous extract of Dunaliella salina and their anticancer potential. J Photochem Photobiol B Biol 166:202–211
Sivalingam MM, Balasubramanian K (2017) Influence of the concentration of reducing agent on gold nanoparticles decorated reduced graphene oxide and its ammonia sensing performance. Appl Phys A Mater Sci Process 123(4):281
Sonker A, Pathak J, Kannaujiya V, Sinha R (2017) Characterization and in vitro antitumor, antibacterial and antifungal activities of green synthesized silver nanoparticles using cell extract of Nostoc sp. strain HKAR-2. Can J Biotechnol 1:26–37
Srivastava V, Jain K (2015) Mechanism of enhancement in NH 3 sensing for surface functionalized WO 3 film. RSC Adv 5(70):56993–56997
Sudha SS, Rajamanickam K, Rengaramanujam J (2013) Microalgae mediated synthesis of silver nanoparticles and their antibacterial activity against pathogenic bacteria. Indian J Exp Biol 52:393–399
Suvith VS, Philip D (2014) Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 118:526–532
Tang Y-L, Li Z-J, Ma J-Y, Su HQ, Guo YJ, Wang L, du B, Chen JJ, Zhou W, Yu QK, Zu XT (2014) Highly sensitive room-temperature surface acoustic wave (SAW) ammonia sensors based on Co3O4/SiO2 composite films. J Hazard Mater 280:127–133
Timmer B, Olthuis W, Van Den Berg A (2005) Ammonia sensors and their applications—a review. Sensors Actuators B Chem 107(2):666–677
Vanlalveni C, Rajkumari K, Biswas A et al (2018) Green synthesis of silver nanoparticles using Nostoc linckia and its antimicrobial activity: a novel biological approach. BioNanoScience 8(2):624–631
Vasquez RD, Apostol JG, de Leon JD et al (2016) Polysaccharide-mediated green synthesis of silver nanoparticles from Sargassum siliquosum JG Agardh: assessment of toxicity and hepatoprotective activity. OpenNano 116–24
Wei N, Hou Y, Lu Z, Yu H, Wang Q (2018) Synthesis of silver nanoparticles stabilized with C-phycocyanin and for fluorimetric detection of copper ions. IOP Conf. Ser. Earth Environ. Sci. IOP Publishing, p 22030
Wetchakun K, Samerjai T, Tamaekong N, Liewhiran C, Siriwong C, Kruefu V, Wisitsoraat A, Tuantranont A, Phanichphant S (2011) Semiconducting metal oxides as sensors for environmentally hazardous gases. Sensors Actuators B Chem 160(1):580–591
Yu L, Xi J, Chan HT, Su T, Phillips DL, Chan WK (2012) The degradation mechanism of methyl orange under photo-catalysis of TiO 2. Phys Chem Chem Phys 14(10):3589–3595
Acknowledgements
We are thankful to the Department of Microbiology, Central University of Rajasthan for providing necessary facilities.
Funding
This work was financially supported by Department of Science and Technology, Rajasthan (P.7(3) DST/BT R&D/EAC/2018/3158).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tomer, A.K., Rahi, T., Neelam, D.K. et al. Cyanobacterial extract-mediated synthesis of silver nanoparticles and their application in ammonia sensing. Int Microbiol 22, 49–58 (2019). https://doi.org/10.1007/s10123-018-0026-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-018-0026-x