Abstract
The midbrain acts as a junction between the brain, lower part of the brainstem, and spinal cord. It functions as a relay station for major white matter tracts (e.g., corticospinal tracts) as well as the origin of two cranial nerve nuclei. Tumors that originate in or involve the midbrain pose a significant challenge, and their treatment usually involves several disciplines including neurosurgery and neurooncology. Surgery for midbrain tumors demands a high surgical technique and multidisciplinary approach in the operating room (e.g., neuromonitoring) and outside of it as well. The surgical approach is tailored for each and every case, taking into account the complex anatomy, specific location of the long tracts using advanced imaging, as well as the adjacent neurovascular structures that surround the surgical location. In recent years, there has been a growing interest and understanding of the molecular and genetic parameters of tumors that invade the midbrain, which, with time, will allow us to tailor more specific treatments for these patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Midbrain
- Thalamus
- Tumor
- Craniotomy
- Choroid
- Cerebellum
- Ventricle
- Corticospinal
- Tracts
- Cranial nerve
- Hydrocephalus
11.1 Introduction: Anatomy and Embryology
The midbrain, which lies between the diencephalon and the pons, contains the motor fibers of the corticospinal tracts (CSTs), important connections for the visual and auditory systems, and the third and fourth cranial nerve nuclei. Embryologically, the midbrain is of ectodermal origin. The anterior portion of the neural tube creates three primordial vesicles, which ultimately form the forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon). Although the prosencephalon and rhombencephalon undergo further segmentation, the mesencephalon does not; it differentiates into the midbrain instead [1].
Anatomically, the midbrain consists of the tectum dorsally and the tegmentum ventrally. The midbrain harbors numerous nuclei and fibers, serving as a relay station via the superior cerebellar peduncles. The cerebral peduncles, cerebral aqueduct and periaqueductal gray, quadrigeminal plate and four colliculi (corpora quadrigemina), and the third and fourth cranial nerve nuclei are all located within the midbrain. The oculomotor nerve (third cranial nerve) can be identified along the ventral midbrain within the interpeduncular fossa, where small blood vessels penetrate the posterior perforated substance [1]. The trochlear nerve (fourth cranial nerve), on the other hand, emerges from the dorsal midbrain near the inferior colliculus [2].
The tectum and the quadrigeminal plate consist of the superior and inferior colliculi. The superior colliculus nuclei are complex-layered structures that play a role in ocular movements and visual reflexes through afferent connections with other regions of the central nervous system (CNS). The inferior colliculi consist of a triad of gray matter nuclei and play a significant role in the auditory pathway. This triad is composed of a central nucleus at the core (responsible for the tonotopic organization of auditory information), a pericentral nucleus, and a more lateral, external nucleus (which receives auditory and non-auditory inputs) [2].
The tegmentum, located at the ventral aspect of the midbrain, harbors white matter fibers en route to and from the cerebral cortex, cerebellum, and spinal cord. The brachium conjunctivum (a.k.a. superior cerebellar peduncle), a bundle of fibers arising from the cerebellum and connecting to the thalamus and red nucleus, traverses the tegmentum and plays an important role in fine motor coordination. The medial lemniscus, trigeminal lemniscus, and spinothalamic tracts also pass through the tegmentum; these are sensory tracts that typically course laterally within the tegmentum, whereas tracts that are responsible for coordinating eye movements, the medial longitudinal fasciculi, run centrally and paracentrally in the tegmentum.
Several groups of nuclei are also located within the tegmentum , particularly at the level of the inferior colliculus. The nucleus parabrachialis pigmentosus is a continuation of the tegmental area of Tsai and is located dorsal to the substantia nigra. The ventral tegmental nucleus is dorsal to the brachium conjunctivum and regulates behavior and emotions via connections with the mammillary bodies. The dorsal tegmental nucleus is located within the periaqueductal gray and sends projections to the reticular formation and autonomic nuclei of the brainstem. The dorsal raphe nucleus (nucleus supratrochlearis) is the largest serotonergic nucleus [2]. The lateral tegmentum harbors the pedunculopontine nucleus (PPN), lateral dorsal tegmental nucleus and parabigeminal areas, all of which are cholinergic nuclei.
The red nucleus resides medially within the rostral tegmentum at the level of the superior colliculus. The red nucleus acts as a relay point for projections from the cerebrum, cerebellum, reticular formation, and inferior olive. The oculomotor nucleus, which is responsible for eye movements, lies ventral to the periaqueductal gray matter within the medial tegmentum [2]; this nucleus is located at the levels of the lower half of the superior colliculus and upper half of the inferior colliculus [3]. The trochlear nuclei are located at the level of the inferior colliculus and reside in the midline and ventral to the cerebral aqueduct. Its axons travel dorsal and lateral to the aqueduct and eventually pierce the dorsal surface of the midbrain, close to the midline. These nuclei are positioned adjacent to the midline and on average are 9.5 mm medial to the surface of the lateral mesencephalic sulcus [3].
The cerebral peduncles contain the corticocerebellar, corticobulbar and corticospinal fibers. The dominant component is the CST, which occupies the central three-fifths of the cerebral peduncle [2]. The tract’s fibers are organized with the efferent cortical motor axons and are arranged somatotopically from medial to lateral: arm, face, then leg representations. The corticobulbar tract resides dorsomedial to the CST.
The midbrain is primarily vascularized via the posterior cerebral circulation, including the basilar, superior cerebellar and posterior cerebral arteries. In cross-section, the medial aspect of the midbrain is supplied by the basilar artery and its paramedian branches, while the lateral aspect is supplied by the superior cerebellar artery at the level of the inferior colliculus and the posterior cerebral artery branches at the level of the superior colliculus [2]. The superior colliculus and the adjacent tectum are vascularized by the superior cerebellar artery.
11.2 Midbrain Lesions
The midbrain is not specifically affected by unique lesions, and the same pathologies that are found in other regions of the brain and brainstem are also found in the midbrain, including but not limited to gliomas, germ cell tumors, cavernous malformations, and arteriovenous malformations. Although not unique regarding pathology, the anatomic location of the midbrain allows for unique patterns of tumor spread, with lesions tending to progress along white matter tracts, thus giving rise to thalamopeduncular tumors. Alternatively, lesions may localize to discrete locations within the midbrain, including the aqueduct, the periaqueductal area, and the quadrigeminal plate.
11.2.1 Thalamopeduncular Tumors
In 2007, Puget et al. presented a series of patients with thalamic tumors that included a newly defined subset of thalamopeduncular tumors [4]. In children, these tumors can present clinically with progressive spastic hemiparesis (secondary to mass effect on the CSTs) and are typically found to be low-grade tumors, such as pilocytic astrocytomas, at the interface of the thalamus and cerebral peduncle [5]. Other potential clinical signs include homonymous hemianopsia, ptosis and/or ophthalmoplegia secondary to mass effect on the optic tracts and oculomotor nerves, and obstructive hydrocephalus, which may arise if there is significant mass effect on the cerebral aqueduct.
Given the low incidence of thalamopeduncular tumors, treatment strategies are being developed while awaiting larger sample sizes and better sub-classification schemes [5]. The majority of these tumors are low-grade gliomas (LGGs), and as such, conventional chemotherapy is associated with a cure rate of only 0–10% [5,6,7,8]. Radiation therapy can cause long-term sequelae, including neurocognitive deficits and endocrinopathies [9]. Therefore, surgical resection remains one of the primary treatment options for these tumors, despite the challenges of the surgery in this region. With careful planning, particularly with respect to the location of the CSTs, optimal surgical outcomes can be achieved, potentially sparing the patient from adjuvant therapy (Figs. 11.1 and 11.2).
Left thalamopeduncular pilocytic astrocytoma. (a) A composite of three axial T1-weighted Gadolinium-enhanced images demonstrates that the tumor arises from the lateral aspect of the peduncle underneath the thalamus, pushing the normal thalamus superiorly. The thalamic displacement made a transcallosal approach to the tumor a poor choice, because the surgeon could have violated the normal thalamus to reach the tumor. (b, c) The optic tract, a structure that must be carefully avoided while removing the tumor, is displaced superior and lateral to the tumor. The arrow designates the CST. (d) An axial DTI of the same tumor shows the CSTs deviated anteriorly and laterally (arrow; CSTs are in blue); this was the most common pattern of CST displacement in the series of patients described by Foley and Boop [52]. This pattern of tract displacement made a transsylvian approach unattractive; one would have to transect the tracts to reach the tumor. (e) An approach was chosen through the middle temporal gyrus by using frameless stereotactic navigation to approach the tumor just posterior to the CSTs. (Modified from Foley and Boop [52]. With permission from Springer Nature)
A 5-year-old child presenting with a 3-week history of progressive hemiparesis – arm worse than leg, and a several-days history of headache, nausea, and vomiting. (a) Preoperative images. (b) Axial DTI. (c) Sagittal DTI. (d) Postoperative axial image following a transtemporal transchoroidal approach. (e) Coronal, post-contrast T1-weighted image at 1 year postop showing complete tumor resection. (Modified from Foley and Boop [52]. With permission from Springer Nature)
Molecular targeted therapies represent another opportunity as we learn more about the molecular drivers of pediatric LGGs. For instance, the KIAA1549-BRAF fusion has been found in pediatric pilocytic astrocytomas [10,11,12], where the Pediatric Cancer Genome Project (PCGP) identified the fusion in 75% of pilocytic astrocytomas, including 80% of brainstem tumors and 59% of diencephalic lesions [13]. Thalamopeduncular tumors, in particular, are also associated with a high frequency of the KIAA1549-BRAF fusion, suggesting that these tumors are more likely to originate from the midbrain rather than the thalamus [5]. This hypothesis is also supported by the anterolateral displacement of the CST in most patients with thalamopeduncular tumors. Regardless of the site of origin, the high frequency of the KIAA1549-BRAF fusion in thalamopeduncular tumors implies that BRAF inhibitors may play a potential role in the treatment of these tumors.
11.2.2 Tectal Gliomas
Tectal gliomas are rare tumors with a predilection to occur in the pediatric population [14]. Tectal gliomas typically present with symptoms of raised intracranial pressure, and the diagnosis may be delayed, with most reports describing onset of symptoms at 3–6 months before the actual diagnosis [15]. Alternatively, tectal gliomas may be an incidental finding, as it was reported in up to 27% of patients in prior series; this percentage may rise with the increasingly widespread use of magnetic resonance (MR) imaging [16, 17].
Tectal gliomas are most commonly LGGs and are almost exclusively identified in children [15]. Tumor growth typically causes tectal expansion and, hence, compression of the aqueduct; it may also extend to the pulvinar region. Tectal tumors are usually isointense on T1- and hyperintense on T2-weighted imaging, and usually do not enhance after contrast administration [18]. Predictors of tumor progression include tumor size greater than 3 cm2, contrast enhancement, and cystic changes at diagnosis [15, 19, 20].
Tectal gliomas have a similar morphology to other LGGs but demonstrate a unique molecular/biological profile: (1) only 25% of tectal gliomas harbor the KIAA1549-BRAF fusion, in contrast to LGGs of the cerebellum (92%) or the supratentorial compartment (59%); (2) the BRAF V600E mutation is uncommon in tectal gliomas, occurring in only around 7–8% of these tumors [21]; (3) although the H3 K27M mutation tends to be found in midline gliomas and portends a more aggressive disease, it is usually absent in tectal gliomas; (4) the methylation profile of tectal gliomas is unique compared to other LGGs [15]. These findings suggest that tectal gliomas are a unique entity and most often represent a chronic disease.
Given the usually indolent course and the risks associated with surgical resection in such an eloquent area, the general recommendation is cerebrospinal fluid (CSF) diversion in patients with hydrocephalus, followed by close observation [15]. The main options for CSF diversion include endoscopic third ventriculostomy (ETV) or a ventricular shunt. ETV has become more widely advocated due to the high rates of success in patients with obstructive hydrocephalus (particularly older children and adults) and the ability to avoid issues such as shunt malfunction, obstruction, and infection. Some authors suggested the use of Ommaya reservoirs, which allows for emergent access to CSF in the event of ETV failure [22]; the authors have also reported two cases in which the presumed tectal gliomas have spontaneously involuted and disappeared following ETV placement for hydrocephalus.
Approximately one-third of patients described in the literature have ultimately undergone surgical resection. Potential complications are significant and include motor deficits, visual deficits, gaze palsies, and intracranial hemorrhage. As a result, biopsy or resection of tectal gliomas is typically reserved for tumors with an atypical radiographic appearance or to guide targeted treatment (such as BRAF and MEK inhibitors) at progression [15, 23,24,25]. Radiation therapy or chemotherapy may be considered in cases of progressive disease. Although tectal gliomas tend to progress in about 25% of children, long-term survival is typically achieved with salvage adjuvant treatment [15]. Tectal gliomas are rarely diagnosed in adults, but some authors hypothesize that these patients had undiagnosed indolent tumors in childhood [26]. As a result, the treatment strategy tends to be similar in adults.
11.2.3 Aqueductal and Periaqueductal Tumors
In most publications, tectal gliomas are grouped along with true aqueductal and periaqueductal tumors, without differentiating between the groups. In recent years, however, some authors described true aqueductal tumors, which is a rare entity [18]. True aqueductal tumors may include both LGGs and high-grade gliomas and are more likely to include enhancing tumors than other types of tectal tumors. It is important that the true aqueductal tumors are recognized as such. Given that the majority are benign and of soft consistency, those located at the rostral aqueduct can be resected from a third ventricular approach, whereas those located at the caudal aqueduct can be approached from a cerebellar fourth ventricular approach. As aqueductal tumors displace the normal tectum dorsally, these tumors should never be approached through the tectum, which is a risk associated with not recognizing aqueductal tumors as a separate entity. Nevertheless, data regarding true aqueductal tumors remain scarce [27].
11.3 Surgical Approaches to Midbrain Tumors
Surgical treatment of midbrain tumors should be guided by an understanding of the underlying anatomy as well as by advanced imaging, including diffusion tensor imaging (DTI), in order to define the location of critical white matter tracts, such as the CSTs, in relation to the tumor. Various safe entry zones have been described: perioculomotor area for anterior lesions, supracollicular, infracollicular and intercollicular zones for posterior lesions, and lateral mesencephalic sulcus for anterolateral lesions [3]. More recently, additional surgical options included obtaining an endoscopic biopsy whenever the tumor extends into the third ventricle [28, 29] and the use of tubular retractors to facilitate resection [30, 31].
11.3.1 Anterior Midbrain
Tumors in the anterior portion of the midbrain usually tend to grow towards either the third ventricle or the interpeduncular cistern. In this setting, the key determinants for the surgical approach are the relation of the tumor to the third ventricle (i.e., how much of the tumor is in the ventricle versus how much is in the cistern) and the relation of the tumor to the oculomotor nerve. For tumors growing mainly into the third ventricle, an intraventricular approach may involve interforniceal, transcallosal, transchoroidal or transforaminal approaches. Transsphenoidal and transclival approaches have also been proposed, particularly for anterior pontine lesions but also for lesions of the anterior mesencephalon [32]. The use of an endoscope is usually reserved for biopsies or for resection of small lesions.
The unique location of the CST (intermediate three-fifths of the peduncle) allows for a possible surgical window from either pure anterior or anterolateral (pterional, orbito-zygomatic, supraorbital) or more lateral (transsylvian, subtemporal or transtemporal) approaches [33]. Combined approaches have also been popularized over the years. In 1911, Krause and colleagues described the subtemporal transtentorial approach, which allows for good visualization of the incisural space, the ambient cistern, and part of the quadrigeminal cistern [3]. In 1980, Sano and colleagues described the temporopolar approach, which allows for good visualization of the anterolateral interpeduncular fossa [34]. Each approach poses specific risks because of the potential neurovascular components that can be compromised. When the trajectory is more lateral, the approach usually includes some degree of tentorial resection and dissection. In particular, these lateral approaches are associated with a risk of injury to the vein of Labbe, as well as possible ophthalmoparesis due to third and fourth cranial nerve injury along the tentorial incisura.
11.3.1.1 Transchoroidal and Subchoroidal Approaches
These approaches will allow to expand the surgical window to the third ventricle, reaching the anterior and, in some cases, the central midbrain. Both approaches are based on safe anatomic passage through the choroidal fissure. The choroidal fissure is a cleft between the body of the fornix and the thalamus in the cavity of the lateral ventricle; it is situated below the choroid plexus, between the tenia fornices and tenia thalami. Anteriorly, it begins at the posterior edge of the foramen of Monro and runs posteriorly between fornix and thalamus. The thalamostriate, septal, caudate and superior choroidal veins run in close proximity to the choroidal fissure; some of these veins cross it as well. The choroidal fissure is the thinnest area in the wall of the lateral ventricle, bordering the basal cisterns and the roof of the third ventricle [35]. Upon entry to the lateral ventricle, either by parasagittal opening through an interhemispheric/corpus callosum dissection or by a transcortical corridor to the lateral ventricle, the choroid plexus and its anatomical complexity is appreciated. It is then coagulated laterally to expose the tenia fornix. The dissection proceeds through the tenia fornix to reach the velum interpositum, and then to the third ventricle. If necessary, the anterior septal vein is coagulated and transected at the entry site to the foramen of Monro [36]. This opening allows for a wide view of the posterior and middle part of the third ventricle, hence to the anterior midbrain. Some surgeons advocate for a wider window, where the choroid plexus is coagulated and dissected laterally to use the tenia fornices as a route for expanding the foramen of Monro.
In the subchoroidal approach, the choroid fissure is opened lateral to the choroid plexus, between the choroid plexus and the thalamus [37]. After the choroid fissure is opened, there are two ways to enter the third ventricle: one route is between the internal cerebral veins, and another route is between the internal cerebral vein and the thalamus [37]. We recommend utilizing this approach in cases where the lesion invades and has a significant component inside the third ventricle. In case the surgeon chooses to further dissect through the velum interpositum along the medial wall of the ipsilateral internal cerebral vein, which allows to further expand the surgical window into the third ventricle, care should be taken to avoid injury to the thalamoperforator arteries running in close proximity to the internal cerebral vein [38]. As mentioned earlier, this approach is mostly useful whenever the midbrain tumor is superficial or exophytic and extending into the third ventricle. The surgical corridor through the third ventricle can provide access to anterior midbrain lesions, periaqueductal area, and some posterior lesions.
11.3.1.2 Peritemporal Approaches
We use this term for approaches that include the classic pterional and orbito-fronto-zygomatic craniotomies, allowing the surgeon to choose between several approaches: subtemporal with or without transtentorial, transtemporal, and transsylvianm (pretemporal) [34]. The detailed description of these classic craniotomies is beyond the scope of this chapter. In general terms, we advocate a regular temporo-frontal or a small question mark skin opening with exposure of the zygomatic root. The temporalis muscle is elevated using interfacial dissection, allowing for more retraction of the muscle and further exposure of the zygomatic arch while protecting facial nerve branches. The temporal craniotomy should be low, just adjacent to the zygomatic root. There is no need to go above the superior temporal line, and the craniotomy flap is cut just above the zygomatic root in the coronal plan [39]. This craniotomy is a bit anterior to the traditional one and will sometimes lead to additional retraction on the temporal lobe, yet it lowers the risk of injuring the vein of Labbé and inducing a significant venous infarct. The dura is exposed, and the dural flap is usually retracted towards the zygomatic arch. Some surgeons advocate zygomatic arch drilling to decrease its prominence so that it does not interfere with the view towards the subtemporal area [39]. In some cases, CSF drainage is needed and can be done using a spinal drain that is placed before surgery or using an external ventricular catheter. In case the transsylvian approach is selected, CSF can be drained from the cisterns. Brain relaxation is necessary before attempting any temporal lobe retraction or manipulation. In the subtemporal approach, once the temporal lobe is retracted and the arachnoid layer of the mesiotemporal region is exposed, one can usually appreciate the pulsation of the P2 segment of the posterior cerebral artery running in the ambient cistern. The arachnoid layer is widely excised using an arachnoid knife, allowing for more CSF drainage and evaluating the perimesencephalic area with a good view of the anterolateral midbrain. The subtemporal approach has several modifications that are basically different perspectives on the angle of attack (anterior, middle or posterior subtemporal), which allows for different views of the ambient cistern. The subtemporal approach does not usually allow for good visualization of the quadrigeminal cistern but provides adequate visualization of the interpeduncular cistern and the first half of the ambient cistern. The main obstacle for more posterior exposure is the presence of vein of Labbé and, in some cases, the parahippocampal gyrus [40]. The transsylvian, a.k.a. pretemporal, approach allows for a relatively quick drainage of CSF once the sylvian fissure is dissected, access to the sylvian cistern, and then to the cisterns around the carotid artery and the optic nerve. This approach usually demands relatively significant lateral and posterior retraction of the temporal lobe. It allows for fairly good control and view of the basilar area, hence the interpeduncular cistern, but lacks good visualization of the ambient cistern components, hence the perimesencephalic safe entry zone [40]. In both cases, the anterolateral midbrain can be exposed, and the perioculomotor safe entry zone can be utilized. At the level of the oculomotor nerve exit site from the midbrain, the CST occupies only the intermediate three-fifths of the cerebral peduncle, which permits only a small corridor between the oculomotor nerve exit site and the CSTs [3, 33]. This narrow window is delimited by the posterior cerebral artery above, the superior cerebellar artery below, the emergence of the third cranial nerve and the basilar artery medially, and the pyramidal tract laterally [3]. Since tumors in the anterior part of the midbrain are usually exophytic in nature, this safe entry zone is rarely used.
An approach that allows for some visualization of the perimesencephalic area, including the transition zone between the ambient and quadrigeminal cisterns, is the transtemporal-transchoroidal approach. In this approach, the surgeon can have a small corridor to attack lesions residing in the anterior and middle parts of the midbrain. The exposure is very similar to that of the subtemporal approach, with the understanding that the temporal horn is usually 2.5–3.5 cm posterior to the temporal tip and 2–2.5 cm deep to the surface [40]. The entry point is along the inferior temporal gyrus, aiming to access the temporal horn and identify the choroid plexus as well as the hippocampal head and body. The temporal choroidal fissure lies between the choroid plexus and the hippocampus. Crossing the fissure from the hippocampal side rather than from the thalamic side will help in avoiding the highly vascularized area just adjacent to the thalamus.
11.3.2 Central Midbrain
Tumors located in the central midbrain region tend to extend either towards the fourth ventricle inferiorly or towards the pineal region superiorly. The direction of extension may guide the surgical approach. If the major component of the tumor extends towards the fourth ventricle, a suboccipital craniotomy and telovelar approach may be appropriate. When the major component of the tumor extends toward the pineal region, the various approaches to the pineal region itself may be utilized. In particular, the midline or paramedian supracerebellar infratentorial approach can open a relatively wide window to the midbrain and pericollicular area, as well as to the transition zone between the upper part of the midbrain and the pineal region [41,42,43]. Alternatively, the occipital transtentorial approach can be considered.
When approaching the pericollicular region, extra care should be taken to protect the fourth cranial nerve, which is situated directly below the inferior colliculus. Safe entry zones to the pericollicular region have been described. Supracollicular access can be obtained by performing a transverse incision above the superior colliculus; dissection continues until the periaqeuductal gray is reached. Alternatively, infracollicular access can be obtained by entering between the trochlear nerve and the inferior colliculus [3].
Similar to anterior midbrain lesions, central midbrain tumors may extend into the third ventricle, in which case they may be accessed via interforniceal, transcallosal, transchoroidal or transforaminal approaches (Fig. 11.3). Most of the approaches described in the Sect. 11.3.1 can be utilized for central midbrain lesions as well. The transchoroidal approach can be very helpful in achieving a good angle of attack to lesions that invade the third ventricle [38]. A similar view can be achieved using the interforniceal approach [36, 37, 44]; this approach works well for patients with a cavum septum pellucidum, but if the surgeon tries to separate the fornices after they have fused, the patients have a high likelihood of developing permanent memory deficits as a consequence of manipulating both fornices. Finally, the different peritemporal approaches are helpful in achieving a good angle of attack to lesions that either exophyte through the lateral midbrain or are fairly close to it.
A 6-year-old girl presented with headaches, right third nerve palsy, and left hemiparesis. (a) Preoperative MRI demonstrated a midbrain tumor with extension into the third ventricle. (b) Postoperative MRI following a transcallosal subchoroidal approach. The third nerve palsy did not improve but she is otherwise intact, and 5 years postop, the residual has not progressed. The patient did not require additional treatment to date
11.3.3 Posterior (Dorsal) Midbrain
The posterior midbrain, or quadrigeminal plate, lies posterior to the cerebral aqueduct [3]. The unique proximity of the posterior part of the midbrain to the aqueduct of Sylvius makes tumors in this location more likely to cause obstructive hydrocephalus. As a result, endoscopic biopsy has become an attractive option, since it can be performed for diagnosis at the same time as an ETV for CSF diversion is performed (Fig. 11.4). The endoscopic procedure can be carried out using a flexible or rigid endoscope and, in some cases, the combination of the two [29]. The entry point is usually more anterior than the regular entry point used for ETV, which is just anterior to the coronal suture [29, 45]. Once inside the lateral ventricle, the endoscope is introduced through the foramen of Monro while aiming to reach the posterior part of the third ventricle. The resection of the tumor can be carried out using endoscopic instruments or the NICO Myriad® (NICO Corporation) in order to achieve meaningful resection.
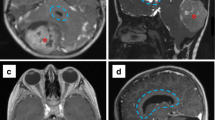
A 14-year-old girl who presented to an outside facility with obstructive hydrocephalus and was diagnosed with a tectal plate mass. The patient underwent an endoscopic third ventriculostomy; given that the tumor was exophytic into the posterior aspect of the 3rd ventricle, an endoscopic biopsy was recommended. (a) Axial T2-weighted and (b) sagittal FIESTA images demonstrate an avoid, well-circumscribed mass centered on the tectum and extending into the posterior aspect of the third ventricle. (c) Sagittal, post-contrast T1-weighted image demonstrates that the mass was not contrast-enhancing
Some tumors ultimately require a more invasive approach for surgical resection. Most of these tumors are exophytic. Surgical corridors include many of those described previously, including the transchoroidal, interforniceal, supracerebellar infratentorial. and occipital transtentorial approaches. When the tumor is extending superiorly and laterally, the occipital transtentorial approach provides a very wide view that includes the pineal region, posterior part of the third ventricle, tectal plate and upper fourth ventricle, posterolateral surface of the mesencephalon, tentorial surface of the cerebellum, and splenium of the corpus callosum. The main pitfall, however, is that excessive retraction on the occipital lobe can injure the visual cortex [46]. The midline approaches for the posterior midbrain, as well as the posterior third ventricle, usually start as interhemispheric, either parietal or occipital. CSF diversion prior to manipulation can significantly reduce potential injury to the brain from manipulation and retraction. In the interhemispheric transcallosal approach, either intervenous or paravenous, the patient is positioned in a supine position with the head in a neutral angle of rotation and considerably flexed. It is advised to even consider a semi-sitting position. Once the interhemispheric approach is achieved, a minimal callosotomy is performed, utilizing the navigation system in order to minimize the opening at the posterior corpus callosum. A midline callosotomy will allow dissection through an avascular plane that runs between the two internal cerebral veins [47]. In order to approach the third ventricle, the surgeon needs to decide on either separating the internal cerebral veins or dissecting next to them; the approach is thus called intervenous or interforniceal, respectively. In the intervenous approach, it is best to aim close to the confluence of the internal cerebral veins and the vein of Galen. Just proximal to this point, there is a natural separation between the two internal cerebral veins [47]. The dissection can be elongated anteriorly as needed. Another important benefit of the intervenous approach is that at this posterior location, the forniceal crura are not fused and always lateral to the internal cerebral veins. After dissecting between the two veins, the surgeon should be cautious not to excessively compress or manipulate the veins to avoid venous injury or thrombosis. This approach allows for direct visualization of the posterior midbrain, periaqueductal area, tectal plate, and pineal region. The drawback of this approach is the potential injury to venous structures, starting with the cortical veins, which may be sacrificed in some cases during the interhemispheric approach (although every effort should be taken to preserve them in order to avoid venous congestion or infarcts), and ending with the internal cerebral veins, which may be attached to tumors originating from the midbrain or pineal region.
The occipital interhemispheric approach to midbrain tumors usually includes the addition of either tentorial transection, falcine trasection, or both. The quoted name in the literature is the occipital interhemispheric transtentorial approach. The angle of attack can be a direct one, from ipsilateral to the center of the tumoral mass, or contralateral permitting a different trajectory. This approach, along with the supracerebellar infratentorial approach, allows for a good trajectory to the quadrigeminal plate and the pericollicular area. Some surgeons advocate the sitting position [48], while others prefer the prone position – in a way that the confluence of sinuses is the highest point, the concord position, or the three-quarters prone position [49]. The tentorial opening is usually done at 1 cm lateral and parallel to the straight sinus. If an ipsilateral approach is chosen, usually only an ipsilateral view of the ambient and quadrigeminal plate can be achieved. Yet, the addition of falcine dural transection usually at around 1 cm above and parallel to the straight sinus allows for a much broader view of the quadrigeminal plate. In recent years, there is growing literature that supports the use of an endoscope to augment the occipital interhemispheric approach [50, 51].
The supracerebellar infratentorial is the most inferior approach to the midbrain. In this approach, the surgeon has a wide view of the quadrigeminal plate. The patient can be positioned in either the sitting or the three-quarters prone position. The opening can be midline or paramedian, preparing for a craniotomy that will be either on the edge of the sinus or crossing the sinus, so that the dural opening will have a horse-shoe pattern with the stalk against the sinus. In this way, the surgeon can elevate the dura upwards, helping to further open the junction between the cerebellum and the tentorium. The dissection is carried out by resecting the arachnoid adhesions at this junction, including the thick arachnoid matter covering the quadrigeminal plate. Care should be taken to avoid injury to the precerebellar veins or the vermian vein. The vascular structures exposed include the posterior cerebral artery, a tentorial branch of the superior cerebellar and medial posterior choroidal arteries, the vein of Galen, and the internal occipital and cerebral veins [49]. The endoscope can be an efficient and important tool in this approach as well.
In recent years, several publications suggested the use of tubular retractors to attack these deep-seated lesions, including midbrain tumors. The use of advanced imaging and understanding of the exact location of the CST is of paramount importance [52].
11.4 Surgical Decision-Making
The main surgical consideration is typically to achieve CSF diversion. ETV, with a possible endoscopic biopsy, is often preferred. In some cases, a ventricular shunt may be necessary (e.g., if the patient’s anatomy is unfavorable to an ETV). In cases in which the initial diagnosis is questionable, or in cases where there is clear tumor progression, surgical biopsy or resection should be considered. Regardless of the surgical approach, DTI to detect the CSTs location is critical for planning the best approach [52] (Fig. 11.5). Most thalamopeduncular tumors, for example, displace the CST anterolaterally, and a transsylvian approach would violate the CST. In these cases, as well as in cases where the CST is displaced medially, a transtemporal transchoroidal approach is appropriate and facilitates complete removal of the tumor while protecting the CST. In cases where the CST is displaced laterally, however, a transcortical middle frontal gyrus approach is more appropriate. In addition to the CSTs, other critical structures that should be identified on preoperative imaging and protected intraoperatively include the optic tract, basal vein of Rosenthal, and the third cranial nerve [52].
A 4-year-old boy who presented with progressive right hemiparesis. (a) Preoperative axial FIESTA image demonstrates a mixed solid and cystic left pontomesencephalic lesion. (b) Axial, post-contrast T1-weighted image demonstrates diffuse enhancement of the center of the lesion. (c) Axial DTI demonstrating medial displacement of the corticospinal tract fibers, facilitating a transtemporal transchoroidal approach. (d) Coronal T2-weighted image. (e) Postoperative axial, post-contrast T1-weighted image
11.5 Prognosis
Prognosis depends on several parameters. Tumor histology (high-grade versus LGG), as well as specific molecular features such as the H3K27 M mutation, are known to significantly affect the prognosis. Although the classic tectal glioma is typically considered indolent with a good prognosis, up to 20% of cases are diagnosed as high-grade gliomas. Radiographic disease progression may occur, with an average duration from diagnosis to progression ranging from 3 months to 7.8 years. Patient outcomes were reported in 28 studies, with 495/508 patients (97.4%) surviving for an average duration that ranged between 2 and 10 years [15].
11.6 Conclusion
Midbrain tumors are complex lesions, and a stepwise approach regarding diagnosis and treatment is critical. Although most tectal gliomas will possibly require CSF diversion and have an otherwise indolent course, care should be taken to ensure that the diagnosis is certain. Additionally, any evidence of tumor progression demands intervention. Most other midbrain tumors will mandate either surgical resection or biopsy and oncological treatment. There are several surgical approaches to the midbrain, which are divided into approaches targeting the anterior, middle and/or posterior areas of the midbrain. For the anterior midbrain, many of the approaches utilize either the transventricular or the peritemporal trajectories. For the central portion of the midbrain, many tumors will invade the third ventricle; hence, the surgeon uses various techniques to expand the view through the third ventricle. For the posterior part of the midbrain, some approaches utilize the interhemispheric corridor, either from the parietal or the occipital areas, whereas some tumors can be approached through the posterior fossa. In recent years, endoscopes and exoscopes have been used more often, the goal of which is to achieve better visualization of the deep areas of the brain, such as the midbrain. Meticulous planning before surgery will assist in choosing the best treatment strategy as well as the right surgical approach for this highly complex area.
Abbreviations
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CST:
-
Corticospinal tract
- DTI:
-
Diffusion tensor imaging
- ETV:
-
Endoscopic third ventriculostomy
- LGGs:
-
Low-grade gliomas
- MR:
-
Magnetic resonance
- PCGP:
-
Pediatric Cancer Genome Project
- PPN:
-
Pedunculopontine nucleus
References
Angeles Fernández-Gil M, Palacios-Bote R, Leo-Barahona M, Mora-Encinas JP. Anatomy of the brainstem: a gaze into the stem of life. Semin Ultrasound CT MR. 2010;31(3):196–219.
Ruchalski K, Hathout GM. A medley of midbrain maladies: a brief review of midbrain anatomy and syndromology for radiologists. Radiol Res Pract. 2012;2012:258524.
Cavalheiro S, Yagmurlu K, da Costa MD, Nicacio JM, Rodrigues TP, Chaddad-Neto F, et al. Surgical approaches for brainstem tumors in pediatric patients. Childs Nerv Syst. 2015;31(10):1815–40.
Puget S, Crimmins DW, Garnett MR, Grill J, Oliveira R, Boddaert N, et al. Thalamic tumors in children: a reappraisal. J Neurosurg. 2007;106(5 Suppl):354–62.
Lee RP, Foster KA, Lillard JC, Klimo P, Ellison DW, Orr B, et al. Surgical and molecular considerations in the treatment of pediatric thalamopeduncular tumors. J Neurosurg Pediatr. 2017;20(3):247–55.
Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–63.
Grigsby PW, Thomas PR, Schwartz HG, Fineberg B. Irradiation of primary thalamic and brainstem tumors in a pediatric population. A 33-year experience. Cancer. 1987;60(12):2901–6.
Gnekow AK, Falkenstein F, von Hornstein S, Zwiener I, Berkefeld S, Bison B, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro-Oncology. 2012;14(10):1265–84.
Armstrong GT, Conklin HM, Huang S, Srivastava D, Sanford R, Ellison DW, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro-Oncology. 2011;13(2):223–34.
Cin H, Meyer C, Herr R, Janzarik WG, Lambert S, Jones DT, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121(6):763–74.
Lin A, Rodriguez FJ, Karajannis MA, Williams SC, Legault G, Zagzag D, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71(1):66–72.
Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ, et al. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro-Oncology. 2010;12(7):621–30.
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–12.
Guillamo JS, Doz F, Delattre JY. Brain stem gliomas. Curr Opin Neurol. 2001;14(6):711–5.
Liu APY, Harreld JH, Jacola LM, Gero M, Acharya S, Ghazwani Y, et al. Tectal glioma as a distinct diagnostic entity: a comprehensive clinical, imaging, histologic and molecular analysis. Acta Neuropathol Commun. 2018;6(1):101.
Gass D, Dewire M, Chow L, Rose SR, Lawson S, Stevenson C, et al. Pediatric tectal plate gliomas: a review of clinical outcomes, endocrinopathies, and neuropsychological sequelae. J Neuro-Oncol. 2015;122(1):169–77.
Pollack IF, Shultz B, Mulvihill JJ. The management of brainstem gliomas in patients with neurofibromatosis 1. Neurology. 1996;46(6):1652–60.
Roth J, Chaichana KL, Jallo G, Mirone G, Cinalli G, Constantini S. True aqueductal tumors: a unique entity. Acta Neurochir. 2015;157(2):169–77.
Ternier J, Wray A, Puget S, Bodaert N, Zerah M, Sainte-Rose C. Tectal plate lesions in children. J Neurosurg. 2006;104(6 Suppl):369–76.
Poussaint TY, Kowal JR, Barnes PD, Zurakowski D, Anthony DC, Goumnerova L, et al. Tectal tumors of childhood: clinical and imaging follow-up. AJNR Am J Neuroradiol. 1998;19(5):977–83.
Bergthold G, Bandopadhayay P, Hoshida Y, Ramkissoon S, Ramkissoon L, Rich B, et al. Expression profiles of 151 pediatric low-grade gliomas reveal molecular differences associated with location and histological subtype. Neuro-Oncology. 2015;17(11):1486–96.
Drake J, Chumas P, Kestle J, Pierre-Kahn A, Vinchon M, Brown J, et al. Late rapid deterioration after endoscopic third ventriculostomy: additional cases and review of the literature. J Neurosurg. 2006;105(2):118–26.
Boydston WR, Sanford RA, Muhlbauer MS, Kun LE, Kirk E, Dohan FC, et al. Gliomas of the tectum and periaqueductal region of the mesencephalon. Pediatr Neurosurg. 1991;17(5):234–8.
Robertson PL, Muraszko KM, Brunberg JA, Axtell RA, Dauser RC, Turrisi AT. Pediatric midbrain tumors: a benign subgroup of brainstem gliomas. Pediatr Neurosurg. 1995;22(2):65–73.
Stark AM, Fritsch MJ, Claviez A, Dörner L, Mehdorn HM. Management of tectal glioma in childhood. Pediatr Neurol. 2005;33(1):33–8.
Yeh DD, Warnick RE, Ernst RJ. Management strategy for adult patients with dorsal midbrain gliomas. Neurosurgery. 2002;50(4):735–8; discussion 8-40.
Bowers DC, Georgiades C, Aronson LJ, Carson BS, Weingart JD, Wharam MD, et al. Tectal gliomas: natural history of an indolent lesion in pediatric patients. Pediatr Neurosurg. 2000;32(1):24–9.
Javadpour M, Mallucci C. The role of neuroendoscopy in the management of tectal gliomas. Childs Nerv Syst. 2004;20(11–12):852–7.
Roth J, Constantini S. Combined rigid and flexible endoscopy for tumors in the posterior third ventricle. J Neurosurg. 2015;122(6):1341–6.
Weiner HL, Placantonakis DG. Resection of a pediatric thalamic juvenile pilocytic astrocytoma with whole brain tractography. Cureus. 2017;9(10):e1768.
Recinos PF, Raza SM, Jallo GI, Recinos VR. Use of a minimally invasive tubular retraction system for deep-seated tumors in pediatric patients. J Neurosurg Pediatr. 2011;7(5):516–21.
Essayed WI, Singh H, Lapadula G, Almodovar-Mercado GJ, Anand VK, Schwartz TH. Endoscopic endonasal approach to the ventral brainstem: anatomical feasibility and surgical limitations. J Neurosurg. 2017;127(5):1139–46.
Bricolo A, Turazzi S. Surgery for gliomas and other mass lesions of the brainstem. Adv Tech Stand Neurosurg. 1995;22:261–341.
Sano K. Temporo-polar approach to aneurysms of the basilar artery at and around the distal bifurcation: technical note. Neurol Res. 1980;2(3–4):361–7.
Fujii K, Lenkey C, Rhoton AL. Microsurgical anatomy of the choroidal arteries: lateral and third ventricles. J Neurosurg. 1980;52(2):165–88.
Wen HT, Rhoton AL, de Oliveira E. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42(6):1205–17; discussion 17-9.
Bozkurt B, Yağmurlu K, Belykh E, Tayebi Meybodi A, Staren MS, Aklinski JL, et al. Quantitative anatomic analysis of the transcallosal-transchoroidal approach and the transcallosal-subchoroidal approach to the floor of the third ventricle: an anatomic study. World Neurosurg. 2018;118:219–29.
Patel P, Cohen-Gadol AA, Boop F, Klimo P. Technical strategies for the transcallosal transforaminal approach to third ventricle tumors: expanding the operative corridor. J Neurosurg Pediatr. 2014;14(4):365–71.
Nakov VS, Spiriev TY, Todorov IT, Simeonov P. Technical nuances of subtemporal approach for the treatment of basilar tip aneurysm. Surg Neurol Int. 2017;8:15.
Ulm AJ, Tanriover N, Kawashima M, Campero A, Bova FJ, Rhoton A. Microsurgical approaches to the perimesencephalic cisterns and related segments of the posterior cerebral artery: comparison using a novel application of image guidance. Neurosurgery. 2004;54(6):1313–27; discussion 27-8.
Yamamoto I. Pineal region tumor: surgical anatomy and approach. J Neuro-Oncol. 2001;54(3):263–75.
Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35(2):197–202.
Stein BM. Supracerebellar-infratentorial approach to pineal tumors. Surg Neurol. 1979;11(5):331–7.
Figueiredo EG, Beer-Furlan A, Welling LC, Ribas EC, Schafranski M, Crawford N, et al. Microsurgical approaches to the ambient cistern region: an anatomic and qualitative study. World Neurosurg. 2016;87:584–90.
Kim IY, Jung S, Moon KS, Jung TY, Kang SS. Neuronavigation-guided endoscopic surgery for pineal tumors with hydrocephalus. Minim Invasive Neurosurg. 2004;47(6):365–8.
Ausman JI, Malik GM, Dujovny M, Mann R. Three-quarter prone approach to the pineal-tentorial region. Surg Neurol. 1988;29(4):298–306.
Patel PG, Cohen-Gadol AA, Mercier P, Boop FA, Klimo P. The posterior transcallosal approach to the pineal region and posterior third ventricle: intervenous and paravenous variants. Oper Neurosurg (Hagerstown). 2017;13(1):77–88.
Sun Q, Zhao X, Gandhi S, Tayebi Meybodi A, Belykh E, Valli D, et al. Quantitative analysis of ipsilateral and contralateral supracerebellar infratentorial and occipital transtentorial approaches to the cisternal pulvinar: laboratory anatomical investigation. J Neurosurg. 2019:1–10.
Akiyama O, Matsushima K, Gungor A, Matsuo S, Goodrich DJ, Tubbs RS, et al. Microsurgical and endoscopic approaches to the pulvinar. J Neurosurg. 2017;127(3):630–45.
Liu JK. Endoscopic-assisted interhemispheric parieto-occipital transtentorial approach for microsurgical resection of a pineal region tumor: operative video and technical nuances. Neurosurg Focus. 2016;40(Video Suppl 1):2016.1.FocusVid.15450.
Tanikawa M, Yamada H, Sakata T, Hayashi Y, Sasagawa Y, Watanabe T, et al. Exclusive endoscopic occipital transtentorial approach for pineal region tumors. World Neurosurg. 2019;131:167. [Epub ahead of print].
Foley R, Boop F. Tractography guides the approach for resection of thalamopeduncular tumors. Acta Neurochir. 2017;159(9):1597–601.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shimony, N., Hersh, D.S., Boop, F.A. (2020). Surgical Approaches to Mesencephalic (Midbrain) Tumors. In: Jallo, G., Noureldine, M., Shimony, N. (eds) Brainstem Tumors. Springer, Cham. https://doi.org/10.1007/978-3-030-38774-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-38774-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38773-0
Online ISBN: 978-3-030-38774-7
eBook Packages: MedicineMedicine (R0)