Abstract
Precision medicine through liquid biopsy represents an emerging approach in the management of cancer. The CTC count in blood samples from patients with advanced breast cancer is a powerful prognostic factor for both progression free and overall survival. Moreover, high levels of CTCs at any time during the treatment can reliably predict progression before imaging studies and/or tumor markers. Furthermore, there are works on the molecular characterization of the CTCs and their potential ability to guide the treatment in a dynamic way. However, their role remains controversial. Detection and enumeration of CTCs is variable among different tumors and is subjected to biases related mainly to their methodology, which is not completely standardized. In addition, they must demonstrate their clinical value to guide the treatment and a translation on patient’s survival.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Metastatic breast cancer
- Circulating tumor cells (CTCs)
- Prognostic value
- Treatment monitoring
- Precision oncology
10.1 Introduction
Advanced or metastatic breast cancer (MBC) is still an incurable disease, although the introduction of modern systemic therapies has improved prognosis. The current median overall survival time is approximately 2 years, varying from a few months to several years, depending on the molecular subtype and treatments received. As more knowledge is gathered regarding the specific molecular alterations of MBC, it becomes essential to define both prognostic (provide information on the evolution of the disease) and predictive factors (report on efficacy to a specific treatment). Likewise, techniques with the capacity to guide the treatment are needed, thus contributing to a better selection of specific therapies.
Detecting and isolating circulating tumor cells (CTCs) in the blood of patients with MBC is possible due to the development of very sensitive techniques. Although several commercially available methods exist for this detection, CellSearch® (Menarini Silicon Biosystems, Inc) is the only one approved in the United States for clinical use. Currently there are two main research lines related to CTCs in MBC. Firstly it was demonstrated that the CTC count before and during systemic treatment is a prognostic factor. This reflects the progression or response of disease to the treatment, so CTCs monitoring could help to identify earlier patients who do not benefit from therapy; however, an early change in treatment based on CTC count has not shown any survival benefit so far. Secondly, on-going clinical trials are looking into patient benefit from receiving targeted therapies based on the molecular profile of isolated CTCs. In this chapter we will revise these questions.
10.2 Prognostic Value of CTCs
10.2.1 Pivotal Study
The first study that confirmed the clinical applicability of CTCs in patients with MBC were published in 2004 [1]. Number of CTCs with a cut-off of 5 per 7.5 ml of blood (CellSearch®) was prognostic factor for progression-free survival (PFS) and overall survival (OS), regardless of other clinical factors. This was a prospective study that included 177 MBC patients with heterogeneous characteristics: all molecular subtypes, different metastatic locations, and pre-treated or not. Minimal follow-up was 38.5 weeks. In the group of 87 patients with basal ≥5 CTCs/7.5 ml (49%), the median PFS and OS were 2.7 (95% CI 2.1–4.4) and 10.1 months (95% CI 6.3–14.6), respectively. In the 90 patients with <5 CTCs (51%), median PFS and OS were 7 (95% CI 5.8–8.9) and more than 18 months, respectively. They also observed that with <5 CTCs at baseline but ≥5 CTCs at the first follow-up visit (n = 5), the results were similar to the poor prognosis group. In contrast, patients with high baseline scores in whom counts decreased below 5 at first follow-up visit (n = 33), had comparable results to the good prognosis group. Finally, in those patients with high baseline CTCs that decreased but not <5 (n = 25), results did not correspond with the good prognosis group.
10.2.2 Other Studies
Although some studies have been published with inconclusive results, the vast majority of subsequent trials (detection ranges 31–61%), and at least two meta-analyses have validated the presence of ≥5 CTCs/7.5 ml as a negative independent prognostic factor in patients with MBC, as well as its value as a dynamic biomarker in different moments of the disease [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Some of these studies will be reviewed in a later section.
A meta-analysis published in 2012 confirmed this prognostic value of CTCs, both in early and advanced disease, at different times of treatment, and using different techniques: immunocytochemistry (CellSearch®) or RT-PCR (“real time polymerase chain reaction”), also suggesting the need to standardize the methodology. In this meta-analysis, both the HR for PFS (12 studies, HR 1.78) and OS (19 studies, HR 2.33) were statistically significant in MBC population (n = 3065) [18]. In a subsequent meta-analysis with 24 studies in MBC patients (n = 3701), it was noted that CTCs are more frequently detected in primary HER2 + tumors with respect to other subtypes (RR = 0.73); and that high counts indicated worse responses to therapy (RR = 0.56), and poorer PFS (RR = 0.64) and OS (RR = 0.69) [19].
10.2.3 Clinical Value of the CTCs
Some studies have been published demonstrating CTC counts have more value than other clinical prognostic markers, such as plasma tumor markers [20]. Correlation between CTC count, radiological evaluation and patient survival has also been studied [21].
In 2014 a retrospective joint analysis from 1944 MBC patients who had participated in 20 studies in several European centers (EPAC Consortium) was published [22]. All patients had a baseline CTC count, prior to starting treatment. In addition, other clinical-pathological variables were collected, as well as new CTC counts. Based on these data, investigators developed a clinical prognostic model for PFS and OS and then assessed the added value of including CTC and serum marker levels to that model. At baseline 47% of patients had ≥5 CTCs/ 7.5 ml. This group presented worse PFS (HR 1.92, 95% CI 1.73–2.14) and OS (HR 2.78, 95% CI 2.42–3.19) than <5 CTC/7.5 ml group (Fig. 10.1). Increase in CTC count reflected tumor burden, but it did not correlate with tumor subtype [22]. The increase in the CTC count 3–5 weeks after starting treatment was also associated with worse PFS (HR 1.85, 95% CI 1.48–2.32) and OS (HR 2.26, 95% CI 1.68–3.03) (Fig. 10.2). Finally, survival prediction improved when adding CTC count to the clinical-pathological models. Furthermore, prediction was even more accurate by adding changes in CTC count at 3–5 and at 6–8 weeks. On the other hand, adding CEA and CA 15-3 changes did not provide significant information. The conclusion is that initial CTC count, as well as early changes after treatment initiation, results in a strong and independent prognostic marker which adds value to the classic clinical variables. So, the authors propose to use prognostic information based on CTC counts to stratify patients within clinical trials, and to check prospectively if efficacy objectives (such as OS and PFS) are improved by CTCs monitorization [22].
Kaplan-Meier analysis of progression-free survival and overall survival, by baseline CTC count. (a) PFS. (b) OS. (Reproduced from [22])
Kaplan-Meier analysis of progression-free survival and overall survival, by early change in CTC count. (a) PFS. (b) OS. (Reproduced from [22])
Furthermore it has been suggested that the prognostic value of CTCs could vary according to MBC subtype. In a retrospective study with 517 patients, baseline CTC count showed prognostic value in all subtypes, more significant in hormone receptor positive (luminal) and triple negative, and less significant in HER2+ tumors, suggesting interaction between CTCs and treatments [23]. These results were reproduced in another retrospective study with 235 patients, confirming the prognostic value of CTC count in the global population and in patients treated with chemotherapy and endocrine therapy. In those treated with bevacizumab or anti-HER2 therapies, the negative prognostic value of baseline elevated CTC levels was lost, suggesting the therapeutic benefit of these drugs [12].
A recent combined analysis of individual data from patients with MBC from the 17 centers of the EPAC Consortium [22] plus a series from MD Anderson Cancer Center in Houston (n = 2436), was done. The authors propose evaluating the aggressiveness (prognosis) of the disease according to CTC count and classifying stage IV into two subgroups: IV-indolent and IV-aggressive. They consider the need to stratify patients based on this classification, and then assessing the molecular and clinical factors to finally evaluate the true impact of treatments [24]. After CTC collection, 44% of patients were treated with chemotherapy; 37% with chemotherapy plus a biologic or targeted therapy; 13% with endocrine monotherapy; and the remaining 6% was classified as others. With a median follow-up of 15 months, there was a statistically significant difference in OS (36.3 vs 16.0 months, p < 0.0001) in patients with IV-indolent versus IV-aggressive stages (Fig. 10.3a). CTC count was also able to stratify patients with de novo disease (OS 41.4 vs 18.7 months, p < 0.0001). Likewise, OS was significantly better in IV-indolent regardless of prior treatments and disease location. According to the molecular subtypes, median OS was also significantly larger in IV-indolent versus IV-aggressive, both in hormone receptor (ER) positive (40.7 vs. 17.3 months) and in triple negative (23.8 vs 9.1 months), as well as in HER2+ (33.2 vs 19.4 months) (Fig. 10.3b–d). CTC count was the most significant predictor of all covariates (HR 2.71, 95% CI 2.35–3.12). Fig. 10.3e shows the forest plot for OS according to the different subgroups. To summarize, CTC count is useful to stratify patients with MBC, independently of tumor subtype, line of therapy and disease location.
OS stage IV-indolent versus IV-agressive, entire (a), ER+ (b), HER2+ (c), and triple negative (d) cohorts; and forest plot of OS according to subgroups (e). (Reproduced from [24])
Finally, the eighth edition of the “AJCC Cancer Staging Manual” recognizes a CTC count ≥5/7.5 mL of plasma in patients with MBC as an unfavorable prognostic factor with a level of evidence II. This type of cancer is a pioneer in the incorporation of liquid biopsy findings to define patient risk groups [25]. However, CTC counts, as well as other molecular factors, have not been systematically included in TNM staging because their analysis is not implemented in most centers.
10.3 Characterization and CTCs Heterogeneity
The molecular characterization of CTCs could contribute to a better understanding of tumor biology and their mechanisms of metastatization and resistance. It could potentially contribute to the development of biomarkers and selection of targeted therapies [26]. The genomic profile of CTCs and primary tumors confirm a shared lineage, with some genetic divergence [27] consistent with the formation of metastasis as a result of a single clonal expansion [28]. It is known that the phenotypes and genotypes of the primary tumor, metastasis and CTCs often differ [29]. Ideally, therapeutic decisions should be based on the characteristics of the predominant disease at the time of relapse and at each progression. The characterization of CTCs in peripheral blood can be an alternative to tissue biopsy, as a less invasive and more dynamic test (repeatable, in real time). Hypothetically, CTCs represent the population of dominant tumor cells of a metastatic disease, so their expression profile could theoretically help us to predict the therapeutic response more accurately [30]. However, the identification and characterization of CTCs is not simple and requires extremely sensitive and specific techniques. CTCs represent a dynamic population that can originate in the primary tumor as well as in the metastasis or in both, and its characterization provides us with information, whose clinical utility is yet to be determined [31].
There are studies that show the possibility of molecularly characterizing CTCs and their prognostic correlation, but it has not yet been proved that this can render a prediction to the corresponding targeted therapy response. Research with cell lines derived from CTCs of MBC ER+ patients made it possible to determine sensitivity to new drugs directed against potentially treatable targets [32]. Gene expression studies with a so-called metastasis-initiating cells phenotype have also been published, reporting the induction of metastasis in xenograft assays [33]. But the greatest development on this field is aimed at characterizing biomarkers in CTCs with clinical implications, or gene expression profiles associated with the proliferation and acquisition of mesenchymal or stem cell phenotypes [34,35,36]. EMT shares some stem-cell properties, including resistance to conventional therapies. More than half of CTCs of patients with MBC show EMT and stem markers, whose presence correlates with a genotype more resistant to drugs [37] and with few responses to conventional treatments [38]. These markers may represent a potential therapeutic goal.
HER2 positivity in CTCs ranges between 27–63%. So, CTCs/HER2+ are frequently detected in tumors (tissue) HER2+; but it has also been described primary (tissue) tumor HER2- and CTCs +, and vice versa, in percentages between 49 and 77% [39,40,41,42,43,44]. In a retrospective study the correlation between CTCs and primary was 69%, and with metastasis 74%. It was also observed that the CTC/HER2 + patients presented a PFS significantly longer than the CTC/HER2-, although with no impact on OS [45]. Conversely, Hayashi et al. observed that patients with CTC/HER2 + had a shorter PFS and OS [46]. Another study in which frequent discordance was found, it did not observe a prognostic impact [47]. We do not know if these discrepancies could be due to the administration of different therapies.
The expression of ER in CTC has been less studied. Despite being by far the most frequent phenotype, in early disease only approximately 25% of CTCs are ER+ [48, 49]. Unlike the expression of HER2, which in CTCs seems to be lost or gained with a similar frequency, ER expression is more frequently lost in the evolution from primary to CTC [50]. However, the lack of a unified methodology to determine ER+ in CTCs and the absence of extensive studies limits the value of these findings. Recently, a group has developed the so-called CTC-Endocrine therapy index (CTC-ETI), a score based on CTCs enumeration and characterization of ER, Bcl-2, HER2 and Ki67 using CellSearch®. A high CTC-ETI index was attributed to patients with high CTC counts and with low expression of ER and Bcl-2 and high levels of HER2 and Ki67 [51]. The Phase II COMETI trial (NCT01701050) is evaluating the value of the CTC-ETI score to identify women with refractory endocrine MBC.
In another study, CTCs are characterized by the presence of mutations in PIK3CA, in addition to HER2 expression, as a biomarker for inhibitory drugs already available for clinical use (Alpelisib). Of 290 patients included, PIK3CA mutations were analyzed in 33 patients with >5 CTC/7.5 ml, with great heterogeneity in mutations of PIK3CA and HER2 expression. Therefore, clinically relevant genomic aberrations such as those of PIK3CA are detectable in a single CTC [52]. Other studies have been published regarding the characterization of the PIK3CA status in CTCs of MBC [53]. Another interesting question is the determination of PD-L1 in CTCs. Immunotherapy (Atezolizumab) has already shown benefit in triple negative MBC PD-L1+. One study shows expression of PD-L1 of CTCs in 11 of 16 cases (68.8%) MBC ER +/HER2- patients [54].
Rossi et al. evaluated the usefulness of combining CTCs and circulating tumor DNA (ctDNA) as a prognostic prediction in MBC. Retrospectively, in 91 patients, CTCs were found in 85%, with mutations in 84% of the samples. The genes most frequently mutated were TP53 (52%), PIK3CA (40%) and ERBB2 (20%). A statistically significant difference was observed in PFS and OS for patients with values < 5 CTCs versus ≥5 or more; and in percent of ctDNA <0.5% versus ≥0.5; and having a number of genetic alterations <2 versus ≥2. They conclude that liquid biopsy can be used as an effective prognostic tool and that the characterization of CTCs is viable [55]. It has also been pointed out that epigenetic silencing in the promoter regions of tumor suppressor genes can be confirmed in CTCs of MBC [56].
10.4 CTCs and Monitoring Treatment in Advanced Breast Cancer
The isolation and quantification of CTCs in early or locally advanced [40, 57,58,59,60,61] or MBC has shown independent prognostic value in several clinical trials and meta-analyses [1, 18, 19, 22]. In addition, changes in CTC counts can reflect early the efficacy of treatment and allows the monitoring of the disease [62]. As an example, in a recent study in patients with stage III-IV breast cancer, differences were observed in CTC responses after treatment according to age groups. The authors propose a combination of baseline CTCs along with age as a new potential criterion for treatment selection [63].
10.4.1 Can Changes in the Quantification of CTCs Be Useful as Early Predictor of Treatment Efficacy?
A multicenter study with 177 MBC patients, in addition to others with advanced colorectal and prostate cancer, was done. The prognostic influence of changes in CTC counts during treatment was studied. In all three tumors, persistence of high CTC counts was related with worse OS, suggesting that treatment was not being effective; unlike those patients who showed a decrease below the unfavorable chosen cut-off (≥5 CTC/7.5 ml for MBC), in whom the prognosis improved [64].
The first study in MBC showing the usefulness of monitoring CTCs as a predictor of response is performed in 68 patients treated with chemo- or hormone therapy. In addition to standard radiological evaluations every 3 months, CTCs were quantified at the beginning of treatment and with each cycle for the first 6 months, and then with each radiological evaluation. A strong correlation was demonstrated between CTC monitoring and radiological progression of the disease. Moreover, changes in CTC counts suggested progression weeks before radiologic evaluation. The authors conclude that serial determination of CTCs can identify treatment efficacy earlier than the standard evaluation [15]. In addition, we have previously mentioned results by Bidard et al., which also demonstrated the clinical value of CTC monitoring in MBC, as well as the limited validity of serum tumor markers [22]. Likewise, in another study in 117 patients with MBC, CTC counts were taken at baseline, before the first cycle of chemotherapy and before the second. Patients with <5 CTC on day 21 had significantly better clinical benefit rate (77 vs 44%), PFS (9.4 vs 3 months) and OS (38.5 vs 8.7 months) versus those with ≥5 CTCs [16]. Other authors report similar results [13, 21].
Finally, a recent meta-analysis including 50 studies with 6712 patients with early and advanced breast cancer confirms CTC levels as predictors of response to treatment [65]. Therefore, it can be concluded that changes in the quantification of CTCs during treatment are predictors of efficacy earlier than standard radiological assessment.
10.4.2 Do Decisions Guided by the Use of CTCs Have an Impact on Treatment Efficacy Outcomes?
Before generalizing its routine clinical use, it must be demonstrated that patients with persistently elevated CTCs during systemic treatment benefit from early change of the therapeutic regimen, in efficacy parameters (PFS, OS), safety (avoiding toxic therapies) and/or in cost reduction (avoiding inefficient and expensive treatments and procedures). Phase III prospective interventional clinical trials investigating these issues in MBC have been designed [66].
In the SWOG 0500 study (NCT00382018), patients in first line of chemotherapy with baseline count ≥5 CTCs/7.5 ml, who maintained high levels (≥5) after the first cycle of treatment, were randomized to continue the same regimen (until radiological or clinical progression) or to change early to a second line. Between 2006 and 2012, 624 patients were screened, of which 288 were randomized. No differences were observed in OS or PFS between the treatment arms: 10.7 vs 12.5 and 3.5 vs 4.6 months, respectively. Investigators conclude that this situation indicates chemoresistance, and the lack of an effective alternative therapy could explain the absence of impact on the outcomes [17].
The French trial CirCe01 (NCT01349842) includes 304 patients in the third line of chemotherapy with CTC levels ≥5/7.5 ml that are randomized to standard management based on clinical-radiological evaluation or based on CTC dynamics. The primary endpoint is OS benefit, with other secondary endpoints including economic analysis. The results of this trial are not available. Another French study, the STIC-CTC METABREAST (NCT01710605), planned a recruitment of 994 patients with endocrine-dependent MBC, where the choice of first-line treatment is based on the levels of CTCs: endocrine therapy for a count of <5 CTCs/7.5 ml or chemotherapy for ≥5. The results are also not available.
These and other smaller similar studies (COMETI/NCT01701050, CTC-EMT/ NCT02025413, PRO OncAssay/NCT01048099, Trastuzumab & Vinorelbine/NCT 01185509) try to demonstrate that the persistence of elevated CTC levels during treatment indicates early ineffectiveness and that patients would benefit from an early change to another effective treatment (if any). On the other hand, toxicities and unnecessary risks for patients and extra costs for the system would be avoided.
10.5 Precision Oncology and CTCs in Advanced Breast Cancer
Previously reviewed approaches would reach their maximum clinical utility used as a dynamic treatment guide, according to the molecular alterations found in the CTCs, and showing a favorable clinical impact for the patient. This should be the ideal expression of the precision oncology. In this sense, a review and classification of genomic alterations of breast cancer according to their level of evidence for actionability has been published, following a scale developed by the European Society of Medical Oncology (ESMO), the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT). Large databases analyzed suggested around 40 recurrent driver alterations. Clinical trials were reviewed following various sources to evaluate the efficacy of drugs matched to these genomic alterations. The targetability for most studied alterations was graded according to the ESCAT scale, which classifies the molecular target at different levels (I–V and X) according to the available evidence. An important limitation of this classification is that it focuses on DNA alterations. In level I, alteration-drug match is associated with benefit in clinical trials, so access to treatment should be considered standard. In level II, it is considered that there is evidence of activity with drugs associated with the alteration, but without information on the magnitude of the benefit due to a lack of prospective data. In this way, amplification of ERBB2, germline mutations of BRCA1/2 and PIK3CA mutations were classified at level of evidence IA. NTRK fusions and microsatellite instability (MSI) were classified as IC. Mutations in ESR1 and loss of PTEN were classified in level IIA; and mutations in ERBB2 and AKT1 in level IIB [67].
10.5.1 Comparison of Primary Tumor Molecular Profile Versus CTCs
The knowledge of the correlation between molecular alterations of CTCs and the primary tumor is essential to support precision oncology. In one study with 62 MBC patients expression levels of 35 genes were studied; and in 48% the profile was discrepant between CTCs and primary tumor. In 24% ER was different, and patients with primary ER- and CTCs/ER+ presented a significant median time to treatment longer (8.5 versus 2.1 months). It is concluded that differences in the ER status could have therapeutic and prognostic implications [68]. Another study showed that some CTCs from patients with tumors originally ER+/HER2- could acquire a HER2+ phenotype and/or activation of different signaling pathways under therapeutic pressure. The coexistence or conversion between these states could make it easier for tumor cells to overcome stressors [69]. However, a phase II study failed to prove benefit with Lapatinib as a single agent in patients with HER2- initial tumors and CTCs/HER2+ [70].
10.5.2 Circulating Stem Cells
It is considered that circulating stem cells represents a particularly aggressive, invasive and proliferative subgroup of MBC, which makes them a target of great value [71]. In vivo xenograft models anti-CD44 antibodies (stem-cell marker) reduced tumor growth [72]. They are currently being investigated inhibitor tirosine-kinase drugs for PAR6A, Notch1, Hedgehog, Wnt, integrins, claudins, and Rho GTPases, all of them signaling pathways activated in stem cells or involved in the regulation of EMT [73].
10.5.3 CTCs, ESR1 and TK1: Importance in the Endocrine Treatment
One of the most studied mechanisms of endocrine acquired resistance is the appearance of specific mutations in the ESR1 gene. It has been related to lower response and resistance to aromatase inhibitors. One study evaluated ESR1 mutations in CellSearch®-enriched CTCs of patients with MBC on endocrine treatment. In cohort 1 were included patients in first line endocrine treatment (n = 43), and in cohort 2 patients progressing in any line of endocrine therapy (n = 40). In a subgroup of them, the mutation status of ESR1 in CTCs and paired cfDNA of each patient was compared. They observed that the mutation of ESR1 in the CTCs was not enriched in cohort 2 (8%) compared to the reference cohort (5%). Instead, in the cfDNA the ESR1 mutation was enriched in cohort 2 (42%) compared to the reference cohort (11%). Therefore, the sensitivity to detect mutations in cfDNA was higher than in the fractions enriched with CTCs. In addition, they concluded that ESR1 mutations are essential in the endocrine treatment resistance [74]. Another work studied the ESR1 methylation in tissue, CTCs and ctDNA of paired plasma in patients with MBC ER+ treated with Everolimus and Exemestane, combination usually used in second or third line. Methylation was detected in 38.5%, 23.3% and 7.4% of tissue samples, CTCs and ctDNA, respectively. Also, correlation was observed between methylated ESR1 and lack of treatment response [75].
Finally, one study analyzed the role of thymidine kinase-1 (TK1, proliferation marker) in blood, CTC counts and mutations of ESR1 and PIK3CA in ctDNA of patients with MBC ER+/HER2-, and the correlation with the benefit of endocrine therapy. A high level of baseline TK1 activity and a high CTC count were observed in the cases with worse PFS rates, as well as a lower response to endocrine treatment. The study concluded that the analysis of TK1 activity together with the CTC count can be considered as a possible prognostic, predictive and monitoring marker for endocrine therapy [76].
10.5.4 Choice of Treatment According to CTCs in Advanced Breast Cancer
Beyond the studies that evaluate the clinical utility of enumeration and characterization of a limited number of markers, CTCs could be a source of tissue for molecular screening. Several groups have demonstrated the feasibility of analyzing enriched fractions or pure CTCs and study the expression of a series of preselected transcripts, what has revealed a wide heterogeneity of CTCs at the transcriptional level [77, 78]. In addition, efforts are directed to identify mutational profiles of CTCs in various types of cancer. We also have evidence that they can be used as a tissue source for drug sensitivity testing. In fact, the ex vivo culture of CTCs allowed the identification of mutations in ESR1 in three of six cell lines derived from CTCs from patients with MBC ER+ pre-treated with aromatase inhibitors [32]. These mutations are very rarely observed in primary tumors or without previous treatment. Using these cell lines derived from CTCs, these mutations were confirmed as conferring resistance to Tamoxifen, Raloxifene and Fulvestrant, and sensitiveness to Raloxifene or Fulvestrant combination with an HSP90 inhibitor [32].
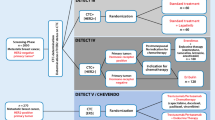
Whether we can choose and/or guide the systemic treatment in patients with MBC according to CTCs and its phenotype is the objective of several interventional on-going trials, whose results are pending. The DETECT trials investigate the therapeutic selection according to levels of CTCs and/or their phenotype. The accompanying translational research of all of them attempt to generate additional knowledge [79]. There are three studies depending on the MBC subtype: DETECT III, DETECT IV and DETECT V. In the first two trials, presence of CTCs is mandatory for inclusion and changes in their levels during treatment are evaluated by several blood samples. DETECT III (NCT01619111) includes patients with HER2- tumors and at least one positive CTC for HER2, randomized to receive standard systemic treatment at the physician’s choice versus +/− Lapatinib. Patients with MBC HER2- and CTCs HER2- were included in DETECT IV trial (NCT02035813), receiving endocrine therapy plus Everolimus in ER+ tumors, or Eribulin (cytotoxic) if ER+ with clinical indication of chemotherapy or triple negatives tumors. In DETECT V study (NCT02344472), HER2+ tumors are included and treated with dual targeted therapy (Pertuzumab/Trastuzumab) in combination with chemotherapy or endocrine therapy (based on their expression of hormone receptors).
10.6 Discussion and Comments
Precision medicine through liquid biopsy represents an emerging and unstoppable approach in the management of cancer, which considers the intra- and inter-tumoral genetic variability, and which is transforming biomedical research [80]. In Spain, a proposal for a national strategy has been developed to regulate its implementation, guaranteeing technical quality and equitable access to its use, while also safeguarding the sustainability of the national health system [81]. Beyond their enumeration, CTC technologies advance towards the use of these cells as an accessible and valid source for dynamic analysis of the tumor. It is particularly interesting to know, as soon as possible and for each progression, the probability of response to treatment as well as the identification of resistances. However, detection and enumeration of CTCs is very variable among different tumors and is subject to biases related mainly to their detection methodology, which is not completely standardized. In addition, they must demonstrate their value to guide the treatment with clinical translation on patient’s survival.
The CellSearch® platform is the only one licensed by the FDA for the isolation of CTCs and their prognostic enumeration in breast, colorectal and prostate cancer [64]. We have not yet reached the maximum benefit that CTCs can offer, and more evidence from prospective studies is needed for its use in another settings. In this way, they must prove to be a representative and relevant sample of the tumor biology versus the tissue samples or other liquid biopsy techniques (ctDNA, ctRNA, exosomes…). Regarding tissue biopsy, CTCs have the advantage of the accessibility of blood or other fluids, which implies the possibility of carrying out samples repeatedly in a non-invasive manner, providing us with real-time information on tumor variability [82].
The detection and measurement of free or tumor DNA (cf/ctDNA) as a biomarker has been widely developed. Dawson et al. evaluated their value in monitoring treatment response in MBC, comparing ctDNA with CA 15-3 and CTCs in 30 patients who received chemotherapy. Both ctDNA and CTC count were associated with worse prognosis, while CA 15-3 levels did not [83]. In a retrospective study from 117 patients with MBC also was reported that CTCs and ctDNA presented a similar prognostic value [84]. The analysis of ctDNA is attractive because the plasma can be easily extracted and analyzed without the prior need to isolate and enrich a small population of cells, and it is possible to identify it in the absence of detectable CTCs. For this reason it is likely that ctDNA analysis is the preferred option for genotyping and monitoring the response to treatment [85]. However, both techniques can provide complementary information.
The analysis of CTCs provides the opportunity to study the entire cell, with its morphological assessment, also providing DNA, RNA, proteins, and the opportunity to perform ex vivo functional studies and cultures. An important limitation of CTCs is that they may not fully reflect the biology of the underlying tumor [86]. In addition, there are several phenotypes within them, epithelial, epithelial-mesenchymal, mesenchymal, and stem-like [87]. However, the standard CellSearch® platform uses epithelial markers expression and excludes those of epithelial-mesenchyme transition [36] and stemness, so that these phenotypes may not be detected. Finally, it is possible that CTCs do not reflect exactly intertumoral heterogeneity but they detect a specific subpopulation [78]. For all of this, some panels of experts have concluded that the CTCs should not be used to influence treatment decisions in MBC at this time [88, 89]. It is necessary to know results of prospective and randomized clinical trials that allow us to confirm the validity of CTCs monitoring and especially the clinical impact of an early change of molecularly and dynamically guided treatment. In addition, according to the hypothesis of the aforementioned study compiled by Cristofanilli et al. despite the significant benefit of the drugs, the joint inclusion in the studies of indolent and aggressive disease can negatively impact the final results. Their findings suggest that clinical and molecular variables are insufficient to adequately stratify patients and that this heterogeneity can be reduced by considering their two subgroups of stage IV, as a step towards a more individualized approach [24].
Recently the presence of clusters of CTCs has been valued. In preclinical models, their oligoclonal nature increases up to fifty times the ability to develop distant metastases against isolated CTCs [90, 91]. It is suggested that these clusters with subclonal alteration profiles can initiate mechanisms of oncogenic cooperation and that their analysis can be highly informative of the biology of the tumor. On the other hand, once isolated CTCs ex vivo, it is possible to expand them in cell lines or in immunocompromised murine models and establish xenograft models (CDX), with molecular profiles identical to those of origin [33, 92].
It has been extensively confirmed that high levels of CTCs at any time during MBC treatment are associated with tumor progression and can reliably predict it before imaging studies and/or classic tumor markers, pointing out resistance earlier. In addition, as we have also indicated, there are works on the molecular characterization of the CTCs and on their potential ability to direct and individualize the treatment in a dynamic way. However until now, the role of CTCs and liquid biopsy techniques in the follow-up of patients with advanced disease is controversial, partly due to the absence of powerful predictive biomarkers and effective treatments.
Different studies suggest that use of molecularly directed agents outside their indications does not necessarily improve outcomes versus standard care in patients with pre-treated MBC. The SAFIR01 trial aimed to define the proportion of patients in whom targeted therapy could be offered based on the results of the genomic analyses. A total of 423 patients with MBC were included; however, only 55 patients (13%) received targeted therapy on a genomic basis. Of these, only 4 had an objective response and 9 showed no evidence of disease progression for ≥16 weeks, assuming a clinical benefit in 13 of 55 patients (23.6%) [93]. Similarly, in the randomized phase II trial SHIVA, of 741 patients with solid tumors pre-treated and refractory to standard therapies, 293 had a specific molecular alteration and 195 (40 with MBC) were assigned to receive an agent directed by molecular alteration or to standard treatment. It was stratified based on three signaling pathways: hormonal receptor, PI3K/AKT/mTOR and RAF/MEK. Among treated patients, median PFS was 2.3 months with targeted therapy versus 2 months in control group [94]. By contrast, a large meta-analysis of 570 phase II studies (32149 patients) showed better results with a personalized versus a non-personalized approach, with significant higher responses ratio (31% vs 10.5%), median PFS and OS (5.9 vs 2.7 and 13.7 vs 8.9 months, respectively) [95]. Together, these data suggest that it is possible to identify genomic alterations in MBC and in other tumors. However, a greater evaluation is necessary on the predictive capacity of these findings; and more important, to have proven drugs against this molecular alterations, before using them in daily clinical practice.
The new NGS and ddPCR technologies have a good analytical validity, but more work is needed to establish their usefulness and the added clinical value of the expansion from individual genetic tests to large genetic panels. Experts agree that we need standardized bioinformatic methods for the interpretation of genomic data and that trials in precision medicine should be stratified according to the level of evidence available for the genomic alterations identified [96]. Thus, in breast cancer five potent markers can currently be used to indicate treatment: expression of the estrogen receptor, progesterone receptor (PgR), Her2 proteins (ERBB2), BRCA mutations, mutations in PIK3CA and expression of PD-L1. According to some authors, an optimal panel for breast cancer clinical trials could add mutations of AKT1, PTEN, ESR1, KRAS, BRAF, NF1, other HRD genes (RAD, ATM, ATR), and amplifications of NOTCH3, CCND1, CDK4, Rb, IGFR1 or FGFR1 [97].
References
Cristofanilli M, Reuben JM, Budd GT, Ellis MJ, Stopeck A, Matera J, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91.
Weigelt B, Bosma AJ, Hart AA, Rodenhuis S, van ’t Veer LJ. Marker genes for circulating tumor cells predict survival in metastasized breast cancer patients. Br J Cancer. 2003;88(7):1091–4. https://doi.org/10.1038/sj.bjc.6600868.
Meng S, Tripathy D, Shete S, Ashfaq R, Saboorian H, Haley B, et al. uPAR and HER-2 gene status in individual breast cancer cells from blood and tissues. Proc Natl Acad Sci U S A. 2006;103(46):17361–5. https://doi.org/10.1073/pnas.0608113103.
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23(7):1420–30. https://doi.org/10.1200/JCO.2005.08.140.
Wong NS, Kahn HJ, Zhang L, Oldfield S, Yang LY, Marks A, et al. Prognostic significance of circulating tumor cells enumerated after filtration enrichment in early and metastatic breast cancer patients. Breast Cancer Res Treat. 2006;99(1):63–9. https://doi.org/10.1007/s10549-006-9181-4.
Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the cell search system. Clin Cancer Res. 2007;13(3):920–8. https://doi.org/10.1158/1078-0432.CCR-06-1695.
Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920–8. https://doi.org/10.1158/1078-0432.CCR-06-1695.
Nole F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol. 2008;19(5):891–7. https://doi.org/10.1093/annonc/mdm558.
Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, et al. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer. 2010;17(3):199–204. https://doi.org/10.1007/s12282-009-0139-3.
Dawood S, Broglio K, Valero V, Reuben J, Handy B, Islam R, et al. Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer. 2008;113(9):2422–30. https://doi.org/10.1002/cncr.23852.
Consoli F, Grisanti S, Amoroso V, Almici C, Verardi R, Marini M, et al. Circulating tumor cells as predictors of prognosis in metastatic breast cancer: clinical application outside a clinical trial. Tumori. 2011;97(6):737–42. https://doi.org/10.1700/1018.11090.
Giuliano M, Giordano A, Jackson S, Hess KR, De Giorgi U, Mego M, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011;13(3):R67. https://doi.org/10.1186/bcr2907.
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–24. https://doi.org/10.1158/1078-0432.CCR-05-2821.
Helissey C, Berger F, Cottu P, Dieras V, Mignot L, Servois V, et al. Circulating tumor cell thresholds and survival scores in advanced metastatic breast cancer: the observational step of the CirCe01 phase III trial. Cancer Lett. 2015;360(2):213–8. https://doi.org/10.1016/j.canlet.2015.02.010.
Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27(31):5153–9. https://doi.org/10.1200/JCO.2008.20.6664.
Martin M, Custodio S, de Las Casas ML, Garcia-Saenz JA, de la Torre JC, Bellon-Cano JM, et al. Circulating tumor cells following first chemotherapy cycle: an early and strong predictor of outcome in patients with metastatic breast cancer. Oncologist. 2013;18(8):917–23. https://doi.org/10.1634/theoncologist.2012-0479.
Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32(31):3483–9. https://doi.org/10.1200/JCO.2014.56.2561.
Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012; https://doi.org/10.1158/1078-0432.CCR-12-1587.
Lv Q, Gong L, Zhang T, Ye J, Chai L, Ni C, et al. Prognostic value of circulating tumor cells in metastatic breast cancer: a systemic review and meta-analysis. Clin Transl Oncol. 2016;18(3):322–30. https://doi.org/10.1007/s12094-015-1372-1.
Pierga JY, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol. 2012;23(3):618–24. https://doi.org/10.1093/annonc/mdr263.
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12(21):6403–9. https://doi.org/10.1158/1078-0432.CCR-05-1769.
Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–14. https://doi.org/10.1016/S1470-2045(14)70069-5.. Figures 10.1 and 10.2. Reprinted from The Lancet Oncology, 15, François-Clément Bidard, Dieter J Peeters, Tanja Fehm, Franco Nolé, Rafael Gisbert-Criado, Dimitrios Mavroudis, Salvatore Grisanti, Daniele Generali, Jose A Garcia-Saenz, Justin Stebbing, Carlos Caldas, Paola Gazzaniga, Luis Manso, Rita Zamarchi et al., Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data, 406–414. Copyright (2014), with permission from Elsevier. Also, Reprinted from The Lancet, 15, François-Clément Bidard, Dieter J Peeters, Tanja Fehm, Franco Nolé, Rafael Gisbert-Criado, Dimitrios Mavroudis, Salvatore Grisanti, Daniele Generali, Jose A Garcia-Saenz, Justin Stebbing, Carlos Caldas, Paola Gazzaniga, Luis Manso, Rita Zamarchi et al., Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data, 406–414. Copyright (2014), with permission from Elsevier
Giordano A, Giuliano M, De Laurentiis M, Arpino G, Jackson S, Handy BC, et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol. 2012;23(5):1144–50. https://doi.org/10.1093/annonc/mdr434.
Cristofanilli M, Pierga JY, Reuben J, Rademaker A, Davis AA, Peeters DJ, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol. 2019;134:39–45. https://doi.org/10.1016/j.critrevonc.2018.12.004.. Figure 10.3. Reprinted from Critical Reviews in Oncology/Hematology, Vol. 134, Massimo Cristofanilli, Jean-Yves Pierga, James Reuben, Alfred Rademaker, Andrew A. Davis, Dieter J. Peeters, Tanja Fehm, Franco Nolé, Rafael Gisbert-Criado, Dimitrios Mavroudis, Salvatore Grisanti, Mario Giuliano, Jose A. Garcia-Saenz, Justin Stebbing et al., The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper, 39–45, (2018), with permission from Elsevier
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, editors. AJCC cancer staging manual. 8th ed. New York: Springer International Publishing; 2017.
Magbanua MJ, Park JW. Advances in genomic characterization of circulating tumor cells. Cancer Metastasis Rev. 2014;33(2–3):757–69. https://doi.org/10.1007/s10555-014-9503-7.
Magbanua MJ, Sosa EV, Roy R, Eisenbud LE, Scott JH, Olshen A, et al. Genomic profiling of isolated circulating tumor cells from metastatic breast cancer patients. Cancer Res. 2013;73(1):30–40. https://doi.org/10.1158/0008-5472.CAN-11-3017.
Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, et al. Tumor evolution inferred by single-cell sequencing. Nature. 2011;472(7341):90–4. https://doi.org/10.1038/nature09807.
Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15(11):1164–8. https://doi.org/10.1634/theoncologist.2010-0059.
Banys-Paluchowski M, Krawczyk N, Fehm T. Potential role of circulating tumor cell detection and monitoring in breast cancer: a review of current evidence. Front Oncol. 2016;6:255. https://doi.org/10.3389/fonc.2016.00255.
Ramos-Medina R, Moreno F, Lopez-Tarruella S, Del Monte-Millan M, Marquez-Rodas I, Duran E, et al. Review: circulating tumor cells in the practice of breast cancer oncology. Clin Transl Oncol. 2016;18(8):749–59. https://doi.org/10.1007/s12094-015-1460-2.
Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC, et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345(6193):216–20. https://doi.org/10.1126/science.1253533.
Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31(6):539–44. https://doi.org/10.1038/nbt.2576.
Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5(180):180–48. https://doi.org/10.1126/scitranslmed.3005109.
Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28(25):4006–12. https://doi.org/10.1200/JCO.2009.27.5388.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. https://doi.org/10.1172/JCI39104.
Gradilone A, Naso G, Raimondi C, Cortesi E, Gandini O, Vincenzi B, et al. Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance and phenotypic characterization. Ann Oncol Off J Eur Soc Med Oncol. 2011;22(1):86–92. https://doi.org/10.1093/annonc/mdq323.
Georgoulias V, Bozionelou V, Agelaki S, Perraki M, Apostolaki S, Kallergi G, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23(7):1744–50. https://doi.org/10.1093/annonc/mds020.
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A. 2004;101(25):9393–8. https://doi.org/10.1073/pnas.0402993101.
Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124(2):403–12. https://doi.org/10.1007/s10549-010-1163-x.
Kallergi G, Agelaki S, Kalykaki A, Stournaras C, Mavroudis D, Georgoulias V. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2008;10(5):R80. https://doi.org/10.1186/bcr2149.
Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, Komor M, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–45. https://doi.org/10.1158/1078-0432.CCR-09-2042.
Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008;14(9):2593–600. https://doi.org/10.1158/1078-0432.CCR-07-4758.
Ignatiadis M, Rothe F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011;6(1):e15624. https://doi.org/10.1371/journal.pone.0015624.
Wallwiener M, Hartkopf AD, Riethdorf S, Nees J, Sprick MR, Schonfisch B, et al. The impact of HER2 phenotype of circulating tumor cells in metastatic breast cancer: a retrospective study in 107 patients. BMC Cancer. 2015;15:403. https://doi.org/10.1186/s12885-015-1423-6.
Hayashi N, Nakamura S, Tokuda Y, Shimoda Y, Yagata H, Yoshida A, et al. Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J Clin Oncol. 2012;17(2):96–104. https://doi.org/10.1007/s10147-011-0260-0.
Beije N, Onstenk W, Kraan J, Sieuwerts AM, Hamberg P, Dirix LY, et al. Prognostic impact of HER2 and ER status of circulating tumor cells in metastatic breast cancer patients with a HER2-negative primary tumor. Neoplasia. 2016;18(11):647–53. https://doi.org/10.1016/j.neo.2016.08.007.
Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009;11(4):R59.
Banys M, Krawczyk N, Becker S, Jakubowska J, Staebler A, Wallwiener D, et al. The influence of removal of primary tumor on incidence and phenotype of circulating tumor cells in primary breast cancer. Breast Cancer Res Treat. 2012;132(1):121–9. https://doi.org/10.1007/s10549-011-1569-0.
Sieuwerts AM, Mostert B, Bolt-de Vries J, Peeters D, de Jongh FE, Stouthard JM, et al. mRNA and microRNA expression profiles in circulating tumor cells and primary tumors of metastatic breast cancer patients. Clin Cancer Res. 2011;17(11):3600–18. https://doi.org/10.1158/1078-0432.CCR-11-0255.
Paoletti C, Muniz MC, Thomas DG, Griffith KA, Kidwell KM, Tokudome N, et al. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer. Clin Cancer Res. 2015;21(11):2487–98. https://doi.org/10.1158/1078-0432.CCR-14-1913.
Gasch C, Oldopp T, Mauermann O, Gorges TM, Andreas A, Coith C, et al. Frequent detection of PIK3CA mutations in single circulating tumor cells of patients suffering from HER2-negative metastatic breast cancer. Mol Oncol. 2016;10(8):1330–43. https://doi.org/10.1016/j.molonc.2016.07.005.
Markou A, Farkona S, Schiza C, Efstathiou T, Kounelis S, Malamos N, et al. PIK3CA mutational status in circulating tumor cells can change during disease recurrence or progression in patients with breast cancer. Clin Cancer Res. 2014;20(22):5823–34. https://doi.org/10.1158/1078-0432.CCR-14-0149.
Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9(9):1773–82. https://doi.org/10.1016/j.molonc.2015.05.009.
Rossi G, Mu Z, Rademaker AW, Austin LK, Strickland KS, Costa RLB, et al. Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast cancer. Clin Cancer Res. 2018;24(3):560–8. https://doi.org/10.1158/1078-0432.CCR-17-2092.
Palmirotta R, Lovero D, Cafforio P, Felici C, Mannavola F, Pelle E, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol. 2018;10:1758835918794630. https://doi.org/10.1177/1758835918794630.
Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106(5) https://doi.org/10.1093/jnci/dju066.
Schochter F, Rack B, Tzschaschel M, Polasik A, Andergassen U, Trapp E, et al. Endocrine treatment with 2 years of tamoxifen versus 2 years of Exemestane in postmenopausal patients with high-risk early breast cancer and persisting circulating tumor cells – first results of the SUCCESS C endocrine treatment sub-study. Oncol Res Treat. 2018;41(3):93–8. https://doi.org/10.1159/000485566.
Schramm A, Schochter F, Friedl TWP, de Gregorio N, Andergassen U, Alunni-Fabbroni M, et al. Prevalence of circulating tumor cells after adjuvant chemotherapy with or without anthracyclines in patients with HER2-negative, hormone receptor-positive early breast cancer. Clin Breast Cancer. 2017;17(4):279–85. https://doi.org/10.1016/j.clbc.2016.11.008.
Goodman CR, Seagle BL, Friedl TWP, Rack B, Lato K, Fink V, et al. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. JAMA Oncol. 2018;4(8):e180163. https://doi.org/10.1001/jamaoncol.2018.0163.
Pierga JY, Bidard FC, Autret A, Petit T, Adre F, Dalenc F, Levy C, Ferrero JM, Romieu G, Bonneterre J, Lerebours F, Bachelot T, Kerbrat P, Campone M, Eymard JC, Mouret-Reynier MA, Gligorov J, Hardy-Bessard AC, Lortholary A, Soulie P, Boher JM, Proudhon C, Charafe-Jaufret E, Lemonnier J, Bertucci F, Viens P. Circulating tumor cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol. 2017;28(1):103–9. https://doi.org/10.1093/annonc/mdw535.
Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192(3):373–82. https://doi.org/10.1083/jcb.201010021.
Qiang Zhang LG, Flaum L, Zhang Y, Gradishar W, Platanias L, Cristofanilli M. Increased circulating tumor cell (CTC) after systemic therapy is associated with younger age in stage III/IV breast cancer patients [abstract]. Cancer Res. 2018. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 Apr 14–18. Chicago/Philadelphia: AACR; 2018.
Zhang Q, Gerratana L, Flaum L, Zhang Y, Gradishar W, Platanias L, et al. Abstract 1597: increased circulating tumor cell (CTC) after systemic therapy is associated with younger age in stage III/IV breast cancer patients. Cancer Res. 2018;78(13 Supplement):1597. https://doi.org/10.1158/1538-7445.am2018-1597.
Yan WT, Cui X, Chen Q, Li YF, Cui YH, Wang Y, et al. Circulating tumor cell status monitors the treatment responses in breast cancer patients: a meta-analysis. Sci Rep. 2017;7:43464. https://doi.org/10.1038/srep43464.
Bidard FC, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabieres C, Janni W, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32(1–2):179–88. https://doi.org/10.1007/s10555-012-9398-0.
Condorelli R, Mosele F, Verret B, Bachelot T, Bedard PL, Cortes J, et al. Genomic alterations in breast cancer: level of evidence for actionability according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2019;30(3):365–73. https://doi.org/10.1093/annonc/mdz036.
Onstenk W, Sieuwerts AM, Weekhout M, Mostert B, Reijm EA, van Deurzen CH, et al. Gene expression profiles of circulating tumor cells versus primary tumors in metastatic breast cancer. Cancer Lett. 2015;362(1):36–44. https://doi.org/10.1016/j.canlet.2015.03.020.
Jordan NV, Bardia A, Wittner BS, Benes C, Ligorio M, Zheng Y, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537(7618):102–6. https://doi.org/10.1038/nature19328.
Pestrin M, Bessi S, Puglisi F, Minisini AM, Masci G, Battelli N, et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res Treat. 2012;134(1):283–9. https://doi.org/10.1007/s10549-012-2045-1.
Faltas B, Zeidan A, Peters K, Das A, Joudeh J, Navaraj A, et al. Identifying circulating tumor stem cells that matter: the key to prognostication and therapeutic targeting. J Clin Oncol. 2011;29(21):2946–7.; author reply 7–8. https://doi.org/10.1200/JCO.2011.36.6179.
Marangoni E, Lecomte N, Durand L, de Pinieux G, Decaudin D, Chomienne C, et al. CD44 targeting reduces tumor growth and prevents post-chemotherapy relapse of human breast cancers xenografts. Br J Cancer. 2009;100(6):918–22. https://doi.org/10.1038/sj.bjc.6604953.
Aalaoui-Jamali M, Bijian K, Batist G. Emerging drug discovery approaches for selective targeting of “precursor” metastatic breast cancer cells: highlights and perspectives. Am J Transl Res. 2011;3(5):434–44.
Beije N, Sieuwerts AM, Kraan J, Van NM, Onstenk W, Vitale SR, et al. Estrogen receptor mutations and splice variants determined in liquid biopsies from metastatic breast cancer patients. Mol Oncol. 2018;12(1):48–57. https://doi.org/10.1002/1878-0261.12147.
Mastoraki S, Strati A, Tzanikou E, Chimonidou M, Politaki E, Voutsina A, et al. ESR1 methylation: a liquid biopsy-based epigenetic assay for the follow-up of patients with metastatic breast cancer receiving endocrine treatment. Clin Cancer Res. 2018;24(6):1500–10. https://doi.org/10.1158/1078-0432.CCR-17-1181.
Bonechi M, Galardi F, Biagioni C, De Luca F, Bergqvist M, Neumuller M, et al. Plasma thymidine kinase-1 activity predicts outcome in patients with hormone receptor positive and HER2 negative metastatic breast cancer treated with endocrine therapy. Oncotarget. 2018;9(23):16389–99. https://doi.org/10.18632/oncotarget.24700.
Markou A, Strati A, Malamos N, Georgoulias V, Lianidou ES. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem. 2011;57(3):421–30. https://doi.org/10.1373/clinchem.2010.154328.
Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One. 2012;7(5):e33788. https://doi.org/10.1371/journal.pone.0033788.
Schramm A, Friedl TW, Schochter F, Scholz C, de Gregorio N, Huober J, et al. Therapeutic intervention based on circulating tumor cell phenotype in metastatic breast cancer: concept of the DETECT study program. Arch Gynecol Obstet. 2016;293(2):271–81. https://doi.org/10.1007/s00404-015-3879-7.
Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. https://doi.org/10.1056/NEJMra1510062.
Garrido P, Aldaz A, Vera R, Calleja MA, de Alava E, Martin M, et al. Proposal for the creation of a national strategy for precision medicine in cancer: a position statement of SEOM, SEAP, and SEFH. Clin Transl Oncol. 2018;20(4):443–7. https://doi.org/10.1007/s12094-017-1740-0.
Smerage JB, Hayes DF. The measurement and therapeutic implications of circulating tumor cells in breast cancer. Br J Cancer. 2006;94(1):8–12. https://doi.org/10.1038/sj.bjc.6602871.
Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199–209. https://doi.org/10.1056/NEJMoa1213261.
Ye Z, Wang C, Wan S, Mu Z, Zhang Z, Abu-Khalaf MM, et al. Association of clinical outcomes in metastatic breast cancer patients with circulating tumor cell and circulating cell-free DNA. Eur J Cancer. 2019;106:133–43. https://doi.org/10.1016/j.ejca.2018.10.012.
Moreno F, Gayarre J, López-Tarruella S, Monte-Millán M, Picornell AC, Álvarez E, et al. Concordance of genomic variants in matched primary breast cancer, metastatic tumor, and circulating tumor DNA: the MIRROR study. JCO Precis Oncol. 2019;3:1–16. https://doi.org/10.1200/po.18.00263.
Chambers AF, Naumov GN, Vantyghem SA, Tuck AB. Molecular biology of breast cancer metastasis. Clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Res. 2000;2(6):400–7.
Barriere G, Fici P, Gallerani G, Fabbri F, Zoli W, Rigaud M. Circulating tumor cells and epithelial, mesenchymal and stemness markers: characterization of cell subpopulations. Ann Transl Med. 2014;2(11):109. https://doi.org/10.3978/j.issn.2305-5839.2014.10.04.
Duffy MJ, Harbeck N, Nap M, Molina R, Nicolini A, Senkus E, et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer. 2017;75:284–98. https://doi.org/10.1016/j.ejca.2017.01.017.
Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J Clin Oncol. 2018;36(16):1631–41. https://doi.org/10.1200/JCO.2017.76.8671.
Macintyre G, Van Loo P, Corcoran NM, Wedge DC, Markowetz F, Hovens CM. How subclonal modeling is changing the metastatic paradigm. Clin Cancer Res. 2017;23(3):630–5. https://doi.org/10.1158/1078-0432.CCR-16-0234.
Cleary AS, Leonard TL, Gestl SA, Gunther EJ. Tumor cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014;508(7494):113–7. https://doi.org/10.1038/nature13187.
Rossi E, Rugge M, Facchinetti A, Pizzi M, Nardo G, Barbieri V, et al. Retaining the long-survive capacity of circulating tumor cells (CTCs) followed by xeno-transplantation: not only from metastatic cancer of the breast but also of prostate cancer patients. Oncoscience. 2014;1(1):49–56.
Andre F, Bachelot T, Commo F, Campone M, Arnedos M, Dieras V, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014;15(3):267–74. https://doi.org/10.1016/s1470-2045(13)70611-9.
Le Tourneau C, Delord JP, Goncalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumor molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–34. https://doi.org/10.1016/s1470-2045(15)00188-6.
Schwaederle M, Zhao M, Lee JJ, Eggermont AM, Schilsky RL, Mendelsohn J, et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J Clin Oncol. 2015;33(32):3817–25. https://doi.org/10.1200/JCO.2015.61.5997.
Swanton C, Soria JC, Bardelli A, Biankin A, Caldas C, Chandarlapaty S, et al. Consensus on precision medicine for metastatic cancers: a report from the MAP conference. Ann Oncol. 2016;27(8):1443–8. https://doi.org/10.1093/annonc/mdw192.
Arnedos M, Vicier C, Loi S, Lefebvre C, Michiels S, Bonnefoi H, et al. Precision medicine for metastatic breast cancer--limitations and solutions. Nat Rev Clin Oncol. 2015;12(12):693–704. https://doi.org/10.1038/nrclinonc.2015.123.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cueva Bañuelos, J.F., Rodríguez López, C., Cortegoso Mosquera, A., Palacios Ozores, P., Curiel García, T. (2020). Clinical Relevance and Therapeutic Application of CTCs in Advanced Breast Cancer. In: Piñeiro, R. (eds) Circulating Tumor Cells in Breast Cancer Metastatic Disease. Advances in Experimental Medicine and Biology, vol 1220. Springer, Cham. https://doi.org/10.1007/978-3-030-35805-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-35805-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35804-4
Online ISBN: 978-3-030-35805-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)