Abstract
Basophils represent approximately 1% of human peripheral blood leukocytes. Their effector functions were initially appreciated in the 1970s when basophils were shown to express the high-affinity receptor (FcεRI) for IgE and to release proinflammatory mediators (histamine and cysteinyl leukotriene C4) and immunoregulatory cytokines (i.e., IL-4 and IL-13). Basophils in the mouse were subsequently identified and immunologically characterized. There are many similarities but also several differences between human and mouse basophils. Basophil-deficient mice have enabled to examine the in vivo roles of basophils in several immune disorders and, more recently, in tumor immunity. Activated human basophils release several proangiogenic molecules such as vascular endothelial growth factor-A (VEGF-A), vascular endothelial growth factor-B (VEGF-B), CXCL8, angiopoietin 1 (ANGPT1), and hepatocyte growth factor (HGF). On the other side, basophils can exert anti-tumorigenic effects by releasing granzyme B, TNF-α, and histamine. Circulating basophils have been associated with certain human hematologic (i.e., chronic myeloid leukemia) and solid tumors. Basophils have been found in tumor microenvironment (TME) of human lung adenocarcinoma and pancreatic cancer. Basophils played a role in melanoma rejection in basophil-deficient mouse model. By contrast, basophils appear to play a pro-tumorigenic role in experimental and human pancreatic cancer. In conclusion, the roles of basophils in experimental and human cancers have been little investigated and remain largely unknown. The elucidation of the roles of basophils in tumor immunity will demand studies on increasing complexity beyond those assessing basophil density and their microlocalization in TME. There are several fundamental questions to be addressed in experimental models and clinical studies before we understand whether basophils are an ally, adversary, or even innocent bystanders in cancers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Angiopoietins
- Antigen-presenting cell
- Basophil
- Chemokines
- Cytokines
- Granzyme
- Hepatocyte growth factor
- IL-4
- IL-13
- Lung cancer
- Melanoma
- Pancreatic cancer
- Tumor immunity
- Tumor microenvironment
- Vascular endothelial growth factor
2.1 General Aspects
Basophils, first described by Paul Ehrlich in 1879 [1], represent less than 1% of human peripheral blood leukocytes. Their effector functions were not appreciated until the 1970s when basophils were shown to express the high-affinity IgE receptor (FcεRI) for IgE and release of histamine [2,3,4]. The difficulties in purifying sufficient numbers of human basophils and the absence of basophil-deficient animals hampered the advance of basophil research. Basophils share some characteristics with mast cells, including the presence of similar but distinct basophilic granules in the cytoplasm [5], surface expression of FcεRI, and release of proinflammatory mediators, such as histamine and cysteinyl leukotrienes [6, 7]. Basophils circulate in the peripheral blood and are rarely present in peripheral tissues unless inflammation occurs in mice [8] and in humans [9,10,11,12,13]. The life span of basophils is relatively short (≅2.5 d in mice) [14], and therefore newly generated basophils are constantly supplied from the bone marrow to the peripheral blood [15]. Mouse basophils were clearly characterized by Dvorak et al. as a granular cell population in murine bone marrow with some ultrastructural characteristics similar to mammalian basophils [16]. Recent development of basophil-deficient mice [17,18,19] has enabled us to examine the in vivo roles of basophils in a variety of immune settings.
In the past, basophils were regarded erroneously as blood-circulating mast cell precursors that could migrate to peripheral tissues and mature into tissue-resident mast cells. There is compelling evidence that basophils and mast cells are distinct cell lineages differentiated from hematopoietic stem cells in the bone marrow [7, 20, 21]. Like other myeloid lineages, basophils develop from hematopoietic stem cells in the bone marrow [15]. It has been suggested that human basophils develop from common basophil-eosinophil progenitors [22, 23]. IL-3 is the most important growth and activating cytokine for human and mouse basophils [24]. Murine basophils can be generated in vitro by culturing bone marrow cells in the presence of IL-3 or thymic stromal lymphopoietin (TSLP) [25]. IL-3-elicited and TSLP-elicited murine basophils differ in terms of gene expression, phenotype, and functions, suggesting heterogeneity among the basophil population [26]. Basophils can be detected in mice deficient for both IL-3 and TSLP signaling, indicating that neither is essential for basophil development. It has been suggested that approximately 10% of human basophils express the TSLP receptor [7] and the TSLP increases histamine release from basophils [27]. By contrast, a collaborative study demonstrated that human basophils do not express the IL-7Rα [28] and do not respond to TSLP [28, 29]. The above findings emphasize some of the differences between human and mouse basophils [7, 30, 31].
2.2 Basophils as a Source of Cytokines, Chemokines, Angiogenic Molecules, and Granzyme B
Human basophils, differently from mast cells, produce a restricted profile of cytokines [7, 21]. A variety of immunologic stimuli induce the release of substantial amounts of IL-4 [32,33,34,35,36]. Activated human basophils also produce IL-13 [37,38,39]. IL-4 and IL-13 are potent mediators for type 2 immunity with both overlapping and distinct functions [40]. Schroeder and collaborators first demonstrated that human basophils secrete IL-3 exerting strong autocrine priming effects on these cells [24]. Activation of human basophils induces the release of several proangiogenic molecules. For instance, immunologically activated human basophils release VEGF-A, the most potent proangiogenic molecule [41, 42]. Angiopoietins (ANGPTs) are a family of growth factors that play a role in angiogenesis and lymphangiogenesis [43]. Human basophils constitutively express ANGPT1 and ANGPT2 mRNAs [44]. ANGPTs were detected in cytoplasmic vesicles of basophils and their activation induced the release of ANGPT1. Human basophils can also release hepatocyte growth factor (HGF) [45]. The latter findings suggest that human basophils can modulate angiogenesis and lymphangiogenesis [42, 46, 47]. Basophils also produce CXCL8 [48] which can contribute to epithelial-to-mesenchymal transition in tumors [49]. Interestingly, human [50] and mouse (Schiavoni and Mattei, unpublished observations) basophils release granzyme B which possesses cytotoxic effects on cancer cells [51, 52]. Mouse, but not human [53], basophils represent an important source of TNF-α [18]. Mouse [54, 55], but not human, basophils produce IL-6 [48]. These findings highlight some of the similarities and differences between human and mouse basophils as a source of cytokines .
2.3 Are Mouse and Human Basophils Antigen-Presenting Cells (APCs)?
Activated human [32, 33] and mouse basophils [25, 53] produce large quantities of IL-4. In mice it has been shown that, under certain experimental conditions, basophils migrate to lymph nodes and secrete IL-4, promoting the differentiation of naive CD4+ T cells toward Th2 cells [56]. Three independent groups reported that murine basophils express MHC class II (MHC-II) and co-stimulatory molecules (i.e., CD80, CD86, and CD40), which are necessary for antigen presentation to naive T cells [57,58,59]. These studies suggested that mouse basophils can function dually as antigen-presenting cells (APCs) and IL-4-producing cells, driving Th2 cell differentiation, even in the absence of classical APCs [i.e., dendritic cells (DCs)]. By contrast, subsequent studies demonstrated the critical role of DCs, but not basophils, in Th2 differentiation [60,61,62]. Thus, the functional significance of basophils as APCs remained highly controversial [63]. The group of Karasuyama recently reported an unexpected mechanism of MHC-II acquisition by mouse basophils [64]. These cells express little or no MHC-II by themselves, but they can capture peptide-MHC-II complexes from DCs through a mechanism called trogocytosis, in a cell contact-dependent manner. Thus, MHC-II-dressed mouse basophils can provide peptide-MHC-II complexes and IL-4 to naive CD4+ T cells that in turn differentiate to Th2 cells. This finding tends to reconcile, at least in part, some of the discrepancies observed in previous studies.
Resting human peripheral blood basophils express little or no HLA-DR, but they can be induced to express it when activated in vitro with stimuli, such as cytokines [59, 65,66,67]. Nevertheless, human basophils did not induce antigen-specific T-cell proliferation [67,68,69]. Human peripheral blood basophils do not express HLA-DR and co-stimulatory molecules (CD80 and CD86) [68, 70, 71]. It would be interesting to investigate whether human basophils can acquire peptide-HLA-DR complexes from DCs through trogocytosis and function as APCs, as observed with murine cells .
2.4 Basophil-Deficient Mice
For decades the absence of basophil-deficient mouse hampered the advance of basophil research. During the last years several models of basophil-deficient mice have been developed. Initial experimental studies employed in vivo administration of antibodies that deplete basophils in mice to study the role of these cells. These antibodies recognize either the FcεRI (clone MAR-1) [72] or the activating receptor CD200 receptor 3 (CD200R3) (clone Ba103), which are both expressed by basophils and mast cells. Although both antibodies can efficiently deplete basophils in vivo, they can also activate mast cells and can cause anaphylaxis [62, 73]. Furthermore, the depletion of basophils by Ba103 is FcR dependent and might therefore activate myeloid cells and natural killer (NK) cells [74]. MAR-1 also depletes a subset of FcεRI-expressing DCs [60]. Several functions have been attributed to basophils based on studies using these depleting antibodies [59, 75]. For example, this experimental approach has led to the conclusion that basophils have a role as APCs during Th2 cell polarization [58, 59]. Similarly, it has been suggested that basophils can cause IgG1-mediated anaphylaxis [76] and that they contribute to protective immunity against Trichuris muris [57]. More recently, several new mouse strains with constitutive or diphtheria toxin (DT)-inducible depletion of basophils have been generated [77]. Genetically engineered basophil-deficient mouse models include Mcpt8DTR [8], Mcpt8Cre [62], Basoph8 [78], BAS-TRECK [79], and Runx1P1N/P1N mice [80]. These new genetically engineered basophil-deficient mice allowed to deepen our knowledge on the in vivo role of these cells in different pathophysiological conditions .
2.5 Peripheral Blood Basophils and Human Cancer
Basophilia is frequently observed during the accelerated phase of chronic myeloid leukemia (CML) [81]. The transcription factor IKAROS is absent or reduced in bone marrow blasts from most patients with advanced CML [82]. Forced expression of the dominant-negative isoform of IKAROS in CD34+ cells from patients with chronic CML resulted in disrupted IKAROS activity and enhanced ability to differentiate into basophils [82]. The latter findings suggest that a loss of IKAROS contributes to myeloid disease progression in CML with basophilia. It has been reported that basophils from patients with CML specifically express abundant HGF, which promotes CML cell expansion in an autocrine fashion [45]. A study using a mouse model of CML demonstrated that basophil-like leukemia cells contribute to CML development by providing the chemokine CCL3 [83]. In this model CML development induced a marked accumulation of basophil-like leukemia cells that produced CCL3 in the bone marrow. Basophil-derived CCL3 negatively regulated the proliferation of normal hematopoietic stem/progenitor cells and supported the predominant expansion of leukemia cells [84]. Indeed, basophil depletion prevented the development of CML. Basophilia appears to be an independent risk factor for evolution of myelodysplastic syndrome to acute myeloid leukemia [85, 86].
Circulating basophils have also been associated with certain solid tumors [87]. For instance, basopenia appears to be associated with worse prognosis of colorectal cancer [88]. By contrast, peripheral blood basophils have no predictive role in breast cancer [89] and oral squamous cell carcinoma [90]. In a mouse model of breast cancer, circulating basophils appeared to exert a protective role in the formation of metastases [91].
2.6 Basophils in Tumor Microenvironment of Human Lung Adenocarcinoma
There is compelling evidence that basophils can migrate into the sites of inflammation in mice [8] and in humans [9,10,11,12, 92]. Basophils can also be recruited into TMEs by several chemotactic molecules produced by tumor and immune cells [6, 41, 93,94,95,96,97] (Fig. 2.1). Lavin and collaborators compared the immune landscape in peripheral blood and in TME of patients with early (stage I) lung adenocarcinoma by single-cell analysis [13]. Basophils were present in both TME and noninvolved lung parenchyma as early as in stage I adenocarcinoma. They found quantitative and qualitative differences in basophils present in peripheral blood when compared to cells in TME and noninvolved lung tissue. Interestingly, a small percentage of basophils in TME and in noninvolved lung parenchyma expressed PD-L1. This study elegantly demonstrated, as early as in stage I disease, that lung adenocarcinoma lesions were accompanied by marked alteration of immune cells, including basophils, in TME .
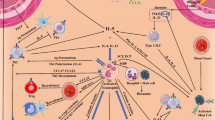
Proinflammatory and immunoregulatory mediators released from human basophils. These cells express a variety of receptors that regulate their development, homeostasis, and effector functions on the cytoplasmic surface. Basophils express the high-affinity receptors for IgE (FcεRI) which bind IgE with high affinity. These cells also express the α-chain (IL-3Rα/CD123) and a common βc (CD131) that bind IL-3, which plays a major role in basophil development [137, 138]. Secretory granules of basophils contain histamine complexed with chondroitin sulfate, basogranulin [139], granzyme B [50], and tryptase at levels of less than 1% of human mast cells. Immunologic activation of basophils leads to the release of histamine, basogranulin, and granzyme B and the production of IL-4 [32, 33, 35, 36, 140], IL-13 [37,38,39], IL-3 [24], VEGF-A and VEGF-B [41], ANGPT1 [44], and HGF [45]. Basophil activation induces the de novo synthesis of cysteinyl leukotriene C4 (LTC4) [141] and platelet-activating factor (PAF) [142]. Human basophils produce several chemokines [48] and, under specific conditions, can release IL-25/IL-17E, IL-31, LL-37, amphiregulin, and B-cell-activating factor (BAFF) [7, 143,144,145]. Human basophils activated by a variety of IgE- and non-IgE-mediated stimuli rapidly release membrane-free granules to the external microenvironment (anaphylactic degranulation). Basophils infiltrating the sites of inflammation can release packets of granule contents (piecemeal degranulation) [5]. Human basophils are also able to form extracellular DNA traps upon IL-3 priming and subsequent immunologic activation [146, 147]
A recent elegant study found that during lung development basophils acquire a unique phenotype, due to local exposure of specific signals (i.e., IL-33, GM-CSF), which regulates alveolar macrophage maturation and function [55]. The authors found that basophils represented a significant proportion of immune cellular composition during lung development. These cells broadly interacted with immune (e.g., monocytes, macrophages, neutrophils, ILCs) and nonimmune cells (e.g., endothelial cells, epithelial cells, fibroblasts) through the production of several cytokines (e.g., IL-4, IL-6, IL-13, TNF-α). Interestingly, the gene expression profile of lung basophils differed from that of blood-circulating basophils and was characterized by a unique gene signature including IL6, IL13, Cxcl2, Tnf, Osm, and Ccl4. The authors attributed the modulation of phenotype of lung basophils mainly to IL-33 and with minor contribution of GM-CSF. Moreover, lung basophils promoted M2 polarization of lung macrophages. Finally, the authors reported that basophils isolated from both the lung and the TME of mice implanted with B16 melanoma cells expressed several cytokines (e.g., IL4, IL6, Osm, IL13). This important study demonstrates that lung basophils acquire the expression of several cytokines and growth factors, critical for immune and nonimmune cell functions due to the exposure to lung-specific signals. Collectively, the results of these two important studies indicate that tissue-resident basophils can acquire distinct features from peripheral blood basophils and can play important roles in lung development and presumably in human lung cancer.
Schroeder and collaborators recently demonstrated that highly purified human basophils release histamine and secrete IL-4/IL-13 when co-cultured with the epithelial cell line, A549, an adenocarcinoma of lung origin [29]. This study further determined that an IgE-binding lectin (expressed on the A549 cells) was likely responsible for this activation of basophils, with all indicators pointing to galectin-3. Indeed, a follow-up study from the same group showed that A549 clones generated to be deficient in galectin-3 protein no longer activated basophils for these responses [98]. In addition, basophils co-cultured with microspheres coated with galectin-3 protein [but not bovine serum albumin (BSA) or galectin-9] likewise secreted IL-4/IL-13. However, when added exogenously as a soluble protein, galectin-3 only marginally activated basophils and only at relatively high concentrations, suggesting that the lectin may better facilitate cellular activation when immobilized on a matrix, whether epithelial cells (A549) or microspheres . While more studies are needed, the significance of these findings currently points to the fact that galectin-3 is now implicated as a biomarker and/or factor contributing to the pathogenesis of a wide range of conditions, particularly in cancer and cardiovascular disease, but also in autoimmunity (lupus erythematosus), wound healing, and asthma [99]. Evidence that galectin-3 modulates the immune responsiveness of basophils (and potentially other IgE-bearing cells) could offer novel insight into how these cells might be activated in the absence of specific IgE/allergen interactions. Indeed, this mechanism of activation could prove relevant to the recent findings showing IL-4-producing basophils in lupus erythematosus [100] and cancer [101].
2.7 Basophils in Experimental Melanoma
The role of basophils has been evaluated in a mouse model of melanoma [102]. A model of Treg depletion was associated with increased production of IL-3, which caused basophil infiltration in the TME. This model was associated with complete rejection of tumors, which was found to be dependent on chemokines (i.e., CCL3 and CCL4) produced by infiltrating basophils. These chemokines caused tumor infiltration of CD8+ T cells, which presumably exerted cytotoxic effect. Administration of MAR-1 (i.e., anti-FcεRI) to deplete basophils prevented the rejection of tumors. The authors concluded that basophils were required for tumor eradication. As previously mentioned, MAR-1 can partially deplete also mast cells and DCs that express FcεRI. Thus, the role of basophils in melanoma rejection will need to be confirmed using genetically engineered basophil-deficient mice .
In a series of ongoing experiments , we have investigated the direct antitumor activities of bone marrow-derived murine basophils following activation with IL-33, an alarmin known to activate the tumoricidal functions in eosinophils [103]. We observed that activation of basophils with IL-33 results in upregulation of granzyme B transcripts (Fig. 2.2a) and surface expression of the degranulation marker CD63 (Fig. 2.2b). In addition, when IL-33-activated basophils were co-cultured with B16.F10 murine metastatic melanoma cells, we found substantial restriction of tumor cell growth, compared to melanoma cells cultured with resting basophils (Fig. 2.2c). These preliminary observations suggest that under proper stimulation basophils can acquire tumoricidal properties and indicate that basophils may orchestrate antitumor immune responses at multiple levels. These interesting findings deserve further investigations in vitro and in vivo .
Activation of basophils with the alarmin IL-33 promotes tumoricidal functions. Basophils were generated by culture of murine bone marrow cells in medium containing IL-3 (2 ng/mL) for 10 days. Basophils were then harvested and cultured in medium alone or with added IL-33 (100 ng/mL) for 18 h. (a) qRT-PCR analysis of expression of granzyme B. Mean expression values in triplicate samples ± SD are shown. ∗∗P < 0.01, Wilcoxon’s t test. (b) Flow cytometry analysis of surface CD63 expression. (c) Growth of B16.F10 melanoma cells after 24 h co-culture with basophils alone or with added IL-33 (100 ng/mL). At the end of the co-culture, adherent tumor cells were stained with crystal violet to visualize tumor-covered area. Scale bar, 150 μm
2.8 Basophils in Experimental and Human Pancreatic Cancer
Ann Dvorak demonstrated the presence of basophils in the stroma of pancreatic cancer showing distinctive ultrastructural morphological features of piecemeal degranulation [5]. The role of basophils and their mediators in experimental and human pancreatic cancer has been elegantly investigated by Protti and collaborators [101]. In a large cohort of pancreatic ductal adenocarcinoma (PDAC), they found basophils expressing IL4 in tumor-draining lymph nodes (TDLNs) of PDAC patients. Basophils in TDLNs served as an independent prognostic biomarker of patient survival after surgery. The authors confirmed the recruitment of basophils in TDLNs in a mouse model of pancreatic cancer. In this model activated cancer-associated fibroblasts (CAFs) released TSLP which activated DCs. These cells induced IL-3 release from CD4+ T cells. IL-3 activated basophils to produce IL-4. CCL7, produced by DCs and CD14+ monocytes, was, at least in part, responsible for basophils migration from arterial blood into TDLNs. In this setting, basophils were the major source of IL-4 presumably contributing to both Th2 and M2 polarization in pancreatic cancer. The authors concluded that basophils and their mediator (i.e., IL-4) play a relevant pro-tumorigenic role in PDAC progression.
2.9 Conclusions and Outstanding Questions
Although peripheral blood basophils represent less than 1% of human leukocytes, there is compelling evidence that they can infiltrate the site of inflammation [9, 10, 18, 92, 104]. Importantly, basophils can be found in TME in human gastric cancer [11, 12] in early lung adenocarcinoma [13] and in PDAC [101]. Moreover, basophils can be identified in experimental melanoma [102] and in TDLNs in a model of pancreatic cancer [101]. The mechanisms regulating the trafficking of basophils into TDLNs, and their contributions to the evolving microenvironment of the metastatic niche, remain poorly understood. Single-cell RNA-seq will be necessary to characterize the basophils in TDLNs.
Human basophils release several angiogenic factors such as VEGF-A and VEGF-B [41], CXCL8 [49], ANGPT1 [44], and HGF [45]. CXCL8 and TNF-α can induce epithelial-to-mesenchymal transition [49, 105]. IL-4 and IL-13 can favor M2 polarization of tumor-associated macrophages [106, 107]. On the other side, basophils can exert anti-tumorigenic effects by releasing granzyme B [51, 52] and TNF-α [18] that possess cytotoxic effects on cancer cells. Moreover, histamine promotes DC maturation and can inhibit experimental tumor growth [108,109,110]. These findings suggest that basophils have the potential to play an anti-tumorigenic or a pro-tumorigenic role in tumor immunity (Fig. 2.3).
Basophils can be recruited into tumor microenvironments (TMEs) by several chemotactic molecules [e.g., VEGFs, histamine, prostaglandin D2 (PGD2), urokinase plasminogen activator (uPA), formyl peptides, CCL5, CCL7, CCL11, CCL13, CCL24, CCL26,CXCL8, CXCL12] produced by tumor or immune cells [6, 41, 93,94,95,96,97]. Basophils in the TMEs can exert anti-tumorigenic and/or pro-tumorigenic roles. Basophils can exert direct tumor cytotoxic effects via granzyme B [50] and TNF-α [18]. Histamine promotes dendritic cell (DC) maturation and inhibits tumor growth [108,109,110]. On the other side, basophils represent a potentially major source of several angiogenic molecules (VEGF-A , VEGF-B, ANGPT1, CXCL8, and HGF) [44, 45, 48]. CXCL8 and TNF-α can induce epithelial-to-mesenchymal transition [49, 105]. IL-4 and IL-13 can favor M2 polarization of tumor-associated macrophages [106]
There is increasing evidence that basophils in peripheral blood differ from those found in TME [13]. This is not surprising because peripheral blood basophils circulate at physiological pH and normoxia, whereas peritumoral and intratumoral basophils are embedded in a hostile microenvironment characterized by increased levels of lactate, PGE2, adenosine, IFN-α, and a low pH [111,112,113,114], which can profoundly influence basophil phenotype [115, 116]. Studies on basophil biology are usually performed at physiological pH and normoxia. It will be important to investigate how the tumor milieu activates/modulates the production of mediators and the expression of receptors in tumor-infiltrating basophils. Analyses of basophils in TDLNs have only recently began [101]. High-dimensional analysis, particularly single-cell RNA-seq, will be necessary to characterize basophils in TDLNs and in TME.
There is increasing evidence that immune cells in TME can play different roles in early and late stages of tumorigenesis [115, 117,118,119,120]. Basophils have been identified in the immune landscape of tumor and noninvolved lung tissue in early lung adenocarcinoma [13]. The hypothesis that basophils and their mediators play diverse roles in different phases of tumor initiation and growth deserves investigation.
Several models of basophil-deficient mice have been described. Initial studies were conducted using administration of antibodies (i.e., MAR-1 and Ba103) that transiently deplete basophils [72, 121]. However, these models can interfere with other immune cells [60, 74]. Recently, several mouse strains with constitutive or inducible depletion of basophils have been described. Studies using antibody-depleted basophils [102] and genetically engineered models [101] yielded apparently discordant findings on the role of basophils in cancer. Results obtained with basophil-deficient mouse models should be interpreted with caution because even new mouse mutants showed some hematological abnormalities. Perhaps, future studies attempting to evaluate the basophil role in a complex and heterogeneous disorder, such as cancer, should be performed using more than one model of basophil deficiency.
IgE is an ancient and highly conserved immunoglobulin isotype found in mammals. There is evidence that IgE has evolved to provide protection against infections and environmental toxins [6, 18, 122, 123]. Basophils express FcεRI which binds IgE [2, 4]. IgE has been suggested to play a protective role in tumor growth [124, 125]. In a mouse model of skin tumorigenesis, topical exposure to a common xenobiotic and carcinogen (i.e., 7,12-dimethylbenzatracene: DMBA) caused a potent IgE response that provided protection against carcinogenesis [126]. Although the mechanism by which IgE inhibited tumor growth in this model remains to be determined, the authors speculated that it “might involve soluble factors and/or cytotoxicity mediated by basophils.” Further studies should investigate the role, if any, of IgE-mediated activation of basophils in experimental and human tumors.
Tumor cells evade host immune attack by expressing several checkpoints, such as programmed cell death-1 (PD-1) and PD-1 ligands (PD-L1 and PD-L2) [127, 128]. Monoclonal antibodies targeting the PD-1/PD-L1 pathway unleash antitumor immunity and have revolutionized the treatment of cancer [129, 130]. PD-L1 is also expressed on the surfaces of various immune cells such as macrophages and DCs [13, 131,132,133], mast cells [13, 134, 135], and basophils in TME [13]. Recent evidence indicates that PD-L1 expressed in immune cells within TME, rather than on tumor cells, plays an essential role in immune checkpoint blockade therapy [132, 133]. Moreover, secreted PD-L1 can interfere with immune checkpoint therapy in cancer [136]. An interesting task will be to investigate the role of PD-L1+ basophils in TME in the context of immune checkpoint blockade.
In conclusion, the roles of basophils in experimental and human cancer have been little investigated and are currently largely unknown. The elucidation of basophils in tumor immunity will demand studies on increasing complexity beyond those assessing basophil density and their microlocalization in TME. There are several unanswered fundamental questions to be addressed in experimental models and clinical studies before we understand whether basophils are an ally, adversary, or even innocent bystanders in cancers.
Abbreviations
- ANGPTs:
-
Angiopoietins
- APCs:
-
Antigen-presenting cells
- BAFF:
-
B-cell-activating factor
- BSA:
-
Bovine serum albumin
- CAFs:
-
Cancer-associated fibroblasts
- CML:
-
Chronic myeloid leukemia
- DCs:
-
Dendritic cells
- DMBA:
-
7,12-Dimethylbenz(a)athracene
- DT:
-
Diphtheria toxin
- FcεRI:
-
High-affinity receptor
- LTC4:
-
Cysteinyl leukotriene C4
- PAF:
-
Platelet-activating factor
- PD-1:
-
Programmed cell death-1
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PD-L1:
-
Programmed death-ligand 1
- PGD2:
-
Prostaglandin D2
- TDLNs:
-
Tumor-draining lymph nodes
- Th2:
-
T helper 2
- TME:
-
Tumor microenvironment
- Treg:
-
T regulatory cell
- TSLP:
-
Thymic stromal lymphopoietin
- uPA:
-
Urokinase plasminogen activator
- VEGF-A:
-
Vascular endothelial growth factor-A
References
Ehrlich P (1879) Ueber die specifischen Granulationen des Blutes. Archiv fuer Anatomie und Physiologie: Physiologische Abteilung 3:571–579
Lichtenstein LM, Marone G, Thomas LL, Malveaux FJ (1978) The role of basophils in inflammatory reactions. J Invest Dermatol 71:65–69
Marone G, Findlay SR, Lichtenstein LM (1979) Adenosine receptor on human basophils: modulation of histamine release. J Immunol 123:1473–1477
Ishizaka T, Tomioka H, Ishizaka K (1971) Degranulation of human basophil leukocytes by anti-gamma E antibody. J Immunol 106:705–710
Dvorak AM (1995) Ultrastructural analysis of human mast cells and basophils. Chem Immunol 61:1–33
Marone G, Borriello F, Varricchi G, Genovese A, Granata F (2014) Basophils: historical reflections and perspectives. Chem Immunol Allergy 100:172–192
Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF (2018) Human mast cells and basophils—how are they similar how are they different? Immunol Rev 282:8–34
Wada T et al (2010) Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest 120:2867–2875
Guo CB et al (1994) Identification of IgE-bearing cells in the late-phase response to antigen in the lung as basophils. Am J Respir Cell Mol Biol 10:384–390
Nouri-Aria KT et al (2001) Basophil recruitment and IL-4 production during human allergen-induced late asthma. J Allergy Clin Immunol 108:205–211
de Paulis A et al (2004) Basophils infiltrate human gastric mucosa at sites of Helicobacter pylori infection, and exhibit chemotaxis in response to H. pylori-derived peptide Hp(2-20). J Immunol 172:7734–7743
de Paulis A et al (2009) Helicobacter pylori Hp(2-20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J Immunol 183:3761–3769
Lavin Y et al (2017) Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169:750–765.e17
Ohnmacht C, Voehringer D (2009) Basophil effector function and homeostasis during helminth infection. Blood 113:2816–2825
Arinobu Y et al (2005) Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A 102:18105–18110
Dvorak AM et al (1982) Ultrastructural identification of the mouse basophil. Blood 59:1279–1285
Voehringer D (2013) Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 13:362–375
Piliponsky AM et al (2019) Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat Immunol 20:129–140
Shibata S et al (2018) Basophils trigger emphysema development in a murine model of COPD through IL-4-mediated generation of MMP-12-producing macrophages. Proc Natl Acad Sci U S A 115:13057–13062
Dwyer DF, Barrett NA, Austen KF (2016) Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol 17:878–887
Varricchi G et al (2019) Physiological roles of mast cells: collegium Internationale Allergologicum update 2019. Int Arch Allergy Immunol 179:247–261
Robida PA, Puzzovio PG, Pahima H, Levi-Schaffer F, Bochner BS (2018) Human eosinophils and mast cells: birds of a feather flock together. Immunol Rev 282:151–167
Varricchi G et al (2018) Eosinophils: the unsung heroes in cancer? Oncoimmunology 7:e1393134
Schroeder JT, Chichester KL, Bieneman AP (2009) Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol 182:2432–2438
Siracusa MC et al (2011) TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477:229–233
Siracusa MC, Wojno ED, Artis D (2012) Functional heterogeneity in the basophil cell lineage. Adv Immunol 115:141–159
Salter BM et al (2015) Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J Allergy Clin Immunol 136:1636–1644
Salabert-Le Guen N et al (2018) Thymic stromal lymphopoietin does not activate human basophils. J Allergy Clin Immunol 141:1476–1479.e6
Schroeder JT, Bieneman AP (2017) Activation of human basophils by A549 lung epithelial cells reveals a novel IgE-dependent response independent of allergen. J Immunol 199:855–865
Varricchi G et al (2018) Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol 9:1595
Afferni C et al (2018) The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front Immunol 9:2601
Genovese A et al (2003) Immunoglobulin superantigen protein L induces IL-4 and IL-13 secretion from human Fc epsilon RI+ cells through interaction with the kappa light chains of IgE. J Immunol 170:1854–1861
Patella V, Florio G, Petraroli A, Marone G (2000) HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J Immunol 164:589–595
Patella V, Giuliano A, Bouvet JP, Marone G (1998) Endogenous superallergen protein Fv induces IL-4 secretion from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J Immunol 161:5647–5655
Galeotti C et al (2019) Intravenous immunoglobulin induces IL-4 in human basophils by signaling through surface-bound IgE. J Allergy Clin Immunol 144:524–535.e8
MacGlashan D Jr et al (1994) Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol 152:3006–3016
Redrup AC et al (1998) Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol 160:1957–1964
Gibbs BF et al (1996) Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol 26:2493–2498
Ochensberger B, Daepp GC, Rihs S, Dahinden CA (1996) Human blood basophils produce interleukin-13 in response to IgE-receptor-dependent and -independent activation. Blood 88:3028–3037
Marone G et al (2019) Intriguing role of Il-13 in the pathophysiology of asthma. Int J Mol Sci. https://doi.org/10.3389/fphar.2019.01387
de Paulis A et al (2006) Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol 177:7322–7331
Varricchi G et al (2018) Innate effector cells in angiogenesis and lymphangiogenesis. Curr Opin Immunol 53:152–160
Thomas M, Augustin HG (2009) The role of the angiopoietins in vascular morphogenesis. Angiogenesis 12:125–137
Prevete N et al (2013) Expression and function of Angiopoietins and their tie receptors in human basophils and mast cells. J Biol Regul Homeost Agents 27:827–839
Cerny-Reiterer S et al (2012) Identification of basophils as a major source of hepatocyte growth factor in chronic myeloid leukemia: a novel mechanism of BCR-ABL1-independent disease progression. Neoplasia 14:572–584
Galdiero MR, Varricchi G, Seaf M, Marone G, Levi-Schaffer F (2017) Bidirectional mast cell-eosinophil interactions in inflammatory disorders and cancer. Front Med (Lausanne) 4:103
Marone G, Varricchi G, Loffredo S, Granata F (2016) Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol 778:146–151
Schroeder JT (2011) Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev 242:144–160
Visciano C et al (2015) Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene 34:5175–5186
Tschopp CM et al (2006) Granzyme B, a novel mediator of allergic inflammation: its induction and release in blood basophils and human asthma. Blood 108:2290–2299
Martinez-Lostao L, Anel A, Pardo J (2015) How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res 21:5047–5056
Voskoboinik I, Whisstock JC, Trapani JA (2015) Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 15:388–400
Motomura Y et al (2014) Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 40:758–771
Yuk CM et al (2017) Basophil-derived IL-6 regulates TH17 cell differentiation and CD4 T cell immunity. Sci Rep 7:41744
Cohen M et al (2018) Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 175:1031–1044.e18
Sokol CL, Barton GM, Farr AG, Medzhitov R (2008) A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 9:310–318
Perrigoue JG et al (2009) MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol 10:697–705
Sokol CL et al (2009) Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 10:713–720
Yoshimoto T et al (2009) Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol 10:706–712
Hammad H et al (2010) Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 207:2097–2111
Phythian-Adams AT et al (2010) CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med 207:2089–2096
Ohnmacht C et al (2010) Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33:364–374
Kambayashi T, Laufer TM (2014) Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol 14:719–730
Miyake K et al (2017) Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc Natl Acad Sci U S A 114:1111–1116
Charles N, Hardwick D, Daugas E, Illei GG, Rivera J (2010) Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med 16:701–707
Reimer JM et al (2006) Isolation of transcriptionally active umbilical cord blood-derived basophils expressing Fc epsilon RI, HLA-DR and CD203c. Allergy 61:1063–1070
Voskamp AL, Prickett SR, Mackay F, Rolland JM, O’Hehir RE (2013) MHC class II expression in human basophils: induction and lack of functional significance. PLoS One 8:e81777
Eckl-Dorna J et al (2012) Basophils are not the key antigen-presenting cells in allergic patients. Allergy 67:601–608
Stephen-Victor E et al (2017) Demystification of enigma on antigen-presenting cell features of human basophils: data from secondary lymphoid organs. Haematologica 102:e233–e237
Sharma M et al (2013) Circulating human basophils lack the features of professional antigen presenting cells. Sci Rep 3:1188
Kitzmuller C et al (2012) Human blood basophils do not act as antigen-presenting cells for the major birch pollen allergen Bet v 1. Allergy 67:593–600
Khodoun MV et al (2013) Rapid polyclonal desensitization with antibodies to IgE and FcepsilonRIalpha. J Allergy Clin Immunol 131:1555–1564
Kojima T et al (2007) Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J Immunol 179:7093–7100
Obata K et al (2007) Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110:913–920
Balam S et al (2019) IL-3 triggers chronic rejection of cardiac allografts by activation of infiltrating basophils. J Immunol 202:3514–3523
Tsujimura Y et al (2008) Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity 28:581–589
Schwartz C, Eberle JU, Voehringer D (2016) Basophils in inflammation. Eur J Pharmacol 778:90–95
Sullivan BM et al (2011) Genetic analysis of basophil function in vivo. Nat Immunol 12:527–535
Sawaguchi M et al (2012) Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol 188:1809–1818
Mukai K et al (2012) Critical role of P1-Runx1 in mouse basophil development. Blood 120:76–85
Denburg JA, Browman G (1988) Prognostic implications of basophil differentiation in chronic myeloid leukemia. Am J Hematol 27:110–114
Beer PA et al (2015) Disruption of IKAROS activity in primitive chronic-phase CML cells mimics myeloid disease progression. Blood 125:504–515
Baba T et al (2013) MIP-1alpha/CCL3-mediated maintenance of leukemia-initiating cells in the initiation process of chronic myeloid leukemia. J Exp Med 210:2661–2673
Baba T et al (2016) MIP-1alpha/CCL3-expressing basophil-lineage cells drive the leukemic hematopoiesis of chronic myeloid leukemia in mice. Blood 127:2607–2617
Matsushima T et al (2003) Prevalence and clinical characteristics of myelodysplastic syndrome with bone marrow eosinophilia or basophilia. Blood 101:3386–3390
Wimazal F et al (2010) Evaluation of the prognostic significance of eosinophilia and basophilia in a larger cohort of patients with myelodysplastic syndromes. Cancer 116:2372–2381
Varricchi G et al (2018) Antineoplastic drug-induced cardiotoxicity: a redox perspective. Front Physiol 9:167
Wei Y et al (2018) The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage—colorectal cancer. Asia Pac J Clin Oncol 14:e243–e251
Cihan YB, Arslan A, Cetindag MF, Mutlu H (2014) Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev 15:4225–4231
Grimm M et al (2016) Standardized pretreatment inflammatory laboratory markers and calculated ratios in patients with oral squamous cell carcinoma. Eur Arch Otorhinolaryngol 273:3371–3384
Wang C et al (2015) Low local blood perfusion, high white blood cell and high platelet count are associated with primary tumor growth and lung metastasis in a 4T1 mouse breast cancer metastasis model. Oncol Lett 10:754–760
Ito Y et al (2011) Basophil recruitment and activation in inflammatory skin diseases. Allergy 66:1107–1113
de Paulis A et al (2004) Urokinase induces basophil chemotaxis through a urokinase receptor epitope that is an endogenous ligand for formyl peptide receptor-like 1 and -like 2. J Immunol 173:5739–5748
Weber M et al (1995) Monocyte chemotactic protein MCP-2 activates human basophil and eosinophil leukocytes similar to MCP-3. J Immunol 154:4166–4172
Jinquan T et al (2000) Chemokine stromal cell-derived factor 1alpha activates basophils by means of CXCR4. J Allergy Clin Immunol 106:313–320
Dahinden CA et al (1994) Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med 179:751–756
de Paulis A et al (1996) Cyclosporin H is a potent and selective competitive antagonist of human basophil activation by N-formyl-methionyl-leucyl-phenylalanine. J Allergy Clin Immunol 98:152–164
Schroeder JT, Adeosun AA, Do D, Bieneman AP (2019) Galectin-3 is essential for IgE-dependent activation of human basophils by A549 lung epithelial cells. J Allergy Clin Immunol 144:312–315.e1
Sciacchitano S et al (2018) Galectin-3: one molecule for an alphabet of diseases, from A to Z. Int J Mol Sci 19:E379
Pellefigues C, Charles N (2013) The deleterious role of basophils in systemic lupus erythematosus. Curr Opin Immunol 25:704–711
De Monte L et al (2016) Basophil recruitment into tumor-draining lymph nodes correlates with Th2 inflammation and reduced survival in pancreatic cancer patients. Cancer Res 76:1792–1803
Sektioglu IM et al (2017) Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells. Cancer Res 77:291–302
Lucarini V et al (2017) IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 6:e1317420
Webb LM et al (2019) The Notch signaling pathway promotes basophil responses during helminth-induced type 2 inflammation. J Exp Med 216:1268–1279
Montfort A et al (2019) The TNF paradox in cancer progression and immunotherapy. Front Immunol 10:1818
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14:399–416
Galdiero MR, Varricchi G, Loffredo S, Mantovani A, Marone G (2018) Roles of neutrophils in cancer growth and progression. J Leukoc Biol 103:457–464
Martner A et al (2015) Histamine promotes the development of monocyte-derived dendritic cells and reduces tumor growth by targeting the myeloid NADPH oxidase. J Immunol 194:5014–5021
Martinel Lamas DJ et al (2013) Therapeutic potential of histamine H(4) receptor agonists in triple-negative human breast cancer experimental model. Br J Pharmacol 170:188–199
Yang XD et al (2011) Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med 17:87–95
Gottfried E, Kreutz M, Mackensen A (2012) Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol 22:335–341
Caslin HL et al (2019) Lactic acid inhibits lipopolysaccharide-induced mast cell function by limiting glycolysis and ATP availability. J Immunol 203:453–464
Calcinotto A et al (2012) Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 72:2746–2756
Kuchuk O et al (2018) pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. Oncoimmunology 7:e1445452
Varricchi G et al (2017) Are mast cells MASTers in cancer? Front Immunol 8:424
Gulliksson M, Carvalho RF, Ulleras E, Nilsson G (2010) Mast cell survival and mediator secretion in response to hypoxia. PLoS One 5:e12360
Eruslanov EB et al (2014) Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 124:5466–5480
Patel S et al (2018) Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol 19:1236–1247
Singhal S et al (2019) Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med 11:eaat1500
Chevrier S et al (2017) An immune atlas of clear cell renal cell carcinoma. Cell 169:736–749.e18
Lubben W et al (2013) IgE knock-in mice suggest a role for high levels of IgE in basophil-mediated active systemic anaphylaxis. Eur J Immunol 43:1231–1242
Marichal T et al (2013) A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity 39:963–975
Varricchi G, de Paulis A, Marone G, Galli SJ (2019) Future needs in mast cell biology. Int J Mol Sci 20:E4397
Jensen-Jarolim E et al (2008) AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy 63:1255–1266
Jensen-Jarolim E et al (2018) AllergoOncology: opposite outcomes of immune tolerance in allergy and cancer. Allergy 73:328–340
Crawford G et al (2018) Epithelial damage and tissue gammadelta T cells promote a unique tumor-protective IgE response. Nat Immunol 19:859–870
Sharma P, Allison JP (2015) Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161:205–214
Kleffel S et al (2015) Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 162:1242–1256
Varricchi G et al (2018) Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Curr Med Chem 25:1327–1339
Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM (2017) Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17:569
Shi L, Chen S, Yang L, Li Y (2013) The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 6:74
Lin H et al (2018) Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 128:805–815
Tang H et al (2018) PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest 128:580–588
Rabenhorst A et al (2016) Expression of programmed cell death ligand-1 in mastocytosis correlates with disease severity. J Allergy Clin Immunol 137:314–318.e5
Nakae S et al (2006) Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol 176:2238–2248
Gong B et al (2019) Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J Exp Med 216:982–1000
Dvorak AM et al (1989) Ultrastructure of eosinophils and basophils stimulated to develop in human cord blood mononuclear cell cultures containing recombinant human interleukin-5 or interleukin-3. Lab Investig 61:116–132
Lantz CS et al (1998) Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 392:90–93
McEuen AR et al (2001) Mass, charge, and subcellular localization of a unique secretory product identified by the basophil-specific antibody BB1. J Allergy Clin Immunol 107:842–848
Patella V et al (1998) Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation 97:971–978
MacGlashan DW Jr, Peters SP, Warner J, Lichtenstein LM (1986) Characteristics of human basophil sulfidopeptide leukotriene release: releasability defined as the ability of the basophil to respond to dimeric cross-links. J Immunol 136:2231–2239
Triggiani M, Schleimer RP, Warner JA, Chilton FH (1991) Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J Immunol 147:660–666
Raap U et al (2017) Human basophils are a source of—and are differentially activated by—IL-31. Clin Exp Allergy 47:499–508
Wang YH et al (2007) IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 204:1837–1847
Qi Y et al (2010) Human basophils express amphiregulin in response to T cell-derived IL-3. J Allergy Clin Immunol 126:1260–1266.e4
Morshed M et al (2014) NADPH oxidase-independent formation of extracellular DNA traps by basophils. J Immunol 192:5314–5323
Yousefi S et al (2015) Basophils exhibit antibacterial activity through extracellular trap formation. Allergy 70:1184–1188
Acknowledgments
The authors apologize to the many researchers who have contributed importantly to this field and whose work has not been cited due to space and citation restrictions. The authors thank Dr. Gjada Criscuolo for critical reading of the manuscript, scientists from the CISI Laboratory and Schiavoni’s Laboratory not listed as authors for invaluable collaborations to the work reviewed, and medical graphic artist Fabrizio Fiorbianco for preparing Figs. 2.1 and 2.3. This work was supported in part by grants from the CISI-Lab Project (University of Naples Federico II), the CRèME Project, and the TIMING Project (Regione Campania) to G.V. and from AIRC IG 21366 to G.S.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Marone, G., Gambardella, A.R., Mattei, F., Mancini, J., Schiavoni, G., Varricchi, G. (2020). Basophils in Tumor Microenvironment and Surroundings. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1224. Springer, Cham. https://doi.org/10.1007/978-3-030-35723-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-35723-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35722-1
Online ISBN: 978-3-030-35723-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)