Abstract
Eosinophils are rare blood-circulating and tissue-infiltrating immune cells studied for decades in the context of allergic diseases and parasitic infections. Eosinophils can secrete a wide array of soluble mediators and effector molecules, with potential immunoregulatory activities in the tumor microenvironment (TME). These findings imply that these cells may play a role in cancer immunity. Despite these cells were known to infiltrate tumors since many years ago, their role in TME is gaining attention only recently. In this chapter, we will review the main biological functions of eosinophils that can be relevant within the TME. We will discuss how these cells may undergo phenotypic changes acquiring pro- or antitumoricidal properties according to the surrounding stimuli. Moreover, we will analyze canonical (i.e., degranulation) and unconventional mechanisms (i.e., DNA traps, exosome secretion) employed by eosinophils in inflammatory contexts, which can be relevant for tumor immune responses. Finally, we will review the available preclinical models that could be employed for the study of the role in vivo of eosinophils in cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Angiogenesis
- Cancer
- Cationic proteins
- CD8+ T cells
- Cytotoxicity
- Eosinophil
- Exosomes
- Extracellular Traps
- Immune regulation
- Lymphangiogenesis
- Mast cell
- Mouse models
- Tumor Immunity
- Tumor Microenvironment
- Tumor prognostic value
1.1 Introduction
Eosinophils are rare blood circulating granulocytic cells representing 1–3% of total leukocyte population under physiological condition. Paul Ehrlich in 1879 first described blood eosinophils by their unique staining properties with acidic dyes, such as eosin and Luxol fast blue [1]. These cells originate and differentiate in the bone marrow in response to IL-5, together with IL-3 and GM-CSF, which support both maturation and survival of eosinophils [2]. In addition, IL-33 sustains eosinophilopoiesis at various levels, promoting survival, maturation, and functional activation [3]. During bone marrow development, IL-33 both expands eosinophil precursors expressing the IL-5Rα and induces systemic IL-5 production, thus fueling the eosinophil maturation [4].
Upon response to certain inflammatory conditions (i.e., allergies, parasitic infections, and autoimmune diseases), eosinophils can rapidly expand and can infiltrate inflamed tissues, where they play diverse roles in inflammatory responses. Eosinophils are well known to infiltrate the tumor microenvironment (TME), and this condition is referred to as tumor-associated tissue eosinophilia (TATE). The role of TATE in human cancers is still controversial [5, 6]. However, recent clinical observations in melanoma patients undergoing immunotherapy targeting the immune checkpoints CTLA-4 and PD-1 have unraveled a predictive role of eosinophil counts for therapeutic response [7]. These findings suggested that eosinophils might be regarded as possible prognostic/predictive biomarkers in cancer immunotherapy, thus repositioning this immune cell population at the forefront of cancer immunology research.
1.2 The Tumor Microenvironment: A Dynamic System with Multiple Interacting Players
The definition of tumor microenvironment (TME) originates from the dynamic interaction of the host immune system with the forming and growing tumor. This continuously evolving milieu is the result of the constant cross-talk between cancer cells and immune cells through the release of soluble factors that shape the phenotype of both cell types [8]. The TME is composed of a number of resident and nonresident cell types, as well as extracellular factors, and each cell component has a distinct role in this complex scenario [9]. When the TME is in its initial stage, resident tumor cells instruct the TME for the formation of blood vessels that allow the access of nutritive factors, cell-derived vesicles, and immune cells. Pericytes and endocytes, key cellular components of the blood vessel architecture, are considered resident cells in the TME [9] and play a relevant role in angiogenesis. In particular, a type-2 (Nestin+) subset of pericytes has been identified that promotes normal and tumoral angiogenesis [10]. Cancer-associated fibroblasts (CAFs), a heterogeneous subset of several cell types, are resident cells that play an important role in tumorigenesis. These cells produce and release several mediators, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and cytokines, important to generate the 3D stromal architecture of blood vessels and of the TME itself [11].
The immune cells infiltrating the TME in solid cancers are heterogeneous, and their roles depend on the site, grade, and stage of malignancy. This is in part due to the fact that within the TME the patterns of soluble mediators (cytokines, chemokines, angiogenic, lymphangiogenic, and growth factors) and cellular receptors dynamically change and thus influence the homing and phenotype of immune cells [9].
Tumor infiltrating lymphocytes (TILs) are associated with antitumor activity, whose frequency often correlates with a favorable prognosis in cancer patients. In particular, CD8+ T cells are often present as infiltrating cells in solid cancers, where they can exert potent and selective cytotoxic action on tumor cells [12]. However, an important fraction of TILs is represented by regulatory CD4+ T (TREG) lymphocytes with opposite effects on cancer progression. Indeed TREG are endowed with potent pro-tumoral effects when infiltrating the TME and are considered a target for immunotherapeutic strategies [13]. Recently, a novel subset of tissue-resident memory CD69+CD103+ T cells (TRM), either CD4+ or CD8+, has been reported to play a crucial role in preventing the development and spread of solid tumors and has been associated with favorable outcomes in cancer patients. TRM cells may mediate tumor protection by promoting tumor-immune equilibrium through the secretion of cytokines and/or via CD103-enhanced tumor cell killing [14]. Natural killer (NK) cells, an innate immune subset with potent cytotoxic function, also contribute to tumor rejection [15].
Several dendritic cell (DC) subsets may be found in variable frequencies in the TME of various solid cancers, where they are deputed to tumor antigen (Ag) presentation and cross-presentation in lymphoid organs and in the TME itself [16]. Certain chemotherapeutic drugs promote the release of immunogenic signals from dying tumor cells, which are perceived by DC and promote a cascade of events that stimulate an anticancer immune response [17]. Among these signals, the ligand Annexin-a1 released by dying tumor cells was shown to bind formyl peptide receptor-1 (FPR-1) , expressed by DC, acting as signal for the correct positioning of DC in proximity of dying cancer cells within TME. This Annexin-a1/FPR-1 axis enabled stable DC-corpse interactions, and subsequent engulfment and Ag cross-presentation by DC [18]. Ag cross-presentation for CD8+ T-cell cross-priming is mainly carried out by Batf3- and Irf8-dependent type 1 conventional DCs, a subset of DC expressing the markers CD103 and CD8α [19]. CD8+ T-cell cross-priming is promoted by type I IFNs signaling on CD8α DC and is required for antitumor immunity in vivo [20]. Type I IFNs act on CD8α DC prolonging Ag retention after engulfment of tumor apoptotic cells leading to efficient CD8+ T-cell cross-priming [21]. In addition, DC can interact with innate and innate-like immune cells, including NK, invariant natural killer T (iNKT), and γδ T cells, amplifying direct and indirect antitumoral responses through a mutual cross-talk [22]. On the other hand, some tolerogenic DC contribute to the generation of TREG and engage in a cross-talk, thus favoring the establishment and maintenance of an immunosuppressive TME that inhibits antitumor immunity [23].
Myeloid cells represent a major fraction of infiltrating immune cells. Tumor-associated macrophages (TAM) play a major role in tumor progression. TAM are distinguished into two major subsets: classically activated M1 with antitumor functions and pro-inflammatory M2 that supports tumor progression [24]. The balance of frequencies in infiltrating M1 and M2 TAMs often dictates the tumor fate and is a prognostic factor for patients [25]. Myeloid-derived suppressor cells (MDSC) are immature-like myeloid cells capable of strong immunosuppressive activity. Based on their phenotype marker expression and morphology, these cells can be subdivided into two subgroups, monocytic MDSC (M-MDSC) and granulocytic or polymorphonuclear MDSC (PMN-MDSC) due to their morphological (but not functional) resemblance with monocytes and granulocytes, respectively [25]. Both types of MDSC infiltrate the TME, where they act as potent suppressors of CD4+ and CD8+ T lymphocytes while favoring recruitment of TREG cells. In addition, MDSC promote tumor cell stemness, angiogenesis, and metastasis [25]. Mast cells, [26], neutrophils [27], eosinophils [6], and basophils [28], historically recognized for their involvement in allergy and inflammation, are now being repositioned for the recently discovered role in cancer. The function and role of eosinophils within the TME will be covered in detail below.
1.3 General Properties of Eosinophils
For many years, eosinophils have been mostly appreciated for two aspects of immune response: the ability to fight parasites and their contribution to allergic inflammation [29, 30]. This is because eosinophils produce a wide array of toxic granule proteins and pro-inflammatory mediators that lead to tissue damage [31]. Indeed, eosinophils exert potent cytotoxic functions through the production and release of cationic proteins, such as major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxide (EPX), and eosinophil-derived neurotoxin (EDN). Furthermore, eosinophils secrete a wide array of soluble mediators, including cytokines, chemokines, and angiogenic and lipid mediators, contributing to immune regulation, tissue remodeling, and many other processes [30].
Eosinophil degranulation can occur via different cellular mechanisms [32]. Eosinophils adherent to parasites have been shown to degranulate through classical exocytosis, a process involving granule fusion with the plasma membrane that creates a pore through which the total granule content is secreted into the target cell. In contrast, piecemeal degranulation enables the release “piece-by-piece” of specific granule-stored proteins, such as cytokines and chemokines , and is thought to be the main secretion mode during chronic inflammatory responses. As a third mode of secretion, eosinophils may undergo cytolysis, a process involving extracellular release of intact granules with rupture of plasma membrane. Eosinophils may also undergo cytolytic cell death with extrusion of nuclear materials, such as histones and DNA, and extracellular expulsion of intact granules entrapped in DNA nets, named DNA traps [33, 34].
According to the LIAR hypothesis formulated by James Lee, eosinophils are homeostatic cells that regulate Local Immunity And/or Remodeling/Repair during both steady state conditions and disease, especially associated with tissue injury [35]. Hence, besides the destructive effects, eosinophils also participate in resolution of inflammation, tissue repair, remodeling, and homeostasis, through the release of a variety of pro-fibrotic (i.e., TGF-β), growth factors (i.e., FGF-2, NGF, and VEGF), and matrix metalloproteinases. In addition, eosinophils participate in the modulation of adaptive immune responses [36]. Eosinophils can induce the recruitment of Th2 cells [37] and TREG cells [38] through the production of the chemokines CCL17 and CCL22. Moreover, eosinophil-derived CXCL9 and CXCL10 recruit Th1 cells [37, 39] and CD8+ T cells [40,41,42]. Eosinophil cationic proteins can have immunostimulatory activities; for example, EDN can both attract and induce the maturation of DC into a Th2-promoting phenotype [43]. Stimulation of eosinophils with CpG-ODN results in degranulation and induction of DC maturation in a cell contact independent manner via MBP [44]. Furthermore, EPX activates DC in vitro and in vivo, inducing mobilization to lymph nodes and Th2 priming [45]. Eosinophils play an active role in the induction and expansion of Th2 type of immune response, through the production of IL-4, IL-5, IL-13, and IL-25 participating in allergic reactions, parasitic infections, and autoimmune disorders [46]. Eosinophils can also produce, store, and secrete Th1-associated pro-inflammatory cytokines (i.e., IFN-γ, TNF-α and IL-12) and TREG-associated mediators (i.e., IDO, IL-10, and TGF-β), thus demonstrating their versatile immunoregulatory role [46]. Following activation with cytokines, such as GM-CSF, IL-4, IL-5, or IFN-γ, eosinophils upregulate MHC class II and co-stimulatory molecules (CD80, CD86, and CD40) and can act as non-professional Ag presenting cells (APC) stimulating Ag-specific CD4+ T-cell proliferation and Th2 cytokine production in vitro and in vivo [47].
Eosinophils are equipped with a variety of surface receptors fundamental for their function and localization within inflamed tissues [30]. These include pattern recognition receptors (PRRs), such as TLR1–5, TLR7, TLR9, NOD1, NOD2, Dectin-1, and RAGE, that recognize specific molecular components associated with pathogens or danger signals and allow a rapid pro-inflammatory response to insults through production of cytokines, chemokines, and granule cationic proteins [48]. Eosinophils also express receptors for many cytokines (IL-2R, IL-3R, IL-4R, IL-5Rα, IL-9R, IL-10R, IL-13R, IL-17R, IL-23R, IL-27R, IL-31R, IL-33/ST2, TSLPR, GM-CSFR, IFNγR, TGF-βR), chemokines (CCR1, CCR3, CCR4, CCR5, CCR6, CCR8, CCR9, CXCR2, CXCR3, CXCR4), formyl peptide receptors-1, −2, −3, and a variety of integrins and adhesion molecules (CD11a/CD18, CD11b/CD18, CD11c/CD18, CD49d/CD29, CD49f/CD29, ICAM-1), which drive eosinophil transmigration from the bloodstream to inflamed tissues [49, 50].
Eosinophils express various receptors for immunoglobulins, complements, proteases, and lipid mediators, such as leukotrienes and prostaglandins. Sialic-binding immunoglobulin-like lectin 8 (Siglec-8) and its ortholog murine Siglec-F are hallmark receptors for eosinophils that function as inducers of apoptosis associated with reactive oxygen species (ROS) production following antibody cross-linking [51, 52], especially when eosinophils are pre-activated with cytokines [53, 54]. Administration of Siglec-F mAb in vivo results in selective ablation of blood and tissue eosinophils in mice through induction of apoptosis [55]. EGF-like module containing mucin-like hormone receptor-1 (EMR1), the human ortholog of mouse F4/80, is a receptor highly specific to mature human eosinophils [56]. Targeting EMR1 with a specific mAb enhanced NK-mediated killing of human eosinophils in vitro and induced eosinophil depletion in monkeys [57]. In addition, eosinophils express various adhesion molecules and integrins (i.e., CD49d/29, CD49f/29, CD11b/18, CD11a/18, CD11c/18, CD11d/18, and CD49d/β7) that are upregulated upon activation and mediate eosinophil migration and effector functions [58]. Overall, these features endow eosinophils with multiple roles as effectors and regulators of different immune responses.
1.4 Eosinophils in Allergic Diseases and Infections
Eosinophils play a prominent role in Th2-related pathologies, and tissue eosinophilia is associated with inflammation in respiratory allergies, atopic dermatitis, eosinophilic esophagitis, and gastroenteritis [30]. Allergic asthma is often associated with skewing of naïve Th cells toward Th2 phenotype and activation of eosinophils. In the latter condition, referred to as eosinophilic asthma [59], eosinophils are recruited in the airways by Th2 cytokines (i.e., IL-5) and chemokines (i.e., eotaxin-1/CCL11). Airway epithelial cells activated by different stimuli (e.g., allergens, superallergens, viral and bacterial proteins, tobacco smoke, and so on) release the alarmins IL-25, IL-33, and TSLP that promote Th2 polarization with massive production of IL-4, IL-5, and IL-13 [60]. Recent evidence suggests that these alarmins stimulate type 2 innate lymphoid cells (ILC2), which also secrete IL-4, IL-5, and IL-13 and subsequently recruit eosinophils to the inflamed tissue [61].
Eosinophils are key players in airway inflammation contributing to the so-called T2 asthma pathogenesis by damaging the epithelium and orchestrating the immune response [62]. It is believed that the pathophysiologic effects of eosinophils in allergic inflammation are caused by the release of cationic proteins, ROS, lipid mediators, proteases, and pro-inflammatory cytokines. The mechanisms triggering eosinophil degranulation in inflamed tissues are not fully understood. However, recent evidences have shown that epithelial cell-derived alarmins may play a role. In mouse models of allergic asthma, accumulation of eosinophils in the lung and ensuing allergic inflammation are strongly inhibited by blockade of IL-33/ST2 signaling pathway [63,64,65,66], while they are exacerbated by administration of recombinant IL-33 [67]. In patients, a rare IL-33 loss-of-function causes reduced number of eosinophils in blood and protects against asthma [68]. Furthermore, IL-33 is a potent activator of eosinophils in vitro, enhancing adhesion and promoting degranulation [69,70,71]. These data strongly suggest that IL-33 not only drives eosinophilia but also stimulates eosinophil effector functions in allergy. TSLP, another epithelial-derived alarmin involved in allergic inflammatory response [60], can promote degranulation and survival of eosinophils through STAT5 phosphorylation [72]. In mice, intradermal administrations of TSLP resulted in the induction of a systemic Th2-skewed inflammatory response, which was dependent on the presence of eosinophils [73]. A monoclonal antibody (mAb) targeting TSLP (tezepelumab) is currently under development for the treatment of different forms of severe type 2 asthma (i.e., eosinophilic and non-eosinophilic) [74, 75].
A canonical function of eosinophils is to provide protection against parasitic helminths. Eosinophils also participate in the host defense against other pathogens, such as bacteria, viruses, and fungi [49, 76]. The mechanisms accounting for the antiparasitic role of eosinophils in vitro include direct killing through the release of cytotoxic proteins (MBP, EPX, ECP, EDN) [77, 78]. Furthermore, eosinophils can present parasite-specific Ags to T cells in vivo, leading to the polarization of Th2 response and increase of Ag-specific IgM concentration [79]. However, studies in mouse models of helminth infection have yielded contrasting results, and the role of eosinophils in host response to parasites in vivo remains controversial [49]. In vitro, eosinophils adhere to the fungus Alternaria alternata by binding of the integrin CD11b to β-glucan, a component of the fungal cell wall, resulting in degranulation and release of the cationic proteins MBP and EDN [80]. In 1978, DeChatelet and coworkers first reported a bactericidal activity of eosinophils, showing that these cells can phagocytize Escherichia coli or Staphylococcus aureus as efficiently as neutrophils via hydrogen peroxide production [81]. Furthermore, human eosinophils can release extracellular DNA traps in response to Aspergillus fumigatus in vitro, via a cytolytic mechanism that depends on the Syk tyrosine kinase pathway and CD11b [82]. Eosinophils exploit extracellular traps of mitochondrial DNA and granule proteins also to kill bacteria both in vitro and in vivo [34]. These catapult-like released traps protected from microbial sepsis in a model of intestinal inflammation, unravelling the importance of eosinophils for maintaining the intestinal barrier function after inflammation-associated epithelial cell damage.
Eosinophils play a role in host defense against single-stranded RNA viruses, such as respiratory syncytial virus (RSV), by exploiting the ribonuclease activity of the granule proteins EDN and ECP [83]. In vitro, degranulation of eosinophils following RSV-infected pulmonary epithelial cells is dependent on CD18-mediated cell contact [84]. In addition, binding of rhinovirus to eosinophils via ICAM-1 causes phenotypic activation of eosinophils promoting their ability to present viral Ags to Ag-specific T cells, causing T-cell proliferation and secretion of IFN-γ [85]. In vivo, eosinophils promote the clearance of RSV by stimulation of the TLR-7-MyD88 pathway, which triggers degranulation and expression of IRF-7, IFN-β, and iNOS [86]. Furthermore, infection of eosinophils with pulmonary viruses can result in the release of proinflammatory mediators, such as IL-6, IP-10, CCL2, and CCL3 [87]. The precise mechanisms by which eosinophils interact with viruses and contribute to host antiviral immunity remain to be clarified.
1.5 Repositioning Eosinophils in Cancer
The increase of eosinophils in cancer patients has been known for over a century. Pioneering studies in the 1980s described tumor-infiltrating eosinophils in human gastric cancers and suggested their good prognostic value for prolonged survival [88, 89]. In addition, eosinophils were reported to exert cytotoxic function against breast cancer cells in vitro [89], and blood eosinophil counts inversely correlated with risk of recurrent disease in breast cancer patients [90]. In cancer patients undergoing immunotherapy with IL-2, eosinophils were expanded and acquired an activated phenotype, with increased degranulation, survival, and antitumor cytotoxicity [91,92,93]. Despite these early evidences supporting the presence of eosinophils in TME of many human cancers, the role of eosinophils in cancer has been largely overlooked for long time. Recent data indicate that these cells are potent immune effectors and regulators within the TME with potential prognostic/predictive role in human cancers.
1.5.1 Role of Eosinophils in Hematologic Tumors
The role of eosinophils in hematologic tumors is still unclear. Andersen and coworkers showed that the eosinophil counts below 0.16 × 109 /L can be associated with acute myeloid leukemia and myelodysplastic syndrome. By contrast, eosinophil counts above 0.16 × 109 /L were associated with myeloproliferative neoplasms [94]. Several studies have reported the association of peripheral blood and tissue eosinophilia with Hodgkin’s disease (HD) [95]. Eosinophilia in peripheral blood is associated with a positive prognostic factor [96]. Eosinophils can be found in lymph nodes of HD patients, but their prognostic relevance remains controversial [95]. It is unclear why tissue eosinophilia can be found only in some subsets of HD patients. Tumor cells can produce different eosinophil-attracting molecules such as IL-5 and eotaxins [97, 98]. In nodular sclerosing Hodgkin’s disease, tissue eosinophilia was shown to represent a poor prognostic indicator. In fact, binding of eosinophil-secreted CD30 ligand to CD30 on tumor cells was shown to trigger NF-kB activation and consequent proliferation of tumor cells [99].
Verstovsek and coworkers highlighted the efficacy of alemtuzumab in the treatment of patients affected by hypereosinophilic syndrome (HES) or chronic eosinophilic leukemia (CEL) refractory to standard therapies [100]. Alemtuzumab induced a relevant decline of the absolute eosinophilic count and the percentage of eosinophils in the peripheral blood in all 11 patients examined. This study demonstrated that alemtuzumab decreased blood eosinophils and improved patients with HES and CEL refractory to standard therapy. These case studies suggest, on one hand, that resistance to standard therapy may be in part due to the hypereosinophilia and, on the other hand, that treatment of these refractory patients with alternative therapies may be successful and is partly associated with a decrease of blood eosinophilia [100]. These data have been confirmed by Strati and coworkers, who reported that alemtuzumab is a useful treatment for CEL patients with hypereosinophilia [101].
1.5.2 Role of the Eosinophils in Solid Tumors
Several studies have shown an improved prognosis of patients with TATE or evidence of eosinophil degranulation in various types of solid tumors . Caruso and coworkers described TATE in human gastric adenocarcinoma, with tumor cells in close proximity to eosinophils displaying signs of autophagic cell death [102]. The same group described the presence of degranulating eosinophils in human advanced gastric carcinoma. In these ultrastructural studies, deposition of extracellular granules from apoptotic eosinophils either free in the tumor stroma [103] or within the cytoplasm of gastric carcinoma cells was observed [104].
An antitumoral role of eosinophils has been described in colon cancer, melanoma, lung cancer, and oral squamous cell carcinoma [6]. In melanoma mouse models, eosinophils play a clear antitumoral role both in restraining tumor growth and in preventing lung metastasis onset [40, 41, 105]. Lucarini et al. demonstrated the positive influence of eosinophils on CD8+ T cells for the local tumor, and their tumor cytotoxicity activity on lung metastasis [41]. Importantly, eosinophil count in peripheral blood is correlated with a good prognosis in patients with metastatic melanoma undergoing immunotherapy with immune checkpoint inhibitors targeting CTLA-4 [106, 107] or PD-1 [108]. Furthermore, in a study involving 173 patients, it was shown that peripheral blood eosinophilia is a good prognostic marker correlating with prolonged survival in patients with metastatic melanoma independently from any treatment [109].
In mouse and human colorectal cancer, Munitz and colleagues elegantly demonstrated an antitumorigenic role of eosinophils during tumor development [110]. By analyzing human biopsies, they found an inverse correlation between tumor stage and intratumoral eosinophil counts. In Apcmin/+ mice, which develop spontaneous intestinal adenomas, eosinophils were recruited into tumors during induction of inflammation-induced colorectal cancer and played an essential role in tumor rejection, independently of CD8+ T cells [110].
In non-small-cell lung cancer (NSCLC), a recent report by Tanizaki and colleagues showed an important correlation between peripheral blood eosinophil counts and patients’ survival. They showed that an increase in absolute eosinophil count (AEC) (≥ 150 /μl) is linked to a better progression-free survival (PSF) and overall survival (OS) in patients treated with nivolumab, an anti-PD-1 mAb. For this reason, they suggested that absolute eosinophil count can be a biomarker for this kind of treatment [111]. Dorta and colleagues reported an antitumor role of eosinophils in oral squamous cell carcinoma (OSCC). Indeed, they demonstrated that patients with a lower number of TATE have lower probability to survive [112].
By contrast, elevated eosinophils in the TME seem to be correlated with poor survival in cervical carcinoma patients [113]. This pro-tumoral activity of eosinophils may be related to the microenvironment of this type of cancer. In this regard, eosinophils may be polarized to a phenotype that promotes tumor growth and reduces tumor cell death [114]. The ensemble of these evidences suggests that the role of eosinophils in tumorigenesis is cancer dependent. Alternatively, as discussed below, different subsets of eosinophils may exist that play divergent roles in tumorigenesis, depending on the tumor histotype.
1.6 Antitumoral Mechanisms of Eosinophils
Tumor-infiltrating eosinophils have the potential to control tumor progression, exerting direct and indirect antitumoral activities, through secretion of a variety of soluble mediators. Recruitment of eosinophils to the TME can be driven by several chemokines (eotaxin-1/CCL11, eotaxin-2/CCL24, eotaxin-3/CCL26, and RANTES) that activate the CCR3 receptor highly expressed on eosinophils [6]. The alarmin IL-33, locally expressed by epithelial and tumor cells, can also promote eosinophil recruitment through stimulation of tumor-derived eosinophil-attracting chemokines [71]. IL-33, together with IL-5, may also prolong the life span of eosinophils at site of tumor growth [6].
Eosinophils accumulate early within tumor necrotic areas of experimental tumors, and this event is accompanied with degranulation and release of MBP and EPX [115, 116]. In fact, danger signals released by necrotic cells, particularly High Mobility Group Box 1 (HMGB1), can induce eosinophil migration, adhesion, survival, and degranulation with release of granule cationic proteins and ROS that promote oxidation and thus inactivation of necrotic material and tumor-promoting inflammation [116]. Moreover, eosinophil-derived MBP can inhibit the activity of heparanase, an endoglycosidase involved in remodeling the extracellular matrix that enhances tumor growth, angiogenesis, and the formation of metastasis [117].
1.6.1 Direct Antitumor Activity of Eosinophils
Eosinophils are equipped with granule proteins endowed with potent cytotoxic activity [118]. MBP can mediate tumor toxicity through disruption of membrane lipid bilayers [119]. ECP is cytotoxic for Hodgkin lymphoma tumor cells [120], inhibits the proliferation of oral squamous cell carcinoma [121], and induces apoptosis of bronchial epithelial cells by caspase-8 activation [122]. Furthermore, EPX and EDN exhibit cytolytic activity against human colorectal carcinoma cell lines [123]. These findings support the hypothesis that eosinophil cationic proteins can exert direct antitumor activities. Vadas and coworkers reported that exposure of eosinophils to CSF or GM-CSF activates antibody-dependent cell-mediated cytotoxicity (ADCC) against EL-4 and BW thymoma cells, as well as P815 mastocytoma cells. Importantly, eosinophils needed direct contact with P815 cells to induce killing, suggesting a contact-dependent cytotoxicity [124]. Murine eosinophils also could induce apoptosis of A20 murine lymphoma cells through the release of granzyme B [125]. Furthermore, eosinophils activated by cross-linking of the 2B4/CD244 receptor exhibited tumoricidal activities against human B lymphoma cells [126]. In addition, human eosinophils can directly kill human colon carcinoma cells via release of granzyme A in a mechanism dependent on the integrin CD11a/CD18 and on IL-18 [123, 127]. These in vitro results suggest that tumor cytotoxic function of eosinophils require cell–cell contact through various adhesion molecules and integrins.
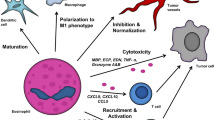
Whether eosinophils mediate tumor cytotoxicity in vivo remains to be demonstrated. Presence of degranulating eosinophils and of eosinophil-specific granules within tumor cytoplasm has been reported in human gastric cancer biopsies [103, 104]. In mouse melanoma models, immunotherapy with the “alarmin” IL-33 promotes the expansion and tumor infiltration of eosinophils, which play an essential role in antitumor responses mediated by IL-33 and prevents the onset of pulmonary metastasis. Terminal differentiation of bone marrow-derived eosinophils with IL-33 results in the generation of highly activated cells, compared to classical eosinophils differentiated with IL-5, revealed by increased aggregation in clusters (Fig. 1.1). IL-33-activated eosinophils highly resembled pulmonary eosinophils recruited by IL-33 in vivo and exhibited upregulation of granzyme B and potent tumor cytotoxicity in vitro [6, 41]. Furthermore, IL-33-activated eosinophils are able to establish a large number of cell conjugates with different tumor cell lines (B16.F10 melanoma, MC38 colon carcinoma, TC-1 lung adenocarcinoma and MCA205 fibrosarcoma) leading to efficient tumor cell killing in vitro and in vivo [71]. IL-33 promoted the tumoricidal functions of eosinophils in a cell adhesion-dependent manner through the integrin CD11b/CD18 and by inducing lytic granule convergence, with polarization of eosinophil effector proteins (ECP, EPX, and granzyme B) to the tumor-eosinophil immune synapse [71], in a similar mechanism operated by NK cells [128]. These observations demonstrate that IL-33 can potently stimulate eosinophil-dependent direct tumor cell killing by targeted degranulation, as schematized in Fig. 1.2.
Terminal differentiation of bone marrow–derived eosinophils with IL-33 yields highly activated cells. Bone marrow cells from tibiae and femurs of C57Bl/6 mice were cultured for 4 days in a medium containing 100 ng/ml SCF and 100 ng/ml FLT-3 L, followed by 10 ng/ml IL-5. From day 10, cells were supplemented every other day with either IL-5 or IL-33 (100 ng/ml) in order to generate IL-5 eosinophils (IL-5 EO) or IL-33 eosinophils (IL-33 EO), as described previously [36]. On day 16, when fully differentiated eosinophils were generated, microphotographs were obtained by EVOS-FL microscope. Images at the indicated magnifications show IL-33 EO aggregating in clusters, as opposed to IL-5 EO, indicative of highly activated phenotype. Scale bars, 100 μm
IL-33 promotes the activation of CD11b/CD18 adhesion-dependent granule polarization in eosinophils within the immune synapse. Upon coculture with tumor cells, eosinophils (EO) activated with IL-33 through its specific receptor complex ST2/IL1RAP form stable EO-tumor cell conjugates. This event is mediated by CD11b/CD18-dependent adhesion and synapse-polarized degranulation of eosinophil toxic proteins (EPX, ECP, granzyme B), resulting in efficient tumor cell killing. By contrast, activation of eosinophils with IL-5, through its receptor complex IL5RA/IL3RB subunit, fails to induce tumor cell adhesion and subsequent degranulation, thus sustaining tumor cell proliferation
1.6.2 Indirect Antitumoral Function of Eosinophils: Interaction with Other Immune Cells within the TME
Studies in preclinical models indicate that tumor-infiltrating eosinophils may affect indirectly tumor growth. In mouse models of melanoma, it has been shown that infiltrating eosinophils promote the recruitment of tumor-reactive CD8+ T cells through expression of the T-cell-attracting chemokines CCL5, CXCL9, and CXCL10 [40, 41]. Another indirect antitumor mechanism operated by eosinophils is their ability of influencing the tumor angiogenesis in TME. Human eosinophils can produce in vitro several proangiogenic molecules [129,130,131,132]. In vivo eosinophils induce vessel normalization by increasing the expression of adhesion molecules, such as VCAM-1, and by polarizing TAM toward M1-like macrophages, which produce smaller amounts of pro-angiogenic factors, compared to M2 macrophages [40]. Finally, as discussed above, eosinophils may function as nonprofessional APC, although whether they do so within the TME remains to be demonstrated.
Both solid and hematologic tumors are associated with the accumulation of peritumoral and/or intratumoral mast cells, suggesting that these cells can help to promote and/or limit tumorigenesis [133]. Interestingly, human mast cells and eosinophils were both identified and named by Paul Ehrlich [134, 135]. These cells have distinct progenitors and differ morphologically, ultrastructurally, immunologically, and biochemically. However, mast cells and eosinophils can form the “allergic effector unit” and can be found in proximity in TME of several tumors [135]. Therefore, it is likely that eosinophils have the capacity to modulate mast cell functions and vice versa. For example, ECP and MBP [136] and VEGFs released by activated eosinophils [137] can modulate mast cell functions. These bidirectional interactions between eosinophils and mast cells and vice versa might be relevant in TME.
1.7 Functional Plasticity of Eosinophils in Cancer
Eosinophils display the potential to interact with the tumor moiety. This feature stems from the ability of eosinophils to change their phenotype in response to stimuli present in the TME, such as cytokines, inducing variable responses. In addition, eosinophils have the capacity to release extracellular vesicles, which may shape the TME. For these reasons, eosinophils can be considered as cells endowed with a certain functional plasticity constantly remodeling the TME.
1.7.1 Role of Cytokines in Shaping Eosinophil Phenotype within the TME
It has been suggested that at least four subsets of murine eosinophils exist depending on their tissue localization, maturation, and type of immune response triggered [138]. The first subset is represented by eosinophil progenitors, which are immature eosinophils undergoing hematopoiesis in situ. They express the receptors for IL-5, IL-33, and TSLP, the latter two regulating eosinophil homing to inflamed tissues and activation. Steady-state or tissue-resident eosinophils, which were only characterized in the lung parenchyma of mice, are resting cells expressing intermediate levels of Siglec-F and with donut-shaped nucleus. A third subset (i.e., type 1 eosinophils) was described as interstitial/stromal cells in morphogenetic and type 1 immunity contexts. These eosinophils display similar surface markers as steady-state eosinophils, such as Siglec-Fint, but have pluri-lobated nucleus without vacuolization. The fourth subset (i.e., type 2 eosinophils) is characterized in the epithelium in type 2 immunity contexts, such as allergic asthma and chronic colitis. These eosinophils exhibit pluri-lobated nucleus, vacuolized cytoplasm and high expression of Siglec-F. The relative roles of these subsets of eosinophils in cancer immunity are unknown. This is because these cell types have been characterized mainly morphologically and by the expression of some surface markers. Thus, gene expression profiles and single-cell RNA sequencing of tumor-infiltrating eosinophils in various settings may help to define these cells in relation to their functional (pro- or antitumorigenic) role.
Some studies have reported that cytokines may shape the phenotype of eosinophils, determining their polarization and tumor immune responses induced. Reichman and colleagues reported that in experimental colorectal cancer, intratumoral eosinophils exhibit an IFN-γ-related signature, which prevented the development of colorectal cancer in mice. Furthermore, activation of resting peritoneal eosinophils with IFN-γ potentiated their ability to kill colorectal cancer cells in vitro [110]. Similarly, activation of eosinophils with IFN-γ plus TNF-α induced upregulation of T-cell-attracting chemokines (CCL5, CXCL9, and CXCL10), IFN-γ, TNF-α, and NOS2. These activated eosinophils reduced tumor growth, through recruitment of tumor-reactive CD8 T cells, when adoptively transferred in melanoma-bearing mice [40]. These data suggest that IFN-γ may skew eosinophils toward a type 1 immunity-promoting phenotype.
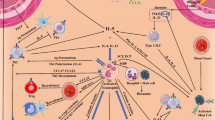
IL-33 is another important cytokine that may affect eosinophil phenotype within the TME. In experimental tumors, IL-33 promotes antitumor immunity through expansion and activation of eosinophils in vivo [41, 105]. In melanoma-bearing mice, depletion of eosinophils by an anti-Siglec-F antibody injections abrogated the therapeutic efficacy of IL-33, indicating that eosinophils were indispensable for IL-33 antitumoral function [41]. Exposure to IL-33 in vivo induced in tumor-infiltrating eosinophil gene transcripts, which differ in the primary tumor site and pulmonary metastasis. At the primary tumor site, IL-33-recruited eosinophils expressed T-cell-attracting chemokines (CCL5, CXCL9, and CXCL10), but not effector molecules. Conversely, at the pulmonary site, eosinophils expressed high levels of granzyme B and IFN-γ, but not T-cell-attracting chemokines. These findings suggested indirect or direct antitumor functions of eosinophils at the primary or pulmonary site, respectively. Furthermore, in vitro activation of eosinophils with IL-33 resulted in upregulation of effector molecules (i.e., granzyme B), Th-1 (i.e., IL-12, TNF-a), and Th-2 cytokines (i.e., IL-10, IL-13) [41, 71]. Thus, IL-33 may polarize eosinophils to a mixed type 1 and 2 immunity phenotype that promotes antitumoral function.
Several lines of evidence indicate that IL-5 may not support antitumor reactions in eosinophils. An early study showed that IL-5 gene-transfected tumors promote eosinophil recruitment but not antitumor immunity [139]. In a Meth-A fibrosarcoma model, intratumoral injection of OK-432 (a derivative of penicillin) and fibrinogen induces local production of IL-5 that recruits in the tumor tissue eosinophils which, however, did not play a relevant role in tumor regression [140]. In a different model, IL-5 could facilitate experimental lung metastasis of various cancer cells by creating an allergic inflammatory environment with CCL22-producing eosinophils that recruited TREG cells [38]. By contrast, IL-5 plays a major role in driving the recruitment of eosinophils at primary and metastatic sites, promoting antitumor responses in models of hepatocellular carcinoma [141], methylcholanthrene-induced fibrosarcoma [98], and melanoma [142]. These apparently contrasting findings are compatible with the hypothesis that IL-5 can play distinct or even opposite roles in modulation of tumorigenesis. The role of IL-5 in shaping the phenotype of eosinophils in TME from different cancers needs to be addressed more extensively . Fig. 1.3 summarizes the possible role of subsets of eosinophils in the TME and their modulation by cytokines (i.e., IFN-γ, IL-5, IL-33).
Modulation of eosinophil phenotype by cytokines. Activation of eosinophils with IFN-γ results in the expression of T-cell-attracting chemokines (CXCL9, CXCL10, and CCL5), effector molecules (TNF-α, IFN-γ, and NOS2), promoting both CD8 T-cell recruitment and tumor cytotoxicity. Similarly, IL-33 triggering leads to upregulation of T-cell-attracting chemokines (CXCL9, CXCL10, and CCL5), effector molecules (granzyme B and TNF-α), Th-1 cytokines (IL-12), and Th-2 cytokines (IL-10 and IL-13), as well as to granule protein polarization toward immune synapses. These traits favor eosinophil-mediated direct and indirect (CD8 T-cell-mediated) antitumor activities. In contrast, activation of eosinophils with IL-5 induce the expression of the chemokine CCL22 that recruits TREG cells, which may promote tumor progression
1.7.2 Role of Extracellular Vesicles in the Biological Activity of Eosinophils within the TME
There is compelling evidence that eosinophils can release exosomes (EXO) and other extracellular vesicles (EV) . EXO represent a small population of vesicles produced by any kind of cell and reflect the molecular signature (made up of lipids, nucleic acids, and proteins) of their producing cells. Through transfer of their bioactive molecules from the cell of origin to the target cell or tissue, EXO contribute to intercellular communication and represent an important diagnostic biomarker in pathological conditions [143].
Although intercellular communication appears to be the one of the most important functions of EV, they also have specific molecules related to their biogenesis. In fact, EXO have been defined by their size, density, and expression of specific biomarkers such as proteins involved in targeting and adhesion (tetraspanins, integrins, adhesion molecules), multivesicular bodies (MVB) biogenesis and secretion-associated proteins (ALIX, Rab GTPase), chaperone proteins, and others. The distinction between eosinophilic granules and EV becomes increasingly difficult, due to many shared molecules expressed, such as CD63 [144], and to the fact that granules can also be found intact extracellularly as membrane-bound, ligand-responsive structures [145]. Eosinophil EXO include/contain a series of cationic proteins (MBP, EPX, EDN, ECP), miRNA, mRNA, cytokines, chemokines, enzymes, and lipid mediators whose activities can mediate and autoregulate eosinophil biological functions. EXO-stored products are released from eosinophils through different mechanisms: classical exocytosis providing for exosome fusion with the plasma membrane and exosome embedding within target cell [146]. The release of eosinophil-derived EXO content in the receiving cell can condition the most important biological cell activities (transcription, translation, regulation by posttranscriptional or translational modifications), leading to drastic phenotypic variations in the receiving cell.
Evidences suggest that eosinophil EXO, secreted into the extracellular microenvironment and delivered to different locations within the body, participate in multiple processes and pathologies, including asthma. Eosinophil EXO from asthmatic patients can influence the functions of structural lung cells, modifying several processes and changing the expression profile of various pro-inflammatory molecules [147]. Furthermore, eosinophil-derived EXO can increase nitric oxide (NO) and ROS production in eosinophils themselves, thus autoregulating eosinophil functions [148]. The role of eosinophil-derived EXO in cancer progression is unknown and deserves investigation. By using electron microscopy, Feng and coworkers demonstrated that pericytes, like eosinophils, are equipped with internal vesicles that can be released outside of the cell [149]. Since pericytes represent an important component of vessels involved in the modulation of angiogenesis , it is conceivable that eosinophils and pericytes interact within the TME through the release of EV.
1.8 Regulatory Functions of Eosinophils: The Complex Role of the Angiogenesis
Angiogenesis and lymphangiogenesis are complex processes requiring a finely tuned balance between stimulatory and inhibitory signals [150,151,152]. The formation of new blood and lymphatic vessels occurs vigorously during embryogenesis but is restricted in adults [150]. In adults, angiogenesis and lymphangiogenesis are limited to sites of chronic inflammation [64], tissue injury or remodeling [153], and cancer [154]. The association between angiogenesis/lymphangiogenesis and tumor growth was of great interest during the last decades for the implications of the nature of tumors and the possibility to inhibit cancer growth and the formation of metastasis by blocking angiogenesis/lymphangiogenesis [155]. The interest in angiogenesis/lymphangiogenesis increased during last years for several reasons. Chronic low-grade inflammation is an essential hallmark of cancer [8] and several immune cells can be involved, directly and indirectly, in the modulation of angiogenesis and lymphangiogenesis [6, 156,157,158,159,160,161,162,163]. The latter observation led to the recognition that the interactions between immune cells and the vascular system are involved in a multitude of cancers [164].
Angiogenesis is initiated by activation of vascular endothelial growth factor receptor 2 (VEGFR2), expressed on blood endothelial cells (BECs) by vascular endothelial growth factor-A (VEGF-A) . Cancer cells are an important source of VEGF-A and other pro-angiogenic mediators [6, 165, 166]. Immune cells in TME increase VEGF-A availability during the angiogenic switch [159]. Angiogenesis and lymphangiogenesis require the participation of additional molecules, such as angiopoietins (ANGPTs) [167]. VEGF-A signaling through VEGFR2 is the major angiogenic pathway. VEGF-C and VEGF-D, mainly through the engagement of VEGFR3 on lymphatic endothelial cells (LECs), induce lymphangiogenesis in tumors and stimulate the formation of metastasis [168,169]. The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor (PlGF) [159]. VEGF-A signaling through VEGFR2 activates angiogenesis by inducing the survival, proliferation, sprouting, and migration of BECs. VEGF-A also increases endothelial permeability [160, 170] and induces inflammation [157, 163, 171]. There are several splicing isoforms of VEGF-A (121, 165, 189, and 206), which differ in their binding to matrix and to co-receptor. VEGF-A121 is freely diffusible, whereas VEGF-A165, VEGF-A189, and VEGF-A206 bind to heparin and heparin proteoglycans on cellular surfaces and extracellular matrices [172].
VEGF-A is primarily known for its essential role in physiologic and pathologic angiogenesis [173] and also retains lymphangiogenic properties [174] by binding to VEGFR2/VEGFR3 heterodimer receptor [175]. VEGF-A modulates lymphangiogenesis also indirectly by recruiting immune cells (e.g., macrophages, mast cells) that produce VEGF-C and VEGF-D [161, 163]. PlGF, expressed in the placenta, heart, and lungs, has four isoforms (PlGF1–4) [176, 177]. VEGF-B is highly expressed in heart, skeletal muscles, and brown fat in adults and has two major isoforms in humans: VEGF-B167 binds to heparin proteoglycans, whereas VEGF-B186 does not bind heparin and is more soluble [178]. PlGF and VEGF-B bind with high affinity to VEGFR1 whose tyrosine kinase (TK) activity is weak and downstream signaling poorly understood [179]. VEGFR1 is expressed on BECs, some immune cells, and pericytes, and its TK activity is required for cell migration toward VEGFs or PlGF [157, 171, 180]. VEGF-B and PlGF are angiogenic in certain pathophysiological settings [181]. VEGF-B can modulate coronary vessel growth and cardiac hypertrophy and lipid metabolism [177, 182].
The VEGF-C/VEGFR3 signaling pathway is the main pathway implicated in lymphangiogenesis [183]. VEGF-C is crucial for the survival, proliferation, and migration of LECs [184]. VEGF-D also binds VEGFR3 to promote lymphangiogenesis [168].
Angiopoietins (ANGPTs) also play an important role in modulating angiogenesis and lymphangiogenesis. In humans, the ANGPT/Tie system consists of two cell-surface TK receptors (TIE1 and TIE2) and two ligands ANGPT1 and ANGPT2. TIE2, primarily expressed on BECs, binds both ANGPTs, whereas TIE1 is an orphan receptor that can modulate ANGPT1, expressed by perivascular cells (i.e., pericytes), and sustains BEC survival. By contrast, ANGPT2, secreted by BECs, acts autocrinally and paracrinally as TIE2 ligand to promote angiogenesis and lymphangiogenesis [185]. Several chemokines, produced by immune and nonimmune cells, also play a role in the modulation of angiogenesis and antiangiogenesis [158].
Human eosinophils produce several angiogenic factors such as VEGF-A [129, 137], fibroblast growth factors (FGF-2) [40, 130], CXCL8/IL-8 [131], and osteopontin [132]. Human eosinophils also produce MMP9 [186,187,188]. Eosinophils have been detected in metastatic lymph nodes of cancer patients, but the production of lymphangiogenic factors by these cells should be further addressed.
1.9 Mouse Models to Investigate the Role of Eosinophils in Cancer
Several mouse models have been developed for the study of the functional role of eosinophils. Most of these experimental models have been employed mainly in the study of respiratory diseases, such as asthma and eosinophilic esophagitis. These models can be transgenic, genetically engineered, target-specific, and humanized.
1.9.1 Transgenic Mouse Models
A relevant transgenic mouse model used to assess phenotypic features of eosinophils in the host is represented by PHIL transgenic mouse model of study. PHIL mice were first described by Lee and colleagues as a transgenic line of mice with a complete ablation of eosinophils and the contemporary presence of a fully functional hematopoietic compartment [189]. These mice have been generated by replacing the eosinophil peroxidase (EPX) with the Diphtheria toxin A chain (DT) and by exploiting the cytocidal property of DT. When host eosinophils undergo maturation or activation of the transcription factors devoted to the expression initiation of EPX in PHIL transgenic mice, the promoter transcribes the replaced DT sequence, thus selectively ablating eosinophils [189]. These mice have allowed to establish the contribution of eosinophils to the resolution of inflammatory responses in experimental pulmonary allergies [190], experimental colitis [191], and to pathology and protection against parasites [78].
An alternative mouse model for studying eosinophil functions in vivo is the ΔdblGATA1 transgenic mice. Deletion of a high-affinity GATA site in the GATA-1 promoter results in a complete ablation of the eosinophil lineage without affecting the development of the other GATA-1-dependent lineages, such as erythroid, megakaryocytic, and mast cell [192]. These mice have been used to demonstrate a protective role for eosinophils in a methylcholanthrene (MCA)-induced fibrosarcoma tumor mouse model [98]. However, besides eosinophil deficiency, ΔdblGATA1 mice were subsequently reported to display numerical and functional aberrancy in basophils [193], thus raising concerns on their specificity for eosinophil-specific studies.
IL-5 transgenic mice display an overrepresented eosinophil compartment due to the insurgence of eosinophilia in the host. These hypereosinophilic mice display abnormally high presence of eosinophils in bone marrow, spleen, and peritoneal exudate, compared to controls. Simson and coworkers proposed a role for eosinophils in tumor immunosurveillance by using the IL-5 transgenic mice with elevated levels of circulating eosinophils [98]. Similarly, Kataoka and coworkers exploited these IL-5 transgenic mice to demonstrate an antitumor activity of eosinophils in hepatocellular carcinoma [141].
1.9.2 Target-Specific in Vivo Models
Depletion mechanisms to generate selected target-specific mouse models have been developed to produce in vivo eosinophil-targeted models of study. Repeated systemic injections of an anti-Siglec-F polyclonal antibody that functionally inhibits the activity of Siglec-F protein and selectively induces apoptosis in eosinophils result in eosinophil ablation in mice [55]. Recently, this Siglec-F-based functional depletion of eosinophils in mice has been employed to study the role of eosinophils in cancer. By using this approach, three independent groups demonstrated the essential role of eosinophils in antitumor response in mouse models of melanoma and other cancers [40, 41, 105].
1.9.3 Genetically Engineered in Vivo Models
Initially, genetically modified mouse models with impaired eosinophil development and/or function, although not eosinophil-specific, were described. These include mice deficient for the eosinophilic cytokine IL-5 [194] or its receptor [195], which are characterized by the absence of eosinophilia upon Th2 cell-inducing stimuli. Furthermore, mice with a double deletion of CCL11 and CCL24 genes are characterized by a severe diminished eosinophil recruitment in response to allergic stimuli [196]. These models were largely employed in allergy and respiratory disease research.
Subsequently, mice deficient for eosinophil-specific granule proteins MBP and EPX were described. MBP-1−/− mice were generated by truncating the MBP-1 gene, thus producing a dysfunctional protein containing the exons 1 and 2 but lacking the exons 2, 3, and 4. This model is characterized by a reorganization of eosinophil secondary granule structures and by a marked reduction of the eosinophil numbers in lung parenchyma and bronchoalveolar lavage [197]. Similarly, EPX−/− mice were generated by a targeted disruption of the EPX gene, in which the normal EPX gene was replaced with a dysfunctional EPX sequence lacking the exons 7, 8, and 9. EPX−/− mice displayed an altered structure of eosinophil secondary granules and a remarkable reduction of eosinophils in lung BAL [198]. Interestingly, a recent report described the generation of a double knockout mouse model for both MBP and EPX (MBP−/-EPX−/− mice). These mice are featured with eosinophil deficiencies similar to those observed in animals deficient of EPX or MBP only, but represent an advance in the implementation of an in vivo model to investigate eosinophil pathophysiology [199]. These models are largely utilized for research in allergy, inflammation, and respiratory diseases and could be successfully employed in anticancer research [200].
1.9.4 Humanized Mouse Models
The development of humanized mouse models best recapitulates the pathology of human diseases and thus represents a major goal to understand the role of eosinophils in human cancer. Recently, a novel IL-3/GM-CSF/IL-5 Tg NOD/Shi-scid-IL2rγnull (NOG) model, a mouse strain in which human eosinophil differentiation is induced from HSC, was reported [201]. In this mouse strain, the authors established a human asthmatic inflammation model by intratracheal administration of human IL-33. This enabled to study the Th2 responses specifically in a human context, including the eosinophil-dependent responses in asthma. By using humanized NSG mice adoptively transferred with human CD34+ hematopoietic stem cells, Arnold and collaborators showed that following infection with gastrointestinal bacteria, eosinophils are recruited to the tissue where they reduce inflammation by suppressing Th1 immune responses [202]. Thus, humanized mouse models represent a valid opportunity to investigate specifically the roles of eosinophils in different human cancers.
1.10 Concluding Remarks and Outstanding Questions
There is compelling evidence that eosinophils are potent effector and immunoregulatory cells in TME of experimental and human cancer [49, 203]. Several studies have reported that eosinophilia can be associated with a favorable prognosis in a variety of solid and hematologic tumors [6]. By contrast, a limited number of studies indicate a protumorigenic role of eosinophils [6, 114]. The potentially dual role (i.e., pro-tumorigenic and antitumorigenic) of eosinophils raises several fundamental questions. First, there is the possibility that the role of eosinophils and their mediator is cancer-specific (e.g., influenced by different TMEs). Alternatively, different subsets of eosinophils and/or different eosinophil-derived mediators can play distinct or even opposite roles in tumorigenesis. There is already evidence, at least in mice, of the existence of different subsets representing different stages of maturation of eosinophils [202, 204, 205]. Recent fate mapping experiments demonstrate that macrophages [206] and mast cells [207, 208] form a highly heterogeneous population of immune cells, similar to T cells [209]. Future studies should address the possible roles of plasticity/hypothetical subtypes of eosinophils by single-cell RNA-seq, together with analyses of encoded proteins.
Studies of eosinophils are usually performed on cells isolated from peripheral blood where O2 and nutrients are abundant and pH neutral. By contrast, eosinophils in TME are embedded in a hostile metabolic setting characterized by hypoxia, accumulation of lactate, potassium and adenosine, and low pH [210,211,212,213,214]. Thus, the biochemical and functional characteristics of peritumoral and intratumoral eosinophils likely differ from those of peripheral blood eosinophils.
Experimental models have started to provide evidence that eosinophils and their mediators can play a protective role by inhibiting tumor growth and the formation of metastasis in different cancers [40, 41]. Several mouse models have been characterized for the evaluation of the pathophysiological roles of eosinophils. Different groups have used target-specific mouse models to demonstrate the antitumorigenic role of eosinophils [40, 41, 105]. Humanized mouse models best recapitulate human disease and will represent a useful tool to evaluate the role of eosinophils and their mediators in different human cancers.
Immunotherapy with mAbs targeting immune checkpoint inhibitors (ICIs) (e.g., CTLA-4, PD-1/PD-L1 network) has revolutionized the therapies of an increasing number of solid and hematologic tumors [215, 216]. Unfortunately, these therapies are effective only in a percentage of patients, and there is urgent need of biomarkers predictive for ICI-based immunotherapy [217]. There is some evidence that baseline peripheral blood eosinophils represent a useful biomarker for prognosis of melanoma [109] and NSCLC [111]. Future studies should evaluate the predictive value of different subsets of peripheral blood eosinophils (e.g., low and high density) in response to ICIs in different cancers.
A deeper insight into the immunological and molecular mechanisms regulating the link between tumor-infiltrating eosinophils and tumor cells could lead to the identification of new prognostic/predictive biomarkers, as well as a wider view of cancer immunotherapy, in an even more personalized therapeutic approach.
Abbreviations
- AEC:
-
Absolute eosinophils count
- ANGPTs:
-
Angiopoietins
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- Ag:
-
Antigen
- APCs:
-
Antigen-presenting cells
- A. fumigatus :
-
Aspergillus fumigatus
- BECs:
-
Blood endothelial cells
- BAL:
-
Bronchoalveolar lavage
- CAFs:
-
Cancer-associated fibroblasts
- CEL:
-
Chronic eosinophilic leukemia
- DCs:
-
Dendritic cells
- ECP:
-
Eosinophil cationic protein
- EDN:
-
Eosinophil-derived neurotoxin
- EPX:
-
Eosinophil peroxidase
- E. coli :
-
Escherichia coli
- EXO:
-
Exosomes
- EV:
-
Extracellular vesicles
- FGF:
-
Fibroblast growth factor
- FPR-1:
-
Formyl peptide receptor-1
- HSC:
-
Hematopoietic stem cell
- HMGB1:
-
High Mobility Group Box 1
- HD:
-
Hodgkin’s disease
- HES:
-
Hypereosinophilic syndrome
- ICIs:
-
Immune checkpoint inhibitors
- ILC2:
-
Innate lymphoid cells
- IFN:
-
Interferon
- IL:
-
Interleukin
- iNKT:
-
Invariant natural killer T
- LIAR:
-
Local immunity and/or remod eling/repair
- LECs:
-
Lymphatic endothelial cells
- MBP:
-
Major basic protein
- MCA:
-
Methylcholanthrene
- mAb:
-
Monoclonal antibody
- M-MDSC:
-
Monocytic myeloid-derived suppressor cells
- MVB:
-
Multivesicular bodies
- MDSC:
-
Myeloid-derived suppressor cells
- NK:
-
Natural killer () cells
- NO:
-
Nitric oxide
- NOG:
-
NOD/Shi-scid/IL-2Rγnull
- NSCLC:
-
Non-small cell lung cancer
- OSCC:
-
Oral squamous cell carcinoma
- OS:
-
Overall survival
- PRR:
-
Pattern recognition receptor
- PlGF:
-
Placenta growth factor
- PDGF:
-
Platelet-derived growth factor
- PMN-MDSC:
-
Polymorphonuclear myeloid- derived suppressor cells
- PD-1:
-
Programmed cell death-1
- PSF:
-
Progression-free survival
- ROS:
-
Reactive oxygen species
- RSV:
-
Respiratory syncytial virus
- Siglec-8:
-
Sialic-binding immunoglobulin like lectin 8
- S. aureus :
-
Staphylococcus aureus
- Th2:
-
T helper 2
- TSLP:
-
Thymic stromal lymphopoietin
- TREG:
-
T regulatory cell
- TAM:
-
Tumor-associated macrophages
- TATE:
-
Tumor-associated tissue eosinophilia
- TILs:
-
Tumor-infiltrating lymphocytes
- TME:
-
Tumor microenvironment
- TK:
-
Tyrosine kinase
- VEGF:
-
Vascular endothelial growth factor
Bibliography
Kay AB (2015) The early history of the eosinophil. Clin Exp Allergy 45:575–582. https://doi.org/10.1111/cea.12480
Willebrand R, Voehringer D (2017) Regulation of eosinophil development and survival. Curr Opin Hematol 24:9–15. https://doi.org/10.1097/moh.0000000000000293
Johnston LK, Bryce PJ (2017) Understanding interleukin 33 and its roles in eosinophil development. Front Med (Lausanne) 4:51. https://doi.org/10.3389/fmed.2017.00051
Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, Berdnikovs S, Bryce PJ (2016) IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol 197:3445–3453. https://doi.org/10.4049/jimmunol.1600611
Simon SCS, Utikal J, Umansky V (2019) Opposing roles of eosinophils in cancer. Cancer Immunol Immunother 68:823–833. https://doi.org/10.1007/s00262-018-2255-4
Varricchi G, Galdiero MR, Loffredo S, Lucarini V, Marone G, Mattei F, Schiavoni G (2018) Eosinophils: the unsung heroes in cancer? Onco Targets Ther 7:e1393134. https://doi.org/10.1080/2162402X.2017.1393134
Buder-Bakhaya K, Hassel JC (2018) Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-a review from the melanoma perspective and beyond. Front Immunol 9:1474. https://doi.org/10.3389/fimmu.2018.01474
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Schiavoni G, Gabriele L, Mattei F (2013) The tumor microenvironment: a pitch for multiple players. Front Oncol 3:90. https://doi.org/10.3389/fonc.2013.00090
Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307:C25–C38. https://doi.org/10.1152/ajpcell.00084.2014
Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R (2019) Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer 18:70. https://doi.org/10.1186/s12943-019-0994-2
Linette GP, Carreno BM (2019) Tumor-infiltrating lymphocytes in the checkpoint inhibitor era. Curr Hematol Malig Rep 14:286–291. https://doi.org/10.1007/s11899-019-00523-x
Tanaka A, Sakaguchi S (2019) Targeting Treg cells in cancer immunotherapy. Eur J Immunol 49:1140–1146. https://doi.org/10.1002/eji.201847659
Park SL, Gebhardt T, Mackay LK (2019) Tissue-resident memory T cells in cancer immunosurveillance. Trends Immunol 40:735–747. https://doi.org/10.1016/j.it.2019.06.002
Larsen SK, Gao Y, Basse PH (2014) NK cells in the tumor microenvironment. Crit Rev Oncog 19:91–105. https://doi.org/10.1615/CritRevOncog.2014011142
Wylie B, Macri C, Mintern JD, Waithman J (2019) Dendritic cells and cancer: from biology to therapeutic intervention. Cancers (Basel) 11:521. https://doi.org/10.3390/cancers11040521
Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G (2008) Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol 20:504–511. https://doi.org/10.1016/j.coi.2008.05.007
Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M et al (2015) Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350:972–978. https://doi.org/10.1126/science.aad0779
Fu C, Jiang A (2018) Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol 9:3059. https://doi.org/10.3389/fimmu.2018.03059
Schiavoni G, Mattei F, Gabriele L (2013) Type I interferons as stimulators of DC-mediated cross-priming: impact on anti-tumor response. Front Immunol 4:483. https://doi.org/10.3389/fimmu.2013.00483
Lorenzi S, Mattei F, Sistigu A, Bracci L, Spadaro F, Sanchez M, Spada M, Belardelli F, Gabriele L, Schiavoni G (2011) Type I IFNs control antigen retention and survival of CD8α+ dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol 186:5142–5150. https://doi.org/10.3389/fimmu.2013.00483
van Beek JJ, Wimmers F, Hato SV, de Vries IJ, Sköld AE (2014) Dendritic cell cross talk with innate and innate-like effector cells in antitumor immunity: implications for DC vaccination. Crit Rev Immunol 34:517–536. https://doi.org/10.1615/CritRevImmunol.2014012204
Janikashvili N, Bonnotte B, Katsanis E, Larmonier N (2011) The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol 2011:430394. https://doi.org/10.1155/2011/430394
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14:399–416. https://doi.org/10.1038/nrclinonc.2016.217
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC et al (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24:541–550. https://doi.org/10.1038/s41591-018-0014-x
Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF (2018) Human mast cells and basophils-how are they similar how are they different? Immunol Rev 282:8–34. https://doi.org/10.1111/imr.12627
Galdiero MR, Varricchi G, Loffredo S, Mantovani A, Marone G (2018) Roles of neutrophils in cancer growth and progression. J Leukoc Biol 103:457–464. https://doi.org/10.1002/JLB.3MR0717-292R
Marone G, Borriello F, Varricchi G, Genovese A, Granata F (2014) Basophils: historical reflections and perspectives. Chem Immunol Allergy 100:172–192. https://doi.org/10.1159/000358734
Robida PA, Puzzovio PG, Pahima H, Levi-Schaffer F, Bochner BS (2018) Human eosinophils and mast cells: birds of a feather flock together. Immunol Rev 282:151–167. https://doi.org/10.1111/imr.12638
Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW (2016) Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol 16:186–200. https://doi.org/10.1097/ACI.0000000000000251
Rothenberg ME, Hogan SP (2006) The eosinophil. Annu Rev Immunol 24:147–174. https://doi.org/10.1146/annurev.immunol.24.021605.090720
Weller PF, Spencer LA (2017) Functions of tissue-resident eosinophils. Nat Rev Immunol 17:746–760. https://doi.org/10.1038/nri.2017.95
Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, Weller PF (2016) Eosinophil ETosis and DNA traps: a new look at eosinophilic inflammation. Curr Allergy Asthma Rep 16:54. https://doi.org/10.1007/s11882-016-0634-5
Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ et al (2008) Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14:949–953. https://doi.org/10.1038/nm.1855
Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA (2010) Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy 40:563–575. https://doi.org/10.1111/j.1365-2222.2010.03484.x
Wen, T.; Rothenberg, M.E. (2016) The regulatory function of eosinophils. Microbiol Spectr 4. https://doi.org/10.1128/microbiolspec.MCHD-0020-2015
Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA (2007) Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol 179:4840–4848. https://doi.org/10.4049/jimmunol.179.7.4840
Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, Connelly L, Dulek D, Peebles RS, Fingleton B et al (2015) Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res 75:1624–1634. https://doi.org/10.1158/0008-5472.can-14-2379
Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ (2008) Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med 205:699–710. https://doi.org/10.1084/jem.20071840
Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ (2015) Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 16:609–617. https://doi.org/10.1038/ni.3159
Lucarini V, Ziccheddu G, Macchia I, La Sorsa V, Peschiaroli F, Buccione C, Sistigu A, Sanchez M, Andreone S, D’Urso MT et al (2017) IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Onco Targets Ther 6:e1317420. https://doi.org/10.1080/2162402x.2017.1317420
Dajotoy T, Andersson P, Bjartell A, Löfdahl CG, Tapper H, Egesten A (2004) Human eosinophils produce the T cell-attracting chemokines MIG and IP-10 upon stimulation with IFN-gamma. J Leukoc Biol 76:685–691. https://doi.org/10.1189/jlb.0803379
Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ (2008) Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med 205:79–90. https://doi.org/10.1084/jem.20062027
Lotfi R, Lotze MT (2008) Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol 83:456–460. https://doi.org/10.1189/jlb.0607366
Chu DK, Jimenez-Saiz R, Verschoor CP, Walker TD, Goncharova S, Llop-Guevara A, Shen P, Gordon ME, Barra NG, Bassett JD et al (2014) Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med 211:1657–1672. https://doi.org/10.1084/jem.20131800
Davoine F, Lacy P (2014) Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol 5:570. https://doi.org/10.3389/fimmu.2014.00570
Schuijs MJ, Hammad H, Lambrecht BN (2019) Professional and ’Amateur’ antigen-presenting cells in type 2 immunity. Trends Immunol 40:22–34. https://doi.org/10.1016/j.it.2018.11.001
Kvarnhammar AM, Cardell LO (2012) Pattern-recognition receptors in human eosinophils. Immunology 136:11–20. https://doi.org/10.1111/j.1365-2567.2012.03556.x
Rosenberg HF, Dyer KD, Foster PS (2013) Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9–22. https://doi.org/10.1038/nri3341
Long H, Liao W, Wang L, Lu Q (2016) A player and coordinator: the versatile roles of eosinophils in the immune system. Transfus Med Hemother 43:96–108. https://doi.org/10.1159/000445215
Nutku E, Aizawa H, Hudson SA, Bochner BS (2003) Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101:5014–5020. https://doi.org/10.1182/blood-2002-10-3058
Nutku E, Hudson SA, Bochner BS (2005) Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun 336:918–924. https://doi.org/10.1016/j.bbrc.2005.08.202
Nutku-Bilir E, Hudson SA, Bochner BS (2008) Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol 38:121–124. https://doi.org/10.1165/rcmb.2007-0154OC
Na HJ, Hudson SA, Bochner BS (2012) IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine 57:169–174. https://doi.org/10.1016/j.cyto.2011.10.007
Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS (2008) Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63:1156–1163. https://doi.org/10.1111/j.1398-9995.2008.01709.x
Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, Lin HH, Gordon S, Kwakkenbos MJ (2007) EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol 37:2797–2802. https://doi.org/10.1002/eji.200737553
Legrand F, Tomasevic N, Simakova O, Lee CC, Wang Z, Raffeld M, Makiya MA, Palath V, Leung J, Baer M et al (2014) The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol 133:1439–1447. https://doi.org/10.1016/j.jaci.2013.11.041
Barthel SR, Johansson MW, McNamee DM, Mosher DF (2008) Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol 83:1–12. https://doi.org/10.1189/jlb.0607344
Carr TF, Zeki AA, Kraft M (2018) Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med 197:22–37. https://doi.org/10.1164/rccm.201611-2232PP
Varricchi G, Pecoraro A, Marone G, Criscuolo G, Spadaro G, Genovese A (2018) Thymic stromal Lymphopoietin isoforms, inflammatory disorders, and Cancer. Front Immunol 9:1595. https://doi.org/10.3389/fimmu.2018.01595
Halim TY (2016) Group 2 innate lymphoid cells in disease. Int Immunol 28:13–22. https://doi.org/10.1093/intimm/dxv050
Kaur R, Chupp G (2019) Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol 144:1–12. https://doi.org/10.1016/j.jaci.2019.05.031
Gabriele L, Schiavoni G, Mattei F, Sanchez M, Sestili P, Butteroni C, Businaro R, Mirchandani A, Niedbala W, Liew FY et al (2013) Novel allergic asthma model demonstrates ST2-dependent dendritic cell targeting by cypress pollen. J Allergy Clin Immunol 132:686–695. https://doi.org/10.1016/j.jaci.2013.02.037
Lee HY, Rhee CK, Kang JY, Byun JH, Choi JY, Kim SJ, Kim YK, Kwon SS, Lee SY (2014) Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp Lung Res 40:66–76. https://doi.org/10.3109/01902148.2013.870261
Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T (2009) Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun 386:181–185. https://doi.org/10.1016/j.bbrc.2009.06.008
Lei Y, Boinapally V, Zoltowska A, Adner M, Hellman L, Nilsson G (2015) Vaccination against IL-33 inhibits airway Hyperresponsiveness and inflammation in a house dust mite model of asthma. PLoS One 10:e0133774. https://doi.org/10.1371/journal.pone.0133774
Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY (2010) IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol 185:3472–3480. https://doi.org/10.4049/jimmunol.1000730
Smith D, Helgason H, Sulem P, Bjornsdottir US, Lim AC, Sveinbjornsson G, Hasegawa H, Brown M, Ketchem RR, Gavala M et al (2017) A rare IL33 loss-of-function mutation reduces blood eosinophil counts and protects from asthma. PLoS Genet 13:e1006659. https://doi.org/10.1371/journal.pgen.1006659
Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H (2008) A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol 121:1484–1490. https://doi.org/10.1016/j.jaci.2008.04.005
Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K et al (2008) Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Investig 88:1245–1253. https://doi.org/10.1038/labinvest.2008.82
Andreone S, Spadaro F, Buccione C, Mancini J, Tinari A, Sestili P, Gambardella AR, Lucarini V, Ziccheddu G, Parolini I et al (1664) IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers 2019:11. https://doi.org/10.3390/cancers11111664
Cook EB, Stahl JL, Schwantes EA, Fox KE, Mathur SK (2012) IL-3 and TNFα increase thymic stromal lymphopoietin receptor (TSLPR) expression on eosinophils and enhance TSLP-stimulated degranulation. Clin Mol Allergy 10:8. https://doi.org/10.1186/1476-7961-10-8
Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, Ziegler SF, Comeau MR (2008) Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol 181:4311–4319. https://doi.org/10.4049/jimmunol.181.6.4311
Varricchi G, Marone G, Spadaro G, Russo M, Granata F, Genovese A (2018) Novel biological therapies in severe asthma: targeting the right trait. Curr Med Chem 26:2801–2822. https://doi.org/10.2174/0929867325666180110094542
Marone G, Spadaro G, Braile M, Poto R, Criscuolo G, Pahima H, Loffredo S, Levi-Schaffer F, Varricchi G (2019) Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs 28:931–940. https://doi.org/10.1080/13543784.2019.1672657
Ravin KA, Loy M (2016) The eosinophil in infection. Clin Rev Allergy Immunol 50:214–227. https://doi.org/10.1007/s12016-015-8525-4
Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL (1990) In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol 144:3166–3173
Cadman ET, Thysse KA, Bearder S, Cheung AY, Johnston AC, Lee JJ, Lawrence RA (2014) Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog 10:e1003988. https://doi.org/10.1371/journal.ppat.1003988
Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D (1844-1851) Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J Infect Dis 2007:196. https://doi.org/10.1086/522968
Yoon J, Ponikau JU, Lawrence CB, Kita H (2008) Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol 181:2907–2915. https://doi.org/10.4049/jimmunol.181.4.2907
DeChatelet LR, Migler RA, Shirley PS, Muss HB, Szejda P, Bass DA (1978) Comparison of intracellular bactericidal activities of human neutrophils and eosinophils. Blood 52:609–617. https://doi.org/10.1182/blood.v52.3.609.609
Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, Hebisawa A, Asano K, Figueiredo RT, Neves JS (2018) Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol 141:571–585. e577. https://doi.org/10.1016/j.jaci.2017.07.048
Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF (1998) Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis 177:1458–1464. https://doi.org/10.1086/515322
Olszewska-Pazdrak B, Pazdrak K, Ogra PL, Garofalo RP (1998) Respiratory syncytial virus-infected pulmonary epithelial cells induce eosinophil degranulation by a CD18-mediated mechanism. J Immunol 160:4889–4895
Handzel ZT, Busse WW, Sedgwick JB, Vrtis R, Lee WM, Kelly EA, Gern JE (1998) Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol 160:1279–1284. https://doi.org/10.1542/peds.104.2.S1.359a
Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI (2007) Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 110:1578–1586. https://doi.org/10.1182/blood-2007-01-071340
Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF (2009) Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood 114:2649–2656. https://doi.org/10.1182/blood-2009-01-199497
Iwasaki K, Torisu M, Fujimura T (1986) Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer 58:1321–1327. https://doi.org/10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o
Jong EC, Klebanoff SJ (1949-1953) Eosinophil-mediated mammalian tumor cell cytotoxicity: role of the peroxidase system. J Immunol 1980:124
Ownby HE, Roi LD, Isenberg RR, Brennan MJ (1983) Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 52:126–130. https://doi.org/10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y
Huland E, Huland H (1992) Tumor-associated eosinophilia in interleukin-2-treated patients: evidence of toxic eosinophil degranulation on bladder cancer cells. J Cancer Res Clin Oncol 118:463–467. https://doi.org/10.1007/bf01629431
Silberstein DS, Schoof DD, Rodrick ML, Tai PC, Spry CJ, David JR, Eberlein TJ (1989) Activation of eosinophils in cancer patients treated with IL-2 and IL-2-generated lymphokine-activated killer cells. J Immunol 142:2162–2167
Rivoltini L, Viggiano V, Spinazzè S, Santoro A, Colombo MP, Takatsu K, Parmiani G (1993) In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. Int J Cancer 54:8–15. https://doi.org/10.1002/ijc.2910540103
Andersen CL, Siersma VD, Hasselbalch HC, Vestergaard H, Mesa R, Felding P, Olivarius ND, Bjerrum OW (2015) Association of the blood eosinophil count with hematological malignancies and mortality. Am J Hematol 90:225–229. https://doi.org/10.1002/ajh.23916
Enblad G, Molin D, Glimelius I, Fischer M, Nilsson G (2007) The potential role of innate immunity in the pathogenesis of Hodgkin’s lymphoma. Hematol Oncol Clin North Am 21:805–823. https://doi.org/10.1016/j.hoc.2007.07.007
Desenne JJ, Acquatella G, Stern R, Muller A, Sánchez M, Somoza R (1992) Blood eosinophilia in Hodgkin’s disease. A follow-up of 25 cases in Venezuela. Cancer 69:1248–1253. https://doi.org/10.1002/cncr.2820690529
Samoszuk M, Nansen L (1990) Detection of interleukin-5 messenger RNA in reed-Sternberg cells of Hodgkin’s disease with eosinophilia. Blood 75:13–16. https://doi.org/10.1182/blood.v75.1.13.13
Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR (2007) Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol 178:4222–4229. https://doi.org/10.4049/jimmunol.178.7.4222
von Wasielewski R, Seth S, Franklin J, Fischer R, Hübner K, Hansmann ML, Diehl V, Georgii A (2000) Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood 95:1207–1213. https://doi.org/10.1182/blood.v95.4.1207.004k34_1207_1213
Verstovsek S, Tefferi A, Kantarjian H, Manshouri T, Luthra R, Pardanani A, Quintás-Cardama A, Ravandi F, Ault P, Bueso-Ramos C et al (2009) Alemtuzumab therapy for hypereosinophilic syndrome and chronic eosinophilic leukemia. Clin Cancer Res 15:368–373. https://doi.org/10.1158/1078-0432.ccr-08-1302
Strati P, Cortes J, Faderl S, Kantarjian H, Verstovsek S (2013) Long-term follow-up of patients with hypereosinophilic syndrome treated with Alemtuzumab, an anti-CD52 antibody. Clin Lymphoma Myeloma Leuk 13:287–291. https://doi.org/10.1016/j.clml.2012.09.018
Caruso RA, Bersiga A, Rigoli L, Inferrera C (2002) Eosinophil-tumor cell interaction in advanced gastric carcinoma: an electron microscopic approach. Anticancer Res 22:3833–3836
Caruso RA, Ieni A, Fedele F, Zuccalà V, Riccardo M, Parisi E, Parisi A (2005) Degranulation patterns of eosinophils in advanced gastric carcinoma: an electron microscopic study. Ultrastruct Pathol 29:29–36. https://doi.org/10.1080/019131290882303
Caruso RA, Branca G, Fedele F, Parisi A, Finocchiaro G, Ieni A, Rigoli L (2015) Eosinophil-specific granules in tumor cell cytoplasm: unusual ultrastructural findings in a case of diffuse-type gastric carcinoma. Ultrastruct Pathol 39:226–230. https://doi.org/10.3109/01913123.2014.991886
Hollande C, Boussier J, Ziai J, Nozawa T, Bondet V, Phung W, Lu B, Duffy D, Paradis V, Mallet V et al (2019) Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat Immunol 20:257–264. https://doi.org/10.1038/s41590-019-0321-5
Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A et al (2015) Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with Ipilimumab. Clin Cancer Res 21:5453–5459. https://doi.org/10.1158/1078-0432.CCR-15-0676
Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M et al (2016) Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with Ipilimumab. Clin Cancer Res 22:2908–2918. https://doi.org/10.1158/1078-0432.CCR-15-2412
Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di Giacomo AM et al (2016) Baseline biomarkers for outcome of melanoma patients treated with Pembrolizumab. Clin Cancer Res 22:5487–5496. https://doi.org/10.1158/1078-0432.CCR-16-0127
Moreira A, Leisgang W, Schuler G, Heinzerling L (2017) Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 9:115–121. https://doi.org/10.2217/imt-2016-0138
Reichman H, Itan M, Rozenberg P, Yarmolovski T, Brazowski E, Varol C, Gluck N, Shapira S, Arber N, Qimron U et al (2019) Activated eosinophils exert Antitumorigenic activities in colorectal Cancer. Cancer Immunol Res 7:388–400. https://doi.org/10.1158/2326-6066.cir-18-0494
Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, Yonesaka K, Kudo K, Kaneda H, Hasegawa Y, Tanaka K et al (2018) Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol 13:97–105. https://doi.org/10.1016/j.jtho.2017.10.030
Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT (2002) Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology 41:152–157. https://doi.org/10.1046/j.1365-2559.2002.01437.x
van Driel WJ, Hogendoorn PC, Jansen FW, Zwinderman AH, Trimbos JB, Fleuren GJ (1996) Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol 27:904–911. https://doi.org/10.1016/s0046-8177(96)90216-6
Xie F, Liu LB, Shang WQ, Chang KK, Meng YH, Mei J, Yu JJ, Li DJ, Li MQ (2015) The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett 364:106–117. https://doi.org/10.1016/j.canlet.2015.04.029
Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O’Neill K, Colbert D et al (2006) Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol 79:1131–1139. https://doi.org/10.1189/jlb.0106027
Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT (2009) Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol 183:5023–5031. https://doi.org/10.4049/jimmunol.0900504
Temkin V, Aingorn H, Puxeddu I, Goldshmidt O, Zcharia E, Gleich GJ, Vlodavsky I, Levi-Schaffer F (2004) Eosinophil major basic protein: first identified natural heparanase-inhibiting protein. J Allergy Clin Immunol 113:703–709. https://doi.org/10.1016/j.jaci.2003.11.038
Sakkal S, Miller S, Apostolopoulos V, Nurgali K (2016) Eosinophils in cancer: favourable or unfavourable? Curr Med Chem 23:650–666. https://doi.org/10.2174/0929867323666160119094313
Kubo H, Loegering DA, Adolphson CR, Gleich GJ (1999) Cytotoxic properties of eosinophil granule major basic protein for tumor cells. Int Arch Allergy Immunol 118:426–428. https://doi.org/10.1159/000024154
Glimelius I, Rubin J, Fischer M, Molin D, Amini RM, Venge P, Enblad G (2011) Effect of eosinophil cationic protein (ECP) on Hodgkin lymphoma cell lines. Exp Hematol 39:850–858. https://doi.org/10.1016/j.exphem.2011.05.006
De Lima PO, Dos Santos FV, Oliveira DT, De Figueiredo RC, Pereira MC (2015) Effect of eosinophil cationic protein on human oral squamous carcinoma cell viability. Mol Clin Oncol 3:353–356. https://doi.org/10.3892/mco.2014.477
Chang KC, Lo CW, Fan TC, Chang MD, Shu CW, Chang CH, Chung CT, Fang SL, Chao CC, Tsai JJ et al (2010) TNF-alpha mediates eosinophil cationic protein-induced apoptosis in BEAS-2B cells. BMC Cell Biol 11:6. https://doi.org/10.1186/1471-2121-11-6
Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, Capron M (2010) Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol 185:7443–7451. https://doi.org/10.4049/jimmunol.1000446
Vadas MA, Nicola NA, Metcalf D (1983) Activation of antibody-dependent cell-mediated cytotoxicity of human neutrophils and eosinophils by separate colony-stimulating factors. J Immunol 130:795–799
Costain DJ, Guha AK, Liwski RS, Lee TD (2001) Murine hypodense eosinophils induce tumour cell apoptosis by a granzyme B-dependent mechanism. Cancer Immunol Immunother 50:293–299. https://doi.org/10.1007/pl00006690
Munitz A, Bachelet I, Fraenkel S, Katz G, Mandelboim O, Simon HU, Moretta L, Colonna M, Levi-Schaffer F (2005) 2B4 (CD244) is expressed and functional on human eosinophils. J Immunol 174:110–118. https://doi.org/10.4049/jimmunol.174.1.110
Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, Lefevre G, Capron M (2015) IL-18 is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J Immunol 195:2483–2492. https://doi.org/10.4049/jimmunol.1402914
Hsu HT, Mace EM, Carisey AF, Viswanath DI, Christakou AE, Wiklund M, Önfelt B, Orange JS (2016) NK cells converge lytic granules to promote cytotoxicity and prevent bystander killing. J Cell Biol 215:875–889. https://doi.org/10.1083/jcb.201604136
Horiuchi T, Weller PF (1997) Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol 17:70–77. https://doi.org/10.1165/ajrcmb.17.1.2796
Hoshino M, Takahashi M, Aoike N (2001) Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol 107:295–301. https://doi.org/10.1067/mai.2001.111928
Yousefi S, Hemmann S, Weber M, Hölzer C, Hartung K, Blaser K, Simon HU (1995) IL-8 is expressed by human peripheral blood eosinophils. Evidence for increased secretion in asthma. J Immunol 154:5481–5490
Puxeddu I, Berkman N, Ribatti D, Bader R, Haitchi HM, Davies DE, Howarth PH, Levi-Schaffer F (2010) Osteopontin is expressed and functional in human eosinophils. Allergy 65:168–174. https://doi.org/10.1111/j.1398-9995.2009.02148.x
Varricchi G, de Paulis A, Marone G, Galli SJ (2019) Future needs in mast cell biology. Int J Mol Sci 20:4397. https://doi.org/10.3390/ijms20184397
Varricchi G, Rossi FW, Galdiero MR, Granata F, Criscuolo G, Spadaro G, de Paulis A, Marone G (2019) Physiological roles of mast cells: collegium Internationale Allergologicum update 2019. Int Arch Allergy Immunol 179:247–261. https://doi.org/10.1159/000500088
Galdiero MR, Varricchi G, Seaf M, Marone G, Levi-Schaffer F (2017) Bidirectional mast cell-eosinophil interactions in inflammatory disorders and Cancer. Front Med (Lausanne) 4:103. https://doi.org/10.3389/fmed.2017.00103
Patella V, de Crescenzo G, Marinò I, Genovese A, Adt M, Gleich GJ, Marone G (1996) Eosinophil granule proteins activate human heart mast cells. J Immunol 157:1219–1225
Nissim Ben Efraim AH, Levi-Schaffer F (2014) Roles of eosinophils in the modulation of angiogenesis. Chem Immunol Allergy 99:138–154. https://doi.org/10.1159/000353251
Abdala-Valencia H, Coden ME, Chiarella SE, Jacobsen EA, Bochner BS, Lee JJ, Berdnikovs S (2018) Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J Leukoc Biol 104:95–108. https://doi.org/10.1002/jlb.1mr1117-442rr
Krüger-Krasagakes S, Li W, Richter G, Diamantstein T, Blankenstein T (1993) Eosinophils infiltrating interleukin-5 gene-transfected tumors do not suppress tumor growth. Eur J Immunol 23:992–995. https://doi.org/10.1002/eji.1830230438
Ishiko T, Sakamoto K, Mita S, Kamohara H, Ogawa M (1997) Evidence that eosinophil infiltration in the OK-432/fibrinogen-injected meth-a tumor in mice is mediated by locally produced IL-5. Int J Immunopharmacol 19:405–412. https://doi.org/10.1016/s0192-0561(97)00086-6
Kataoka S, Konishi Y, Nishio Y, Fujikawa-Adachi K, Tominaga A (2004) Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol 23:549–560. https://doi.org/10.1089/dna.2004.23.549
Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S et al (2012) Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol 188:703–713. https://doi.org/10.4049/jimmunol.1101270
Mazzeo C, Cañas JA, Zafra MP, Rojas Marco A, Fernández-Nieto M, Sanz V, Mittelbrunn M, Izquierdo M, Baixaulli F, Sastre J et al (1603-1613) Exosome secretion by eosinophils: a possible role in asthma pathogenesis. J Allergy Clin Immunol 2015:135. https://doi.org/10.1016/j.jaci.2014.11.026
Carmo LA, Bonjour K, Ueki S, Neves JS, Liu L, Spencer LA, Dvorak AM, Weller PF, Melo RC (2016) CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils. J Leukoc Biol 100:391–401. https://doi.org/10.1189/jlb.3A1015-480R
Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R et al (2008) Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A 105:18478–18483. https://doi.org/10.1073/pnas.0804547105
Sastre B, Rodrigo-Muñoz JM, Garcia-Sanchez DA, Cañas JA, Del Pozo V (2018) Eosinophils: old players in a new game. J Investig Allergol Clin Immunol 28:289–304. https://doi.org/10.18176/jiaci.0295
Cañas JA, Sastre B, Rodrigo-Muñoz JM, Fernández-Nieto M, Barranco P, Quirce S, Sastre J, Del Pozo V (2018) Eosinophil-derived exosomes contribute to asthma remodelling by activating structural lung cells. Clin Exp Allergy 48:1173–1185. https://doi.org/10.1111/cea.13122
Cañas JA, Sastre B, Mazzeo C, Fernández-Nieto M, Rodrigo-Muñoz JM, González-Guerra A, Izquierdo M, Barranco P, Quirce S, Sastre J et al (2017) Exosomes from eosinophils autoregulate and promote eosinophil functions. J Leukoc Biol 101:1191–1199. https://doi.org/10.1189/jlb.3AB0516-233RR
Feng D, Flaumenhaft R, Bandeira-Melo C, Weller P, Dvorak A (2001) Ultrastructural localization of vesicle-associated membrane protein(s) to specialized membrane structures in human pericytes, vascular smooth muscle cells, endothelial cells, neutrophils, and eosinophils. J Histochem Cytochem 49:293–304. https://doi.org/10.1177/002215540104900303
Zheng W, Aspelund A, Alitalo K (2014) Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124:878–887. https://doi.org/10.1172/jci71603
Marone G, Granata F (2014) Angiogenesis, lymphangiogenesis and clinical implications. Preface. Chem Immunol Allergy 99:XI–XII. https://doi.org/10.1159/000352074
Zachary I (2014) Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy 99:37–70. https://doi.org/10.1159/000354169
Lee SJ, Park C, Lee JY, Kim S, Kwon PJ, Kim W, Jeon YH, Lee E, Yoon YS (2015) Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci Rep 5:11019. https://doi.org/10.1038/srep11019
Karaman S, Detmar M (2014) Mechanisms of lymphatic metastasis. J Clin Invest 124:922–928. https://doi.org/10.1172/jci71606
Welti J, Loges S, Dimmeler S, Carmeliet P (2013) Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest 123:3190–3200. https://doi.org/10.1172/jci70212
Kim H, Kataru RP, Koh GY (2014) Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest 124:936–942. https://doi.org/10.1172/jci71607
de Paulis A, Prevete N, Fiorentino I, Rossi FW, Staibano S, Montuori N, Ragno P, Longobardi A, Liccardo B, Genovese A et al (2006) Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol 177:7322–7331. https://doi.org/10.4049/jimmunol.177.10.7322
Bosisio D, Salvi V, Gagliostro V, Sozzani S (2014) Angiogenic and antiangiogenic chemokines. Chem Immunol Allergy 99:89–104. https://doi.org/10.1159/000353317
Varricchi G, Granata F, Loffredo S, Genovese A, Marone G (2015) Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol 73:144–153. https://doi.org/10.1016/j.jaad.2015.03.041
Loffredo S, Borriello F, Iannone R, Ferrara AL, Galdiero MR, Gigantino V, Esposito P, Varricchi G, Lambeau G, Cassatella MA et al (2017) Group V secreted phospholipase A2 induces the release of proangiogenic and antiangiogenic factors by human neutrophils. Front Immunol 8:443. https://doi.org/10.3389/fimmu.2017.00443
Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, Oslund R, Gelb MH, Lambeau G, Marone G et al (2010) Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol 184:5232–5241. https://doi.org/10.4049/jimmunol.0902501
Marone G, Varricchi G, Loffredo S, Granata F (2016) Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol 778:146–151. https://doi.org/10.1016/j.ejphar.2015.03.088
Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, Ribatti D, Genovese A, Triggiani M, Marone G (2009) Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol 123:1142–1149. https://doi.org/10.1016/j.jaci.2009.01.044
De Palma M, Biziato D, Petrova TV (2017) Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 17:457–474. https://doi.org/10.1038/nrc.2017.51
Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N, Rossi FW, Basolo F, Ugolini C, de Paulis A et al (2010) Mast cells have a protumorigenic role in human thyroid cancer. Oncogene 29:6203–6215. https://doi.org/10.1038/onc.2010.348
Prevete N, Liotti F, Visciano C, Marone G, Melillo RM, de Paulis A (2015) The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene 34:3826–3838. https://doi.org/10.1038/onc.2014.309
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146:873–887. https://doi.org/10.1016/j.cell.2011.08.039
Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, Alitalo K (2001) Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 61:1786–1790
Achen MG, Williams RA, Baldwin ME, Lai P, Roufail S, Alitalo K, Stacker SA (2002) The angiogenic and lymphangiogenic factor vascular endothelial growth factor-D exhibits a paracrine mode of action in cancer. Growth Factors 20:99–107. https://doi.org/10.1080/08977190290031969
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985. https://doi.org/10.1126/science.6823562
Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D (1996) Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87:3336–3343. https://doi.org/10.1182/blood.v87.8.3336.bloodjournal8783336
Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N (1992) Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 267:26031–26037
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936. https://doi.org/10.1038/nature04478
Wuest TR, Carr DJ (2010) VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med 207:101–115. https://doi.org/10.1084/jem.20091385
Simons M, Gordon E, Claesson-Welsh L (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17:611–625. https://doi.org/10.1038/nrm.2016.87
Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG (1991) Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A 88:9267–9271. https://doi.org/10.1073/pnas.88.20.9267
Bry M, Kivelä R, Leppänen VM, Alitalo K (2014) Vascular endothelial growth factor-B in physiology and disease. Physiol Rev 94:779–794. https://doi.org/10.1152/physrev.00028.2013
Maglione D, Guerriero V, Viglietto G, Ferraro MG, Aprelikova O, Alitalo K, Del Vecchio S, Lei KJ, Chou JY, Persico MG (1993) Two alternative mRNAs coding for the angiogenic factor, placenta growth factor (PlGF), are transcribed from a single gene of chromosome 14. Oncogene 8:925–931
Shibuya M, Claesson-Welsh L (2006) Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 312:549–560. https://doi.org/10.1016/j.yexcr.2005.11.012
Clauss M, Weich H, Breier G, Knies U, Röckl W, Waltenberger J, Risau W (1996) The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 271:17629–17634. https://doi.org/10.1074/jbc.271.30.17629
Fischer C, Mazzone M, Jonckx B, Carmeliet P (2008) FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 8:942–956. https://doi.org/10.1038/nrc2524
Karpanen T, Bry M, Ollila HM, Seppänen-Laakso T, Liimatta E, Leskinen H, Kivelä R, Helkamaa T, Merentie M, Jeltsch M et al (2008) Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res 103:1018–1026. https://doi.org/10.1161/circresaha.108.178459
Heinolainen K, Karaman S, D’Amico G, Tammela T, Sormunen R, Eklund L, Alitalo K, Zarkada G (2017) VEGFR3 modulates vascular permeability by controlling VEGF/VEGFR2 signaling. Circ Res 120:1414–1425. https://doi.org/10.1161/circresaha.116.310477
Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, Oike Y, Pajusola K, Thurston G, Suda T et al (2005) Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 105:4642–4648. https://doi.org/10.1182/blood-2004-08-3327
Eklund L, Saharinen P (2013) Angiopoietin signaling in the vasculature. Exp Cell Res 319:1271–1280. https://doi.org/10.1016/j.yexcr.2013.03.011
Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA (2001) Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol 167:4008–4016. https://doi.org/10.4049/jimmunol.167.7.4008
Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM (1997) Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol 17:519–528. https://doi.org/10.1165/ajrcmb.17.4.2877
Schwingshackl A, Duszyk M, Brown N, Moqbel R (1999) Human eosinophils release matrix metalloproteinase-9 on stimulation with TNF-alpha. J Allergy Clin Immunol 104:983–989. https://doi.org/10.1016/s0091-6749(99)70079-5
Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA et al (2004) Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305:1773–1776. https://doi.org/10.1126/science.1099472
Takeda K, Shiraishi Y, Ashino S, Han J, Jia Y, Wang M, Lee NA, Lee JJ, Gelfand EW (2015) Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge. J Allergy Clin Immunol 135:451–460. https://doi.org/10.1016/j.jaci.2014.08.014
Masterson JC, McNamee EN, Fillon SA, Hosford L, Harris R, Fernando SD, Jedlicka P, Iwamoto R, Jacobsen E, Protheroe C et al (2015) Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut 64:1236–1247. https://doi.org/10.1136/gutjnl-2014-306998
Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH (2002) Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 195:1387–1395. https://doi.org/10.1084/jem.20020656
Nei Y, Obata-Ninomiya K, Tsutsui H, Ishiwata K, Miyasaka M, Matsumoto K, Nakae S, Kanuka H, Inase N, Karasuyama H (2013) GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci U S A 110:18620–18625. https://doi.org/10.1073/pnas.1311668110
Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG et al (1996) IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15–24. https://doi.org/10.1016/s1074-7613(00)80294-0
Yoshida T, Ikuta K, Sugaya H, Maki K, Takagi M, Kanazawa H, Sunaga S, Kinashi T, Yoshimura K, Miyazaki J et al (1996) Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity 4:483–494. https://doi.org/10.1016/s1074-7613(00)80414-8
Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME (2005) The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol 175:5341–5350. https://doi.org/10.4049/jimmunol.175.8.5341
Denzler KL, Farmer SC, Crosby JR, Borchers M, Cieslewicz G, Larson KA, Cormier-Regard S, Lee NA, Lee JJ (2000) Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol 165:5509–5517. https://doi.org/10.4049/jimmunol.165.10.5509
Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, Cormier SA, Lindenberger KA, Song W, Wu W et al (2001) Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol 167:1672–1682. https://doi.org/10.4049/jimmunol.167.3.1672
Ochkur SI, Doyle AD, Jacobsen EA, LeSuer WE, Li W, Protheroe CA, Zellner KR, Colbert D, Shen HH, Irvin CG et al (2017) Frontline science: eosinophil-deficient MBP-1 and EPX double-knockout mice link pulmonary remodeling and airway dysfunction with type 2 inflammation. J Leukoc Biol 102:589–599. https://doi.org/10.1189/jlb.3HI1116-488RR
Munitz A, Hogan SP (2019) Alarming eosinophils to combat tumors. Nat Immunol 20:250–252. https://doi.org/10.1038/s41590-019-0318-0
Ito R, Maruoka S, Soda K, Katano I, Kawai K, Yagoto M, Hanazawa A, Takahashi T, Ogura T, Goto M et al (2018) A humanized mouse model to study asthmatic airway inflammation via the human IL-33/IL-13 axis. JCI insight 3:e121580. https://doi.org/10.1172/jci.insight.121580
Arnold IC, Artola-Borán M, Tallón de Lara P, Kyburz A, Taube C, Ottemann K, van den Broek M, Yousefi S, Simon HU, Müller A (2018) Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J Exp Med 215:2055–2072. https://doi.org/10.1084/jem.20172049
Davis BP, Rothenberg ME (2014) Eosinophils and cancer. Cancer Immunol Res 2:1–8. https://doi.org/10.1158/2326-6066.cir-13-0196
Percopo CM, Brenner TA, Ma M, Kraemer LS, Hakeem RM, Lee JJ, Rosenberg HF (2017) SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol 101:321–328. https://doi.org/10.1189/jlb.3A0416-166R
Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M et al (2016) Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 126:3279–3295. https://doi.org/10.1172/jci85664
Martner A, Wiktorin HG, Lenox B, Ewald Sander F, Aydin E, Aurelius J, Thorén FB, Ståhlberg A, Hermodsson S, Hellstrand K (2015) Histamine promotes the development of monocyte-derived dendritic cells and reduces tumor growth by targeting the myeloid NADPH oxidase. J Immunol 194:5014–5021. https://doi.org/10.4049/jimmunol.1402991
Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC (2005) Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol 174:7665–7675. https://doi.org/10.4049/jimmunol.174.12.7665
Li Z, Liu S, Xu J, Zhang X, Han D, Liu J, Xia M, Yi L, Shen Q, Xu S et al (2018) Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity 49:640–653.e645. https://doi.org/10.1016/j.immuni.2018.09.023
Clarke J, Panwar B, Madrigal A, Singh D, Gujar R, Wood O, Chee SJ, Eschweiler S, King EV, Awad AS et al (2019) Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med 216:2128–2149. https://doi.org/10.1084/jem.20190249
Liu N, Luo J, Kuang D, Xu S, Duan Y, Xia Y, Wei Z, Xie X, Yin B, Chen F et al (2019) Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression. J Clin Invest 129:631–646. https://doi.org/10.1172/jci123027
Kuchuk O, Tuccitto A, Citterio D, Huber V, Camisaschi C, Milione M, Vergani B, Villa A, Alison MR, Carradori S et al (2018) pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. Onco Targets Ther 7:e1445452. https://doi.org/10.1080/2162402x.2018.1445452
Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, Lee PH, Shin M, Patel SJ, Yu Z et al (2019) T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363:eaau0135. https://doi.org/10.1126/science.aau0135
Corbet C, Feron O (2017) Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 17:577–593. https://doi.org/10.1038/nrc.2017.77
Nissim Ben Efraim AH, Eliashar R, Levi-Schaffer F (2010) Hypoxia modulates human eosinophil function. Clin Mol Allergy 8:10. https://doi.org/10.1186/1476-7961-8-10
Havel JJ, Chowell D, Chan TA (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19:133–150. https://doi.org/10.1038/s41568-019-0116-x
Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L et al (2017) Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 17:286–301. https://doi.org/10.1038/nrc.2017.17
Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17:e542–e551. https://doi.org/10.1016/s1470-2045(16)30406-5
Acknowledgments
The authors apologize to the many researchers who have contributed importantly to this field and whose work has not been cited due to space and citation restrictions. The authors thank Dr. Gjada Criscuolo for critical reading of the manuscript, scientists from the CISI Laboratory and Schiavoni’s Laboratory not listed as authors for invaluable collaborations to the work reviewed, and medical graphic artist Fabrizio Fiorbianco for preparing Fig. 1.1. This work was supported in part by grants from CISI-Lab Project (University of Naples Federico II), CRèME Project, and TIMING Project (Regione Campania) to G.M. and from AIRC IG 21366 to G.S.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mattei, F. et al. (2020). Eosinophils in the Tumor Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1273. Springer, Cham. https://doi.org/10.1007/978-3-030-49270-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-49270-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49269-4
Online ISBN: 978-3-030-49270-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)