Abstract
Mushrooms have been used since centuries as food and medicine in many ancient cultures. This is based on their high content of carbohydrates, proteins, minerals, and many bioactive ingredients. Of different types of mushrooms, Pleurotus ostreatus is widely used and becomes nowadays the third important type in terms of worldwide production. P. ostreatus have been cultivated historically on tree logs, wood chops, and many other lignocellulosic biomass residues. This is based on the high capacity of mushroom to degrade complex organic lignin materials and convert them to utilizable carbohydrates. Therefore, this mushroom has been considered as high potential source for the production of lignin-degrading enzymes such as laccases, manganese peroxidases, veratryl alcohol oxidases, versatile peroxidases, heme-thiolate peroxidases, and many other biocatalysts. However, the production of these enzymes is regulated by different genes, and the enzyme yield is highly dependent on the type of substrate used, cultivation conditions, and addition of some minerals which act as enzyme cofactors. This chapter will provide up-to-date information about the enzyme system of P. ostreatus which is used for the degradation of lignocellulosic materials to support mushroom growth. Furthermore, these enzymes have a wide range of potential applications in different industries. Therefore, these types of enzymes can be used individually or in the form of enzyme cocktail in bleaching of pulp, removal of toxic phenolic compounds and toxic dyes from wastewater, biorefinery for biofuel production, and many other applications. In addition, as this type of mushroom is considered as Generally Regarded As Safe (GRAS) according to the Food and Drug Administration (FDA), thus, the produced enzyme has no limitations in terms of safety issues for application in food and feed industries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Mushrooms have been recognized as important food and medicine in many ancient civilizations (El Enshasy et al. 2013). These are based on their high nutrient contents of carbohydrates, proteins, vitamins, minerals, and many other growth-promoting ingredients (Eleftherios et al. 2014; Maftoun et al. 2015). Nowadays, of thousands types of mushroom studied, Pleurotus sp. is considered as one of the top three economically important and widely grown mushrooms beside Agaricus and Lentinula. Pleurotus ostreatus (widely known as oyster mushroom) gained more interest in the recent years not only because of their high nutritional value but also due to the high content of bioactive polysaccharides and other metabolites of high medicinal values. These compounds exhibited immunomodulatory, antitumor, antioxidant, anti-inflammatory, antihyperglycemic, antihypocholesterolemic, antimicrobial, and antithrombotic properties (El Enshasy et al. 2012; El Enshasy and Hatti-Kaul 2013; Mohamed and Farghaly 2014; Correa et al. 2016). The therapeutic effect of oyster mushroom extract and pure bioactive compounds has been studied and proved in many in vivo and in vitro studies (Ryu et al. 2014; Elsayed et al. 2014; Younis et al. 2015; Masri et al. 2017).
In addition, P. ostreatus gained more interest than other types of mushrooms based on their ability to grow on large number of substrates and under different environmental conditions (tropical and subtropical region), high capacity to produce a large number of hydrolases, and ease of cultivation both in solid-state fermentation and in submerged cultivation system as well as higher growth rate compared to other types of mushrooms (El Enshasy et al. 2010; Maftoun et al. 2013). However, to survive in nature, mushrooms should be able to degrade complex lignocellulosic materials by different hydrolytic enzymes to break the complex structure of wood and utilize the produced sugars as substrate for growth and metabolite production. Therefore, mushrooms are well known for their high capacity to produce and excrete different types of enzymes of wide range of hydrolytic activities. Therefore, this chapter will provide the latest information about the enzyme systems of this type of mushroom and their potential application in different industries.
5.2 Understanding Lignin Structure
Lignins constitute with cellulose and hemicelluloses the three major components of lignocellulosic biomass. They are the second most abundant terrestrial polymers and carbon source after cellulose (Evers et al. 1999; Boerjan et al. 2003). In 1813, A. P. de Candolle has firstly named the substance as “lignine” based on the Latin word lignum which means “wood.” He described lignin as a fibrous, tasteless material, insoluble in water and alcohol, but soluble in weak alkaline solutions and which can be precipitated from solution using acid. The typical composition of the dry weight of wood, considered as a whole, is about 50% cellulose, 25% lignin, 20–25% hemicellulose, and 1–4% pectin (Camarero et al. 2014).
Cellulose forms basically a skeleton that is bounded by hemicelluloses and lignin (Sakakibari 1980). Lignin, a polyphenolic amorphous polymer, is the essential natural glue that fills the spaces between cellulose and hemicellulose and acts like a resin that holds the plants’ lignocellulose matrix together (Ritter 2008; Carmen 2009). Through this cross-linking with cellulose, lignin not only confers the strength, rigidity, and flexibility as well as aids in water transport to the plant but represents also the most significant barrier to wood decay by insects/microorganisms attacks and prevents the access of low molecular weight diffusible agents (Zakzeski et al. 2010).
In chemical point of view, lignin is a heterogeneous complex class of compounds that changes according to biomass source and isolation technique (Johnson 2002). However, many aspects in the chemistry of lignin still remain unclear due to its high complex structure (Pouteau et al. 2003); However, the lignin classification as grass, hardwood, and softwood is based on the ratios of three major phenylpropene monomers which vary according to the plant species, plant tissue, individual cell types, and cell wall layers (Faix 1991). In general, both native lignin (as present in biomass) and technical lignin (isolated from biomass through various processes) have three-dimensional amorphous polymer made up of methoxylated phenylpropane aromatic units structures of p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (Chakar and Ragauskas 2004). These phenylpropene monomers are randomly cross-linked polymers arising from an enzyme-mediated dehydrogenative polymerization of three phenylpropane monomer precursors (cinnamyl alcohol: coniferyl, synapyl, and p-coumaryl alcohols) (Kleinert and Barth 2008; Wong 2009). These three monolignols (phenylpropene units of lignins) shown in Fig. 5.1 are as follows:
-
Guaiacyl (G) units from the precursor trans-coniferyl-alcohol
-
Syringyl (S) units from trans-sinapyl-alcohol
-
p-Hydroxyphenyl (H) units from the precursor trans-p-coumaryl alcohol
Then, grass lignin is built up by the three monomeric units (G, S, and H), hardwood lignin contains roughly equal amounts of (G) and (S) units, and softwood lignin is composed mainly of (G) units (Faix 1991).
5.2.1 Lignocellulose Processing and Industrial Applications
For more economic profitability, lignocellulosic biorefinery produces multiple products, including fuels, and bulk or fine chemicals, from biomass, analogically compared to a petroleum refinery, which produces fuels and chemicals from crude oil (Zhang 2008; Rastegari et al. 2019). In order to achieve a high energy impact and an economically viable biorefinery, the valorization of all components of lignocellulosic biomass should be carried out (Abdel-Hamid et al. 2013). With various aromatic structures, many authors claim that lignin has a special industrial interest offering the feasibility of replacing relevant aromatic polymeric and fine chemicals (Duval and Lawoko 2014; Norgren and Edlund 2014; Laurichesse and Avérous 2014; Thakur and Thakur 2015; Thakur et al. 2014).
Lignins are usually considered as waste products of pulp and paper industry and had limited industrial uses. However, in 1998, only 1% of the lignin produced was used in valuable industrial processes. About 50 million tons of lignin were estimated to be extracted annually from woody biomass for the pulp and paper industry and applied as dispersants, adhesives, and surfactants (Cohen et al. 2002; Shah and Nervd 2002; Karam and Nicell 1997). However, new technologies are currently in use to valorization and converting lignin into value-added chemicals; then, a biomass fractionation technology has been developed by PureVision Technology, Inc., to produce value-added low molecular weight lignin as a coproduct to the cellulose stream and also as fuel substrate (Chheda et al. 2007).
In energy production process, the lignin fraction acts as a barrier against enzyme or microbial penetration through lignocelluloses decreasing the fermentable sugar yields and affecting negatively the overall biofuel development, making it an uneconomical process (Margeot et al. 2009; Menon and Rao 2012). Essential mechanical, thermomechanical, and thermochemical pretreatment strategies to overcome this limitation have been reported by many authors (Margeot et al. 2009; Frigon and Guiot 2010; Kumar et al. 2009) and could be summarized as follows:
-
Mechanical particle size reduction including both of wet, dry, ball, or vibratory ball milling and other forms of biomass grinding (Sarkar et al. 2012; Agbor et al. 2011).
-
Thermochemical hydrolysis at temperatures between 140 °C and 180 °C, using a dilute solution of sulfuric acid (0.5–2%) for 10–30 minutes as residence times for rendering the carbohydrate fraction (Yang and Lu 2010).
-
Thermomechanical steam explosion heating briefly the biomass to high temperatures (~200 °C) under high pressure followed by a rapid pressure drop that makes the biomass more penetrable for subsequent fermentation (Chandra et al. 2007).
-
Microwaves heating at short time create localized hotspots and open up the lignocelluloses biomass, facilitating enzyme access for saccharification (Canam et al. 2013).
-
Organosolv process modifying chemically and removing low molecular weight lignin fractions from biomass using alcohols such as ethanol, methanol, or other solvents at high-temperature extraction. In this process, some dilute acids like hydrochloric and sulfuric acid are used as a catalyst (Agbor et al. 2011).
-
As natural modification and degradation of the lignin component, biological pretreatments can reduce the severity requirements of obvious pretreatment strategies. The exploitation of the capacity to access and to modify the lignocellulosic biomass by microorganisms was studied by several authors such as Itoh et al. (2003) who used a variety of lignin-degrading white-rot fungi pretreatments followed by extracting lignin by an organosolv method from wood chips. This process leads to saving of electricity up to 15% and increases the ethanol yield obtained from the solid fraction.
The enzymatic degradation of lignocellulose by microbial consortia or lignin-degrading fungi involves both oxidative and hydrolytic mechanisms based on OH radical reactivities. Attack on the hydroxyl group in lignin subunits by abstracting aliphatic hydrogens and adding to aromatic rings results in benzyl ketone production and hydroxylated cyclohexadienyl radical generation occurring on subsequent C-C bond cleavage and degradative chain reactions (Gierer 1990). This leads to decreasing energy requirements, less producing of fermentation-inhibiting substances. Thus, biological pretreatment of lignocellulosic materials is very useful when incorporated into any economical biorefinery strategies for biofuels and/or metabolite production (Isroi et al. 2011; Chen et al. 2010). In pulp and paper industries, processed lignin is produced traditionally through three distinct processes: kraft lignin, lignosulfonate lignin, and organosolv lignin (Doherty et al. 2011). Other than renewable bioenergy resources and pulp and paper industry supplement, significant progress is being made in research for the feasibility of a variety of applications of lignin or lignin-related product as a resource for chemicals and materials. For this, two different strategies are followed for lignin conversion to value-added components, either controlled depolymerization into small molecules (Li et al. 2015; Behling et al. 2016) or building block to synthesize new functional materials (Kai et al. 2016; Upton and Kasko 2016). Possessing multiple functional groups that can form inter- and intramolecular hydrogen bonding, lignin is considered as an alternative source for the production of more value-added chemicals according to its compatibility with host matrices (Karam and Nicell 1997; Doherty et al. 2011; Wang et al. 2016). This compatibility which is improved by chemical modification (oxyalkylation or hydroxyalkylation) minimizing lignin molecules’ auto-association (Zhao et al. 2016; Feldman et al. 2001; Maldhure et al. 2012). Gallezot (2007) indicated that three potential strategies for lignin valorization to produce fine chemicals with a high degree of functionality can be adopted.
The first strategy acts on gasifying lignin allowed to synthesize gas or degraded by pyrolysis to a mixture of small molecules. The second strategy turns, in the first step, the functional groups present into the lignin monomers’ simple aromatic compounds such as phenol, benzene, toluene, and xylene. In a second step, bulk and fine chemicals are produced using catalytic technology developed for petroleum refineries. Finally, using highly selective catalysts in a one-pot approach, the third strategy converses directly the biomass to valuable chemicals (Karam and Nicell 1997; Doherty et al. 2011; Thakur et al. 2014; Chatterjee and Saito 2015; Liu et al. 2015). Then, several value-added chemicals are produced including:
-
1.
Lignin: as a filler or additive in polymers and biopolymers – usually at less than 20–30% of total weight
-
2.
Lignin-derived functional materials such as carbon fibers, activated carbon, adhesives, and foams
-
3.
Lignosulfonates: as dispersants, water reducer in concrete, additive in coal-water slurry, or viscosity reducer
In addition, in light of renewed interest in promoting value-added applications of lignin, it is expected recently lignin nanoparticles will play a vital role in promoting lignin valorization in polymer industry (Stark et al. 2015).
5.2.2 Lignin Degradation
Lignin is a complex polymer with very low degradation rate in nature. The biodegradation which is carried out by wood-rotting fungi constitutes a key step for carbon cycling in nature, as well as for the industrial use of plant biomass by increasing accessibility to cellulose. It is an oxidative process that has been investigated for decades as a model for biotechnological application in the pulp and paper industries, animal feeding, and bioethanol production. Ligninolytic oxidoreductases (laccases and different types of peroxidases) secreted by wood-rotting fungi are the sole enzymes able to oxidize the phenylpropane lignin units (Schwarze 2007; Yadav et al. 2016, 2018). However, different enzymes have been reported for their role in lignin degradation such as lignin peroxidase (LiP), manganese peroxidase (MnP), aryl alcohol oxidase (AAO), and glyoxal oxidase (GLOX) as shown in Fig. 5.2.
Lignin-degrading enzymes’ actions. LiP lignin peroxidase; MnP manganese peroxidase; AAO aryl alcohol oxidase; GLOX glyoxal oxidase; VP versatile peroxidase; MED mediator. (Modified from Abdel-Hamid et al. 2013)
The main enzymes associated with lignin degradation are laccases, lignin peroxidases, and manganese peroxidases; while some white-rot fungi produce all the three classes of enzymes, others produce only one or two (Hatakka 1994). Laccases are multicopper enzymes, which catalyze the oxidation of phenolic compounds including a range of dyes with concomitant reduction of oxygen (Eggert et al. 1996; Chivukula and Renganathan 1995; Munoz et al. 1997). Recent interest in laccase is, in part, a consequence of the findings that the substrate range of laccase can be expanded to include non-phenolic dyes, eventually in the presence of suitable mediators (Bourbonnais and Paice 1990).
5.3 Laccase (EC 1.1.2.2)
Mushrooms excrete different oxidative, hydrolytic, and non-hydrolytic enzymes that act synergistically to hydrolyze the complex chemical structure which is composed of cellulose, hemicelluloses, and lignin (Guerriero et al. 2015). Laccases and class II peroxidases belong to the oxidative enzymes including lignin peroxidase, manganese peroxidase, and hybrid lignin/manganese versatile peroxidase which catalyze the cleavage of C-C and C-O-C bonds in a wide variety of organic compounds, such as lignin and polyphenolic structures (Ertan et al. 2012; Siddiqui et al. 2014). Laccases (or phenoloxidase) were firstly described and identified in 1883 by Yoshida, from the Japanese lacquer tree Rhus vernicifera. They are phenol-oxidizing enzymes and listed as ecofriendly catalyzing variety of reactions involving one-electron oxidation with water generation (Hao et al. 2007; Javed et al. 2017). Laccases are widespread in nature and have been found in non-microbial sources (plants and insects) and in microbial sources (Thurston 1994; Brijwani et al. 2010; Mayer and Staples 2002; Santhanam et al. 2011; Yavuz et al. 2014). As shown in Table 5.1, the genus Pleurotus showed strong laccase activity (Silva et al. 2012; Alexandrino et al. 2007; Sethuraman et al. 1999; Ardon et al. 1996). P. ostreatus produces many extracellular enzymes with strong ability for lignin degradation and substrate oxidation. Therefore, this mushroom attracted attention because of its high potential for biofactory laccase production for many biotechnology applications (Karas et al. 2011; Faraco et al. 2009; Pezzella et al. 2013).
5.3.1 Laccases Structure and Mechanism of Reaction
Laccase (EC 1.10.3.2), or benzenediol: oxygen oxidoreductases, belongs to a group of phenol oxidizers enzymes called blue multicopper oxidases, which are considered generally as part of the monomeric glycoprotein group. This group of enzyme is mainly exocellular enzyme that can oxidize phenols and aromatic compounds including amines, esters, and ethers (Rathinasamy and Thayumanavan 2010; Solomon et al. 1996). They act by reducing the molecular oxygen to water and catalyzing the oxidation of ortho- and para-aminophenols, diphenols, aryl diamines, polyphenols, polyamines, and lignin (Shleev et al. 2006a, b). Therefore, laccases can involve in the degradation of a wide range of xenoaromatics substrates such as textile dyes, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, pesticides, and synthetic polymers (Mester and Tien 2000; Bezalel et al. 1997; Novotny et al. 2000; Riva 2006; Yadav et al. 2019a, b). Regarding this high nonspecific oxidation capacity, laccases derive interests and attraction from researchers to be used as biocatalysts for many industrial and biotechnological applications, from food processing to environmental technologies through textile and paper industry. Recent research showed also the potential applications of laccases in nanobiotechnology and biomedicine (Mayer and Staples 2002; Jurado et al. 2009; Zhuo et al. 2011; Javed et al. 2017).
Based on genetic research, four different genes and their corresponding cDNAs have been identified from P. ostreatus and named pox1 (laccase isoenzyme not yet identified) (Giardina et al. 1995), pox2 or poxc (Giardina et al. 1996), poxa1b (Giardina et al. 1999), and poxa3 (Palmieri et al. 2003). According to Mot and Silaghi-Dumitrescu (2012), the structure of laccases is held up by monomeric units consisting of three domains which are arranged in a sequence to form a barrel-type structure. The same study reported that fungal laccases lack high-order oligomeric assemblies making the crystal lattice and are found generally as monomers.
According to Hoegger et al. (2006), laccases have four catalytic copper atoms in their structure organized in two individual sites that bind the reducing substrate and O2. The substrate oxidation site contains a paramagnetic type 1 copper (T1 or T1Cu) responsible for the characteristic blue color of the enzyme in the reduced resting environment. Another copper (T2 or T2Cu) contributes in catalytic and redox activity site coordinated by three histidines. The two other coppers (T3 or T3Cu) are responsible for the activation and O2 transport and substrate oxygenation and coordinated by three histidines (Hoegger et al. 2006). Both T2Cu and the two T3Cu are clustered at 12 Å from the T1Cu and form a tri-nuclear site as shown in Fig. 5.3, where O2 is reduced to two molecules of water, receiving four consecutive electrons from four independent mono-oxidation reactions at the T1Cu site through a strictly conserved His-Cys-His electron transfer route (Mot and Silaghi-Dumitrescu 2012).
Representation of a native fungus laccase from Trametes hirsute (Left), showing the copper ions organization (Right). http://www.rcsb.org/pdb/explore/jmol.do?structureId=3FPXopt=3
Beyond this molecular configuration, other enzymes can include also 2, 3, or 6 copper atoms (Gil et al. 2009; Messerschmidt and Huber 1990). The high potential of copper T1 is of great interest to biotechnologists because of its geometry. In this site, around the T1 copper atom, two histidines and one cysteine which are organized three-dimensionally with two other residues are weakly coordinated from the axial position. One of these residues is isoleucine, and the other can be phenylalanine, leucine, or methionine depending on the type of laccase characterized and reduction potential (Rodgers et al. 2010; Rivera-Hoyos et al. 2013). Several authors have reported that more than one laccase isozyme is secreted by fungi (Soden and Dobson 2001; Hoshida et al. 2001; Palmieri et al. 2003; Rodrïguez et al. 2008). At least, eight different laccase isoenzymes are produced by P. ostreatus and six of which have been isolated and fully characterized (Giardina et al. 1999; Pezzella et al. 2009; Palmieri et al. 1993, 1997, 2003). The most abundant protein present in the P. ostreatus cultures is POXC (59-kDa with pI 2.7). The enzymes POXA1b and POXA1w have a similar molecular weight around 61 kDa, while POXA2, POXB1, and POXB2 isoenzymes are of higher molecular weight around 67 kDa. POXA3a and POXA3b are heterodimers and composed of small (16 or 18 kDa) and large (61 kDa) subunits (Palmieri et al. 1997; Giardina et al. 1999).
However, several studies reported that P. ostreatus produces mainly three laccase isoenzymes called POXCs (Giardina et al. 1996): POXA1w, the white laccase isoenzyme with a singular metal content (Palmieri et al. 1997); POXA1b, the more stable isoenzyme at alkaline pH (Giardina et al. 1999); and the heterodimeric laccase isoenzyme POXA3 (Palmieri et al. 2003; Giardina et al. 2007; Faraco et al. 2008) with two forms, POXA3a and POXA3b (Palmieri et al. 2003; Giardina et al. 2007), that exhibit unusual structural features, being heterodimeric enzymes (Wahleithner et al. 1996; Yaver et al. 1996). These laccase isoenzymes are endowed with quaternary structure, consisting of two subunits of different molecular weights (Faraco et al. 2008). Palmieri et al. (1997) reported that the two laccase isoenzymes POXA1 and POXA2 are monomeric glycoproteins containing 3% and 9% carbohydrate with pI values of 6.7 and 4.0, respectively. The sequencing of the N terminus and three tryptic peptides of POXA1 reveals clear homology with laccases from other microorganisms, while POXA2 showed a blocked N terminus. However, POXA1 showed remarkable high stability at relatively wide range of temperature and pH values, whereas POXA2 is less stable at high temperature (Palmieri et al. 1997). The molecular weights of the two laccase isoenzymes were determined with three different methods. Sodium dodecyl sulfate polyacrylamide gel electrophoresis shown that the Mwt of POXA1 and POXA2 are of about 61 and 67 kDa; Gel filtration in native conditions shows a Mwt of about 54 and 59 kDa; Finally, matrix-assisted laser desorption ionization mass spectrometry was applied for POXA1 and give 61 kDa (Palmieri et al. 1997). Substrates with specific linkages and structural similarity with lignin, an amorphous polyphenolic polymer, can induce laccase activity. In addition, a wide range of aromatic compounds can be oxidized by laccases with concomitant water generation by reduction of molecular oxygen (Thurston 1994; Ullah et al. 2000).
In general, laccase is not able to oxidize substrates with lower redox potential like non-phenolic compounds. However, Call and Mücke (1997) have reported that the oxidation can be catalyzed in the presence of electron transfer mediators. Oxidation of substrates with chemical structure smilar to lignin like amino-phenols, orth- and para-diphenols, polyamines, aryl diamines, lignins and polyphenols is also carried out using the same mechanism. Then, laccases can act on many substances such as agricultural inputs, pesticides and herbicides, and dyes used in many industrial processes which must be degraded before being discarded (Morozova et al. 2007).
According to Baldrian (2006), more than 100 fungal laccases are purified and characterized and usually with optimal activity at acidic pH range with a temperatures range from 50 to 70 °C. Table 5.1 summarizes some other characteristics of laccase isoenzymes from Pleurotus ostreatus strains. As reported by Palmieri et al. (1993, 1997), generally, for the P. ostreatus laccase POXC, the optimal pH is already acidic for both ABTS and DMP degradation but around 6 for guaiacol and syringaldazine with a range of optimum temperature from 50 to 60 °C (Palmieri et al. 1993, 1997). Studying the two laccase isoenzymes POXA1 and POXA2 from P. ostreatus, Palmieri et al. (1997) reported that POXA2 acts with an acidic optimum pH of 3 for ABTS as substrate but an optimal pH of around 6 with other substrates like DMP, guaiacol, and syringaldazine, with optimum range of temperature between 25 and 35 °C. Both POXA1and POXA2 isoenzymes oxidize ABTS and syringaldazine, but POXA1 is unable to oxidize guaiacol (Palmieri et al. 1997).
5.3.2 Laccase Production and Bioprocessing
P. ostreatus grows naturally on tree stumps and can be easily cultured in the laboratory scale. However, most of mushroom cultivars usually use cereal straw-based substrates (Arjona et al. 2009). Different parameters influence the laccase production which is linked to a complex regulation of nutrients that affects directly the molecular expression. These include the type and concentration of carbon and nitrogen sources used, C/N ratio, and also the presence of some inducers and their concentrations (Terrón et al. 2004). Beside the nutritional factors, the production of laccase by P. ostreatus is influenced by other factors such as pH, the fermentation technique applied, and cultivation conditions like agitation, aeration, cultivation time, and many other factors (Terrón et al. 2004; Soden and Dobson (2001); Periasamy and Palvannan 2010; Janusz et al. 2007).
Different studies were focused on the optimization of the fermentation medium for laccase production by P. ostreatus to facilitate economic design of the full-scale fermentation operation systems. Studies include the investigation of the effect of carbon and nitrogen source (Stajic et al. 2006a; Liu et al. 2009), addition of some metal ions (Soden and Dobson 2001; Collins and Dobson 1997; Galhaup and Haltrich 2001; Galhaup et al. 2002; Baldrian and Gabriel 2002), and addition of aromatic compounds as inducers (González et al. 2003; De Souza et al. 2004; Terrón et al. 2004; Krishna et al. 2005a).
5.3.2.1 Media Composition and Cultivation Conditions
It has been reported that increasing the concentrations of glucose, wheat bran, urea, yeast extract, KH2PO4 and inoculums can increase laccase production (Krishna et al. 2005a; Fernández-Fernández et al. 2013). The works of Ardon et al. (1998) and Krishna et al. (2005b) show that the maximal laccase production by P. ostreatus was obtained within the pH range of 5.0–5.5 in submerged culture. Another study carried out by Terrón et al. (2004) reported that the presence of nine different aromatic compounds, including p-coumaric acid, guaiacol, and ferulic acid, can enhance laccase activity. Zhuo et al. (2017) studied the effect of aromatic compounds on the transcript levels of laccase genes, and the results have confirmed the previous results of Nyanhongo et al. (2002) who used ferulic acid as an effective laccase inducer and show that ferulic acid and vanillic acid have the most pronounced stimulatory effect on laccase gene transcription (Zhuo et al. 2017; Nyanhongo et al. 2002). Krishna et al. (2005a) reported on the positive effect of 2,5-xylidene addition to culture media after 96 h on laccase production. However, the inducers’ effects depend on the chemical structure, concentration, and time of its introduction into the production medium (Pointing et al. 2000; Sethuraman et al. 1998). The production of laccases in P. ostreatus is regulated by the presence of copper. Therefore, the two dimeric isoenzymes POXC and POXA1b have been detected only in the presence of copper (Palmieri et al. 2000, 2003).
5.3.2.2 Cultivation Process and Purification
Solid-state fermentation is an interesting operational mode for the production of laccases by P. ostreatus. For solid-state fermentation, Karp et al. (2012) have reported a productivity of 151.6 Ug−1 after 5 days in sugarcane bagasse-based medium. Several studies have showed high laccase productivity by P. ostreatus in submerged culture conditions; a productivity of 3500 U L−1 was reported by Lenz and Hölker (2004). Tlecuitl-Beristain et al. (2008) have reported 80 UmL−1 after 12 days in liquid culture without exogenous inducers (Lettera et al. 2011); also 12.2 UmL−1 has been reached after 18 days according to Tlecuitl-Beristain et al. (2008). Optimizing the laccase production in submerged culture conditions, Krishna et al. (2005a) and Rathinasamy and Thayumanavan (2010) have increased the laccase expression by 32.9%, from 538.8 to 803.3 U, and by 86.8%, from 485.0 to 906.3 U, using P. ostreatus 1804 and P. ostreatus IMI 395545, respectively.
Mansur et al. (2003) studied the secretion of laccase isozymes by P. ostreatus V-184 with different substrate specificities. They showed that laccase activity reached 2.4 U mL−1 using submerged culture for 14–16 days, at pH 6.5. Krishna et al. (2005b) have also studied the feasibility of Pleurotus ostreatus 1804 immobilization on polyurethane foam (PUF) cubes in the objective of laccase production in three different cases of submerged culture system. After 192 hours of cultivation at 5.6 pH, the enzyme yields of free mycelia and immobilized and packed bed reactor were 272.2 U, 312.6 U, and 392.9 U, respectively.
Rodrigues da Luz et al. (2012) reported also on the potential use of agrowastes such as coffee husks, eucalyptus sawdust, and eucalyptus bark as substrate in combination with rice bran for laccase production by P. ostreatus as substrates in combination with 20% rice bran. Parenti et al. (2013) have used water polluted with wheat straw extracts as substrate in submerged cultures incubated in the dark at 25 °C for 24 days with orbital shaking (150 rpm) and reported a laccase activity of 200 UL−1. To separate laccase isoenzymes, Palmieri et al. (1997) have fractioned P. ostreatus culture broth after 70 h of growth using ammonium sulfate precipitation followed by anionic exchange chromatography. Five different laccase fractions were separated (POXA1, POXA2, POXB1, POXB2, and POXC). Elution with a saline gradient at around 0.17, 0.18, and 0.32 M NaCl allowed to separate three isoenzymes POXB1, POXB2, and POXC, respectively, whereas the major laccase peak activity, corresponding to POXA1, and a fraction of POXA2 were recovered using the equilibrating buffer (Palmieri et al. 1997).
5.3.3 Applications of Laccase
Since the nineteenth century, laccases are considered as the most effective green catalyst by many researchers (Javed et al. 2017). Due to their nonspecific and high oxidative capacity, laccases present a high potential in many industrial and biotechnological applications with a great market demand for commercial applications in detergent, food, cosmetic, paper/pulp, and textile industries. In addition, they have also many applications in soil bioremediation and wastewater treatment (Shah and Nervd 2002). Recent studies showed also the potential use in pharmaceutical industries based on the enzyme ability for transformation of antibiotics and steroids (Cohen et al. 2002; Wu and Nian 2014; Pezzella et al. 2013; Yadav et al. 2017a, b).
5.3.3.1 Bioremediation of Environmental Contaminants in Soil
Soil bioremediation is a process applied to recover pollutant from contaminated soils using bacteria and fungi which degrade these organic compounds, transforming them into compounds that are less or nontoxic. Bioremediation is considered a safer, cheaper, and more efficient method compared to other physicochemical methods (Chibuike 2013). Acting on substrates, which are insoluble and very linked to the soil particles and nonaccessible to bacteria, makes the application of laccases more attractive. These exocellular enzymes are able to degrade some pollutant compounds similar to lignin such as polyaromatic hydrocarbons, chlorophenols, and nitrophenols (Chibuike 2013). Action of laccases on the xenobiotics releases intermediate products with more bioavailability and less toxic, which can be more efficiently removed by physicochemical and mechanical processes (Javed et al. 2017; Viswanath et al. 2014; Zucca et al. 2015). Anthracene, benzopyrene, and organophosphorus compounds, like the nerve agents VX or Russian VX, have been removed by microbial and fungal laccases (Amitai et al. 1998; Zeng et al. 2016). Other studies showed also that effluents from sewage treatment containing estrogenic hormones have been treated using enzymatic systems containing laccases. Fungal laccase has been used to oxidize the estrogens like estrone, 17b-estradiol, and 17a-ethynylestradiol (Tanaka et al. 2009).
5.3.3.2 Wastewater Treatment
The appropriate wastewater treatment is important regarding that industrial sewage can contain many carcinogenic, mutagenic, and teratogenic potential substances with toxic effects to human, fish, microorganisms, and plant species (Aljeboree et al. 2014). Using enzymatic processes in wastewater treatment is relatively a new approach decreasing reagents’ consumption and allowing degradation of many persistent and toxic substances such as dyes, solvents, inks, fertilizers, and pesticides (Niebisch et al. 2014). One of the applications of laccases in combination with peroxidases in wastewater treatment is related to the discoloration of wastewater from textile industry that contains different types of dyes such as azo, triphenylmethane, and anthraquinone (Fig. 5.4). Laccases act on the degradation of the chemical dyes before the final disposal. In this process, immobilized laccases in alginate beads are commonly used (Niebisch et al. 2014; Peralta-Zamora et al. 1997). Another study by Zhuo et al. (2017) investigated the ability of laccase from P. ostreatus HAUCC 162 to decolorize six synthetic dyes belonging to different categories (methyl orange, crystal violet, malachite green, bromophenol blue, Reactive Blue 4, and Remazol Brilliant Blue R (RBBR )). The results showed that within 24 hours, and at an initial concentration of 100 mg/L, methyl orange, crystal violet, malachite green, and bromophenol blue could be decolorized by 81.3%, 87.6%, 85.1%, and 98%, respectively. For Reactive Blue 4 and RBBR, the removal of 64.6% and 89.1% was achieved, respectively, when using initial concentration up to 800 mg/L (Rui Zhuo et al. 2017).
Decolorization of crystal violet by laccase from P. ostreatus MTCC142 has also been studied by Kunjadia et al. (2012), and the results show that 92% of dye decolorization can be achieved at an initial concentration of 20 mg/L. Palmieria et al. (2005) have reported the decolorization of the recalcitrant anthraquinonic dye Remazol Brilliant Blue R (RBBR ) by P. ostreatus on agar plate and also in liquid media supplemented with veratryl alcohol, where RBBR was completely decolorized in 3 days. Two isoenzyme laccases in mixture (POXC and POXA3) were found to be responsible for this RBBR transformation under acidic conditions reducing its toxicity by 95% (Palmieria et al. 2005). Another study by Hongman et al. (2004) found that the anthraquinone dye SN4R (Remazol Brilliant Blue SN4R) decolorization rate was increased by 90% in the presence of ABTS as a mediator of laccase. The crude laccase alone in the concentration of 30 Uml−1 can effectively decolorize the dye by 66%. Reactive Blue HFRL dye decolorization was also achieved by using mushroom laccase after 3 days (Devi et al. 2012).
5.3.3.3 Food Industry
Laccases can catalyze homo- and hetero-polymerization reactions needed in fruit juice processing, in sugar beet pectin gelation, and for wine/beer stabilization (Osma et al. 2010). In addition, laccases are currently being utilized in baking to cross-link biopolymers (Javed et al. 2017). It was also used to preserve and to increase juice stability by reducing the oxidation of phenolic compounds, the formation of polyphenol and protein is delayed, and there are many studies which show that laccases can be used for food preservation/stabilization process (Osma et al. 2010; Sammartino et al. 1998).
5.3.3.4 Paper and Textile Industries
In paper industry, the pretreatment of wood pulp with ligninolytic oxidoreductases shows special attention to replace the conventional chlorine-based delignification processes. Unlike peroxidase, the benefit of using laccase is that it requires O2 rather than H2O2 (Javed et al. 2017). In addition, use of laccase for phenols grafting to flax fibers in the production process of paper has been recently studied (Fillat et al. 2012; Aracri et al. 2010; Virk et al. 2012). Laccases can be used in textile industry for bleaching processes and they have now replaced the conventional peroxide bleaching to enhancing the whiteness of cotton (Yavuz et al. 2014; Iracheta-Cardenas et al. 2016; Tzanov et al. 2003). Laccase-based products are also applied for denim bleaching and against fabrics odor (Rodriguez-Couto 2012; Kunamneni et al. 2008). Different textile dyes like phenoxazine and azo dyes have been produced using laccases based on their ability to oxidize the aromatic compounds (Sousa et al. 2013).
5.3.3.5 Biofuels
Lignocellulosic materials are the most reliable feedstock for bioethanol and other organic alcohol production in biofuel industries (Kour et al. 2019a). However, based on the complexity of the material, it requires pretreatment of biomass to eliminate lignin and thus expose the cellulose/hemicellulose to hydrolytic enzymes. An interesting research showed that P. ostreatus enzymes have potential application in bioethanol production process (Yu et al. 2009). The results showed that the combination of ultrasonic pretreatment with enzymatic hydrolysis of rice hull showed a maximal yield by using laccases followed by lignin peroxidase and Mn peroxidase (Yu et al. 2009).
5.3.3.6 Biomedical, Pharmaceutical, and Cosmetic Industries
Based on the ability of laccases to catalyze reactions by direct electron transfer, developing biosensors based on laccases systems attracts scientists for many biomedicine application like in insulin, morphine, and codeine analysis (Rodriguez-Delgado et al. 2015). In addition, this enzyme can play a potential role in pharmaceutical industries in many processes such as antibiotic transformation, amino acid derivatization, and synthesis of metabolically stable analogues (Piscitelli et al. 2012). Complex medical products have been synthesized by laccases, like immunosuppressors (e.g., cyclosporin A), antibiotics (e.g., penicillin X dimer and cephalosporins), and anticancer drugs (e.g., vinblastine, mitomycin, and actinomycin) (Kunamneni et al. 2008). By exploiting the oxidative potential of laccases, many products have been developed in the field of cosmetic industry and personal care products (Javed et al. 2017). In addition, laccases have been applied for hair bleaching and dying. Therefore, some cosmetics and dermatological formulations containing laccases were patented and used for skin lightening (Morel and Christie 2011).
5.4 Manganese Peroxidase (EC 1.11.1.13)
One of the most widely used substrates for P. ostreatus cultivation is wheat straw, especially in European country. As a white-rot fungus, it can be cultivated of agro-industrial lignocellulosic wastes (Sanchez 2010). It also has been described as selective and simultaneous lignocellulose degrader (Banfi et al. 2015). P. ostreatus start to grow at first by easily utilizable and soluble materials from intra- and intercellular spaces from the plant substrate tissues. After nutrient depletion, the fungal biomass starts to degrade the plant cellulose and lignin polymers for further growth (Banfi et al. 2015. Lignin degradation is an oxidative and nonspecific process usually carried out by white-rot fungi. Ligninolytic enzymes, LiP, manganese peroxidase (MnP), and laccase, catalyze the one-electron oxidation of lignin units producing aromatic radicals that lead to non-enzymatic depolymerization. Ligninolytic enzymes, LiP, are the only enzyme able to oxidize directly non-phenolic ones in the presence of certain compounds (Sarkar et al. 1997). However, P. ostreatus produces two manganese peroxidase (MnP) isoenzymes when grown in solid stationary condition on poplar sawdust (Giardina et al. 2000).
5.4.1 Characterization of MnP
MnP was first described in P. chrysosporium (Kuwahara et al. 1984). Peroxidases with Mn2-independent activity on phenolic and non-phenolic aromatic compounds were first reported from Pleurotus species (Martínez et al. 1996; Camarero et al. 1996; Sarkar et al. 1997; Palma et al. 2000). These enzymes have been considered as MnP, despite differences in catalytic properties with P. chrysosporium MnP, because they show the highest affinity for Mn and the existence of a characteristic Mn2 interaction site has been shown in the molecular model of P. eryngii Mn2-oxidizing peroxidase (Ruiz-Dueñas et al. 1999). Manganese peroxidase (MnP) is the most common lignin-modifying peroxidase produced by almost all wood-colonizing basidiomycetes causing white-rot and various soil-colonizing litter-decomposing fungi (Hotrichter 2002; Hatakka 1994; Peláez et al. 1995).
MnP is developed by multiple forms of glycosylated heme protein with molecular weight between 40 and 50 kDa and secreted by ligninolytic fungi (Fig. 5.5). From that point, MnP preferentially oxidizes manganese (II) ions (Mn2+), commonly present in wood and soils, into highly reactive Mn3+. The trivalent Mn3+ is then stabilized by fungal chelators such as oxalic acid. Like other peroxidases, MnP is sensitive to high concentrations of H2O2, but it can be rescued by Mn3+ ions but become quite unstable in aqueous media. To overcome this drawback, they form complexes with organic acids naturally secreted by the fungus, such as malonic or oxalic acid (Hofrichter 2002; Wong 2009). There are three mnp genes, namely mnp1, mnp2, and mnp3, that have been isolated, and their products were characterized from P. ostreatus (Asada et al. 1995; Giardina et al. 2000; Irie et al. 2001). Besides, both MnP2 and MnP3 have been purified (Kamitsuji et al. 2004; Sarkar et al. 1997). However, it is worth to note that MnP2 from P. ostreatus (strain Florida) did not oxidize non-phenolic compounds such as veratryl alcohol (Giardina et al. 2000). Therefore, the difference in enzymatic properties between these two MnP2 isozymes might be due to the differences in posttranslational modification (Kamitsuji et al. 2005).
3D Crystal structure of manganese peroxidase IV from Pleurotus ostreatus. (Source: http://www.rcsb.org/pdb/explore.do?structureId=4BM4)
In fact, the homologous expression system developed by Tsukihara et al. (2006b) reflects the posttranscriptional modifications, secretion, and stability in the physiological condition during the enzyme production process in P. ostreatus. Irie et al. (2001) pointed out the successful molecular breeding of a P. ostreatus strain with high MnP productivity, using an expression system employing the promoter and terminator sequence of sdi1.
5.4.2 Mechanism of Action of MnP
Manganese peroxidase is a heme-containing glycoprotein which requires hydrogen peroxide as an oxidant. Manganese peroxidase basically oxidases Mn2+ to Mn3+ in the presence of H2O2 and organic acid chelators, such as lactic acid. Mn3+, in turn, oxidizes a variety of phenolic substrates (Hotrichter 2002). MnP catalyze the oxidation of several monoaromatic phenol and dyes but depends on both divalent manganese and certain types of buffer. This enzyme has been shown to have the ability to oxidize non-phenolic substrates in the presence of mediators. Due to the intense activity in oxidizing a wide variety of aromatic compounds, it exhibited many potential industrial applications. Mushrooms also produce aryl alcohol oxidase (AAO), an enzyme participating in hydrogen peroxide production. MnP production by different Pleurotus species, which were grown in both submerged and solid-state culture, was shown (Stajic et al. 2006a, b). MnP are considered to be the key enzymes in the lignin degradation system (Irie et al. 2001). The ability to synthesize MnP is common among distinct taxonomical groups of basidiomycetes. In addition, there are fungal MnP producers from rather exotic habitats such as decaying sea grass, cooling tower wood, and brown coal. The molecular weight of MnP is usually within the range between 38 and 62.5 kDa, but most purified enzymes have Mwt around 45 kDa. MnP is often produced in multiple forms, and these isoforms differ in their isoelectric points which are usually rather acidic (pH 3–4).
5.4.3 Applications of MnP
Manganese peroxidase (MnP) is one of the two extracellular peroxidases secreted by the lignin-degrading fungus P. ostreatus. Manganese peroxidase has great potential for industrial waste remediation application such as degrading refractory industrial pollutants (Asgher et al. 2013; Hotrichter 2002). As such, direct disposal of olive mills waste to aquatic bodies’ results in environmental deterioration due to the large amount of organic loading discharged (Fountoulakis et al. 2002). MnP was very helpful in bioremediation of olive mills’ waste. In other applications, MnP is also valuable for decolorization of synthetic dyes and significant potential application for textile industry (Susla et al. 2008; Praveen et al. 2012; Saravanakumar et al. 2013). These complexes function as diffusible oxidants that, in turn, can oxidize terminal phenolic substrates and also possibly non-phenolic substituents via a radical mediator (Giardina et al. 2000).
5.5 Versatile Peroxidase (EC 1.11.1.16)
The first study reported on the presence of versatile peroxidases (VP) in Pleurotus eryngii was revealed by Martinez et al. (1996), and the complete purification of the enzyme was carried out by Min et al. (2010). To date, little evidence has been found associating versatile peroxidase in Pleurotus sp. except in few researches established for P. ostreatus and P. pulmonarius (Moreira et al. 2007). Versatile peroxidases are glycoprotein with hybrid properties, included in a group of H2O2-dependent ligninolytic heme peroxidases (POXs) also known as lignin manganese peroxidases, since it combines together with catalytic properties of lignin peroxidases (LiP, EC 1.11.1.14) and manganese peroxidases (MnP, EC 1.11.1.13) (Busse et al. 2013). It is characterized by the similar properties of MnP in oxidizing phenolic and non-phenolic aromatic compounds as well as its efficient oxidation of Mn2+ to Mn3+ (Tsukihara et al. 2006a). In another major studies, their capabilities in oxidizing veratryl alcohol, the typical lignin peroxidase (LiP) substrate (Camarero et al. 1999) and simple phenols, which are the substrates of Coprinopsis cinerea peroxidase (CIP) has been reported (Ruiz-Dueñas et al. 2009). These could conceivably then recognized that versatile peroxidase as a new family of ligninolytic peroxidases. In consequence of this, while sharing almost identical heme environment together with lignin peroxidase (LiP) and manganese peroxidase (MnP), VP differentiates in the catalytic sites in their molecular structure. VPs form an attractive ligninolytic enzyme group due to their dual oxidative ability to oxidize Mn(II) and also phenolic and non-phenolic aromatic compounds (Dashtban et al. 2010). This dual substrate specificity makes VP powerful to oxidize a variety of high and low redox potential substrates (Ruiz-Dueñas et al. 2011).
5.5.1 Biochemical Characteristics of VP
The biochemical properties of only a few versatile peroxidases of Pleurotus sp. have been analyzed and studied in details especially in P. eryngii, which is superior than VP reported in P. chrysosporium (Camarero et al. 1999; Min et al. 2010). However, the Pleurotus VP isoenzymes were first described as MnP isoenzymes due to their similarity to Mn-oxidizing activity (Martínez et al. 1996). This has shown that VPs have high affinity for manganese and dyes, and strongly oxidized 6-dimethoxyphenol (DMP) and veratryl alcohol (VA) in a manganese-independent reaction (Moreira et al. 2005). The catalytic cycle of VP consists of three steps as described clearly in Bjerkandera adusta influenced by pH-independent and pH-dependent steps in the conversion process (Ertan et al. 2012). The cleavage of H2O2 by heme Fe3+ to Fe4+ led to the formation and reduction of compound I and II intermediates. With the excessive of H2O2 in the absence of reducing substrate at low pH (3.0-3.5), the conversion of compound II to compound III will be carried out which lead to the inactivation of the enzyme (Fig. 5.6). This supports the theory of involvement of LiP and MnP in the catalytic cycle activities of VP (Wong 2009).
Catalytic cycle of VP involving the activities of LiP and MnP; VA veratryl alcohol; VAD veratryl aldehyde (Ertan et al. 2012)
5.5.2 Structural Characteristics of VP
Comprehensive and complete genome sequences for P. ostreatus were done in JGI (U.S. Department of Energy, Office of Science, Joint Genome Institute; http://genome.jgi-psf.org). This facilitated the identification of nine genes encoding members of short MnP- and VP-encoding gene family in the inventory of heme peroxidases of this mushroom (Ruiz-Dueñas et al. 2011). Gene-expression analysis of P. ostreatus has revealed to have five Mn2+-dependent peroxidases encoded with mnp3, 6, 7, 8, and 9, while for versatile peroxidases encoded with MNP1, 2, 4, and 5, VPs all have the related gene and protein structure (Salame et al. 2012). Among these VPs, MNP3 and MNP2 were demonstrated to be unique in their ability to oxidize high molecular weight compounds such as Poly R-478 and RNaseA (Kamitsuji et al. 2005). According to Ruiz-Dueñas et al. (2011), two model types of VPs (137760 and 137766) show genetic variations in only one or two amino acids from the whole sequence of P. ostreatus MnP1 (GenBank AAA84396) (Asada et al. 1995) and MnP2 (GenBank CAB51617) (Giardina et al. 2000), respectively. A recent review reported that P. ostreatus genome exhibited three versatile peroxidases (VPs) and six manganese peroxidases (MnPs) in which two of these crystal structures, VP1 and MnP4, designated dissimilarities at 1.0 to 1.1 Å (Fernández-Fueyo et al. 2014). The significant differences between these two isoenzymes involved not to their kinetic constants only, however including the activity T50 and residual activity at both acidic and alkaline pH. The findings from the previous studies make several contributions to a growing body of literatures on VP in Pleurotus sp. in which the presence of VP has strongly replaced the role of LiP in lignin degradation for P. ostreatus. However, complete purification of VPs from P. ostreatus is not fully reported yet. Versatile peroxidases from P. eryngii are gaining much attention so far as it is first revealed to have high redox potential compared to other Pleurotus group. Generally, versatile peroxidase from P. eryngii was reported to have 40 kDa molecular weight and isoelectric point of 4.1. ABTS (2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) is the best to be used as a substrate, and the maximal enzyme activity was achieved at 50 °C, pH 3.0 (Min et al. 2010).
5.5.3 Applications of VP
Pleurotus sp. has been described as being able to degrade lignin selectively. The limited attack to cellulose makes them very interesting in different physicochemical and biotechnological pretreatment applications related to the use of plant biomass, including the integrated lignocellulose biorefineries for the future production of chemicals, materials, and biofuels. The versatile catalytic of VP in biotechnological and industrial application is due to its high redox potential and unique characteristics in degrading the aromatic compounds without the use of redox mediators and the presence of polyvalent catalytic sites (Ravichandran and Sridhar 2016). The ability to direct degradation of a variety of recalcitrant compounds that other peroxidases are not able to oxidize directly represents VPs as unique enzymes. Their potential applications are not limited to Mn (II) oxidation only but also extend to VA; phenolic, non-phenolic, high molecular weight compounds; as well as dyes in Mn-independent reactions (Wong 2009).
In dye treatment, the capacity of P. ostreatus in the degree of decolorization is depending on the type and concentration of dyes, the ligninolytic enzymes produced, and reaction conditions. An example of this is the study carried out by Vishwakarma et al. (2012) in which the treatment of azo dye (direct blue 14) with enzymes of P. ostreatus has been reported. They reported that immobilized enzymes can reduce color up to 99% only in 18 hours. However, in contrast to Kahraman et al. (2012), the color reduction of indigo carmine dye varied from 93% to 64% as concentration was increased from 50 to 500 mg/L when using dead biomass. The ability of P. ostreatus to degrade aromatic pollutants such as 2,4-dichlorophenol (2,4-DCP) and benzo(a)pyrene ([B(a)P] was reported by Rodrıguez et al. (2004). An increasing number of studies have found that versatile peroxidase from Pleurotus represents an important preference with respect to LiP since no mediator such as veratryl alcohol could be used.
5.6 Aryl Alcohol Oxidase (EC 1.1.3.7)
Aryl alcohol (AAO) is an extracellular flavoprotein (flavin adenine dinucleotide (FAD)-dependent proteins) providing the H2O2 required by ligninolytic peroxidases for fungal degradation of lignin (Xiao et al. 2017). Aryl alcohol oxidase (AAO) which is thermodynamically favorable (Hamdane et al. 2015) has other names in common use including veratryl alcohol oxidase and aromatic alcohol oxidase. Aryl alcohol oxidase was first detected in the culture liquid of Polystictus versicolor (a synonym of Trametes versicolor) (Farmer et al. 1960) before it is known as best produced in three species of the genus Pleurotus: P. sajor-caju (Bourbonnais and Paice 1988), P. eryngii (Guillen et al. 1992), and P. ostreatus (Sannia et al. 1991). The isolation of the enzyme from basidiomycetes has been first carried out by Janssen et al. (1965) which later designed this enzyme as “alcohol oxidase” included as the prosthetic group of the enzymes (Janssen and Ruelius 1968).
Preliminary work in this field was focused mainly on alcohol oxidase in mycelial extracts of a basidiomycete grown in submerged culture (Kerwin and Ruelius 1969). In 2000, Varela et al. published a paper in which they described a gene of aryl alcohol enzymes in the species of Pleurotus and Bjerkandera. Rodrıguez et al. (2004) define evidence that supported the involvement of AAO in lignin degradation as they observed the simultaneous production of AAO and VP in Pleurotus cultures. However, previous studies have not studied AAO in much detail, and the molecular characteristics are not yet clearly elucidated except in P. eryngii (Ferreira et al. 2009). P. eryngii is the first Pleurotus species that has been cloned (Martínez et al. 1994), and a prokaryotic heterologous expression system has been developed in order to obtain fully non-glycosylated AAO for further biochemical and structural studies (Ruiz-Dueñas et al. 2006). However, further study within the species had discovered the best producer of AAO is P. ostreatus compared to other species (Kumar and Rapheal 2011).
5.6.1 Biochemical Characteristics of AAO
Aryl alcohol oxidase cooperates with laccase and other peroxidase in the production of hydroxyl radical, which is believed to be involved in the initial attack of lignocellulosic materials. This is to prevent the repolymerization of laccase oxidation products to occur in order to sustain the lignin degradation process (Goswami et al. 2013). During degradation by Pleurotus species, H2O2 is generated from the expense of benzylic or other p system-containing primary alcohols such as p-anisaldehyde and p-anisyl alcohol into corresponding aldehydes (Ferreira et al. 2015). They point out that in addition to p-anisyl alcohol, the enzyme also oxidizes other polyunsaturated primary alcohols; where flavin reduction by substrate oxidation and re-oxidation of the reduce enzymes by oxygen before the release of the aldehyde product. Aromatic radicals that are produced during the reaction then act to catalyze subsequent degradation, which further generated potentially toxic molecules as defensive tool for the cells from the environment (Li et al. 2015).
5.6.2 Structure of AAO
Depending on the substrate specificity, group of alcohol oxidase (alcohol: O2 oxidoreductase; EC 1.1.3.x) may contain four different categories: AAO which was categorized as aromatic alcohol oxidase and others which are short-chain alcohol oxidase (SCAO), long-chain alcohol oxidase (LCAO), and secondary alcohol oxidase (SAO) (Goswami et al. 2013). However, SCAO and LCAO are reported as intracellular in nature. The crystalline structure of AAO was revealed in P. eryngii by Fernández et al. (2009) which confirmed to share similar fold topology with other members of the glucose oxidase from Aspergillus niger (Frederick et al. 1990) and choline oxidase from Arthrobacter globiformis (Quaye et al. 2008). The use of single-wavelength anomalous diffraction of a selemethionine derivative obtained by Escherichia coli expression and in vitro folding had identified that this monomeric enzyme has two additional structural elements existing in the surroundings of its active site that modulate the access of substrates, which is absent in the structure of model GMC oxidoreductase glucose oxidase (Fernández et al. 2009). These two domains were defined as FAD-binding domains, which interact non-covalently with the proteins, and substrate-binding domain, with a type of funnel-shaped channel that led the connection between the solvent and the flavin cofactor (Hernández-Ortega et al. 2012). The unique properties of AAO associated with its active site, which is able to bind over a wide range of aromatic ligands, include competitive inhibitors such as chavicol (4-allylphenol) and p-anisic (4-methoxybenzoic) acid, while 4-methoxybenzylamine was observed to be the best uncompetitive inhibitor (Ferreira et al. 2005). Until recently, there has been little interest in AAO. Aryl alcohol oxidases from P. ostreatus monomeric glycoproteins were reported to have 67 kDa molecular weight, with optimum pH, and the temperature was found to be around 6 and 40 °C, while Km value of AAO for oxidizing veratryl alcohol was determined to be 0.6 mM, respectively (Kumar and Rapheal 2011).
5.6.3 Applications of AAO
The advantage characteristic of veratryl alcohol oxidase to oxidize various alcohols irreversibly and selectively without the necessity of cofactors in the catalysis has gained industrial interest for large-scale production (Goswami et al. 2013; Kerwin and Ruelius 1969). It is widely known that cellulose and hemicellulose in lignocellulose are the main carbon sources for P. ostreatus (Xiao et al. 2017). In a study by Kumar and Rapheal (2011), AAO activity was observed to be induced by aromatic amino acids and aryl alcohols up to a level of 289 U L−1. However, another component of lignocelluloses, lignin, was reported to inhibit the growth P. ostreatus (Barakat et al. 2012). The presence of lignin plays restricting roles in the efficiency of enzymatic hydrolysis of cellulose and hemicellulose (Kumar et al. 2012). This is supported by Feldman et al. (2015) who reported on the formation of toxic degradation products such as 5-hydroxymethylfurfural (HMF). However, by the involvement of aryl alcohol oxidase and other yeast dehydrogenases via heterologous gene expression, P. ostreatus was capable to metabolize and detoxify HMF while converting it into 2,5-bis-hydroxymethylfuran (HMF alcohol) and 2,5-furandicarboxylic acid (FDCA) (Feldman et al. 2015). The involvement of AAO in lignin degradation requires either substrate from lignin-derived compounds; phenolic aromatic aldehydes and acids that being reduced to alcohol substrates, and aromatic fungal metabolites, respectively (Hernández-Ortega et al. 2012).
5.7 Heme-Thiolate Peroxidase (HTP)
Heme-thiolate (or haem-thiolate) proteins belong to the class of thiolate-containing hemoproteins. Omura (2005) reported that heme-thiolate proteins contain six different classes: cytochromes P450, chloroperoxidase (CPO), nitric oxide synthases, cystathionine β-synthase, protein CoA, and eIF2α. The specialty for the heme-thiolate proteins is based on their function as oxidoreductases in the biological systems and the most versatile biocatalyst. The thiolate group usually comes from cysteine residue and is the axial ligand of heme iron, and the cysteine residue in heme-thiolate peroxidases acts as peroximal axial ligand.
The only known heme-thiolate peroxidases (HTP) are CPO from ascomycete Leptoxyphium fumago that has been extensively studied (Smith et al. 2015; Ruiz-Dueñas et al. 2011). CPO was first discovered by Hager and team from marine fungus Caldariomyces fumago (Shaw and Hager 1959; Morris and Hager 1966; Hofrichter and Ullrich 2006). CPO is composed of 299 amino acids and uses hydrogen peroxide for catalytic process under acidic environment. Research in genome sequences of ascomycete and basidiomycete has gathered many putative heme-thiolate CPO sequences, where many were being included in PeroxiBase, a database that has been created for heme and non-heme peroxidases (Ruiz-Dueñas et al. 2011; Koua et al. 2009). However, a second heme-thiolate peroxidase has been reported. It is an aromatic peroxygenase (APO) and first reported in Agrocybe aegerita (Anh et al. 2007; Ullrich et al. 2004). APO and CPO values are based on their characteristics such as wide substrate specificity, ability to catalyze halogenation reactions, shared catalases and cytochrome P450 monooxygenases, and ability to oxygenate aromatic substrates.
Although they shared the same catalytic properties of peroxidases, these two enzymes differ from typical heme peroxidases in terms of their amino acid sequence, catalytic activity, and structure. Therefore, they were classified under new heme-thiolate peroxidase superfamily. Three heme-thiolate peroxidase gene models were also found in fungus P. ostreatus from genus Pleurotus. All genes were found at sequences 2–4 with high amino acid identities that are similar to the unpublished putative CPO of A. bisporus (GenBankCAC03461) (Ruiz-Dueñas et al. 2011).
5.7.1 Biochemical Characteristics of Structure of HTP
CPO of L. fumago have been identified with 21 amino acid N-terminal signal peptide and 52 amino acid C-terminal propeptide (have chaperon-like function). Its molecular structure has also been experimentally established and is available in Protein Data Bank. Therefore, despite the low amino acid sequence (approximately 20%), its structure has been used as template to get the theoretical models for the three putative P. ostreatus heme-thiolate peroxidases. Ruiz-Dueñas et al. (2011) studied homology models of the three P. ostreatus heme-thiolate peroxidase and the crystallographic model of CPO from L. fumago in detail. It has been shown that all models have cysteine as axial heme ligand. The surrounding residues which stabilize the cysteine-ligand loop by hydrogen bonding are conserved at the proximal side. The presence of Ala and Cys residues contiguous to the axial Cys in 114464 could reinforce the hydrogen-bonds structure at this site. The models also show that Glu, Ser, and His are responsible for cation coordination in CPO and suggest different catalytic properties (activation rate and mechanism, substrate specificity, etc.) from P. ostreatus heme-thiolate peroxidases (Ruiz-Dueñas et al. 2011).
5.7.2 Applications of HTP
Peroxidases and peroxygenases are beneficial biocatalyst and can play big role in the chemical modification of wide range of organic substrates, including regioselective and stereoselective oxygenations, which are difficult to do using conventional chemical reaction (Martínez et al. 2014). As a green technology, enzymes can act as catalyst in chemical syntheses to perform reactions under mild conditions, environmental friendly, and characterized by high specificity and selectivity compared to traditional chemical methods. The advantages of this enzyme are involvement in the production of fine chemical and the possibility to catalyze unspecific chlorination reaction. In addition, it also suitable in bromination and iodation of electrophilic organic substrates via hypohalous acid as actual halogenating agent (Hofrichter and Ullrich 2006).
5.8 Other Enzymes Produced by Pleurotus ostreatus
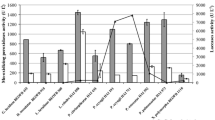
Beside lignin-degrading enzymes, mushrooms have high capacity to produce a wide range of biocatalysts which were not reported in one type of microorganism. Previous studies reported on the production of different types of lipases, proteases, amylases, and polyphenol oxidases (Deepalakshmi and Mirunalini 2014). Nowadays, Pleurotus sp. has become a major cell factory in the enzyme cocktail production based on the ease of cultivation and ability to grow on most of agro-industrial wastes considered as sustainable and green substrate (Saini et al. 2014). Pleurotus ostreatus can produce and excrete a large number of enzymes including xylanases (Getachew et al. 2016; Masutti et al. 2015; Raymond et al. 2015; Karthikeyan 2015; Rodrigues da Luz et al. 2013; Lim et al. 2013; Rodrigues da Luz et al. 2012; Morais et al. 2005; Qinnghe et al. 2004; Elisashvili et al. 2003), cellulases (Raymond et al. 2015; Lim et al. 2013; Radhika et al. 2013; Rodrigues da Luz et al. 2013; Daba et al. 2011; Sherief et al. 2010), pectinases (Masutti et al. 2015; Raymond et al. 2015; Morales Huerta et al. 2014; Sherief et al. 2010), proteases (Ergun and Urek 2017; Genier et al. 2015; Lebedeva and Proskuryakov 2009; Palmieri et al. 2001), amylases (Ergun and Urek 2017; Lim et al. 2013; Akinyele et al. 2010; Rashad et al. 2009), and lipases (Wijayati et al. 2017; Guarino and Sannia 2013).
This part describes other biocatalyst produced by P. ostreatus under different cultivation strategies as well as the purifications process. The secretion of these cocktail of enzymes is dependent on the medium composition, pH, and temperature as well as the mycelial growth. For many researchers, solid-state fermentation (SSF) is the most appropriate method for mushroom cultivation to scale up and the production of extracellular enzymes such as laccase, manganese peroxidase, xylanase, cellulose, and amylase (Koyani and Rajput 2015). This is supported by the utilization of inexpensive lignocellulosic which can stimulate enzyme synthesis and supporting fungal growth (Koyani and Rajput 2015).
5.8.1 Cellulases
Cellulases (EC 3.2.1.4) are known as hydrolase enzymes that cleave cellulose by catalyzing a series of cellulolytic reactions. Cellulases can be classified under different types such as (i) endoglucanases, endo-1,4-β-glucanase, carboxymethyl cellulose (CMCase), β-1,4-glucanase, endo-1,4-β-D-glucanase, β-1-4-endoglucan hydrolase, β-1,4-glucanase, cellobiohydrolases (CBH), and exogluconases, exocellulases, and β-glucosidases. Generally, cellulases were produced by most of the fungi and have the potential to degrade cellulose; at the same time, the same fungus can secrete other enzymes to hydrolyze lignin and hemicelluloses (Khalil et al. 2011). Different cellulases have been produced and isolated from P. ostreatus as reported by many authors using SSF and submerged cultivation system and summarized in Table 5.1. Several studies reported the potential use of green substrate, which is in the form of agriculture wastes, for the production of cellulases by P. ostreatus (Radhika et al. 2013; Rodrigues da Luz et al. 2012). However, growth phase is critical factor governing the enzyme production in SSF. Singh et al. (2003) reported that cellulase production was highest during fruiting phase of strain Pleurotus sp. Furthermore, cellulases are widely used in textile industries, as well as detergent industries. In addition, they have also many applications in paper/pulp industries and pharmaceutical industries.
5.8.2 Xylanases
Xylanases are enzymes which are able to break down the linear polysaccharide β-1,4-xylan into xylose monomer and were classified into two major families based on their protein sequence alignment. The two protein sequence alignments are Family 10 (F) and Family 11 (G). According to Alvarez-Cervantes et al. (2016), the xylanases of GH10 family of P. ostreatus, BOFX60 are related to the GH10 and GH11 families, bBased on the smilimarities in amino acids, 233–318 and 180–193, respectively. Xylanases have gained attention because of their high potential applications in many industrial processes (Yadav et al. 2015). Xylan plays very important role in insect nutrition and ruminant animal since xylan is the major component of hemicelluloses of most of the plants biomass and the polymer can be easily bioconverted into small digestible molecules. Applications for xylanase can be found in the pulp, feed, food, and paper industries (El-Enshasy et al. 2016; Kour et al. 2019b; Rana et al. 2019a, b). For instance, xylanases are beneficial in improving the quality of bread. It enhances the digestibility of ruminant feeds (El-Enshasy et al. 2016) and also applied widely in the pre-bleaching of kraft pulp.
In general, the xylanase activities were higher when mushroom cultivated on waste material is compared to medium containing glucose or other low molecular weight fermentable carbohydrates. Different researchers reported that xylanase can be produced by P. ostreatus as summarized in Table 5.1. The study of Hazra et al. (1997) showed that 90% of the xylanase enzyme from the fungus was secreted out by mushroom T. clypeatus. Other research reported that P. ostreatus SYJ042 was able to produce xylanase. This xylanase was characterized by optimum temperature (40 °C) and stability at pH range between 3.0 and 9.0 (Qinnnghe et al. 2004). Getachew et al. (2016) studied xylanase purification by using ammonium sulfate in concentration between 30% and 80% (wt vol−1), and the best yield was obtained at 40%. The produced enzyme exhibited maximal activity at 50 °C and pH 6.0. This enzyme showed high affinity toward substrate used and can further be used for animal feed processing or other industrial applications.
5.8.3 Pectinases
Pectinase (EC 3.2.1.5) or pectinolytic enzyme is a heterogeneous group of enzymes which is used to hydrolyze pectin, pectic acid, and oligo-D-galacturonate (Tapre and Jain 2014). In general, pectic substances can be found broadly in the plant cell wall including fruits and vegetables. The pectic substances are usually acidic and characterized as very high molecular weight (about 25–360 kDa) and negatively charged (Lakshminarasimha Reddy and Sreeramulu 2012). Pectinase is comprised of many groups and types. It is classified into three major types which are pectin pectinesterases (PE), protopectinase, and depolymerizing enzymes (Jayani et al. 2005; Garg et al. 2016). Usually, PE is used for pectic acid formation, whereas protopectinase is used for soluble pectin formation. Different research reported on the ability of P. ostreatus to produce and excrete pectinases using different substrates and cultivation systems (Table 5.1). Most of the studies were focused on increasing of the production yield and secretion of pectinase.
The presence of extractive substances, derived from wheat bran and grape stalks (Massutti et al. 2015), sawdust (Sherief et al. 2010), sisal leaf (Raymond et al. 2015) as well as cell immobilization in polyurethane foam (PUF) (Morales et al. 2014), in culture medium can increase the production of pectinase by P. ostreatus. Sherief et al. (2010) reported on the cultivation of commercial strain of P. ostreatus on lignocellulosic rice straw and sawdust in plastic bags. The highest pectinase obtained was 21.42 U g−1 after 35 days in case of saw dust, and 13.80 U g−1 after 20 days incubation in rice straw culture. However, pectinase production by P. ostreatus is not widely studied like other enzymes in spite of its importance based on its wide use in textile industry, food processing industries, and for wine and juice clarification (Tapre and Jain 2014; Pasha et al. 2013; Jayani et al. 2005).
Pectinylase (PL) EC 4.2.2.10 can only be produced by a small number of microorganisms such as Aspergillus, Penicillium, and Fusarium (Rashad et al. 2011; Buyukkileci and Fernandez-Lahore 2011). Pectinylase is a pectinase which is only able to degrade highly esterified pectin into small molecules via β-elimination without methanol production. It was reported that P. ostreatus has also the capacity to produce PL. The produced enzyme had molecular weight of 23 KDa (Rashad et al. 2011). This pectin lyase showed high level of activity, with biochemical characteristic with alkaline pH (7.5), high optimum temperature (60 °C), and good affinity toward citrus pectin. Higher pectinase production was obtained by substrates from food processing at different varieties which is orange peel, lemon peel, apple pomace, and sugarcane bagasse using submerged fermentation by P. ostreatus NRRL-366. The best substrate is lemon peel, giving 1750 U of exopolygalacturonase (exo-PGase and 750 U of pectin lyase (PL) per 1 g wet substrate after cultivation for only 4 days (Rashad et al. 2010).
5.8.4 Amylases
Amylases (EC 3.2.1.x) or amylolytic enzymes are widely used for starch hydrolysis into glucose oligomers and glucose monomer. Amylases can be divided into four types of enzymes which hydrolyze different linkages and form various products. They are (i) α-amylase, β-amylase, glucoamylase, and pullulanase. Amylase can secrete from plants, microorganism and animals and share at least 30% of the world enzyme market (Deljou and Arezi 2016). However, enzymes from mushroom sources have subjected applications in industrial part. Amylase has a great potential application in industrial such as food, textile, paper, fermentation, detergent, and pharmaceutical industries.
The production of β-amylase (Akinyele et al. 2010) and α-amylase (Ergun and Urek 2017; Lim et al. 2013: Rashad et al. 2009) has been reported from the species P. ostreatus (Table 5.1). α-Amylase is an enzyme with a potential to breakdown the α-1,4-glucosidic linkage of starch to produce small molecules such as maltose and malto-oligosaccharides (Deljou and Areze 2016). The isolation and characterization of the β-amylase was reported from the edible mushroom, P. ostreatus by Akinyele et al. (2010). The purification of amylase enzyme has been achieved by ion exchange chromatography method. The final finding was proven that the purified α-amylase by ion exchange chromatography on DEAE SephadexA 50 and gel filtration is thermostable with high potential for industrial use. The recent study of Ergun and Urek (2017) showed that the maximal amylase activities were obtained in SSF after 7 days of cultivation using dry potato peel waste after pretreatment with potassium hydroxide.
5.8.5 Proteases
Proteases refer to a group of enzymes which catalyze hydrolytic reactions responsible for the degradation of protein into peptides and amino acids. Proteases are among widely used enzymes, which share about 65% of the global market based on catalytic properties (Yin et al. 2014). It was reported that Pleurotus ostreatus can secrete different types of proteases, which are metalloprotease (Shen et al. 2007; Liu et al. 2014; Dohmae et al. 1995); serine protease (ProA, EC 3.4.22.9), amino acid residues containing serine, aspartic acid, and histidine in the active center (Lebedeva and proskuryakov 2009; Palmieri et al. 2001; Dohmae et al. 1995); cysteine protease or thiol protease, containing cysteine and histidine residues at active center (Shin and Choi 1998); and aspartic protease (Yin et al. 2014). Researchers isolated different types of proteases from fruiting bodies, mycelia, and fermentation broth of P. ostreatus (Dohmae et al. 1995; Palmieri et al. 2001; Shen et al. 2007). Interestingly, the aspartic protease showed modeling crystal structure containing Po-Asp D110 and D295, with Asp110 and Asp295 in the active site. This study also has clearly shown that the structure had specific N- and C-terminal sequence (Yin et al. 2014). An aspartic protease from P. ostreatus (Pro-Asp) with Mwt of 35.3 KDa and isoelectric point of 4.57 consisted of 1324 nucleotides with an open reading frame (ORF) of 1212 bp encoding 403 amino acid residues (Yin et al. 2014). In addition to that, the crude aspartic protease had milk clotting activity and can be used in cheese making industry. MEROPS database defined that Aps was engaged into 16 different families according to amino acid sequence homology. Cysteine proteases or thiol proteases are enzymes that degrade protein containing a Cys-His-Asn at the active site. These proteases have histidine residue at active site acting as proton donor to enhance the nucleophilicity of the cysteine residue. Shin and Choi (1998) successfully purified cysteine protease and did full characterization of the enzyme. Cysteine protease from P. ostreatus is well known as leucine pNA (LPNA) consisting of ASGLXXAIL amino acid. This enzyme showed thermal stability up to 37 °C and the pH optimum in the range of 5.5–6.5.
A serine protease (ProA, EC 3.4.22.9) and two metalloproteases, which are ProB (EC 3.4.99.32) and ProC (EC 3.4.24.4), were isolated from the fruiting bodies of P. ostreatus (Dohmae et al. 1995). Paminieri et al. (2001) reported that serine protease, present in the submerged culture of P. ostreatus, is different from other proteases purified from the fruiting bodies done by Dohmae et al. (1995). Interestingly, these enzymes involved in POXA1b degradation and play a key role in the regulation of laccase activity and thus involved in lignin degradation by P. ostreatus. Furthermore, this finding was similar in the regulation of laccase enzyme in cultures of Trametes versicolor and Phanerochaete chrysosporium (Dosoretz et al. 1990). The purified serine protease (PoSI) was reported to have an isoelectric point of 4.5 and Mwt of 75 kDa, which is larger than other fungal proteases (Palmieri et al. 2001; Dohmae et al. 1995; Mellon and Cotty 1996).
Metalloprotease is defined as protease enzymes with catalytic mechanism involve metal ions and mostly require zinc or cobalt for activity. PoFE (a fibrinolytic protease) consisting of 19 amino acids with N-terminal sequence of ALRKGGAAALNIYSVGFTS. Enzyme activity was increased by the addition of Ca2+, Zn2+, and Mg2+ ions, which is similar to the metalloprotease, extracted from fruiting body of P. ostreatus (GenBank Accession No. AAU94648.1) (Shen et al. 2007). In another study by Liu et al. (2014), they successfully purified fibrinolytic protease enzyme which exhibited thermostability up to 40 °C and pH optimum of 7.4. This result for optimum pH was similar to other fibrinolytic enzymes from Cordyceps militaris, Armillaria mellea, and Tricholoma saponaceum and application for medical applications, for thrombolytic treatment (Liu et al. 2014). In general, proteases from P. ostreatus have molecular weight between 18.2 and 75 kDA (Liu et al. 2014; Yin et al. 2014; Lebedeva and Proskuryakov 2009; Shen et al. 2007; Palmieri et al. 2001; Shin and Choi 1998; Dohmae et al. 1995). However, different types of protease produced by P. ostreatus are presented in Table 5.2.
5.8.6 Lipases
Lipases (EC 3.1.1.3) constitute a very important class of hydrolytic enzymes. Lipases were classified into two types: (a) lipases those of enhanced enzyme activity in emulsion form and (b) lipases with the active site full of protein (Karigar and Rao 2011; Sharma et al. 2011). Generally, lipases are varied in size and sequence of amino acids in primary structure. Lipases are produced in all organisms, and natural function of lipases is to hydrolyze the fats into glycerol and fatty acids (Sharma et al. 2011). Therefore, they also play an important role in food, chemical, cosmetic, detergent, and paper making industries. In addition, they were used as indicator to determine hydrocarbon degradation for soil (Karigar and Rao 2011). Currently, P. ostreatus genome was successfully isolated and characterized. Sixty seven putative lipase coding genes have been annotated in this species (Guarino and Sannia 2013). Moreover, the ability to produce lipase enzymes by P. ostreatus was investigated by many authors. The ability P. ostreatus to produce extracellular lipolytic enzymes was studied by Guarino and Sannia (2013). They reported on the successful production of lipase by using 5% olive mill waste as the main carbon source to achieve a final titer of 30 U L−1 after only 5 days of cultivation. Mushroom enzymes were extracted from the SSF cultures, and the extracted enzymes were successfully immobilized using sodium alginate beads for continuous operation (Wijayati et al. 2017).
5.9 Conclusion and Future Prospects
As presented in this chapter, P. ostreatus have strong production and excretion capacity for large number of enzymes from different groups. The process can be easily shifted to the targeted enzyme through proper design of the cultivation medium and optimization of the cultivation parameters. In addition, this mushroom has long history as food without any previously reported negative impact on human and animal health. Therefore, it received GRAS status from FDA which makes it very attractive for any food, feed, cosmetic, and pharmaceutical industries. In addition, based on the high capacity for concomitant production of hydrolase enzyme cocktail, P. ostreatus is considered as a very attractive biofactory in biorefinery for the hydrolysis of complex lignocellulosic biomass structure to fermentable sugars which is subsequently used for the economic production of different primary and secondary metabolites. However, further research is required for better understanding of the combined factors effect on the induction, production, and excretion of mushroom enzymes during large-scale cultivation.
References
Abdel-Hamid AM, Solbiati JO, Cann IKO (2013) Insights into Lignin degradation and its potential industrial applications. In: Sariaslani S, Gadd GM (eds) Advances in applied microbiology. Academic Press, pp 1–28. https://doi.org/10.1016/B978-0-12-407679-2.00001-6
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 6:675–685
Akinyele BJ, Arotupin DJ, Fagbohungbe YD (2010) Production and purification of β-amylase from the mushroom, Pleurotus ostreatus. Appl Trop Agr 15:22–29
Alexandrino AM, Faria HGD, Souza CGMD, Peralta RM (2007) Reutilisation of orange waste for production of lignocellulolytic enzymes by Pleurotus ostreatus (Jack: Fr). Food Sci Technol 27:364–368
Aljeboree AM, Alshirifi AN, Alkaim AF (2014) Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab J Chem 10:3381–3393
Álvarez-Cervantes J, Díaz-Godínez G, Mercado-Flores Y, Gupta VK, Anducho-Reyes MA (2016) Phylogenetic analysis of β-xylanase SRXL1 of Sporisorium reilianum and its relationship with families (GH10 and GH11) of Ascomycetes and Basidiomycetes. Sci Rep 6:24010
Amitai G, Adani R, Sod-Moriah G, Rabinovitz I, Vincze A, Leader H, Chefetz B, Leibovitz-Persky L, Friesem D, Hadar Y (1998) Oxidative biodegradation of phosphorothiolates by fungal laccase. FEBS Lett 438:195–200
Anh DH, Ullrich R, Benndorf D, Svatos A, Muck A, Hofrichter M (2007) The Coprophilous mushroom Coprinus radians secretes a haloperoxidase that catalyzes aromatic peroxygenation. Appl Environ Microbiol 73:5477–5485
Aracri E, Fillat A, Colom JF, Gutiérrez A, José C, Martínez ÁT, Vidal T (2010) Enzymatic grafting of simple phenols on flax and sisal pulp fibres using laccases. Bioresour Technol 101:8211–8216
Ardon O, Karem Z, Hadar Y (1996) Enhancement of laccase activity in liquid cultures of the ligninolytic fungus Pleurotus ostreatus by cotton stalk extract. J Biotechnol 51:201–207
Ardon O, Kerem Z, Hadar Y (1998) Enhancement of lignin degradation and laccase activity in Pleurotus ostreatus by cotton stalk extract. Can J Microbiol 44:676–680
Arjona D, Aragón C, Aguilera JA, Ramírez L, Pisabarro AG (2009) Reproducible and controllable light induction of in vitro fruiting of the white rot basidiomycete Pleurotus ostreatus. Mycol Res 113:552–558
Asada Y, Watanabe A, Irie T, Nakayama T, Kuwahara M (1995) Structures of genomic and complementary DNAs coding for Pleurotus ostreatus manganese (II) peroxidase. Biochim Biophys Acta 1251:205–209
Asgher M, Aslam B, Iqbal HNM (2013) Novel catalytic and effluent decolorization functionalities of sol gel immobilized Pleurotus ostreatus IBL 02 manganese peroxidase produced from bioprocessing of wheat straw. Chin J Catal 34:1756–1761
Baldrian P (2006) Fungal laccases: occurrence and properties. FEMS Microbiol Rev 30:215–242
Baldrian P, Gabriel J (2002) Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiol Lett 206:69–74
Banfi R, Pohner Z, Kovacs J, Luzics S, Nagy A, Dudas M, Tanos P, Marialigeti K, Vajna B (2015) Characterisation of the large scale production process of oyster mushroom (Pleurotus ostreatus) with the analysis of succession and spatial heterogeneity of lignocellulolytic enzyme activities. Fungal Microbiol 119:1354–1363
Barakat A, Monlau F, Steyer JP, Carrere H (2012) Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour Technol 104:90–99
Behling R, Valange S, Chatel G (2016) Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: what results? What limitations? What trends? Green Chem 18:1839–1854
Bezalel L, Hadar Y, Cerniglia CE (1997) Enzymatic mechanism involved in phenanthrene degradation by white rot fungus Pleurotus ostreatus. Appl Environ Microbiol 63:2495–2501
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bourbonnais R, Paice MG (1988) Veratryl alcohol oxidases from the lignin-degrading basidiomycete Pleurotus sajor-caju. Biochem J 255:445–450
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates: an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function and applications in food processing. Enzym Res 2010:1–10
Busse N, Wagner D, Kraume M, Czermak P (2013) Reaction kinetics of versatile peroxidase for the degradation of lignin compounds. Am J Biochem Mol Biol 4:365–394
Buyukkileci AO, Fernandez-Lahore TCM (2011) Enhanced production of exo-polygalacturonase from agro-based products by Aspergillus sojae. Bioresources 6:3452–3468
Call H, Mücke I (1997) History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems. J Biotechnol 53:163–202
Camarero S, Böckle B, Martínez MJ, Martínez AT (1996) Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl Environ Microbiol 62:1070–1072
Camarero S, Sarkar S, Ruiz-Dueñas FJ, Martı́nez MAJ, Martı́nez ÁT (1999) Description of a versatile peroxidase involved in the natural degradation of lignin that has both manganese peroxidase and lignin peroxidase substrate interaction sites. J Biol Chem 274:10324–10330
Camarero S, Martínez MJ, Martínez AT (2014) Understanding lignin biodegradation for the improved utilization of plant biomass in modern biorefineries. Biofuels Bioprod Biorefin 8:615–625
Canam T, Town J, Iroba K, Tabil L, Dumonceaux T (2013) Pretreatment of Lignocellulosic biomass using microorganisms: approaches, advantages, and limitations. In: Chandel AK, da Silva SS, Rijeka (eds) Sustainable degradation of lignocellulosic biomass-techniques, applications and commercialization. InTech, Croatia, pp 181–206
Carmen S (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194
Chakar FS, Ragauskas AJ (2004) Review of current and future softwood Kraft lignin process chemistry. Ind Crop Prod 20:131–141
Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Saddler JN (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics. Adv Biochem Eng Biotechnol 108:67–93
Chatterjee S, Saito T (2015) Lignin-derived advanced carbon materials. ChemSusChem 8:3941–3958
Chen S, Zhang X, Singh D, Yu H, Yang X (2010) Biological pretreatment of lignocellulosics: potential, progress and challenges. Biofuels 1:177–199
Chheda JN, Huber GW, Dumesic JA (2007) Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew Chem Int Ed 46:7164–7183
Chibuike GU (2013) Use of mycorrhiza in soil remediation: a review. Academic J 8:1679–1687
Chivukula M, Renganathan V (1995) Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl Environ Microbiol 61:4374–4377
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood degrading mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol 58:582–594
Collins PJ, Dobson A (1997) Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol 63:3444–3450
Correa RCG, Brugnari T, Bracht A, Peralta RM, Isabel CFRF (2016) Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: a review on the past decade finding. Trends Food Sci Technol 50:103–117
Da Luz JMR, Paes SA, Nunes MD, da Silva MDCS, Kasuya MCM (2013) Degradation of oxo-biodegradable plastic by Pleurotus ostreatus. PLoS One 8:1–8
Daba AS, Youssef GA, Kabeil SS, Hafez EE (2011) Production of recombinant cellulase enzyme from Pleurotus ostreatus (Jacq.) P.Kumm. (type NRRL-0366). Afr J Microbiol Res 5:1197–1202
Dashtban M, Schraft H, Syed TA, Qin W (2010) Fungal biodegradation and enzymatic modification of lignin. Int J Biochem Mol Biol 1:36–50
De Souza CGM, Tychanowicz GK, De Souza DF, Peralta RM (2004) Production of laccase isoforms by Pleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J Basic Microbiol 44:129–136
Deepalakshmi K, Mirunalini S (2014) Pleurotus ostreatus: an oyster mushroom with nutritional and medicinal properties. J Biochem Technol 5:718–726
Deljou A, Arezi I (2016) Production of the thermostable extracellular α-amylase by a moderate thermophilic Bacillus licheniformis-AZ2 isolated from Qinarje Hot Spring (Ardebil prov.of Iran). Period Biol 118:405–416
Dosoretz CG, Chen HC, Grethlein HE (1990) Effect of environmental conditions on extracellular protease activity in ligninolytic cultures of Phanerochaete chrysosporium. Appl Environ Microbiol 56:395–400
Doherty WOS, Mousavioun P, Fellows CM (2011) Value-adding to cellulosic ethanol: Lignin polymers. Ind Crop Prod 33:259–276
Dohmae N, Hayashi K, Miki K, Tsumuraya Y, Hashimoto Y (1995) Purification and characterization of intracellular proteinases in Pleurotus ostreatus fruiting bodies. Biosci Biotechnol Biochem 59:2074–2080
Duval A, Lawoko M (2014) A review on lignin-based polymeric, micro- and nano-structured material. React Funct Polym 85:78–96
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus. Appl Env Microbiol 62:1151–1158
El Enshasy HA, Maftoun P, Abd Malek R (2012) Pleuran: immunomodulotor polysaccharide from Pleurotus ostreatus, structure, production and application In: Andres S, Baumann N (eds) Mushrooms: types, properties and nutrition. Nova Publisher, New York, pp 153 –172. ISBN: 978-1-61470- 130-9. https://www.novapublishers.com/catalog/product_info.php?products_id=26333
El Enshasy H, Hatti-Kaul R (2013) Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol 31:668–677
El Enshasy H, Daba A, El Demellawy M, Ibrahim A, El Sayed S, El Badry I (2010) Bioprocess development for large scale production of anticancer exo-polysaccharide by Pleurotus ostreatus in submerged culture. J Appl Sci 10:2523–2529
El Enshasy H, Elsayed EA, Aziz R, Wadaan MA (2013) Mushrooms and truffles: historial biofactories for complementary medicine in African and in the Middle East. eCAM 2013:Article ID 620451, 10 pages
El Enshasy HA, Kandiyil SK, Malek R, Othman NZ (2016) Microbial Xylanases: sources, types, and their applications. In: Gupta VK (ed) Microbial enzymes in bioconversions of biomass, biofuel and biorefinery technologies 3. Springer International Publishing Switzerland, Cham
Eleftherios E, Vassilis MG, Israilidis C (2014) The potential use of mushrooms β-glucans in the food industry. Int J Biotechnol Well Ind 3:15–18
Elisashvili V, Chichua D, Kachlishvili E, Siklauri N, Khardziani T (2003) Lignocellulolytic enzyme activity during growth and fruiting of the edible and medicinal mushroom Pleurotus ostreatus (Jacq.:Fr.) Kumm. (Agaricomycetidae). Int J Med Mushrooms 5:193–198
Elsayed EA, El Enshasy HA, Al Wadaan MA, Aziz R (2014) Mushrooms: a potential natural source of anti-inflammatory compounds for medical applications. Mediat Inflamm 2014:Article ID 805841, 15 Pages
Ergun SO, Urek RO (2017) Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. AOAS 15:273–277
Ertan H, Siddiqui KS, Muenchhoff J, Charlton T, Cavicchioli R (2012) Kinetic and thermodynamic characterization of the functional properties of a hybrid versatile peroxidase using isothermal titration calorimetry: insight into manganese peroxidase activation and lignin peroxidase inhibition. Biochimie 94:1221–1231
Evers A, Blakeney A, O’Brien L (1999) Cereal structure and composition. Aus J Agric Res 50:629–650
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45:21–27
Faraco V, Ercole C, Festa G, Giardina P, Piscitelli A, Sannia G (2008) Heterologous expression of heterodimeric laccases from Pleurotus ostreatus in Kluyveromyces lactis. Appl Microbiol Biotechnol 77:1329–1335
Faraco V, Pezzella C, Miele A, Giardina P, Sannia G (2009) Bio-remediation of colored industrial wastewaters by the white-rot fungi Phanerochaete chrysosporium and Pleurotus ostreatus and their enzymes. Biodegradation 20:209–220
Farmer V, Henderson ME, Russell J (1960) Aromatic-alcohol-oxidase activity in the growth medium of Polystictus versicolor. Biochem J 74:257–262
Feldman D, Banu JD, Campanelli J, Zhu H (2001) Blends of vinylic copolymer with plasticized lignin: thermal and mechanical properties. J Appl Polym Sci 81:861–874
Feldman D, Kowbel DJ, Glass NL, Yarden O, Hadar Y (2015) Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnol Biofuels 8:1–11
Fernández IS, Ruiz-Dueñas, Santillana E, Neila PF, Martinez MJ, Martinez AT, Romero A (2009) Novel structural features in the GMC family of oxidoreductases revealed by the crystal structure of fungal aryl-alcohol oxidase. Acta Crystallogr D Biol Crystallogr 65(pt 11):1196–1205
Fernández-Fernández M, Sanromán MA, Moldes D (2013) Recent developments and applications of immobilized laccase. Biotechnol Adv 31:1808–1825
Fernández-Fueyo E, Ruiz-Dueñas FJ, Martínez AT (2014) Engineering a fungal peroxidase that degrades lignin at very acidic pH. Biotechnol Biofuels 7:114
Ferreira P, Medina M, Guillén F, Martínez MJ, Van Berkel WJ, Martínez ÁT (2005) Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem J 389:731–738
Ferreira P, Hernandez-Ortega A, Herguedas B, Martínez ÁT, Medina M (2009) Aryl-alcohol oxidase involved in lignin degradation. A mechanistic study based on steady and pre-steady state kinetics and primary and solvent isotope effects with two alcohol substrates. J Biol Chem 284:24840–24847
Ferreira P, Hernández-Ortega A, Lucas F, Carro J, Herguedas B, Borrelli KW, Medina M (2015) Aromatic stacking interactions govern catalysis in aryl-alcohol oxidase. FEBS J 282:3091–3106
Fillat U, Prieto A, Camarero S, Martínez ÁT, Martínez MJ (2012) Biodeinking of flexographic inks by fungal laccases using synthetic and natural mediators. Biochem Eng J 67:97–103
Fountoulakis MS, Dokianakis SN, Kornaros ME, Aggelis GG, Lyberatos G (2002) Removal of phenolics in olive mill wastewaters using the white-rot fungus Pleurotus ostreatus. Water Res 36:4735–4744
Frederick KR, Tung J, Emerick RS, Masiarz FR, Chamberlain SH, Vasavada A, Rosenberg S, Chakraborty S, Schopfer LM, Schopter L, Massey V (1990) Glucose oxidase from Aspergillus niger. Cloning, gene sequence, secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. J Biol Chem 265:3793–3802
Frigon JC, Guiot SR (2010) Biomethane production from starch and lignocellulosic crops: a comparative review. Biofuels Bioprod Biorefin 4:447–458
Galhaup C, Haltrich D (2001) Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biotechnol 56:225–232
Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D (2002) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148:2159–2169
Gallezot P (2007) Catalytic routes from renewables to fine chemicals. Catal Today 121:76–91
Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R (2016) Microbial pectinases: an eco-friendly tool of nature for industries. 3. Biotech 6:1–13
Genier HLA, de Freitas Soares FE, de Queiroz JH, de Souza Gouveia A, Araújo JV, Braga FR, Pinheiro IR, Kasuya MCM (2015) Activity of the fungus Pleurotus ostreatus and of its proteases on Panagrellus sp. larvae. Afr J Biotechnol 14:1496–1503
Getachew F, Alemu M, Kebede A (2016) Production, purification and characterization of xylanase from oyster mushroom (Pleurotus Sp.). J Nat Sci Res 6:2224–3186
Giardina P, Cannio R, Martirani L, Marzullo L, Palmieri G, Sannia G (1995) Cloning and sequencing of a laccase gene from the lignin-degrading basidiomycete Pleurotus ostreatus. Appl Environ Microbiol 61:2408–2413
Giardina P, Aurilia V, Cannio R, Marzullo L, Amoresano A, Siciliano R, Pucci P, Sannia G (1996) The gene, protein and glycan structures of laccase from Pleurotus ostreatus. Eur J Biochem 235:508–515
Giardina P, Palmieri G, Scaloni A, Fontanella B, Faraco V, Cennamo G, Sannia G (1999) Protein and gene structure of a blue laccase from Pleurotus ostreatus. Biochem J 341:655–663
Giardina P, Palmieri G, Fontanella B, Rivieccio V, Sannia G (2000) Manganese peroxidase isoenzymes produced by Pleurotus ostreatus grown on wood sawdust. Arch Biochem Biophys 376:171–179
Giardina P, Autore F, Faraco V, Festa G, Palmieri G, Piscitelli A, Sannia G (2007) Structural characterization of heterodimeric laccases from Pleurotus ostreatus. Appl Microbiol Biotechnol 7:1293–1300
Gierer J (1990) Basic principles of bleaching. Part 1. Cationic and radical processes. Holzforschung 44:387–394
Gil ES, Muller L, Santiago MF, Garcia TA (2009) Biosensor based on Brut Extract from laccase analysis of phenolic compounds. Port Eletrochim Acta 27:215–225
González T, Terrón MC, Zapico EJ, Téllez A, Yagüe S, Carbajo JM, González AE (2003) Use of multiplex reverse transcription-PCR to study the expression of a laccase gene family in a basidiomycetous fungus. Appl Environ Microbiol 69:7083–7090
Goswami P, Chinnadayyala SSR, Chakraborty M, Kumar AK, Kakoti A (2013) An overview on alcohol oxidases and their potential applications. Appl Microbiol Biotechnol 97:4259–4275
Guarino L, Sannia G (2013) Pleurotus ostreatus: biosystems for industrial applications. X PhD-Chem Day. Pisa, April 23th. 2013
Guerriero G, Hausman JF, Strauss J, Ertan H, Siddiqui KS (2015) Destructuring plant biomass: focus on fungal and extremophilic cell wall hydrolases. Plant Sci 234:180–193
Guillen F, Martinez AT, Martinez MJ (1992) Substrate-specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem 209:603–611
Hamdane D, Bou-Nader C, Cornu D, Hui-Bon-Hoa G, Fontecave M (2015) Flavin–protein complexes: aromatic stacking assisted by a hydrogen bond. Biochemist 54:4354–4364
Hao J, Song F, Huang F, Yang C, Zhang Z, Zheng Y, Tian X (2007) Production of laccase by a newly isolated deuteromycetes fungus Pestalotiopsis sp. and its decolorization of azo dye. J Ind Microbiol Biotechnol 34:233–240
Hatakka A (1994) Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev 13:125–136
Hazra PP, Sengupta T, Mukhopadhyay A, Ghosh AK, Mukherjee M, Sengupta S (1997) Regulation of protein secretion by mycelial culture of the mushroom Termitomyces clypeatus. FEMS Microbiol Lett 154:239–243
Hernández-Ortega A, Ferreira P, Martínez AT (2012) Fungal aryl-alcohol oxidase: a peroxide-producing flavoenzyme involved in lignin degradation. Appl Microbiol Biotechnol 93:1395–1410
Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U (2006) Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J 273:2308–2326
Hofrichter M, Ullrich R (2006) Heme-thiolate haloperoxidases: versatile biocatalyst with biotechnological and environmental significance. Appl Microbiol Biotechnol 71:276–288
Hongman H, Jiti Z, Jing W, Cuihong D, Bin Y (2004) Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39:1415–1419
Hoshida H, Nakao M, Kanazawa H, Kubo K, Hakukawa T, Morimasa K, Akada R, Nishizawa Y (2001) Isolation of five laccase gene sequences from the white-rot fungus Trametes sanguinea by PCR, and cloning, characterization and expression of the laccase cDNA in yeasts. J Biosci Bioeng 92:372–380
Hotrichter M (2002) Review: Lignin conversion by manganese peroxidase (MnP). Enzym Microb Technol 30:454–466
Iracheta-Cardenas MM, Rocha-Peña MA, Galán-Wong LJ, Arévalo-Niño K, Tovar-Herrera OE (2016) A Pycnoporus sanguineus laccase for denim bleaching and its comparison with an enzymatic commercial formulation. J Environ Mang 177:93–100
Irie T, Honda Y, Watanabe T, Kuwahara M (2001) Homologous expression of recombinant manganese peroxidanse genes in ligninolytic fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 55:566–570
Irie T, Honda Y, Watanabe T, Kuwahara M (2001) Isolation of cDNA and genome fragments the major manganese peroxidase isozyme from the white rot basidiomycete Pleurotus ostreatus. J Wood Sci 46:230–233
Isroi MR, Syamsiah S, Niklasson C, Cahyanto MN, Lundquist K, Taherzadeh MJ (2011) Biological pretreatment of lignocelluloses with white-rot fungi and its applications: a review. BioResources 4:5224–5259
Itoh H, Wada M, Honda Y, Kuwahara M, Watanabe T (2003) Bioorganosolve pretreatments for simultaneous saccharification and fermentation of beech wood by ethanolysis and white rot fungi. J Biotechnol 3:273–280
Janssen FW, Ruelius HW (1968) Alcohol oxidase, a flavoprotein from several basidiomycetes species: crystallization by fractional precipitation with polyethylene glycol. Biochim Biophys Acta 151:330–342
Janssen FW, Kerwin RM, Ruelius HW (1965) Alcohol oxidase, a novel enzyme from a basidiomycete. Biochem Biophys Res Commun 20:630–634
Janusz G, Rogalski J, Szczodrak J (2007) Increased production of laccase by Cerrena unicolor in submerged liquid cultures. World J Microbiol Biotechnol 23:1459–1464
Javed A, Ali S, Abid W, Ali N (2017) A review on the potential industrial applications of microbial laccases. Euro J Pharm Med Res 4:238–246
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944
Johnson DK (2002) Lignin, a source of bioethanol co-products. Environ Sci Technol 8:1665–1670
Jurado M, Prieto A, Martínez-Alcalá Á, Martínez ÁT, Martínez MJ (2009) Laccase detoxification of steam exploded wheat straw for second generation bioethanol. Bioresour Technol 100:6378–6384
Kahraman S, Kuru F, Dogan D, Yesilada O (2012) Removal of indigo carmine from an aqueous solution by fungus Pleurotus ostreatus. Arch Environ Protect 38:51–57
Kai D, Tan MJ, Chee PL, Chua YK, Yap YL, Loh XJ (2016) Towards lignin-based functional materials in a sustainable world. Green Chem 18:1175–1200
Kamitsuji H, Honda Y, Watanabe T, Kuwahara M (2004) Production and induction of manganese peroxidase isozymes in a white rot fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 65:287–294
Kamitsuji H, Honda Y, Watanabe T, Kuwahara M (2005) Mn2+ is dispensable for the production of active MnP2 by Pleurotus ostreatus. Biochem Biophys Res Commun 327:871–876
Karam J, Nicell JA (1997) Potential applications of enzymes in waste treatment. J Chem Technol Biotechnol 69:141–153
Karas PA, Perruchon C, Exarhou K, Ehaliotis C, Karpouzas DG (2011) Potential for bioremediation of agro-industrial effluents with high loads of pesticides by selected fungi. Biodegradation 22:215–228
Karigar CS, Rao SS (2011) Role of microbial enzymes in the bioremediation of pollutants: a review. Enzym Res 2011:1–11
Karp SG, Faraco V, Amore A, Birolo L, Giangrande C, Soccol VT, Pandey A, Soccol CR (2012) Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour Technol 114:735–739
Karthikeyan P (2015) Optimization of cellulase enzyme production from Pleurotus ostreatus and Calocybe indica. Int J Pharm Biol Sci 5:11–16
Kerwin RM, Ruelius HW (1969) Production of alcohol oxidase by several basidiomycetes. Appl Microbiol 17:347–351
Khalil MI, Hoque MM, Basunia MA, Alam N, Khan MA (2011) Production of cellulase by Pleurotus ostreatus and Pleurotus sajor-caju in solid state fermentation of lignocellulosic biomass. Turk J Agric For 35:333–341
Kleinert M, Barth T (2008) Phenols from lignin. Chem Eng Technol 5:736–745
Koua D, Cerutti L, Falquet L, Sigrist CJA, Theiler G, Hulo H, Dunand C (2009) PeroxiBase: a database with new tools for peroxidase family classification. Nucleic Acids Res 37:261–266
Kour D, Rana KL, Yadav N, Yadav AN, Rastegari AA, Singh C, Negi P, Singh K, Saxena AK (2019a) Technologies for biofuel production: current development, challenges, and future prospects. In: Rastegari AA, Yadav AN, Gupta A (eds) Prospects of renewable bioprocessing in future energy systems. Springer International Publishing, Cham, pp 1–50. https://doi.org/10.1007/978-3-030-14463-0_1
Kour D, Rana KL, Yadav N, Yadav AN, Singh J, Rastegari AA, Saxena AK (2019b) Agriculturally and industrially important fungi: current developments and potential biotechnological applications. In: Yadav AN, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi, Volume 2: perspective for value-added products and environments. Springer International Publishing, Cham, pp 1–64. https://doi.org/10.1007/978-3-030-14846-1_1
Koyani RD, Rajput KS (2015) Solid state fermentation: comprehensive tool for utilization of lignocellulosic through biotechnology. J Bioprocess Biotech 5:1–15
Krishna KP, Mohana SV, Rao RS, Pati BR, Sarma PN (2005a) Laccase production by Pleurotus ostreatus 1804: optimization of submerged culture conditions by Taguchi DOE methodology. Biochem Eng J 24:17–26
Krishna KP, Mohana SV, Vijaya BY, Ramanaiah SV, Lalit BV, Pati BR, Sarma PN (2005b) Laccase production using Pleurotus ostreatus 1804 immobilized on PUF cubes in batch and packed bed reactors: influence of culture conditions. J Microbiol 43:301–307
Kumar VV, Rapheal VS (2011) Induction and purification by three-phase partitioning of aryl alcohol oxidase (AAO) from Pleurotus ostreatus. Appl Biochem Biotechnol 163:423–432
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 8:3713–3729
Kumar L, Arantes V, Chandra R, Saddler J (2012) The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour Technol 103:201–208
Kunamneni A, Plou FJ, Ballesteros A, Alcalde M (2008) Laccases and their applications: a patent review. Recent Pat Biotechnol 2:10–24
Kunjadia PD, Patel FD, Nagee A, Mukhopadhyaya PN, Dave GS (2012) Crystal violet (triphenylmethane dye) decolorization potential of Pleurotus ostreatus (MTCC 142). BioResources 7:1188–1199
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Lakshminarasimha RP, Sreeramulu A (2012) Isolation, identification and screening of pectinolytic fungi from different soil samples of Chittoor district. Int J Life Sci Biotechnol Pharma Res 1:186–193
Laurichesse S, Avérous L (2014) Chemical modification of lignins: towards biobased polymers. Prog Polym Sci 39:1266–1290
Lebedeva GV, Proskuryakov MT (2009) Purification and characterization of milk-clotting enzymes from oyster mushroom (Pleurotus ostreatus (Fr.) Kumm). Appl Biochem Microbiol 45:623–625
Lenz J, Hölker U (2004) Trickle-film processing: an alternative for producing fungal enzymes. BioForum Eur 6:55–57
Lettera V, Del Vecchio C, Piscitelli A, Sannia G (2011) Low impact strategies to improve ligninolytic enzyme production in filamentous fungi: the case of laccase in Pleurotus ostreatus. C R Biol 334:781–788
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624
Lim SH, Lee YH, Kang HW (2013) Efficient recovery of lignocellulolytic enzymes of spent mushroom compost from oyster mushrooms, Pleurotus spp; and potential use in dye decolorization. Mycobiology 41:214–220
Liu L, Lin Z, Zheng T, Lin L, Zheng C, Lin Z, Wang S, Wang Z (2009) Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969. Enzym Microb Technol 44:426–433
Liu XL, Zheng XQ, Qian PZ, Kopparapu NK, Deng YP, Nonaka M, Harada N (2014) Purification and characterization of a novel fibrinolytic enzyme from culture supernatant of Pleurotus ostreatus. J Microbiol Biotechnol 24:245–253
Liu WJ, Jiang H, Yu HQ (2015) Thermochemical conversion of lignin to functional materials: a review and future directions. Green Chem 17:4888–4907
Maftoun P, Malek R, Abbas M, El Enshasy H (2013) Bioprocess for semi-industrial production of immunomodulatory polysaccharide Pleuran by Pleurotus ostreatus in submerged culture. J Sci Ind Res 72:655–662
Maftoun P, Johari H, Soltani M, Malik R, Othman NZ, El Enshasy HA (2015) The edible mushroom Pleurotus sp.: I. biodiversity and nutritional values. Int J Biotechnol Well Ind 4:67–83
Maldhure AV, Ekhe JD, Deenadayalan E (2012) Mechanical properties of polypropylene blended with esterified and alkylated lignin. J Appl Polym Sci 125:1701–1712
Mansur M, Arias ME, Copa-Patiño JL, Flärdh M, González AE (2003) The white-rot fungus Pleurotus ostreatus secretes laccase isozymes with different substrate specificities. Mycologia 6:1013–1020
Margeot A, Hahn-Hagerdal B, Edlund M, Slade R, Monot F (2009) New improvements for lignocellulosic ethanol. Curr Opin Biotechnol 3:372–380
Marsi HJ, Maftoun P, Abd Malek R, Boumehira AZ, Pareek A, Hanapi SZ, Ling OM, El Enshasy H (2017) The edible mushroom Pleurotus spp.: II. Medicinal values. Int J Biotech Well Ind 6:1–11
Martínez AT, Camarero S, Guillén F, Gutiérrez A, Muñoz C, Varela E, Pelayo JM (1994) Progress in biopulping of non-woody materials: chemical, enzymatic and ultrastructural aspects of wheat straw delignification with ligninolytic fungi from the genus Pleurotus. FEMS Microbiol Rev 13:265–273
Martínez M, Bockle B, Camarero S, Guillén F, Martínez AT (1996) MnP isoenzymes produced by two Pleurotus species in liquid culture and during wheat-straw solid-state fermentation. ACS Symp Ser 655:183–196
Martinez MJ, Ruiz-Dueñas FJ, Guillén F, Martinez AT (1996) Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem 237:424–432
Martinez AT, Ruiz-Dueñas FJ, Gutierrez A, del Rio JC, Alcalde M, Liers C, Ullrich R, Hofrichter M, Scheibner K, Kalam L, Vind J, Lund H (2014) Search, engineering, and applications of new oxidative biocatalyst. Biofuels Bioprod Biorefin 8:819–835
Masutti DC, Borgognone A, Scardovi F, Vaccari C, Setti L (2015) Effects on the enzymes production from different mixes of agro-food wastes. Chem Eng Trans 43:487–492
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Mellon JE, Cotty PJ (1996) Purification and partial characterization of an elastinolytic proteinase from Aspergillus flavus culture filtrates. Appl Microbiol Biotech 46:138–142
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 4:522–550
Messerschmidt A, Huber R (1990) The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Eur J Biochem 187:341–352
Mester T, Tien M (2000) Oxidation mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants. Int Biodeter Biodegr 46:51–59
Min C, Shanjing Y, Zhang H, Liang X (2010) Purification and characterization of a versatile peroxidase from edible mushroom Pleurotus eryngii. Chin J Chem Eng 18:824–829
Mohamed EM, Farghaly FA (2014) Bioactive compounds of fresh and dried Pleurotus ostreatus mushroom. Int J Biotech Well Ind 3:4–14
Morais H, Forgacs E, Cserhati T (2005) Enzyme production of the edible mushroom Pleurotus ostreatus in shaken cultures completed with agro-industrial wastes. Eng Life Sci 5:152–157
Morales Huerta E, Cruz Chilado MM, Díaz Godínez G (2014) Pectinase activity of Pleurotus ostreatus grown in solid-state fermentation. J Chem Biol Phys Sci 4:100
Moreira PR, Duez C, Dehareng D, Antunes A, Almeida-Vara E, Frère JM, Duarte J (2005) Molecular characterisation of a versatile peroxidase from a Bjerkandera strain. J Biotechnol 118:339–352
Moreira PR, Almeida-Vara E, Malcata FX, Duarte JC (2007) Lignin transformation by a versatile peroxidase from a novel Bjerkandera sp. strain. Int Biodeter Biodegr 59:234–238
Morel OJ, Christie RM (2011) Current trends in the chemistry of permanent hair dyeing. Chem Rev 111:2537–2561
Morozova O, Shumakovich G, Gorbacheva M, Shleev S, Yaropolov A (2007) “Blue” laccases. Biochem Mosc 72:1136–1150
Morris DR, Hager LP (1966) Chloroperoxidase. I. Isolation and properties of the crystalline glycoprotein. J Biol Chem 241:1763–1768
Mot AC, Silaghi-Dumitrescu R (2012) Laccases: complex architectures for one-electron oxidations. Biochem Mosc 77:1395–1407
Munoz G, Gillen F, Martinez AT, Marinez MJ (1997) Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of molecular oxygen and Mn2+ oxidation. Appl Environ Microbiol 63:2166–2174
Niebisch CH, Foltran C, Domingues RCS, Paba J (2014) Assessment of Heteroporus biennis secretion extracts for decolorization of textile dyes. Int Biodeter Biodegr 88:20–28
Norgren M, Edlund H (2014) Lignin: recent advances and emerging applications. Curr Opin Colloid Interface Sci 19:409–416
Novotny C, Erbanova P, Cajthaml T, Rothschild N, Dosoretz C, Sasek V (2000) Irpex lacteus a white rot fungus applicable to water and soil bioremediation. Appl Environ Microbiol 54:850–853
Nyanhongo G, Gomes J, Gübitz G, Zvauya R, Read J, Steiner W (2002) Production of laccase by a newly isolated strain of Trametes modesta. Bioresour Technol 84:259–263
Omura T (2005) Heme-thiolate proteins. Biochem Biophys Res Commun 338(1):404–409.
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Uses of laccases in the food industry. Enzym Res 2010:1–8
Palma C, Martínez AT, Lema JM, Martínez MJ (2000) Different fungal manganese-oxidizing peroxidases: a comparison between Bjerkandera sp. and Phanerochaete v chrysosporium. J Biotechnol 77:235–245
Palmieri G, Giardina P, Marzullo L, Desiderio B, Nitti G, Cannio R, Sannia G (1993) Stability and activity of a phenol oxidase from the lignolytic fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 39:632–636
Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, Sannia G (1997) A novel white laccase from Pleurotus ostreatus. J Biol Chem 272:31301–31307
Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G (2000) Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl Environ Microbiol 66:920–924
Palmieri G, Bianco C, Cennamo G, Giardina P, Marino G, Monti M, Sannia G (2001) Purification, characterization, and functional role of a novel extracellular protease from Pleurotus ostreatus. Appl Environ Microbiol 67:754–2759
Palmieri G, Cennamo G, Faraco V, Amoresano A, Sannia G, Giardina P (2003) Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzym Microb Technol 33:220–230
Palmieri G, Cennamob G, Sannia G (2005) Remazol Brilliant Blue R decolourisation by the fungus Pleurotus ostreatus and its oxidative enzymatic system. Enzym Microb Technol 36:17–24
Parenti A, Muguerza E, Iroz AR, Omarini A, Conde E, Alfaro M, Castanera R, Santoyo F, Ramirez L, Pisabarro AG (2013) Induction of laccase activity in the white rot fungus Pleurotus ostreatus using water polluted with wheat straw extracts. Bioresour Technol 133:142–149
Pasha KM, Anuradha P, Subarao D (2013) Application of pectinase in industrial sector. Int J Pure Appl Sci Technol 16:89–95
Peláez F, Martínez MJ, Martínez AT (1995) Screening of 68 species of basidiomycetes for enzymes involved in lignin degradation. Mycol Res 99:37–42
Peralta-Zamora P, Esposito E, Reyes J, Durãn N (1997) Remediação de efluentes derivados da indústria de papel e celulose. Tratamento biológico e fotocatalítico. Quím Nova 20:186–190
Periasamy R, Palvannan T (2010) Optimization of laccase production by Pleurotus ostreatus IMI 395545 using the Taguchi DOE methodology. J Basic Microbiol 50:548–556
Pezzella C, Autore F, Giardina P, Piscitelli A, Sannia G, Faraco V (2009) The Pleurotus ostreatus laccase multi-gene family: isolation and heterologous expression of new family members. Curr Genet 55:45–57
Pezzella C, Lettera V, Piscitelli A, Giardina P, Sannia G (2013) Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl Microbiol Biotechnol 97:705–717
Piscitelli A, Amore A, Faraco V (2012) Last advances in synthesis of added value compounds and materials by laccase mediated biocatalysis. Curr Org Chem 16:2508–2524
Pointing SB, Jones EBG, Vrijmoed LLP (2000) Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia 92:139–144
Pouteau C, Dole P, Cathala B, Averous L, Boquillon N (2003) Antioxidant properties of lignin in polypropylene. Polym Degrad Stab 81:9–18
Praveen K, Usha KY, Viswanath B, Reddy BR (2012) Kinetic properties of manganese peroxidase from the mushroom Stereum ostrea and its ability to decolorize dyes. J Microbiol Biotechnol 22:1540–1548
Qinnghe C, Xiaoyu Y, Tiangui N, Cheng J, Qiugang M (2004) The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Process Biochem 39:1561–1566
Quaye O, Lountos GT, Fan F, Orville AM, Gadda G (2008) Role of Glu312 in binding and positioning of the substrate for the hydride transfer reaction in choline oxidase. Biochemist 47:243–256
Radhika R, Jebapriya GR, Gnanadoss JJ (2013) Production of cellulase and laccase using Pleurotus sp. under submerged and solid-state fermentation. Int J Curr Sci 6:7–13
Rana KL, Kour D, Sheikh I, Dhiman A, Yadav N, Yadav AN, Rastegari AA, Singh K, Saxena AK (2019a) Endophytic fungi: biodiversity, ecological significance, and potential industrial applications. In: Yadav AN, Mishra S, Singh S, Gupta A (eds) Recent advancement in white biotechnology through fungi: Volume 1: diversity and enzymes perspectives. Springer International Publishing, Cham, pp 1–62. https://doi.org/10.1007/978-3-030-10480-1_1
Rana KL, Kour D, Sheikh I, Yadav N, Yadav AN, Kumar V, Singh BP, Dhaliwal HS, Saxena AK (2019b) Biodiversity of endophytic fungi from diverse niches and their biotechnological applications. In: Singh BP (ed) Advances in endophytic fungal research: present status and future challenges. Springer International Publishing, Cham, pp 105–144. https://doi.org/10.1007/978-3-030-03589-1_6
Rashad MM, Abdou HM, Shousha WGH, Ali MM, El-Sayed NN (2009) Utilization of some food processing wastes for production of Pleurotus ostreatus pectinases. Adv Food Sci 31:151–157
Rashad MM, Abdou HM, Shousha WGH, Ali MM, El-Sayed NN (2010) Purification and characterization of extracellular polygalacturonase from Pleurotus ostreatus using Citrus limonium waste. J Appl Sci Res 6:81–88
Rashad MM, Abdou HM, Shousha WGH, Ali MM, El-Sayed NN (2011) Purification and characterization of the pectin lyase produced by Pleurotus ostreatus. Aust J Basic Appl Sci 5:1377–1384
Rastegari AA, Yadav AN, Gupta A (2019) Prospects of renewable bioprocessing in future energy systems. Springer International Publishing, Cham
Rathinasamy P, Thayumanavan P (2010) Optimization of laccase production by Pleurotus ostreatus IMI 395545 using the Taguchi DOE methodology. J Basic Microbiol 6:548–556
Ravichandran A, Sridhar M (2016) Versatile peroxidases: super peroxidases with potential biotechnological applications-A mini review. J Adv Vet Anim Res 4:1–5
Raymond P, Mshandete AM, Kivaisi AK (2015) Enzyme profiles of Pleurotus HK-37during mycelia vegetative growth and fruiting on solid sisal waste fractions supplemented with cow manure. Adv Biochem 3:57–65
Ritter SK (2008) Lignocellulose: a complex biomaterial. Chem Eng News 86:15
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226
Rivera-Hoyos CM, Morales-Alvarez ED, Poutou-Piñales RA, Pedroza-Rodríguez AM, Rodríguez-Vázquez R, Delgado-Boada JM (2013) Fungal laccases. Fungal Biol Rev 27:67–82
Rodgers CJ, Blanford CF, Giddens SR, Skamnioti P, Armstrong FA, Gurr SJ (2010) Designer laccases: a vogue for high-potential fungal enzymes. Trends Biotechnol 28:63–72
Rodráguez E, Ruiz-Duenas FJ, Kooistra R, Ramb A, Martánez AT, Martánez MJ (2008) Isolation of two laccase genes from the white-rot fungus Pleurotus eryngii and heterologous expression of the pel3 encoded protein. J Biotechnol 134:9–19
Rodrigues da Luz JM, Nunes MD, Albino Paes S, Torres DP, de CássiaSoares da Silva M, Kasuya MCM (2012) Lignocellulolytic enzyme production of Pleurotus ostreatus growth in agroindustrial wastes. Braz J Microbiol 43:1508–1515
Rodríguez E, Nuero O, Guillén F, Martínez A, Martínez M (2004) Degradation of phenolic and non-phenolic aromatic pollutants by four Pleurotus species: the role of laccase and versatile peroxidase. Soil Biol Biochem 36:909–916
Rodriguez-Couto S (2012) Laccases for denim bleaching: an eco-friendly alternative. Sigma 1:10–12
Rodriguez-Delgado MM, Alemán-Nava GS, Rodríguez-Delgado JM, Dieck-Assad G, Martínez-Chapa SO, Barceló D, Parra R (2015) Laccase-based biosensors for detection of phenolic compounds. Trends Anal Chem 74:21–45
Ruiz-Dueñas FJ, Martínez MJ, Martínez AT (1999) Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol Microbiol 31:223–235
Ruiz-Dueñas FJ, Ferreira P, Martínez MJ, Martínez AT (2006) In vitro activation, purification, and characterization of Escherichia coli expressed aryl-alcohol oxidase, a unique H2O2-producing enzyme. Protein Expr Purif 45:191–199
Ruiz-Dueñas FJ, Morales M, García E, Miki Y, Martínez MJ, Martínez AT (2009) Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases. J Exp Bot 60:441–452
Ruiz-Dueñas FJ, Fernández E, Martínez MJ, Martínez AT (2011) Pleurotus ostreatus heme peroxidases: an in silico analysis from the genome sequence to the enzyme molecular structure. C R Biol 334:795–805
Ryu H-S, Kim K-O, Liu Y, Yoon L, Kim H-S (2014) Effects of edible mushrooms (Pleurotus ostreatus (Jacq.) P. Mumm. Pleurotus eryngii, Flammulina velutipes) extracts on immune cell activation in mice. FASEB J 28:830.17
Saini JK, Saini R, Tewari L (2014) Lignocellulosic agriculture wastes as biomass feedstocks for second generation bioethanol production: concepts and recent developments. 3 Biotech 5:337–353
Sakakibari A (1980) A structural model of softwood lignin. Wood Sci Technol 14:89–100
Salame TM, Knop D, Levinson D, Mabjeesh SJ, Yarden O, Hadar Y (2012) Release of Pleurotus ostreatus versatile-peroxidase from Mn2+ repression enhances anthropogenic and natural substrate degradation. PLoS One 7:1–10
Sammartino M, Piacquadio P, Stefano GD, Sciancalepore V (1998) Apple juice stabilization by conventional and innovative methods. Industrie delle Bevande 27:367–369
Sanchez C (2010) Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 85:1321–1337
Sannia G, Limongi P, Cocca E, Buonocore F, Nitti G, Giardina P (1991) Purification and characterization of a veratryl alcohol oxidase enzyme from the lignin degrading basidiomycete Pleurotus ostreatus. Biochim Biophys Acta 1073:114–119
Santhanam N, Vivanco JM, Decker SR, Reardon KF (2011) Expression of industrially relevant laccases: Prokaryotic style. Trends Biotechnol 29:480–489
Saravanakumar T, Palvannan T, Kim D, Park SM (2013) Manganese peroxidase H4isozyme mediated degradation and detoxification of triarylmethane dye malachite green: optimization of decolorization by response surface methodology. Appl Biochem Biotechnol 17:1178–1193
Sarkar S, Martínez AT, Martínez MJ (1997) Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. Biochim Biophys Acta 1339:23–30
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 1:19–27
Schwarze FWMR (2007) Wood decay under the microscope. Fungal Biol Rev 21:133–170
Sethuraman A, Akin DE, Eisele JG, Eriksson KEL (1998) Effect of aromatic compounds on growth and ligninolytic enzymes production of two white-rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus. Can J Microbiol 44:872–885
Sethuraman A, Akin DE, Eriksson KEL (1999) Production of ligninolytic enzymes and synthetic lignin mineralization by the birds nest fungus Cyathus stercoreus. Appl Microbiol Biotechnol 52:689–697
Shah V, Nervd F (2002) Lignin degrading system of white rot fungi and its exploitation of dye decolorization. Can J Microbiol 48:857–870
Sharma D, Sharma B, Shukla K (2011) Biotechnological approach of microbial lipase: a review. Biotechnology 10:23–40
Shaw PD, Hager LP (1959) Biological chlorination. III. beta-Ketoadipate chlorinase: a soluble enzyme system. J Biol Chem 234:2565–2569
Shen MH, Kim JS, Sapkota K, Park SE, Choi BS, Kim S, Lee HH, Kim CH, Chun HS, Ryoo CI, Kim SJ (2007) Purification, characterization, and cloning of fibrinolytic metalloprotease from Pleurotus ostreatus mycelia. J Microbiol Biotechnol 17:1271–1283
Sherief AA, El-Tanash AB, Temraz AM (2010) Lignocellulolytic enzyme and substrate utilization during growth and fruiting of Pleurotus ostreatus on some solid wastes. J Environ Sci Technol 3:18–34
Shin H-H, Choi H-S (1998) Purification and characterization of cysteine protease from Pleurotus ostreatus. Biosci Biotechnol Biochem 62:1416–1418
Shleev S, Persson P, Shumakovich G, Mazhugo Y, Yaropolov A, Ruzgas T, Gorton L (2006a) Interaction of fungal laccases and laccase-mediator systems with lignin. Enzym Microb Technol 39:841–847
Shleev S, Persson P, Shumakovich G, Mazhugo Y, Yaropolov A, Ruzgas T, Gorton L (2006b) Laccase-based biosensors for monitoring lignin. Enzym Microb Technol 39:835–840
Siddiqui KS, Ertana H, Charltona T, Poljakc A, Daud Khaleda AK, Yanga X, Marshallf G, Cavicchioli R (2014) Versatile peroxidase degradation of humic substances: use of isothermal titration calorimetry to assess kinetics and applications to industrial wastes. J Biotechnol 178:1–11
Silva JJ, Santana TT, Oliveira ACC, Almeida PH, Souza SGH, Linde GA, Colauto NB, Silveira do Valle J (2012) Produçáo de lacase de fungos basidiomicetos por fermentac¸áo submersa com cascas de café. Arquivos de Ciências Veterinárias e Zoologia da UNIPAR 2012(2):191–196
Singh AD, Abdullah N, Vikineswary S (2003) Optimization of extraction of bulk enzymes from spent mushroom compost. J Chem Technol Biotechnol 78:743–752
Smith AT, Pazicni S, Marvin KA, Stevens DJ, Paulsen KM, Burstyn JN (2015) Functional divergence of heme-thiolate proteins: a classification based on spectroscopic attribute. Chem Rev 115:2532–2558
Soden DM, Dobson ADW (2001) Differential regulation of laccase gene expression in Pleurotus sajor-caju. Microbiology 147:1755–1763
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2606
Sousa AC, Martins LO, Robalo MP (2013) Laccase catalysed homocoupling of primary aromatic amines towards the biosynthesis of dyes. Adv Synth Catal 355:2908–2917
Stajić M, Persky L, Friesem D, Hadar Y, Wasser SP, Nevo E, Vukojevi J (2006a) Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enzym Microb Technol 38:65–73
Stajić M, Persky L, Hadar Y, Friesem D, Duletić-Laušević S, Solomon PW, Eviatar N (2006b) Effect of copper and manganese ions on activities of laccase and peroxidase in three Pleurotus species grown on agricultural wastes. Appl Biochem Biotechnol 128:87–97
Stark WJ, Stoessel PR, Wohlleben W, Hafner A (2015) Industrial applications of nanoparticles. Chem Soc Rev 44:5793–5805
Susla M, Novotny C, Erbanová P, Svobodová K (2008) Implication of Dichomitus squalens manganese-dependent peroxidase in dye. Decolorization and cooperation of the enzyme with laccase. Folia Microbiol 53:479–485
Tanaka T, Tamura T, Ishizaki Y, Kawasaki A, Kawase T, Teraguchi M, Taniguchi M (2009) Enzymatic treatment of estrogens and estrogen glucuronide. J Environ Sci 21:731–735
Tapre AR, Jain RK (2014) Optimization of an enzyme assisted banana pulp clarification process. Int Food Res J 21:2043–2048
Terrón MC, González T, Carbajo JM, Yagüe S, Arana-Cuenca A, Téllez A, Dobson AD, González AE (2004) Structural close-related aromatic compounds have different effects on laccase activity and on lcc gene expression in the ligninolytic fungus Trametes sp. I-62. Fungal Genet Biol 41:954–962
Thakur VK, Thakur MK (2015) Recent advances in green hydrogels from lignin: a review. Int J Biol Macromol 72:834–847
Thakur VK, Thakur MK, Raghavan P, Kessler MR (2014) Progress in green polymer composites from lignin for multifunctional applications: a review. ACS Sustain Chem Eng 2:1072–1092
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–26
Tlecuitl-Beristain S, Sánchez C, Loera O, Robson GD, Díaz-Godínez G (2008) Laccases of Pleurotus ostreatus observed at different phases of its growth in submerged fermentation: production of a novel laccase isoform. Mycol Res 112:1080–1084
Tsukihara T, Honda Y, Sakai R, Watanabe T (2006a) Exclusive overproduction of recombinant versatile peroxidase MnP2 by genetically modified white rot fungus, Pleurotus ostreatus. J Biotechnol 126:431–439
Tsukihara T, Honda Y, Watanabe T, Watanabe T (2006b) Molecular breeding of white rot fungus Pleurotus ostreatus by homologous expression of its versatile peroxidase MnP2. Appl Microbiol Biotechnol 71:114–120
Tzanov T, Basto C, Gübitz GM, Cavaco-Paulo A (2003) Laccases to improve the whiteness in a conventional bleaching of cotton. Macromol Mat Eng 288:807–810
Ullah MA, Bedford CT, Evans CS (2000) Reactions of pentachlorophenol with laccase from Coriolus versicolor. Appl Microbiol Biotechnol 53:230–234
Ullrich R, Nuske J, Scheibner K, Spantzel J, Hofrichter M (2004) Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl Environ Microbiol 70:4575–4581
Upton BM, Kasko AM (2016) Strategies for the conversion of lignin to high-value polymeric. Chem Rev 116:2275–2306
Varela E, Martinez AT, Martinez MJ (2000) Southern blot screening for lignin peroxidase and aryl-alcohol oxidase genes in 30 fungal species. J Biotechnol 83:245–251
Virk AP, Sharma P, Capalash N (2012) Use of laccase in pulp and paper industry. Biotechnol Prog 28:21–32
Vishwakarma SK, Singh MP, Srivastava AK, Pandey VK (2012) Azo dye (direct blue 14) decolorization by immobilized extracellular enzymes of Pleurotus species. Cell Mol Biol (Noisy-le-grand) 58:21–25
Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Res 2014:1–21
Wahleithner JA, Xu F, Brown KM, Brown SH, Golightly EJ, Halkier T, Kauppinen S, Pederson A (1996) The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr Genet 29:395403
Wang C, Kelley SS, Venditti RA (2016) Lignin-based thermoplastic materials. ChemSusChem 9:770–783
Wijayati N, Masubah K, Supartono (2017) Oyster mushroom’s lipase enzyme entrapment on calcium alginate as biocatalyst in the synthesis of lauryl diethanolamide. IOP Conf Ser Mat Sci Eng 172:1–7. https://doi.org/10.1088/1757-899X/172/1/012034
Wong DWS (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209
Wu Y, Nian DL (2014) Production optimization and molecular structure characterization of a new isolated novel laccase from Fusarium solani MAS2, an anthracene-degrading fungus. Int Biodeter Biodegr 86:382–389
Xiao Q, Ma F, Li Y, Yu H, Li C, Zhang X (2017) Differential proteomic profiles of Pleurotus ostreatus in response to lignocellulosic components provide insights into divergent adaptive mechanisms. Front Microbiol 8:1–14
Yadav AN, Sachan SG, Verma P, Saxena AK (2015) Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J Biosci Bioeng 119:683–693
Yadav AN, Sachan SG, Verma P, Kaushik R, Saxena AK (2016) Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. J Basic Microbiol 56:294–307
Yadav A, Verma P, Kumar R, Kumar V, Kumar K (2017a) Current applications and future prospects of eco-friendly microbes. EU Voice 3:21–22
Yadav AN, Kumar R, Kumar S, Kumar V, Sugitha T, Singh B, Chauhan VS, Dhaliwal HS, Saxena AK (2017b) Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J Appl Biol Biotechnol 5:1–13
Yadav AN, Verma P, Kumar V, Sangwan P, Mishra S, Panjiar N, Gupta VK, Saxena AK (2018) Biodiversity of the genus Penicillium in different habitats. In: Gupta VK, Rodriguez-Couto S (eds) New and future developments in microbial biotechnology and bioengineering, Penicillium system properties and applications. Elsevier, Amsterdam, pp 3–18. https://doi.org/10.1016/B978-0-444-63501-3.00001-6
Yadav AN, Mishra S, Singh S, Gupta A (2019a) Recent advancement in white biotechnology through fungi Volume 1: diversity and enzymes perspectives. Springer International Publishing, Cham
Yadav AN, Mishra S, Singh S, Gupta A (2019b) Recent advancement in white biotechnology through fungi. Volume 2: perspective for value-added products and environments. Springer International Publishing, Cham
Yang B, Lu Y (2010) Wyman CE cellulosic ethanol from agricultural residues. In: Balscheck HP, Ezeji TC, Scheffran J (eds) Biofuels from agricultural wastes and byproducts. Wiley-Blackwell, Ames, pp 175–200
Yaver DS, Xu F, Golightly EJ, Brown KM, Brown SH, Rey MW, Schneider P, Halkier T, Mondorf K, Dalbïge H (1996) Purification, characterization, molecular cloning, and expression of two lactase genes from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol 62:834–841
Yavuz M, Kaya G, Aytekin Ç (2014) Using Ceriporiopsis subvermispora cz-3 laccase for indigo carmine decolourization and denim bleaching. Int Biodeter Biodegr 88:199–205
Yin C, Zheng L, Chen L, Tan Q, Shang X, Ma A (2014) Cloning, expression, and characterization of a milk-clotting aspartic protease gene (Pro-Asp) from Pleurotus ostreatus. Appl Biochem Biotechnol 172:2119–2131
Younis AM, Wu F-S, El Shikh HH (2015) Antimicrobial activity of extracts of the oyster culinary medicinal mushroom Pleurotus ostreatus (higher basidiomycetes) and identification of a new antimicrobial compound. Int J Med Mushrooms 17:579–590
Yu J, Zhang J, He J, Liu Z, Yu Z (2009) Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour Technol 100:903–908
Zakzeski J, Bruijnincx PC, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 6:3552–3599
Zeng J, Zhu Q, Wu Y, Lin X (2016) Oxidation of polycyclic aromatic hydrocarbons using bacillus subtilis cota with high laccase activity and copper independence. Chemosphere 148:1–7
Zhang Y-HP (2008) Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J Ind Microbiol Biotechnol 35:367–375
Zhao WW, Simmons B, Singh S, Ragauskas A, Cheng G (2016) From lignin association to nano-/micro-particle preparation: extracting higher value of lignin. Green Chem 18:5693–5700
Zhuo R, Ma L, Fan F, Gong Y, Wan X, Jiang M, Zhang X, Yang Y (2011) Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp. En3 and cloning and functional analysis of its laccase gene. J Hazard Mater 192:855–873
Zhuo R, Yuan P, Yang Y, Zhang S, Ma F, Zhang X (2017) Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem Eng J 117:62–72
Zucca P, Cocco G, Sollai F, Sanjust E (2015) Fungal laccases as tools for biodegradation of industrial dyes. Biocatalysis 1:82–108
Acknowledgments
The authors would like to express their sincere acknowledgment for the support of MOE and UTM-RMC (Malaysia) through HICOE grant no. R.J130000.7846.4J262.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
El Enshasy, H. et al. (2019). Pleurotus ostreatus: A Biofactory for Lignin-Degrading Enzymes of Diverse Industrial Applications. In: Yadav, A., Singh, S., Mishra, S., Gupta, A. (eds) Recent Advancement in White Biotechnology Through Fungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-25506-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-25506-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25505-3
Online ISBN: 978-3-030-25506-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)