Abstract
Alzheimer’s disease (AD) is an age-related detrimental neurodegenerative disorder with no effective treatment, which is clinically characterized by progressive memory decline and cognitive dysfunction, altered decision making, apraxia, language disturbances, etc., and often histologically manifested by the deposition of amyloid-beta (Aβ) plaques and the formation of neurofibrillary tangles. AD is a global health crisis, currently, more than 35 million people worldwide were estimated to be afflicted by AD, and the number is expect to increase with the aging of the society. Current therapy is based on neurotransmitter or enzyme replacement/modulation, and recently, stem cells therapy is proposed as a promising strategy for AD. However, effective strategies for AD treatment has not been achieved. One of the major problems is the blood–brain barrier (BBB), which hampers drug delivery into the brain. Intranasal (IN) route will overcome this obstacle by delivering drugs or cells directly to the central nervous system (CNS) through the olfactory and trigeminal neural pathways. Here, we demonstrate how intranasal delivery systems works and its advantages and disadvantages. Moreover, we discuss and summarize some latest findings on IN delivery of drug and cell in AD models, with a focus on the potential efficacy of treatments for AD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Alzheimer’s disease (AD) is an age-dependent neurodegenerative disorder that is pathologically characterized by intracellular neurofibrillary tangles and extracellular amyloid beta (Aβ) plaque, neural apoptosis and neuron loss in the brain. Moreover, disturbance of metals homeostasis, extensive oxidative stress, mitochondrial damage and distribution, neuroinflammatory and calcium imbalance also contribute to the pathogenesis [1]. AD is the most common type of dementia and clinically characterized by progressive decline in learning and memory, aphasia, disuse, agnosia, spatial skills and executive dysfunction, as well as personality and behavior change. AD is the fifth cause of death among people over 65 years [2], its threats to life and reducing life quality of the patient and their families brings serious social and economic problems to the world. However, AD is a complex disease, the etiology and pathogenesis of AD is still unclear and effective therapeutic strategies remain unavailable.
Currently, acetylcholinesterase inhibitors (AChEIs), such as tacrine, donepezil, galantamine and rivastigmine are the main drugs for AD treatment. Besides, chelators that selectively bind to transition metals and reduce oxidative stress are also attractive approach to combat AD. In addition, nuclear factor kB (NF-kB), GSK3, peroxisome proliferator-activated receptor-g (PPAR-g) are suggested to regulate Aβ deposition, tau hyperphosphorylation and NFTs formation, oxidation, inflammation, demyelination and excitotoxicity, are potential targets for neuroprotective therapies. Despite major advances in neurotherapeutics, poor brain penetration due to the blood-brain barrier (BBB) pose a big challenge. Intranasal (IN) delivery, therefore, is emerged as a promising way since it bypasses the BBB in a non-invasive way, allowing direct drug delivery to the brain via a large surface area in the olfactory region and respiratory epithelium with less systemic side effects. In this chapter, we review IN delivery of AChEIs, natural anti-oxidants, insulin, nerve growth factor (NGF), peptides and several other molecules and the application to translational and clinical studies for AD treatment.
10.2 IN Delivery
10.2.1 Advantages and Challenges
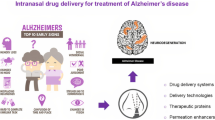
IN delivery is a promising strategy to deliver drugs directly to the brain. Compared to oral administration, IN delivery of drugs achieves fast effects, avoids first-pass metabolism, reduces the side effects of systemic exposure, enhances practicality and compliance because it is noninvasive. However, the problems with IN delivery are mucociliary clearance of drugs and poor nasal permeability. To overcome this, mucoadhesive formulations or chemical penetration enhancers were explored and summarized in Fig. 10.1 [3]. These formula are generally safe and could enhance the stability of drugs, improve the drug absorption, protect the drugs from enzymes and chemical degradation and/or efflux back into the nasal cavity, prevent drug irritant effects, control drug release and reduce their ciliary clearance. Meanwhile, the molecular weight of polymers, free chain length, cross-link density as well as the hydration, pH, swelling, etc. should be taken into consideration for enhanced mucoadhesion.
10.2.2 Pathways of Transport from Nose to Brain
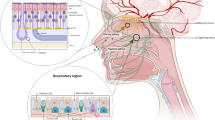
Major cerebral routes of IN delivery are olfactory pathway, rostral migratory stream pathway, and trigeminal pathway (shown in Fig. 10.2) [4].
Schema showing major routes of entry utilized after intranasal delivery of therapeutics in mice. Intranasally administered material (yellow deposits) is picked up by sensory neurons of Grueneberg ganglion, septal organ (green arrows), olfactory epithelium (blue arrow), and ventro-nasal organ (red arrow). The sensory neurons of Grueneberg ganglion, septal organ (green arrows), and olfactory epithelium (blue arrow)—all projecting to the granule cells of the olfactory lobe—eventually drain intranasally-administered material into the rostral migratory stream (RMS) (yellow arrowheads) and olfactory track at the base of the mid-brain (blue and red arrows). The material tracked into the RMS reaches the lateral and third ventricle in the close vicinity of hippocampus. The sensory neurons of ventro-nasal organ (red arrows) project to the accessory olfactory lobe, which further combine with the olfactory track at the base of the mid-brain. The material trafficked along the trigeminal nerve also combines with the olfactory track delivering to pons and hind brain, reaching to the fourth ventricle
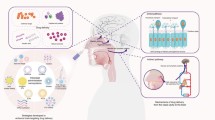
Drugs were transported from nose to brain in intracellular or extracellular ways as shown in Fig. 10.3. The first step in intracellular transport across the olfactory and respiratory epithelia includes endocytosis into olfactory sensory neurons and trigeminal ganglion cells, respectively. This is followed by intracellular transport to olfactory bulb and brain stem, including transcytosis or transcellular transport of drug into lamina propria. Transcytosis involves the permeation of lipid soluble molecules across the apical cell membrane, intracellular space and basolateral membrane either by passive diffusion or receptor-mediated endocytosis. In terms of extracellular transport, it has been estimated to take 0.73–2.3 h to diffuse from olfactory epithelium to olfactory bulb along olfactory associated extracellular pathway and 17–56 h from respiratory epithelium to brain stem along trigeminal associated extracellular pathway. This is an important pathway for the absorption of polar or hydrophilic substances, peptides and proteins. These molecules diffuse slowly from nasal membrane into the blood stream, later into the olfactory mucosa and finally transported into CNS. This pathway is less efficient with respect to transcellular pathway and is strongly dependent on drug molecular weight and size. Moreover, this mechanism is quite fast and responsible for transport of low molecular weight drugs to CNS within minutes of administration. The drugs may also be transported by rapid extracellular delivery through intercellular clefts in the olfactory and respiratory epithelium and extracellular transport along the olfactory and trigeminal neural pathway to reach the brain. Once the drug reaches lamina propria it may transport to systemic circulation; enter deep cervical lymph vessels; enter cranial compartments associated with olfactory nerve bundles.
10.3 IN Delivery Strategies for AD
IN delivery for AD treatment was first proposed by Frey in 1989. And accumulating evidence showed that IN route is a promising approach for delivery of drugs, molecules and cells in AD and is more effective than oral and intravenous (IV) route.
10.3.1 Tacrine
Tacrine (1, 2, 3, 4-tetrahydro-9-aminoacridine) is the first reversible AChEI approved for AD treatment. However, its clinical application has been limited due to low oral bioavailability, extensive hepatic first-pass effect, rapid clearance from the systemic circulation, and hepatotoxicity. To deal with these problems, Jogani et al. [5] investigated the IN delivery of tacrine, and found it could be directly transported into the brain from the nasal cavity and resulted in higher bioavailability with reduced distribution into non-targeted tissues. This selective localization of tacrine in the brain may be helpful in reducing dose , frequency of dosing and dose-dependent side effects, and proved to be an interesting new approach in delivery of the drug to the brain for the treatment of AD. Additionally, IN mucoadhesive microemulsion of tacrine improve brain targeting and fastest retrieval of memory in scopolamine-induced amnesic mice [6]. Luppi et al. reported that albumin nanoparticles carrying native and hydrophilic derivatives β-cyclodextrin derivatives can be employed for the formulation of mucoadhesive nasal formulations to modulate the mucoadhesion and permeation at the administration site [7]. Using these methods, tacrine was promising to be re-introduced for AD treatment.
10.3.2 Galantamine
Galantamine is another AChEI, however, it was discontinued for AD treatment for low aqueous solubility, dose volume limitations, and side effects such as nausea and vomiting. Therefore, researchers investigated addition of co-solvents, cyclodextrins and counter-ion exchange to enhance its solubility. Among which, galantamine-lactate represents a viable candidate for IN delivery [8]. Researchers further reported IN formulations of galantamine containing methylated-β-cyclodextrin as a stabilizer. L-a-phosphatidylcholine didecanoyl, a lipid surfactant and disodium edetate as a chelator [9] resulted in greater permeation without toxic effects to cells. In addition, galantamine hydrobromide combined with cationic chitosan nanoparticles were successfully delivered to different brain regions shortly after intranasal administration, improved pharmacological efficacy and in vivo safety, suggesting a promising way to improve AD management [10].
10.3.3 Rivastigmine
Rivastigmine is also a AChEI for AD treatment. However, the extensive first-pass metabolism and low aqueous solubility lead to poor bioactivity of the drug in vivo. Researchers found that IN administration of rivastigmine showed higher concentration in CNS regions and longer action on inhibiting the activity of AChE than intravenous (IV) administration [11]. What’s more, IN administration of rivastigmine could improve distribution and pharmacological effects in CNS, especially in hippocampus, cortex and cerebrum [11]. Moreover, Shah et al. formulated rivastigmine with microemulsion (ME) and mucoadhesive microemulsions (MMEs) and found that MMEs with 0.3% w/w chitosan showed higher diffusion. Also, chitosan-modified ME are free from nasal ciliotoxicity and stable for 3 months [12]. Arumugam et al. [13]. investigated multilamellar liposomes for IN delivery of rivastigmine using soy lecithin and cholesterol by the lipid layer hydration, and showed higher AUC and Cmax compared with oral-treated group and also suggested that liposomal formulations accumulated in nasal mucosa and released the drug slowly. Fazil et al. [14]investigated IN delivery of rivastigmine loaded chitosan (CS) nanoparticles, and found the brain/blood ratio of rivastigmine was highest in the nanoparticles IN group. These results indicated that the intranasal route was a promising strategy for delivering rivastigmine and rivastigmine nanoparticles into brain.
10.3.4 Physostigmine
Physostigmine , an AChEI, is ineffective when administrated orally as it undergoes extensive first-pass metabolism. IN delivery of physostigmine combined with arecoline, a muscarinin agonist, has shown to be efficient to improve cognition. The nasal BA of physostigmine was 100% compared with IV administration and that of arecoline was 85% compared with intramuscular administration [15]. NXX-066, a physostigmine analogue, could be absorbed rapidly and completely into systemic circulation after nasal administration with Tmax of 1.5 min which was lesser than physostigmine [16]. However, the concentration of drug in CSF was very low after IN administration indicating that uptake into CSF was not enhanced by nasal administration. Therefore, the transport of drugs to CNS via IN administration may be better for poorly soluble drugs but insignificant for drugs which are completely and rapidly absorbed into systemic circulation.
10.3.5 Huperizin A
Huperizin A (Hup A) , an unsaturated sesquiterpene alkaloid, is a powerful and reversible AChEI. It could easily penetrates the BBB, however, it influences peripheral cholinergic system and leads to side effects. To overcome these limitations, Zhao et al. [17] investigated nasal delivery of Hup A by means of in situ gel of gellan gum, and found that concentration of the drug after 6 h in the cerebrum, hippocampus, cerebellum, left olfactory bulb and right olfactory bulb were 1.5, 1.3, 1.0, 1.2 and 1.0 times of those after IV administration, and 2.7, 2.2, 1.9, 3.1 and 2.6 times of those after oral administration. The results revealed that IN route was a viable option for improving the brain-targeting efficiency of Hup A and also reduced the side effects to peripheral tissues. Moreover, nanoparticles have been found to improve drug transport across the epithelium due to the small particle size and the large total surface area [18].
10.3.6 Tarenflurbil
Tarenflurbil (TFB) is an Aβ42 and γ-secretase modulator. Poor brain penetration of TFB was one of the major reasons for its failure in phase III clinical trials conducted on AD patients. Thus it is urgent to improve drug delivery to brain through intranasally delivered nanocarriers. In vitro release studies proved the sustained release of TFB from nanoparticles loaded TFB (TFB-NPs and TFB-SLNs), indicating prolonged residence times of drug at targeting site. Pharmacokinetics suggested improved circulation behavior of nanoparticles and the absolute bioavailability, as well as the brain targeting efficiency. These encouraging results proved that therapeutic concentrations of TFB could be transported directly to brain via olfactory pathway after intranasal administration of polymeric and lipidic nanoparticles [19]
10.3.7 Quercetin
Quercetin , an antioxidative agent, could eliminate free radicals and protect the brain from injury. However, its therapeutic efficacy has been hampered by low solubility in the blood, rapid metabolism in the intestine and liver, and limited ability to cross the BBB. Researchers found that IN administration of quercetin liposomes modulate cognitive impairment and inhibit acetylcholinesterase activity in hippocampus of AD. This may be attributed to its antioxidant property as evidenced by decreased lipid peroxidation and increased level of antioxidant enzymes superoxide dismutase and glutathione peroxidase. Moreover, IN administration of quercetin liposomes significantly increased the survival of neurons and cholinergic neurons in hippocampus of the AD model.
10.3.8 Insulin
AD is associated with abnormal metabolism, and IV insulin administration in AD patients has been shown to improve memory recovery [20]. However, high dose is required to achieve sufficient concentration in the brain and this may lead to hypoglycemia. IN administration of insulin is a promising approach to overcome these limitations. IN administration was suggested to be safe and effective for increasing brain insulin levels, and exerts rapid effects on EEG parameters, memory, attention, mood and self-confidence without any systemic side effects [21]. IN insulin also reduced biomarker of neurodegeneration [22] and the CSF Aβ 40/42 ratio [20]. However, sex and ApoE genotype should be considered as suggested in a controlled clinical trial that only ApoE-e4-negative individuals showed significantly improvements in cognitive performance and functional abilities were relatively preserved for women [20]. In addition, glucagon-like peptide-1 (GLP-1) could stimulate insulin secretion, enhance insulin responsiveness, stimulate neuritic growth and protect against glutamate-mediated excitotoxity, oxidative stress, trophic factor withdrawal, and cell death. What’s more, GLP-1 can cross BBB, and effectively reduce brain APP-Aβ burden in AD. Therefore, developing synthetic long-lasting analogues (receptor agonists) of GLP-1, e.g. Geniposide or Extendin-4, can help to preserve cholinergic neuron function. Additionally, a future approach could be to genetically mesenchymal or stem cells to provide sustained delivery of neuro-stimulatory and neuro-protective agonists to restore insulin levels and functions in the brain [23].
10.3.9 Deferoxamine
Accumulation of metal leads to oxidative stress, inflammation, and contribute to neurodegenerative such as AD. Deferoxamine (DFO), a natural prototype iron chelator/radical scavenger, has been clinically applied to slow down the progression of the cognitive decline associated with iron-induced AD, however, targeting to the brain remained an issue. Hason reported that intranasal administration of DFO (2.4 mg) in C57 mice resulted in micromolar concentrations at 30 min within brain, and IN administration of 10% DFO (2.4 mg) three times a week for three in 48-week-old APP/PS1 mice significantly reduced the escape latencies in Morris water maze [24]. Guo et al. [25] reported iron-induced abnormal tau phosphorylation in cortical and hippocampal regions was suppressed by IN administration of DFO. In another study they found that IN administration of DFO reduced neuritic plaque formation, inhibited iron-induced amyloidogenic APP processing, rescued synapse loss and reversed behavioural alterations in APP/PS 1 mice [25]. And recently Fine et al. reported that IN deferoxamine affects memory loss, oxidation, and the insulin pathway in streptozotocin induced rat model of Alzheimer’s disease [26].
10.3.10 R-Flurbiprofen
R-flurbiprofen was found to offer neuroprotective effects by inhibiting mitochondrial calcium overload induced by β-amyloid peptide toxicity in Alzheimer’s disease (AD). However, poor brain penetration after oral administration posed a challenge to its further development for AD treatment. Study suggested that serum albumin-based nanoparticles administered via the nasal route may be a viable approach in delivering R-flurbiprofen to the brain to alleviate mitochondrial dysfunction in AD [27].
10.3.11 Curcumin
Curcumin (diferuloyl methane) has been found to exert beneficial effects on experimental models of AD by inhibiting Aβ aggregation, inflammation, tau phosphorylation in the brain, and improve memory and cognitive deficits in rats [28]. However, the poor aqueous solubility, chemical instability in alkaline medium, rapid metabolism and poor absorption from gastrointestinal tract limited its application. Chen et al. found that IN delivery of curcumin thermosensitive hydrogel resulted in short gelation time, longer mucociliary transport time and prolonged residence in nasal cavity of rats, without significant toxicity and integrity of mucocilia [29]. What’s more, distribution of curcumin thermosensitive hydrogel via IN administration in cerebrum, cerebellum, hippocampus and olfactory bulb were enhanced. Some researched found that curcumin mucoadhesive nanoemulsions had a significantly higher release, higher flux and permeation across sheep nasal mucosa, with no obvious toxicity [30].
10.3.12 Piperine
Piperine (PIP) is a phytopharmaceutical with neuroprotective potential in Alzheimer’s disease (AD). Oral PIP delivery is disadvantageous for the hydrophobicity and pre-systemic metabolism. Therefore, researchers developed mono-disperse intranasal chitosan nanoparticles (CS-NPs) for brain targeting of PIP and found that PIP-NPs could significantly improve cognitive functions as efficient as standard drug (donpezil injection) with additional advantages of dual mechanism (Ach esterase inhibition and antioxidant effect). Meanwhile, CS-NPs could significantly alleviate PIP nasal irritation with no brain toxicity. Mucoadhesive CS-NPs were successfully tailored for effective, safe, and non-invasive PIP delivery with significant decrease in oral dose [31].
10.3.13 Angiotensin Receptor Blocker
The Renin-angiotensin system in the brain has been implicated in pathogenesis of cognitive decline. Danielyan et al. found that IN administration of losartan, an angiotensin receptor blocker, at sub-antihypertensive dose (10 mg/kg every other day for 2 months) exhibited neuroprotective effect in the APP/PS1 transgenic mouse model. There was a significantly reduction in Aβ plaques, interleukin-12, p40/p70, IL-1β, granulocytemacrophage colony-stimulating factor and increased IL-10 in mice treated with IN losartan compared with the vehicle group. The authors concluded that IN administration of losartan had direct anti-inflammatory and neuroprotective effect in CNS at concentration below than that would cause hypotensive reaction in AD patients [32].
10.3.14 Neurotrophic Factors
Neurotrophic factors plays a critical role in neural growth, regeneration and repair. IN delivery was proposed as a non-invasive technique for application of neurotrophic factors. IN delivery of NGF to the brain was rapid and efficient, and was found to decrease cholinergic deficits, phosphorylated tau and Aβ in AD11 mice [33]. Besides, some researchers found that the intranasal administration was significantly more effective than the ocular one, in rescuing the neurodegenerative phenotypic hallmarks in AD11 mice [34]. Capsoni et al. also studied the form of NGF mutated at R100 called “painless” hNGFER100 to overcome limitations of NGF due to its potent nociceptive action [35]. The mutant showed neurotrophic and anti-amyloidogenic activity in neuronal culture and a reduced nociceptive activity in vivo. Its IN administration in App X PS1 mice prevented the progress of neurodegeneration and behavioral deficits, indicating that hNGFR100 mutants variants as a new generation of therapeutics for neurodegenerative diseases.
Human acidic fibroblast growth factor (haFGF) plays significant roles in development, differentiation and regeneration of brain neurons. It regulates synaptic plasticity and processes attributed to learning and memory by improving cholinergic nerve functions [36]. However, its transport to brain is limited by BBB barrier. Lou et al. [28] investigated a novel technique of delivering haFGF14-154 to brain by fusing it with transactivator of transcription protein transduction domain, a cell penetrating peptide. And the efficacy of Tat-haFGF14-154 is markedly increased when loaded cationic liposomes for intranasal delivery in APP/PS1 mice as evidenced by ameliorated behavioral deficits, relieved brain Aβ burden, and increased the expression and activity of disintegrin and metal loproteinase domain-containing protein 10 in the brain [37].
Basic fibroblast growth factor (bFGF) promotes the survival and neurite growth of brain neurons, and modulates synaptic transmission in the hippocampus [22]. Intranasal administration of bFGF solution could help to improve the memory impairments of AD model rats, but limitations are the poor stability in nasal cavity and small transport amount. Researchers used nanoparticles conjugated with Solanum tuberosum lectin (STL), which selectively binds to N-acetylglucosamine on the nasal epithelial membrane for its brain delivery. The areas under the concentration-time curve of 125I-bFGF in the olfactory bulb, cerebrum, and cerebellum of rats following nasal application of STL modified nanoparticles (STL-bFGF-NP) were 1.79–5.17 folds of that of rats with intravenous administration, and 0.61–2.21 and 0.19–1.07 folds higher compared with intranasal solution and unmodified nanoparticles, respectively. The spatial learning and memory of AD rats in STL-bFGF-NP group were significantly better. Together with the value of choline acetyltransferase activity of rat hippocampus, the histological observations of rat hippocampal region, their study indicated that STL-NP was a promising drug delivery system for peptide and protein drugs such as bFGF to enter the CNS and play the therapeutic role.
Intranasal administration of plasma rich in growth factor PRGF Endoret to APP/PS1 mice for 4 weeks effectively reduced Aβ accumulation, tau hyperphosphorylation, astroglial activation, synaptic loss, and inflammatory responses, while promoted Aβ degradation, stimulated global improvements in anxiety, learning, and memory behaviors [38], suggesting that IN delivery of PRGF-Endoret may hold promise as an innovative therapy in AD.
10.3.15 Peptide
Vasoactive intestinal peptide (VIP) is a major neuropeptide has been found to be neuroprotective and plays important role in learning and memory. Gozes et al. synthesized a potent lipohilic analogue of VIP [stearyl-norleucinel7] VIP ([St-Nle17]VIP) and found it prevented Aβ-induced cell death in rat cerebral cortical cultures with greater potency than VIP. Daily i.c.v. injections of [St-Nle17]VIP significantly improved performance of animal in Morris water maze test in animals treated with the cholinergic blocker [39]. Another study showed that daily intranasal administration of PEI-conjugated R8-Aβ(25–35) peptide significantly reduced Aβ amyloid accumulation and ameliorated the memory deficits of the transgenic mice [40]. Peptides corresponding to the NF-κB essential modifier (NEMO)-binding domain (NBD) of IκB kinase (IKK) or IκB kinase (IKK) specifically inhibit the induction of NF-κB activation without inhibiting the basal NF-κB activity. After intranasal administration, NBD peptide entered into the hippocampus, reduced hippocampal activation of NF-κB, suppressed hippocampal microglial activation, lowered the burden of Aβ in the hippocampus, attenuated apoptosis of hippocampal neurons, protected plasticity-related molecules, and improved memory and learning in 5XFAD mice [41]. IN delivery of H102 (a novel β-sheet breaker peptide) liposomes could significantly ameliorate spatial memory impairment of AD rats, increase the activities of ChAT and IDE and inhibit plaque deposition, with no toxicity on nasal mucosa [42]. Nasal administration of the β sheet breaker peptide AS 602704 was also suggested as an approach for treatment of Alzheimer’s disease [27]. Taken together, these studies suggests that intranasal administration is a feasible route for peptide delivery.
10.3.16 Hormone
Melatonin , an indole amide neurohormone, has been found to protect neurons against Aβ toxicity and inhibit the progressive formation of β-sheets and amyloidfibrils, however, it has been found to have low oral BA, short biological half-life and erratic pharmacokinetic profile. Jayachandra Babu et al. [43] studied IN transport of melatonin using polymeric gel suspensions prepared with carbopol, carboxymethyl cellulose (CMC) and PEG400, and found that the concentration of melatonin in olfactory bulbs after IN administration were higher.
17β-estradiol and its brain-selective 17β-estradiol prodrug were proved to be an effective early-stage intervention in an AD mouse [44]. However, adverse peripheral effects and low estradiol water solubility were the main problems for its application. Water-soluble prodrugs, 3-N, N-dimethylamino butyl ester hydrochloride, 3-N, N-diethylamino propionyl ester hydrochloride and 3-N, N-trimethylamino butyl ester iodide, 17-N, N-dimethylamino butyl ester hydrochloride have been proposed to increase the solubility of 17β-estradiol [45]. In another preclinical study, estradiol solubility was enhanced by chitosan nanoparticles, which behaves as a bioadhesive material and binds strongly to the negatively charged mucin through electrostatic interactions, thus increasing significantly the half-time of clearance of estradiol. Moreover, the CSF concentration of estradiol following IN administration than that of IN administration [46].
Allopregnanolone (Allo), a neurosteroid, was proved to enhance neurogenesis in the hippocampus and restored learning and memory of AD mouse. However, low solubility pose a challenge for oral administration. Some researcher demonstrated that intranasal Allo increased hippocampal BrdU-labeled nuclei and PCNA protein levels in both aged wild type mice and young 3xTg AD mice [47].
10.3.17 Immunization
Vaccination with Aβ1-42 has been found to prevent Aβ accumulation and clearance of amyloid plaques [48]. Cattepoel et al. [49]. studied immunization of APP transgenic mice with single-chain variable fragment (scFv) derived from full IgG antibody raised against C-terminus of Aβ. scFv was found to enter brain after IN application and bind to amyloid plaques in cortex and hippocampus of APP transgenic mice, and inhibit Aβ fibril formation and neurotoxicity. Chronic IN administration of scFv was found to reduce congophilic amyloid angiopathy and Aβ plaques in cortex of transgenic AD mice. Another investigation confirmed that oligomeric amyloid- antibody (NU4) was able to enter the brain and maintain for 96 h post IN administration, and showed evidence of perikaryal and parenchymal uptake of NU4 in 5XFAD mouse brain, confirming the intranasal route as a non-invasive and efficient way of delivering therapeutics to the brain. In addition, this study demonstrated that intranasal delivery of NU4 antibody lowered cerebral amyloid- and improved spatial learning in 5XFAD mice [4]. Moreover, Wheat germ agglutinin enhanced cerebral uptake of antibody after intranasal administration in 5XFAD mice, resulted in greater reduction of cerebral Aβ compared to the unconjugated anti-Aβ antibody delivered intranasally in Alzheimer’s 5XFAD model [50].
10.3.18 Cell-Based Therapy
Cell transplantation is a promising strategy for nervous system (CNS) disorders for the paracrine effect and multi-differential potential. However, the poor migration and homing of cells to the brain after IV delivery are the main barriers for effective treatment, IN provides a more efficient and targeted method for delivering cells to the brain than systemic administration. Moreover, IN delivery of therapeutic cells helps to avoid problems associated with surgical transplantation, such as the low survival rate of transplanted cells, limitations in cell dosage, immunological response and the impracticality of repeated surgical administration. Danielyan et al. reported that 7 days after IN delivery, MSCs were detected in the olfactory bulb (OB), cortex, amygdala, striatum, hippocampus, cerebellum, and brainstem of (Thy1)-h[A30P] αS transgenic mice. IN delivered macrophages could be detected in the OB, hippocampus, cortex, and cerebellum of 13-month-old APP/PS1 mice [51]. However, additional work is needed to determine the optimal dosage to achieve functional improvement in these mouse models. In another report , repeated intranasal delivery of soluble factors secreted by hMSCs in culture, in the absence of intravenous hMSCs injection, was also sufficient to diminish cerebral amyloidosis and neuroinflammation in the mice, suggesting that these may be used in combination or as a maintenance therapy after IV delivery of hMSCs [52].
10.4 Conclusion and Future Perspectives
AD is a multifactorial disease with complex pathogenesis. Various neuroprotective molecules, growth factors, viral vectors, and even stem cells, or other alternatives ways have been explored to intervene AD, however, the efficacy to deliver these agents to the brain was still low. IN administration bypasses the BBB and delivers a wide range of agents to the brain through olfactory, rostral migratory stream, and trigeminal routes. It provides a more effective approach to deliver drugs or cells. However, despite the progress made in area of IN delivery of drugs to brain, IN delivery for AD is still under preclinical stage for the safety and toxicity concerns. The extended contact of formulations with nasal mucosa may lead to irritation, tissue damage, epithelial/sub epithelial toxicity or ciliotoxicity and may result in environment suitable for microbial growth. In addition, IN drug formulation should be developed not to damage the primary olfactory nerves and the sense of smell. Moreover, long-term studies in animals and humans need to be carried out to confirm the effectiveness and drawbacks.
References
Sindi S, Mangialasche F, Kivipelto M. Advances in the prevention of Alzheimer’s disease. F1000Prime Rep. 2015;7:50.
Hu J, Lin T, Gao Y, et al. The resveratrol trimer miyabenol C inhibits beta-secretase activity and beta-amyloid generation. PLoS One. 2015;10(1):e0115973.
Sood S, Jain K, Gowthamarajan K. Intranasal therapeutic strategies for management of Alzheimer’s disease. J Drug Target. 2014;22(4):279–94.
Xiao C, Davis FJ, Chauhan BC, et al. Brain transit and ameliorative effects of intranasally delivered anti-amyloid-beta oligomer antibody in 5XFAD mice. J Alzheimers Dis. 2013;35(4):777–88.
Jogani VV, Shah PJ, Mishra P, et al. Nose-to-brain delivery of tacrine. J Pharm Pharmacol. 2007;59(9):1199–205.
Jogani VV, Shah PJ, Mishra P, et al. Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis Assoc Disord. 2008;22(2):116–24.
Luppi B, Bigucci F, Corace G, et al. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur J Pharm Sci. 2011;44(4):559–65.
Leonard AK, Sileno AP, MacEvilly C, et al. Development of a novel high-concentration galantamine formulation suitable for intranasal delivery. J Pharm Sci. 2005;94(8):1736–46.
Leonard AK, Sileno AP, Brandt GC, et al. In vitro formulation optimization of intranasal galantamine leading to enhanced bioavailability and reduced emetic response in vivo. Int J Pharm. 2007;335(1–2):138–46.
Hanafy AS, Farid RM, ElGamal SS. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: preparation and detection in rat brain. Drug Dev Ind Pharm. 2015;41(12):2055–68.
Yang ZZ, Zhang YQ, Wu K, et al. Tissue distribution and pharmacodynamics of rivastigmine after intranasal and intravenous administration in rats. Curr Alzheimer Res. 2012;9(3):315–25.
Shah BM, Misra M, Shishoo CJ, et al. Nose to brain microemulsion-based drug delivery system of rivastigmine: formulation and ex-vivo characterization. Drug Deliv. 2015;22(7):918–30.
Arumugam K, Subramanian GS, Mallayasamy SR, et al. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008;58(3):287–97.
Fazil M, Md S, Haque S, et al. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci. 2012;47(1):6–15.
Hussain MA, Mollica JA. Intranasal absorption of physostigmine and arecoline. J Pharm Sci. 1991;80(8):750–1.
Dahlin M, Bjork E. Nasal administration of a physostigmine analogue (NXX-066) for Alzheimer’s disease to rats. Int J Pharm. 2001;212(2):267–74.
Zhao Y, Yue P, Tao T, et al. Drug brain distribution following intranasal administration of Huperzine A in situ gel in rats. Acta Pharmacol Sin. 2007;28(2):273–8.
Illum L. Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems? J Pharm Sci. 2007;96(3):473–83.
Muntimadugu E, Dhommati R, Jain A, et al. Intranasal delivery of nanoparticle encapsulated tarenflurbil: a potential brain targeting strategy for Alzheimer’s disease. Eur J Pharm Sci. 2016;92:224–34.
Craft S, Newcomer J, Kanne S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17(1):123–30.
Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29(10):1326–34.
Zhang C, Chen J, Feng C, et al. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int J Pharm. 2014;461(1–2):192–202.
de la Monte SM. Early intranasal insulin therapy halts progression of neurodegeneration: progress in Alzheimer’s disease therapeutics. Aging Health. 2012;8(1):61–4.
Hanson LR, Fine JM, Renner DB, et al. Intranasal delivery of deferoxamine reduces spatial memory loss in APP/PS1 mice. Drug Deliv Transl Res. 2012;2(3):160–8.
Guo C, Wang T, Zheng W, et al. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34(2):562–75.
Fine JM, Forsberg AC, Stroebel BM, et al. Intranasal deferoxamine affects memory loss, oxidation, and the insulin pathway in the streptozotocin rat model of Alzheimer’s disease. J Neurol Sci. 2017;380:164–71.
Wong LR, Ho PC. Role of serum albumin as a nanoparticulate carrier for nose-to-brain delivery of R-flurbiprofen: implications for the treatment of Alzheimer’s disease. J Pharm Pharmacol. 2017;70(1):59–69.
Lou G, Zhang Q, Xiao F, et al. Intranasal administration of TAT-haFGF((1)(4)(-)(1)(5)(4)) attenuates disease progression in a mouse model of Alzheimer’s disease. Neuroscience. 2012;223:225–37.
Chen X, Zhi F, Jia X, et al. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol. 2013;65(6):807–16.
Sood S, Jain K, Gowthamarajan K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf B: Biointerfaces. 2014;113:330–7.
Elnaggar YSR, Etman SM, Abdelmonsif DA, et al. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci. 2015;104(10):3544–56.
Danielyan L, Klein R, Hanson LR, et al. Protective effects of intranasal losartan in the APP/PS1 transgenic mouse model of Alzheimer disease. Rejuvenation Res. 2010;13(2–3):195–201.
Chen XQ, Fawcett JR, Rahman YE, et al. Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimer’s Dis. 1998;1(1):35–44.
Capsoni S, Covaceuszach S, Ugolini G, et al. Delivery of NGF to the brain: intranasal versus ocular administration in anti-NGF transgenic mice. J Alzheimer’s Dis. 2009;16(2):371–88.
Capsoni S, Marinelli S, Ceci M, et al. Intranasal “painless” human Nerve Growth Factor [corrected] slows amyloid neurodegeneration and prevents memory deficits in App X PS1 mice. PLoS One. 2012;7(5):e37555.
Reuss B, und Halbach OVB. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313(2):139–57.
Meng T, Cao Q, Lei P, et al. Tat-haFGF14-154 upregulates ADAM10 to attenuate the Alzheimer phenotype of APP/PS1 mice through the PI3K-CREB-IRE1alpha/XBP1 pathway. Mol Ther Nucleic Acids. 2017;7:439–52.
Anitua E, Pascual C, Antequera D, et al. Plasma rich in growth factors (PRGF-Endoret) reduces neuropathologic hallmarks and improves cognitive functions in an Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35(7):1582–95.
Gozes I, Bardea A, Reshef A, et al. Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proc Natl Acad Sci U S A. 1996;93(1):427–32.
Cheng YS, Chen ZT, Liao TY, et al. An intranasally delivered peptide drug ameliorates cognitive decline in Alzheimer transgenic mice. EMBO Mol Med. 2017;9(5):703–15.
Rangasamy SB, Corbett GT, Roy A, et al. Intranasal delivery of NEMO-binding domain peptide prevents memory loss in a mouse model of Alzheimer’s disease. J Alzheimer’s Dis. 2015;47(2):385–402.
Zheng X, Shao X, Zhang C, et al. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm Res. 2015;32(12):3837–49.
Jayachandra Babu R, Dayal PP, Pawar K, et al. Nose-to-brain transport of melatonin from polymer gel suspensions: a microdialysis study in rats. J Drug Target. 2011;19(9):731–40.
Tschiffely AE, Schuh RA, Prokai-Tatrai K, et al. A comparative evaluation of treatments with 17beta-estradiol and its brain-selective prodrug in a double-transgenic mouse model of Alzheimer’s disease. Horm Behav. 2016;83:39–44.
Al-Ghananeem AM, Traboulsi AA, Dittert LW, et al. Targeted brain delivery of 17 beta-estradiol via nasally administered water soluble prodrugs. AAPS PharmSciTech. 2002;3(1):E5.
Wang X, Chi N, Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm. 2008;70(3):735–40.
Stutzmann GE, Irwin RW, Solinsky CM, et al. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer’s disease. PLoS One. 2015;10(6):e0128313.
Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9.
Cattepoel S, Hanenberg M, Kulic L, et al. Chronic intranasal treatment with an anti-Abeta(30-42) scFv antibody ameliorates amyloid pathology in a transgenic mouse model of Alzheimer’s disease. PLoS One. 2011;6(4):e18296.
Chauhan NB, Davis F, Xiao C. Wheat germ agglutinin enhanced cerebral uptake of anti-Abeta antibody after intranasal administration in 5XFAD mice. Vaccine. 2011;29(44):7631–7.
Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(Suppl 1):S123–39.
Harach T, Jammes F, Muller C, et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2017;51:83–96.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wang, X., Guan, F. (2019). Therapeutic Intranasal Delivery for Alzheimer’s Disease. In: Chen, J., Wang, J., Wei, L., Zhang, J. (eds) Therapeutic Intranasal Delivery for Stroke and Neurological Disorders. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-030-16715-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-16715-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16713-4
Online ISBN: 978-3-030-16715-8
eBook Packages: MedicineMedicine (R0)