Abstract
Effective control of core body temperature and producing hypothermia is the standard of care for comatose patients with cardiac arrest and also in neurogenic fevers. Nasopharyngeal space has been a region of great interest to induce therapeutic hypothermia for a long time. This is primarily due to the favorable location of the nasal heat exchanger directly beneath the brain, the main target for hypothermia. This chapter focuses on achieving therapeutic hypothermia of the brain and core body temperature by using transnasal dry air.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Nasopharyngeal space has been a region of great interest to induce therapeutic hypothermia for a long time. This is primarily due to the favorable location of the nasal heat exchanger directly beneath the brain, the main target for hypothermia. Nasal heat exchanger is a highly evolved system and has undergone significant evolutionary changes. The architecture of the nasal passages, the size of the turbinates (primary site for heat exchange) vary significantly among species and within the same species based on the environment. In human beings, people inhabiting the polar zones often have long nasal passages and complex nasal turbinate morphology that enables optimal conditioning of the inspired air, a prerequisite for the health of the lower respiratory tract. People in the tropical climates typically have shorter and wider nasal passages as conditioning is less of a burden in hot humid climates. The nasal mucosa is also highly evolved and is supplied by a rich plexus of venous channels which form submucosal sinusoidal spaces that are optimal for heat exchange [1]. Both the internal and external carotid arteries supply the nasal cavity. The anterior and posterior ethmoid arteries, both branches of the internal carotid artery system supply the upper nasal septum and nasal sidewalls. The superior labial branch of the facial artery supplies the front part of the nose. The sphenopalatine artery, a branch of the external carotid system supplies most of the back of the nasal cavity.
The nasal turbinates have high capacity to engorge and increase significantly in surface area to promote heat exchange. Nasal mucosa also has a rich submucosal goblet cell layer that secretes nasal mucus. Evaporation of nasal mucus is the primary mechanism that drives the heat exchange function of the nose. Nasal mucosal glands are capable of secreting up to 1 mL/min of mucus fluid. An average person breathing 6–7 L/min consumes evaporates up to 600 mL of water in the nasal cavity. Ninety percent of this evaporated water condenses back on to the nasal mucosa during expiration as the expired air is colder than the mucosal surface. Thus, the nasal mucosa conserves water loss. Mucus production is significantly increased on exposure to irritants, dry air, increased nasal osmolarity and cold weather, thus increasing humidification in such settings. The cranial sinuses such as the maxillary and ethmoid sinus also participate in heat exchange. The internal carotid artery enters the spend air sinus and traverses the venous sinusoidal space in this region. In animals with “carotid rate” an intricate network of connections between the venous sinuses and the carotid artery, this arrangement favors significant exchange of heat from the cerebral arteries to the venous sinuses resulting in selective cerebral cooling. This has not been demonstrated in mammals without carotid rate, which includes humans. However, it is undeniable that the proximity of the nasal heat exchanger does favor local conductive cooling to critical deep brain structures and makes it enticing to exploit this for inducing hypothermia.
Several investigators have explored the possibility of inducing hypothermia through the nasopharynx. Most of the methods relied on instilling cold fluids in to the nasal cavities to cool the nasal mucosa which will in turn cool the paranasal space. Covaciu et al. used cold saline irrigated through a series of thin walled balloons deployed in the nasal cavities of awake volunteers [2]. Balloons were inserted under local anesthesia. Saline at 4 °C was circulated through the balloons. Volunteers underwent MR thermography to assess changes in brain temperature. Over a period of 2 h, up to 2 °C reduction in brain temperature was noted. Except for nasal erythema and discomfort there were no adverse events. No changes were noted in core rectal temperatures in the volunteers suggesting that this was selective cerebral cooling. This was the first study to use MR thermography to assess regional brain temperatures during nasopharyngeal cooling.
Andrews and Harris et al. [3] used flow of ambient air twice the patients minute ventilation in brain injured patients and noted a small decline in brain temperature. The authors used nitric oxide (NO) to promote vasodilation of the nasal mucosa. The temperature of the air was 23 °C with a relative humidity of ~30–35%. Brain temperature was measured using a Camino catheter placed in the prefrontal cortex. Core temperature was measured using an esophageal probe. The intervention lasted for 5 min and the mean airflow was 17.7 LPM. A small but clinically insignificant of 0.2 °C decline in cerebral temperature was note by the investigators which led them to conclude that selective cerebral cooling does not occur. It will be clear in the later part of this report as to why this study failed to show changes in brain temperature.
1.1 Rhinochill
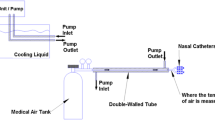
The most promising technology to date that has been studied well in acute brain injury is “Rhinochill” which uses a coolant spray delivered to the nasal cavity that is evaporated by high flow of oxygen [4]. The Rhinochill device (Fig. 1.1) consists of a control unit, a source of compressed oxygen and the coolant bottle which is a proprietary perflurocarbon (PFC ). A pair of elongated nasal tubes that are inserted in to the nostrils delivers PFC directly to the turbinates. High flow of oxygen delivered through the tubing set evaporates the PFC that is sprayed in to the nostrils. Rhinochill device has been tested in cardiac arrest [4,5,6,7] and in the neurocritical care [8] (NCCU) settings and shows promise in reducing brain temperatures selectively at least in the NCCU cohort. The coolant, a perflurocarbon, has a very low boiling point and belongs to a class of chemicals that are biologically non-reactive. Latent heat of vaporization of the coolant is approximately 85 kJ/kg which is the dominant mechanism behind the heat loss by evaporative cooling in the nasopharynx. In a randomized large out of hospital cardiac arrest study in 200 patients, Rhinochill showed the ability to initiate cooling during resuscitation prior to return of spontaneous circulation, and showed small but significant lowering of tympanic and core temperature in the cooled subjects. No significant adverse effects were observed except for a “white nose” due to excessive cooling which returned to baseline with discontinuation of cooling. In a study in the neurocritical care setting where brain temperature monitoring was feasible, Rhinochill demonstrated a gradient of cooling with maximal cooling occurring in the brain tissue followed by the core body temperature measured in the bladder with a difference of up to 0.5 °C. Further there was also a favorable reduction in the intracranial pressure in brain cooled patients.
Despite positive encouraging studies, and the feasibility studies some concerns with the use of PFC remain a hindrance to its widespread use. PFC chemicals are among the least acutely toxic compounds known, although they are known to be potent immunomodulators and inhibit white blood cell chemotaxis at femtogram concentrations [9,10,11]. PFC has also been linked to carcinogenicity in smaller studies and the EPA considers PFC as a toxic substance with detrimental biologic effects [12]. Other than the biologic effects, the PFC has resulted in inadvertent freezing injury of the nasal mucosa which does not completely resolve in all patients with cessation of cooling [6]. Other complications include direct injury of the nasal mucosa by the long nasal cannula and pneumocephalus as the cannulas are inserted by non-specialized medical personnel [13].
Finally, the cost of PFC is prohibitive for use in the ambulance, which is generally a low resource setting. Duration of nasopharyngeal cooling in their clinical trial has been 60 min, and the amount of refrigerant per-patient used was approximately 3.5 L [5]. This would amount to at least $2000 for the consumables alone per hour of use which poses significant strain on the EMS. Rhinochill device is currently not approved for use in the United States.
1.2 Transnasal Evaporative Cooling Using Dry Air: (CoolStat Device)
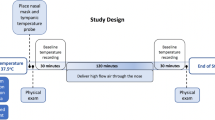
Evaporative heat loss is a very well understood thermodynamic phenomenon and the human body is uniquely designed to exploit this mechanism for thermoregulation. Evaporation of nasal mucus is fundamental to the humidification of the inspired air by the nasal turbinates and this process can be harnessed to promote heat loss. The latent heat of evaporation of water is 2257 kJ/kg which is among the highest in all known fluids. Einer-Jensen et al. [14] were the first to demonstrate cerebral cooling in intubated pigs by flushing the nostrils with air at high flow rates. However, the authors concluded that this was due to the presence unique vascular plexus in the pigs (“rete mirabile”) and the underlying determinants of the cooling were not appreciated. We performed similar experiments in intubated porcine animals and showed that the primary mechanism behind heat loss is the evaporation of nasal mucosal water [15]. The determinants of water evaporation by a dry gas such as the air flow rate and the air humidity govern the cooling response. We not only showed that uniform cerebral cooling occurs (Fig. 1.2), but also systemic cooling ensues with continued exposure to dry air. Humidification of the inspired air abolished the cooling response thus proving that water evaporation is key to this process. Rates of cerebral and systemic cooling in pigs using dry air are comparable to the published perflurocarbon based cooling results. Based on the success of cooling in animals, we performed a human proof of concept study using a prototype device (CoolStat, Fig. 1.3) in 16 intubated subjects in the peri-operative setting (unpublished data) which showed 0.7 °C of reduction in core esophageal temperature over a 1-h period which is comparable to the human data published using the PFC based cooling device. A clinical trial in patients is currently underway to validate this method in the setting of fevers in the neurocritical care unit using this novel device.
Uniform cooling of a porcine brain using high flow dry air. Shown in the panel in the left top corner is an MRI image of a pig brain in the black circle. On the right is shown the same image over 10 min of dry air flow at 60 LPM through the nostrils. The brain gradually and uniformly cools (black arrows) and note the cooling of the oral nasal cavity. In the bottom left corner is direct measurements of temperature via thermocouples placed in frontal, parietal and temporal cortex showing uniform and rapid brain cooling
In conclusion, transnasal cooling has gained significant interest and acceptance mainly due to emergence of PFC based cooling in the recent years. This has the potential to induce hypothermia in an out of hospital setting that is particularly attractive in conditions such as cardiac arrest and traumatic brain injury where early intervention has shown significant neuroprotection at least in animal studies. Large scale clinical trials are needed to evaluate the impact of these new devices on survival and neuroprotection in threatened brain injury.
References
Geurkink N. Nasal anatomy, physiology, and function. J Allergy Clin Immunol. 1983;72(2):123–8.
Covaciu L, Allers M, Enblad P, et al. Intranasal selective brain cooling in pigs. Resuscitation. 2008;76:83–8.
Andrews PJD, Harris B, Murray GD. A randomised cross-over trial of the effects of airflow through the upper respiratory tract of intubated, brain injured patients on brain temperature and selective brain cooling. Br J Anaesth. 2005;94:330–5.
Buscha H, Eichwedeb F, Födisch M, Taccone FS, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation. 2010;81:943–9.
Castren M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling a randomized, prehospital, multicenter study (PRINCE: pre-ROSC intranasal cooling effectiveness). Circulation. 2010;122:729–36.
Bollera M, Lampea JW, Katza JM, Barbutc D, Beckera LB. Feasibility of intra-arrest hypothermia induction: a novel nasopharyngeal approach achieves preferential brain cooling. Resuscitation. 2010;81:1025–30.
Guan J, Barbut D, Wang H, Li Y, Tsai M, Sun S, Inderbitzen B, Weil MH, Tang W. Rapid induction of head cooling by the intranasal route during cardiopulmonary resuscitation improves survival and neurological outcomes. Crit Care Med. 2008;36(suppl):S428–33.
Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke. 2011;42(8):2164–9.
Edwards C, Lowe KC, Röhlke W, Geister U, Reuter P, Meinert H. Effects of a novel perfluorocarbon emulsion on neutrophil chemiluminescence in human whole blood in vitro. Artif Cells Blood Substit Immobil Biotechnol. 1997;25:255–60.
Flaim S. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:1043–54.
Bucala R, Kawakami M, Cerami A. Cytotoxicity of a perfluorocarbon blood substitute to macrophages in vitro. Science. 1983;27:965–7.
OECD/UNEP Global PFC Group. Synthesis paper on per- and polyfluorinated chemicals (PFCs). Paris: Environment, Health and Safety, Environment Directorate, OECD; 2013.
Harris S, Bansbach J, Dietrich I, Kalbhenn J, Schmutz A. RhinoChill(®)-more than an “ice-cream headache (1)” serious adverse event related to transnasal evaporative cooling. Resuscitation. 2016;103:e5–6.
Einer-Jensen N, Khorooshi MH. Cooling of the brain through oxygen flushing of the nasal cavities in intubated rats: an alternative model for treatment of brain injury. Exp Brain Res. 2000;130:244–7.
Chava R, Zviman M, Raghavan MS, Halperin H, et al. Rapid induction of therapeutic hypothermia using transnasal high flow dry air. Ther Hypothermia Temp Manag. 2017;7(1):50–6.
Acknowledgements
This work was supported by a NIH SBIR grant to Harikrishna Tandri (1 R44 HL108542-01A1).
Disclosures
Dr. Tandri holds patents for transnasal cooling and invented the transnasal dry air cooling device (CoolStat).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chava, R., Tandri, H. (2019). Transnasal Induction of Therapeutic Hypothermia for Neuroprotection. In: Chen, J., Wang, J., Wei, L., Zhang, J. (eds) Therapeutic Intranasal Delivery for Stroke and Neurological Disorders. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-030-16715-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-16715-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16713-4
Online ISBN: 978-3-030-16715-8
eBook Packages: MedicineMedicine (R0)