Abstract
NO and H2O2 act as key regulators in a broad range of physiological processes in algae and higher plants. A large amount of research highlights multiple roles for NO/H2O2 in plant defence. They function as protectants but also as signaling molecules that mediate various metabolic processes and activate further systematic plant defence reactions through the regulation of genes involved in pathogen defence. This chapter summarises the current knowledge on NO and H2O2 necessity in plant cell resistance response to biotic stressors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Reactive oxygen species
- Reactive nitrogen species
- Signal molecule
- Biotic stress
- Gene expression
- Crosstalk
1 Introduction
Plants and algae possess different signaling molecules (e.g. growth regulators, proteins, amino acids, nucleotides, hormones) which are essential for their growth, development as well as for their response and adaptation to a variety of abiotic and biotic stresses (Agostoni and Montgomery 2014; Bickerton et al. 2016; Dobrikova 2017; Górka and Wieczorek 2017). In view of the increasing pollution and more pronounced climate changes that affect plant organisms at the level of molecular function, developmental processes, morphological traits, and physiology (Gray and Brady 2016), influence the severity of plant disease and further coevolution of plants and their pathogens, the investigation of signaling molecules in algae and plants is becoming increasingly important.
Nitric oxide (NO) has been recognised as one of the central players in the cell signaling and attracted great attention of researchers (Mallick et al. 2002; Chung et al. 2008; Neill et al. 2002; Jeandroz et al. 2016). NO is a small gaseous molecule with low density and lipophilic quality (Cevahir et al. 2007). Its production arises from different enzymatic and nonenzymatic pathways (Rőszer 2014; Astier et al. 2018a). The well documented reductive NO production routes in plants are nitrate reductase (NR) and the plasma membrane-bound nitrite: NO reductase (NiNOR) systems. Also, NO can be produced in an arginine-dependent pathway, similar to the nitric oxide synthase (NOS) activity present in animals. Recent investigations showed that terrestrial plants do not possess animal NOS-like enzymes, while these enzymes were found in few algal species (Santolini et al. 2017; Weisslocker-Schaetzel et al. 2017; Astier et al. 2018b). Foresi et al. (2010) isolated protein from the alga Ostreococcus tauri (OtNOS) that is about 45% similar to human NOS enzyme and has in vitro NOS activity. Kumar et al. (2015) found NOS-like sequences from the marine green algae Bathycoccus prasinos and Ostreococcus lucimarinus that exhibited 62% similarity with OtNOS and several conserved residues in cofactor-binding sites. Each monomer of OtNOS homodimer enzyme possesses two domains: a C-terminal reductase domain (NOSred) and an N-oxygenase domain (NOSoxy) which harbours the catalytic site. Furthermore, Weisslocker-Schaetzel et al. (2017) made in-depth structural and functional analysis of OtNOS NOSoxy domain and found weak homology with NOS enzymes from other clades. Still, residues that form the catalytic core of the domain were conserved.
Nonenzymatic pathways include NO synthesis from nitrite NO2 − under acidic conditions or from hydroxylamine and salicylhydroxamate (Gupta et al. 2011a; Procházková et al. 2014). NO generation has been clearly demonstrated in a several different cell organelles including protoplasts, chloroplasts, mitochondria and peroxisomes (Gupta et al. 2011b; Tewari et al. 2013; Corpas and Barroso 2014), but it also could be synthesised in the cytoplasm, cell membrane, endoplasmic reticulum as well as apoplast (Frohlich and Durner 2011; Sahay and Gupta 2017). Due to the simple structure and small dimensions, NO freely diffuses through biological membranes provoking both beneficial and harmful effects in algae and plant cells (Arasimowicz and Floryszak-Wieczorek 2007). This dual role depends on the NO local concentration, as well as its ability to directly interact with other molecules and signals. Generally, at low concentrations, NO is a signaling molecule with a variety of physiological roles (Galatro and Puntarulo 2014). It plays an important role in many developmental processes of plants (Mur et al. 2013) including seed and pollen germination (Beligni and Lamattina 2000; Pasqualini et al. 2015), pollen tube growth (Prado et al. 2004), root growth (Corpas and Barroso 2015; Moni et al. 2018; Singh and Bhatla 2018; Sun et al. 2018), flowering (Senthil Kumar et al. 2016; Salachna and Zawadzińska 2018), photomorphogenesis (Lozano-Juste and León 2011; Melo et al. 2016), stomatal closure (García-Mata and Lamattina 2001), leaf senescence (Yang et al. 2018), gravitropism (Hu et al. 2005; París et al. 2018) and fertilisation (Domingos et al. 2015). Furthermore, it acts as a signal molecule for the activation of plant and algal defence response. The antioxidant role of NO is mainly based on its ability to maintain the cellular redox homeostasis and regulate the toxicity of reactive oxidative species (ROS) (Sheokand and Kumari 2015). For example, peroxynitrite (OONO−) results from the reaction between NO and superoxide radical (O2 ·−). It is a relatively short-lived reactive nitrogen species (RNS) which may readily migrate through biological membranes and interact with proteins, lipids and DNA via direct oxidation reactions or indirectly through the formation of highly reactive radicals (Radi 2013; Procházková et al. 2015). However, in systems where the toxicity is predominantly from peroxides, these compounds are much more toxic than NO and OONO−, making NO protective against them (Misra et al. 2011).
ROS have also been shown to play an important role as signaling molecules in response to environmental conditions (Mallick and Mohn 2000; del Río 2015; Mullineaux et al. 2018). Among the ROS compounds, H2O2 is generally recognised as one of the most important signaling molecules which has relatively long half-life and small size and thus may migrate in different cell compartments through aquaporin channels (Hooijmaijers et al. 2012; Srivastava et al. 2014; Rodrigues et al. 2017). H2O2 is a product of aerobic metabolism in plants and could be generated by several pathways such as photorespiration, electron transport chains and redox reaction (Møller 2001; Voss et al. 2013). It can be synthesised by different enzymes including cell wall peroxidases (Mittler 2002), oxalate oxidases (Hu et al. 2003), amine oxidases (An et al. 2008) and flavin-containing enzymes (Cona et al. 2006), as well as by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases coupled with superoxide dismutases (SOD; Vianello and Macri 1991). At lower concentrations, it acts as a long-distance signaling molecule (Matilla-Vázquez and Matilla 2014) which has a role in various physiological processes, including photosynthesis, respiration, translocation and transpiration (Vranová et al. 2002; Ślesak et al. 2007; Ismail et al. 2015). It is involved in the formation and development of adventitious roots (Li et al. 2007), stomatal closure (Zhang et al. 2001), senescence process (Bieker et al. 2012), protection against pathogen attack (Shetty et al. 2008) as well as in the regulation of various abiotic stresses (Mittler and Berkowitz 2001; Cuypers et al. 2016).
Abiotic and biotic factors induce rapid accumulation of variety of reactive nitrogen species (e.g. nitrosonium cation (NO+), nitroxyl anion (NO−), free radical (NO·), dinitrogen trioxide (N2O3), nitrogen dioxide (NO2) and S-nitrosothiols (SNOs)) causing nitrosative stress (Corpas et al. 2007; Procházková et al. 2015). In response to different stress factors including infections by pathogenic fungi, bacteria, and viruses, rapid accumulation of a variety of ROS also occurs (Mittler 2017). One of the earliest events following elicitation characterised by high ROS and NO concentrations is generally known as oxidative burst (Wojtaszek 1997). H2O2 generated during oxidative bursts under biotic stress may reduce pathogen growth or may cause the expression of genes encoding proteins involved in antioxidant and defensive processes but also may induce programmed cell death (PCD) that occurs during hypersensitive response (HR) in plants (Bhattacharjee 2005).
At higher concentration, all reactive species including NO and H2O2 interact with different macromolecules affecting their functionalities (Gadjev et al. 2008; Habibi 2014; Corpas and Palma 2018). The differential reactivity of RNS defines the diversity of the NO potential target molecules and its chemical reactions including nitrosylation, nitration and oxidation (Arasimowicz and Floryszak-Wieczorek 2007; Krasylenko et al. 2017).
Plants have developed an antioxidative system that encompasses both the enzymatic and nonenzymatic components to minimise nitro-oxidative stress caused by RNS and ROS (Corpas and Barroso 2013). The enzymatic components include very efficient various enzymes such as SOD, catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), guaiacol peroxidase (GPOX) and the ascorbate-glutathione cycle enzymes: ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDAR) and glutathione reductase (GR). Nonenzymatic antioxidants such as ascorbic acid (ASH), glutathione (GSH), phenolic compounds, alkaloids, nonprotein amino acids and α-tocopherols can quench all kinds of ROS directly but also may assist the enzymes to efficiently detoxify ROS (Perl-Treves and Perl 2002; Gill and Tuteja 2010; Štolfa et al. 2016, 2018).
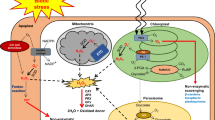
In response to development and different abiotic and biotic factors, both NO and H2O2 may interact with a variety of other signaling molecules and phytohormones (Ferreira and Cataneo 2010; Mostofa et al. 2015; Saxena et al. 2016) (Fig. 1). Also, crosstalk between the NO and H2O2 has been demonstrated in plants and algae (González et al. 2012; Niu and Liao 2016). These molecules may interact in their different developmental and physiological processes. Thus, it has been found that NO and H2O2 are essential signal molecules that mediate abscisic acid (ABA)-induced stomatal closure (Li et al. 2017) and that H2O2 and NO signaling pathways are involved in adventitious rooting in mung bean seedlings (Li and Xue 2010) as well as in cell cycle of green alga Chlamydomonas reinhardtii (Pokora et al. 2017). During the last decades, the roles of NO and H2O2, as well as their crosstalk in mediating plant defence mechanisms, have been largely studied. For example, it has been found that H2O2 and NO-signaling pathway overlap during the citrus plant acclimation on salinity (Tanou et al. 2009).
The mechanisms of stresses response triggered by NO and H2O2 include the enhancement of antioxidant systems and specific stress mechanisms, depending on the stress type (e.g. drought, temperature, heavy metals, etc.), and demand the interaction with other signaling molecules, such as mitogen-activated protein kinase (MAPK), plant hormones and calcium (Molassiotis and Fotopoulos 2011; Farnese et al. 2016). MAPK cascades are present in plants and algae (Mohanta et al. 2015) and consist of a few protein kinase modules including MAP kinase kinase (MAP2Ks, also called MKKs, MEKs or MAPKK), MAP kinase kinase kinases (MAP3Ks, also called MAPKKKs or MEKKs) and MAP kinase kinase kinase kinases (MAP4K; Rodriguez et al. 2010; Çakır and Kılıçkaya 2015). Generally, MAPK is activated via phosphorylation of threonine and tyrosine residues in the catalytic subdomain (Ichimura et al. 2002) and then translocated into the cytoplasm or nucleus to trigger the cellular responses (Nadarajah and Sidek 2010). They are involved in cellular responses to hormones, plant growth and development, regulation of the cell cycle and responses to biotic and abiotic stresses (Sinha et al. 2011; Zhao et al. 2013; Hettenhausen et al. 2015; Chardin et al. 2017). MAPKs can phosphorylate many target molecules including other kinases, enzymes and transcription factors (TFs). The responses mediated by MAPKs involve the induction of a variety of enzymes and may attenuate or amplify the original signal triggered by ROS and NO (Asai et al. 2008; Opdenakker et al. 2012). NO and H2O2 may also increase the concentration of some other signaling molecules such as cyclic guanosine 3′,5′-monophosphate (cGMP). This cyclic nucleotide is generated from guanosine 5′-triphosphate (GTP) by enzymes with guanylate cyclase (GCs) activity (Mulaudzi et al. 2011). These molecules have long been known to be present in plants and algae, playing important roles in many biological processes including growth and differentiation, photosynthesis as well as biotic and abiotic defence (Dubovskaya et al. 2011; Nan et al. 2014; Gehring and Turek 2017; Demidchik et al. 2018). Responses associated with cGMP depend on the time between the perception of the stimulus and the peak in nucleotide concentration and consequently involve fast responses, which include the ion channels modulation (e.g. Ca2+ channels) or long-term adaptive responses, which result in changes in the transcriptome and in the proteome (Pasqualini et al. 2009).
Thus, considering the very important role of NO and H2O2 molecules in a variety of biological process in plant and algae as well as in their response and adaptation to the environmental changes, the present review aims to summarise the recent data concerning the role of NO and H2O2 in plant defence mechanisms.
2 Enrolment of NO and H2O2 in Plant Stress Response
2.1 Sources, Signaling and Interaction
Elicitation by abiotic or biotic stress factors induces oxidative burst as one of the earliest events following plant challenge with pathogenic micro-organisms, including fungi, bacteria and viruses, as well as in cultured cells treated with elicitor preparations, pathogens or plant cell-wall fragments or in response to mechanical wounding (Wojtaszek 1997). To protect themselves against pathogens and herbivores, plants have evolved a complex network of immune responses – HR. It is defined as a programmed execution of infected cells and sometimes additional surrounding cells with a function to restrict pathogen infection (Lamb and Dixon 1997; Greenberg and Yao 2004). Beside local HR, plants react to pathogen attack by establishing systemic-acquired resistance (SAR), a long-lasting systemic immunity that protects the entire plant from the subsequent invasion of a broad range of pathogens associated with the systemic expression of defence gene families encoding pathogenesis-related proteins (Yu et al. 2014). One of the earliest events in the HR is the rapid accumulation of ROS (Zurbriggen et al. 2010) and NO (Delledonne et al. 1998; Durner et al. 1998). Plant species differentiate according to the major ROS building the oxidative burst. New and highly sensitive methods enabled the real-time detection of ROS generation in response to stress. Recently, the use of catalytic amperometric biosensors tested on in vivo spinach (Spinacia oleracea) leaf sample showed the continuous generation of O2 ·− for minutes after wounding, followed by a decline, while the production increased considerably with the dose of mechanical injury. The total O2 ·− concentration was found to be equivalent to 40 nM and 200 nM for a minimal dose of injury and injury at multiple sites, respectively (Prasad et al. 2017). Seaweed species released strong oxidants within 1 min of wounding and/or showed cellular accumulation of strong oxidants over an hour post-wounding (McDowell et al. 2014). However, the inherent interrelationship between H2O2 and O2 ·− makes it sometimes difficult to clearly identify the ROS behind the oxidative burst (Wojtaszek 1997). Stress treatment induces an oxidative burst in barley (Hordeum vulgare) anthers, as revealed by the formation of O2 ·− that gives rise to H2O2. Among ROS, H2O2 may have a central role in anthers as a diffusible signal molecule allowing selective induction of defence-related genes (Varnova et al. 2002).
The bulk of active oxygen species, mainly O2 ·−, H2O2, singlet oxygen (1O2) as well as NO, are produced to serve not only as protectants but also as signals activating further plant defence reactions (Bolwell 1999). H2O2 production is indispensable during plant growth, development and resistance responses. Its great capacity to buffer other ROS molecules and balance the capacity of oxygen scavenging enzymes changes oxygen’s relative impact on cells altering its resistance to stress (Quan et al. 2008). However, its beneficial role in integrating signaling network in response to biotic and abiotic stress and during developmental processes is well established (Wojtyla et al. 2016). Due to the large plethora of different signal messages triggered by H2O2, plants can sense, transport and induce cellular responses (Quan et al. 2008). The network of signals includes other reactive species such as NO and H2S, plant phytohormones jasmonic acid (JA), salicylic acid (SA), ABA, ethylene, auxin and gibberellins, as well as ions regulating diverse cellular processes like Ca2+ and K+ (Quan et al. 2008; Kaurilind et al. 2015).
One of the most important roles of H2O2 is in the reaction with the invading organisms. After the detection of an invader at the cell wall, the NADPH oxidase at the plasma membrane adjacent to the invasion site produces O2 ·− and ultimately H2O2 outside the plasma membrane.
Aquaporins in the plasma membrane are crucial for the efficiency of H2O2 signaling between cells. Their capacity to facilitate the H2O2 diffusion may have physiological significance in many organisms and be important in communication between different species (Bienert et al. 2006).
A number of possible functions for H2O2 have been proposed in plant-pathogen reactions: direct killing of pathogens, involvement in cell-wall reinforcing processes, promotion of PCD at the site of infection, phytoalexin synthesis, induction of defence gene expression and signaling in the induction of SAR (Bolwell 1999; Kuźniak and Urbanek 2000). During the pathogen attack on the tea plant (Camellia sinensis) leaves, H2O2 can accumulate in mesophyll and epidermal cells (Wang et al. 2018). Cellular and subcellular localisation analyses revealed that H2O2 was mainly localised in wound zones and spread throughout the veins and tissues. Preferentially, H2O2 was found in cell walls of spongy and mesophyll cells facing intercellular spaces in the herbivore-wounded lima bean (Phaseolus lunatus) leaves, even though confocal laser scanning microscopy analyses also revealed the presence of H2O2 in mitochondria/peroxisomes (Maffei et al. 2006). Like in the case of wounding, H2O2 also accumulates in the Capsicum leaf abscission zone throughout plant growth, it increases with age and during the execution phase, and its role in leaf abscission is associated with ethylene both in vitro and in planta (Sakamoto et al. 2008).
In the unicellular green alga Micrasterias denticulata, chloroplasts and mitochondria are generally the main production sites of H2O2 independent of the treatment (salt and osmotic stress), followed by cytoplasm, while levels remained unchanged or even slightly decreased in cell walls of treated cells and elevated concentration at the plasma membrane of KCl-treated cells (Darehshouri and Lütz-Meindl 2010). Increased H2O2 production can also be a defensive mechanism against epiphytism in algae. Green macroalga Caulerpa taxifolia enhanced the production of H2O2 and toxin caulerpenyne as well as the antioxidant enzymes activities to compete against epiphytic rhodophyte Lophocladia lallemandii (Box et al. 2008). H2O2 is also engaged in signal transduction and defence reactions in the nonhost-pathogen interactions, like the formation of callose-containing papilla for cell wall reinforcement and HR. HR and papilla deposition was highly associated with H2O2 accumulation in pepper epidermal cells, which has a key role in nonhost resistance against Blumeria graminis f. sp. tritici preventing its colonisation and disease development (Hao et al. 2011). Although being the most prominent oxidant in the algal oxidative burst cocktail (Potin 2008), some seaweeds show the smaller role of H2O2 upon wounding and oxidant release. For example, in several species of Antarctic brown and red macroalgae, other oxidant(s) besides H2O2 are released upon wounding (McDowell et al. 2014). H2O2 was a component of immediate oxidant release in one of five species, while in some it was not detected at all. Even the two close sister algal taxa may produce different amounts of ROS and have a different composition of chemical species in the oxidative burst. While after sonic injury endosymbiotic dinoflagellate Symbiodinium sp. produced a small oxidative burst of 0.042 ± 0.0045 pmol H2O2 min−1 cell−1 composed of nearly 100% H2O2, the oxidative burst in Heterocapsa pygmaea, the species similar in size and genetically related, was nearly 80 times greater (3.37 ± 0.26 pmol H2O2 min−1 cell−1) and also produced a fraction of other free radicals (Mydlarz and Jacobs 2004). The amount of produced H2O2 can vary with the different type of wounding, e.g. herbivore-wounded lima bean (Phaseolus lunatus) leaves produced higher H2O2 concentrations compared to mechanically damaged leaves (Maffei et al. 2006). As a response to oxidative burst, many species of marine algae produce volatile organic compounds, such as halogenated, sulphur containing, aldehydes, non-methane hydrocarbons and oxygenated species as one of the ways to lower the content of produced H2O2 (Potin 2008). An oxidative burst of H2O2 and the consequent production of volatile organic compounds in brown alga Laminaria digitata after elicitation induce defence response which can even be transmitted as a warning message to neighbouring conspecifics (Thomas et al. 2011).
Exogenously applied H2O2 is known as a good microbicidal agent which can benefit seed health and performance. Soaking the zinnia (Zinnia elegans) seeds regardless of time (10, 20, 30 and 60 min) and H2O2 concentration (3, 6, 9 and 12%, respectively) significantly reduced seed infestation with Alternaria spp. and Fusarium spp. fungi, while 3% solution positively influenced seed germination and vigour at 20 min and 10 min application, respectively (Szopińska 2014).
Foliar application of H2O2 (18 mM) in chili pepper plants (Capsicum chinense) significantly increased the amount of phenolics, flavonoids and capsaicinoids contents in fruits, thus concomitantly increasing antimicrobial activity of its extracts against bacteria and yeast (Vargas-Hernández et al. 2017). H2O2 is also utilised as an efficient algicide for the management of waste waters. The dose of 3.0 × 10−3 g H2O2 μg−1 phytoplankton chlorophyll-a was the most effective in the removal of nuisance phytoplankton, including toxic cyanobacteria (Barrington and Ghadouani 2008). Sensing the increased H2O2 concentrations can also be one of the phytoplankton survival strategies under unfavourable environmental conditions. The encystment of some dinoflagellate species, an effective strategy that enables them to survive, is redox-mediated, presumably by H2O2 (Ganini et al. 2013).
NO burst can be detected very early after inoculation with pathogens and directly precedes H2O2 generation. NO production in affected cells can be monitored with the application of fluorescent dyes (Foissner et al. 2000; Prats et al. 2005).
One of the most important NO signaling mechanism during plant defence is based on its chemical reaction with proteins (Leitner et al. 2009). The fine balance between S-nitrosylation and denitrosylation is critical for NO signaling. S-Nitrosylation regulates numerous defence-related proteins such as enzymes, transcriptional activators, or co-activators involved in plant immune response in the way of protein relocalisation or activity modulation (Bellin et al. 2013). S-nitrosylation is a chemical reaction in which a NO moiety is covalently added to the sulfhydryl group of reactive cysteine residues in target proteins to form an S-nitrosothiol (SNO) (Stamler et al. 2001). The SNO turnover represents an alternative mechanism to control the process of protein S-nitrosylation. Namely, the main enzymatic systems which mediate denitrosylation in plants are described: the glutathione/S-nitrosoglutathione reductase (GSH/GSNOR) and the thioredoxin-h5 (Trx-h5) (Kneeshaw et al. 2014). An S-nitrosylated form of glutathione, S-nitrosoglutathione (GSNO) is a global reservoir of NO bioactivity (Liu 2001). Experiments with Arabidopsis knockout mutants of this enzyme, known as GSNO Reductase 1 (GSNOR1), accumulate high levels of protein-SNO and consequently have impaired SA-dependent immune signaling and are highly disease susceptible (Feechan et al. 2005). In contrast, plants expressing antisense GSNOR1 show enhanced SAR, likely due to a partial reduction in GSNOR1 activity, which increases NO levels to the extent that is ideal for stimulating SAR (Rustérucci et al. 2007; Espunya et al. 2012). In plants, GSNOR has been found to be important in resistance to bacterial and fungal pathogens like Blumeria graminis, Hyaloperonospora parasitica, and Pseudomonas syringae (Feechan et al. 2005) and possibly also involved in plant-herbivore interactions (Wünsche et al. 2011). The enzyme GSNOR does not directly reverse the S-nitrosylation of Cys residue but rather turns over GSNO thereby reducing the cells store of NO bioactivity and controls intracellular levels of both GSNO and SNO affecting the global level of S-nitrosylation (Feechan et al. 2005; Malik et al. 2011). On the other hand, the mechanism of Trx denitrosylation directly interacts with SNO-proteins by the formation of an intermolecular disulphide intermediate in which Trx is covalently linked to the substrate protein through a disulphide bridge or transnitrosylation in which Trx is transiently S-nitrosylated. This mechanism is described in detail in animal cells (Benhar et al. 2008), while Kneeshaw et al. (2014) proved the similar mechanism of Trx-h5 in plant cells.
A number of experiments using NO donors, scavengers and NOS inhibitors proved that NO plays a central role in plant defence against biotic stress, in combination with ROS (Delledonne et al. 1998, 2001; Yun et al. 2011). Keshavarz-Tohid et al. (2016) found the positive interaction of NO and H2O2 in bean plant defence against Pseudomonas fluorescens and Rhizoctonia solani. Namely, NO donor S-nitroso-N-acetyl D-penicillamine increased the production of H2O2, thereby increasing the activity of antioxidant enzymes as well as the activity of phenylalanine ammonia lyase (PAL). NO action upstream of H2O2 was also shown in plants responses to herbivore attacks and Tobacco Mosaic Virus infection (Orozco-Cárdenas and Ryan 2002; Liao et al. 2013). Reversely, Qiao et al. (2015) found that Ca2+ and H2O2 are involved in upstream of NO production to induce the HR cell death in the wheat cells during Puccinia triticina infection.
H2O2 and NO display both prooxidant and antioxidant properties. For example, they serve as prooxidant agents and putative redox signals for in vitro encystment of the dinoflagellate Lingulodinium polyedrum. Oxidative stress induced by high H2O2 dose (500 mM) forced L. polyedrum cells to rapidly encyst (<30 min), while slower cyst formation was observed in lower H2O2 concentrations (Ganini et al. 2013). NO may induce scavenging of excess H2O2 and inhibit peroxide signaling pathways but also may collaborate with H2O2 to switch on SAR or stress tolerance. Two faced NO molecule can act as an antioxidant and an antiapoptotic modulator that prevents cell death and in the same time can have a cytotoxic effect. These cytotoxic and protective effects of NO are often concentration dependent (Wink and Mitchell 1998).
The mechanism of NO/H2O2 interaction in the induction of cell death is largely unknown. The in vitro chemical reaction between NO and H2O2 produces either 1O2 or hydroxyl radicals (·OH) which can mediate cell killing (Noronha-Dutra et al. 1993). Although 1O2 formation in plant system is merely dependent on light, especially pronounced in the highlight, temperature, heavy metal stress or wounding, its light-independent generation in plant cells under multiple stresses has also been proposed (Mor et al. 2014; Chen and Fluhr 2018; Prasad et al. 2018). NO can also interact with O2 ·− to produce another highly reactive species, OONO−. Alamillo and Garcia-Olmedo (2001) found that direct application of OONO− induced plant cell death, which was not observed in the case when urea (OONO− scavenger) was added. OONO− may have a toxic effect on microorganisms, although so far it has not been clarified whether NO and its derivatives are directly toxic to pathogens in plants (Garcia-Olmedo et al. 2001). In tobacco cell suspensions, only a simultaneous increase in NO and H2O2 activated cell death, whereas an independent increase of only one of the factors mentioned above induced cell death only slightly (de Pinto et al. 2002). Moreover, only the synergistic effect of H2O2 and NO was effective in control of tomato bacterial wilt (Hong et al. 2013).
2.2 Regulation of Gene Expression
Both NO and H2O2 influence gene expression from transcription to protein synthesis. They regulate genes involved in the induction of pathogen defence, such as genes encoding pathogenesis-related (PR) proteins, genes involved in accumulation of phenylpropanoid compounds, genes encoding H2O2 detoxifying enzymes, enzymes involved in JA biosynthesis (Jacquard et al. 2009), upregulate cellulase expression (Sakamoto et al. 2008) and control genes involved in the hypersensitive cell death (Kaurilind et al. 2015). The large-scale gene expression analysis in Arabidopsis performed using the paired-end RNA-seq technology showed a different response to GSNO treatment in leaves and roots, suggesting that NO signaling mechanisms are organ specific (Begara-Morales et al. 2014). Another study on Arabidopsis transcriptome also showed that NO modulates gene expression in a concentration-dependent manner (Parani et al. 2004).
NO and ROS signaling is important in the establishment of plant disease resistance through modulation of defence-related genes encoding phenylalanine ammonia lyase (PAL) and pathogenesis-related protein 1 (PR1), markers for phenylpropanoid biosynthesis and SA-mediated signaling, respectively.
The key redox control of SAR exerts through nitrosylation of NPR1 (pathogenesis-related (PR)1), the key protein in plant immunity that co-activates defence gene expression and is an important component of SA-mediated signal transduction (Mou et al. 2003; Tada et al. 2008). Moreover, Wu et al. (2012) showed this protein to bind SA, working as an SA receptor directly. In unaffected cells, NPR1 is normally present as a high molecular weight oligomer formed with intermolecular disulphide bridges. After the pathogen attack as well as accumulation of SA, changes in cellular redox potential lead to the reduction of cysteines through the activity of thioredoxins (TRX-h3 and TRX-h5) and translocation of NPR1 monomers to the nucleus. In the nucleus, NPR1 interacts with the TGACG motif-binding factor (TGA) that binds to elements of the PR1 promoter, promoting PR gene expression and defence (Tada et al. 2008; Bellin et al. 2013). On the other hand, Tada et al. (2008) also found that S-nitrosylation in vivo promotes disulphide bond formation and oligomerisation. This reaction may be required to maintain NPR1 oligomer/monomer homeostasis, thereby facilitating the steady supply of monomeric protein to support SA-dependent gene expression. Beside NO, increased levels of H2O2 also inhibit NPR1 translocation (Peleg-Grossman et al. 2010). On the other hand, an oxidative burst and accumulation of H2O2 induced by the fungus C. gloeosporioides in the resistant cowpea genotype TE97 enhanced the levels of PR proteins (GLU and CHI) as a defence strategy against pathogen attack (Oliveira et al. 2014).
However, Lindermayr et al. (2010) found that NO also promotes NPR1-dependent defence responses by facilitating the translocation of NPR1 into the nucleus. Notably, TGA, an interaction partner of NPR1, can also be oxidised and S-nitrosylated. The oxidised form carries disulphide bonds that block interactions with NPR1, while the GSNO-mediated S-nitrosylation of cysteine residues protects TGA proteins from oxidative modification and improves its binding activity to PR1 promoter region (Lindermayr et al. 2010). This additional positive regulatory mechanism also involves the NO-dependent regulation of GSH biosynthesis and accumulation, which increases SA levels and thus activates NPR1-dependent defence responses (Kovacs et al. 2015). However, the nuclear translocation of NPR1 was much slower when driven by GSNO instead of SA. This finding suggests that this process was not the direct consequence of NPR1 S-nitrosylation but, instead, was dependent on a signaling pathway involving GSH biosynthesis. These findings revealed evidence of additional crosstalks among NO, GSH and SA pathways in the establishment of immunity in plants.
Besides NPR, S-nitrosylation modulates the activity of another SA-binding protein AtSABP3 (A. thaliana SA-binding protein 3). Namely, it suppresses both binding of SA and also its carbonic anhydrase activity that could contribute to negative feedback that modulates plant defence response and cell death (Wang et al. 2009).
Another important defence-related gene PAL is also modulated via NO signaling (Durner et al. 1998; de Pinto et al. 2002). Moreover, inhibition of NOS activity significantly reduces the accumulation of transcripts encoding PAL and chalcone synthase enzymes important for flavonoids and isoflavonoid synthesis (Delledonne et al. 1998). Additionally, an increase in cinnamate-4-hydroxylase transcripts, a key enzyme in the synthesis of phenolic compounds, has been found in Arabidopsis treated with a NO donor (Polverari et al. 2003). Likewise, exogenous H2O2 treatment induces PAL1 mRNA expression in Arabidopsis where this enzyme mediates biosynthesis of lignin and SA (Desikan et al. 1998).
NO is involved in the regulation of gene expression of NO-responsive TFs such as MYB, WRKY, C2H2, Aux/IAA, bZIP, etc. included in the mediation of abiotic and biotic stress responses (Imran et al. 2018). Novel information about a large number of genes involved in NO signaling is discovered with the use of next-generation sequencing (NGS) technologies. Imran et al. (2018) showed that almost 30% of total NO-responsive TFs were related to stress tolerance, among which 95.5% were related to biotic stress. For example, bZIP-binding elements associated with defence formed by octopine synthase (OCS) are very important for the expression of above-mentioned PR1 gene in Arabidopsis where OCS element-binding factor interacts with NPR1 (Lebel et al. 1998; Zhou et al. 2000). Likewise, WRKY transcription factors involved in plant response to wounding and pathogen infection were upregulated in seedlings of Larix olgensis Henry treated with NO (Hu et al. 2015). Expression of transcription factor WRKY8 can also be activated through ROS-mediated signaling mechanism (Chen et al. 2010). Also, a large number of genes encoding TFs involved in NO signaling are characterised by RNA-Seq in root nodules formed in the symbiotic relationship of Medicago truncatula and its bacterial symbiont Sinorhizobium meliloti (Boscari et al. 2013).
The NO/ROS crosstalk during plant defence was also reflected through modulation of NO reactivity by S-nitrosylation of ROS producers and ROS or RNS scavengers. NADPH oxidase AtRBOHD is a major participant in ROS production induced by pathogens in Arabidopsis (Torres et al. 2005) and in grass roots infected with fungal phytopathogen Rhizoctonia solani (Liu et al. 2018). Yun et al. (2011) found that cysteine 890 S-nitrosylation of AtRBOHD during plant defence decreases NADPH oxidase activity and consequently reduces ROS accumulation, limiting the later stages of HR cell death. Also, different NO-responsive TFs knockout Arabidopsis mutants showed significantly higher expression of the NADPH oxidase 1 (NOX1) and consequently more ROS accumulation. However, these mutants also showed higher expression of important antioxidative enzyme genes CAT1, 2 and 3 and APX1 and 2 (Imran et al. 2018). Another way of oxidative burst inhibition triggered by NO is through its reaction with O2 ·− to form ONOO− and thus work directly as an antioxidant. Through this interaction, NO direct competes with SOD activity that could, therefore, inhibit the oxidative burst and decrease the accumulation of H2O2. Also, Holzmeister et al. (2015) found that NO modifies and inhibits three of the seven A. thaliana SODs by tyrosine nitration. The relevance of this modification in plant defence is still undefined, but it could be important for regulating ROS levels in HR cell death. Also, different antioxidant enzymes involved in H2O2 detoxification are exposed to S-nitrosylation or nitration according to in vitro studies, like APX, MDAR, CAT and peroxiredoxins, all of which are involved in H2O2 detoxification (Romero-Puertas et al. 2007; de Pinto et al. 2013; Begara-Morales et al. 2015; Camejo et al. 2015). Another antioxidative enzyme, Peroxidase 2 (PA 2) gene, may be a negative regulator of H2O2 production and is suppressed by an unknown TF to increase H2O2 levels in an anthracnose-resistant tea plant (Camellia sinensis) cultivar as a defence to Colletotrichum fructicola. H2O2 also upregulated a cell signaling receptor (wall-associated kinase 3) connected with cell wall reinforcement in C. sinensis at the penetration sites of pathogen hyphae (Wang et al. 2018). Activated resistance genes also regulate the thickening of cell wall tissue to defend against hyphal growth.
H2O2 accumulation also regulates the expression of cellulase genes. H2O2 is involved in the abscission signaling downstream of ethylene to upregulate cellulase expression. Moreover, salinity induced the production of H2O2 before leaf abscission, indicating that H2O2 might generally be involved in stress-induced leaf abscission (Sakamoto et al. 2008). Increased H2O2 accumulation in herbivore-wounded leaves was also correlated with increased SOD enzyme activity and gene expression (Maffei et al. 2006).
Besides NO, ROS metabolism localised in the peroxisome is usually controlled by the protein peroxin 11a (PEX11a) under stress conditions which was distinctly upregulated in the resistant tea plant during C. fructicola infection and may also be associated with H2O2 production (Wang et al. 2018).
In the recent years, the physiological relevance of H2S signaling and its tight connection with H2O2 and NO gained considerable interest (Zhang 2016). As a part of ROS/NO antioxidative network, H2S is involved in plant responses to different abiotic stress factors, although its role in plant response to a pathogen is still largely unknown (Hancock 2018). For example, spermidine-induced H2O2 in leaves of white clover could be an upstream signal molecule of NO and H2S, and derived H2S might act as the downstream signal of NO. Moreover, accumulation of H2S can significantly improve antioxidant enzyme (SOD, CAT, GPOX, APX, GR, DAR and MDAR) activities and transcript levels of dehydration-regulated TFs (bZIP37, bZIP107, DREB2, DREB4 and WRKY108715) and genes encoding antioxidant enzymes (Li et al. 2017).
3 Conclusion
The review highlights important roles and tight relationship between NO and H2O2 signaling in plants subjected to biotic stress. Stressors (e.g. pathogen attack, wounding) initiate a myriad of reactions resulting in the oxidative burst, i.e. bulk production of reactive species such as NO and H2O2. These trigger the defence signaling through different pathways including phytohormones, MAPKs, NR, cGMP, cADPR and Ca2+, and further activate transcriptional factors inducing the expression of genes involved in plant defence (secondary metabolism, SAR, HR, antioxidative system). New studies are awaited to identify further biochemical and functional characteristics of NO and H2O2, their interactions and mechanisms which lead to plant defence responses.
References
Agostoni M, Montgomery BL (2014) Survival strategies in the aquatic and terrestrial world: the impact of second messengers on cyanobacterial processes. Life 4:745–769
Alamillo JM, Garcia-Olmedo F (2001) Effects of urate, a natural inhibitor of peroxynitrite-mediated toxicity, in the response of Arabidopsis thaliana to the bacterial pathogen Pseudomonas syringae. Plant J 125:529–540
An Z, Jing W, Liu Y, Zhang W (2008) Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J Exp Bot 59:815–825
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887
Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20:390–1406
Astier J, Gross I, Durner J (2018a) Nitric oxide production in plants: an update. J Exp Bot 69:3401–3411
Astier J, Jeandroz S, Wendehenne D (2018b) Nitric oxide synthase in plants: the surprise from algae. Plant Sci 268:64–66
Barrington DJ, Ghadouani A (2008) Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Environ Sci Technol 42:8916–8921
Begara-Morales JC, Sánchez-Calvo B, Luque F, Leyva-Pérez MO, Leterrier M, Corpas FJ, Barroso JB (2014) Differential transcriptomic analysis by RNA-Seq of GSNO-responsive genes between Arabidopsis roots and leaves. Plant Cell Physiol 55:1080–1095
Begara-Morales JC, Sánchez-Calvo B, Chaki M, Mata-Pérez C, Valderrama RP, Adilla MN, López-Jaramillo J, Luque F, Corpas FJ, Barroso JB (2015) Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J Exp Bot 66:5983–5996
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210:215–221
Bellin D, Asai S, Delledonne M, Yoshioka H (2013) Nitric oxide as a mediator for defense responses. Mol Plant Microb Interact 26:271–277
Benhar M, Forrester MT, Hess DT, Stamler JS (2008) Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320:1050–1054
Bhattacharjee S (2005) Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plants. Curr Sci 89:1113–1121
Bickerton P, Sello S, Brownlee C, Pittman JK, Wheeler GL (2016) Spatial and temporal specificity of Ca2+ signalling in Chlamydomonas reinhardtii in response to osmotic stress. New Phytol 212:920–933
Bieker S, Riester L, Stahl M, Franzaring J, Zentgraf U (2012) Senescence-specific alteration of hydrogen peroxide levels in Arabidopsis thaliana and oilseed rape spring variety Brassica napus L. cv. Mozart. J Integr Plant Biol 54:540–554
Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994–1003
Bolwell GP (1999) Role of active oxygen species and NO in plant defence responses. Curr Opin Plant Biol 2:287–294
Boscari A, Del Giudice J, Ferrarini A, Venturini L, Zaffini AL, Delledonne M, Puppo A (2013) Expression dynamics of the Medicago truncatula transcriptome during the symbiotic interaction with Sinorhizobium meliloti: which role for nitric oxide? Plant Physiol 161:425–439
Box A, Sureda A, Terrados J, Pons A, Deudero S (2008) Antioxidant response and caulerpenyne production of the alien Caulerpa taxifolia (Vahl) epiphytized by the invasive algae Lophocladia lallemandii (Montagne). J Exp Mar Biol Ecol 364:24–28
Çakır B, Kılıçkaya O (2015) Mitogen-activated protein kinase cascades in Vitis vinifera. Front Plant Sci 6:556
Camejo D, Ortiz-Espin A, Lazaro JJ, Romero-Puertas MC, Lazaro-Payo A, Sevilla F, Jiménez A (2015) Functional and structural changes in plant mitochondrial PrxII F caused by NO. J Protom 119:112–125
Cevahir G, Aytamka E, Erol Ç (2007) The role of nitric oxide in plants. Biotechnol Biotechnol Equip 21:13–17
Chardin C, Schenk ST, Hirt H, Colcombet J, Krapp A (2017) Review: Mitogen-activated protein kinases in nutritional signaling in Arabidopsis. Plant Sci 260:101–108
Chen T, Fluhr R (2018) Singlet oxygen plays an essential role in the root’s response to osmotic stress. Plant Physiol 177:1717–1727
Chen L, Zhang L, Yu D (2010) Wounding-induced WRKY8 is involved in basal defense in Arabidopsis. Mol Plant Microb Int 23:558–565
Chung CC, Hwang SPL, Chang J (2008) Nitric oxide as a signaling factor to upregulate the death-specific protein in a marine diatom, Skeletonema costatum, during blockage of electron flow in photosynthesis. Appl Environ Microbiol 74:6521–6527
Cona A, Rea G, Botta M, Corelli F, Federico R, Angelini R (2006) Flavin-containing polyamine oxidase is a hydrogen peroxide source in the oxidative response to the protein phosphatase inhibitor cantharidin in Zea mays L. J Exp Bot 57:2277–2289
Corpas FJ, Barroso JB (2013) Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol 199:633–635
Corpas FJ, Barroso JB (2014) Peroxisomal plant nitric oxide synthase (NOS) protein is imported by peroxisomal targeting signal type 2 (PTS2) in a process that depends on the cytosolic receptor PEX7 and calmodulin. FEBS Lett 588:2049–2054
Corpas FJ, Barroso JB (2015) Functions of nitric oxide (NO) in roots during development and under adverse stress conditions. Plants 4:240–252
Corpas FJ, Palma JM (2018) Assessing nitric oxide (NO) in higher plants: an outline. Nitrogen 1:12–20
Corpas FJ, Carreras A, Valderrama R, Chaki M, Palma JM, del Río LA, Barroso JB (2007) Reactive nitrogen species and nitrosative stress in plants. Plant Stress 1:37–41
Cuypers A, Hendrix S, Amaral dos Reis R, De Smet S, Deckers J, Gielen H, Jozefczak M, Loix C, Vercampt H, Vangronsveld J, Keunen E (2016) Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front Plant Sci 7:470
Darehshouri A, Lütz-Meindl U (2010) H2O2 localization in the green alga Micrasterias after salt and osmotic stress by TEM-coupled electron energy loss spectroscopy. Protoplasma 239:49–56
de Pinto MC, Tomassi F, de Gara L (2002) Changes in the antioxidant systems as a part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco bright-yellow 2 cells. Plant Physiol 130:689–708
de Pinto MC, Locato V, Sgobba A, Romero-Puertas M d C, Gadaleta C, Delledonne M, De Gara L (2013) S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco bright yellow-2 cells. Plant Physiol 163:1766–1775
del Río LA (2015) ROS and RNS in plant physiology: an overview. J Exp Bot 66:2827–2837
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci U S A 98:13454–13459
Demidchik V, Maathuis F, Voitsekhovskaja O (2018) Unravelling the plant signalling machinery: An update on the cellular and genetic basis of plant signal transduction. Funct Plant Biol 45:1–8
Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330:115–120
Dobrikova AG (2017) Signaling molecules in plants: exogenous application. Acta Sci Agri 1:38–41
Domingos P, Prado AM, Wong A, Gehring C, Feijo JA (2015) Nitric oxide: A multitasked signaling gas in plants. Mol Plant 8:506–520
Dubovskaya LV, Bakakina YS, Kolesneva EV, Sodel DL, McAinsh MR, Hetherington AM, Volotovski ID (2011) cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol 191:57–69
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci U S A 95:10328–10333
Espunya MC, De MRG-CA, Martinez M (2012) S-nitrosoglutathione is a component of wound- and salicylic acid induced systemic responses in Arabidopsis thaliana. J Exp Bot 63:3219–3227
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA (2016) When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic Stress. Front Plant Sci 7:471
Feechan A, Kwon E, Yun BW, Wang Y, Pallas JA, Loake GJ (2005) A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci U S A 102:8054–8059
Ferreira LC, Cataneo AC (2010) Nitric oxide in plants: A brief discussion on this multifunctional molecule. Sci Agric (Piracicaba Braz) 67:236–243
Foissner ID, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23:817–824
Foresi N, Correa-Aragunde N, Parisi G, Caló G, Salerno G, Lamattina L (2010) Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 22:3816–3830
Frohlich A, Durner J (2011) The hunt for plant nitric oxide synthase (NOS): is one really needed? Plant Sci 181:401–404
Gadjev I, Stone JM, Gechev TS (2008) Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int Rev Cell Mol Biol 270:87–144
Galatro A, Puntarulo S (2014) An update to the understanding of nitric oxide metabolism in plants. In: Khan MN, Mobin M, Mohammad F, Corpas JF (eds) Nitric oxide in plants: metabolism and role in stress physiology. Springer, Basel, pp 3–16
Ganini D, Hollnagel HC, Colepicolo P, Barros MP (2013) Hydrogen peroxide and nitric oxide trigger redox-related cyst formation in cultures of the dinoflagellate Lingulodinium polyedrum. Harmful Algae 27:121–129
García-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204
Garcia-Olmedo F, Rodrigguez-Palenzulea P, Molina A, Alamillo JM, Lopez-Solanilla E, Berrocal-Lobo M, Poza-Carrion C (2001) Antibiotic activities of peptides, hydrogen peroxide and peroxynitrite in plant defence. FEBS Lett 489:219–222
Gehring C, Turek IS (2017) Cyclic nucleotide monophosphates and their cyclases in plant signaling. Front Plant Sci 8:1704
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
González A, de los Ángeles Cabrera M, Henríquez MJ, Contreras RA, Morales B, Moenne A (2012) Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol 158:1451–1462
Górka B, Wieczorek PP (2017) Simultaneous determination of nine phytohormones in seaweed and algae extracts by HPLC-PDA. J Chromatogr B Analyt Technol Biomed Life Sci 1057:32–39
Gray SB, Brady SM (2016) Plant developmental responses to climate change. Dev Biol 419:64–77
Greenberg JT, Yao N (2004) The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 6:201–211
Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT (2011a) On the origins of nitric oxide. Trend Plant Sci 16:160–168
Gupta KJ, Igamberdiev AU, Manjunatha G, Segu S, Moran JF, Neelawarne B, Bauwe H, Kaiser WM (2011b) The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci 181:520–526
Habibi G (2014) Hydrogen peroxide (H2O2) generation, scavenging and signaling in plants. In: Ahmad P (ed) Oxidative damage to plants: antioxidant networks and signaling. Academic, San Diego, pp 557–584
Hancock JT (2018) Hydrogen sulfide and environmental stresses. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2018.08.034
Hao X, Yu K, Ma Q, Song X, Li H, Wang M (2011) Histochemical studies on the accumulation of H2O2 and hypersensitive cell death in the nonhost resistance of pepper against Blumeria graminis f. sp. tritici. Physiol Mol Plant Pathol 76:104–111
Hettenhausen C, Schuman MC, Wu J (2015) MAPK signalling: a key element in plant defense response to insects. Insect Sci 22:157–164
Holzmeister C, Gaupels F, Geerlof A, Sarioglu H, Sattler M, Durner J, Lindermayr C (2015) Differential inhibition of Arabidopsis superoxide dismutases by peroxynitrite-mediated tyrosine nitration. J Exp Bot 66:989–999
Hong JK, Kang SR, Kim YH, Yoon DJ, Kim DH, Kim HJ, Sung CH, Kang HS, Choi CW, Kim DH, Kim YS (2013) Hydrogen peroxide- and nitric oxide-mediated disease control of bacterial wilt in tomato plants. Plant Pathol J 29:386–396
Hooijmaijers C, Rhee JY, Kwak KJ, Chung GC, Horie T, Katsuhara M, Kang H (2012) Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J Plant Res 125:147–153
Hu X, Bidney DL, Yalpani N, Duvick JP, Crasta O, Folkerts O, Lu G (2003) Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol 133:170–181
Hu X, Neill SJ, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137:663–670
Hu X, Yang J, Li C (2015) Transcriptomic response to nitric oxide treatment in Larix olgensis Henry. Int J Mol Sci 16:28582–28597
Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang S, Hirt H, Wilson C, Heberle-Bors E, Ellis BE, Morris PC, Innes RW, Ecker JR, Scheel D, Klessig DF, Machida Y, Mundy J, Ohashi Y, Walker JC (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trend Plant Sci 27:301–308
Imran QM, Hussain A, Lee SU, Mun BG, Falak N, Loake GJ, Yun BW (2018) Transcriptome profile of NO-induced Arabidopsis transcription factor genes suggests their putative regulatory role in multiple biological processes. Sci Rep 8:771
Ismail SZ, Khandaker MM, Mat N, Boyce AN (2015) Effects of hydrogen peroxide on growth, development and quality of fruits: a review. J Agron 14:331–336
Jacquard C, Mazeyrat-Gourbeyre F, Devaux P, Boutilier K, Baillieul F, Clément C (2009) Microspore embryogenesis in barley: anther pre-treatment stimulates plant defence gene expression. Planta 229:393–402
Jeandroz S, Wipf D, Stuehr DJ, Lamattina L, Melkonian M, Tian Z, Zhu Y, Carpenter EJ, Wong GKS, Wendehenne D (2016) Occurrence, structure, and evolution of nitric oxide synthase–like proteins in the plant kingdom. Sci Signal 9:re2
Kaurilind E, Xu E, Brosché M (2015) A genetic framework for H2O2 induced cell death in Arabidopsis thaliana. BMC Genom 16:837
Keshavarz-Tohid V, Taheri P, Taghavi SM, Tarighi S (2016) The role of nitric oxide in basal and induced resistance in relation with hydrogen peroxide and antioxidant enzymes. J Plant Physiol 199:29–38
Kneeshaw S, Gelineau S, Tada Y, Loake GJ, Spoel SH (2014) Selective protein denitrosylation activity of thioredoxin-h5 modulates plant immunity. Mol Cell 56:153–162
Kovacs I, Durner J, Lindermayr C (2015) Crosstalk between nitric oxide and glutathione is required for NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1)-dependent defense signaling in Arabidopsis thaliana. New Phytol 208:860–872
Krasylenko YA, Yemets AI, Blume YB (2017) Cell mechanisms of nitric oxide signaling in plants under abiotic stress conditions. In: Pandey G (ed) Mechanism of plant hormone signaling under stress. Wiley, Hoboken, NJ
Kumar A, Castellano I, Patti FP, Palumbo A, Buia MC (2015) Nitric oxide in marine photosynthetic organisms. Nitric Oxide 47:34–39
Kuźniak E, Urbanek H (2000) The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol Plant 22:195–203
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Ann Rev Plant Physiol Plant Mol Biol 48:251–275
Lebel E, Heifetz P, Thorne L, Uknes S, Ryals J, Ward E (1998) Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J 16:223–233
Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M (2009) NO signals in the haze: nitric oxide signalling in plant defence. Curr Opin Plant Biol 12:451–458
Li SW, Xue L (2010) The interaction between H2O2 and NO, Ca2+, cGMP, and MAPKs during adventitious rooting in mung bean seedlings. In Vitro Cell Dev Biol Plant 46:142–148
Li SW, Xue L, Xu S, Feng H, An L (2007) Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul 52:173–180
Li Q, Wang YJ, Liu CK, Pei ZM, Shi WL (2017) The crosstalk between ABA, nitric oxide, hydrogen peroxide, and calcium in stomatal closing of Arabidopsis thaliana. Biologia 72:1140–1146
Liao YWK, Sun ZH, Zhou YH, Shi K, Li X, Zhang GQ, Xia XJ, Chen ZX, Yu JQ (2013) The role of hydrogen peroxide and nitric oxide in the induction of plant-encoded RNA-dependent RNA polymerase 1 in the basal defense against Tobacco Mosaic Virus. PLoS One 8:e76090
Lindermayr C, Sell S, Muller B, Leister D, Durner J (2010) Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell 22:2894–2907
Liu L (2001) A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410:490–494
Liu B, Wang H, Ma Z, Gai X, Sun Y, He S, Liu X, Wang Y, Xuan Y, Gao Z (2018) Transcriptomic evidence for involvement of reactive oxygen species in Rhizoctonia solani AG1 IA sclerotia maturation. Peer J 6:e5103
Lozano-Juste J, León J (2011) Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis. Plant Physiol 156:1410–1423
Maffei ME, Mithöfer A, Arimura G, Uchtenhagen H, Bossi S, Bertea CM, Starvaggi Cucuzza L, Novero M, Volpe V, Quadro S, Boland W (2006) Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol 140:1022–1035
Malik SI, Hussain A, Yun BW, Spoel SH, Loake GJ (2011) GSNOR-mediated de-nitrosylation in the plant defence response. Plant Sci 181:540–544
Mallick N, Mohn FH (2000) Reactive oxygen species: response of algal cells. J Plant Physiol 157:183–193
Mallick N, Mohn FH, Soeder CJ, Grobbelaar JU (2002) Ameliorative role of nitric oxide on H2O2 toxicity to a chlorophycean alga Scenedesmus obliquus. J Gen Appl Microbiol 48:1–7
Matilla-Vázquez MA, Matilla AJ (2014) Role of H2O2 as signaling molecule in plants. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, Heidelberg, pp 361–380
McDowell RE, Amsler CD, Dickinson DA, McClintock JB, Baker BJ (2014) Reactive oxygen species and the Antarctic macroalgal wound response. J Phycol 50:71–80
Melo NKG, Bianchetti RE, Lira BS, Oliveir PMR, Zuccarelli R, Dias DLO, Demarco D, Peres LEP, Rossi M, Freschi L (2016) Nitric oxide, ethylene, and auxin cross talk mediates greening and plastid development in deetiolating tomato seedlings. Plant Physiol 170:2278–2294
Misra AN, Misra M, Singh R (2011) Nitric oxide ameliorates stress responses in plants. Plant Soil Environ 57:95–100
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trend Plant Sci 7:405–410
Mittler R (2017) ROS are good. Trend Plant Sci 22:11–19
Mittler R, Berkowitz G (2001) Hydrogen peroxide, a messenger with too many roles? Redox Rep 6:69–72
Mohanta TK, Arora PK, Mohanta N, Parida P, Bae H (2015) Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genom 16:58
Molassiotis A, Fotopoulos V (2011) Oxidative and nitrosative signaling in plants two branches in the same tree? Plant Signal Behav 6:210–214
Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Moni A, Islam MN, Uddin MJ (2018) Role of auxin and nitric oxide on growth and development of lateral root of plants: possible involvement of exogenously induced Phot1. J Adv Biotechnol Exp Ther 1:61–64
Mor A, Koh E, Weiner L, Rosenwasser S, Sibony-Benyamini H, Fluhr R (2014) Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant Physiol 165:249–261
Mostofa MG, Fujita M, Tran LSP (2015) Nitric oxide mediates hydrogen peroxide- and salicylic acid induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 77:265–277
Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113:935–944
Mulaudzi N, Ludidi O, Ruzvidzo M, Morse N, Hendricks E, Iwuoha C, Gehring C (2011) Identification of a novel Arabidopsis thaliana nitric oxide-binding molecule with guanylate cyclase activity in vitro. FEBS Lett 585:2693–2697
Mullineaux PM, Exposito-Rodriguez M, Laissue PP, Smirnoff N (2018) ROS-dependent signalling pathways in plants and algae exposed to high light: comparisons with other eukaryotes. Free Radic Biol Med 122:52–64
Mur LAJ, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJM, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plant 5:pls052
Mydlarz LD, Jacobs RS (2004) Comparison of an inducible oxidative burst in freeliving and symbiotic dinoflagellates reveals properties of the pseudopterosins. Phytochemistry 65:3231–3241
Nadarajah K, Sidek H (2010) The green MAPKS. Asian J Plant Sci 9:1–10
Nan W, Wang X, Yang L, Hu Y, Wei Y, Liang X, Mao L, Bi Y (2014) Cyclic GMP is involved in auxin signalling during Arabidopsis root growth and development. J Exp Bot 65:1571–1583
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247
Niu L, Liao W (2016) Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front Plant Sci 7:230
Noronha-Dutra AA, Epperlein MM, Woolf N (1993) Reaction of nitric oxide with hydrogen peroxide to produce potentially cytotoxic singlet oxygen as a model for nitric oxide-mediated killing. FEBS Lett 321:59–62
Oliveira JTA, Barreto ALH, Vasconcelos IM, Eloy YRG, Gondim DMF, Fernandes CF, Freire-Filho FR (2014) Role of antioxidant enzymes, hydrogen peroxide and PR-proteins in the compatible and incompatible interactions of cowpea (Vigna unguiculata) genotypes with the fungus Colletotrichum gloeosporioides. J Plant Physiol Pathol 2:3
Opdenakker K, Remans T, Vangronsveld J, Cuypers A (2012) Mitogenactivated protein (map) kinases in plant metal stress: regulation and responses in comparison to other biotic and abiotic stresses. Int J Mol Sci 13:7828–7853
Orozco-Cárdenas ML, Ryan CA (2002) Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol 130:487–493
Parani M, Rudrabhatla S, Myers R, Weirich H, Smith B, Leaman DW, Goldman SL (2004) Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol J 2:359–366
París R, Vazquez MM, Graziano M, Terrile MC, Miller ND, Spalding EP, Otegui MS, Casalongué CA (2018) Distribution of endogenous NO regulates early gravitropic response and PIN2 localization in Arabidopsis roots. Front Plant Sci 9:495
Pasqualini S, Meier S, Gehring C, Madeo L, Fornaciari M, Romano B, Ederli L (2009) Ozone and nitric oxide induce cGMP-dependent and independent transcription of defence genes in tobacco. New Phytol 181:860–870
Pasqualini S, Cresti M, Del Casino C, Faleri C, Frenguelli G, Tedeschini E, Ederli L (2015) Roles for NO and ROS signalling in pollen germination and pollen-tube elongation in Cupressus arizonica. Biol Plant 59:735–744
Peleg-Grossman S, Melamed-Book N, Cohen G, Levine A (2010) Cytoplasmic H2O2 prevents translocation of NPR1 to the nucleus and inhibits the induction of PR genes in Arabidopsis. Plant Signal Behav 5:1401–1406
Perl-Treves R, Perl A (2002) Oxidative stress: an introduction. In: Inze D, Montago M (eds) Oxidative stress in plants. Taylor and Francis, New York, NY, pp 1–32
Pokora W, Aksmann A, Baścik-Remisiewicz A, Dettlaff-Pokora A, Rykaczewski M, Gappa M, Tukaj Z (2017) Changes in nitric oxide/hydrogen peroxide content and cell cycle progression: study with synchronized cultures of green alga Chlamydomonas reinhardtii. J Plant Physiol 208:84–93
Polverari A, Molesini B, Pezzotti M, Buonaurio R, Marte M, Delledonne M (2003) Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Mol Plant Mic Int 16:1094–1105
Potin P (2008) Oxidative burst and related responses in biotic interactions of algae. In: Amsler CD (ed) Algal chemical ecology. Springer, Heidelberg
Prado AM, Marshall Porterfield D, Feijó JA (2004) Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131:2707–2714
Prasad A, Kumar A, Matsuoka R, Takahashi A, Fujii R, Sugiura Y, Kikuchi H, Aoyagi S, Aikawa T, Kondo T, Yuasa M, Pospíšil P, Kasai S (2017) Real-time monitoring of superoxide anion radical generation in response to wounding: electrochemical study. Peer J 5:e3050
Prasad A, Sedlářová M, Pospíšil P (2018) Singlet oxygen imaging using fuorescent probe singlet oxygen sensor green in photosynthetic organisms. Sci Rep 8:13685
Prats E, Mur LAJ, Sanderson R, Carver TLW (2005) Nitric oxide contributes both to papilla-based resistance and the hypersensitive response in barley attacked by Blumeria graminis f. sp. hordei. Mol Plant Pathol 6:65–78
Procházková D, Haisel D, Pavlíková D (2014) Nitric oxide biosynthesis in plants – the short overview. Plant Soil Environ 60:129–134
Procházková D, Wilhelmová N, Pavlík M (2015) Reactive nitrogen species and nitric oxide. In: Khan MN, Mobin M, Mohammad F, Corpas FJ (eds) Nitric oxide action in abiotic stress responses in plants. Springer, Heidelberg, pp 3–19
Qiao M, Sun J, Liu N, Sun T, Liu G, Han S, Hou C, Wang D (2015) Changes of nitric oxide and its relationship with H2O2 and Ca2+ in defense interactions between wheat and Puccinia triticina. PLoS One 10:e0132265
Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol 50:2–18
Radi R (2013) Peroxynitrite, a stealthy biological oxidant. J Biol Chem 288:26464–26472
Rodrigues O, Reshetnyak G, Grondin A, SaijoY LN, Maurel C, Verdoucq L (2017) Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc Natl Acad Sci U S A 114:9200–9205
Rodriguez MCS, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649
Romero-Puertas MC, Laxa M, Matte A, Zaninotto F, Finkemeier I, Jones AM, Perazzolli M, Vandelle E, Dietz KJ, Delledonne M (2007) S-nitrosylation of peroxiredoxin II E promotes peroxynitrite mediated tyrosine nitration. Plant Cell 19:4120–4130
Rőszer T (2014) Biosynthesis of nitric oxide in plants. In: Khan MN, Mobin M, Mohammad F, Corpas JF (eds) Nitric oxide in plants: metabolism and role in stress physiology. Springer, Heidelberg, pp 17–33
Rustérucci C, Espunya MC, Díaz M, Chabannes M, Martínez MC (2007) S-nitrosoglutathione reductase affords protection against pathogens in Arabidopsis, both locally and systemically. Plant Physiol 143:1282–1292
Sahay S, Gupta M (2017) An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 67:39–52
Sakamoto M, Munemura I, Tomita R, Kobayashi K (2008) Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. Plant J 56:13–27
Salachna P, Zawadzińska A (2018) Effect of nitric oxide on growth, flowering and bulb yield of Eucomis autumnalis. Acta Hortic 1201:635–640
Santolini J, André F, Jeandroz S, Wendehenne D (2017) Nitric oxide synthase in plants: where do we stand? Nitric Oxide 63:30–38
Saxena I, Srikanth S, Chen Z (2016) Cross talk between H2O2 and interacting signal molecules under plant stress response. Front Plant Sci 7:570
Senthil Kumar R, Shen CH, Wu PY, Suresh Kumar S, Sang Hua M, Yeh KW (2016) Nitric oxide participates in plant flowering repression by ascorbate. Sci Rep 6:35246
Sheokand S, Kumari A (2015) Nitric oxide and abiotic stress-induced oxidative stress. In: Khan M, Mobin M, Mohammad F, Corpas F (eds) Nitric oxide action in abiotic stress responses in plants. Springer, Heidelberg
Shetty NP, Jørgensen HJL, Jensen JD, Collinge DB, Shetty HS (2008) Roles of reactive oxygen species in interactions between plants and pathogens. Eur J Plant Pathol 121:267–280
Singh N, Bhatla SC (2018) Nitric oxide regulates lateral root formation through modulation of ACC oxidase activity in sunflower seedlings under salt stress. Plant Signal Behav 25:1–7
Sinha AK, Jagg M, Raghuram B, Tuteja N (2011) Mitogen-activated protein kinase signalling in plants under abiotic stress. Plant Signal Behav 6:196–203
Ślesak I, Libik M, Karpinska B, Karpinski S, Miszalski Z (2007) The role of hydrogen peroxide in regulation of plant metabolism and cellular signaling in response to environmental stresses. Acta Biochim Pol 54:39–50
Srivastava AK, Penna S, Van Nguyen D, Tran LSP (2014) Multifaceted roles of aquaporins as molecular conduits in plant responses to abiotic stresses. Crit Rev Biotechnol 28:1–10
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106:675–683
Štolfa CI, Špoljarić MD, Žuna PT, Lončarić Z (2016) Glutathione and related enzymes in response to abiotic stress. In: Gupta DK, Palma JM, Corpas FJ (eds) Redox state as a central regulator of plant cell stress responses. Springer, Heidelberg, pp 183–211
Štolfa ČI, Žuna PT, Špoljarić MD (2018) Abiotic stress response in plants: the relevance of tocopherols. In: Gupta DK, Palma JM, Corpas FJ (eds) Antioxidants and antioxidant enzymes in higher plants. Springer, Cham, pp 233–251
Sun H, Feng F, Liu J, Zhao Q (2018) Nitric oxide affects rice root growth by regulating auxin transport under nitrate supply. Front Plant Sci 9:659
Szopińska D (2014) Effects of hydrogen peroxide treatment on the germination, vigour and health of Zinnia elegans seeds. Folia Hort 26:19–29
Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X (2008) Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 321:952–956
Tanou G, Job C, Rajjou L, Arc E, Belghazi M, Diamantidis G, Molassiotis A, Job D (2009) Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J 60:795–804
Tewari RK, Prommer J, Watanabe M (2013) Endogenous nitric oxide generation in protoplast chloroplasts. Plant Cell Rep 32:31–44
Thomas F, Cosse A, Goulitquer S, Raimund S, Morin P, Valero M, Leblanc C, Potin P (2011) Waterborne signaling primes the expression of elicitor-induced genes and buffers the oxidative responses in the brown alga Laminaria digitata. PLoS One 6:e21475
Torres MA, Jones JD, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37:1130–1134
Vargas-Hernández M, Torres-Pacheco I, Gautier F, Álvarez-Mayorga B, Cruz-Hernández A, García-Mier L, Jiménez-García SN, Ocampo-Velázquez RV, Feregrino-Perez AA, Guevara-Gonzalez RG (2017) Influence of hydrogen peroxide foliar applications on in vitro antimicrobial activity in Capsicum chinense Jacq. Plant Biosys 151:269–275
Varnova E, Inze D, van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Vianello A, Macri FJ (1991) Generation of superoxide anion and hydrogen peroxide at the surface of plant cells. Bioenerg Biomemb 23:409–423
Voss I, Sunil B, Scheibe R, Raghavendra AS (2013) Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol 5:713–722
Vranová E, Inzé D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Wang YQ, Feechan A, Yun BW, Shafiei R, Hofmann A, Taylor P, Xue P, Yang FQ, Xie ZS, Pallas JA, Chu CC, Loake GJ (2009) S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J Biol Chem 284:2131–2137
Wang Y, Hao X, Lu Q, Wang L, Qian W, Li N, Ding C, Wang X, Yang Y (2018) Transcriptional analysis and histochemistry reveal that hypersensitive cell death and H2O2 have crucial roles in the resistance of tea plant (Camellia sinensis (L.) O. Kuntze) to anthracnose. Hortic Res 5:18
Weisslocker-Schaetzel M, André F, Touazi N, Foresi N, Lembrouk M, Dorlet P, Frelet-Barrand A, Lamattina L, Santolini J (2017) The NOS-like protein from the microalgae Ostreococcus tauri is a genuine and ultrafast NO-producing enzyme. Plant Sci 265:100–111
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25:434–456
Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322:681–692
Wojtyla L, Lechowska K, Kubala S, Garnczarsk M (2016) Different modes of hydrogen peroxide action during seed germination. Front Plant Sci 7:66
Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 8:639–647
Wünsche H, Baldwin IT, Wu J (2011) S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata. J Exp Bot 62:4605–4616
Yang H, Kim HJ, Chen H, Lu Y, Lu X, Wang C, Zhou B (2018) Reactive oxygen species and nitric oxide induce senescence of rudimentary leaves and the expression profiles of the related genes in Litchi chinensis. Hort Res 5:23
Yu M, Lamattina L, Spoe SH, Loake GJ (2014) Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol 202:1142–1156
Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, Pallas JA, Loake GJ (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478:264–268
Zhang H (2016) Hydrogen sulfide in plant biology. In: Lamattina L, García-Mata C (eds) Gasotransmitters in plants. Signaling and communication in plants. Springer, Cham
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Zhao FY, Hu F, Zhang SY, Wang K, Zhang CR, Liu T (2013) MAPKs regulate root growth by influencing auxin signaling and cell cycle-related gene expression in cadmium-stressed rice. Environ Sci Pollut Res 28:5449–5460
Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF (2000) NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant Microbe 13:191–202
Zurbriggen MD, Carrillo N, Hajirezaei MR (2010) ROS signaling in the hypersensitive response: when, where and what for? Plant Signal Behav 5:393–396
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Štolfa Čamagajevac, I., Špoljarić Maronić, D., Žuna Pfeiffer, T., Bek, N., Lončarić, Z. (2019). Nitric Oxide and Hydrogen Peroxide in Plant Response to Biotic Stress. In: Gupta, D., Palma, J., Corpas, F. (eds) Nitric Oxide and Hydrogen Peroxide Signaling in Higher Plants. Springer, Cham. https://doi.org/10.1007/978-3-030-11129-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-11129-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11128-1
Online ISBN: 978-3-030-11129-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)