Abstract
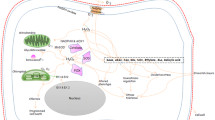
Hydrogen peroxide (H2O2), an active oxygen species, is widely generated in many biological systems and mediates various physiological and biochemical processes in plants. In the present study, we present a signaling network involving H2O2, nitric oxide (NO), calcium (Ca2+), cyclic guanosine monophosphate (cGMP), and the mitogen-activated protein kinase (MAPK) cascade during adventitious rooting in mung bean seedlings. Both exogenous H2O2 and the NO donor sodium nitroprussiate were capable of promoting the formation and development of adventitious roots. H2O2 and NO signaling pathways were elicited in parallel in auxin-induced adventitious rooting. Cytosolic Ca2+ was required for adventitious rooting, and Ca2+ served as a downstream component of H2O2, as well as cGMP or MAPK, signaling cascades. cGMP and MAPK cascades function downstream of H2O2 signaling and depend on auxin responses in adventitious root signaling processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many years, H2O2, a form of reactive oxygen, was mainly viewed as a toxic cellular metabolite. However, it is now clear that it functions as a signaling molecule that mediates responses to various stimuli in plant cells (Neill et al. 2002). Hydrogen peroxide is continually generated from various sources during normal metabolism in plant cells. It mediates various physiological and biochemical processes, including systemic acquired resistance and the hypersensitive response (Melillo et al. 2006), senescence (Hung et al. 2006), programmed cell death (Levine et al. 1994), stomatal closure (Pei et al. 2000; Bright et al. 2006), root gravitropism (Joo et al. 2001), lateral root development (Su et al. 2006), cell wall development (Potikha et al. 1999), and pollen–stigma interactions and development (McInnis et al. 2006). Recently we demonstrated that H2O2 is a messenger involved in auxin-induced adventitious rooting in cucumber (Li et al. 2007) and mung bean (Li et al. 2009).
Adventitious roots are post-embryonic roots that arise from the stem and leaves, as well as from non-pericyclic tissues in existing roots. Adventitious root formation is one of the most important means of vegetative plant propagation. The formation of adventitious roots involves a process of redifferentiation in which predetermined cells switch from their morphogenetic path to act as mother cells for the root primordia (Aeschbacher et al. 1994). Many environmental and endogenous factors regulate adventitious rooting. Some endogenous factors have been identified, such as calcium (Ca2+) (Bellamine et al. 1998), sugar (Takahashi et al. 2003), phenolics (Rout 2006), ethylene (Liu et al. 1990), polyamines (Nag et al. 2001), nitric oxide (NO) (Pagnussat et al. 2003, 2004), carbon monoxide (Xu et al. 2006), cyclic guanosine monophosphate (cGMP), and mitogen-activated protein kinase (MAPKs) (Pagnussat et al. 2003, 2004), phytohormones (De Klerk et al. 1999), and peroxidase (Syros et al. 2004). Some of these molecules may function in signaling and mediate auxin-induced adventitious rooting and auxin-response gene expression. To date, the intricate signaling network that participates in adventitious root formation remains poorly understood.
In recent years, significant progress in the elucidation of auxin and adventitious root response signaling pathways has been made, e.g., IAA induces adventitious rooting via a pathway that involves NO. cGMP and MAPK cascades are downstream signals in the NO signaling pathway during adventitious rooting (Pagnussat et al. 2003, 2004). Although a variety of components participating in auxin transport and signal transduction have been identified, the molecular and biochemical mechanisms and intermediates underlying the signal transduction cascades in auxin-promoted root formation remain poorly understood.
In a previous study, we demonstrated that H2O2 might function as a signaling molecule in auxin-induced adventitious root formation in cucumber and mung bean seedlings (Li et al. 2007, 2009). Further investigations are needed to conclusively confirm the presence of downstream H2O2 signaling in adventitious rooting. The main aim of the present study was to elucidate the interactions between H2O2 and other signaling molecules such as NO, Ca2+, cGMP, and the MAPK cascade in the formation and development of adventitious roots in mung bean.
Materials and Methods
Plant material.
Seeds of mung bean, Vigna radiata (L.) R. Wilczek, were washed in distilled water and immersed in 70% ethanol for 1 min. After five washes in sterile distilled water, seeds were germinated in Petri dishes on perlite soaked in distilled water and maintained at 25 ± 1°C for 2 d in the dark, followed by 4 d in a 14-h photoperiod (PAR of 100 µmol m−2 s−1) provided by white fluorescent lamps. Seedlings with their primary roots removed were used as explants and were maintained under the same temperature and photoperiod conditions described above for another 5 d in the presence of different test solutions.
Explant treatments.
After the primary roots were removed, seedling explants, 10 per beaker, were inserted into the holes of filter paper in 50-ml beakers containing 20 ml of distilled water (control) or 20 ml of test solution. The test solutions utilized are described in the caption of Fig. 1. The beakers were kept in the same conditions as during germination. The chemicals used in the treatments were AR or biochemical standard reagents, i.e., indole-3-butyric acid (IBA), hydrogen peroxide (H2O2) (Chinese supplier, Beijing), sodium nitroprusside (SNP), diphenylene iodonium (DPI), N ω-nitro-l-arginine (l-NNA), ruthenium red, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), 6-anilino-5,8-quinolinedione (LY83583), 2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one (PD98059), ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), and 8-bromoguanosine 3,5-cyclic monophosphate (8-Br-cGMP) (Sigma-Aldrich Co., St. Louis, MO).
Effects of different treatments on the formation and growth of adventitious roots in 5-d-old mung bean seedling explants. Explants were incubated in the medium for 5 d. H2O2 was used at 10 mM and IBA 10 μM. Bar = 1 cm. 1, H2O; 2, 10 mM H2O2; 3, 10 μM IBA; 4, 300 μM SNP; 5, 200 μM cPTIO; 6, 200 μM cPTIO + H2O2; 7, 200 μM cPTIO + 10 μM IBA; 8, 10 mM l-NNA; 9, 10 mM l-NNA + 10 mM H2O2; 10, 10 mM l-NNA + IBA; 11, 20 μM LY83583; 12, 20 μM LY83583 + H2O2; 13, 20 μM LY83583 + IBA; 14, 20 μM LY83583 + 5 μM 8-Br-cGMP; 15, 20 μM LY83583 + H2O2 + 5 μM 8-Br-cGMP; 16, 20 μM LY83583 + IBA + 5 μM 8-Br-cGMP; 17, 20 μM PD98059; 18, 20 μM PD98059 + H2O2; 19, 20 μM PD98059 + IBA; 20, 2 mM EGTA; 21, 50 μM CaCl2; 22, 1 mM EGTA + 50 μM CaCl2; 23, 1 mM EGTA + H2O2; 24, 1 mM EGTA + IBA; 25, 1 mM EGTA + H2O2 + 50 μM CaCl2; 26, 1 mM EGTA + IBA + 50 μM CaCl2; 27, 10 μM ruthenium red; 28, 20 μM PD98059 + 50 μM CaCl2; 29, 20 μM LY83583 + 50 μM CaCl2; 30, 20 μM LY83583 + 5 μM 8-Br-cGMP + 50 μM CaCl2; 31, 5 μM DPI; 32, 5 μM DPI + 50 μM CaCl2; 33, 5 μM DPI + H2O2.
Data collection and statistical analyses.
The number and fresh weight of adventitious roots were quantified after a 5-d treatment. Data presented are the means ± SE of at least three independent experiments, with 10 explants per treatment. Data were analyzed using ANOVA, and comparisons between the mean values were evaluated by the least significant difference test at P < 0.05 (indicated with the letters above the bars in the figures). Statistical analyses were performed using SPSS/PC Ver. 14.0 software (SPSS Inc., Chicago, IL).
Results
Adventitious roots grow through the epidermis of the hypocotyls within 3 d after excision of the primary roots and incubation with water (control).
H2O2 involvement in adventitious rooting in the NO-independent signaling pathway.
The results obtained in preliminary experiments show that exogenous H2O2 caused a significant and dose-dependent increase in adventitious root number and fresh weight, similar to the promoting effect observed for treatment with IBA (Fig. 1: 1–3) (Li et al. 2009). When compared with the control, treatment with 1–100 mM H2O2 for 8 h significantly enhanced the number of adventitious roots per explant, with optimal concentrations of H2O2 ranging between 50 and 100 mM (data not shown). We confirmed that treatment with 300 μM NO donor SNP significantly increased the number of adventitious roots per explant (Fig. 1: 4, data not shown), as reported by She and Huang (2004).
Treatment of seedlings with 10 μM DPI, a specific inhibitor of H2O2 production (Desikan et al. 2004), completely inhibited adventitious rooting. When DPI was used in combination with SNP, SNP could not mitigate the inhibitory effect of DPI on rooting (data not shown). However, treatment with cPTIO (a specific NO scavenger) or l-NNA (a NO synthase inhibitor) (Hu et al. 2005; Bright et al. 2006) did not inhibit adventitious rooting (Fig. 1: 5, 8; Fig. 2). When either cPTIO or l-NNA was used in combination with H2O2 or IBA, cPTIO or l-NNA had no inhibitory effect on H2O2- or IBA-induced increases in the number (and fresh weight; data not shown) of adventitious roots (Fig. 1: 6, 7, 9, 10; Fig. 2). These results indicate that H2O2 is involved in the NO-independent signaling pathway or acts as a downstream component in the NO signaling pathway suggested by Bright et al. (2006), during adventitious rooting.
cGMP functions as a downstream component of H2O2 signaling involved in adventitious rooting.
Treatment with 3 μM LY83583, the guanylate cyclase inhibitor (Alessi et al. 1995), neither inhibited the formation and growth of adventitious roots nor suppressed the promoting effects of H2O2 or IBA on adventitious rooting (data not shown). However, treatment with 5 μM LY83583 markedly inhibited the formation and growth of adventitious roots (Fig. 1: 11–16; Fig. 3). When 5 μM LY83583 was used together with H2O2, IBA, or 8-Br-cGMP, the cell-permeable cGMP derivative (Hu et al. 2005), the inhibitory effects of LY83583 on rooting were wholly or partially reversed. The results suggested that cGMP is involved in adventitious rooting, and it is worthy of study that H2O2 and IBA may induce the increase of cGMP in cells.
MAPK is a downstream mediator of H2O2- and IBA-induced adventitious root formation.
PD98059 is a MAPK kinase (MAPKK) inhibitor (Alessi et al. 1995). PD98059 (50 μM) treatment strongly inhibited the formation and growth of adventitious roots in cucumber via inhibition of the MAPKK cascade (Pagnussat et al. 2004). In our experiments, treatment with 10 μM PD98059 inhibited neither adventitious root formation nor H2O2- or IBA-induced adventitious rooting (data not shown). However, treatment with 20 μM PD98059 strongly inhibited adventitious rooting (Fig. 1: 17; Fig. 4). H2O2- or IBA-induced adventitious rooting was also suppressed by the addition of 20 μM PD98059 (Fig. 1: 18, 19). These results indicate that the MAPK cascade mediated adventitious rooting as a downstream component of H2O2 and IBA signaling.
Cytosolic Ca2+ functions as a downstream component in H2O2 signaling involved in adventitious rooting.
Ruthenium red is an inhibitor of the endomembrane Ca2+ permeable channel (Toyota et al. 2008), and EGTA is a chelator of extracellular Ca2+ (Pei et al. 2000). Treatment with 10 μM ruthenium red completely inhibited adventitious rooting (Fig. 1: 27). When 10 μM ruthenium red was used together with either 10 mM H2O2 or 10 μM IBA, the inhibitory effect of ruthenium red on adventitious rooting remained unchanged (data not shown). When 10 μM ruthenium red was used together with CaCl2, the inhibitory effect of ruthenium red on adventitious rooting was not abrogated by CaCl2 (data not shown). Furthermore, 1 mM EGTA treatment had no effect on the formation of adventitious roots but markedly inhibited their growth (Figs. 4, 5). Treatment with 2 mM EGTA significantly inhibited the formation and growth of adventitious roots (Fig. 1: 20). In addition, treatment with 50 μM CaCl2 had no effect on either adventitious rooting or H2O2- or IBA-induced adventitious rooting (Fig. 1: 21–26; Figs. 4, 5). These results indicate that the formation of adventitious roots depended mainly on the cytosolic Ca2+ concentration, rather than the extracellular Ca2+ concentration. Furthermore, when DPI was used in combination with CaCl2, CaCl2 partially reversed the inhibitory effect of DPI on adventitious rooting (Fig. 1: 31, 32; Fig. 6). H2O2 can partially reverse the inhibitory effect of DPI on adventitious rooting when DPI was used in combination with H2O2 (Fig. 1: 31, 33; Fig. 6). As DPI suppresses adventitious rooting by inhibiting the H2O2 production, and Ca2+ can partially reverse this inhibition, we concluded that Ca2+ is a downstream component of H2O2 signaling.
Ca2+ also mediates the cGMP or MAPK signaling pathways during adventitious rooting.
Neither treatment with 1 mM EGTA nor CaCl2 alone (Figs. 4, 5) had an effect on adventitious rooting. Similarly, treatment with EGTA plus 8-Br-cGMP and EGTA plus CaCl2 and 8-Br-cGMP failed to affect adventitious rooting. In contrast, treatment with LY83583 alone significantly inhibited adventitious rooting (Fig. 1: 11; Fig. 7), and when LY83583 was used in combination with either CaCl2 or CaCl2 plus 8-Br-cGMP, the inhibitory effects of LY83583 were partially reversed (Fig. 1: 29, 30; Fig. 7). Furthermore, when PD98059 was used in combination with CaCl2, the inhibitory effect of PD98059 on adventitious rooting was partially reversed (Fig. 1: 28; Fig. 4). These results indicate that Ca2+ is also involved in the cGMP or MAPK signaling pathways during adventitious rooting.
Discussion
Based on the above results, we conclude that (1) both H2O2 and NO act as essential components of the signaling network that induces adventitious root formation after excision of primary roots; (2) H2O2 and NO are involved in two parallel downstream signaling pathways in auxin-induced adventitious root formation; and (3) H2O2 is also a downstream component of NO signaling during adventitious rooting. Similar conclusions were reported in a number of studies on the roles of NO and H2O2 in plant development and defense responses. For instance, NO serves to modulate H2O2 production and to downregulate its effects on the expression of defense-related genes (Orozco-Cardenas and Ryan 2002). H2O2 and NO generation occur in parallel or in short succession to each other and function synergistically and independently (Clarke et al. 2000). H2O2 and NO are essential components of the complex signaling network responsible for stomatal closure (Desikan et al. 2004).
cGMP is involved in plant development processes and responses to both biotic and abiotic stresses, such as stomatal closure (Neill et al. 2002), adventitious root development (Pagnussat et al. 2003), and Arabidopsis cell death (Clarke et al. 2000). cGMP regulates its targets, the cyclic nucleotide-gated channels involved in Na+ and K+ transport during cation uptake in roots, and influences salt tolerance in Arabidopsis (Guo et al. 2008). cGMP is involved in Ca2+ accumulation and ion flux that can produce a localized signal capable of regulating the pollen tip growth (Frietsch et al. 2007). In this study, LY83583 strongly inhibited the formation and growth of adventitious roots, and this inhibitory effect can be partially reversed by H2O2 or 8-Br-cGMP, suggesting cGMP functions as a downstream signal involved in H2O2-promoting adventitious rooting. Furthermore, the inhibitory effects of LY83583 were partially reversed by CaCl2, suggesting that Ca2+ is also involved in the cGMP signaling pathway during adventitious rooting.
Cross-talk between H2O2 and Ca2+ occurs in plant cells. Ca2+, or Ca2+ fluxes, induce the generation of H2O2, and H2O2 activity requires Ca2+ (Chen and Li 2001; Agarwal et al. 2005). H2O2 activates Ca2+ channels in guard cells, and the increase in cytosolic Ca2+ concentration in response to H2O2 has been observed and is necessary for stomatal closure (Pei et al. 2000). H2O2 treatment induces an increase in cytosolic Ca2+ concentration (Rentel and Knight 2004). Cross-talk between Ca2+–CaM and H2O2 plays a pivotal role in ABA signaling (Hu et al. 2007). Our study demonstrated that Ca2+ is involved in adventitious rooting. Chelation of extracellular Ca2+ by EGTA was shown to have no clear inhibitory effect on adventitious rooting. However, ruthenium red completely inhibited adventitious rooting, suggesting that cytosolic Ca2+ fluxes are required for adventitious rooting and that Ca2+ serves as a downstream component in the H2O2 signaling pathway.
H2O2 is known to activate MAPK cascades in various tissues (Kovtun et al. 2000; Desikan et al. 2004), and H2O2 and NO may converge on MAPK signaling pathways involved in regulating stomatal closure (Desikan et al. 2004). A MAPK signaling cascade is activated during the adventitious rooting process induced by IAA (Morris 2001; Pagnussat et al. 2004). In the present study, PD98059 treatment strongly inhibited the formation of adventitious roots and completely suppressed the adventitious root-promoting effects of H2O2 or IBA, indicating that the MAPK cascade functions as a downstream component in signaling of H2O2 promotion of adventitious rooting.
Altogether, adventitious rooting is regulated by a complex set of cellular messengers, among which MAPK and cGMP are activated by upstream components that involve H2O2, NO, and Ca2+. H2O2 and NO signaling represent two parallel pathways in this process. Activation of both pathways seems to be required for the development of adventitious roots since if one pathway is blocked, no adventitious roots develop. Ca2+ signaling plays a key role in adventitious root formation and functions as a downstream component in both the H2O2/NO and MAPK/cGMP pathways, although the network responsible for root development remains to be elucidated.
References
Aeschbacher R. A.; Schiefelbein J. W.; Benfey P. N. The genetic and molecular basis of root development. Ann. Rev. Plant Physiol. Plant Mol. Biol. 45: 25–45; 1994.
Agarwal S.; Sairam R. K.; Srivastava G. C.; Tyagi A.; Meena R. C. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 169: 559–570; 2005.
Alessi D. R.; Cuenda A.; Cohen P.; Dudley D. T.; Saltiel A. R. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270: 27489–27494; 1995.
Bellamine J.; Penel C.; Greppin H.; Gaspar T. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regul. 26: 191–194; 1998.
Bright J.; Desikan R.; Hancock J. T.; Weir I. S.; Neill S. J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 45: 113–122; 2006.
Chen W. P.; Li P. H. Chilling induced Ca2+ over load enhances production of active oxygen species in maize (Zea mays L.) cultured cells: the effect of abscisic acid treatment. Plant Cell Environ. 24: 791–800; 2001.
Clarke A.; Desikan R.; Hurst R. D.; Hancock J. T.; Neill S. J. NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 24: 667–677; 2000.
De Klerk G. J.; Krieken W. V. D.; De Jong J. C. The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev. Biol-Plant 35: 189–199; 1999.
Desikan R.; Cheung M. K.; Bright J.; Henson D.; Hancock J. T.; Neill S. J. ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J. Exp. Bot. 55: 205–212; 2004.
Frietsch S.; Wang Y. F.; Sladek C.; Poulsen L. R.; Romanowsky S. M.; Schroeder J. I.; Harper J. F. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA 104: 14531–14536; 2007.
Guo K. M.; Babourina O.; Christopher D. A.; Borsics T.; Rengel Z. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol. Plant. 134: 499–507; 2008.
Hu X.; Neill S. J.; Tang Z.; Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 137: 663–670; 2005.
Hu X. L.; Jiang M. Y.; Zhang J. H.; Zhang A. Y.; Lin F.; Tan M. P. Calcium–calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytol. 173: 27–38; 2007.
Hung K. T.; Hsu Y. T.; Kao C. H. Hydrogen peroxide is involved in methyl jasmonate-induced senescence of rice leaves. Physiol. Plant. 127: 293–303; 2006.
Joo J. H.; Bae Y. S.; Lee J. S. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 126: 1055–1060; 2001.
Kovtun Y.; Chiu W. L.; Tena G.; Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl Acad. Sci. USA 97: 2940–2945; 2000.
Levine A.; Tenhaken R.; Dixon R. A.; Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive response. Cell 79: 583–593; 1994.
Li S. W.; Xue L. G.; Xu S. J.; Feng H. Y.; An L. Z. Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul. 52: 173–180; 2007.
Li S. W.; Xue L. G.; Xu S. J.; Feng H. Y.; An L. Z. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ. Exp. Bot. 65: 63–71; 2009.
Liu J. H.; Mukherjee I.; Reid D. M. Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings. III. The role of ethylene. Physiol. Plant. 78: 268–276; 1990.
McInnis S. M.; Desikan R.; Hancock J. T.; Hiscock S. J. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytol. 172: 221–228; 2006.
Melillo M. T.; Leonetti P.; Bongiovanni M.; Astagnone S. P.; Bleve Z. T. Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato–root-knot nematode interactions. New Phytol. 170: 501–512; 2006.
Morris P. C. MAP kinase signal transduction pathway in plants. New Phytol. 151: 67–89; 2001.
Nag S.; Saha K.; Choudhuri M. A. Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J. Plant Growth Regul. 20: 182–194; 2001.
Neill S. J.; Desikan R.; Hancock J. T. Hydrogen peroxide signaling. Curr. Opin. Plant Biol. 5: 388–395; 2002.
Orozco-Cardenas M. L.; Ryan C. A. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 130: 487–493; 2002.
Pagnussat G. C.; Lanteri M. L.; Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 132: 1241–1248; 2003.
Pagnussat G. C.; Lanteri M. L.; Lombardo M. C. Nitric oxide mediates the indole acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 135: 279–286; 2004.
Pei Z. M.; Murata Y.; Benning G.; Thomine S.; Klusener B.; Allen G. T.; Grill E.; Schroeder J. I. Calcium channels activated by hydrogen peroxide mediate abscisic signaling in guard cells. Nature 406: 731–734; 2000.
Potikha T. S.; Collins C. C.; Johnson D. I.; Delmer D. P.; Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 119: 849–858; 1999.
Rentel M. C.; Knight M. R. Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 135: 1471–1479; 2004.
Rout G. R. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regul. 48: 111–117; 2006.
She X. P.; Huang A. X. Change of nitric oxide and NADPH-diaphorase during the generation and the development of adventitious roots in mung bean hypocotyls cuttings. Act Bot Sin 46: 1049–1055; 2004.
Su G. X.; Zhang W. H.; Liu Y. L. Involvement of hydrogen peroxide generated by polyamine oxidative degradation in the development of lateral roots in soybean. J. Integr. Plant Biol. 48: 426–432; 2006.
Syros T.; Yupsanis T.; Zafiriadis H.; Economou A. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J. Plant Physiol. 161: 69–77; 2004.
Takahashi F.; Sato-Nara K.; Kobayashi K.; Suzuki M.; Suzuki H. Sugar-induced adventitious roots in Arabidopsis seedlings. J. Plant Res. 116: 83–91; 2003.
Toyota M.; Furuichi T.; Tatsumi H.; Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 146: 505–514; 2008.
Xu J.; Xuan W.; Huang B. K.; Zhou Y. H.; Ling T. F.; Xu S.; Sheng W. B. Carbon monoxide-induced ARF of hypocotyl cutting from mung bean seedling. China Sci. Bull. 51: 668–674; 2006.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (30960063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: D. T. Tomes
Rights and permissions
About this article
Cite this article
Li, SW., Xue, L. The interaction between H2O2 and NO, Ca2+, cGMP, and MAPKs during adventitious rooting in mung bean seedlings. In Vitro Cell.Dev.Biol.-Plant 46, 142–148 (2010). https://doi.org/10.1007/s11627-009-9275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-009-9275-x